Redescription and First Nucleotide Sequences of Opecoeloides pedicathedrae (Digenea: Opecoelidae), a Parasite of Cynoscion leiarchus (Cuvier, 1830) (Eupercaria: Sciaenidae) from Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Morphological Analysis
2.2. DNA Extraction, PCR Amplification, and DNA Sequencing
3. Results
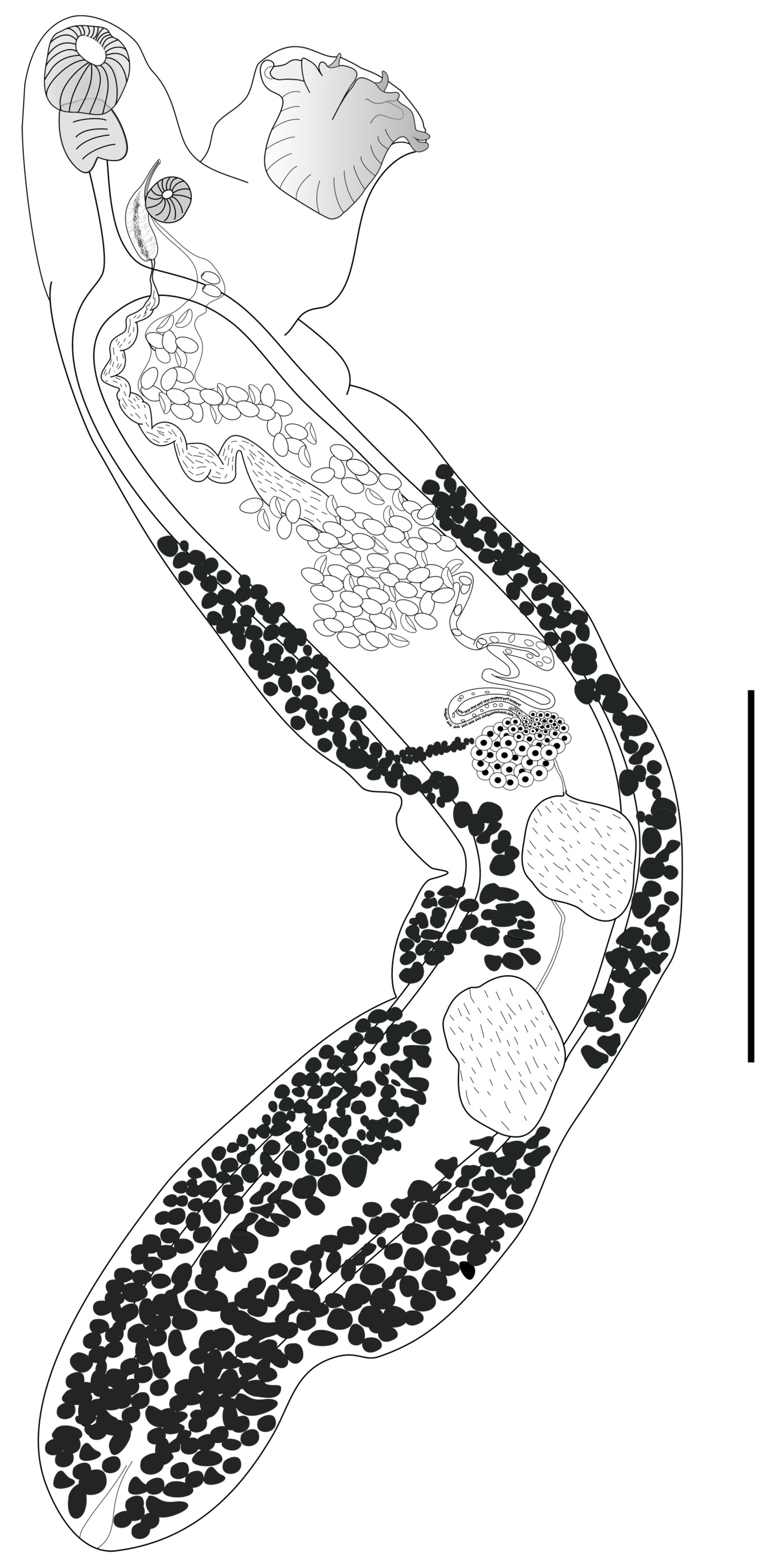
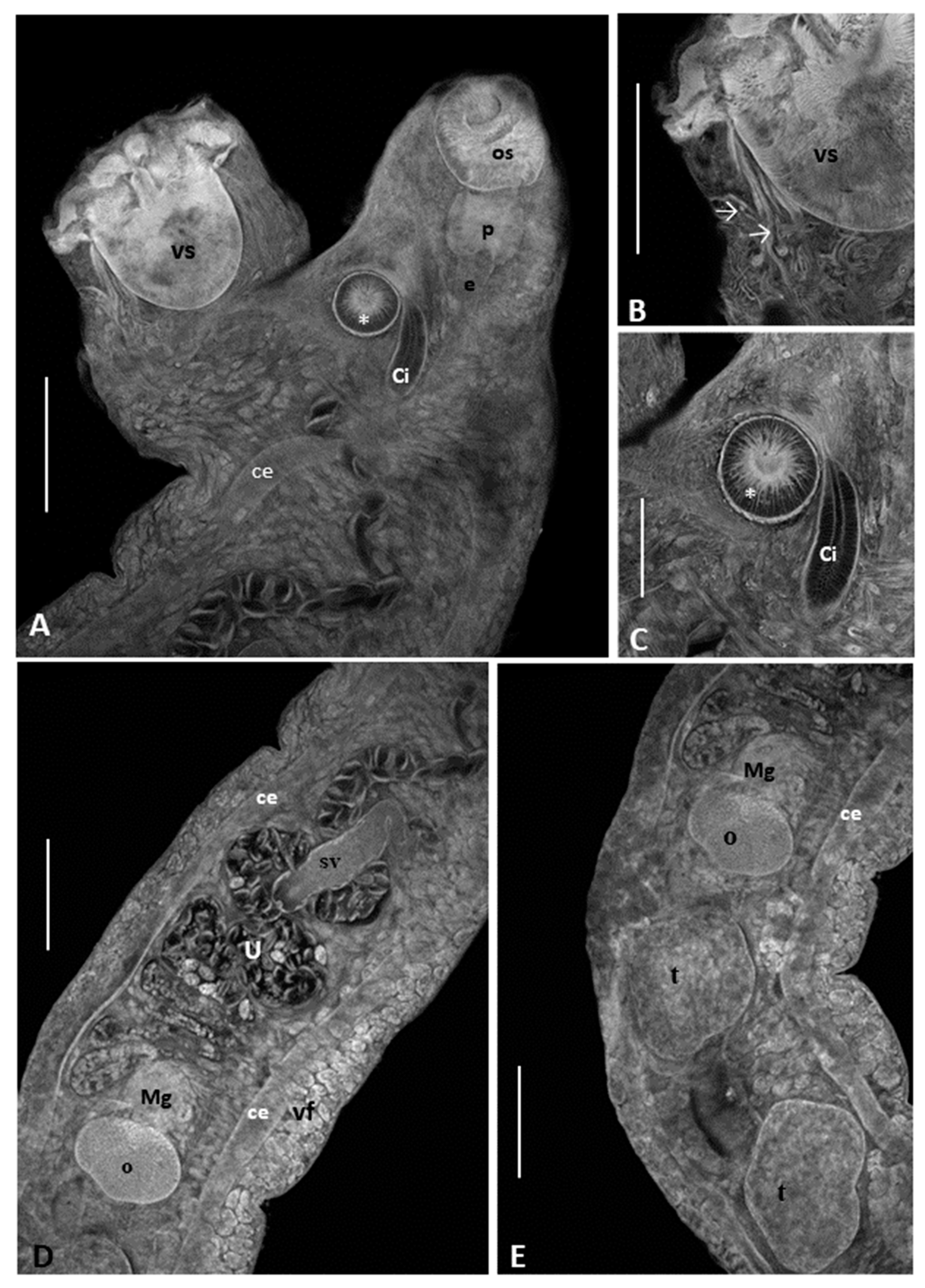
3.1. Morphological Analysis
Remarks
3.2. Molecular Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cribb, T.H. Family Opecoelidae Ozaki, 1925. In Keys to the Trematoda; Jones, A., Bray, R.A., Gibson, D.I., Eds.; CABI Publishing and The Natural History Museum: Wallingford, UK, 2005; Volume 2, pp. 443–531. [Google Scholar]
- WoRMS. Opecoeloides Odhner, 1928. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=108534 (accessed on 20 September 2022).
