The Role of Vegetation in Elevational Diversity Patterns of Tenebrionid Beetles in Central Italy
Abstract
1. Introduction
- (1)
- Prediction 1. If tenebrionid richness varies along the elevational gradient in response to a filtering process [39,40], declining patterns in species richness should be observed in both forest and secondary open vegetations. To test this prediction, richness was calculated separately for natural and secondary vegetations, and the respective elevational patterns compared.
- (2)
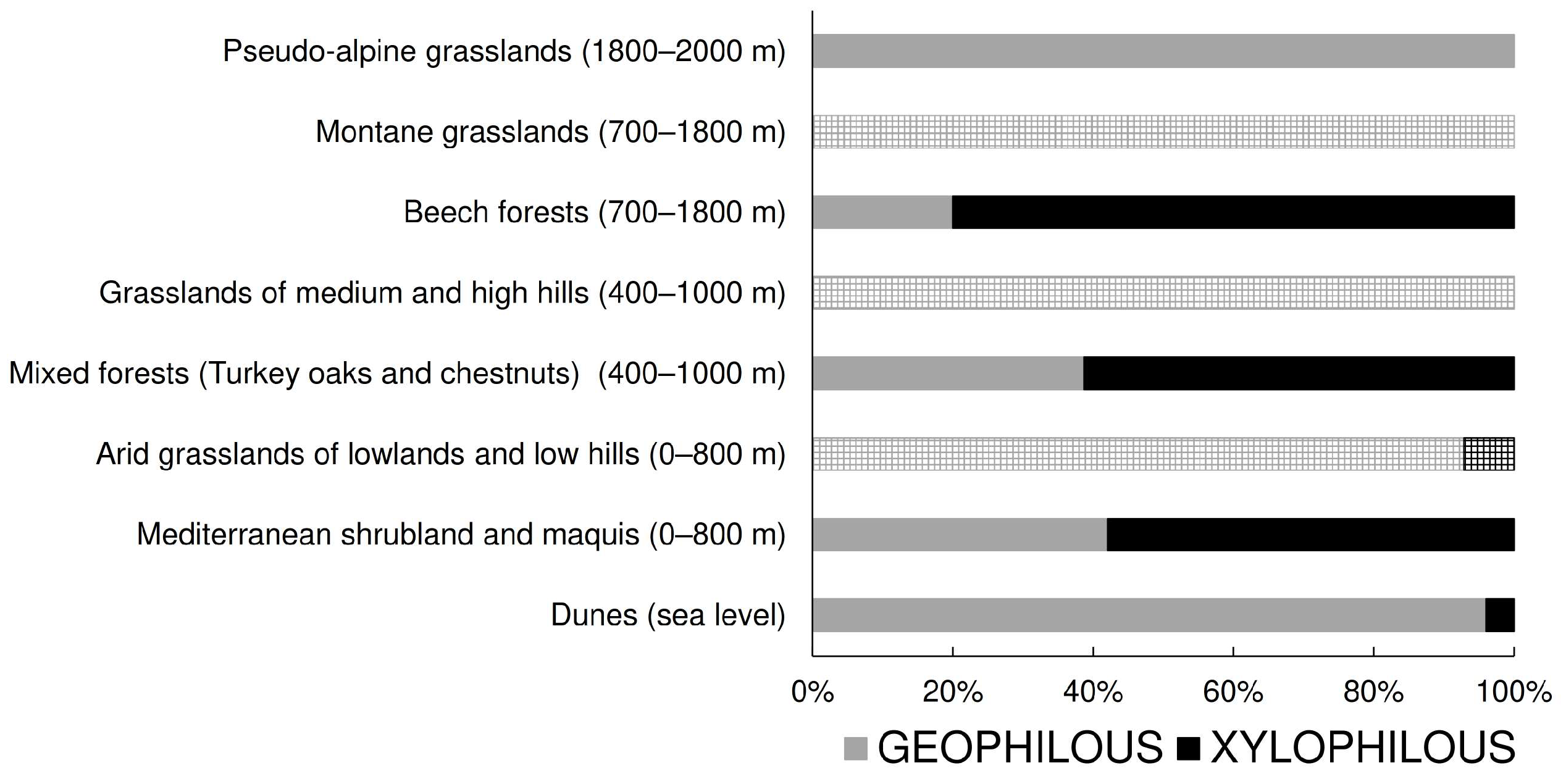
- Prediction 2. If tenebrionids of secondary open vegetations are not an impoverished version of the communities inhabiting former forests, but are composed of species associated with natural open vegetations, we expect a low similarity in species composition between tenebrionid of secondary and natural vegetations even within the same elevational belt, but a high similarity between secondary and open natural vegetations. Additionally, we expect a high relative abundance of geophilous species even at elevations in which xylophilous species should be more frequent. To test this prediction, similarities in species composition among vegetation types and levels of association between ecological groups (geophilous vs. xylophilous) and vegetation types were investigated.
- (3)
- Prediction 3. Previous research showed that biogeographical composition of tenebrionid beetles in Latium changed with elevation, with species mainly distributed in the Mediterranean basin declining with elevation, being less tolerant to decreasing temperatures [38]. As a result of this filtering process, it can be expected that their proportion declines with elevation in both secondary and natural vegetations. Variation in the incidence of Mediterranean species in different vegetation types was analyzed for testing this prediction.
