Abstract
To make the distinction against pharmaceuticals, natural product medicines are more accurately denoted as nutritional therapies. In the context of topical therapies targeting dermatological conditions, nutritional therapy may explain the mechanism of ethnocosmetic plants used in hair treatment and care. Inspired by emerging theories of a connection between dysregulated glucose metabolism and hair loss, the current review of the literature focused on African plants used to target hair conditions in general, such as alopecia or scalp dermis infections, with a cross-examination of the potential of the species to alleviate issues with glucose metabolism. We distinguish between topical nutrition and sterilization (i.e., dandruff and lice). Sixty-eight plants were identified as an African treatment for alopecia, dandruff, lice, and tinea. Fifty-eight of the species have potential as antidiabetic treatments when taken orally. The family Lamiaceae was the most represented (six spp.), followed by Fabaceae and Asteraceae (five spp. each). Most species are herbs, and the most used plant part is the leaf. Thirty of the sixty species have research associated with hair growth and general hair care, with studies focused on 5α-reductase inhibition, biomarkers such as vascular endothelial growth factor, and the rate of telogen to anagen phase transition. While studies tend to conceptualize the mechanisms of these medicinal species similarly to pharmaceuticals, the current review argues that a nutritional interpretation is more appropriate, where a general improvement to local glucose metabolism may play a role.
1. Introduction
People of the 21st century are demonstrating a renewed interest in plant-based cosmetic products for beautification and care. This globe-wide trend is due to the potential detrimental effects experienced by users of cosmetic products comprised of synthetic compounds [1]. In the context of hair care, products or therapies tend to prevent or manage conditions such as androgenetic alopecia, telogen effluvium, other types of alopecia, dandruff, and other infections caused by fungi, bacteria, parasites, or ectoparasites [2,3]. Cosmetic treatments for hair are also focused on aesthetic outcomes by conditioning, cleansing, and increasing the growth rate of otherwise healthy hair [3].
With better recognition and understanding of the mechanisms of topical nutricosmetics [4], the indigenous plant use industry, if sustainably practiced, may be better promoted and embraced by societies and consumers, thereby helping to build on the sustainable development goals. To an extent, it may lead to poverty eradication through job creation, promotion of good health and well-being, and empowerment of women due to a stronger inclination toward the cosmetics industry compared to men [1]. For example, in South Africa, Citrullus lanatus (Thunb.) Matsum., and Nakai’s brand name, “Royal Honey and Kalahari Desert Melon” is now used to produce a natural hair care product [5], empowering small communities economically. Also, the dried pulp of the unripe fruit of Citrullus colocynthis Schrad is an ingredient of a commercial serum used in the treatment of hair loss in India (Colocynth of Commerce) [6].
Ethnobotanical studies on cosmetic plants, especially in Africa, have always focused on general beautification, skin, and oral care [7,8], with less attention to hair care. This is gradually changing due to the increasing prevalence of scalp and hair pathologies in both men and women, concomitant with the rise of cardiovascular disease and diabetes [9,10]. Given the high demand for plant-based products in the industry for hair care and nourishment, it is important to promote a better understanding of their potential as an adjuvant. Since ethnobotanical studies on nutricosmetic plants for hair care are very scarce in Africa, it is also necessary to comprehensively summarize this knowledge.
Research focused on the mechanism of traditional hair therapies often seeks to explain mechanisms similarly to the pharmaceutical industry by following the single-target or “magic bullet” paradigm. While this approach can sometimes identify natural products that have the potential to become pharmaceuticals, it is more common for traditional therapies to confer systematic effects that can be loosely called nutrition. In the context of androgenetic alopecia, alopecia areata, telogen effluvium, or scarring alopecias, there is a growing body of research that links nutritional shortcomings with their phenotypic presentation [4,9,10,11].
Research is now starting to demonstrate that the etiology of androgenetic alopecia involves problems with glucose metabolism in the scalp tissue [9,10]. In this regard, a review of the literature demonstrated from a global perspective that 44% of traditional plants used specifically for androgenetic alopecia have ethnobotanical records for diabetes treatment [11]. This finding excluded species that had been researched and demonstrated to have antidiabetic potential but had no ethnobotanical records making the connection. If such studies are included in that metric, a significantly higher correlation would be observed.
Generally, most traditional therapies for hair in Africa are applied topically, whereas the same species, when used for diabetic complaints, are taken orally. While this may seemingly obscure the link between local diabetes (scalp diabetes) and hair loss, natural treatments for hair loss may be conceptualized as a form of topical nutrition that improves glucose metabolism locally rather than systemically.
While there is much information of traditional therapies for hair in other countries and continents of the world, information related to plants used in Africa is quite scarce. Furthermore, by summarizing such species, an opportunity presents itself by enabling the global coincidence of hair care plants and antidiabetic potential to be tested in Africa.
The current study provides a comprehensive summary of the indigenous knowledge of African plants that are being used for hair care. The research on efficacy or mechanism is critically discussed, with particular reference to the similarity in mechanism compared to phytochemical diversity and its role in nutrition [12]. The link to glucose metabolism and the potential antidiabetic activity of the species is discussed. Knowledge gaps are highlighted with the objective of renewing research interest and encouraging the rising numbers of natural product scientists in Africa to consider potentially commercially viable projects that benefit regional communities by creating grassroots-level industries.
2. Methodology
The data accumulated for this review were extracted from websites, search engines, and published journal databases such as African Journal Online (AJOL), Google Scholar, ScienceDirect, Scopus Web of Science, and Scopus. Keywords used to search were “ethnobotany”, the name of every African country (Angola, Cameroon, Congo, Egypt, Nigeria, South Africa, Zambia, etc.), “hair care”, “hair condition”, “dandruff”, “lice”, “hair infection”, “alopecia”, “Indigenous knowledge”, and “traditional medicinal plants”. The plant names were verified on the Plant List website and World Flora Online.
The literature search on different databases resulted in 129 articles from 143 downloaded and published between 1991 and 2023. The selection of the downloaded articles was based on the following criteria: (1) the article is published in English or translated to English; (2) at least one plant listed or mentioned in the article is used in hair care or treatment; (3) if the study is on the bioactivity related to hair care of any plant listed; (4) if the study listed an African species of plant that is used in another country outside Africa. Articles excluded from this review are based on the following: (1) studies that use chemical agents or non-plant-based agents for the treatment of hair; (2) studies on hair care that were not peer reviewed; (3) studies that mentioned that a plant is used for hair care in Africa without information on how and where it is used; (4) duplicated studies similar to already reviewed articles.
Lastly, the authors then adopted the “2SR” acronym (search, screen, and review) during the review of the articles.
The vernacular names of the plants and the tribes (cultures, civilizations) associated with the traditional use report were recorded as mentioned in the publications. Where the tribe was not available, the country was recorded. The species used in regions other than Africa but distributed in Africa are also included in this paper; in this case, the vernacular names of the plants are based on the local language of the first author, when available (Yoruba).
The species names that were compiled were then searched on Google Scholar using the search words “genus, species, antidiabetic”. Species were considered to have antidiabetic potential if there were in vitro or in vivo studies of hypoglycemic effects in mice or cultured cell lines, or if there were ethnobotanical records associated with the treatment of diabetes, insulin resistance, or metabolic syndrome.
3. Results and Discussion
3.1. Medicinal Plants for Hair Treatment
In the current review, 68 plants distributed in Africa were compiled. Traditional use targets included alopecia, dandruff, lice removal, and tinea treatment (Table 1). Most of the reported plants are from Nigeria, Egypt, Cameroon, Tunisia, and South Africa, while those species that are used in countries like India and Thailand are included in Table 1 if the plant is also distributed in Africa. For example, species like Ipomoea aquatica, Senna siamea, and Cymbopogon citratus are reportedly used for hair in Thailand, while Abrus precatorius, Azadirachta indica, and Melia azedarach are used in India. Furthermore, the people from Haiti, Samoa, and Tonga used Calophyllum inophyllum for hair and scalp care [13].
The 68 species are distributed between 39 angiosperm families, with Lamiaceae having the highest number of species at 6, then Fabaceae and Asteraceae with 5 species each (Figure 1).
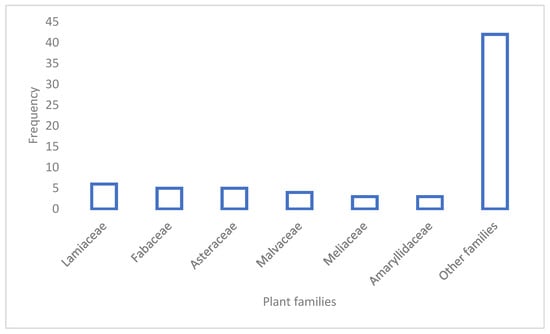
Figure 1.
Most recorded families of plants used for hair care in Africa.
The family Lamiaceae has been previously reported as the leading family in terms of cosmetical usage in the Eastern Cape region of South Africa [5], while Asteraceae and Fabaceae have been reported as the leading families for cosmetical use among the Vhavenda women from South Africa [7] and peoples of West Africa [8].
The family Lamiaceae is known for its high yield of essential oils after distillation, with many that are valuable in the cosmetic and perfume industry [14]. Evidently, when the macerated leaves are extracted into a fixed oil, the volatile organic compounds are included, among other lipophilic compounds [15], creating a mixture of biomolecules with transdermal potential [16]. The dominance of the families Asteraceae and Fabaceae is also unsurprising as they are often the second and largest families of angiosperms, respectively, according to many other ethnobotanical studies [17,18,19]. At the level of genera, the aromatic species from Lavandula (Lamiaceae) and the alkaloid-rich species from Pterocarpus (Fabaceae) are the most utilized genera, with two species each.

Table 1.
African species used for hair care or hair loss.
Table 1.