- Travassos, L.; Freitas, J.F.T.; Bührnheim, P.F. Trematódeos de peixes do litoral capixaba: Opecoeloides pedicathedrae sp. n., parasita de pé de banco. Atas Soc. Biol. 1966, 4, 1–4. [Google Scholar]
- Amato, J.F.R. Digenetic trematodes of percoid fishes of Florianópolis, southern Brazil—Homalometridae, Lepocreadiidae, and Opecoelidae, with the description of seven new species. Rev. Brasil. Biol. 1983, 43, 73–98. [Google Scholar] [PubMed]
- Figueiredo, J.D.; Menezes, N.A. Manual de Peixes Marinhos Do Sudeste Do Brasil. VI. Teleostei (5); Museu de Zoologia, USP: São Paulo, Brazil, 2000. [Google Scholar]
- Nóbrega, M.F.D.; Lessa, R.P. Descrição e composição das capturas da frota pesqueira artesanal da região nordeste do Brasil. Arq. Ciênc. Mar. 2007, 40, 64–74. [Google Scholar]
- Fábio, S.P. Estudo de dois trematódeos parasitos de peixes marinhos. Mem. Inst. Oswaldo Cruz 1976, 74, 71–75. [Google Scholar] [CrossRef][Green Version]
- Fernandes, B.M.M.; Kohn, A.; Pinto, R.M. New morphological data on Opecoeloides pedicathedrae Travassos, Freitas & Bührnheim, 1966 (Digenea: Opecoelidae). Mem. Inst. Oswaldo Cruz 1985, 80, 359–361. [Google Scholar] [CrossRef][Green Version]
- Cordeiro, A.S.; Luque, J.L. Metazoan parasites of flying gurnards, Dactylopterus volitans (Linnaeus, 1758) (Osteichthyes: Dactylopteridae) from the coastal zone of the State of Rio de Janeiro, Brazil. Acta Sci. Biol. Sci. 2005, 27, 119–123. [Google Scholar] [CrossRef]
- Cárdenas, M.Q.; Fernandes, B.M.M.; Justo, M.C.N.; Santos, A.L.; Cohen, S.C. Helminth parasites of Ctenosciaena gracilicirrhus (Perciformes: Sciaenidae) from the coast of Angra dos Reis, Rio de Janeiro State, Brazil. Rev. Mex. Biodiv. 2012, 83, 31–35. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. 2023. Available online: http://www.fishbase.org (accessed on 20 June 2023).
- Chisholm, L.A.; Morgan, J.A.T.; Adlard, R.D.; Whittington, I.D. Phylogenetic analysis of the Monocotylidae (Monogenea) inferred from 28S rDNA sequences. Int. J. Parasitol. 2001, 31, 1253–1263. [Google Scholar] [CrossRef]
- Levy, M.; Flowers, J.; Poore, M.; Khoo, L.; Pote, L.M.; Paperna, I.; Dzikowski, R.; Litaker, R.W. Morphologic, pathologic, and genetic investigations of Bolbophorus spp. (Diplostomatida, Trematoda) affecting cultured Ictalurus punctatus in the Mississippi Delta. J. Aquat. Anim. Health 2002, 14, 235–246. [Google Scholar] [CrossRef]