- (4)
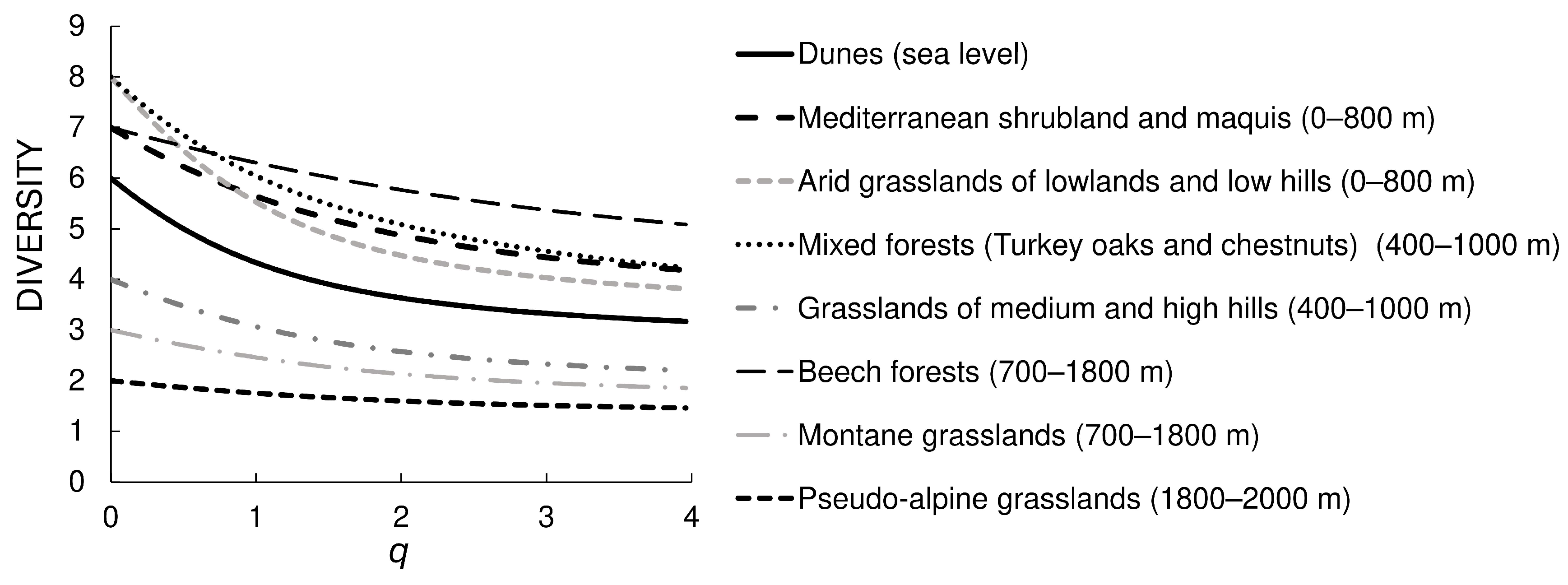
- Prediction 4. Not only tenebrionid species richness, but also biogeographical diversity declines with increasing elevation [38]. Under the filtering hypothesis, this decline in biogeographical diversity should be observed in both natural and secondary vegetations. To test this prediction, diversity profiles based on the biogeographical composition of tenebrionid assemblages were constructed for the different vegetation types.
- (5)
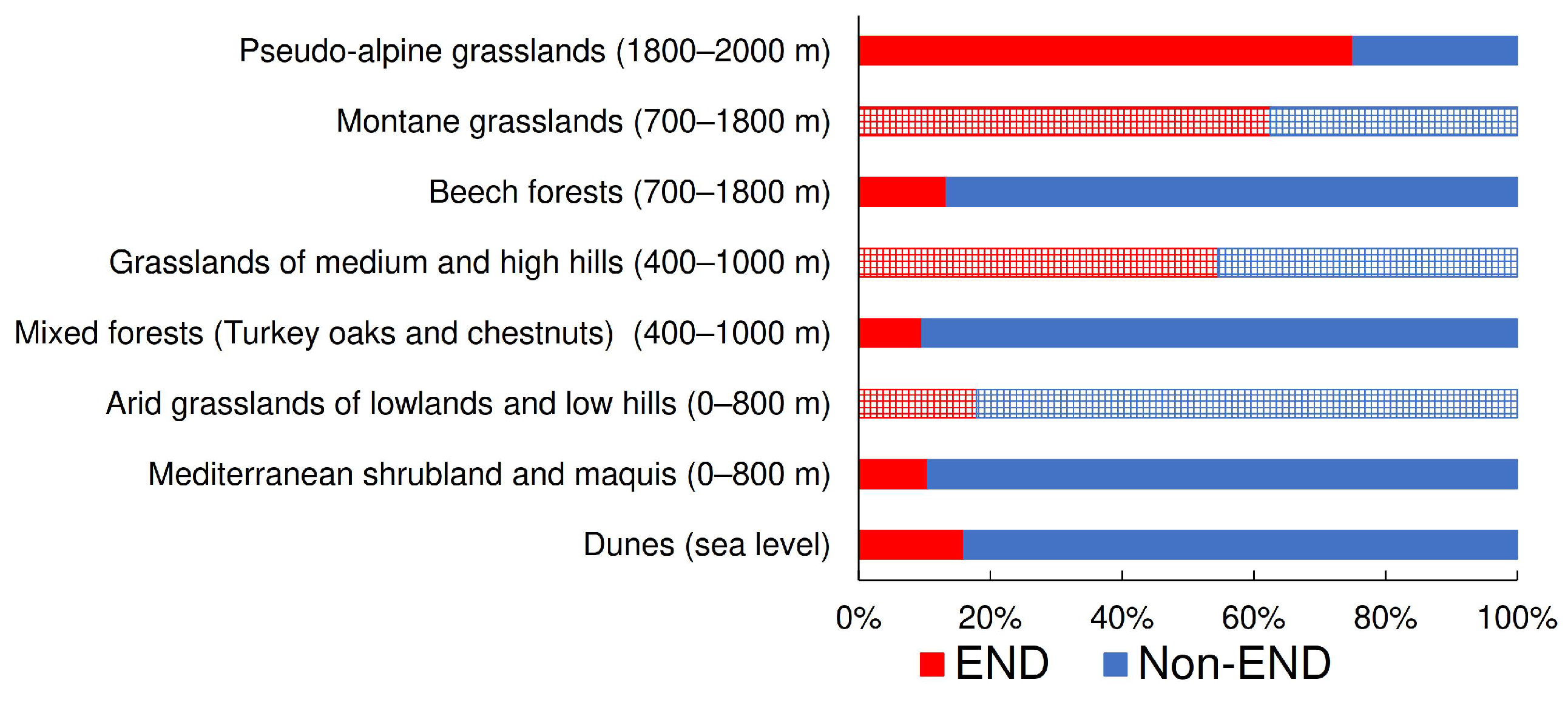
- Prediction 5. The proportion of tenebrionid endemic species is known to increase with elevation [38]. This may be explained by the fact that they are almost entirely represented by geophilous tenebrionids, which should be more associated with open habitats that naturally dominate the highest elevations. If this assumption is correct, endemics should also represent a conspicuous component of tenebrionid assemblages of secondary open vegetations at high elevations. For this prediction, frequencies of endemics were compared among vegetation types.