African species used for hair care or hair loss.
| Family | Species | Country Where Report Was Conducted | Local Name | Part(s) Used | Ailment Targeted, Mode of Administration, and Antidiabetic Potential | Growth Habit | References |
|---|---|---|---|---|---|---|---|
| Adiantaceae | * Adiantum capillus-veneris L | Egypt | Not determined | Aerial part | Baldness or alopecia: whole plant, aqueous then topical. Antidiabetic? Yes [11] | Herb | [20] |
| Annonaceae | Xylopia aethiopica (Dunal) A.Rich. | Nigeria | Eeru | Fruit | Baldness or alopecia: extract from the fruit is applied to the scalp. Antidiabetic? Yes [21] | Tree | [22] |
| Asteraceae | Artemisia afra Jacq. | South Africa; Setswana | Lengana/umhlonyane | Leaf | Baldness or alopecia: leaves are mixed with the leaves of rosemary to wash hair. Antidiabetic? Yes [23] | Shrub | [24] |
| Asteraceae | * Eclipta prostrata (L.) L. (Synonym: Eclipta alba L. Hassak) | Not determined | Not determined | Whole plant | Baldness or alopecia: the juice extract is applied to the scalp. Antidiabetic? Yes [11] | Herb | [25] |
| Asteraceae | Gymnanthemum amygdalinum (Delile) Sch. | Cameroon | Panboka | Leaf | General hair care: extract from the macerated or crushed leaves or infusion of the leaves is applied to the hair. Antidiabetic? Yes [26] | Shrub | [1] |
| Asteraceae | Eriocephalus africanus L. | South Africa; Setswana | Not determined | Twig and inflorescence | Baldness and hair conditioning: extract from the boiled twigs and inflorescence is applied to the scalp. Antidiabetic? Yes [27] | Shrub | [24] |
| Asteraceae | Tridax procumbens L. | Nigeria | Imi esu (Yoruba) | Leaf | Baldness or alopecia: extract from the leaves is applied to the scalp. Antidiabetic? Yes [28] | Herb | [3] |
| Arecaceae | Cocos nucifera L. | Nigeria | Agbon (Yoruba) | Fruit | General hair care: oil extract from the fruit is applied to the scalp. Antidiabetic? Yes [11] | Tree | [3] |
| Arecaceae | Elaeis guineensis Jacq. | Cameroon and Nigeria | Tî Mbanga | Seed | General hair care: oil extract from the fruit is applied to the scalp. Antidiabetic? Yes [29] | Tree | [1] |
| Acoraceae | Acorus calamus L. | South Africa (Zoa, Kannaland) | Makkalmoes (Afrikaans) | Rhizome | Baldness or alopecia: infused rhizome is used to wash the scalp, or oil extract is applied to the scalp. Antidiabetic? Yes [30] | Herb | [31] |
| Amaryllidaceae | Allium ascalonicum L. | Nigeria (Yoruba) | Alubosa elewe | Bulb | Baldness or alopecia: juice extract of the bulb is applied to the scalp. Antidiabetic? Yes [32] | Herb | [33] |
| Amaryllidaceae | Allium cepa L. | Not determined | Alubosa (Yoruba) | Bulb | Baldness and dandruff: bulb is used to rub the scalp, or juice extract is applied to the scalp. Antidiabetic? Yes [11] | Herb | [3] |
| Amaryllidaceae | Allium sativum L. | Nigeria | Alubosa Ayyu | Bulb | Baldness or alopecia: juice extract of the bulb is applied to the scalp. Antidiabetic? Yes [11] | Herb | [33] |
| Amaranthaceae | * Achyranthes aspera L. | Not determined | Abora (Yoruba) | Root | Baldness and dandruff: paste from the fresh root is applied to the scalp overnight. The ash from the whole plant is also used in making hair dye. Antidiabetic? Yes [34] | Herb | [35] |
| Asparagaceae | Asparagus africanus Lam. | South Africa (Eastern Cape) | Ubumhlope/umathunga | Aerial part | Baldness or alopecia: the aerial part is used to rub the scalp. Antidiabetic? Yes [36] | Shrub | [5] |
| Calophyllaceae | * Calophyllum inophyllum L | Haiti, Samoa, & Tonga | Flower and nut | Baldness and scalp care: oil extracted from the nut is applied to the scalp alone or mixed with coconut oil. Flowers are used as a fragrance in coconut oil for scalp care. Antidiabetic? Yes [37] | Tree | [13] | |
| Caricaceae | * Carica papaya L. | Nigeria | Ibepe | Fruit | General hair: fruit is used as a poultice on the scalp. Antidiabetic? Yes [38] | Tree | [39] |
| Cannabaceae | * Cannabis sativa L. | Cameron, South Africa (Venda), and Nigeria | Mbângué (Gbaya, Cameroon), Igbo, (Nigeria), banzhe (Venda, South Africa | Leaf and seed | Baldness and general hair care: extract from crushed seeds or leaves is applied to the scalp for hair care in Cameroon. Powder from the dried leaves mixed with cream is applied to the scalp for baldness in Nigeria. Extract from the macerated seed is applied to the scalp for baldness in South Africa. Antidiabetic? Yes [11] | Herb | [1,7] |
| Cornaceae | * Alangium salviifolium (L.f.) Wang | Not determined | Not determined | Seed and stem bark | Baldness and dandruff: paste made from seed is applied to the scalp. Paste made from fresh stem bark is applied to the scalp for dandruff. Antidiabetic? Yes [40] | Tree | [35] |
| Convolvulaceae | * Ipomoea aquatica Forssk. | Not determined | Not determined | Leaf and stem | Baldness and hair conditioning: decoction of leaves and stem is applied to the scalp. Antidiabetic? Yes [41] | Creeper | [42] |
| Convolvulaceae | Ipomoea batatas (L.) Lam | Cameroon | Mouké | Leaf | General hair care: extract from the macerated or crushed leaves or infusion is applied to the scalp. Antidiabetic? Yes [43] | Creeper | [1] |
| Cucurbitaceae | * Citrullus colocynthis Schrad | Not determined | Not determined | Fruit and seed | Baldness or alopecia: oil extracted from the seeds is applied to the scalp. Dried pulp from the unripe fruit is applied to the scalp. Antidiabetic? Yes [44] | Trailer | [6] |
| Cyperaceae | Cyperus longus L. | Egypt | Su’d | Leaf | Baldness or alopecia: powder from the dried leaves mixed with cream is applied to the scalp. Antidiabetic? No records found | Herb | [45] |
| Cyperaceae | Cyperus rotundus L. | Egypt | Not determined | Tuber | Hirsutism: oil extracted is applied to the scalp. Antidiabetic? Yes [46] | Herb | [47] |
| Dilleniaceae | Tetracera alnifolia Willd. | Nigeria | Igi opon | Leaf and fruit | Baldness or alopecia: powder from the ground leaves or fruits is applied to the scalp. Antidiabetic? Yes [48] | Climber | [33] |
| Ericaceae | Erica multiflora L | Tunisia and Morocco | Not determined | Not determined | Baldness or alopecia: extract from the plant is applied to the scalp. Antidiabetic? No records found | Shrub | [49] |
| Euphorbiaceae | Ricinus communis L | Tunisia | Not determined | Seed | General hair care: oil extract from the seeds is applied to the scalp. Antidiabetic? [11] | Shrub | [50] |
| Euphorbiaceae | Spirostachys africana Sond. | South Africa (Amandwe) | Umthombothi | No records | Hairdressing and lice: method of administration not determined. Antidiabetic? No records found | Tree | [51] |
| Fabaceae | Abrus precatorius L. | Reported in India | Not determined | Seed and root | Baldness and hair tinea: the seed paste is applied to the scalp for baldness or alopecia. The seed oil is applied to the scalp to prevent hair from falling as a result of a Tinnea capitis infection. Paste made from the root, seed, and honey is applied to the scalp for alopecia or baldness. Antidiabetic? Yes [11] | Climber | [35] |
| Fabaceae | Pterocarpus erinaceus Poir. | Togo | Tém | Latex | Hair tinea: latex is applied to the scalp. Antidiabetic? Yes [52] | Tree | [53] |
| Fabaceae | Pterocarpus soyauxii Taub. (Padouk) | Cameroon | Koula | Bark | Hair colourant: bark is macerated or crushed and applied to the scalp used as a natural dye. Antidiabetic? Yes [54] | Tree | [1] |
| Fabaceae | Senna siamea (Lam.) H.S.Irwin & Barneby | Not determined | Not determined | Leaf | Dandruff, oily scalp, and general hair care: decoction of the leaves is applied to the scalp. Antidiabetic? Yes [55] | Tree | [42] |
| Fabaceae | Trigonella foenum-graecum L. | Not determined | Not determined | Seed | Baldness or alopecia: ground seeds are mixed with oil and applied to the scalp. Antidiabetic? Yes [11] | herb | [11] |
| Lamiaceae | Ajuga iva (L.) Schreb | Tunisia | Chendgoura | Leaf | Hair softener: oil macerated from the leaves is applied to the scalp. Antidiabetic? Yes [56] | Herb | [57] |
| Lamiaceae | Lavandula coronopifolia Poir | Tunisia | ‘Khzema | Flowers | Hair softener and perfume: oil macerated from the flowers is applied to the scalp. Antidiabetic? Yes [58] | Shrub | [57] |
| Lamiaceae | Lavandula multifida L | Tunisia | Kammoun | Flowers | Hair softener and perfume: oil macerated from the flowers is applied to the scalp. Antidiabetic? Yes [59] | Shrub | [57] |
| Lamiaceae | Leonotis leonurus (L.) R.Br | South Africa (Sestwana) | Utshwala-bezinyoni (isiZulu) | Leaf | Baldness or alopecia: extracts of the leaves is taken orally. Antidiabetic? Yes [60] | Shrub | [24] |
| Lamiaceae | * Ocimum sanctum L. | Not determined | Not determined | Leaf | Baldness and general hair care: oil macerated from the leaves is applied to the scalp. Antidiabetic? Yes [61] | Herb | [3] |
| Lamiaceae | Salvia aegyptiaca L. | Tunisia | ‘Kammouna | Flower | Hair perfume: oil macerated from the flowers. Antidiabetic? Yes [62] | Herb | [57] |
| Lauraceae | Cassytha ciliolata Nees | South Africa (Zoar and Vanwyksdorp Kannaland | Nooienshaar | Whole plant | Baldness or alopecia: whole plant decoction is applied to the scalp. Antidiabetic? No records found | Climbing or twinning herb | [31] |
| Lauraceae | Persea americana Mill | Cameroon, South Africa (Venda), Tanzania | Afukhuda (Venda) | Fruit | General hair care, baldness, dandruff, and hair moisturizer: oil macerated from the fruit is applied to the scalp and used for hair care in Cameroon. Fruit is applied to the scalp for hair nourishment in South Africa. The mesocarp of the fruit is mixed with the egg yolk and used as a hair moisturizer, for dandruff, and to prevent hair loss. Antidiabetic? No; leaves and seeds: yes [63] | Tree | [1,7,64] |
| Lythraceae | * Lawsonia inermis L. | Not determined | Not determined | Leaf | Dandruff: paste made from fresh leaves is applied to the scalp. Leaves extract is used in making hair oil for hair tonics and dyes. Antidiabetic? Yes [11] | Shrub | [3] |
| Malvaceae | Hermania spp. | Mbeere Kenya (Karundu) | Not determined | Leaf | General hair care: leaves are added to water and used as shampoo to wash the hair. Antidiabetic? No records found | Shrub | [65] |
| Malvaceae | * Hibiscus rosa-sinensis L. | Not determined | Not determined | Leaf | Baldness or alopecia: extract from the leaves is applied to the scalp. Antidiabetic? Yes [11] | Shrub | [3] |
| Malvaceae | Malva parviflora L. | South Africa (Eastern Cape) | Umajikanelanga/ijongilanga | Leaf and root | Dandruff and hair softener: decoction of the roots or leaves is applied to the scalp. Antidiabetic? Yes [66] | Herb | [5] |
| Malvaceae | Triumfetta pentandra A. Rich. | Cameroon | Mbol | Bark | General hair care: extract from macerated or crushed bark or infusion of bark is applied to the scalp. Antidiabetic? Yes [67] | Herb | [1] |