- Dzikowski, R.; Levy, M.G.; Poore, M.F.; Flowers, J.R.; Paperna, I. Use of rDNA polymorphism for identification of Heterophyidae infecting freshwater fishes. Dis. Aquat. Organ 2004, 59, 35–41. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Vaughan, T.V.; Barido-Sottan, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; de Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Tkach, V.; Pawlowski, J.; Mariaux, J. Phylogenetic analysis of the suborder plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. Int. J. Parasitol. 2000, 30, 83–93. [Google Scholar] [CrossRef]
- Andres, M.J.; Pulis, E.E.; Overstreet, R.M. New genus of opecoelid trematode from Pristipomoides aquilonaris (Perciformes: Lutjanidae) and its phylogenetic affinity within the family Opecoelidae. Folia Parasitol. 2014, 61, 223–230. [Google Scholar] [CrossRef]
- Pérez-Ponce de León, G.; Hernández-Mena, D. Testing the higher-level phylogenetic classification of Digenea (Platyhelminthes, Trematoda) based on nuclear rDNA sequences before entering the age of the ‘next-generation’ Tree of Life. J. Helminthol. 2019, 93, 260–276. [Google Scholar] [CrossRef]
- Bray, R.A.; Cribb, T.H.; Littlewood, D.T.J.; Waeschenbach, A. The molecular phylogeny of the digenean family Opecoelidae Ozaki, 1925 and the value of morphological characters, with the erection of a new subfamily. Folia Parasitol. 2016, 63, 1. [Google Scholar] [CrossRef]
- Sokolov, S.G.; Shchenkov, S.V.; Gordeev, I.I. Records of opecoeline species Pseudopecoelus cf. vulgaris and Anomalotrema koiae Gibson & Bray, 1984 (Trematoda, Opecoelidae, Opecoelinae) from fish of the North Pacific, with notes on the phylogeny of the family Opecoelidae. J. Helminthol. 2019, 93, 475–485. [Google Scholar] [CrossRef]
- Shedko, M.B.; Sokolov, S.G.; Atopkin, D.M. The first record of Dimerosaccus oncorhynchi (Trematoda: Opecoelidae) in fishes from rivers of Primorsky Territory, Russia, with a discussion on its taxonomic position using morphological and molecular data. Parazitologiya 2015, 49, 171–189. [Google Scholar]
- Martin, S.B.; Huston, D.C.; Cutmore, S.C.; Cribb, T.H. A new classification for deep-sea, opecoelid trematodes based on the phylogenetic position of some unusual taxa from shallow-water, herbivorous fishes off south-west Australia. Zool. J. Linn. Soc. 2019, 186, 385–413. [Google Scholar] [CrossRef]
- Bray, R.A.; Waeschenbach, A.; Dyal, P.; Littlewood, D.T.J.; Morand, S. New digeneans (Opecoelidae) from hydrothermal vent fishes in the south eastern Pacific Ocean, including one new genus and five new species. Zootaxa 2014, 3768, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T.J. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Bray, R.A.; Littlewood, D.T.J.; Pichelin, S.; Herniou, E.A. The Digenea. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; Taylor & Francis: London, UK, 2001; pp. 185–186. [Google Scholar]