2. Materials and Methods
2.1. Study Area
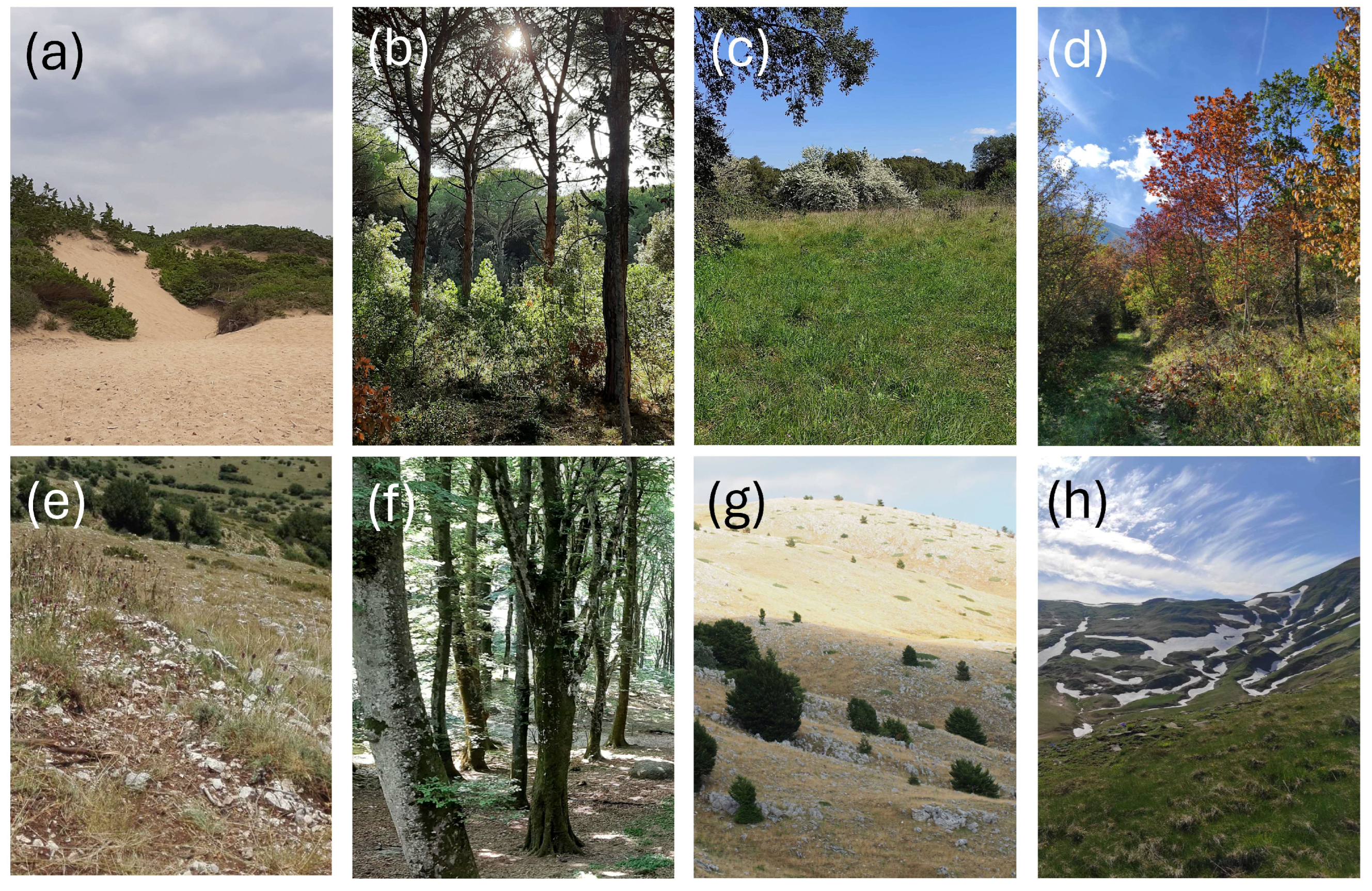
- Dunes (about sea level). This belt includes the vegetation of mobile and fixed dunes and extends inland to a very limited extent. Because of coastal exploitation, this habitat is now currently highly fragmented and under severe threats, including those posed by climate change [52,53]. It represents a natural open vegetation.
