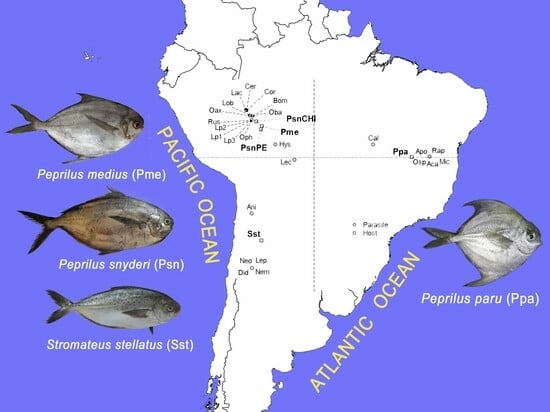
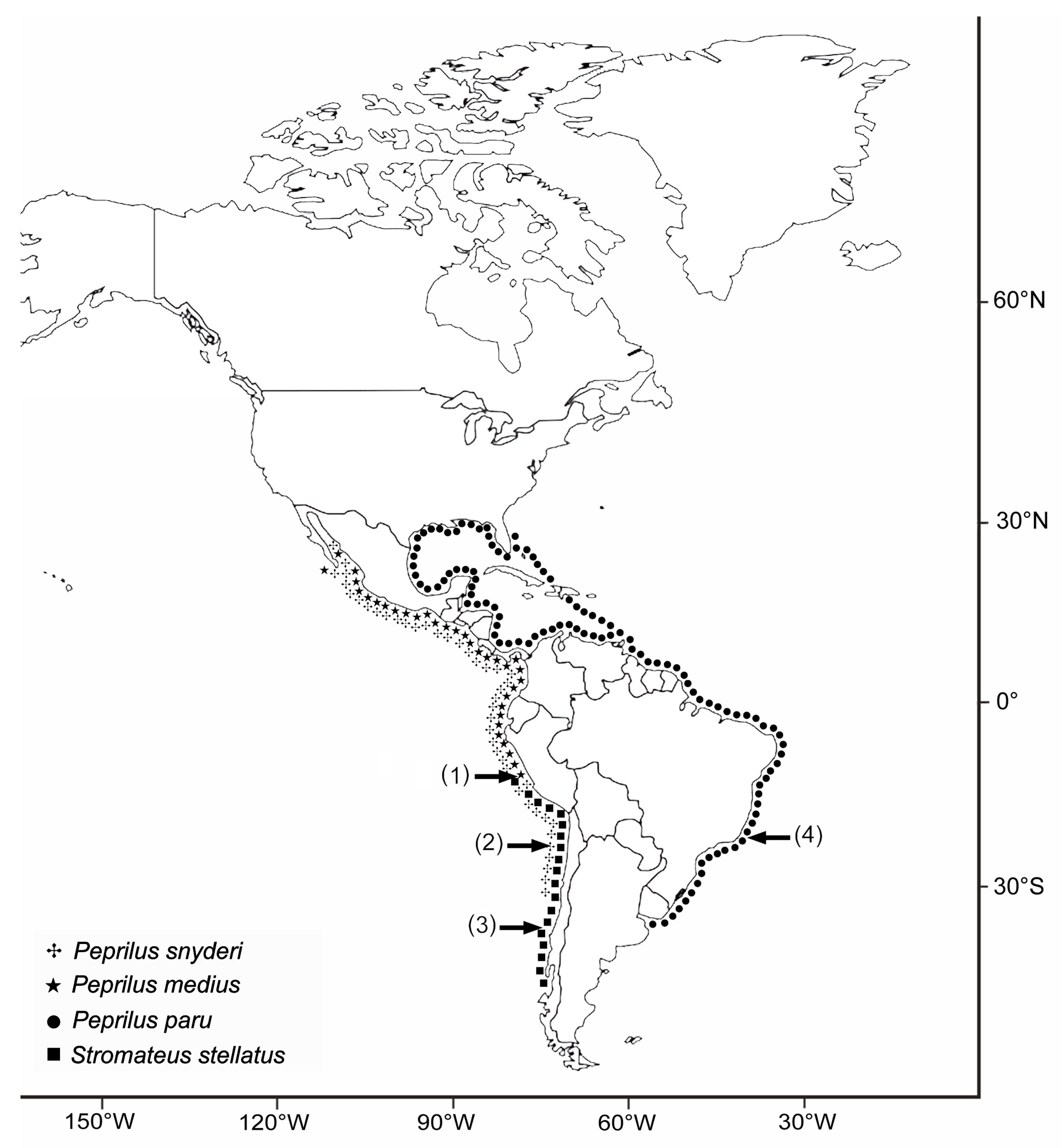
The Diversity of Metazoan Parasites of South American Stromateidae (Pisces: Teleostei) Is Related to Marine Biogeography
Abstract
1. Introduction
2. Materials and Methods
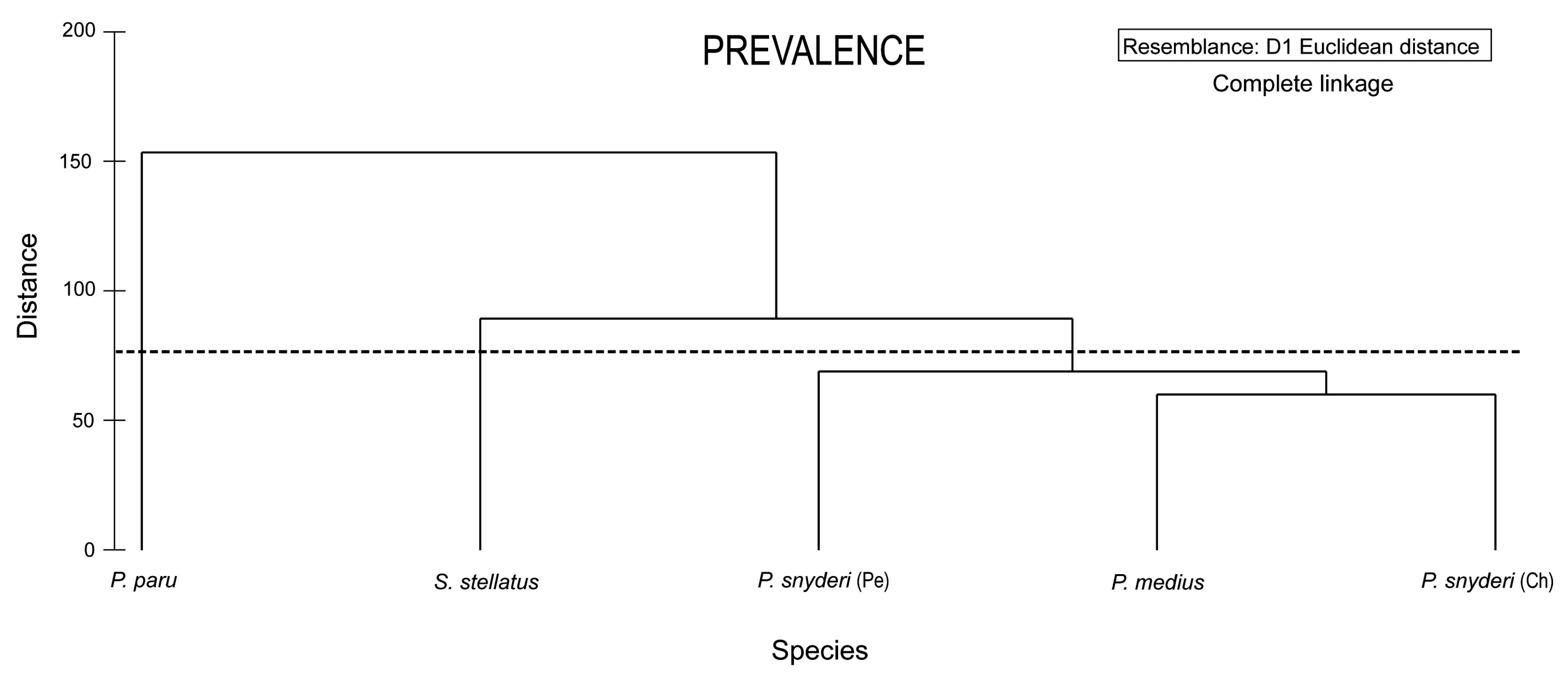
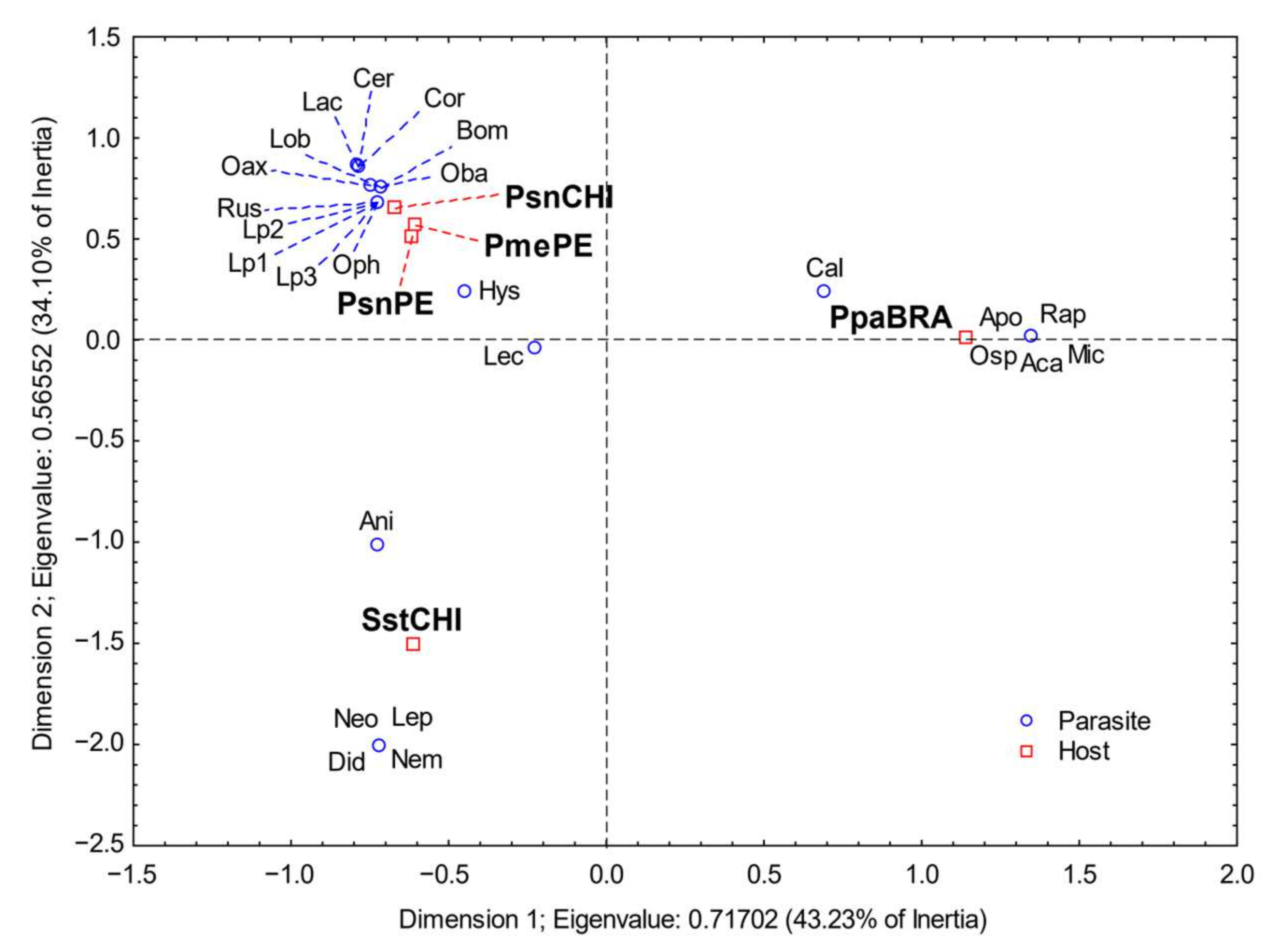
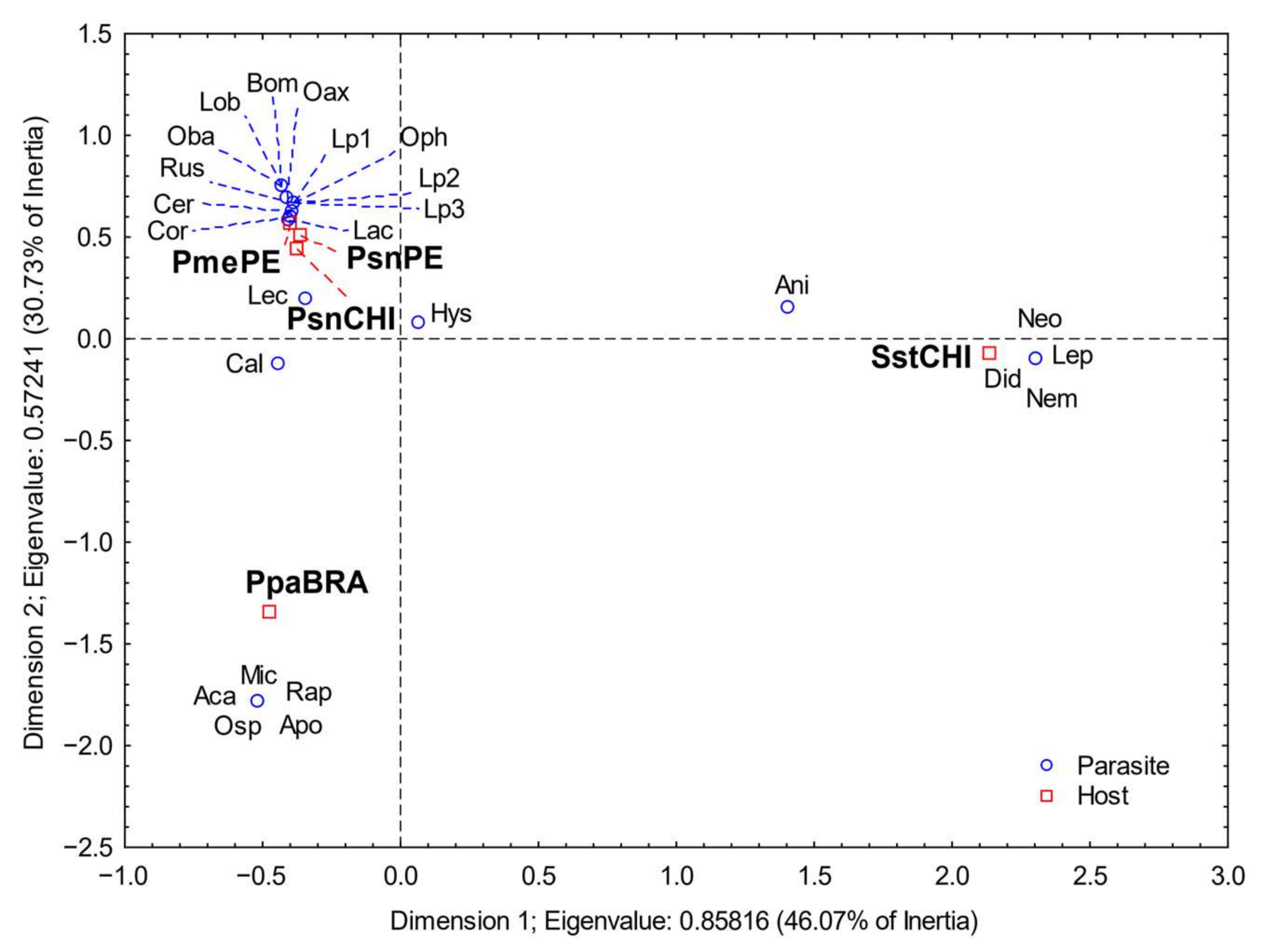
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Poulin, R. Phylogeny, ecology, and the richness of parasite communities in vertebrates. Ecol. Monogr. 1995, 65, 283–302. [Google Scholar] [CrossRef]
- Chávez, R.A.; Gonzalez, M.T.; Oliva, M.E.; Valdivia, I.M. Endoparasite fauna of five Gadiformes fish species from the coast of Chile: Host ecology versus phylogeny. J. Helminthol. 2012, 86, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ñacari, L.A.; Oliva, M.E. Metazoan parasites of deep-sea fishes from the Southeastern Pacific: Exploring the role of ecology and host phylogeny. Deep. Sea Res. Part I Oceanogr. Res. Pap. 2016, 115, 123–130. [Google Scholar] [CrossRef]
- MacLeod, C.J.; Paterson, A.M.; Tompkins, D.M.; Duncan, R.P. Parasites lost—Do invaders miss the boat or drown on arrival? Ecol. Lett. 2010, 13, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Poulin, R.; Rohde, K. Comparing the richness of metazoan ectoparasite communities of marine fishes: Controlling for host phylogeny. Oecologia 1997, 110, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Cribb, T.H.; Kulbicki, M.; Rigby, M.C.; Chauvet, C.; Dufour, V.; Faliex, E.; Galzin, R.; Lo, C.M.; Lo-Yat, A.; et