- Constenla, M.; Carrassón, M.; Moyà, C.M.; Fernàndez-Chacón, A.; Padrós, F.; Repullés-Albelda, A.; Montero, F.E. Parasitisation by Bathycreadium elongatum (Digenea, Opecoelidae) in pyloric caeca of Trachyrincus scabrus (Teleostei, Macrouridae). Dis. Aquat. Org. 2011, 96, 239–247. [Google Scholar] [CrossRef]
- Fayton, T.J.; Andres, M.J. New species of Plagioporus Stafford, 1904 (Digenea: Opecoelidae) from California, with an amendment of the genus and a phylogeny of freshwater plagioporines of the Holarctic. Syst. Parasitol. 2016, 93, 731–748. [Google Scholar] [CrossRef]
- Curran, S.S.; Overstreet, R.M.; Tkach, V.V. Phylogenetic affinities of Plagiocirrus Van Cleave and Mueller, 1932 with the description of a new species from the Pascagoula River, Mississippi. J. Parasitol. 2007, 93, 1452–1458. [Google Scholar] [CrossRef]
- Odhner, T. Weitere Trematoden mit anus. Ark. Foè R Zool. 1928, 20, 1–6. [Google Scholar]
- Martin, S.B.; Sasal, P.; Cutmore, S.C.; Ward, S.; Aeby, G.S.; Cribb, T.H. Intermediate host switches drive diversification among the largest trematode family: Evidence from the Polypipapiliotrematinae n. subf. (Opecoelidae), parasites transmitted to butterflyfishes via predation of coral polyps. Int. J. Parasitol. 2018, 48, 1107–1126. [Google Scholar] [CrossRef]
- Martin, S.B.; Cutmore, S.C.; Cribb, T.H. The Pseudoplagioporinae, a new subfamily in the Opecoelidae Ozaki, 1925 (Trematoda) for a small clade parasitizing mainly lethrinid fishes, with three new species. J. Zool. Syst. Evol. 2020, 58, 79–113. [Google Scholar] [CrossRef]
- Martin, S.B.; Downie, A.J.; Cribb, T.H. A new subfamily for a clade of opecoelids (Trematoda: Digenea) exploiting marine fishes as second-intermediate hosts, with the first report of opecoelid metacercariae from an elasmobranch. Zool. J. Linn. Soc. 2020, 188, 455–472. [Google Scholar] [CrossRef]
- Sokolov, S.G.; Shchenkov, S.V.; Frolov, E.V.; Gordeev, I.I. A Phylogenetic Re-Evaluation of the Stenakrine Opecoelids (Trematoda, Digenea: Opecoeloidea) with Some Taxonomic Novelties. Diversity 2022, 14, 949. [Google Scholar] [CrossRef]