- Mediterranean shrubland and maquis: holm-oak forests, cork oak forests, coastal pinewoods, Mediterranean maquis with xerophilic species (~0–800 m). This belt is represented by a mosaic of vegetation types, ranging from garrigues to Mediterranean forests. Coastal pinewoods were mostly planted centuries ago and are mixed with natural vegetation. This belt roughly corresponds to the Mediterranean belt of Pignatti [49]. It represents a natural and more or less closed (forest) vegetation.
- Arid grasslands of lowlands and low hills (~0–800 m). This form of secondary open vegetation is the result of anthropic activities (low intensity agriculture and grazing) within the former belt.
- Mixed forests (Turkey oaks and chestnuts) (~400–1000 m). This belt, which roughly corresponds to the Samnite belt of Pignatti [49], is characterized by mixed deciduous forests. The presence of large chestnut groves is anthropogenic, but ancient (for example, on the Abani Hills, they date back to the 17th century); currently they are managed as coppices or orchards for fruit production [54,55]. It represents a substantially natural closed vegetation.
- Grasslands of medium and high hills (~400–1000 m). This form of secondary open vegetation is the result of anthropic activities (especially grazing) within the former belt.
- Beech forests (~700–1800 m). This belt is characterized by pure or mixed forests of Fagus sylvatica and roughly corresponds to the sub-Atlantic belt of Pignatti [49]. In fact, in peninsular Italy, old-growth primary forest remnants can only be found at high elevation (say, >1600 m), whereas most beech forests are secondary old-growth forests, but occupy areas where they would be the natural vegetation anyway [56,57]. This vegetation represents a substantially natural closed vegetation.
- Montane grasslands (~700–1800 m). This form of secondary open vegetation is the result of anthropic activities (especially grazing) within the former belt.
- Pseudo-alpine grasslands (beyond the tree line) (~1600–2000 m). This belt is represented by the grassland vegetation above the tree line and corresponds to the Mediterraneo-altomontane belt of Pignatti [49]. It represents a substantially natural open vegetation.
2.2. Data Collection
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poschlod, P.; WallisDeVries, M.F. The historical and socioeconomic perspective of calcareous grasslands—Lessons from the distant and recent past. Biol. Conserv. 2002, 104, 361–376. [Google Scholar] [CrossRef]
- Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Beneš, J. Origin and history of grasslands in Central Europe—A review. Grass Forage Sci. 2013, 68, 345–363. [Google Scholar] [CrossRef]
- Giarrizzo, E.; Burrascano, S.; Chiti, T.; de Bello, F.; Lepš, J.; Zavattero, L.; Blasi, C. Re-visiting historical semi-natural grasslands in the Apennines to assess patterns of changes in species composition and functional traits. Appl. Veg. Sci. 2017, 20, 247–258. [Google Scholar] [CrossRef]
- Hobohm, C.; Bruchmann, I. Endemische Gefäßpflanzen und ihre Habitate in Europa: Plädoyer für den Schutz der Grasland-Ökosysteme. Berichte Reinhold-Tüxen-Ges. 2009, 21, 142–161. [Google Scholar]
- WallisDeVries, M.F.; van Swaay, C.A.M. Grasslands as habitats for butterflies in Europe. In Grasslands in Europe of High Nature Value; Veen, P., Jefferson, R., de Smidt, J., van der Straaten, J., Eds.; KNNV Publishing: Zeist, The Netherlands, 2009; pp. 27–34. [Google Scholar]
- Wilson, J.B.; Peet, R.K.; Dengler, J.; Pärtel, M. Plant Species Richness: The World Records. J. Veg. Sci. 2012, 23, 796–802. [Google Scholar] [CrossRef]
- Habel, J.C.; Dengler, J.; Janišová, M.; Török, P.; Wellstein, C.; Wiezik, M. European grassland ecosystems: Threatened hotspots of biodiversity. Biodivers. Conserv. 2013, 22, 2131–2138. [Google Scholar] [CrossRef]
- Öckinger, E.; Eriksson, A.K.; Smith, H.G. Effects of grassland abandonment, restoration and management on butterflies and vascular plants. Biol. Conserv. 2006, 133, 291–300. [Google Scholar] [CrossRef]
- Assini, S.; Filipponi, F.; Zucca, F. Land cover changes in an abandoned agricultural land in the Northern Apennine (Italy) between 1954 and 2008: Spatio-temporal dynamics. Plant Biosyst. 2014, 149, 807–817. [Google Scholar] [CrossRef]
- Bonari, G.; Fajmon, K.; Malenovský, I.; Zelený, D.; Holuša, J.; Jongepierová, I.; Kočárek, P.; Konvička, O.; Uřičář, J.; Chytrý, M. Management of semi-natural grasslands benefiting both plant and insect diversity: The importance of heterogeneity and tradition. Agric. Ecosyst. Environ. 2017, 246, 243–252. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Médail, F.; Myers, N. Mediterranean Basin. In Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; Mittermeier, R.A., Gil, P.R., Hoffman, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., Da Fonseca, G.A.B., Eds.; CEMEX: Agrupación Sierra Madre, Mexico, 2004; pp. 144–147. [Google Scholar]
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global biodiversity conservation: The critical role of hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Heidelberg, Germany, 2011; pp. 2–22. [Google Scholar]
- Birdlife International. Ecosystem Profile. Mediterranean Basin Biodiversity Hotspot. Available online: https://www.cepf.net/sites/default/files/mediterranean-basin-2017-ecosystem-profile-english_0.pdf (accessed on 25 November 2023).
