Integrative Morphological and Genetic Characterisation of the Fish Parasitic Copepod Ergasilus mirabilis Oldewage & van As, 1987: Insights into Host Specificity and Distribution in Southern Africa
Abstract
1. Introduction
| Species | Hosts | Distribution | Host Families | Water Body | Genetic data | References |
|---|---|---|---|---|---|---|
| Ergasilus brevimanus (Sars, 1909) Syn: Ergasiloides brevimanus Sars 1909 | TH: Unknown | TLOC: Mbete, south shore of Lake Tanganyika | - | Freshwater | - | Sars [32] |
| - | Lake Malawi | - | Freshwater | - | Sars [32] | |
| - | Angola: Dilolo Lake | - | Freshwater | - | Marques [33] | |
| Ergasilus caparti Míč, Řehulková & Seifertová, 2023 | TH: Neolamprologus brichardi (Poll, 1974) | TLOC: Magara, Lake Tanganyika, Burundi | Cichlidae | Freshwater | - | Míč et al. [34] |
| Eretmodus marksmithi Burgess, 2012; Lamprologus callipterus Boulenger, 1906; Neolamprologus mondabu (Boulenger, 1906); Perissodus microlepis Boulenger, 1898; Spathodus erythrodon Boulenger, 1900 | Burundi: Mukuruka, Mvugo, Nyaruhongoka (Lake Tanganyika) | Cichlidae | Freshwater | OQ407469 (18S); OQ407474 (28S) | Míč et al. [34] | |
| Ergasilus cunningtoni Capart, 1944 | TH: Campylomormyrus elephas (Boulenger, 1898) | TLOC: Lake Tumba, Ubangi River, Democratic Republic of the Congo | Mormyridae | Freshwater | - | Capart [35] |
| Cyphomyrus psittacus (Boulenger, 1897); Distichodus atroventralis Boulenger, 1898; Marcusenius greshoffii (Schilthuis, 1891); M. moorii (Günther, 1867); Mormyrops nigricans Boulenger, 1899; Petrocephalus grandoculis Boulenger, 1916; Pollimyrus isidori (Valenciennes, 1847); Pterochromis congicus (Boulenger, 1897), Schilbe laticeps (Boulenger, 1899); S. tumbanus (Pellegrin, 1926), Synodontis nigriventris David, 1936; Tylochromis microdon Regan, 1920 | Democratic Republic of the Congo: Lake Tumba, Ubangi River, Ikela, Tshuapa River & Mokombe River | Cichlidae; Distichodontidae; Mochokidae; Mormyridae; Schilbeidae | Freshwater | - | Fryer [36,37] | |
| Brycinus leuciscus (Günther, 1867); B. nurse (Rüppell, 1832); Distichodus rostratus Günther, 1864; Pellonula leonensis Boulenger, 1916 | Ghana: Lake Volta | Alestidae; Distichodontidae; Dorosomatidae | Freshwater | - | Paperna [38] | |
| Brycinus nurse (Rüppell, 1832); Enteromius macrops (Boulenger, 1911); Hydrocynus vittatus Castelnau, 1861; Mormyrops anguilloides (Linnaeus, 1758); Mormyrus macrophthalmus Günther, 1866; Raiamas senegalensis (Steindachner, 1870) | Nigeria: Galma River, Zaria | Alestidae; Cyprinidae; Mormyridae | Freshwater | - | Shotter [39] | |
| Chrysichthys auratus (Geoffroy Saint-Hilaire, 1809) | Nigeria: Tiga Lake, Kano | Claroteidae | Freshwater | - | Ndifon & Jimeta [40] | |
| Ergasilus egyptiacus Abdel-Hady, Bayoumy & Osman, 2008 | TH: Coptodon zillii (Gervais, 1848) | TLOC: Lake Temsah | Cichlidae | Freshwater | - | Abdel-Hady et al. [41] |
| Ergasilus flaccidus Fryer, 1965 | TH: Oreochromis tanganicae (Günther, 1894) | TLOC: Lake Tanganyika | Cichlidae | Freshwater | - | Fryer [42] |
| Ergasilus ilani Oldewage & Van As, 1988 | TH: Mugil cephalus Linnaeus, 1758 | TLOC: Mgobezeleni Estuary, Sodwana Bay, South Africa | Mugilidae | Brackish; Freshwater | - | Oldewage & van As [3] |
| M. cephalus Linnaeus, 1758 | South Africa: Kowie River Estuary, Eastern Cape | Mugilidae | Brackish; Freshwater | - | Oldewage & van As [4] | |
| Chelon richardsonii (Smith, 1846) | South Africa: Berg River and Verlorevlei River, Western Cape | Mugilidae | Freshwater | - | Oldewage & van As [4] | |
| Ergasilus inflatipes Cressey in Cressey & Collette, 1970 | TH: Strongylura senegalensis (Valenciennes, 1864) | TLOC: Volta River, Ghana | Belonidae | Freshwater | - | Cressey & Collette [43] |
| S. senegalensis (Valenciennes, 1864) | Ivory Coast: Ébrié Lagoon | Belonidae | Brackish; Marine | - | Cressey & Collette [43] | |
| Ergasilus kandti van Douwe, 1912 | TH: Unknown | TLOC: Lake Albert | Freshwater | - | van Douwe [44] | |
| Pseudosimochromis curvifrons (Poll, 1942) | Lake Tanganyika | Cichlidae | Freshwater | - | Capart [35] | |
| Lates niloticus (Linnaeus, 1758) | Mali: Niger River | Latidae | - | Capart [45] | ||
| Pterochromis congicus (Boulenger, 1897) | Democratic Republic of the Congo: Lake Tumba, Ubangi River | Cichlidae | Freshwater | - | Fryer [36] | |
| Lamprologus lemairii Boulenger, 1899; Lates niloticus (Linnaeus, 1758); Limnotilapia dardennii (Boulenger, 1899); Oreochromis tanganicae (Günther, 1894); Plecodus paradoxus Boulenger, 1898 | Lake Albert & Lake Tanganyika | Cichlidae; Latidae | Freshwater | - | Fryer [42] | |
| Tylochromis bangwelensis Regan, 1920; T. mylodon Regan, 1920; | Democratic Republic of the Congo: Lake Mweru and Luapula River | Cichlidae | Freshwater | - | Fryer [37] | |
| T. polylepis (Boulenger, 1900) | Tanzania: Malagarasi Delta | Cichlidae | Freshwater | - | Fryer [37] | |
| Citharinus citharus (Geoffroy St. Hilaire, 1809); Lates niloticus (Linnaeus, 1758); Synodontis membranaceus (Geoffroy Saint-Hilaire, 1809); Schilbe intermedius Rüppell, 1832 | Ghana: Lake Volta | Citharinidae; Mochokidae; Latidae; Schilbeidae | Freshwater | - | Paperna [38] | |
| Bagrus bajad (Forsskål, 1775); Lates niloticus (Linnaeus, 1758) | Lake Albert | Bagridae | Freshwater | - | Thurston [46] | |
| L. niloticus (Linnaeus, 1758) | Egypt: Lake Nasser | Latidae | Freshwater | - | Hamouda et al. [47] | |
| Ergasilus lamellifer Fryer, 1961 | TH: Various Haplochromis species | TLOC: Lake Victoria and the Victoria Nile | Cichlidae | Freshwater | - | Fryer [48] |
| Parailia pellucida (Boulenger, 1901) | Ghana: Lake Volta | Schilbeidae | Freshwater | - | Paperna [38] | |
| Astatoreochromis alluaudi Pellegrin, 1904; Haplochromis bicolor Boulenger, 1906; H. degeni (Boulenger, 1906); H. guiarti (Pellegrin, 1904); H. longirostris (Hilgendorf, 1888); H. nuchisquamulatus (Hilgendorf, 1888); H. obesus (Boulenger, 1906); H. obliquidens (Hilgendorf, 1888); H. retrodens (Hilgendorf, 1888) | Lake Victoria and the Victoria Nile | Cichlidae | Freshwater | - | Thurston [46] | |