- Baker, T.G.; Morand, S.; Wenner, C.A.; Roumillat, W.A.; de Buron, I. Stock identification of the sciaenid fish Micropogonias undulatus in the western North Atlantic Ocean using parasites as biological tags. J. Helminthol. 2007, 81, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Sogandares-Bernal, F.; Hutton, R.F. Studies on helminth parasites from the coast of Florida. III. Digenetic trematodes of marine fishes from Tampa and Boca Ciega Bays. J. Parasitol. 1959, 45, 337–346. [Google Scholar] [CrossRef]
- Bray, R.A.; Diaz, P.E.; Cribb, T.H. Knowledge of marine fish trematodes of Atlantic and Eastern Pacific Oceans. System. Parasitol. 2016, 93, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ponce de León, G.; García-Prieto, L.; Mendoza-Garfias, B.; León-Règagnon, V.; Pulido-Flores, G.; Aranda-Cruz, C.; García-Vargas, F. Listados Faunísticos de México IX. In Biodiversidad de Helmintos Parásitos de peces Marinos y Estuarinos de la Bahía de Chamela, Jalisco; Instituto de Biología, Universidad Nacional Autónoma de México: Mexico City, Mexico, 1999; 51p. [Google Scholar]
- Jousson, O.; Bartoli, P. The life cycle of Opecoeloides columbellae (Pagenstecher, 1863) n. comb. (Digenea, Opecoelidae): Evidence from molecules and morphology. Int. J. Parasitol. 2000, 30, 747–760. [Google Scholar] [CrossRef] [PubMed]
- Tkach, V.V.; Pawlowski, J.; Mariaux, J.; Swiderski, Z. Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In Interrelationships of the Platyhelminthes; Littlewood, D.T.J., Bray, R.A., Eds.; Taylor & Francis: London, UK, 2001; pp. 186–193. [Google Scholar]
- Gijon-Botella, H.; Lopez-Roman, R. Aportacion al catalogo de Digenea de peces marinos del Archipelago de Canarias. Rev. Ibér. Parasitol. 1989, 49, 137–138. [Google Scholar]
- Van Beneden, P.J. Les poissons des côtes de Belgiques, leurs parasites et leurs commensaux. In Mémoires de l’Académie Royal des Sciences; des Lettres et des Beaux-Arts de Belgique: Bruxelles, Belgium, 1870; Volume 38, pp. 1–100. [Google Scholar]
- Yamaguti, S. Studies on the helminth fauna of Japan. Part 39. Trematodes of fishes mainly from Naha. Trans. Biogeograph. Soc. Jap. 1942, 3, 329–398. [Google Scholar]

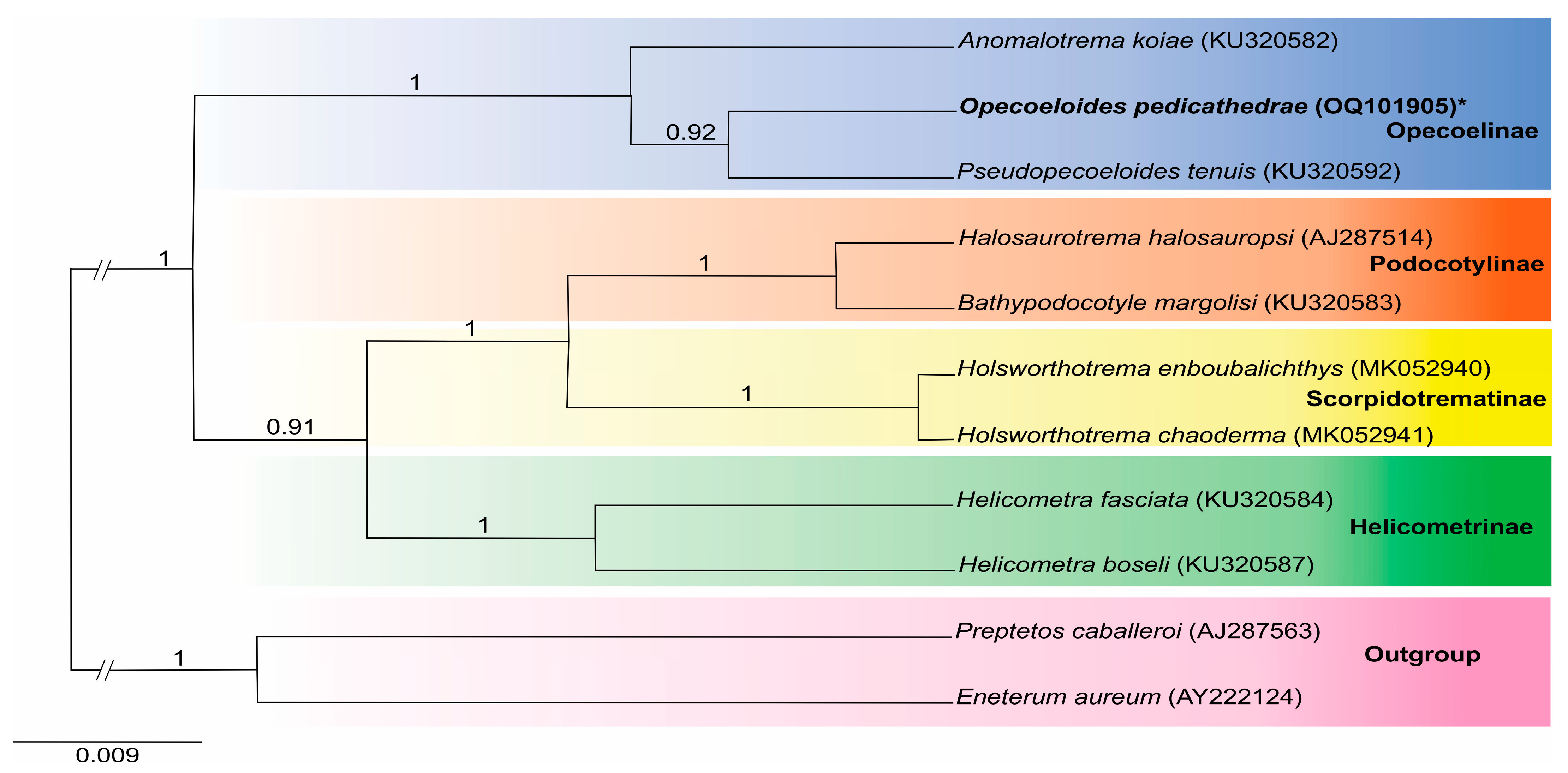
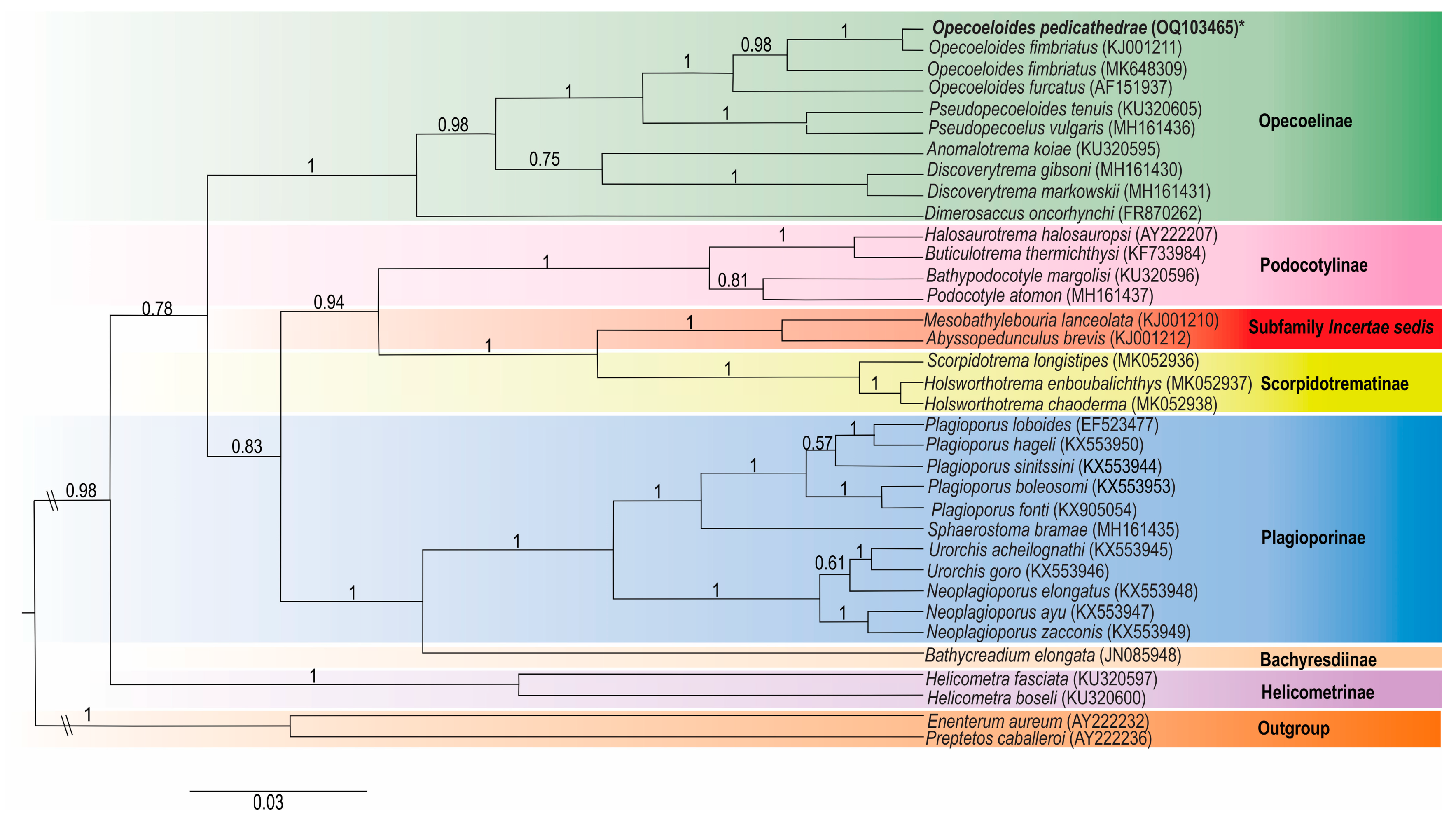
| Opecoelidae | 28S rDNA | 18S rDNA | Reference |
|---|---|---|---|
| Opecoelinae | |||
| Opecoeloides furcatus (Bremser in Rudolphi, 1819) | AF151937 | - | [21] |
| Opecoeloides fimbriatus (Linton, 1934) | KJ001211 | - | [22] |
| Opecoeloides fimbriatus (Linton, 1934) | MK648309 | [23] | |
| Anomalotrema koiae Gibson & Bray, 1984 | KU320595 | KU320582 | [24] |
| Pseudopecoeloides tenuis Yamaguti, 1940 | KU320605 | KU320592 | [24] |
| Pseudopecoelus vulgaris (Manter, 1934) | MH161436 | - | [25] |
| Discoverytrema gibsoni Zdzitowiecki, 1990 | MH161430 | - | [25] |