al. Endoparasite species richness of New Caledonian butterfly fishes: Host density and diet matter. Parasitology 2000, 121, 65–73. [Google Scholar] [CrossRef]
- Bush, A.O.; Aho, J.M.; Kennedy, C.R. Ecological versus phylogenetic determinants of helminth parasite community richness. Evol. Ecol 1990, 4, 1–20. [Google Scholar] [CrossRef]
- Poulin, R. Decay of similarity with host phylogenetic distance in parasite faunas. Parasitology 2010, 137, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Grutter, A.S.; Cribb, T.H. Endoparasite communities of five fish species (Labridae: Cheilininae) from Lizard Island: How important is the ecology and phylogeny of the hosts? Parasitology 2006, 132, 363–374. [Google Scholar] [CrossRef]
- Alarcos, A.J.; Timi, J.T. Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae) Similar ecological filters driving toward repeatable assemblages. Parasitol. Res. 2012, 110, 2155–2166. [Google Scholar] [CrossRef]
- Oliva, M.E.; González, M.T. The decay of similarity over geographic distance in parasite communities of marine fishes. J. Biogeogr. 2005, 32, 1327–1332. [Google Scholar] [CrossRef]
- Luque, J.L.; Oliva, M.E. Metazoan Parasite Infracommunities of Menticirrhus (Teleostei: Sciaenidae): An Amphi-Oceanic Approximation. J. Parasitol. 