| Haplochromis spp.; Haplochromis heusinkveldi Witte & Witte-Maas, 1987; H. hiatus Hoogerhoud & Witte, 1981; H. iris Hoogerhoud & Witte, 1981; H. macrognathus Regan, 1922; H. ptistes Greenwood & Barel, 1978; H. pyrrhocephalus Witte & Witte-Maas, 1987; H. teegelaari Greenwood & Barel, 1978 | Lake Victoria | Cichlidae | Freshwater | - | Witte & van Oijen [49] | |
| H. nyererei Witte-Maas & Witte, 1985 | Tanzania: Makobe Island in the western Speke Gulf, Lake Victoria | Cichlidae | Freshwater | - | Maan et al. [50] | |
| H. nyererei Witte-Maas & Witte, 1985; H. pundamilia (Seehausen & Bouton, 1998) | Tanzania: Makobe Island, south-eastern Lake Victoria | Cichlidae | Freshwater | - | Maan et al. [51] | |
| Haplochromis chilotes (Boulenger, 1911); Haplochromis mbipi (Lippitsch & Bouton, 1998); Haplochromis nyererei Witte-Maas & Witte, 1985; Haplochromis omnicaeruleus (Seehausen & Bouton, 1998); Haplochromis pundamilia (Seehausen & Bouton, 1998); Haplochromis rufocaudalis (Seehausen & Bouton, 1998); Haplochromis sauvagei (Pfeffer, 1896); Neochromis sp.; Pundamilia sp. | Tanzania: Lake Victoria | Cichlidae | Freshwater | - | Karvonen et al. [52]; Gobbin et al. [53] | |
| Clarias gariepinus (Burchell, 1822); Haplochromis spp.; Oreochromis esculentus (Graham, 1928); Protopterus aethiopicus Heckel, 1851 | Kenya: Lake Kanyaboli | Cichlidae; Clariidae; Protopteridae | Freshwater | - | Mwamburi et al. [54] | |
| Oreochromis niloticus (Linnaeus, 1758) | Kenya: Lake Victoria | Cichlidae | Freshwater | - | Mwainge et al. [55]; Outa et al. [56] | |
| Ergasilus latus Fryer, 1960 | TH: Oreochromis niloticus (Linnaeus, 1758); Sarotherodon galilaeus (Linnaeus, 1758) | TLOC: Lake Turkana, Kenya | Cichlidae | Freshwater | - | Fryer [57] |
| S. nigripinnis (Guichenot, 1861); Pelmatolapia cabrae (Boulenger, 1899) | Kitona, Moanda, and Bulambemba, near the Congo River mouth; Nile River | Cichlidae | Brackish; Freshwater | - | Fryer [37,58] | |
| Coptodon guineensis (Günther, 1862); C. zillii (Gervais, 1848); Oreochromis niloticus (Linnaeus, 1758); Sarotherodon melanotheron Rüppell, 1852 | Ghana: Volta Basin and Peshi Lagoon | Cichlidae | Brackish; Freshwater | - | Paperna [38] | |
| Auchenoglanis occidentalis (Valenciennes, 1840); Coptodon zillii (Gervais, 1848); Oreochromis niloticus (Linnaeus, 1758); Sarotherodon galilaeus (Linnaeus, 1758); Schilbe mystus (Linnaeus, 1758) | Nigeria: Galma River | Claroteidae; Cichlidae; Schilbeidae | Freshwater | - | Shotter [39] | |
| Chrysichthys nigrodigitatus (Lacepède, 1803) | Nigeria: Cross River estuary | Claroteidae | Brackish | - | Obiekezie et al. [59] | |
| Mugil cephalus Linnaeus, 1758; Neochelon falcipinnis (Valenciennes, 1836) | Republic of Benin: Ganvie, Djdje and Zogbo, Lake Nokoue Lagoon | Mugilidae | Brackish | - | Aladetohun et al. [60] | |
| M. cephalus Linnaeus, 1758; N. falcipinnis (Valenciennes, 1836) | Nigeria: Makoko, Mcquin, and University of Lagos lagoon | Mugilidae | Brackish | - | Aladetohun et al. [61] | |
| Sarotherodon melanotheron Rüppell, 1852 | Ghana: Oyibi, Fosu, Apabaka, Kpeshie, Sakumo, and Keta Lagoons | Cichlidae | Brackish | - | Rokicki et al. [62] | |
| Lates niloticus (Linnaeus, 1758) | Egypt: Lake Nasser | Latidae | Freshwater | - | Hamouda et al. [47] | |
| Sarotherodon melanotheron Rüppell, 1852 | Côte d’Ivoire: Ebrie Lagoon | Cichlidae | Brackish | - | Adou et al. [63] | |
| Ergasilus lizae Krøyer, 1863 Syn: Ergasilus nanus Beneden, 1870 | TH: Mugil liza Valenciennes, 1836 | TLOC: New Orleans, USA | Mugilidae | Marine | - | Krøyer [64] |
| Alosa fallax (Lacepéde, 1803); Barbus barbus (Linnaeus, 1758); Chelon ramada (Risso, 1827); C. saliens (Risso, 1810); Mugil cephalus Linnaeus, 1758; Solea solea (Linnaeus, 1758) | Tunisia: Gulf of Gabès & Lake Ichkeul | Alosidae; Cyprinidae; Mugilidae; Soleidae | Brackish; Marine | - | Raïbaut et al. [65] | |
| M. cephalus Linnaeus, 1758 | Algeria: Gulf of Annaba, East coast | Mugilidae | Marine | - | Boualleg et al. [66] | |
| M. cephalus Linnaeus, 1758; Neochelon falcipinnis (Valenciennes, 1836) | Republic of Benin: Ganvie, Djdje and Zogbo, Lake Nokoue Lagoon | Mugilidae | Brackish | - | Aladetohun et al. [60] | |
| Mugil cephalus Linnaeus, 1758; Neochelon falcipinnis (Valenciennes, 1836) | Nigeria: Makoko, Mcquin, and University of Lagos lagoon | Mugilidae | Brackish | - | Aladetohun et al. [61] | |
| Synodontis schall (Bloch & Schneider, 1801) | Nigeria: Nsidung beach, Cross River Estuary | Mochokidae | Brackish | - | Eyo & Effanga [67] | |
| Clarias gariepinus (Burchell, 1822) | Nigeria: Lake Gerio, Yola, Adamawa | Clariidae | Freshwater | - | Amos et al. [68] | |
| Coptodon zillii (Gervais, 1848) | Egypt: Lake Maruit | Cichlidae | Freshwater | - | Mitwally et al. [69] | |
| Ergasilus macrodactylus (Sars, 1909) Syn: Ergasiloides macrodactylus Sars, 1909 | TH: Unknown | TLOC: Sumbu, south-western shore of Lake Tanganyika | Freshwater | - | Sars [32] | |
| Brycinus imberi (Peters, 1852); Haplochromis spp.; Lethrinops spp.; Tilapia spp. | Lake Malawi | Alestidae; Cichlidae | Freshwater | - | Fryer [70] | |
| Eretmodus marksmithi Burgess, 2012; Gnathochromis permaxillaris (David, 1936); Lamprologus callipterus Boulenger, 1906; Perissodus microlepis Boulenger, 1898; Tanganicodus irsacae Poll, 1950 | Burundi: Magara, Mvugo, Nyaruhongoka (Lake Tanganyika) | Cichlidae | Freshwater | OQ407465 (18S) OQ407470 (28S) | Míč et al. [34] | |
| Ergasilus megacheir (Sars, 1909) Syn: Ergasiloides megacheir Sars, 1909 | TH: Unknown | TLOC: Sumbu, south-western shore of Lake Tanganyika | - | Freshwater | - | Sars [32] |
| Pseudosimochromis curvifrons (Poll, 1942) | Lake Tanganyika | Cichlidae | Freshwater | - | Capart [35] | |