| Meliaceae | * Azadirachta indica A. Juss. | Dogo Yaro (Yoruba) | Leaf, seed, and sap | Baldness, dandruff, and lice: leaves and seeds are crushed and applied to the scalp for lice. Infusion of fresh leaves for dandruff. The mixture of the seed and the sap from the tree growing near the water is used to massage bald heads for baldness. Antidiabetic? Yes [68] | Tree | [35] | |
| Meliaceae | * Melia azedarach L. | Not determined | Not determined | Flower | Dandruff: the crushed flowers are applied to the scalp. Antidiabetic? Yes [69] | Tree | [35] |
| Meliaceae | Trichilia dregeana Sond | South Africa (Eastern Cape) | Umkhuhlu | Seed | General hair care: ointment made from the seeds is applied to the scalp. Antidiabetic? No records found | Tree | [5] |
| Mesembryanthemaceae | Psilocaulon coriarium (Burch. ex N.E.Br. | South Africa (Eastern Karoo) | Loogbossie | Whole plant | Pimples or pustules: infusion of the whole plant is used to wash the scalp or as a poultice. Antidiabetic? No records found | Herb | [70] |
| Moraceae | Ficus sur Forssk | Togo | Kaliay | Leaf | Hair tinea: leaves are used to rub the scalp. Antidiabetic? Yes [71] | Tree | [53] |
| Myrtaceae | Eugenia nigerina A. Chev | Nigeria | Kanafuru | Fruit | Baldness or alopecia: powder from the ground dried fruit is mixed with oil and applied to the scalp. Antidiabetic? Yes [72] | Shrub | [33] |
| Myrtaceae | Myrtus communis L. | Tunisia | Not determined | Leaf and twig | General hair care: essential oil extracted from leaves and twigs is applied to the scalp. Antidiabetic? Yes [11] | Shrub | [73] |
| Passifloraceae | Adenia gummifera Harms. | South Africa; Sestawana | Mfulwa (isiZulu) | Leaf | Baldness and hair conditioning: leaves mixed with rosemary plant to wash the hair. Antidiabetic? No records found | Climber | [24] |
| Pedaliaceae | Sesamum senecioides (Klotzsch) Byng & Christenh. | South Africa and Zimbabwe | Museto (South Africa; Tshivenda) | Leaf | Baldness and hair softener: extract from the leaves is applied to the scalp. Antidiabetic? No records found | Creeper | [74] |
| Pedaliaceae | Sesamum indicum L. | Cameroon | Soundou | Seed | General hair care: extract from the crushed seeds or infusion of the seeds is applied to the scalp. Antidiabetic? Yes [11] | Herb | [1] |
| Poaceae | * Cymbopogon citratus Stapf. | Not determined | Ewe tea/kooko oba (Yoruba) | Whole plant | Oil scalp and general hair care: decoction of the whole plant is used to wash the hair. Antidiabetic? Yes [11] | Herb | [42] |
| Phyllanthaceae | * Phyllanthus emblica L. | Not determined | Not determined | Fruit | Baldness or alopecia: oil extracted from the dried fruit is applied to the scalp individually or when fried in sesame oil. Antidiabetic? Yes [11] | Tree | [3,42] |
| Rubiaceae | Chassalia kolly (Schumach.) Hepper | Togo | Tiyah | Root | Hair tinea: paste made from the roots is applied to the scalp. Antidiabetic? No records found | Shrub | [53] |
| Rubiaceae | Gardenia ternifolia Schumach and Thonn. | Togo | Kaou | Root | Hair tinea: calcinate made from the root is applied to the scalp. Antidiabetic? No records found | Small tree | [53] |
| Rutaceae | Ruta graveolens L. | South Africa; Sestwana | Not determined | Stem | Baldness or alopecia: decoction of the stem is used to wash hair. Antidiabetic? Yes [75] | Herb | [24] |
| Sapotaceae | Baillonella toxisperma Pierre | Cameroon | Guibi | Seed | General hair care: oil from the macerated or crushed seeds is applied to the scalp. Antidiabetic? Yes [76] | Tree | [1] |
| Sapotaceae | Vitellaria paradoxa C.F. Gaertn. | Cameroon and Nigeria | Igi oori (Yoruba, Nigeria) | Seed | General hair care: oil from the macerated or crushed seeds is applied to the scalp. Antidiabetic? Yes [77] | Tree | [1] |
| Solanaceae | Nicotiana tabacum L. | Cameroon and Nigeria | Ndalka (Cameroon) Taaba (Yoruba; Nigeria) | Leaf | Baldness or alopecia: extract from crushed leaves is applied to the scalp in Cameroon. Dried leaves are ground to powder, mixed with oil, and applied to the scalp in Nigeria. Antidiabetic? Yes [78] | Herb | [1,33] |
| Sterculiaceae | Theobroma cacao L. | Cameroon and Nigeria | Koko (Nigeria) | Seed | Baldness and general hair care: extract from crushed or macerated seeds is used for hair care in Cameroon. Dried seeds are ground into powder and mixed with cream for baldness in Nigeria. Antidiabetic? Yes [79] | Tree | [1] |
| Xanthorrhoeaceae | Aloe ferox Mill. | Lesotho | Lekhala-la-Quthing | Leaf | General hair care: extract from leaves is applied to the scalp. Antidiabetic? Yes [80] | Tree | [81] |
| Xanthorrhoeaceae | Aloe vera (L.) Burm.f. | Cameroon, Nigeria and Tunisia | Eti eerin (Nigeria) | leaf | General hair care: macerated gel from the leaves is applied to the scalp. Antidiabetic? Yes [11] | Herb | [1,50] |
| Zingiberaceae | * Zingiber officinale Roscoe | Not determined | Ataale (Yoruba | Rhizome | Baldness and oily scalp: juice extracted from fire-grilled rhizomes is applied to the scalp. Antidiabetic? Yes [11] | Herb | [42] |
Plants with * are not used in Africa but distributed in Africa.
3.2. Habit of Plants, Plant Part Used, and Preparation Methods
Regarding the habits of the species recorded in this review, most of the plants are herbs (24), followed by trees (21), shrubs (15), and climbers/creepers/trailers (8) (Figure 2). Herbaceous plants have been reported as the most common habit due to the ease of collection and global distribution, and they will also be easier to use topically for cosmetic purposes because of their soft organs [8,82].
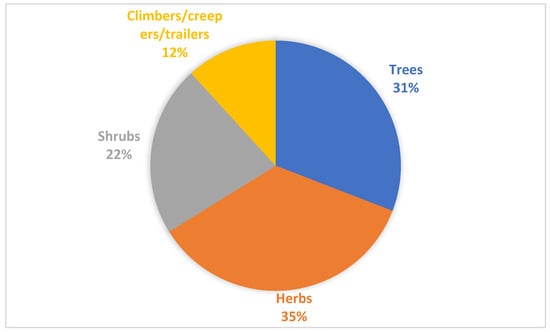
Figure 2.
Habit of the recorded plants used in hair treatment and care in Africa.
The plant parts that are mostly used in the preparation of medicinal plants for hair treatment in Africa are the leaves (24), followed by seeds (13), and then fruits (8). Other plant parts such as root, stem, rhizome, twig, nut, and the whole plant are also used, albeit less frequently. Plant parts are mostly prepared through maceration and decoction and are sometimes used as poultices or extracted into a carrier oil. The observation of a high frequency of use of leaves in the context of cosmetic benefit is consistent with other reports [7,8]. Furthermore, the high frequency of seeds and fruits may be because they contain a high concentration of essential oils (sterilizing effects and anti-inflammatory effects), with fatty acid components such as arachidic, linoleic acid, oleic, and stearic acids. The fatty acid and triglyceride components are natural preservative ingredients commonly used in the manufacturing of cosmetics for skin and hair care and rejuvenation [83,84]. Fatty acids tend to change the aesthetic feel of hair and improve the dryness of scalps afflicted with dermatological complaints.
As expected, nearly all the extracts of species are administered topically, except for Leonotis leonurus. In this case, the leaf extract is drunk for hair growth [24]. A probe into the oral toxicological profile of L. leonurus revealed that its aqueous extract caused cardiovascular changes in male rats at doses ranging from 125–500 mg/kg [85], and histopathological and haematological changes in female rats from 125–3200 mg/kg body weight, with death occurring at the higher concentration [86]. While the studies emphasized dose as key to these safety concerns, the material collected for these studies should be examined for similarity to the chemical profile used in other regions of the country. It is common for chemical variation across geography to be a significant factor in toxicity and non-toxicity [87]. It should also be noted that these doses in humans equate to 10–256 g for an 80 kg mass, which may exceed the dose taken in traditional medicine. But even at lower doses, chronic toxicity from long-term use should be considered.
Nevertheless, it is clear that topical administration of medicinal plant extracts is the most preferred choice in cosmetic applications for hair and skin care [7]. This is suggestive that practitioners of nutritional medicine are unaware of the importance of the two-thronged approach recommended by researchers, where both a topical and an oral approach is recommended [10].
3.3. Perspectives on the Culture of Hair Care in Africa
Allegedly, the sub-Saharan African people are less concerned with hair loss by comparison with the other cultures of the world. Until recently, these same people were unlikely to experience pattern hair loss, also known as androgenetic alopecia or “male pattern baldness” [88]. However, studies are demonstrating an increase in occurrence, such as in Nigeria, where prevalence has reached 30% in men compared to approximately 50% in Europeans [89].
The lower frequency of topical and oral treatments for AGA in southern and southwestern parts of Africa is rumored to be due to a cultural indifference to balding, since many sub-Saharan Africans shave their hair off. An alternative perspective is that AGA was not prevalent in earlier generations, giving less time for traditional medicines to be discovered through iterative trial and error selection.
The apparent generational change in the frequency of AGA in Africans has confounded perspectives on the genetic aspect of AGA. This is because cultures that previously had minimal occurrence of the phenotype in recent or ancient history are now demonstrating baldness. In Nigeria, familial history of AGA accounts for approximately 50% of incidences, with the remainder having no apparent genetic basis for its occurrence [89]. This is evident in other cultures, too, such as Korea [90] and China [91], with family history of AGA accounting for only 48.5% and 30% of those with the phenotype, respectively.
The rising prevalence of AGA has also been observed in several countries but epidemiological studies that confirm this observation are scarce. A study of Korean men documented that the average age of men who developed AGA in the years from 2006 to 2010 decreased from 34.1 to 31.6 years [92]. A study of a population in Israel over the course of a decade (2010–2020) measured an increased rate of AGA from 17% to 32% [93] in the general population.
Due to the apparent contradictions to the theory of genetic origins of AGA, scholars are now shifting their focus to epigenetics, where diet and lifestyle factors may be driving epigenetic changes that accumulate along family lineages [9]. This may explain why family history does have an association with AGA development but does not account for most cases. It is speculated that the same diet and lifestyle factors leading to cardiovascular disease and diabetes may be responsible for the emergence of AGA in family lineages with no history of this phenotype.