1999, 85, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Luque, J.L.; Mouillot, D.; Poulin, R. Parasite biodiversity and its determinants in coastal marine teleost fishes of Brazil. Parasitology 2004, 128, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Cortés, Y. Parasite communities of a fish assemblage from the intertidal rocky zone of central Chile: Similarity and host specificity between temporal and resident fish. Parasitology 2009, 136, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Ñacari, L.A.; Sanchez, L. Helminth fauna of Peprilus snyderi Gilbert & Starks, 1904 (Stromateidae) of Chorrillos fishmarket, Lima, Perú. Neotrop. Helminthol. 2014, 8, 1–17. [Google Scholar]
- Azevedo, R.K.; Abdallah, V.D.; Luque, J.L. Aspectos quantitativos da comunidade de metazoários parasitos do gordinho Peprilus paru (Linnaeus, 1758) (Perciformes: Stromateidae), do litoral do estado do Rio de Janeiro, Brasil. Rev. Bras. Parasitol. Vet. 2007, 16, 10–14. [Google Scholar] [PubMed]
- Iannacone, J.; Alvarino, L. Influencia del tamaño y sexo de Peprilus medius (Peters) (Stromateidae: Perciformes) capturados en Chorrillos, Lima, Perú, sobre su comunidad parasitaria. Neotrop. Helminthol. 2008, 2, 62–70. [Google Scholar] [CrossRef]
- Pritchard, M.H.; Kruse, G.O. The Collection and Preservation of Animal Parasites; Technical Bulletin 1; The Harold W. Manter Laboratory, University of Nebraska Press: Lincoln, NE, USA, 1982; p. 141. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer v6: User Manual/Tutorial. Primer-E; Plymouth Marine Laboratory: Plymouth, UK, 2006; p. 193. [Google Scholar]