| Pterochromis congicus (Boulenger, 1877) | Democratic Republic of the Congo: Lake Tumba | Cichlidae | Freshwater | - | Fryer [36] | |
| Bathybates fasciatus Boulenger, 1901; Bathybates minor Boulenger, 1906; Cyphotilapia frontosa (Boulenger, 1906); Haplotaxodon microlepis Boulenger, 1906; Limnotilapia dardennii (Boulenger, 1899); Plecodus paradoxus Boulenger 1898; Synodontis granulosus Boulenger, 1900; S. multipunctatus Boulenger, 1898 | Lake Tanganyika | Cichlidae; Mochokidae | Freshwater | - | Fryer [42] | |
| Shuja horei (Günther, 1894); Simochromis diagramma (Günther, 1894) | Burundi: Magara, Nyaruhongoka (Lake Tanganyika) | Cichlidae | Freshwater | OQ407466 (18S) OQ407471 (28S) | Míč et al. [34] | |
| Ergasilus mirabilis Oldewage & van As, 1987 | TH: Synodontis leopardinus Pellegrin, 1914 | TLOC: Phongolo flood plains on the Makatini Flats, South Africa | Mochokidae | Freshwater | - | Oldewage & Van As [29] |
| Brycinus imberi (Peters, 1852); Clarias gariepinus (Burchell, 1822); C. ngamensis Castelnau, 1861; Enteromius afrohamiltoni (Crass, 1960); Glossogobius giuris (Hamilton, 1822); Hydrocynus vittatus Castelnau, 1861; Labeo rosae Steindachner, 1894; Schilbe intermedius Rüppell, 1832; Synodontis zambezensis Peters, 1852 | South Africa: Limpopo River & Phongolo River System | Alestidae; Clariidae; Cyprinidae; Gobiidae; Schilbeidae | Freshwater | - | Oldewage & Van As [4] | |
| Clarias gariepinus (Burchell, 1822); C. ngamensis Castelnau, 1861; Hemichromis elongatus (Guichenot, 1861); Hepsetus odoe (Bloch, 1794); Marcusenius macrolepidotus (Peters, 1852); Schilbe intermedius Rüppell, 1832; S. mystus (Linnaeus, 1758); Synodontis leopardinus Pellegrin, 1914; S. macrostigma Boulenger, 1911; S. nigromaculatus Boulenger, 1905 | Namibia: Zambezi River, Caprivi | Cichlidae; Clariidae; Hepsetidae; Mochokidae; Mormyridae; Schilbeidae | Freshwater | - | Oldewage & Van As [4] | |
| Synodontis zambezensis Peters, 1852 | Mozambique: Lake Malawi | Mochokidae | Freshwater | - | Oldewage & Van As [4] | |
| Cyphomyrus discorhynchus (Peters, 1852) | Zimbabwe: Lake Kariba | Mormyridae | Freshwater | - | Oldewage & Van As [4] | |
| Clarias gariepinus (Burchell, 1822); Marcusenius macrolepidotus (Peters, 1852); Petrocephalus catostoma (Günther, 1866); Synodontis nigromaculatus Boulenger, 1905 | Namibia: Kwando River, Caprivi | Clariidae; Mochokidae; Mormyridae | Freshwater | - | Avenant-Oldewage & Oldewage [5] | |
| Cyphomyrus discorhynchus (Peters, 1852) | Zimbabwe: Lake Kariba | Mormyridae | Freshwater | - | Douëllou & Erlwanger [30] | |
| Clarias gariepinus (Burchell, 1822) | South Africa: Kushokwe Pan | Clariidae | Freshwater | - | Present study | |
| C. gariepinus (Burchell, 1822) | South Africa: Vaal River | Clariidae | Freshwater | OR449753 (18S); OR449755 (28S); OR448769 (COI) | Present study | |
| C. gariepinus (Burchell, 1822) | Zambia: Zambezi River | Clariidae | Freshwater | OR449754 (18S); OR449756 (28S); OR448770 (COI) | Present study | |
| Ergasilus nodosus Wilson, 1924 | TH: Bagrus bajad (Forsskål, 1775) | TLOC: White Nile, Omdurman, Sudan | Bagridae | Freshwater | - | Wilson [71] |
| Bagrus sp. | Ghana: Sielo Tuni Stream | Bagridae | Freshwater | - | Fryer [36] | |
| Ergasilus parasarsi Míč, Řehulková & Seifertová, 2023 | TH: Simochromis diagramma (Günther, 1894) | TLOC: Magara, Lake Tanganyika, Burundi | Cichlidae | Freshwater | - | Míč et al. [34] |
| Eretmodus marksmithi Burgess, 2012; Gnathochromis permaxillaris (David, 1936); Lamprologus callipterus Boulenger, 1906; Ophthalmotilapia nasuta (Poll & Matthes, 1962); Perissodus microlepis Boulenger, 1898; Tanganicodus irsacae Poll, 1950 | Burundi: Mukuruka, Nyaruhongoka (Lake Tanganyika) | Cichlidae | Freshwater | OQ407467 (18S) OQ407473 (28S) | Míč et al. [34] | |
| Ergasilus parvus Míč, Řehulková & Seifertová, 2023 | TH: Spathodus erythrodon Boulenger, 1900 | TLOC: Magara, Lake Tanganyika, Burundi | Cichlidae | Freshwater | - | Míč et al. [34] |
| Bathybates ferox Boulenger, 1898; Eretmodus marksmithi Burgess, 2012; Lamprologus callipterus Boulenger, 1906; Neolamprologus brichardi (Poll, 1974); Neolamprologus mondabu (Boulenger, 1906) | Burundi: Bujumbura fish market, Nyaruhongoka (Lake Tanganyika) | Cichlidae | Freshwater | OQ407468 (18S) OQ407472 (28S) | Míč et al. [34] | |
| Ergasilus sarsi Capart, 1944 | TH: Tylochromis mylodon Regan, 1920 | TLOC: Katanga, Democratic Republic of the Congo | Cichlidae | Freshwater | - | Capart [35] |
| Clarias ngamensis Castelnau, 1861; Marcusenius macrolepidotus (Peters, 1852); Synodontis nigromaculatus Boulenger, 1905 | Lake Bangwelu | Clariidae; Mochokidae; Mormyridae | Freshwater | - | Fryer [72] | |
| Thoracochromis moeruensis (Boulenger, 1899); Tylochromis bangwelensis Regan, 1920; T. mylodon Regan, 1920 | Democratic Republic of the Congo: Lake Mweru and Luapula River | Cichlidae | Freshwater | - | Fryer [37] | |
| Clarias gariepinus (Burchell, 1822) | Ghana: Mawli River | Clariidae | Freshwater | - | Paperna [38] | |
| Clarias anguillaris (Linnaeus, 1758); Heterobranchus bidorsalis Geoffroy Saint-Hilaire, 1809 | Nigeria: River Galma, small lakes around Zaria | Clariidae | Freshwater | - | Shotter [39] | |
| Clarias gariepinus (Burchell, 1822) | Nigeria: Bagauda fish farm, Kano | Clariidae | Freshwater | - | Bichi & Yelwa [73] | |
| Lamprichthys tanganicanus (Boulenger, 1898) | Democratic Republic Congo: Lake Tanganyika | Procatopodidae | Freshwater | - | Kilian & Avenant-Oldewage [12] | |
| Oreochromis niloticus (Linnaeus, 1758) | Egypt: Mariotteya Stream | Cichlidae | Freshwater | - | Mahmoud et al. [74] | |
| O. niloticus (Linnaeus, 1758) | Egypt: River Nile Branch (Bahr Nashart), Drainage canal (Damroo Drainage canal), and Fish farm | Cichlidae | Freshwater | - | El-Seify et al. [75] | |