Challenges to this perspective include the documentation of baldness during the times of ancient Rome and Greece. However, the theory of epigenetic factors in AGA gives cognizance to the potential for such changes to be reversed when negative lifestyle and dietary factors are removed [94,95], meaning that AGA can be created in or removed from family lineages. While this theory is still in early development, it is currently the best explanation for the changing epidemiology of AGA in societies that have a more recent history of progression into the “western diet”.
Thus, the changed diet and lifestyle of the sub-Saharan African people may be considered a factor in the rising prevalence of AGA and the infrequency of records of flora used to reduce the severity of the phenotype. For example, most records in Table 1 are for hair problems that are not AGA.
It is also common for women to want their hair to grow or regrow faster, so a unique approach is utilized to find a species that does not resolve hair loss per se but promotes hair growth. An example is the southern African climber, Cassytha ciliolata, which is selected for this application based on a “doctrine of signatures” approach. It grows atop other bushes and colonizes the canopy, creating the appearance of a thick head of hair over its host. The rapid and thick growth of the species is conceptually replicated if an extract of its aerial parts is applied topically.
While studies of medicinal plants used to treat hair loss tend to focus on identifying single active metabolites with selective mechanisms of action, natural products are not pharmaceuticals and should, therefore, not be thought of as having single targets and mechanisms. The studies listed in Table 2, for example, tend to focus on isolated hair follicles or cells and measure biomarkers of growth, changes to the growth rate of hair fibers, or proliferation of cells. While these studies give some level of credence to the medicinal species and may also demonstrate a lack of toxicity, the systematic nature of AGA is ignored in those studies. Therefore, the current review argues that it makes more sense to consider that benefits to hair are mainly due to the use of nutritional species that are prophylactic to lifestyle diseases and can work locally to alleviate the damaging effects of dietary components in a matrix of cells that have a pathophysiological difference compared to non-balding tissues.
3.4. Topical Nutrition: Biological Activities of the Recorded Plant Species
An earlier review of natural therapies for hair loss, from a global perspective, recognized that a high number of remedies are also used to treat diabetic complaints, such as insulin resistance and metabolic syndrome, as well as the symptoms of diabetes mellitus type two [11]. From these observations, multiple theories of hair loss etiology and pathogenesis have been proposed where androgenetic alopecia is partly mediated by issues of glucose metabolism leading to mitochondrial fatigue and reactive oxygen species accumulation in the cells at the base of the hair follicle [9,10].
Research has also demonstrated that hair loss of all types is linked to eight key etiological components, including inflammation, accelerated lipogenesis, androgen imbalance, glucose metabolism, prostaglandin imbalance, microbial overgrowth of the scalp, fibrosis, and nutritional deficiency [10]. Hence, topical therapies can ameliorate factors leading to hair loss by contributing nutrients to the scalp, reducing inflammation, reducing microbial density, and harmonizing glucose metabolism.
Transcriptomic studies have identified that cells at the base of the hair follicle (dermal papilla cells) demonstrate a malfunction of the electron transport chain and a high rate of reactive oxygen species generation [96]. This indicates that even penetrating antioxidants from plants can be supportive of hair growth [97]; however, a greater effect can be achieved if natural products support or promote the production of endogenous antioxidants, such as superoxide dismutase, catalase, or glutathione.
Isoflavones have been linked to endogenous antioxidant promotion [98,99,100]. A significant number of species used to treat hair loss express isoflavones in their metabolome [11]. Furthermore, the mechanism of diabetes attenuation is often linked to the promotion of endogenous antioxidants [101]. Thus, the nutritional effects of oral or transdermal hair nutraceuticals may be related to the attenuation of redox imbalance, explaining the link between attenuating diabetic symptoms and hair rejuvenation.
The restoring of redox balance may also be linked to a more efficient metabolism of androgens, cholesterol, and endogenous artifacts of mitochondrial overactivity. For example, the isothiocyanates in species of Allium or Brassica are associated with improved cellular antioxidant status [102] and improve the activity of dermal metabolizing enzymes, including 3α-hydroxysteroid dehydrogenase [103], an enzyme that eliminates dihydrotestosterone, which is an androgen linked to androgenetic alopecia [10].
While the mechanisms of hair loss therapies are not immediately obvious, published bioassays may assist with reinforcing the efficacy of natural products and demonstrating safety if tolerated by animals or humans [18]. Of the 68 plants recorded, 30 have been tested for activities related to managing alopecia (hair growth) and hair care, like dandruff and scalp care (Table 2). To the latter, while these items are not directly linked to hair growth, it is the contention of some scholars that alleviating microbial overgrowth, resolving inflammation, and conditioning will directly contribute to the quality and quantity of hair strands on the scalp [10].

Table 2.
Studies and findings of species used for hair care and rejuvenation.
Table 2.
Studies and findings of species used for hair care and rejuvenation.
| Plant Species | Plant Part | Extract Used/Isolated Compound | Type of Study and Result | Phytochemicals | References |
|---|---|---|---|---|---|
| Abrus precatorius | Leaf | Petroleum ether and ethanolic extract | Preclinical (in vivo): promotes hair growth by accelerating hair follicles in up to 71% hair population from the telogen phase to the anagen phase. | NA | [104] |
| Adiantum capillus-veneris | Aerial part | Ethanol | Preclinical (in vivo): follicular density in the treated group was higher than in the testosterone-treated group (1.92 vs. 1.05). Anagen/telogen ratio is also higher (0.92 vs. 0.23). | Triterpenes, flavonoids, phenylpropanoids, and carotenoids | [20] |
| Allium ascalonicum | Bulb | Methanol | Preclinical (in vitro): promotes hair growth by inhibiting the androgen gene expression and amplification of the genes associated with Wnt/β-catenin, sonic hedgehog and angiogenesis pathways. | p-coumaric acid, quercetin, and rosmarinic acid | [105] |
| Allium cepa | Bulb | Juice | Clinical: improved hair re-growth in people with alopecia areata by up to 93%. | NA | [106] |
| Azadirachta indica | Seed | Shampoo based on seed extract | Clinical: shampoo was effective on head lice with a mortality rate of up to 93% | NA | [107] |
| Calophyllum inophyllum | Leaf | Polyphenol, flavonoid, anthocyanin, and saponin | Preclinical (in vitro): promotes hair follicle proliferation up to 25 µg mL−1 and reduces the expression of genetic biomarkers associated with reduced hair growth, namely DKK1 and TGFB1. | Procyanidin, sinapic acid, and 3,4,5-tri-O-caffeoylquinic acid | [13] |
| Cannabis sativa | Seed | Oil | Clinical: the oil markedly improved hair growth in 25% of the patients with alopecia areata between 4 and 8 weeks. | Cannabidiol | [108] |
| Capsicum annum | Fruit | Capsaicin and isoflavone | Clinical and preclinical (in vitro and in vivo): capsaicin and isoflavone also significantly promote hair growth by up to 64.5% in humans with alopecia. Capsaicin increased IGF-1 in hair follicles and hair development. | Capsaicin and isoflavone | [109,110] |
| Carica papaya | Fruit | Isopropyl 2,5-dihydroxyben-zoate | Preclinical (in vitro): the compound significantly promotes growth of human hair follicle dermal papilla cells by 112% | Isopropyl 2,5-dihydroxyben-zoate | [111] |
| Citrullus colocynthis | Fruit | Petroleum ether and ethanol | Preclinical (in vivo): reduction in hair growth initiation time and time required to complete hair growth in rats through promotion of the hair follicles from the telogen to anagen phase by more than 70%. | NA | [6] |
| Cocos nicifera | Oil | Vatika enriched coconut hair oil | Clinical: oil is effective in the management of dandruff and hair fall by increasing hair health parameters. | NA | [112] |
| Cymbopogon citratus | Whole plant | Ethanol | Preclinical (in vitro): inhibited 5α-reductase in vitro and improved hair growth. | NA | [42] |
| Cyperus rotundus | Tuber | Oil extract | Clinical: extract significantly reduced axillary hair growth in humans at p < 0.5. | Flavonoids, lignans, and polyphenols | [47] |
| Dicerocaryum senecioides | Leaf | Flavonoid glycosides extract | Preclinical (in vivo): increase in hair follicle length of individual hairs up to 12 mm compared to control, which is 5 mm. | Steroidal glycosides, triterpenoid glycosides, and flavonoid glycosides | [74] |
| Eclipta prostrata | Whole plant | Methanol | Preclinical and clinical (in vivo and human trial): promotes hair follicle counts in mice by inducing anagen transition from the telogen (resting) phase in hair follicles. It also showed 54% efficacy in a human trial. | Flavonoids, glycosides, and thiophene | [25] |
| Erica multiflora | Ethanol | Preclinical (in vivo): the extract promotes dermal papilla cell proliferation and hair growth up to 144% at 5000 μg/mL | NA | [49] | |
| Hibiscus rosa-sinensis | Leaves | Petroleum ether extract | Preclinical (in vivo): leaf extract increased hair light up to 17 mm after 30 days compared to control (13.6 mm). It also reduced the rate of telogen hair follicles in mice. | Flavonoids, glycosides, lipids, and citrus and oxalic acids | [113,114] |
| Ipomoea aquatica | Leaf and stem | Ethanol extract | Preclinical (in vitro): inhibited 5α-reductase activity with IC50 of 13.16. | NA | [42] |
| Ipomoea batatas | Rhizome | Oil extract | Preclinical (in vitro): promoted hair growth in mice through expression of vascular endothelial growth factor up to 392 pg/mL compared to control (246 pg/mL). | Ethyl α-linolenate, ethyl linoleate, and ethyl palmitate | [115] |
| Lawsonia inermis | Leaf | Ethanol | Preclinical (in vitro): inhibited 5α-reductase activity with IC50 of 12.58. | NA | [42] |
| Malus domestica | Fruit | Procyanidin B-2 (epicatechin-(4b-8)-epicatechin) | Preclinical (in vitro and in vivo): Procyanidin B-2, procyanidin B-3, and procyanidin C-1, inhibited protein kinase C and promoted hair growth. | Procyanidin B-2, procyanidin B-3, and procyanidin C-1 | [116,117,118] |
| Myrtus communis | NA | Aqueous extract plus vinegal | Clinical: the solution was effective against scaly scalps and dandruff at p < 0.001. | NA | [119] |
| Ocimum sanctum | Whole plant | Fermented product from the powder | Preclinical (in vitro): inhibited different strains of Malassezia furfur, causing dandruff up to the 17 mm zone. | NA | [120] |
| Persea americana | Fruit | Oil | Preclinical (in vitro): the compounds improved damaged hair cells in zebrafish. | (2R,4R,6Z)-1,2,4-trihydroxynonadec-6-ene and (2R,4R)- 1,2,4-trihydroxyheptadecadi-14,16-ene | [121] |
| Phyllanthus emblica | NA | Ellagic acid | Preclinical (in vitro): inhibited 5α-reductase activity, and it is associated with the expression of vascular endothelial growth factor (a biomarker for hair growth). | Ellagic acid | [122] |
| Ricinus communis | Seed | Oil | Preclinical (in vitro): promotes the shining of the hair. It improves the dilation of blood vessels to the hair follicle. Decreases the expression of prostaglandin D2. | Ricinoleic acid and fatty acids | [123] |
| Senna siamea | Leaf | Ethanol | Preclinical (in vitro): inhibited 5α-reductase inhibitory activity with IC50 12.87. | NA | [42] |
| Sesamum indicum | Seed | Oil | Preclinical (in vitro): inhibited 5α-reductase by 37% | Sesamin | [124] |
| Theobroma cacao | Fruit peel | Ethanol | Preclinical (in vivo): extract improved hair growth in rabbits at concentrations above 15%. | NA | [125] |
| Tridax procumbens | Aerial part | Ethanol | Preclinical (in vivo): extract in the form of 10% ointment promotes hair growth and development | Flavonoids, beta-sitosterol, alkaloids, tannin, luteolin, glucolteolin, and isoquercetin | [3,126] |
| Trigonella foenum-graecum | Leaf | Ethanol and petroleum ether | Preclinical (in vivo): it significantly improved hair length in mice at p < 0.05. | Vitexin | [127] |
| Xylopiaaethiopica | Fruit | Mixture of Xylopia aethiopica and sulphur | Preclinical (in vivo): topical application promoted hair re-growth and cured psoroptic mange in rabbits at p < 0.05. | NA | [22] |
| Zingiber officinale | Rhizome | Ethanol | Preclinical (in vitro): inhibited 5α-reductase activity with IC50 18.32. | NA | [42] |
The list in Table 2 demonstrates a deficit of research in West Africa that is focused on bioassays of hair loss therapies. Nevertheless, from the publications summarized, several potential mechanisms have been proposed. For example, ricinoleic acid from R. communis oil allegedly decreases the expression of prostaglandin D2 in the scalp [123], which is a negative growth factor.