- Carrassón, M.; Dallarés, S.; Cartes, J.E.; Constenla, M.; Pérez-del-Olmo, A.; Zucca, L.; Kostadinova, A. Drivers of parasite community structure in fishes of the continental shelf of the Western Mediterranean: The importance of host phylogeny and autecological traits. Int. J. Parasitol. 2019, 49, 668–669. [Google Scholar] [CrossRef]
- Poulin, R. Parasite species richness in New Zealand fishes: A grossly underestimated component of biodiversity? Diversity Distrib. 2004, 10, 31–37. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; Wiley: Hoboken, NJ, USA, 2006; p. 752. [Google Scholar]
- Yin, G.; Pan, Y.; Sarker, A.; Baki, M.A.; Kim, J.-K.; Wu, H.; Li, C. Molecular systematics of Pampus (Perciformes: Stromateidae) based on thousands of nuclear loci using target-gene enrichment. Mol. Phyl. Evol. 2019, 140, 106595. [Google Scholar] [CrossRef]
- Marceniuk, A.P.; Caires, R.; Siccha-Ramirez, R.; Oliveira, C. Review of the harvestfishes, genus Peprilus (Perciformes: Stromateidae), of the Atlantic coast of South America. Zootaxa 2016, 4098, 311–332. [Google Scholar] [CrossRef]
- Doiuchi, R.; Sato, T.; Nakabo, T. Phylogenetic relationships of the stromateoid fishes (Perciformes). Ichthyol. Res. 2004, 51, 202–212. [Google Scholar] [CrossRef]
- Marques, J.F.; Santos, M.J.; Cabral, H.N. Zoogeographical patterns of flatfish (Pleuronectiformes) parasites in the Northeast Atlantic and the importance of the Portuguese coast as a transitional area. Sci. Mar. 2009, 73, 461–471. [Google Scholar] [CrossRef][Green Version]