- Fattorini, S.; Ulrich, W. Spatial distributions of European Tenebrionidae point to multiple postglacial colonization trajectories. Biol. J. Linn. Soc. 2012, 105, 318–329. [Google Scholar] [CrossRef]
- Schmitt, T.; Fritz, U.; Delfino, M.; Ulrich, W.; Habel, J.C. Biogeography of Italy revisited: Genetic lineages confirm major phylogeographic patterns and a pre-Pleistocene origin of its biota. Front. Zool. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Odonate diversity patterns in Italy disclose intricate colonization pathways. Biology 2022, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, S. Biogeographical patterns of earwigs in Italy. Insects 2023, 14, 235. [Google Scholar] [CrossRef] [PubMed]
- Stoch, F. How many endemic species? Species richness assessment and conservation priorities in Italy. Belg. J. Entomol. 2000, 2, 125–133. [Google Scholar]
- Ruffo, S.; Stoch, F. (Eds.) Checklist and Distribution of the Italian Fauna. 10,000 Terrestrial and Freshwater Species. 2 Serie, Sez. Scienze della Vita, 2nd ed.; Memorie del Museo Civico di Storia Naturale di Verona: Verona, Italy, 2007; Volume 17. [Google Scholar]
- Blasi, C.; Biondi, E. La Flora in Italia; Ministero dell’Ambiente e della Tutela del Territorio e del Mare, Sapienza Università Editrice: Rome, Italy, 2017; pp. 1–704. [Google Scholar]
- Blasi, C.; Boitani, L.; La Posta, S.; Manes, F.; Marchetti, M. Stato della Biodiversità in Italia; Palombi Editori: Roma, Italy, 2005; pp. 1–468. [Google Scholar]
- Stinca, A.; Musarella, C.M.; Rosati, L.; Laface, V.L.A.; Licht, W.; Fanfarillo, E.; Wagensommer, R.P.; Galasso, G.; Fascetti, S.; Esposito, A.; et al. Italian vascular flora: New findings, updates and exploration of floristic similarities between regions. Diversity 2021, 13, 600. [Google Scholar] [CrossRef]
- Sestini, A. Conosci l’Italia. Volume VII. Il Paesaggio; Touring Club Italiano: Milano, Italy, 1963; pp. 1–232. [Google Scholar]
- Bonapace, U. (Ed.) I Paesaggi Umani; Touring Club Italiano: Milano, Italy, 1977; pp. 1–224. [Google Scholar]
- Pignatti, S. Ecologia del Paesaggio; UTET: Torino, Italy, 1994; pp. 1–228. [Google Scholar]
- Touring Club Italiano. Il Paesaggio Italiano: Idee Contributi Immagini; Touring Club Italiano: Milano, Italy, 2000; pp. 1–224. [Google Scholar]
- ISTAT. Annuario Statistico Italiano; Istituto Nazionale di Statistica: Roma, Italy, 2023; pp. 1–857. Available online: https://www.istat.it/storage/ASI/2022/ASI_2022.pdf (accessed on 10 December 2023).
- Caveri, L.; Da Polenza, A.; Salvatori, F.; Smiraglia, C. (Eds.) Montagne d’Italia; Touring Club Italiano: Milano, Italy, 2002; pp. 1–320. [Google Scholar]
- Fattorini, S.; Di Biase, L.; Chiarucci, A. Recognizing and interpreting vegetational belts: New wine in the old bottles of a von Humboldt’s legacy. J. Biogeogr. 2019, 46, 1643–1651. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Di Biase, L.; Pace, L. Mountain biodiversity and sustainable development. In Encyclopedia of the UN Sustainable Development Goals. Life on Land; Leal Filho, W., Azul, A., Brandli, L., Özuyar, P., Wall, T., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–31. [Google Scholar]
- Ortisi, A. Boschi e legno in Europa. In Storia dell’Agricoltura in Europa; ETAS-BNL: Roma, Italy, 1980; pp. 64–115. [Google Scholar]
- Kelly, M.G.; Huntley, B. An 11,000-year record of vegetation and environment from Lago di Martignano, Latium, Italy. J. Quat. Sci. 1991, 6, 209–224. [Google Scholar] [CrossRef]
- Sadori, L.; Giardini, M.; Giraudi, C.; Mazzini, I. The plant landscape of the imperial harbour of Rome. J. Archaeol. Sci. 2010, 37, 3294–3305. [Google Scholar] [CrossRef]
- Sadori, L.; Mercuri, A.M.; Mariotti Lippi, M. Reconstructing past cultural landscape and human impact using pollen and plant macroremains. Plant Biosyst. 2010, 144, 940–951. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Latini, M.; Nicolella, G.; Abbate, G. Development of a new GIS-based method to detect high natural value farmlands: A case study in Central Italy. Ann. Bot. 2017, 7, 25–31. [Google Scholar]
- Mensing, S.A.; Schoolman, E.M.; Tunno, I.; Noble, P.J.; Sagnotti, L.; Florindo, F.; Piovesan, G. Historical ecology reveals landscape transformation coincident with cultural development in central Italy since the Roman Period. Sci. Rep. 2018, 8, 2138. [Google Scholar] [CrossRef]