Studies that explore the efficacy or mechanism of species often measure the expression of vascular endothelial growth factor (VEGF) in human hair follicle dermal papilla cells (HFDPC) as a biomarker of improvement in growth. Such studies include ellagic acid from Phyllanthus emblica, isopropyl 2,5-dihydroxybenzoate (Figure 3) from the fermented fruits of Carica papaya, shochu oil from Ipomoea batatas, and a crude ethanol extract from Erica multiflora [49,111,122]. While these studies tend to associate the measured increase in the expression of growth factors as a mechanism of action leading to improved hair growth, it is not likely to have been directly related. These changes are more correctly interpreted as part of the downstream cascade of biochemical changes caused by another more general mechanism, quite likely related to the promotion of the electron transport chain through phytochemical nutrition.
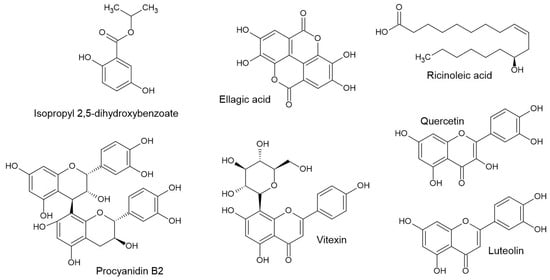
Figure 3.
Structures of some metabolites responsible for hair care activity in African plants.
Generally, when studies focus on inhibition of 5α-reductase, the IC50 values are not realistically met in vivo to actualize the outcome unless applied topically. For example, the study of P. emblica reported that the extract can reduce the expression of 5α-reductase while increasing VEGF at 50 µg/mL [122]. While this concentration is unfeasible with oral ingestion, it is achievable with topical application and transdermal absorption of the active constituents [16]. Changes to the expression of 5α-reductase may be via any number of hypothetical mechanisms not necessarily familiar to the mechanism of the pharmaceutical 5α-reductase inhibitor finasteride.
Similar comments can be made in relation to studies of other species, such as the whole plant of Cymbopogon citratus, leaves of Senna siamea, Lawsonia inermis, Ipomoea aquatica (leaf and stem), rhizome of Zingiber officinale, and sesamin from Sesamum indicum L [42,122,124]. Concentrations required to inhibit 5α-reductase are many orders higher than the standard pharmaceutical 5α-reductase inhibitor finasteride, but due to the advantages of transdermal penetration, the in vivo outcome is feasible.
Nevertheless, due to the difficulty in defining the mechanism of action or the potential for improper interpretation of such studies, it is often better to conduct in vivo studies. For example, capsaicin and isoflavones from the fruit of Capsicum annum, juice from Allium cepa, the extract from the aerial parts of Adiantum capillus-veneris, the leaves from Abrus precatorius, the whole plant of Eclipta prostrata, the leaves of Hibiscus rosa-sinensis, and the leaves of Citrullus colocynthis promoted hair growth in mice and people by shortening the time for hair follicles to transition from the telogen to anagen phase [6,20,25,104,109,110,113,114]. While these studies are not about mechanisms, they do convey phenotypic changes that are consistent with claims about being supportive of hair growth. Nevertheless, because these studies are commonly conducted in mice with testosterone-induced hair loss, the pathological state of the dermis of the mouse cannot be compared to that of the human with dissimilar pathophysiology caused by androgenetic alopecia or other hair loss pathologies.
For example, human scalps with a true hair loss pathology often benefit from disinfection. This is because hair loss pathologies may involve potentiating factors, such as bacterial overgrowth due to excessive sebum production, Malassezia furfur overgrowth leading to dandruff, or ectoparasite infection by Demodex spp. [10]. These microbes contribute to inflammation and can interfere with the health of the scalp dermis, creating a barrier to the efficacy of other lines of therapy. They can also create cosmetic effects that are undesirable, such as dandruff. Hence, other extracts from plants such as Azadirachta indica, Cocos nicifera, Myrtus communis, and Ocimum sanctum may benefit hair by nurturing the health of the dermis, in the management of dandruff, lice, inflammation, and scaly scalps [107,112,119,120].
3.5. Comment on the Geography of Species and Reports
The countries in Africa from where the reports originated, and the species used in hair care are summarized in Table 1. The information conveys that the countries with the highest number of reports are Nigeria, Tunisia, and South Africa. While this may be an artifact of the numbers of ethnobotanical studies conducted in each country, it also gives a very general overview of cultural differences across the continent.
Nevertheless, due to the high possibility of chemical differences in species according to geography, it is necessary to acknowledge that species with efficacy in hair care from one country might not be replicated in another if the raw material is sourced locally. The geographical specificity of chemistry has been extensively documented in the published literature [87,128,129]. While such variation has not been comprehensively explored in the species relevant to the current review, the chemovariability of medicinal and edible species is a widely established phenomenon.
4. Conclusions
The paper presents, for the first time, an overview of medicinal plants used in hair treatment and care in Africa. The 68 reported plants are distributed among 39 angiosperm families, with Lamiaceae having the highest number of species. Regarding the habits of the species recorded in this review, most of the plants are herbs (23), followed by trees (21), shrubs (16), and climbers/creepers/trailers (8). The frequently used plant parts are leaves (24), followed by seeds (13) and fruits (8), and they are mostly prepared through maceration, oil extraction, and decoction, and are sometimes used as poultices. Of the 68 plants recorded, 30 have been evaluated for different biological activities, particularly in relation to phenotypic changes demonstrated by in vivo studies.
The beneficial effects of these therapies may be conceptualized as a type of topical nutrition since the mechanisms are not easily explained in the pharmaceutical paradigm. In most cases, such species restore redox balance, support the electron transport chain in mitochondria, and reduce triggers of inflammation. This lends credence to the folkloric use of plants in hair treatments and care.
Furthermore, 58 of the 68 species have demonstrated the potential to be used as antidiabetic therapies, so the link between hair loss and dysregulated glucose metabolism is reinforced. While potent antioxidation can alleviate diabetic symptoms, restore redox balance, and rejuvenate levels of NADPH, the same extracts can also confer sterilizing and anti-inflammatory effects that support their efficacy in a systematic way.
Future studies of the species used to support hair growth should, therefore, focus on natural products and their pharmacophores that sensitize insulin as an extrapolation of efficacy linked to hair growth. The same studies, if focused on yield and potency of metabolites, may reveal commercial opportunities. Currently, the strongest research deficit on natural products, particularly as candidates for hair care, is in West Africa; therefore, this part of Africa may represent the least explored region in this context, with the highest potential for novel findings to be made.