| Ergasilus sieboldi von Nordmann, 1832 Syn: Ergasilus baicalensis Messjatzeff, 1928 Syn: Ergasilus depressus Sars, 1863 Syn: Ergasilus esocis Sumpf, 1871 Syn: Ergasilus hoferi Borodin, 1915 Syn: Ergasilus surbecki Baumann, 1913 Syn: Ergasilus trisetaceus von Nordmann, 1832 | TH: pike, bream, and carp | TLOC: Europe | Cyprinidae; Percidae | Marine | - | von Nordmann [76] |
| - | Angola: Dilolo Lake | - | Freshwater | - | Marques [34] | |
| Cyprinus carpio (Linnaeus, 1758) | Algeria: Foum El Khanga reservoir, Souk Ahras | Cyprinidae | Freshwater | - | Boucenna et al. [77] | |
| Luciobarbus callensis (Valenciennes, 1842) | Algeria: Beni-Haroun Dam, Mila city | Cyprinidae | Freshwater | - | Boucenna et al. [78] | |
| Bagrus bajad (Fabricius, 1775) | Egypt: Lake Nasser | Bagridae | Freshwater | - | Hamouda [79] | |
| Sparus aurata Linnaeus, 1758 | Egypt: Semi-intensive marine fish farms | Sparidae | Marine | OM812074 (28S) | Abdel-Radi et al. [80] | |
| Carassius carassius (Linnaeus, 1758) | Algeria: Beni-Haroun Dam, Mila city | Cyprinidae | Freshwater | - | Berrouk et al. [25,81] |
2. Materials and Methods
2.1. Sampling
2.2. Morphological Analysis
2.3. Infestation Rates
2.4. Molecular Analysis
| Taxon | Host | Locality | GenBank Accession Numbers | Reference | ||
|---|---|---|---|---|---|---|
| 18S | 28S | COI | ||||
| Acusicola margulisae | Amphilophus citrinellus, Parachromis managuensis, Oreochromis sp., Poecilia exicana | Nicaragua | MN852694 | MN852851 | MN854870 | Santacruz et al. [96] |
| Ergasilus anchoratus | Pseudobagrus fulvidraco | China | DQ107564 | DQ107528 | - | Song et al. [85] |
| Ergasilus briani | Misgurnus anguillicaudatus | China | DQ107572 | DQ107532 | - | Song et al. [85] |
| Ergasilus caparti | Neolamprologus brichardi | Burundi | OQ407469 | OQ407474 | - | Míč et al. [34] |
| Ergasilus hypomesi | Acanthogobius hasta | China | DQ107573 | DQ107539 | - | Song et al. [85] |
| * Ergasius lizae | Fundulus diaphanus | Canada | - | - | ECTCR024-14 | BOLD [97] |
| Ergasilus macrodactylus | Gnathochromis permaxillaris | Burundi | OQ407465 | OQ407470 | - | Míč et al. [34] |
| Ergasilus megacheir | Simochromis diagramma | Burundi | OQ407466 | OQ407471 | - | Míč et al. [34] |
| Ergasilus mirabilis | Clarias gariepinus | Vaal River, South Africa | OR449753 | OR449755 | OR448769 | Present study |
| Ergasilus mirabilis | Clarias gariepinus | Zambezi River, Zambia | OR449754 | OR449756 | OR448770 | Present study |
| Ergasilus parasarsi | Simochromis diagramma | Burundi | OQ407467 | OQ407473 | - | Míč et al. [34] |
| Ergasilus parvus | Spathodus erythrodon | Burundi | OQ407468 | OQ407472 | - | Míč et al. [34] |
| ** Ergasilus parasiluri | Tachysurus fulvidraco | China | DQ107567 | DQ107536 | - | Song et al. [85] |
| Ergasilus peregrinus | Siniperca chuatsi | China | DQ107577 | DQ107531 | - | Song et al. [85] |
| Ergasilus scalaris | Tachysurus dumerili | China | DQ107565 | DQ107538 | - | Song et al. [85] |
| Ergasilus sieboldi | Perca fluviatilis | Czech Republic | MW810238 | MW810242 | - | Kvach et al. [98] |
| Ergasilus sieboldi | Sparus aurata | Egypt | - | OM812074 | - | Abdel-Radi et al. [80] |
| Ergasilus sp. | Free-living | South Korea | - | - | KR049035 | Baek et al. [99] |
| Ergasilus sp. | Mugil liza | Argentina | - | - | KU557411 | Castro-Romero et al. [100] |
| Ergasilus tumidus | Acanthorhodeus taenianalis | China | DQ107569 | DQ107535 | - | Song et al. [85] |
| Ergasilus wilsoni | Free-living | South Korea | - | - | KR049036 | Baek et al. [99] |
| Ergasilus yaluzangbus | Gymnocypris stewartii | China | DQ107578 | DQ107540 | - | Song et al. [85] |
| *** Ergasilus yandemontei | Odontesthes hatcheri | Argentina | MT969345 | - | - | Waicheim et al. [23] |
| Neoergasilus japonicus | Lepomis gibbosus | Czech Republic | MH167969 | MH167967 | - | Ondračková et al. [101] |
| Neoergasilus japonicus | Lepomis gibbosus | Czech Republic | MH167970 | MH167968 | - | Ondračková et al. [101] |
| Neoergasilus japonicus | Lepomis gibbosus | Czech Republic | MW810236 | MW810240 | - | Kvach et al. [98] |
| Neoergasilus japonicus | Lepomis gibbosus, Scardinius erythrophthalmus | Czech Republic | MW810237 | MW810241 | - | Kvach et al. [98] |
| Neoergasilus japonicus | Collected by plankton net | USA | - | - | MZ964935 | Vasquez et al. [102] |
| Neoergasilus japonicus | Free-living | South Korea | - | - | KR049037 | Baek et al. [99] |
| Paraergasilus brevidigitus | Cyprinus carpio | China | DQ107576 | DQ107530 | - | Song et al. [85] |
| Paraergasilus longidigitus | Abramis brama, Perca fluviatilis, Scardinius erythrophthalmu | Czech Republic | MW810239 | MW810243 | - | Kvach et al. [98] |
| Paraergasilus medius | Ctenopharyngodon idellus | China | DQ107574 | DQ107529 | - | Song et al. [85] |
| Sinergasilus major | Ctenopharyngodon idella | China | DQ107560 | DQ107524 | - | Song et al. [85] |
| Sinergasilus major | Silurus glanis | Hungary | MZ047814 | MZ047815 | - | Dos Santos et al. [103] |
| Sinergasilus polycolpus | Hypophthalmichthys molitrix | China | DQ107563 | DQ107525 | - | Song et al. [85] |
| Sinergasilus polycolpus | Hypophthalmichthys molitrix | China | - | - | KR263117 | Feng et al. [104] |
| Sinergasilus undulatus | Cyprinus carpio | China | DQ107561 | DQ107526 | - | Song et al. [85] |
| Sinergasilus undulatus | Cyprinus carpio | China | - | - | MW080644 | Hua et al. [105] |
| Lernaea cyprinacea | Carassius auratus, Cyprinus carpio, Chanodichthys ilishaeformis | China | MH982195 | MH982204 | MH982220 | Hua et al. [106] |
3. Results
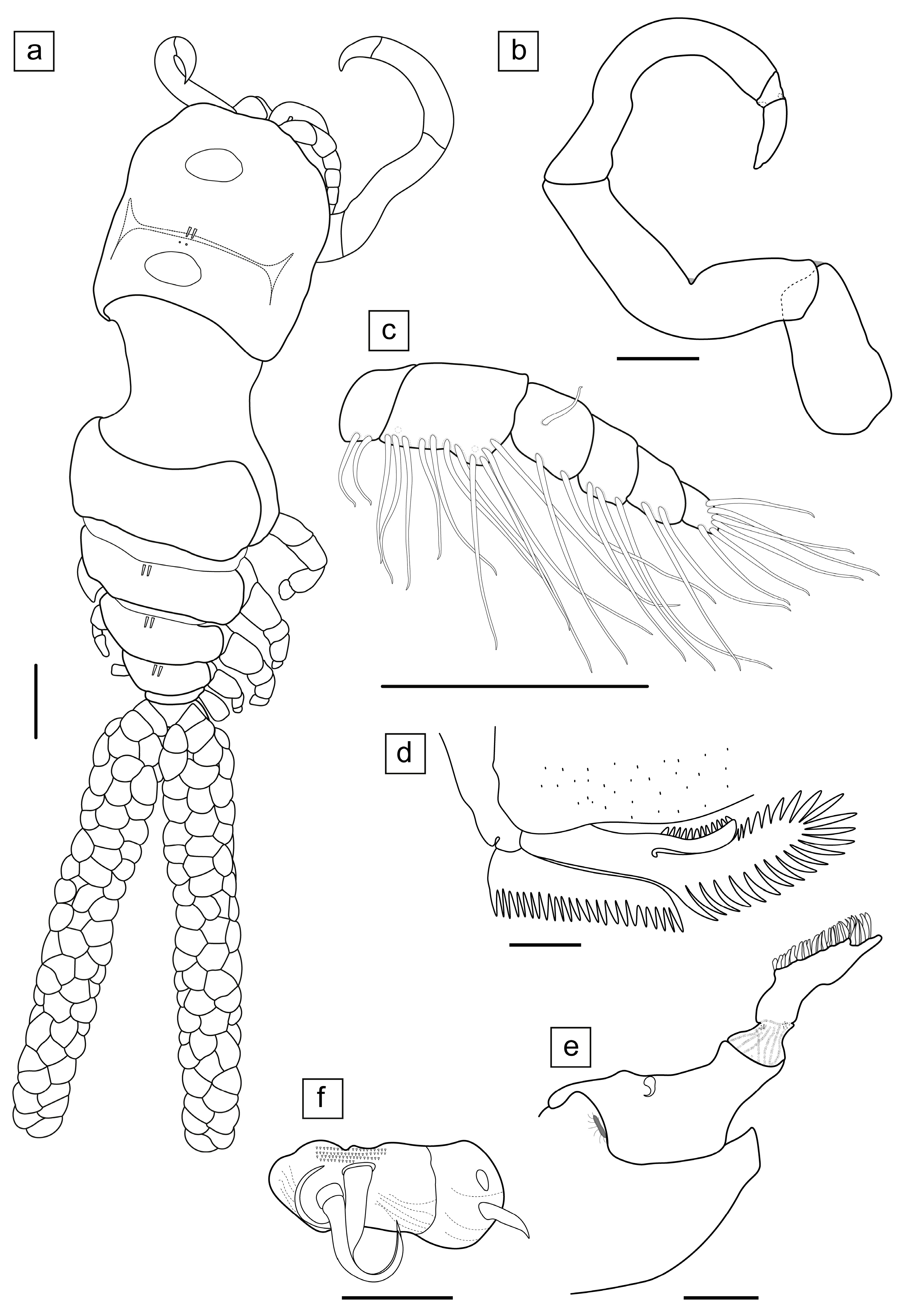
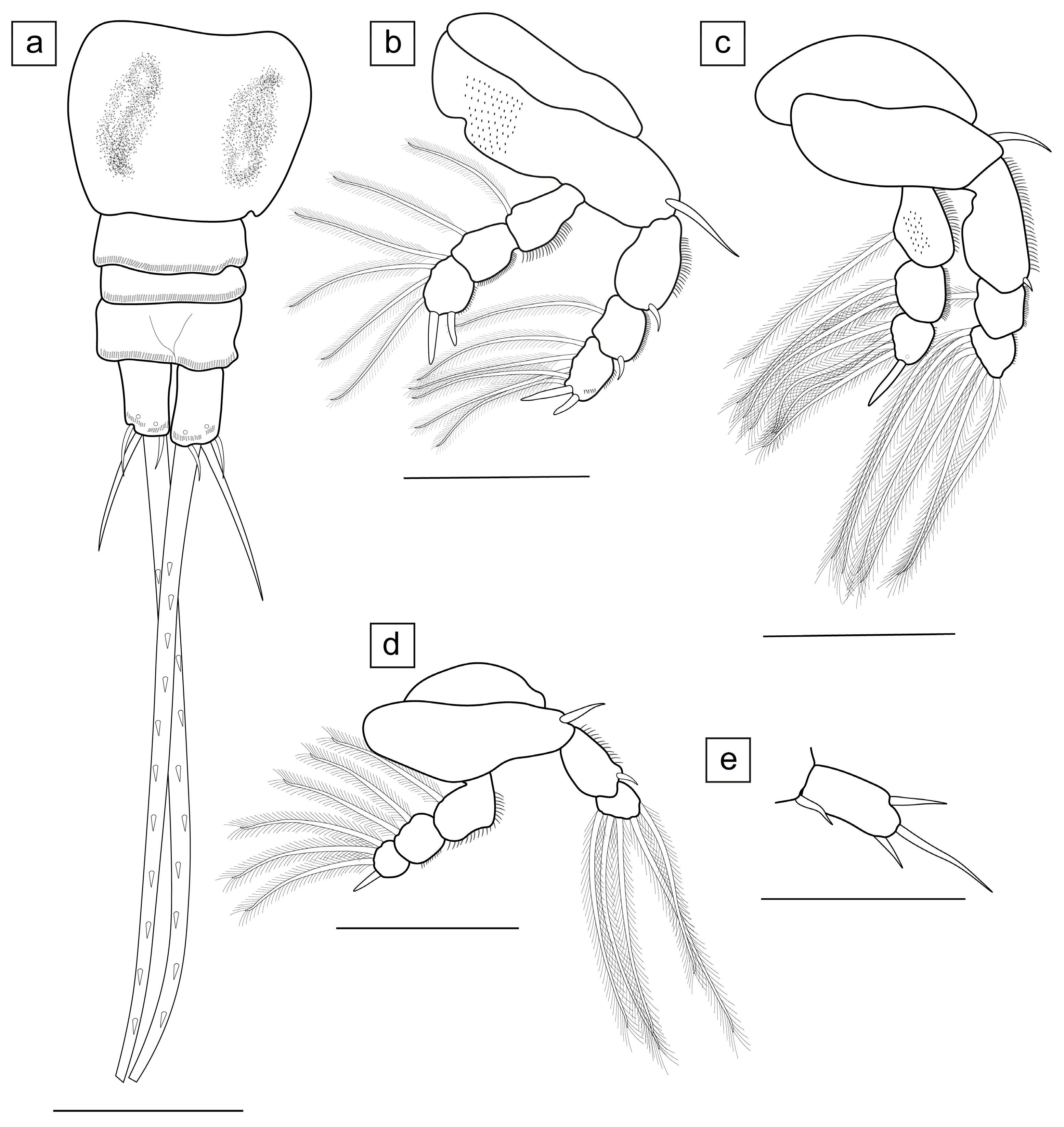
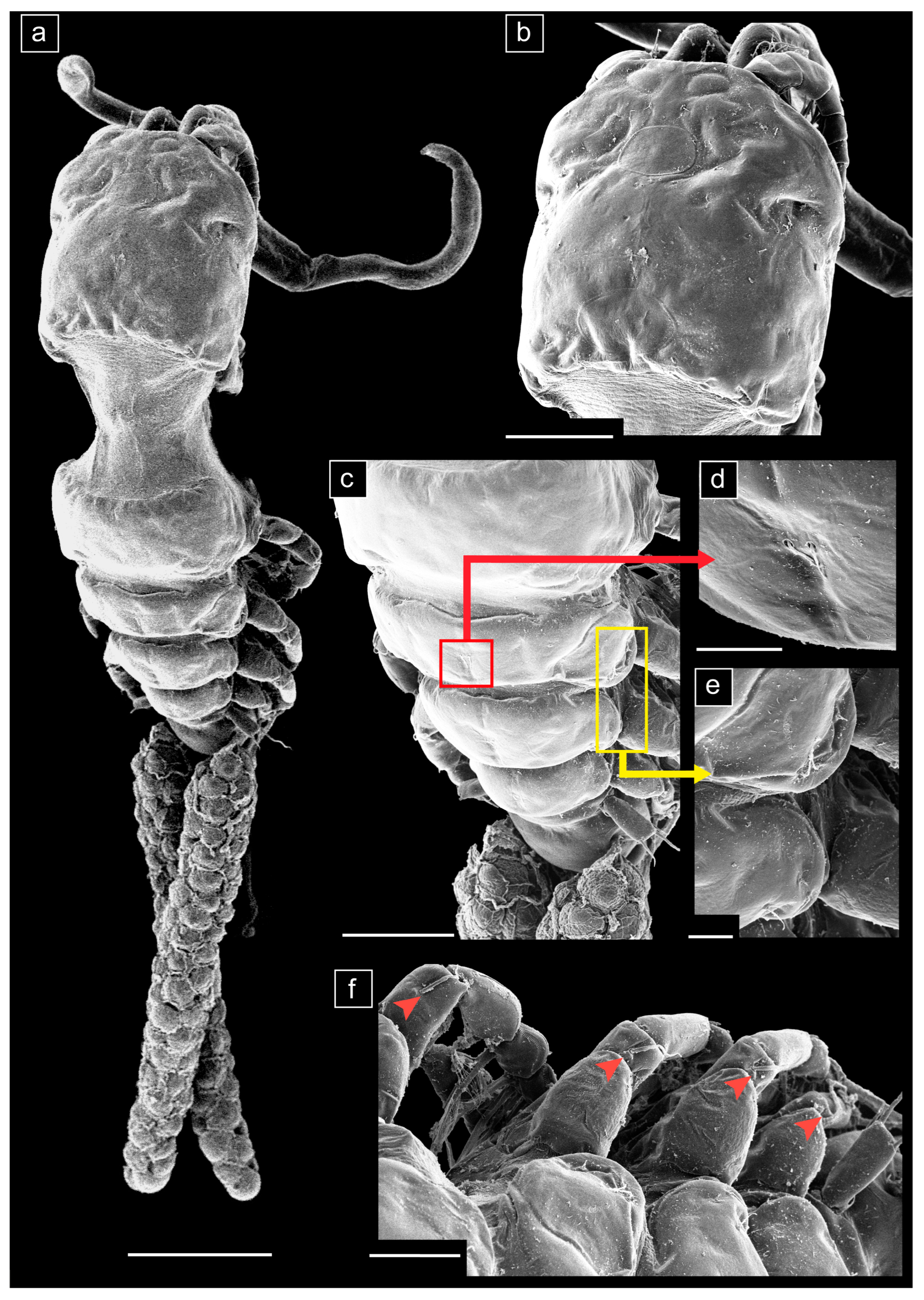
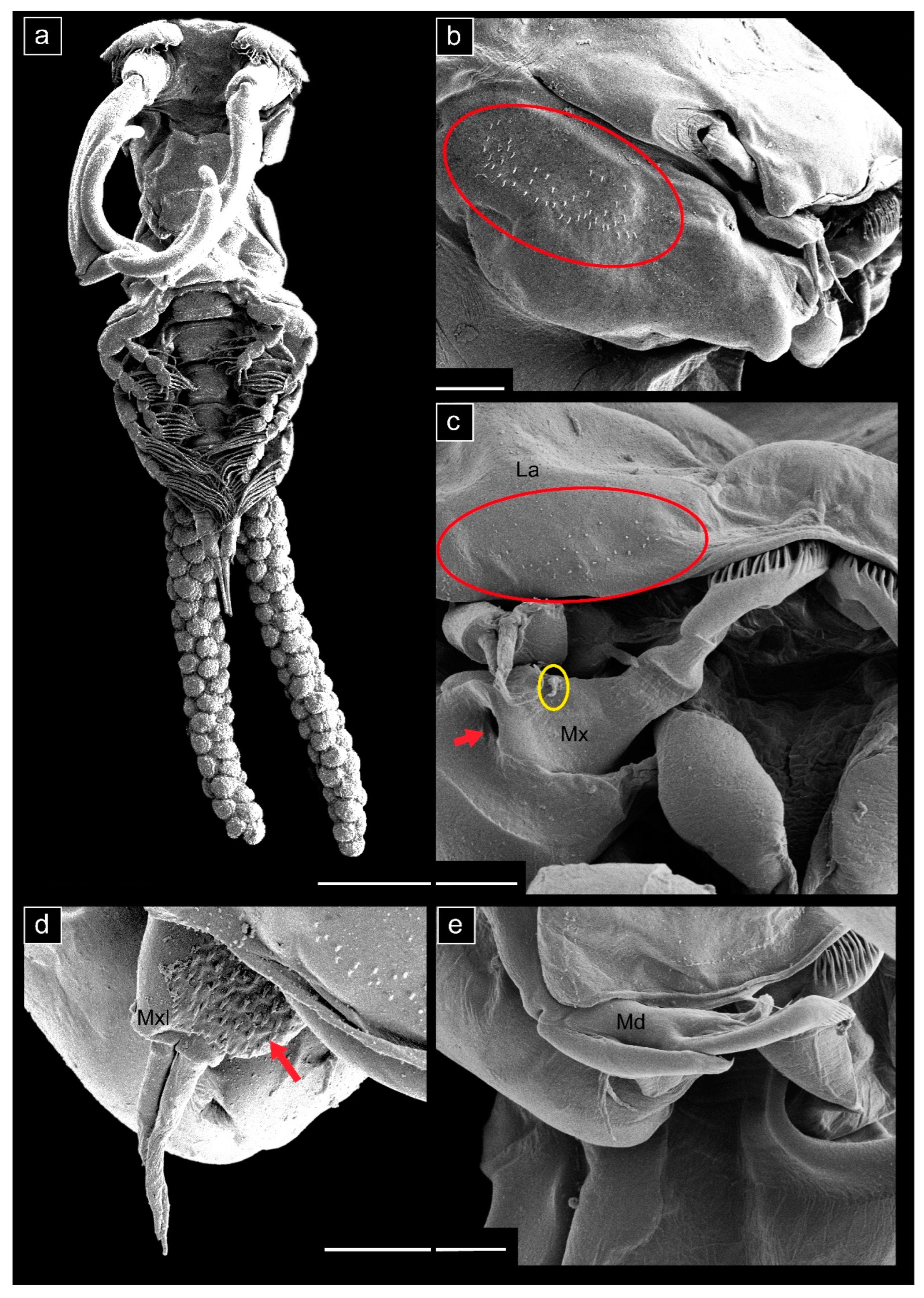
3.1. Taxonomy
- Order Cyclopoida Burmeister, 1834
- Family Ergasilidae Burmeister, 1835
- Genus Ergasilus von Nordmann, 1832
- Type species: Ergasilus gibbus von Nordmann, 1832 and Ergasilus sieboldi von Nordmann, 1832.