| Discoverytrema markowskii Gibson, 1976 | MH161431 | - | [25] |
| Dimerosaccus oncorhynchi (Eguchi, 1931) | FR870262 | - | [26] |
| Helicometrinae | |||
| Helicometra fasciata (Rudolphi, 1819) | KU320597 | KU320584 | [24] |
| Helicometra boseli Nagaty, 1956 | KU320600 | KU320587 | [24] |
| Scorpidotrematinae | |||
| Holsworthotrema enboubalichthys Martin, Huston, Cutmore & Cribb, 2018 | MK052937 | MK052940 | [27] |
| Holsworthotrema chaoderma Martin, Huston, Cutmore & Cribb, 2018 | MK052938 | MK052941 | [27] |
| Scorpidotrema longistipes Aken’Ova & Cribb, 2003 | MK052936 | - | [27] |
| Podocotylinae | |||
| Buticulotrema thermichthysi Bray, Waeschenbach, Dyal, Littlewood & Morand, 2014 | KF733984 | - | [28] |
| Podocotyle atomon (Rudolphi, 1802) | MH161437 | - | [25] |
| Bathypodocotyle margolisi (=Allopodocotyle margolisi) (Gibson, 1995) | KU320596 | KU320583 | [24] |
| Halosaurotrema halosauropsi (=Gaevskajatrema halosauropsi) (Bray & Campbell, 1996) | AY222207 | AJ287514 | [29,30] |
| Bathycreadiinae | |||
| Bathycreadium elongata (Maillard, 1970) | JN085948 | - | [31] |
| Plagioporinae | |||
| Neoplagioporus ayu (Takahashi, 1928) | KX553947 | - | [32] |
| Neoplagioporus zacconis (Yamaguti, 1934) | KX553949 | - | [32] |
| Neoplagioporus elongatus (Goto & Ozaki, 1930) | KX553948 | - | [32] |
| Urorchis goro Ozaki, 1927 | KX553946 | - | [32] |
| Urorchis acheilognathi Yamaguti, 1934 | KX553945 | - | [32] |
| Sphaerostoma bramae (Müller, 1776) | MH161435 | - | [25] |
| Plagioporus fonti Fayton, Choudhury, McAllister & Robison, 2017 | KX905054 | - | [32] |
| Plagioporus boleosomi (Pearse, 1924) | KX553953 | - | [32] |
| Plagioporus sinitsini Mueller, 1934 | KX553944 | - | [32] |
| Plagioporus hageli Fayton & Andres, 2016 | KX553950 | - | [32] |
| Plagioporus loboides (=Plagiocirrus loboides) (Curran, Overstreet & Tkach, 2007) (Incertae sedis) | EF523477 | - | [33] |
| Subfamily Incertae sedis | |||
| Abyssopedunculus brevis (=Podocotyloides brevis) (Andres & Overstreet, 2013) | KJ001212 | - | [22] |
| Mesobathylebouria lanceolata (=Neolebouria lanceolata) (Price, 1934) | KJ001210 | - | [22] |
| Outgroup | |||
| Enenterum aureum Linton, 1910 | AY222232 | AY222124 | [29] |
| Preptetos caballeroi Pritchard, 1960 | AY222236 | AJ287563 | [30] |
| Travassos et al. [3] | Fabio [7] | Fernandes et al. [8] | Present Study ** | |
|---|---|---|---|---|
| Body | 2.37–6.56 × 0.82–0.83 mm | 4.25–4.9 × 0.45–0.78 mm | 2.35–6.83 × 0.26–0.71 mm | 3.56–4.98 × 400–740 mm |