Author Contributions
Conceptualization, A.A.-n.A. and N.J.S.; writing—original draft preparation, A.A.-n.A. and N.J.S.; writing—review and editing, A.A.-n.A. and N.J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the University of Johannesburg for support while compiling this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fongnzossie, E.F.; Tize, Z.; Fogang Nde, P.J.; Nyangono Biyegue, C.F.; Bouelet Ntsama, I.S.; Dibong, S.D.; Nkongmeneck, B.A. Ethnobotany and pharmacognostic perspective of plant species used as traditional cosmetics and cosmeceuticals among the Gbaya ethnic group in Eastern Cameroon. S. Afr. J. Bot. 2017, 112, 29–39. [Google Scholar] [CrossRef]
- Butler, H.; Poucher, W.A. Perfumes Cosmetics and Soaps; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Bharti, M.; Shrivastav, A.; Abid, M.; Khan, N.A. A Review on Hair Growth Regulator. J. Drug Deliv. Ther. 2020, 10, 368–375. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Simmonds, M.S.J. Topical and nutricosmetic products for healthy hair and dermal anti-aging using ‘dual-acting’ (2 for 1) plant-based peptides, hormones, and cannabinoids. FASEB BioAdvances 2021, 3, 610. [Google Scholar] [CrossRef] [PubMed]
- Sagbo, I.J.; Mbeng, W.O. Are plants used in the Eastern Cape province for cosmetics fully commercialized? Indian J. Pharmacol. 2019, 51, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.K.; Thakur, M.; Dixit, V.K. Effect of Citrullus colocynthis. on Hair Growth in Albino Rats. Pharm. Biol. 2007, 45, 739–744. [Google Scholar] [CrossRef]
- Ndhlovu, P.T.; Mooki, O.; Otang Mbeng, W.; Aremu, A.O. Plant species used for cosmetic and cosmeceutical purposes by the Vhavenda women in Vhembe District Municipality, Limpopo, South Africa. S. Afr. J. Bot. 2019, 122, 422–431. [Google Scholar] [CrossRef]
- Manka, S.; Mapadimeng, M.S.; Mokgadi, R. Indigenous Foods in Decline? A Study on Changing Consumption Patterns within the Barolong Boo Ratshidi Community, Northwest Province of South Africa. S. Afr. Rev. Sociol. 2023, 53, 67–84. [Google Scholar] [CrossRef]
- Sadgrove, N.J. The ‘bald’ phenotype (androgenetic alopecia) is caused by the high glycaemic, high cholesterol and low mineral ‘western diet’. Trends Food Sci. Technol. 2021, 116, 1170–1178. [Google Scholar] [CrossRef]
- Sadgrove, N.; Batra, S.; Barreto, D.; Rapaport, J. An updated etiology of hair loss and the new cosmeceutical paradigm in therapy: Clearing ‘the big eight strikes’. Cosmetics 2023, 10, 106. [Google Scholar] [CrossRef]
- Sadgrove, N.J. The new paradigm for androgenetic alopecia and plant-based folk remedies: 5α-reductase inhibition, reversal of secondary microinflammation and improving insulin resistance. J. Ethnopharmacol. 2018, 227, 206–236. [Google Scholar] [CrossRef] [PubMed]
- Sadgrove, N.J. Are South African wild foods the answer to rising rates of cardiovascular disease? Diversity 2022, 14, 1014. [Google Scholar] [CrossRef]
- Hughes, K.; Ho, R.; Greff, S.; Filaire, E.; Ranouille, E.; Chazaud, C.; Herbette, G.; Butaud, J.-F.; Berthon, J.-Y.; Raharivelomanana, P. Hair Growth Activity of Three Plants of the Polynesian Cosmetopoeia and Their Regulatory Effect on Dermal Papilla Cells. Molecules 2020, 25, 4360. [Google Scholar] [CrossRef]
- Khoury, M.; Stien, D.; Eparvier, V.; Ouaini, N.; El Beyrouthy, M. Report on the Medicinal Use of Eleven Lamiaceae Species in Lebanon and Rationalization of Their Antimicrobial Potential by Examination of the Chemical Composition and Antimicrobial Activity of Their Essential Oils. Evid.-Based Complement. Altern. Med. 2016, 2016, 2547169. [Google Scholar] [CrossRef]
- Sadgrove, N.J. Southern Africa as a ‘cradle of incense’ in wider African aromatherapy. Sci. Afr. 2020, 9, e00502. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Jones, G.L. From petri dish to patient: Bioavailability estimation and mechanism of action for antimicrobial and immunomodulatory natural products. Front. Microbiol. 2019, 10, 2470. [Google Scholar] [CrossRef]
- Ajao, A.A.; Sibiya, N.P.; Moteetee, A.N. Sexual prowess from nature: A systematic review of medicinal plants used as aphrodisiacs and sexual dysfunction in sub-Saharan Africa. S. Afr. J. Bot. 2019, 122, 342–359. [Google Scholar] [CrossRef]
- Ajao, A.A.-n.; Mukaila, Y.O.; Kenkpen, D.Y. An ethnobotanical study of medicinal plants used to treat and manage diabetes mellitus in Ede, Osun State Nigeria. Ethnobot. Res. Appl. 2023, 25, 1–18. [Google Scholar] [CrossRef]
- Maroyi, A. Medicinal Uses of the Fabaceae Family in Zimbabwe: A Review. Plants 2023, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Noubarani, M.; Rostamkhani, H.; Erfan, M.; Kamalinejad, M.; Eskandari, M.R.; Babaeian, M.; Salamzadeh, J. Effect of Adiantum capillus veneris Linn on an Animal Model of Testosterone-Induced Hair Loss. Iran. J. Pharm. Res. IJPR 2014, 13, 113–118. [Google Scholar] [PubMed]
- Mohammed, A.; Koorbanally, N.A.; Islam, M.S. Anti-diabetic effect of Xylopia aethiopica (Dunal) A. Rich. (Annonaceae) fruit acetone fraction in a type 2 diabetes model of rats. J. Ethnopharmacol. 2016, 180, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, O.; Ajasin, F.; Ola-Davies, E.; Taiwo, O. Investigations into the use of Xylopia aethiopica in the treatment of psoroptic mange in rabbits. J. Nat. Remedies 2001, 1, 140–143. [Google Scholar]
- Sunmonu, T.O.; Afolayan, A.J. Evaluation of Antidiabetic Activity and Associated Toxicity of Artemisia afra Aqueous Extract in Wistar Rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 929074. [Google Scholar] [CrossRef] [PubMed]
- Monakisi, C.M. Knowledge and Use of Traditional Medicinal Plants by the Setswana-Speaking Community of Kimberley, Northern Cape of South Africa. Master’s thesis, Stellenbosch University, Stellenbosch, South Africa, 2007. [Google Scholar]
- Datta, K.; Singh, A.T.; Mukherjee, A.; Bhat, B.; Ramesh, B.; Burman, A.C. Eclipta alba extract with potential for hair growth promoting activity. J. Ethnopharmacol. 2009, 124, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Vonia, S.; Hartati, R.; Insanu, M. In Vitro Alpha-Glucosidase Inhibitory Activity and the Isolation of Luteolin from the Flower of Gymnanthemum amygdalinum (Delile) Sch. Bip ex Walp. Molecules 2022, 27, 2132. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Kerebba, N.; Oguntibeju, O.O. UPLC-ESI-QTOF-MS Profiling of Phenolic Compounds from Eriocephalus africanus: In Vitro Antioxidant, Antidiabetic, and Anti-Inflammatory Potentials. Molecules 2022, 27, 8912. [Google Scholar] [CrossRef] [PubMed]
- Petchi, R.R.; Parasuraman, S.; Vijaya, C. Antidiabetic and antihyperlipidemic effects of an ethanolic extract of the whole plant of Tridax procumbens (Linn.) in streptozotocin-induced diabetic rats. J. Basic Clin. Pharm. 2013, 4, 88–92. [Google Scholar] [CrossRef]
- Sharif, F.; Hamid, M.; Ismail, A.; Adam, Z. Antihyperglycemic Activity of Oil Palm Elaeis guineensis Fruit Extract on Streptozotocin-induced Diabetic Rats. Malays. J. Health Sci./J. Sains Kesihat. Malays. 2015, 13, 37–43. [Google Scholar]
- Prisilla, D.H.; Balamurugan, R.; Shah, H.R. Antidiabetic activity of methanol extract of Acorus calamus in STZ induced diabetic rats. Asian Pac. J. Trop. Biomed. 2012, 2, S941–S946. [Google Scholar] [CrossRef]
- Hulley, I.M.; Van Wyk, B.E. Quantitative medicinal ethnobotany of Kannaland (western Little Karoo, South Africa): Non-homogeneity amongst villages. S. Afr. J. Bot. 2019, 122, 225–265. [Google Scholar] [CrossRef]
- Luangpirom, A.; Kourchampa, W.; Junaimuang, T.; Somsapt, P.; Sritragool, O. Effect of shallot (Allium ascalonicum L.) bulb juice on hypoglycemia and sperm quality in streptozotocin induced diabetic mice. Anim. Biol. Anim. Husb. 2013, 5, 49–54. [Google Scholar]
- Fred-Jaiyesimi, A.; Ajibesin, K.K.; Tolulope, O.; Gbemisola, O. Ethnobotanical studies of folklore phytocosmetics of South West Nigeria. Pharm. Biol. 2015, 53, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Sha, A.; Mohapatra, A.K. Evaluation of antidiabetic and antioxidant activities of Achyranthes aspera leaf extracts: An in vitro study. J. Pharmacogn. Phytochem. 2021, 10, 103–110. [Google Scholar]
- Punjani, B.L.; Kumar, V. Plants used in traditional phytotherapy for hair care by tribals in Sabarkantha district, Gujarat, India. India J. Indig. Knowl. 2003, 2, 74–78. [Google Scholar]
- Okolie, O.D. An Evaluation of the Anti-Diabetic Properties Asparagus africanus Lam. Root Extracts. Master’s thesis, Central University of Technology, Bloemfontein, Free State, South Africa, 2014. [Google Scholar]
- abbas Abbas, J.; Minarti, M.; Artanti, N. Antioxidant and antidiabetes activity from the fruit shell of Calophyllum inophyllum. J. Kim. Terap. Indones. 2021, 23, 73–78. [Google Scholar] [CrossRef]
- Venkateshwarlu, E.; Dileep, P.; Sandhya, P. Evaluation of anti diabetic activity of Carica papaya seeds on streptozotocin-induced type-II diabetic rats. J. Adv. Sci. Res. 2013, 4, 38–41. [Google Scholar]
- Muanya, C.; Akpunonu, C.; Onyenucheya, A. Scientists Validate More Herbs for Hair Growth. Available online: https://guardian.ng/features/scientists-validate-more-herbs-for-hair-growth/ (accessed on 12 December 2023).