- Generic remarks.
- Ergasilus mirabilis Oldewage & Van As, 1987
- Type host: Synodontis zambezensis Peters, 1851 (incorrectly identified as Synodontis leopardinus Pellegrin, 1914).
- Other hosts: Brycinus imberi (Peters, 1852); Clarias gariepinus (Burchell, 1822); Clarias ngamensis Castelnau, 1861; Cyphomyrus discorhynchus (Peters, 1852); Enteromius afrohamiltoni (Crass, 1960); Glossogobius giuris (Hamilton, 1822); Hemichromis elongatus (Guichenot, 1861); Hepsetus cuvieri (Castelnau, 1861); Hydrocynus vittatus Castelnau, 1861; Labeo rosae Steindachner, 1894; Marcusenius macrolepidotus (Peters, 1852); Petrocephalus catostoma (Günther, 1866); Schilbe intermedius Rüppell, 1832; Schilbe mystus (Linnaeus, 1758); Synodontis macrostigma Boulenger, 1911; Synodontis nigromaculatus Boulenger, 1905.
- Type locality: Phongolo River, northern Natal, South Africa.
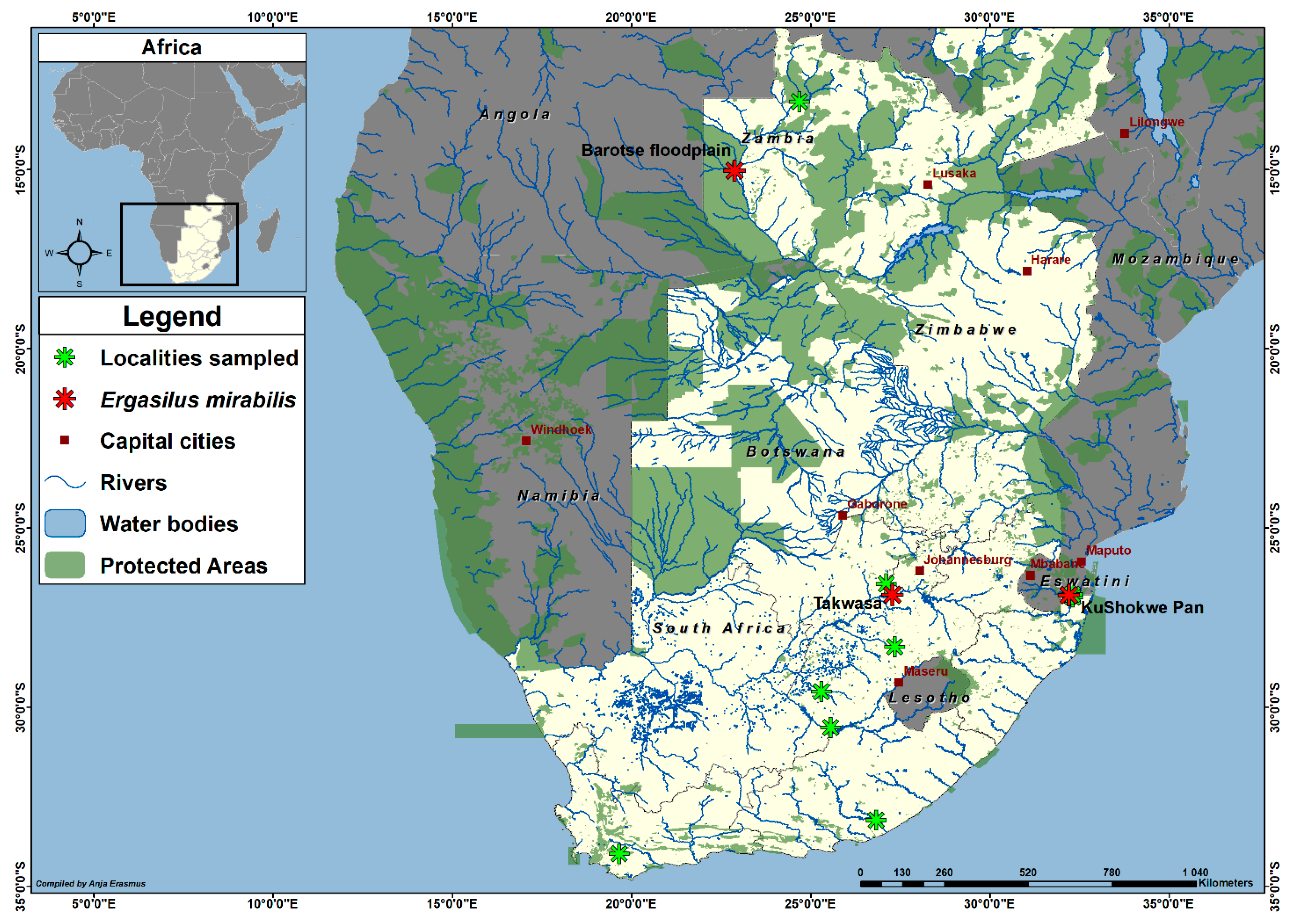
- Other localities: Mozambique—Lake Malawi; South Africa—Kushokwe Pan (present study), Limpopo River; Vaal River (present study); Namibia—the Zambezi region (previously known as Caprivi strip): Chobe River, Kwando River, Lake Liambezi, Lake Lisikili, Zambezi River; Zambia—Barotse floodplain (present study); Zimbabwe—Lake Kariba [3,4,5].
- Material examined.
- Zambia: One hundred and sixty-four copepods (164; 146 females, 25 examined) were collected from the Barotse floodplain, Zambezi River, Western Province, Zambia (15°12′01.59″ S 22°58′09.27″ E), from four C. gariepinus, col. 2019 M. Truter.
- South Africa: Seventeen copepods (17; three females, three examined) were collected from the Vaal River (Takwasa Youth Camp), Venterskroon, North West Province, South Africa (26°52′02.7″ S 27°17′36.0″ E) from nine C. gariepinus, col. 2019 M. Truter. Another three copepods (two females, two examined) copepods were collected from the KuShokwe Pan, Phongolo floodplain in the Ndumo Game Reserve, KwaZulu-Natal Province, South Africa (26°52′19.5″ S 32°12′53.1″ E) from three C. gariepinus, col. 2018 M. Truter.
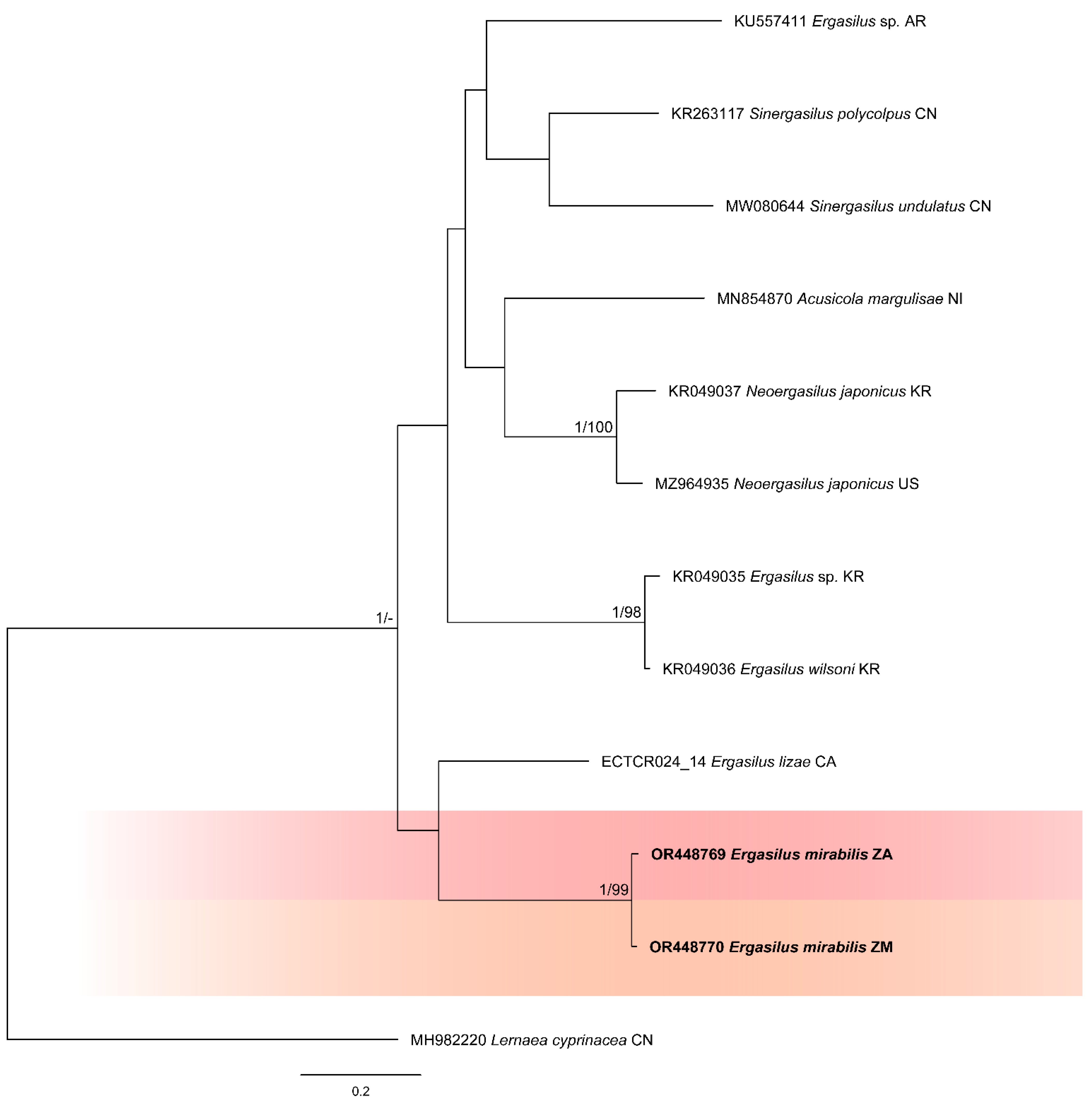
- Representative DNA sequences. GenBank accession numbers and numbers of bases (bp) for Vaal River and Barotse floodplain, Zambezi River specimens are given as follows: (18S)—1367 & 1373 bp long sequences of two specimens, OR449753–OR449754; (28S)—668 & 694 bp long sequences of two specimens, OR449755–OR449756; (COI)—692 & 693 bp long sequences of two specimens, OR448769–OR448770.
- Infestation rates. From all the localities sampled, E. mirabilis was only collected from three sites and the infestation rates (of copepodites and adults) are given as follows:
- South Africa: Kushokwe Pan—prevalence 20% (3/15), mean intensity 1 (3/3), mean abundance 0.2 (3/15); Vaal River—prevalence 50% (9/18), mean intensity 1.8 (17/9), mean abundance 0.9 (17/18).
- Zambia: Barotse floodplain—prevalence 23.5% (4/17), mean intensity 41 (164/4), mean abundance 9.6 (164/17).