| Oral sucker | 210–270 × 200–280 | 180–220 × 180–200 | 180–270 × 120–250 | 200–225 × 150–220 |
| Ventral sucker | 350–480 × 270–430 | 250–390 × 220–270 | 340–470 × 260–590 * | 275–375 × 235–300 |
| Ratio OS: VS | 1: 1.51–1: 1.65 | 1: 1.26–1: 1.57 | 1:1.37–1: 1.83 | |
| Accessory sucker | 80–130 × 110–130 | 80–90 × 90 –100 | 0.6–0.12 × 0.07 × 0.13 mm | 82–115 × 85–145 |
| Pharynx | 110–160 × 100–130 | 110–120 × 110–140 | 0.08–0.15 × 0.08–0.12 mm | 112–125 × 92–145 |
| Seminal vesicle | 400 × 130 | 300–380 × 70–100 | 0.11–0.43 × 0.13 × 0.15 mm | 160–360 |
| Ejaculatory duct | 1120 × 400 | |||
| Cirrus | 270 × 70 | |||
| Anterior testis | 210–480 × 400–450 | 300–390 × 260–320 | 0.18–0.42 × 0.15–0.35 mm | 275–405 × 215–330 |
| Posterior testis | 270–480 × 400–450 | 330–340 × 280–320 | 0.20–0.47 × 0.16–0.38 mm | 310–435 × 225–305 |
| Ovary | 130–240 × 290–330 | 160–190 × 200–270 | 0.06–0.23 × 0.08–0.32 mm | 125–200 × 180–280 |
| Eggs | 60–63 × 37–40 | 52–66 × 31–46 | 0.059–0.068 × 0.026–0.034 mm | 47–60 × 25–37 |
| Host | Umbrina coroides | Menticirrhus americanus | Menticirrhus americanus | Cynoscion leiarchus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas, M.Q.; Cohen, S.C.; Oliveira, A.G.L.d.; Justo, M.C.N.; Santos, C.P. Redescription and First Nucleotide Sequences of Opecoeloides pedicathedrae (Digenea: Opecoelidae), a Parasite of Cynoscion leiarchus (Cuvier, 1830) (Eupercaria: Sciaenidae) from Brazil. Diversity 2024, 16, 197. https://doi.org/10.3390/d16040197
Cárdenas MQ, Cohen SC, Oliveira AGLd, Justo MCN, Santos CP. Redescription and First Nucleotide Sequences of Opecoeloides pedicathedrae (Digenea: Opecoelidae), a Parasite of Cynoscion leiarchus (Cuvier, 1830) (Eupercaria: Sciaenidae) from Brazil. Diversity. 2024; 16(4):197. https://doi.org/10.3390/d16040197
Chicago/Turabian StyleCárdenas, Melissa Querido, Simone Chinicz Cohen, Amanda Gleyce Lima de Oliveira, Marcia Cristina Nascimento Justo, and Cláudia Portes Santos. 2024. "Redescription and First Nucleotide Sequences of Opecoeloides pedicathedrae (Digenea: Opecoelidae), a Parasite of Cynoscion leiarchus (Cuvier, 1830) (Eupercaria: Sciaenidae) from Brazil" Diversity 16, no. 4: 197. https://doi.org/10.3390/d16040197
APA StyleCárdenas, M. Q., Cohen, S. C., Oliveira, A. G. L. d., Justo, M. C. N., & Santos, C. P. (2024). Redescription and First Nucleotide Sequences of Opecoeloides pedicathedrae (Digenea: Opecoelidae), a Parasite of Cynoscion leiarchus (Cuvier, 1830) (Eupercaria: Sciaenidae) from Brazil. Diversity, 16(4), 197. https://doi.org/10.3390/d16040197