- Fattorini, S. Variation in zoogeographical composition along an elevational gradient: The tenebrionid beetles of Latium (Central Italy). Entomologia 2013, 1, 33–40. [Google Scholar] [CrossRef][Green Version]
- Fattorini, S. Disentangling the effects of available area, mid-domain constraints, and species environmental tolerance on the altitudinal distribution of tenebrionid beetles in a Mediterranean area. Biodivers. Conserv. 2014, 23, 2545–2560. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Di Biase, L.; Strona, G.; Pace, L.; Biondi, M. Elevational patterns of generic diversity in the tenebrionid beetles (Coleoptera Tenebrionidae) of Latium (Central Italy). Diversity 2020, 12, 47. [Google Scholar] [CrossRef]
- Fattorini, S.; Mantoni, C.; Audisio, P.; Biondi, M. Taxonomic variation in levels of endemism: A case study of Italian tenebrionid beetles. Insect. Conserv. Divers. 2019, 12, 351–361. [Google Scholar] [CrossRef]
- Lucchese, F. Atlante della Flora Vascolare del Lazio, Cartografia, Ecologia e Biogeografia. Vol. 1. Parte Generale e Flora Alloctona; Regione Lazio, Direzione Capitale Naturale, Parchi e Aree Protette: Rome, Italy, 2017; pp. 1–356. [Google Scholar]
- Lucchese, F. Atlante della Flora Vascolare del Lazio, Cartografia, Ecologia e Biogeografia. Vol. 2. La Flora di Maggiore Interesse Conservazionistico; Regione Lazio, Direzione Capitale Naturale, Parchi e Aree Protette: Rome, Italy, 2018; p. 400. [Google Scholar]
- Blasi, C. Fitoclimatologia del Lazio; Università “La Sapienza”-Roma, Regione Lazio: Roma, Italy, 1994; pp. 1–58. [Google Scholar]
- Salvati, L.; Perini, L.; Bajocco, S.; Sabbi, A. Climate aridity and landuse change: A regional-scale analysis. Geogr. Res. 2012, 50, 193–203. [Google Scholar] [CrossRef]
- Fenaroli, L.; Giacomini, V. Conosci l’Italia. Vol. II. La Flora; Touring Club Italiano: Milano, Italy, 1958; pp. 1–276. [Google Scholar]
- Fenaroli, L. Note Illustrative della Carta della Vegetazione Reale d’Italia; Ministero dell’Agricoltura e delle Foreste: Roma, Italy, 1970; pp. 1–128. [Google Scholar]
- Tomaselli, R. Note Illustrative della Carta della Vegetazione Naturale Potenziale d’Italia; Ministero dell’Agricoltura e delle Foreste: Roma, Italy, 1970; pp. 1–64. [Google Scholar]
- Pignatti, S. I piani di vegetazione in Italia. Giorn. Bot. Ital. 1979, 113, 411–428. [Google Scholar] [CrossRef]
- Ubaldi, D. Le fasce della vegetazione italiana su basi fitosociologiche. Giorn. Bot. ital. 1989, 123 (Suppl. 1), 106. [Google Scholar]
- Carpaneto, G.M. Note sulla distribuzione geografica ed ecologica dei Coleotteri Scarabaeoidea Laparosticti nell’Italia Appenninica (I Contributo). Boll. Ass. Romana. Entomol. 1986, 40, 31–58. [Google Scholar]
- Stanisci, A.; Acosta, A.; Ercole, S.; Blasi, C. Plant communities on coastal dunes in Lazio (Italy). Ann. Bot. 2004, 2, 39–48. [Google Scholar]
- Prisco, I.; Carboni, M.; Acosta, A.T.R. The fate of threatened coastal dune habitat in Italy under climate change scenarios. PLoS ONE 2013, 8, e68850. [Google Scholar] [CrossRef] [PubMed]
- De Felici, S.; Vigna Taglianti, A. I Coleotteri Carabidi dei Colli Albani (Coleoptera, Carabidae). Boll. Ass. Romana Entomol. 1994, 49, 3–96. [Google Scholar]
- Angelini, A.; Mattioli, W.; Merlini, P.; Corona, P.; Portoghesi, L. Empirical modelling of chestnut coppice yield for Cimini and Vicani mountains (Central Italy). Ann. Silvic. Res. 2013, 37, 7–12. [Google Scholar]
- Ziaco, E.; Alessandrini, A.; Blasi, S.; Di Filippo, A.; Dennis, S.; Piovesan, G. Communicating old-growth forest through an educational trail. Biodivers. Conserv. 2012, 21, 131–144. [Google Scholar] [CrossRef]
- Piovesan, G.; Alessandrini, A.; Baliva, M.; Chiti, T.; D’Andrea, E.; De Cinti, B.; Di Filippo, A.; Hermanin, L.; Lauteri, M.; Scarascia Mugnozza, G.; et al. Structural patterns, growth processes, carbon stocks in an Italian network of old-growth beech forests. Ital. J. For. Mt. Environ. 2010, 65, 557–590. [Google Scholar] [CrossRef]
- Direzione Regionale Capitale Naturale, Parchi e Aree Protette. Carta delle formazioni naturali e seminaturali al IV e V livello Corine Land Cover. Available online: http://dati.lazio.it/catalog/dataset/cus-lazio-approfondimento-delle-formazioni-naturali-e-seminaturali-iv-e-v-livello-corine-land-cover (accessed on 25 January 2024).