- Measurements (n = 20) are given as total length (anterior margin of prosome to posterior margin of caudal rami, excluding caudal rami setae) 1.35 ± 0.14 (1.05–1.58) mm, cephalosome length 0.51 ± 0.07 (0.36–0.63) mm, cephalosome width 0.42 ± 0.04 (0.34–0.50) mm.

3.2. Molecular Analysis
4. Discussion
4.1. Morphology and Phylogenetics
4.2. Host Preference and Distribution Range
4.3. Infestation Intensities and Parasitisation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabata, Z. Parasitic Copepoda of British Fishes; Ray Society: London, UK, 1979; pp. 87–89. [Google Scholar]
- Fryer, G. The parasitic Crustacea of African freshwater fishes; their biology and distribution. J. Zool. 1968, 15, 45–95. [Google Scholar] [CrossRef]
- Oldewage, W.H.; van As, J.G. Two new species of Ergasilidae (Copepoda: Poecilostomatoida) parasitic on Mugil cephalus L. from southern Africa. Hydrobiologia 1988, 162, 135–139. [Google Scholar] [CrossRef]
- Oldewage, W.H.; van As, J.G. The occurrence and distribution of African Ergasilidae (Crustacea: Copepoda). J. Afr. Zool. 1988, 102, 177–187. [Google Scholar]
- Avenant-Oldewage, A.; Oldewage, W.H. The occurrence of fish parasites in the Kwando River, Caprivi, Namibia. MADOQUA 1993, 18, 182–185. [Google Scholar]
- Oldewage, W.H.; Avenant-Oldewage, A. Checklist of the parasitic Copepoda (Crustacea) of African fishes. K. Mus. Voor Midden-Afr. -Zool. Doc. 1993, 23, 2–28. [Google Scholar]
- Rosim, D.F.; Boxshall, G.A.; Ceccarelli, P.S. A novel microhabitat for parasitic copepods: A new genus of Ergasilidae (Copepoda: Cyclopoida) from the urinary bladder of a freshwater fish. Parasitol. Int. 2013, 62, 347–354. [Google Scholar] [CrossRef]
- Shinn, A.P.; Avenant-Oldewage, A.; Bondad-Reantaso, M.G.; Cruz-Laufer, A.J.; García-Vásquez, A.; Hernández-Orts, J.S.; Kuchta, R.; Longshaw, M.; Metselaar, M.; Pariselle, A.; et al. A global review of problematic and pathogenic parasites of farmed tilapia. Rev. Aquac. 2023, 15, 92–153. [Google Scholar] [CrossRef]
- Oldewage, W.H.; Van As, J.G. Observations on the attachment of a piscine gill parasitic ergasilid (Crustacea: Copepoda). S. Afr. J. Zool. 1987, 22, 313–317. [Google Scholar] [CrossRef]
- Dezfuli, B.S.; Giari, L.; Konecni, R.; Jaeger, P.; Manera, M. Immunohistochemistry, ultrastructure and pathology of gills of Abramis brama from Lake Mondsee, Austria, infected with Ergasilus sieboldi (Copepoda). Dis. Aquat. Org. 2003, 53, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dezfuli, B.S.; Squerzanti, S.; Fabbri, S.; Castaldelli, G.; Giari, L. Cellular response in semi-intensively cultured sea bream gills to Ergasilus sieboldi (Copepoda) with emphasis on the distribution, histochemistry and fine structure of mucous cells. Vet. Parasitol. 2010, 174, 359–365. [Google Scholar] [CrossRef]
- Kilian, E.; Avenant-Oldewage, A. Infestation and pathological alterations by Ergasilus sarsi (Copepoda) on the Tanganyika killifish from Africa. J. Aquat. Anim. Health 2013, 25, 237–242. [Google Scholar] [CrossRef]
- Roberts, L.S. Ergasilus (Copepoda: Cyclopoida): Revision and key to species in North America. Trans. Am. Micros. Soc. 1970, 89, 134–161. [Google Scholar] [CrossRef]
- Einszporn, T. Nutrition of Ergasilus sieboldi Nordmann. I. Histological structure of the alimentary canal. Acta Parasitol. 1965, 13, 151–160. [Google Scholar]
- Abdelhalim, A.I.; Lewis, J.W.; Boxshall, G.A. The life cycle of Ergasilus sieboldi Nordmann (Copepoda: Poecilostomatoida), parasitic on British freshwater fish. J. Nat. Hist. 1991, 25, 559–582. [Google Scholar] [CrossRef]
- Abdelhalim, A.I.; Lewis, J.W.; Boxshall, G.A. The external morphology of adult female ergasilid copepods (Copepoda: Poecilostomatoida): A comparison between Ergasilus and Neoergasilus. Syst. Parasitol. 1993, 24, 45–52. [Google Scholar] [CrossRef]
- Kim, I.H. Copepodid stages of Ergasilus hypomesi Yamaguti (Copepoda, Poecilostomatoida, Ergasilidae) from a brackish lake in Korea. Korean J. Biol. Sci. 2004, 8, 1–12. [Google Scholar] [CrossRef]
- Piasecki, W.; Goodwin, A.E.; Eiras, J.C.; Nowak, B.F. Importance of Copepoda in freshwater aquaculture. Zool. Stud. 2004, 43, 193–205. [Google Scholar]
- Suárez-Morales, E.; Santana-Piñeros, A.M. A new species of Ergasilus (Copepoda: Cyclopoida: Ergasilidae) from coastal fishes of the Mexican Pacific. Folia Parasitol. 2008, 55, 224–230. [Google Scholar] [CrossRef]
- Boxshall, G.A. A new species of Ergasilus von Nordmann, 1832 (Copepoda: Cyclopoida) from the gills of a dasyatid ray, Himantura oxyrhyncha (Sauvage, 1878) from West Kalimantan, Indonesia. Zootaxa 2016, 4174, 93–103. [Google Scholar] [CrossRef]
- Jiménez-Garciá, M.I.; Suárez-Morales, E. Complementary description of Ergasilus arthrosis Roberts, 1969 (Copepoda: Poecilostomatoida: Ergasilidae), a new parasite of cichlid teleosts in southeast Mexico. Syst. Parasitol. 2017, 94, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Varella, A.M.B.; Morey, G.A.M.; de Oliveira Malta, J.C. Ergasilus tipurus n. sp. (Copepoda: Ergasilidae), A Parasite of Brazilian Amazon fish species. Acta Parasitol. 2019, 64, 187–194. [Google Scholar] [CrossRef]
- Waicheim, M.A.; Mendes Marques, T.; Rauque, C.A.; Viozzi, G. New species of Ergasilus von Nordmann, 1832 (Copepoda: Ergasilidae) from the gills of freshwater fishes in Patagonia, Argentina. Syst. Parasitol. 2021, 98, 131–139. [Google Scholar] [CrossRef]
- Walter, T.C.; Boxshall, G. World of Copepods Database. Ergasilidae Burmeister, 1835. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=128571 (accessed on 14 May 2023).
- Berrouk, H.; Tolba, M.; Boucenna, I.; Touarfia, M.; Bensouilah, M.; Kaouachi, N.; Boualleg, C. Copepod parasites of the gills of Luciobarbus callensis (Valencienne, 1842) and Carassius carassius (Linnaeus, 1758) (Cyprinid Fish) collected from Beni Haroun Dam (Mila, Algeria). World J. Environ. Biosci. 2018, 7, 1–7. [Google Scholar]
- Berrouk, H.; Tolba, M.; Touarfia, M.; Boualleg, C. A study of parasitic copepod infesting two freshwater fish populations (Cyprinus carpio and Abramis brama) from Beni-Haroun Dam (Mila) North-East of Algeria. Annu. Res. Rev. Biol. 2020, 34, 1–11. [Google Scholar] [CrossRef]
- Walter, T.C.; Boxshall, G. World of Copepods Database. Ergasilus von Nordmann, 1832. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=128641 (accessed on 1 July 2023).
- Smit, N.J.; Hadfield, K.A. Chapter 4.9: Crustacea. In A Guide to the Parasites of African Freshwater Fishes; Scholz, M.P.M.V.T., Smit, N., Jayasundera, Z., Gelnar, M., Eds.; RBINS’ Scientific Publication Unit, Charlotte Gérard (RBINS): Brussels, Belgium, 2018; Volume 18, pp. 333–355. [Google Scholar]
- Oldewage, W.H.; Van As, J.G. A new fish-ectoparasitic ergasilid (Crustacea: Copepoda) from the Pongola River system. S. Afr. J. Zool. 1987, 22, 62–65. [Google Scholar] [CrossRef]
- Douëllou, L.; Erlwanger, K.H. Crustacean parasites of fishes in Lake Kariba, Zimbabwe, preliminary results. Hydrobiologia 1994, 287, 233–242. [Google Scholar] [CrossRef]
- Truter, M.; Hadfield, K.A.; Smit, N.J. Parasite diversity and community structure of translocated Clarias gariepinus (Burchell) in South Africa: Testing co-introduction, parasite spillback and enemy release hypotheses. IJP-PAW 2023, 20, 170–179. [Google Scholar] [CrossRef]
- Sars, G.O. Report on the Copepoda. Zoological results of the third Tanganyika expedition, conducted by Dr. W.A. Cunnington, F.Z.S., 1904–1905. Proc. Zool. Soc. Lond. 1909, 79, 31–77. [Google Scholar] [CrossRef]
- Marques, E. Copepodes e bran quiuros das aguas do logo Dilolo. Garcia Orta Sér. Zool. 1978, 7, 1–6. [Google Scholar]
- Míč, R.; Řehulková, E.; Seifertová, M. Species of Ergasilus von Nordmann, 1832 (Copepoda: Ergasilidae) from cichlid fishes in Lake Tanganyika. Parasitology 2023, 150, 579–598. [Google Scholar] [CrossRef]
- Capart, A. Notes sur les copépodes parasites des poissons d’eau douce du Congo Belge. Bull. Mus. R. Hist. Nat. Belg. 1944, 22, 1–24. [Google Scholar]
- Fryer, G. Further studies on the parasitic Crustacea of African freshwater fishes. Proc. Zool. Soc. Lond. 1964, 143, 79–102. [Google Scholar]
- Fryer, G. Parasitic copepods from African cichlids fishes in the Musée Royal de l’Afrique centrale. Rev. Zool. Bot. Afr. 1967, 76, 357–363. [Google Scholar]
- Paperna, I. Parasitic Crustacea from fishes of the Volta Basin, Ghana. Rev. Zool. Bot. Afr. 1969, 80, 208–216. [Google Scholar]
- Shotter, R.A. Copepod parasites of fishes from Northern Nigeria. Bull. Inst. Fr. Afr. Noire 1977, 39, 583–600. [Google Scholar]
- Ndifon, G.T.; Jimeta, S. Preliminary observations of the parasites of Chrysichthys auratus Geoffory in Tiga Lake, Kano, Nigeria. Niger. J. Parasitol. 1990, 9–11, 139–144. [Google Scholar]
- Abdel-Hady, O.K.; Bayoumy, E.M.; Osman, H.A.M. New copepodal ergasilid parasitic on Tilapia zilli from Lake Temsah with special reference to its pathological effect. Glob. Vet. 2008, 2, 123–129. [Google Scholar]
- Fryer, G. Crustacean parasites of African freshwater fishes, mostly collected during the expeditions to Lake Tanganika, and to Lakes Kivu, Edward, and Albert by the Institut Royal des Sciences Naturelles de Belgique. Bull. Inst. R. Sci. Nat. Belg. 1965, 41, 1–22. [Google Scholar]
- Cressey, R.F.; Collette, B.B. Copepods and needlefishes: A study in host-parasite relationships. Fish. Bull. 1970, 68, 347–432. [Google Scholar]