- Kumar, R.; Pate, D.K.; Prasad, S.K.; Sairam, K.; Hemalatha, S. Antidiabetic activity of alcoholic leaves extract of Alangium lamarckii Thwaites on streptozotocin–nicotinamide induced type 2 diabetic rats. Asian Pac. J. Trop. Med. 2011, 4, 904–909. [Google Scholar] [CrossRef]
- Hefny Gad, M.; Demeyer, K.; Vander Heyden, Y.; Mangelings, D. Cytotoxic, Antioxidant, and Antidiabetic Activities versus UPLC-ESI-QTOF-MS Chemical-Profile Analysis of Ipomoea aquatica Fractions. Planta Med. 2021, 87, 1089–1100. [Google Scholar] [CrossRef]
- Kumar, N.; Rungseevijitprapa, W.; Narkkhong, N.-A.; Suttajit, M.; Chaiyasut, C. 5α-reductase inhibition and hair growth promotion of some Thai plants traditionally used for hair treatment. J. Ethnopharmacol. 2012, 139, 765–771. [Google Scholar] [CrossRef]
- KUSANO, S.; ABE, H.; TAMURA, H. Isolation of Antidiabetic Components from White-Skinned Sweet Potato (Ipomoea batatas L.). Biosci. Biotechnol. Biochem. 2001, 65, 109–114. [Google Scholar] [CrossRef]
- Ghauri, A.O.; Ahmad, S.; Rehman, T. In Vitro and in vivo anti-diabetic activity of Citrullus colocynthis pulpy flesh with seeds hydro-ethanolic extract. J. Complement. Integr. Med. 2020, 17, 20180228. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Mohamed, A.A. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomed. 2011, 7, 18. [Google Scholar] [CrossRef]
- Tran, H.H.T.; Nguyen, M.C.; Le, H.T.; Nguyen, T.L.; Pham, T.B.; Chau, V.M.; Nguyen, H.N.; Nguyen, T.D. Inhibitors of α-glucosidase and α-amylase from Cyperus rotundus. Pharm. Biol. 2014, 52, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, G.F.A.E.-K. Topical Cyperus rotundus Oil: A New Therapeutic Modality With Comparable Efficacy to Alexandrite Laser Photo-Epilation. Aesthetic Surg. J. 2014, 34, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Oriakhi, K.; Ibeji, C.U.; Essien, E.E.; Eluehike, N.; Orumwensodia, K.; Uadia, P.; Choudhary, I.M. In Vitro and computational studies on the antiglycation activity of compounds isolated from antidiabetic Tetracera alnifolia stem bark. J. Biomol. Struct. Dyn. 2022, 40, 9742–9751. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Han, J.; Kchouk, M.E.; Isoda, H. Hair growth regulation by the extract of aromatic plant Erica multiflora. J. Nat. Med. 2009, 63, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ben-Salah, M.; Barhoumi, T.; Abderraba, M. Ethnobotanical study of medicinal plant in Djerba island, Tunisia. Arab. J. Med. Aromat. Plants 2019, 5, 31. [Google Scholar] [CrossRef]
- Mhlongo, L.S.; Van Wyk, B.E. Zulu medicinal ethnobotany: New records from the Amandawe area of KwaZulu-Natal, South Africa. S. Afr. J. Bot. 2019, 122, 266–290. [Google Scholar] [CrossRef]
- Atchou, K.; Lawson-Evi, P.; Metowogo, K.; Eklu-Gadegbeku, K.; Aklikokou, K.; Gbeassor, M. Hypoglycemic effect and antioxidant potential of Pterocarpus erinaceus Poir. stem bark and Amaranthus spinosus L. roots extracts. J. Pharm. Sci. Res. 2020, 12, 340–350. [Google Scholar]
- Alfa, T.; Anani, K.; Adjrah, Y.; Batawila, K.; Ameyapoh, Y. Ethnobotanical survey of medicinal plants used against fungal infections in prefecture of sotouboua central region, Togo. Eur. Sci. J. ESJ 2018, 14, 342. [Google Scholar] [CrossRef][Green Version]
- Tchamadeu, M.C.; Dzeufiet, P.D.; Blaes, N.; Girolami, J.P.; Kamtchouing, P.; Dimo, T. Antidiabetic Effects of Aqueous and Dichloromethane/Methanol Stem Bark Extracts of Pterocarpus soyauxii Taub (Papilionaceae) on Streptozotocin-induced Diabetic Rats. Pharmacogn. Res. 2017, 9, 80–86. [Google Scholar] [CrossRef]
- Yakubu, J.; Isah, T.; Medugu, A.; Andema, K.; Abdulrahman, F.; Sodipo, O.; Balami, V. Acute toxicity evaluation and antidiabetic efficacy of Senna siamea Lam. methanol leaf extract in mice. Open J. Biosci. Res. 2022, 3, 18–27, ISSN: 2734-2069. [Google Scholar] [CrossRef]
- Wang, J.-J.; Jin, H.; Zheng, S.-L.; Xia, P.; Cai, Y.; Ni, X.-J. Phytoecdysteroids from Ajuga iva act as potential antidiabetic agent against alloxan-induced diabetic male albino rats. Biomed. Pharmacother. 2017, 96, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Karous, O.; Ben Haj Jilani, I.; Ghrabi-Gammar, Z. Ethnobotanical Study on Plant Used by Semi-Nomad Descendants’ Community in Ouled Dabbeb—Southern Tunisia. Plants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Elsbaey, M.; Mwakalukwa, R.; Shimizu, K.; Miyamoto, T. Pentacylic triterpenes from Lavandula coronopifolia: Structure related inhibitory activity on α-glucosidase. Nat. Prod. Res. 2021, 35, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Hamad Al-Mijalli, S.; Elsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Alshahrani, M.M.; Mrabti, H.N.; Bouyahya, A. Determination of Volatile Compounds of Mentha piperita and Lavandula multifida and Investigation of Their Antibacterial, Antioxidant, and Antidiabetic Properties. Evid.-Based Complement. Altern. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, S.; Yakubu, M.; Afolayan, A. Antidiabetic activities of aqueous leaves extract of Leonotis leonurus in streptozotocin induced diabetic rats. J. Med. Plants Res. 2011, 5, 119–125. [Google Scholar]
- Patil, R.; Patil, R.; Ahirwar, B.; Ahirwar, D. Isolation and characterization of anti-diabetic component (bioactivity-guided fractionation) from Ocimum sanctum L. (Lamiaceae) aerial part. Asian Pac. J. Trop. Med. 2011, 4, 278–282. [Google Scholar] [CrossRef]
- Mamache, W.; Amira, S.; Ben Souici, C.; Laouer, H.; Benchikh, F. In Vitro antioxidant, anticholinesterases, anti-α-amylase, and anti-α-glucosidase effects of Algerian Salvia aegyptiaca and Salvia verbenaca. J. Food Biochem. 2020, 44, e13472. [Google Scholar] [CrossRef] [PubMed]
- Makopa, M.; Mangiza, B.; Banda, B.; Mozirandi, W.; Mombeshora, M.; Mukanganyama, S. Antibacterial, Antifungal, and Antidiabetic Effects of Leaf Extracts from Persea americana Mill. (Lauraceae). Biochem. Res. Int. 2020, 2020, 8884300. [Google Scholar] [CrossRef]
- Maregesis, S.M.; Kagashe, G.A.; Felix, F. Documentation and Phytochemical Screening of Traditional Beauty Products Used in Missenyi District of Tanzania. J. Cosmet. Dermatol. Sci. Appl. 2014, 4, 355–364. [Google Scholar] [CrossRef][Green Version]
- Kareru, P.; Kenji, G.; Gachanja, A.; Keriko, J.; Mungai, G. Traditional medicines among the Embu and Mbeere people of Kenya. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 75–86. [Google Scholar] [CrossRef]
- Perez Gutierrez, R.M. Evaluation of hypoglycemic activity of the leaves of Malva parviflora in streptozotocin-induced diabetic rats. Food Funct. 2012, 3, 420–427. [Google Scholar] [CrossRef]
- Sanilkumar, R.; Muthu, A.K. Evaluation of in-vivo Antioxidant Activity of Methanolic Extract of Triumfetta rotundifolia (Linn.) on Streptozotocin induced Oxidative Stress in Wistar Rats. J. Pharm. Sci. Res. 2013, 5, 249. [Google Scholar]
- Sonia, B.; Srinivasan, B.P. Investigations into the anti-diabetic activity of Azadirachia indica. Indian J. Pharmacol. 1999, 31, 138. [Google Scholar]
- Seifu, D.; Gustafsson, L.E.; Chawla, R.; Genet, S.; Debella, A.; Holst, M.; Hellström, P.M. Antidiabetic and gastric emptying inhibitory effect of herbal Melia azedarach leaf extract in rodent models of diabetes type 2 mellitus. J. Exp. Pharmacol. 2017, 9, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.E.; de Wet, H.; Van Heerden, F.R. An ethnobotanical survey of medicinal plants in the southeastern Karoo, South Africa. S. Afr. J. Bot. 2008, 74, 696–704. [Google Scholar] [CrossRef]
- Akomas, S.C.; Ikechukwu, O.A.; Ijioma, S.N. Glucose level hematological parameters and lipid profile in Ficus sur treated diabetic rats. J. Agric. Biol. Sci. 2014, 2, 5–11. [Google Scholar]
- Sangeetha, S.; Archit, R.; SathiaVelu, A. Phytochemical testing, antioxidant activity, HPTLC and FTIR analysis of antidiabetic plants Nigella sativa, Eugenia jambolana, Andrographis paniculata and Gymnema sylvestre. J. Biotechnol. 2014, 9, 65. [Google Scholar]
- Dhouibi, I.; Flamini, G.; Bouaziz, M. Comparative Study on the Essential Oils Extracted from Tunisian Rosemary and Myrtle: Chemical Profiles, Quality, and Antimicrobial Activities. ACS Omega 2023, 8, 6431–6438. [Google Scholar] [CrossRef] [PubMed]
- Rambwawasvika, H.; Dzomba, P.; Gwatidzo, L. Hair Growth Promoting Effect of Dicerocaryum senecioides Phytochemicals. Int. J. Med. Chem. 2019, 2019, 7105834. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Moneim, A.A.; Yazid, I.A.; Mahmoud, A.M. Antihyperglycemic, antihyperlipidemic and antioxidant effects and the probable mechanisms of action of Ruta graveolens infusion and rutin in nicotinamide-streptozotocin-induced diabetic rats. Diabetol. Croat. 2010, 39, 15–35. [Google Scholar]
- Roussel, T.N.G.; Martin, F.; Aimé, Y.F.J.; Lanvin, E.E.F.; Edwige, D.K.R.; Boris, A.K.; Laure, N.J.; Enyong, O.J. Antihyperglycemic and antihyperlipidemic activities of hydroethanolic extract of the fruit of Baillonella toxisperma in streptozotocin-induced diabetic rats. Metab. Open 2022, 15, 100199. [Google Scholar] [CrossRef] [PubMed]
- Miaffo, D.; Guessom Kamgue, O.; Ledang Tebou, N.; Maa Maa Temhoul, C.; Kamanyi, A. Antidiabetic and antioxidant potentials of Vitellaria paradoxa barks in alloxan-induced diabetic rats. Clin. Phytosci. 2019, 5, 44. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Ogungbe, S.M.; Saibu, G.M.; Aboyade, O.M. In Vitro study on the hypoglycemic potential of Nicotiana tabacum leaf extracts. Bangladesh J. Pharmacol. 2014, 9, 140–145. [Google Scholar] [CrossRef]
- Sarmadi, B.; Aminuddin, F.; Hamid, M.; Saari, N.; Abdul-Hamid, A.; Ismail, A. Hypoglycemic effects of cocoa (Theobroma cacao L.) autolysates. Food Chem. 2012, 134, 905–911. [Google Scholar] [CrossRef]
- Loots, D.T.; Pieters, M.; Islam, M.S.; Botes, L. Antidiabetic effects of Aloe ferox and Aloe greatheadii var. davyana leaf gel extracts in a low-dose streptozotocin diabetes rat model. S. Afr. J. Sci. 2011, 107, 6. [Google Scholar] [CrossRef]
- Seleteng-Kose, L.E.; Likoetla, P.; Motjotji, L. Plants of Commercial Importance in Lesotho: Ethnobotanical and Pharmacological Insights. Cosmetics 2023, 10, 28. [Google Scholar] [CrossRef]
- Ahmad, H.; Khan, S.M.; Ghafoor, S.; Ali, N. Ethnobotanical Study of Upper Siran. J. Herbs Spices Med. Plants 2009, 15, 86–97. [Google Scholar] [CrossRef]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Zepernick, B. Arzneipflanzen der Polynesier; D. Reimer: Berlin, Germany, 1972; Volume 8. [Google Scholar]