- Trichas, A. The genus Dendarus Latreille, 1829 (Coleoptera, Tenebrionidae: Dendarini) in Greece (A systematic account of the genus with description of a new species and four new systematic combinations). In Advances in Arachnology and Developmental Biology; Makarov, S.E., Dimitrijević, R.N., Eds.; SASA, Belgrade and UNESCO MAB Serbia: Belgrade, Serbia, 2008; pp. 417–462. [Google Scholar]
- Ferrer, J. Contribución al conocimiento de los Asidini iberobaleares. Segunda nota. Las Alphasida (Glabrasida) del grupo Tricostatae Escalera 1922 (Coleoptera, Tenebrionidae, Pimeliinae). Boletín Soc. Entomológica Aragonesa A 2008, 43, 61–73. [Google Scholar]
- Condamine, F.L.; Soldati, L.; Rasplus, J.Y.; Kergoat, G.J. New insights on systematics and phylogenetics of Mediterranean Blaps species (Coleoptera: Tenebrionidae: Blaptini), assessed through morphology and dense taxon sampling. Syst. Entomol. 2011, 36, 340–361. [Google Scholar] [CrossRef]
- Ferrer, J. Contribución al conocimiento del Género Phylan Dejean, 1821, y descripción de una specie nueva del género Heliopates Dejean, 1834 (Coleoptera, Tenebrionidae, Pedinini). Boletín Soc. Entomológica Aragonesa 2011, 49, 75–82. [Google Scholar]
- Pons, J.; Bruvo, B.; Petitpierre, E.; Plohl, M.; Ugarkovic, D.; Juan, C. Complex structural features of satellite DNA sequences in the genus Pimelia (Coleoptera: Tenebrionidae): Random differential amplification from a common ‘satellite DNA library’. Heredity 2004, 9, 418–427. [Google Scholar] [CrossRef]
- Soldati, F.; Soldati, L. Species delimitation using morphological and molecular tools in the Asida (Polasida) jurinei Solier, 1836 species complex. Preliminary results. (Coleoptera: Tenebrionidae: Tentyrinae). Cah. Sci. Muséum Lyon 2006, 10, 111–116. [Google Scholar]
- Stroscio, S.; Baviera, C.; Frati, F.; Lo Paro, G.; Nardi, F. Deep genetic divergence in the darkling beetle Pimelia rugulosa (Coleoptera, Tenebrionidae) reflects Plio-Pleistocenic paleogeographic history of Sicily. J. Zool. Syst. Evol. Res. 2011, 49, 196–203. [Google Scholar] [CrossRef]
- Fattorini, S. Regional insect inventories require long time, extensive spatial sampling and good will. PLoS ONE 2013, 8, e62118. [Google Scholar] [CrossRef]
- Fattorini, S.; Salvati, L. Tenebrionid beetles as proxy indicators of climate aridity in a Mediterranean area. Ecol. Indic. 2014, 38, 256–261. [Google Scholar] [CrossRef]
- Fattorini, S. Use of insect rarity for biotope prioritisation: The tenebrionid beetles of the Central Apennines (Italy). J. Insect Conserv. 2010, 14, 367–378. [Google Scholar] [CrossRef]
- Fattorini, S. Biotope prioritisation in the Central Apennines (Italy): Species rarity and cross-taxon congruence. Biodivers. Conserv. 2010, 19, 3413–3429. [Google Scholar] [CrossRef]
- Fattorini, S. I Coleotteri Tenebrionidi di Roma (Coleoptera Tenebrionidae). Fragm. Entomol. 2013, 45, 87–142. [Google Scholar] [CrossRef][Green Version]
- Fattorini, S.; Maltzeff, P.; Salvati, L. Use of insect distribution across landscape-soil units to assess conservation priorities in a Mediterranean coastal reserve: The tenebrionid beetles of Castelporziano (Central Italy). Rend. Lincei-Sci. Fis. 2015, 26, 353–366. [Google Scholar] [CrossRef]
- Iwan, D.; Löbl, I. Catalogue of Palaearctic Coleoptera. Vol. 5. Revised and Updated Second Edition. Tenebrionoidea; Brill: Leiden, The Netherlands, 2020; p. 946. [Google Scholar]
- Ferrer, J. Revisión del género Leptoderis Billberg, 1820 y comentarios sobre el origen, composición, anatomía y necrofagia de la tribu Elenophorini (Coleoptera, Tenebrionidae). Boln. SEA 2015, 57, 19–38. [Google Scholar]
- Ferrer, J. Contribución al conocimiento del género Pachychila Eschscholtz, 1831 y descripción de dos especies nuevas de la Península Ibérica y una de Italia (Coleoptera, Tenebrionidae, Pimeliinae). Boln. SEA 2018, 62, 35–54. [Google Scholar]
- Fattorini, S. On the concept of chorotype. J. Biogeogr. 2015, 42, 2246–2251. [Google Scholar] [CrossRef]
- Fattorini, S. A history of chorological categories. Hist. Philos. Life Sci. 2016, 38, 12. [Google Scholar] [CrossRef] [PubMed]
- Gatto, C.A.F.R.; Cohn-Haft, M. Spatial Congruence Analysis (SCAN): A method for detecting biogeographical patterns based on species range congruences. PLoS ONE 2021, 16, e0245818. [Google Scholar] [CrossRef] [PubMed]
- Olivero, J.; Real, R.; Márquez, A.L. Fuzzy chorotypes as a conceptual tool to improve insight into biogeographic patterns. Syst. Biol. 2011, 60, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Vigna Taglianti, A.; Audisio, P.A.; Biondi, M.; Bologna, M.A.; Carpaneto, G.M.; De Biase, A.; Fattorini, S.; Piattella, E.; Sindaco, R.; Venchi, A.; et al. A proposal for a chorotype classification of the Near East fauna, in the framework of the Western Palearctic region. Biogeographia 1999, 20, 31–59. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Baselga, A.; Leprieur, F. Comparing methods to separate components of beta diversity. Methods Ecol. Evol. 2015, 6, 1069–1079. [Google Scholar] [CrossRef]
- Moulpied, M.; Smith, C.H.; Robertson, C.R.; Johnson, N.A.; Lopez, R.; Randklev, C.R. Biogeography of freshwater mussels (Bivalvia: Unionida) in Texas and implications on conservation biology. Divers. Distrib. 2022, 28, 1458–1474. [Google Scholar] [CrossRef]
- Jost, L. The relation between evenness and diversity. Diversity 2010, 2, 207–232. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chiu, C.H.; Chao, A. Distance-based functional diversity measures and their decomposition: A framework based on Hill numbers. PLoS ONE 2020, 9, e100014. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef] [PubMed]
- Petrén, H.; Köllner, T.G.; Junker, R.R. Quantifying chemodiversity considering biochemical and structural properties of compounds with the R package CHEMODIV. New Phytol. 2023, 237, 2478–2492. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 15 March 2022).