- Van Douwe, C. Copepoden des ostafrikanischen Seengebietes. Wissenschaftliche Ergebnisse der Deutsche Zentral Afrika Expedition 1907/08. Zool. Res. 1912, 3, 487–496. [Google Scholar]
- Capart, A. Quelques Copepodes parasites de poisons du Niger. (Gourao) récoltés par Th. Monod. Bull. Inst. Français Afr. Noire 1956, 58, 485–494. [Google Scholar]
- Thurston, J.P. The incidence of Monogenea and parasitic Crustacea on the gills of fish in Uganda. Rev. Zool. Bot. Afr. 1970, 82, 111–130. [Google Scholar]
- Hamouda, A.H.; Sorour, S.S.; El-Habashi, N.M.; Adam, E.-H.A. Parasitic infection with emphasis on Tylodelphys spp. as new host and locality records in Nile perch; Lates niloticus from Lake Nasser, Egypt. World’s Vet. J. 2018, 8, 19–33. [Google Scholar]
- Fryer, G. The parasitic Copepoda and Branchiura of the fishes of Lake Victoria and the Victoria Nile. Proc. Zool. Soc. Lond. 1961, 137, 41–60. [Google Scholar] [CrossRef]
- Witte, F.; van Oijen, M.J.P. Taxonomy, ecology and fishery of Lake Victoria haplochromine trophic groups. Zool. Verh. Leiden 1990, 262, 1–47. [Google Scholar]
- Maan, M.E.; van der Spoel, M.; Jimenez, P.Q.; van Alphen, J.J.M.; Seehausen, O. Fitness correlates of male coloration in a Lake Victoria cichlid fish. Behav. Ecol. 2006, 17, 691–699. [Google Scholar] [CrossRef]
- Maan, M.E.; Rooijen, A.M.C.V.; Alphen, J.J.M.V.; Seehausen, O. Parasite-mediated sexual selection and species divergence in Lake Victoria cichlid fish. Biol. J. Linn. 2008, 94, 53–60. [Google Scholar] [CrossRef]
- Karvonen, A.; Wagner, C.E.; Selz, O.M.; Seehausen, O. Divergent parasite infections in sympatric cichlid species in Lake Victoria. J. Evol. Biol. 2018, 31, 1313–1329. [Google Scholar] [CrossRef]
- Gobbin, T.P.; Vanhove, M.P.M.; Seehausen, O.; Maan, M.E. Microhabitat distributions and species interactions of ectoparasites on the gills of cichlid fish in Lake Victoria, Tanzania. Int. J. Parasitol. 2021, 51, 201–214. [Google Scholar] [CrossRef]
- Mwamburi, J.; Yongo, E.; Aura, M.C.; Babu, M.J.; Basweti, M.G.; Gichuru, N.N.; Guya, F.; Nyaboke, H.; Nyamweya, C.; Nyaundi, K.J.; et al. Balancing community needs and resource protection: The case of Lake Kanyaboli, Kenya. J. Biodivers. Endanger. Species 2018, 6, 2. [Google Scholar]
- Mwainge, V.M.; Ogwai, C.; Aura, C.M.; Mutie, A.; Ombwa, V.; Nyaboke, H.; Oyier, K.N.; Nyaundi, J. An overview of fish disease and parasite occurrence in the cage culture of Oreochromis niloticus: A case study in Lake Victoria, Kenya. Aquat. Ecosyst. Health Manag. 2021, 24, 43–55. [Google Scholar] [CrossRef]
- Outa, J.O.; Dos Santos, Q.M.; Avenant-Oldewage, A.; Jirsa, F. Parasite diversity of introduced fish Lates niloticus, Oreochromis niloticus and endemic Haplochromis spp. of Lake Victoria, Kenya. Parasitol. Res. 2021, 120, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Fryer, G. Studies on some parasitic crustaceans on African freshwater fishes, with descriptions of a new copepod of the genus Ergasilus and a new branchiuran of the genus Chonopeltis. Proc. Zool. Soc. Lond. 1960, 133, 629–647. [Google Scholar] [CrossRef]
- Fryer, G. Crustacean parasites from cichlid fishes of the genus Tilapia in the Musee Royal de l′Afrique centrale. Rev. Zool. Bot. Afr. 1963, 68, 386–392. [Google Scholar]
- Obiekezie, A.I.; MÖer, H.; Anders, K. Diseases of the African estuarine catfish Chrysichthys nigrodigitatus (Lacépède) from the Cross River estuary, Nigeria. J. Fish Biol. 1988, 32, 207–221. [Google Scholar] [CrossRef]
- Aladetohun, N.F.; Sakiti, N.G.; Babatunde, E.E. Copepoda parasites in economically important fish, Mugilidae (Mugil cephalus and Liza falcipinnis) from Lac Nokoue Lagoon in Republic of Benin, West Africa. Afr. J. Environ. Sci. Technol. 2013, 7, 799–807. [Google Scholar] [CrossRef][Green Version]
- Aladetohun, N.F.; Sakiti, N.G.; Babatunde, E.E. Copepod parasites in gills of economically important fish Mugilidae (Mugil cephalus and Liza falcipinnis) from Lagos Lagoon, West Africa, Nigeria. J. Am. Sci. 2013, 9, 392–401. [Google Scholar] [CrossRef]
- Rokicki, J.; Armah, A.K.; Sywula, T.; Skorkowski, E.; Hristovski, N.; Stojanowski, S. Environmental influence on infestation of the parasitic copepods, Ergasilus latus Fryer, 1960, in Sarotherodon mmelanotheron (Actinopterygii: Cichlidae), from coastal lagoons in Ghana. Ann. Parasitol. 2016, 62, 65. [Google Scholar]
- Adou, Y.E.; Blahoua, K.G.; Yeo, K.; Konate, S.; Tiho, S. Parasitofauna of blackchin tilapia Sarotherodon melanotheron (Teleostei: Cichlidae) from Ebrie Lagoon, Côte d’Ivoire. Int. J. Fish. Aquat. Sci. 2021, 9, 354–360. [Google Scholar] [CrossRef]
- Krøyer, H. Bidrag til Kundskab om Snyltekrebsene. Naturhistorisk Tidsskr. III 1863, 2, 75–320. (In Swedish) [Google Scholar]
- Raïbaut, A.; Ben-Hassine, O.K.; Maamouri, K. Copepodes parasites des poissons de tunisie (première série). Bull. Inst. Natl. Sci. Tech. Oceanogr. Peche Salammbo 1971, 2, 169–197. [Google Scholar]
- Boualleg, C.; Kaouachi, N.; Seridi, M.; Ternango, S.; Bensouilah, M.A. Copepod parasites of gills of 14 teleost fish species caught in the gulf of Annaba (Algeria). Afr. J. Microbiol. Res. 2011, 5, 4253–4259. [Google Scholar] [CrossRef]
- Eyo, V.O.; Effanga, E.O. Ectoparasitic infestation of the Nile squeaker, Synodontis schall (Bloch and Schneider, 1801) from the Cross River Estuary, Nigeria. Int. J. Aquat. Biol. 2018, 6, 37–43. [Google Scholar] [CrossRef]
- Amos, S.O.; Eyiseh, T.E.; Michael, E.T. Parasitic infection and prevalence in Clarias gariepinus in Lake Gerio, Yola, Adamawa state. MOJ Anat. Physiol. 2018, 5, 376–381. [Google Scholar] [CrossRef]
- Mitwally, H.; Rashidy, H.E.; Montagna, P. Biota interactions for ecological assessment of a deteriorated Coastal Lake following a brief period of restoration. Environ. Monit. Assess. 2023. [Google Scholar] [CrossRef]
- Fryer, G. A report on the parasitic Copepoda and Branchiura of the fishes of Lake Nyasa. Proc. Zool. Soc. Lond. 1956, 127, 293–344. [Google Scholar] [CrossRef]
- Wilson, C.B. Parasitic copepods from the White Nile and the Red Sea. In Results of the Swedish Zoological Expedition to Egypt and the White Nile 1901; Jägerskiöld, A.L.K.E., Ed.; The library of the University of Upsala: Upsala, Sweden, 1924; Volume 5, pp. 1–17. [Google Scholar]
- Fryer, G. A report on the parasitic Copepoda and Branchiura of the fishes of Lake Bangweulu (Northern Rhodesia). Zool. Soc. Lond. 1959, 132, 517–550. [Google Scholar] [CrossRef]
- Bichi, A.H.; Yelwa, S.I. Incidence of piscine parasites on the gills and gastrointestinal tract of Clarias gariepinus (Teugels) at Bagauda fish farm, Kano. Bayero J. Pure Appl. Sci. 2010, 3, 104–107. [Google Scholar] [CrossRef]
- Mahmoud, N.E.; Fahmy, M.; Badawy, M.F. Investigations on Mass Mortalities among Oreochromis niloticus at Mariotteya Stream, Egypt: Parasitic infestation and Environmental Pollution Impacts. Fish. Aquac. J. 2014, 5, 1–7. [Google Scholar] [CrossRef]
- El-Seify, M.A.; Zaki, M.S.; Desouky, A.R.Y.; Abbas, H.H.; Abdel Hady, O.K.; Abou Zaid, A.A. Seasonal variations and prevalence of some external parasites affecting freshwater fishes reared at upper Egypt. In Phytobiont and Ecosystem Restitution; Kumar, V., Kumar, M., Prasad, R., Eds.; Springer: Singapore, 2018; pp. 175–183. [Google Scholar] [CrossRef]
- Von Nordmann, A. Mikrographische Beiträge zur Naturgeschichte der wirbellosen Thiere, XVIII; Zweites Heft. Mit zehn Kupfertafeln, G. Reimer: Berlin, Germany, 1832; pp. 1–150. Available online: https://archive.org/details/mikrographische00nordgoog (accessed on 1 April 2023).
- Boucenna, I.; Boualleg, C.; Kaouachi, N.; Allalgua, A.; Menasria, A.; Maazi, M.C.; Barour, C.; Bensouilah, M. Infestation of a population of Cyprinus carpio (Linnaeus, 1758) by parasitic copepods at the dam of Foum El Khanga (Souk-Ahras, Algeria). Bull. Soc. Zool. Fr. 2015, 140, 163–179. [Google Scholar]
- Boucenna, I.; Khelifi, N.; Boualleg, C.; Allalgua, A.; Bensouilah, M.; Kaouachi, N. Infestation of Luciobarbus callensis (Cyprinidae) by parasitic copepods at the reservoir of Foum El Khanga (Souk-Ahras, Algeria). Bull. Soc. Zool. Fr. 2018, 143, 199–212. (In French) [Google Scholar]
- Hamouda, A. Epizootiological Studies on Some Parasitic Infections in Bagrus bajad from Lake Nasser, Egypt. Alex. J. Vet. Sci. 2018, 58, 40–47. [Google Scholar] [CrossRef]
- Abdel-Radi, S.; Rashad, M.M.; Ali, G.E.; Eissa, A.E.; Abdelsalam, M.; Abou-Okada, M. Molecular characterization and phylogenetic analysis of parasitic Copepoda; Ergasilus sieboldi isolated from cultured gilthead sea bream (Sparus aurata) in Egypt, associated with analysis of oxidative stress biomarkers. J. Parasit. Dis. 2022, 46, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Berrouk, H.; Sid, A.; Lahoual, A.; Sahtout, F.; Kaouachi, N.; Boualleg, C. Effect of parasitic copepods on the length-weight relationship and the condition factor of crucian carp (Carassius carassius) in the Beni-Haroun Dam, Mila City, Northeast Algeria. Anim. Res. Int. 2022, 19, 4625–4633. [Google Scholar]
- Truter, M.; Hadfield, K.A.; Smit, N.J. Chapter Two—Review of the metazoan parasites of the economically and ecologically important African sharptooth catfish Clarias gariepinus in Africa: Current status and novel records. In Advances in Parasitology; Rollinson, D., Stothard, R., Eds.; Academic Press: Cambridge, MA, USA, 2023; Volume 119, pp. 65–222. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication, Version 02/2023. 2023. Available online: www.fishbase.org (accessed on 1 May 2023).