- Camus, P.A. Biogeografía marina de Chile continental. Rev. Chil. Hist Nat. 2001, 74, 587–617. [Google Scholar] [CrossRef]
- González, M.T.; Barrientos, C.; Moreno, C.A. Biogeographical patterns in endoparasite communities of a marine fish (Sebastes capensis Gmelin) with extended range in the Southern Hemisphere. J. Biogeogr. 2006, 33, 1086–1095. [Google Scholar] [CrossRef]
- González, M.T.; Moreno, C.A. The distribution of the ectoparasite fauna of Sebastes capensis from the southern hemisphere does not correspond with zoogeographical provinces of free-living marine animals. J. Biogeogr. 2005, 32, 1539–1547. [Google Scholar] [CrossRef]
- Braicovich, P.E.; Pantoja, C.; Pereira, A.N.; Luque, J.L.; Timi, J.T. Parasites of the Brazilian flathead Percophis brasiliensis reflect West Atlantic biogeographic regions. Parasitology 2017, 144, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Garfias, B.; Garcia-Prieto, L.; Perez-Ponce de Leon, G. Checklist of the monogenea (Platyhelminthes) parasitic in mexican aquatic vertebrates. Zoosystema 2017, 39, 501–598. [Google Scholar] [CrossRef]
- Hernández-Orts, J.S.; Alama-Bermejo, G.; Crespo, E.A.; Raga, J.A.; Montero, F.E. A revision of the genus Neogrubea Dillon & Hargis, 1968 (Monogenea: Mazocraeidae): New morphological and molecular data from off the Patagonian coast of Argentina. Syst. Parasitol. 2014, 89, 59–72. [Google Scholar] [PubMed]