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C.H. SpadeR: Species-Richness Prediction and Diversity Estimation with R; Version 0.1.1. 2016. Available online: https://CRAN.R-project.org/package=SpadeR (accessed on 30 May 2021).
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E. Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 April 2022).
- Dapporto, L.; Ramazzotti, M.; Fattorini, S.; Vila, R.; Talavera, G.; Dennis, R.L.H. recluster: Ordination Methods for the Analysis of Beta-Diversity Indices. Available online: https://CRAN.R-project.org/package=recluster (accessed on 15 March 2022).
- ESRI. ArcGis Desktop: Version 10.4; Environmental Systems Research Institute (ESRI): Redlands, CA, USA, 2016. [Google Scholar]
- Tarquini, S.; Isola, I.; Favalli, M.; Battistini, A.; Dotta, G. TINITALY, a Digital Elevation Model of Italy with a 10 Meters Cell Size, Version 1.1. Istituto Nazionale di Geofisica e Vulcanologia (INGV). 2023. Available online: https://tinitaly.pi.ingv.it/ (accessed on 3 October 2023).
- Fattorini, S. Tenebrionid beetle distributional patterns in Italy: Multiple colonisation trajectories in a biogeographical crossroad. Insect Conserv. Divers. 2014, 7, 144–160. [Google Scholar] [CrossRef]
- Aliquò, V.; Soldati, F. Updating the CD-rom on Coleoptera Tenebrionidae of Italy and the check-list of the same family. Biodivers. J. 2014, 5, 429–442. [Google Scholar]
- Leo, P.; Fancello, L. Observaciones sobre Akis bacarozzo (Schrank, 1786) y Akis tuberculata Kraatz, 1865, y nota sinonímica (Coleoptera, Tenebrionidae). Rev. Gaditana De Entomol. 2019, 10, 31–46. [Google Scholar]
- Leo, P.; Fancello, L. Clitobius subplumbeus (Fairmaire, 1856) bona species: Nuovo inquadramento sistematico delle popolazioni mediterranee attribuite a Clitobius ovatus (Erichson, 1843) (Coleoptera, Tenebrionidae). Ann. Mus. Civ. Stor. Nat. “Giacomo Doria” 2020, 112, 155–166. [Google Scholar]
- Ponel, P.; Lo Cascio, P.; Leo, P. A new species of Catomus Allard, 1876 (Coleoptera: Tenebrionidae: Helopini) from the Aeolian archipelago (Sicily, Italy). Zootaxa 2020, 4743, 2. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, M.J.; Bouchard, P.; Leo, P.; Iwan, D. On the type species of the genus Dendarus Dejean, 1821 (Coleoptera: Tenebrionidae). Zootaxa 2021, 5048, 575–580. [Google Scholar] [CrossRef]
- Leo, P.; Pezzi, G.; Riolo, F. Prima segnalazione per l’Italia di Cryphaeus cornutus (Fischer von Waldheim, 1823) e della tribù Toxicini con osservazioni sulla distribuzione della specie in Europa (Coleoptera Tenebrionidae Toxicini). Boll. Soc. Entomol. Ital. 2021, 153, 123–128. [Google Scholar] [CrossRef]
- Fattorini, S. Ecology and conservation of tenebrionid beetles in Mediterranean coastal areas. In Insect Ecology and Conservation; Fattorini, S., Ed.; Research Signpost: Trivandrum, Kerala, India, 2008; pp. 165–297. [Google Scholar]
- Fattorini, S. Adaptations of tenebrionid beetles to Mediterranean sand dune environments and the impact of climate change (Coleoptera: Tenebrionidae). Fragm. Entomol. 2023, 55, 1–20. [Google Scholar]
- Carpaneto, G.M.; Baviera, C.; Biscaccianti, A.B.; Brandmayr, P.; Mazzei, A.; Mason, F.; Battistoni, A.; Teofili, C.; Rondinini, C.; Fattorini, S.; et al. A Red List of Italian Saproxylic Beetles: Taxonomic overview, ecological features and conservation issues (Coleoptera). Fragm. Entomol. 2015, 47, 53–126. [Google Scholar] [CrossRef]
- ISTAT. Rapporto sul Territorio 2020. Ambiente, Economia e Società; Istituto Nazionale di Statistica: Roma, Italy, 2020; pp. 1–201. Available online: https://www.istat.it/storage/rapporti-tematici/territorio2020/Rapportoterritorio2020.pdf (accessed on 10 December 2023).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattorini, S. The Role of Vegetation in Elevational Diversity Patterns of Tenebrionid Beetles in Central Italy. Diversity 2024, 16, 110. https://doi.org/10.3390/d16020110
Fattorini S. The Role of Vegetation in Elevational Diversity Patterns of Tenebrionid Beetles in Central Italy. Diversity. 2024; 16(2):110. https://doi.org/10.3390/d16020110
Chicago/Turabian StyleFattorini, Simone. 2024. "The Role of Vegetation in Elevational Diversity Patterns of Tenebrionid Beetles in Central Italy" Diversity 16, no. 2: 110. https://doi.org/10.3390/d16020110
APA StyleFattorini, S. (2024). The Role of Vegetation in Elevational Diversity Patterns of Tenebrionid Beetles in Central Italy. Diversity, 16(2), 110. https://doi.org/10.3390/d16020110