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Song, Y.; Wang, G.T.; Yao, W.J.; Gao, Q.; Nie, P. Phylogeny of freshwater parasitic copepods in the Ergasilidae (Copepoda: Poecilostomatoida) based on 18S and 28S rDNA sequences. Parasitol. Res. 2008, 102, 299–306. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hayes, P.M.; Christison, K.W.; Vaughan, D.B.; Smit, N.J.; Boxshall, G.A. Sea lice (Copepoda: Caligidae) from South Africa, with descriptions of two new species of Caligus. Syst. Parasitol. 2021, 98, 369–397. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Pfeiffer, W.T.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. Proc. Gatew. Comput. Environ. Workshop Workshop 2010, 14, 1–8. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1.4; Institute of Evolutionary Biology, University of Edinburgh: Edinburgh, UK, 2012; Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 April 2023).
- Santacruz, A.; Morales-Serna, F.N.; Leal-Cardín, M.; Barluenga, M.; Pérez-Ponce de León, G. Acusicola margulisae n. sp. (Copepoda: Ergasilidae) from freshwater fishes in a Nicaraguan crater lake based on morphological and molecular evidence. Syst. Parasitol. 2020, 97, 165–177. [Google Scholar] [CrossRef]
- Barcode of Life Database BOLD, Ergasilus Lizae, BOLD SYSTEMS. Available online: http://www.boldsystems.org/index.php/TaxBrowser_TaxonPage?taxid=598126 (accessed on 7 June 2023).
- Kvach, Y.; Tkachenko, M.Y.; Seifertová, M.; Ondračková, M. Insights into the diversity, distribution and phylogeny of three ergasilid copepods (Hexanauplia: Ergasilidae) in lentic water bodies of the Morava River basin, Czech Republic. Limnologica 2021, 91, 125922. [Google Scholar] [CrossRef]
- Baek, S.Y.; Jang, K.H.; Choi, E.H.; Ryu, S.H.; Kim, S.K.; Lee, J.H.; Lim, Y.J.; Lee, J.; Jun, J.; Kwak, M.; et al. DNA barcoding of metazoan zooplankton copepods from South Korea. PLoS ONE 2016, 11, e157307. [Google Scholar] [CrossRef] [PubMed]
- Castro-Romero, R.; Montes, M.M.; Martorelli, S.R.; Sepulveda, D.; Tapia, S.; Martínez-aquino, A. Integrative taxonomy of Peniculus, Metapeniculus, and Trifur (Siphonostomatoida: Pennellidae), copepod parasites of marine fishes from Chile: Species delimitation analyses using DNA barcoding and morphological evidence. System. Biodivers. 2016, 14, 466–483. [Google Scholar] [CrossRef]
- Ondračková, M.; Fojtů, J.; Seifertová, M.; Kvach, Y.; Jurajda, P. Non-native parasitic copepod Neoergasilus japonicus (Harada, 1930) utilizes non-native fish host Lepomis gibbosus (L.) in the floodplain of the River dyje (Danube basin). Parasitol. Res. 2019, 118, 57–62. [Google Scholar] [CrossRef]
- Vasquez, A.A.; Bonnici, B.L.; Kashian, D.R.; Trejo-Martinez, J.; Miller, C.J.; Ram, J.L. The Biodiversity of Freshwater Crustaceans Revealed by Taxonomy and Mitochondrial DNA Barcodes; Physiology Faculty Research Publications: Wayne State University: Detroit, MI, USA, 2021; Volume 3, Available online: https://digitalcommons.wayne.edu/physio_frp/3 (accessed on 1 April 2023).
- Dos Santos, Q.M.; Avenant-Oldewage, A.; Piasecki, W.; Molnar, K.; Sellyei, B.; Szekely, C. An alien parasite affects local fauna-Confirmation of Sinergasilus major (Copepoda: Ergasilidae) switching hosts and infecting native Silurus glanis (Actinopterygii: Siluridae) in Hungary. IJP-PAW 2021, 15, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.L.; Wang, L.X.; Huang, J.; Jiang, J.; Tang, D.; Fang, R.; Su, Y.B. Complete mitochondrial genome of Sinergasilus polycolpus (Copepoda: Poecilostomatoida). Mitochondrial DNA A DNA Mapp. Seq. Anal. 2016, 27, 2960–2962. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.J.; Su, M.Y.; Sun, Z.W.; Lu, Y.H.; Feng, J.M. Complete mitochondrial genome of the copepod Sinergasilus undulates (Copepoda: Poecilostomatoida). Mitochondrial DNA B Resour. 2021, 6, 1226–1228. [Google Scholar] [CrossRef]
- Hua, C.J.; Zhang, D.; Zou, H.; Li, M.; Jakovlic, I.; Wu, S.G.; Wang, G.T.; Li, W.X. Morphology is not a reliable taxonomic tool for the genus Lernaea: Molecular data and experimental infection reveal that L. cyprinacea and L. cruciata are conspecific. Parasit. Vectors 2019, 12, 579–591. [Google Scholar] [CrossRef]
- Mugridge, R.E.R.; Stallybrass, H.G.; Hollman, A. Neoergasilus japonicus (Crustacea: Ergasilidae). A parasitic copepod new to Britain. J. Zool. 1982, 197, 551–557. [Google Scholar] [CrossRef]
- Hayden, K.J.; Rogers, W.A. Neoergasilus japonicus (Poecilostomatoida: Ergasilidae), a parasitic copepod new to North America. J. Parasitol. 1998, 84, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Cristescu, M.E.A.; Hebert, P.D.N. Phylogeny and adaptive radiation in the Onychopoda (Crustacea, Cladocera): Evidence from multiple gene sequences. J. Evol. Biol. 2002, 15, 838–849. [Google Scholar] [CrossRef]
- FishBase. Available online: https://www.fishbase.se/summary/synodontis-leopardinus.html (accessed on 2 June 2023).
- Skelton, P. A Complete Guide to the Freshwater Fishes of Southern Africa; Struik Publishers: Cape Town, South Africa, 2001; p. 395. [Google Scholar]
- Acosta, A.A.; Netherlands, E.C.; Retief, F.; de Necker, L.; du Preez, L.; Truter, M.; Alberts, R.; Gerber, R.; Wepener, V.; Malherbe, W.; et al. Conserving freshwater biodiversity in an African subtropical wetland: South Africa’s lower Phongolo River and Floodplain. In Managing Wildlife in a Changing World; Kideghesho, J.R., Ed.; IntechOpen: London, UK, 2020; pp. 1–36. [Google Scholar] [CrossRef]
- Arif, S.M.; Sheriff, H.A. Study of infection intensity of Copepods parasites from the genus (Ergasilus) on gills of carp fishes (Cyprinus carpio L) (endoparasites), and on fish’s tail region (exoparasites) for big sizes and small sizes (fingerlings) at three seasons (summer, winter and autumn). Int. J. Health Sci. 2022, 6, 671–677. [Google Scholar] [CrossRef]
- Paperna, I.; Zwerner, D.E. Parasites and diseases of striped bass, Morone saxatilis (Walbaum), from the lower Chesapeake Bay. J. Fish Biol. 1976, 9, 267–287. [Google Scholar] [CrossRef]
- Paperna, I.; Zwerner, D.E. Studies on Ergasilus labracis Krøyer (Cyclopidea: Ergasilidae) parasitic on striped bass, Morone saxatilis, from the lower Chesapeake Bay. I. Distribution, life cycle, and seasonal abundance. Can. J. Zool. 1976, 54, 449–462. [Google Scholar] [CrossRef]
- Paperna, I. Parasites and diseases of the grey mullet (Mugilidae) with special reference to the seas of the Near East. Aquaculture 1975, 5, 65–80. [Google Scholar] [CrossRef]


| Gene Regions | Primers | Sequences | Sources |
|---|---|---|---|
| 18S | 18SF | 5′-AAG GTG TGM CCT ATC AAC T-3′ | Song et al. [85] |
| 18SR | 5′-TTA CTT CCT CTA AAC GCT C-3′ | ||
| 28S | 28SF | 5′-ACA ACT GTG ATG CCC TTA G-3′ | |
| 28SR | 5′-TGG TCC GTG TTT CAA GAC G-3′ | ||
| COI | LCO1490 | 5′-GGT CAA CAA ATC ATA AAG ATA TTG G-3′ | Folmer et al. [86] |
| HCO2198 | 5′-TAA ACT TCA GGG TGA CCA AAA AAT CA-3′ |
| Coxa | Basis | Exopod | Endopod | |
|---|---|---|---|---|
| Leg 1 | 0-0 | I-0 | I-0; I-1; II-5 | 0-1; 0-1; II-4 |
| Leg 2 | 0-0 | I-0 | I-0; 0-1; 0-6 | 0-1; 0-2; I-4 |
| Leg 3 | 0-0 | I-0 | I-0; 0-1; 0-6 | 0-1; 0-2; I-4 |
| Leg 4 | 0-0 | I-0 | I-0; 0-5 | 0-1; 0-2; I-3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fikiye, P.P.; Smit, N.J.; Van As, L.L.; Truter, M.; Hadfield, K.A. Integrative Morphological and Genetic Characterisation of the Fish Parasitic Copepod Ergasilus mirabilis Oldewage & van As, 1987: Insights into Host Specificity and Distribution in Southern Africa. Diversity 2023, 15, 965. https://doi.org/10.3390/d15090965
Fikiye PP, Smit NJ, Van As LL, Truter M, Hadfield KA. Integrative Morphological and Genetic Characterisation of the Fish Parasitic Copepod Ergasilus mirabilis Oldewage & van As, 1987: Insights into Host Specificity and Distribution in Southern Africa. Diversity. 2023; 15(9):965. https://doi.org/10.3390/d15090965
Chicago/Turabian StyleFikiye, Precious P., Nico J. Smit, Liesl L. Van As, Marliese Truter, and Kerry A. Hadfield. 2023. "Integrative Morphological and Genetic Characterisation of the Fish Parasitic Copepod Ergasilus mirabilis Oldewage & van As, 1987: Insights into Host Specificity and Distribution in Southern Africa" Diversity 15, no. 9: 965. https://doi.org/10.3390/d15090965
APA StyleFikiye, P. P., Smit, N. J., Van As, L. L., Truter, M., & Hadfield, K. A. (2023). Integrative Morphological and Genetic Characterisation of the Fish Parasitic Copepod Ergasilus mirabilis Oldewage & van As, 1987: Insights into Host Specificity and Distribution in Southern Africa. Diversity, 15(9), 965. https://doi.org/10.3390/d15090965