| Host Species | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. stellatus (n = 25) | P. medius (n = 115) | P. snyderi (Pe) (n = 214) | P. snyderi (Ch) (n = 9) | P. paru (n = 81) | ||||||
| Parasite Species | P | MI | P | MI | P | MI | P | MI | P | MI |
| Monogenea | ||||||||||
| Oaxacotyle oaxacensis (Oax) (Caballero ands ero and Bravo, 1964) | 33.9 | 8.2 | 86.4 | 8.8 | 78 | 3.0 | ||||
| Neogrubea seriolellae (Neo) Dillon and Hargis, 1968 | 20 | 1.8 | ||||||||
| Microcotyle sp. (Mic) | 81 | 4.9 | ||||||||
| Trematoda | ||||||||||
| Aponurus laguncula (Apo) Looss, 1907 | 26 | 2.5 | ||||||||
| Lecithocladium cristatum (Lec) (Rudolphi, 1819) | 80 | 2.8 | 33.2 | 18.2 | 92.5 | 39.4 | 67 | 15.8 | 89 | 17.2 |
| Opechona bacillaris (Oba) (Molin, 1859) | 4.3 | 1.2 | ||||||||
| Opechona pharingodactyla Oph) Manter, 1940 | 31.3 | 6.9 | ||||||||
| Acanthocolphoides sp. (Aca) | 40 | 2.8 | ||||||||
| Dydimozoidae gen. sp. (Did) | 8 | 2.0 | ||||||||
| Lepidapedon sp. 1 (Lp1) | 9.3 | 1.6 | ||||||||
| Lepidapedon sp. 2. (Lp2) | 1.9 | 3.3 | ||||||||
| Lepidapedon sp. 3. (Lp3) | 1.9 | 6.3 | ||||||||
| Lepocreadiidae gen. sp. (Lep) | 76 | 24.1 | ||||||||
| Opechona sp. (Osp) | 81 | 15.7 | ||||||||
| Lobatostoma sp. (Lob) | 7.8 | 7.2 | ||||||||
| Nematoda | ||||||||||
| Anisakis simplex (Ani) Davey 1971 | 8 | 2.0 | 5 | 1.0 | ||||||
| Hysterothylacium sp. (Hys) | 4 | 1.0 | 0.9 | 1 | 5.6 | 1.5 | 11 | 1.0 | 4.0 | 1.0 |
| Raphidascaris sp. (Rap) | 68 | 4.1 | ||||||||
| Rusguniella sp. (Rus) | 0.5 | 1.0 | ||||||||