- Oyedemi, S.O.; Yakubu, M.T.; Afolayan, A.J. Effect of aqueous extract of Leonotis leonurus (L.) R. Br. leaves in male Wistar rats. Hum. Exp. Toxicol. 2010, 29, 377–384. [Google Scholar] [CrossRef]
- Maphosa, V.; Masika, P.; Adedapo, A. Safety evaluation of the aqueous extract of Leonotis leonurus shoots in rats. Hum. Exp. Toxicol. 2008, 27, 837–843. [Google Scholar] [CrossRef]
- Ramos, Y.J.; Gouvêa-Silva, J.G.; de Brito Machado, D.; Felisberto, J.S.; Pereira, R.C.; Sadgrove, N.J.; de Lima Moreira, D. Chemophenetic and chemodiversity approaches: New insights on modern study of plant secondary metabolite diversity at different spatiotemporal and organizational scales. Rev. Bras. Farmacogn. 2023, 33, 49–72. [Google Scholar] [CrossRef]
- Setty, L.R. Hair patterns of the scalp of white and Negro males. Am. J. Phys. Anthropol. 1970, 33, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Oiwoh, S.O.; Akinboro, A.O.; Olasode, O.A.; Onayemi, E.O. Androgenetic Alopecia: Prevalence and Clinical Characteristics in a South-West Nigerian Population. Niger. J. Med. 2021, 30, 507–513. [Google Scholar] [CrossRef]
- Paik, J.H.; Yoon, J.B.; Sim, W.Y.; Kim, B.S.; Kim, N.I. The prevalence and types of androgenetic alopecia in Korean men and women. Br. J. Dermatol. 2001, 145, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Lee, H.-J. Characteristics of Androgenetic Alopecia in Asian. Ann. Dermatol. 2012, 24, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Son, I.P.; Yeo, I.K.; Park, K.Y.; Li, K.; Kim, B.J.; Seo, S.J.; Kim, M.N.; Hong, C.K. The Annual Changes of Clinical Manifestation of Androgenetic Alopecia Clinic in Korean Males and Females: A Outpatient-Based Study. Ann. Dermatol. 2013, 25, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Lyakhovitsky, A.; Tzanani, I.; Gilboa, S.; Segal, O.; Galili, E.; Baum, S.; Barzilai, A. Changing spectrum of hair and scalp disorders over the last decade in a tertiary medical centre. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Fahy, G.M.; Brooke, R.T.; Watson, J.P.; Good, Z.; Vasanawala, S.S.; Maecker, H.; Leipold, M.D.; Lin, D.T.S.; Kobor, M.S.; Horvath, S. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 2019, 18, e13028. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A. Epigenetic changes during aging and their reprogramming potential. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 61–83. [Google Scholar] [CrossRef]
- Chew, E.G.Y.; Lim, T.C.; Leong, M.F.; Liu, X.; Sia, Y.Y.; Leong, S.T.; Yan-Jiang, B.C.; Stoecklin, C.; Borhan, R.; Heilmann-Heimbach, S.; et al. Observations that suggest a contribution of altered dermal papilla mitochondrial function to androgenetic alopecia. Exp. Dermatol. 2022, 31, 906–917. [Google Scholar] [CrossRef]
- Beoy, L.A.; Woei, W.J.; Hay, Y.K. Effects of tocotrienol supplementation on hair growth in human volunteers. Trop. Life Sci. Res. 2010, 21, 91–98. [Google Scholar]
- Van der Eecken, H.; Joniau, S.; Berghen, C.; Rans, K.; De Meerleer, G. The Use of Soy Isoflavones in the Treatment of Prostate Cancer: A Focus on the Cellular Effects. Nutrients 2023, 15, 4856. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M. Dietary Antioxidants and Chronic Diseases. Antioxidants 2023, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Sethumathi, P.P.; Uddandrao, V.V.S.; Chandrasekaran, P.; Sengottuvelu, S.; Tamilmani, P.; Ponmurugan, P.; Vadivukkarasi, S.; Santhanakumar, M.; Begum, M.S.; Saravanan, G. Biochanin-A attenuates high-fat diet and streptozotocin-induced hyperlipidemia and oxidative stress in rats by improving antioxidant status and lipid metabolic markers. Asian Pac. J. Trop. Biomed. 2023, 13, 460–468. [Google Scholar]
- Abbasi, E.; Khodadadi, I. Antidiabetic effects of genistein: Mechanism of action. Endocr. Metab. Immune Disord.-Drug Targets (Former. Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2023, 23, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Shinozaki, S.; Shimokado, K. Sulforaphane promotes murine hair growth by accelerating the degradation of dihydrotestosterone. Biochem. Biophys. Res. Commun. 2016, 472, 250–254. [Google Scholar] [CrossRef]
- Omhare, N.; Sahu, P.; Gautam, S.; Jain, N. Evaluation of hair growth promoting activity of petroleum ether extract of Abrus precatorius Linn. on Wistar Albino rats. J. Drug Deliv. Ther. 2019, 9, 330–335. [Google Scholar] [CrossRef]
- Ruksiriwanich, W.; Khantham, C.; Muangsanguan, A.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Sommano, S.R.; Sringarm, K.; Ferrer, E.; et al. Phytochemical Constitution, Anti-Inflammation, Anti-Androgen, and Hair Growth-Promoting Potential of Shallot (Allium ascalonicum L.) Extract. Plants 2022, 11, 1499. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Al-Obaidi, H.K. Onion Juice (Allium cepa L.), A New Topical Treatment for Alopecia Areata. J. Dermatol. 2002, 29, 343–346. [Google Scholar] [CrossRef]
- Heukelbach, J.; Oliveira, F.A.S.; Speare, R. A new shampoo based on neem (Azadirachta indica) is highly effective against head lice in vitro. Parasitol. Res. 2006, 99, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Farhood, I.G.; Mamoori, A.; Sahib, Z.H. Clinical and Pathological Evaluation of Hemp Seeds Oil Effectiveness in the Treatment of Alopecia Areata. Med. J. Babylon 2023, 20, 109. [Google Scholar]
- Paus, R.; Heinzelmann, T.; Schultz, K.D.; Furkert, J.; Fechner, K.; Czarnetzki, B.M. Hair growth induction by substance P. Lab. Investig. J. Tech. Methods Pathol. 1994, 71, 134–140. [Google Scholar]
- Harada, N.; Okajima, K.; Arai, M.; Kurihara, H.; Nakagata, N. Administration of capsaicin and isoflavone promotes hair growth by increasing insulin-like growth factor-I production in mice and in humans with alopecia. Growth Horm. IGF Res. 2007, 17, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.; Amen, Y.; Nakagawa, T.; Niwa, Y.; Mira, A.; Ohnuki, K.; Murakami, S.; Imao, M.; Shimizu, K. A new aliphatic ester of hydroxysalicylic acid from fermented Carica papaya L. preparation with a potential hair growth stimulating activity. Nat. Prod. Res. 2020, 34, 1750–1755. [Google Scholar] [CrossRef]
- Kura, M.; Gupta, A.; Srivastava, R.; Luthra, S. A Randomized Double Blind Controlled Study Evaluating Efficacy & Safety of Vatika Enriched Coconut Hair Oil on Hair Health in Women with Hair Fall and Dandruff. Anc. Sci. Life 2017, 37, 281. [Google Scholar]
- Adhirajan, N.; Ravi Kumar, T.; Shanmugasundaram, N.; Babu, M. In Vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensis Linn. J. Ethnopharmacol. 2003, 88, 235–239. [Google Scholar] [CrossRef]
- Thorat, R.; Jadhav, V.; Kadam, V. Development and evaluation of polyherbal formulations for hair growth-promoting activity. Int. J. PharmTech Res. 2009, 1, 1251–1254. [Google Scholar]
- Sho, C.; Kawano, K.; Kurata, R.; Yoshimoto, M.; Okuno, H. Hair growth-promoting activity of components derived from sweet potato shochu. J. Biosci. Bioeng. 2021, 131, 405–411. [Google Scholar] [CrossRef]
- Takahashi, T.; Kamiya, T.; Yokoo, Y.; Hasegawa, A. Procyanidin Oligomers Selectively and Intensively Promote Proliferation of Mouse Hair Epithelial Cells In Vitro and Activate Hair Follicle Growth In Vivo. J. Investig. Dermatol. 1999, 112, 310–316. [Google Scholar] [CrossRef]
- Kamimura, A.; Takahashi, T. Procyanidin B-3, isolated from barley and identified as a hair-growth stimulant, has the potential to counteract inhibitory regulation by TGF-beta1. Exp. Dermatol. 2002, 11, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, A.; Takahashi, T. Procyanidin B-2, extracted from apples, promotes hair growth: A laboratory study. Br. J. Dermatol. 2002, 146, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Chaijan, M.R.; Handjani, F.; Zarshenas, M.; Rahimabadi, M.S.; Tavakkoli, A. The Myrtus communis L. solution versus ketoconazole shampoo in treatment of dandruff: A double blinded randomized clinical trial. J. Pak. Med. Assoc. 2018, 68, 715–720. [Google Scholar] [PubMed]
- Punyoyai, C.; Sirilun, S.; Chantawannakul, P.; Chaiyana, W. Development of Antidandruff Shampoo from the Fermented Product of Ocimum sanctum Linn. Cosmetics 2018, 5, 43. [Google Scholar] [CrossRef]
- Park, S.; Jeong, S.Y.; Nam, Y.H.; Park, J.H.; Rodriguez, I.; Shim, J.H.; Yasmin, T.; Kwak, H.J.; Oh, Y.; Oh, M.; et al. Fatty Acid Derivatives Isolated from the Oil of Persea americana (Avocado) Protects against Neomycin-Induced Hair Cell Damage. Plants 2021, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-H.; Kim, M.-J.; Wee, J.-H.; Kim, J.-T.; Choi, W.-H. Effects of amla (Phyllanthus embilica L.) extract on hair growth promoting. Korean Soc. Biotechnol. Bioeng. J. 2018, 33, 299–305. [Google Scholar]
- Nieves, A.; Garza, L.A. Does prostaglandin D2 hold the cure to male pattern baldness? Exp. Dermatol. 2014, 23, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, J.; Jantrawut, P.; Manosroi, W.; Kongtawelert, P.; Manosroi, A. 5α-reductase inhibition and melanogenesis activity of sesamin from sesame seeds for hair cosmetics. Chiang Mai J. Sci. 2015, 42, 669–680. [Google Scholar]
- Mustarichie, R.; Hasanah, A.N. Anti-alopecia activity of waste cacao (Theobroma cacao L.) peels. Drug Invent. Today 2019, 11, 2194. [Google Scholar]
- Saraf, S.; Pathak, A.; Dixit, V. Hair growth promoting activity of Tridax procumbens. Fitoterapia 1991, 62, 495–498. [Google Scholar]
- Imtiaz, F.; Islam, M.; Saeed, H.; Saleem, B.; Asghar, M.; Saleem, Z. Impact of Trigonella foenum-graecum leaves extract on mice hair growth. Pak. J. Zool. 2017, 49, 1405–1412. [Google Scholar] [CrossRef]
- Padilla-González, G.F.; Diazgranados, M.; Oliveira, T.B.; Chagas-Paula, D.; Da Costa, F.B. Chemistry of the subtribe Espeletiinae (Asteraceae) and its correlation with phylogenetic data: An in silico chemosystematic approach. Bot. J. Linn. Soc. 2018, 186, 18–46. [Google Scholar] [CrossRef]
- Žiarovská, J.; Padilla-González, G.F.; Viehmannová, I.; Fernández, E. Genetic and chemical diversity among yacon [Smallanthus sonchifolius (Poepp. et Endl.) H. Robinson] accessions based on iPBS markers and metabolomic fingerprinting. Plant Physiol. Biochem. 2019, 141, 183–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).