| Nematoda gen. sp. (Nem) | 4 | 1.0 | ||||||||
| Cestoda | ||||||||||
| Lacistorhynchus tenuis (Lac) (Van Beneden, 1858) | 11 | 1.0 | ||||||||
| Acanthocephala | ||||||||||
| Corynosoma sp. (Cor) | 11 | 3.0 | ||||||||
| Copepoda | ||||||||||
| Bomolochus sp. (Bom) | 0.9 | 1 | ||||||||
| Caligus sp. (Cal) | 0.9 | 1 | 4.5 | 0.4 | 11 | 1.0 | 6 | 1.0 | ||
| Isopoda | ||||||||||
| Ceratothoa gaudichaudii (Cer) H. Milne Edwards, 1840 | 3.2 | 0.3 | 22 | 1.0 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, M.E.; Ñacari, L.A.; Escribano, R.; Luque, J.L. The Diversity of Metazoan Parasites of South American Stromateidae (Pisces: Teleostei) Is Related to Marine Biogeography. Diversity 2024, 16, 108. https://doi.org/10.3390/d16020108
Oliva ME, Ñacari LA, Escribano R, Luque JL. The Diversity of Metazoan Parasites of South American Stromateidae (Pisces: Teleostei) Is Related to Marine Biogeography. Diversity. 2024; 16(2):108. https://doi.org/10.3390/d16020108
Chicago/Turabian StyleOliva, Marcelo E., Luis A. Ñacari, Ruben Escribano, and José L. Luque. 2024. "The Diversity of Metazoan Parasites of South American Stromateidae (Pisces: Teleostei) Is Related to Marine Biogeography" Diversity 16, no. 2: 108. https://doi.org/10.3390/d16020108
APA StyleOliva, M. E., Ñacari, L. A., Escribano, R., & Luque, J. L. (2024). The Diversity of Metazoan Parasites of South American Stromateidae (Pisces: Teleostei) Is Related to Marine Biogeography. Diversity, 16(2), 108. https://doi.org/10.3390/d16020108