Relationships between Environmental Factors and Functional Traits of Macrophyte Assemblages in Running Waters of Greece
Abstract
:1. Introduction
2. Materials and Methods
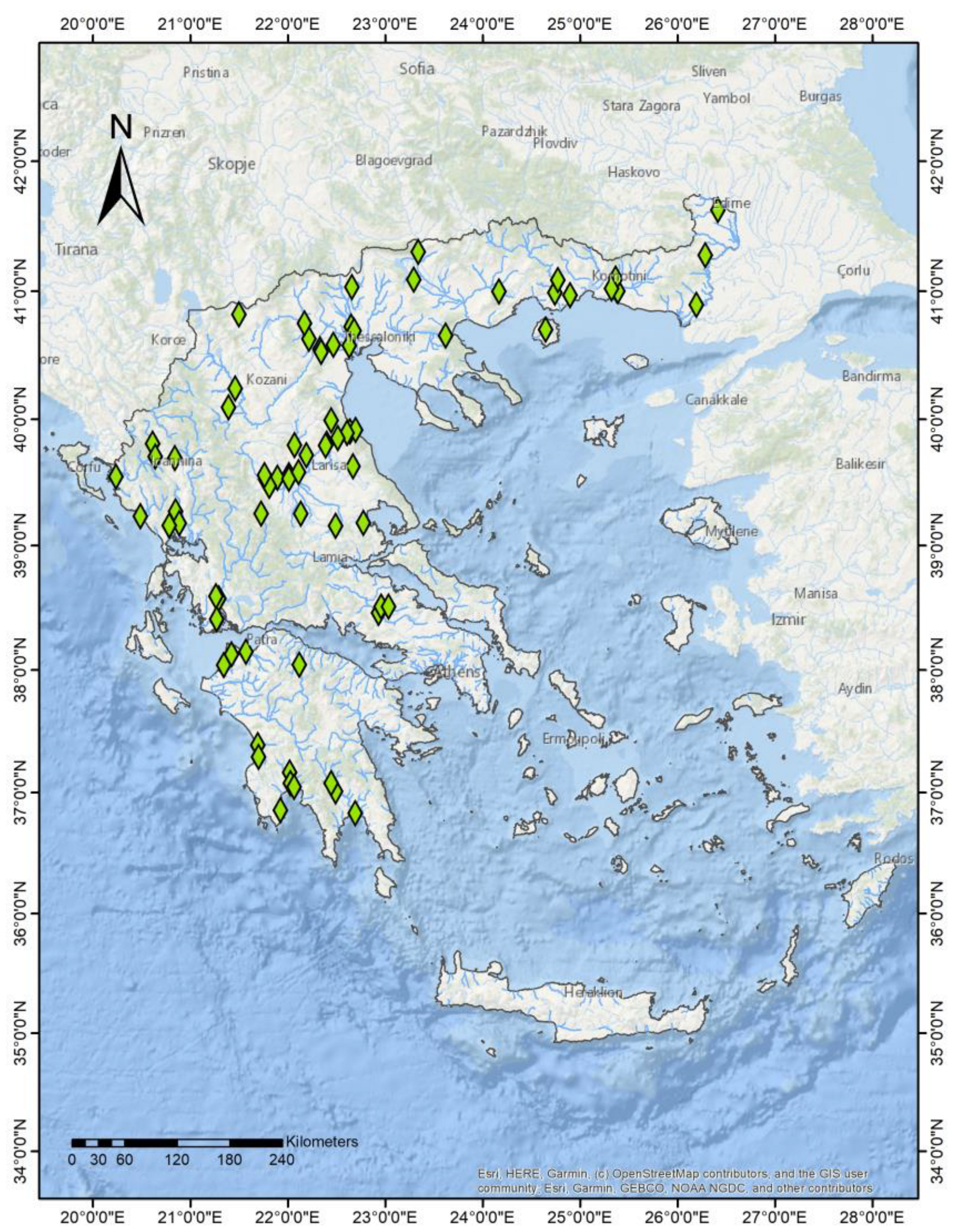
2.1. Field Samplings and Data Compilation
2.2. Allocation of Traits
2.3. RLQ and Fourth-Corner Analysis
3. Results
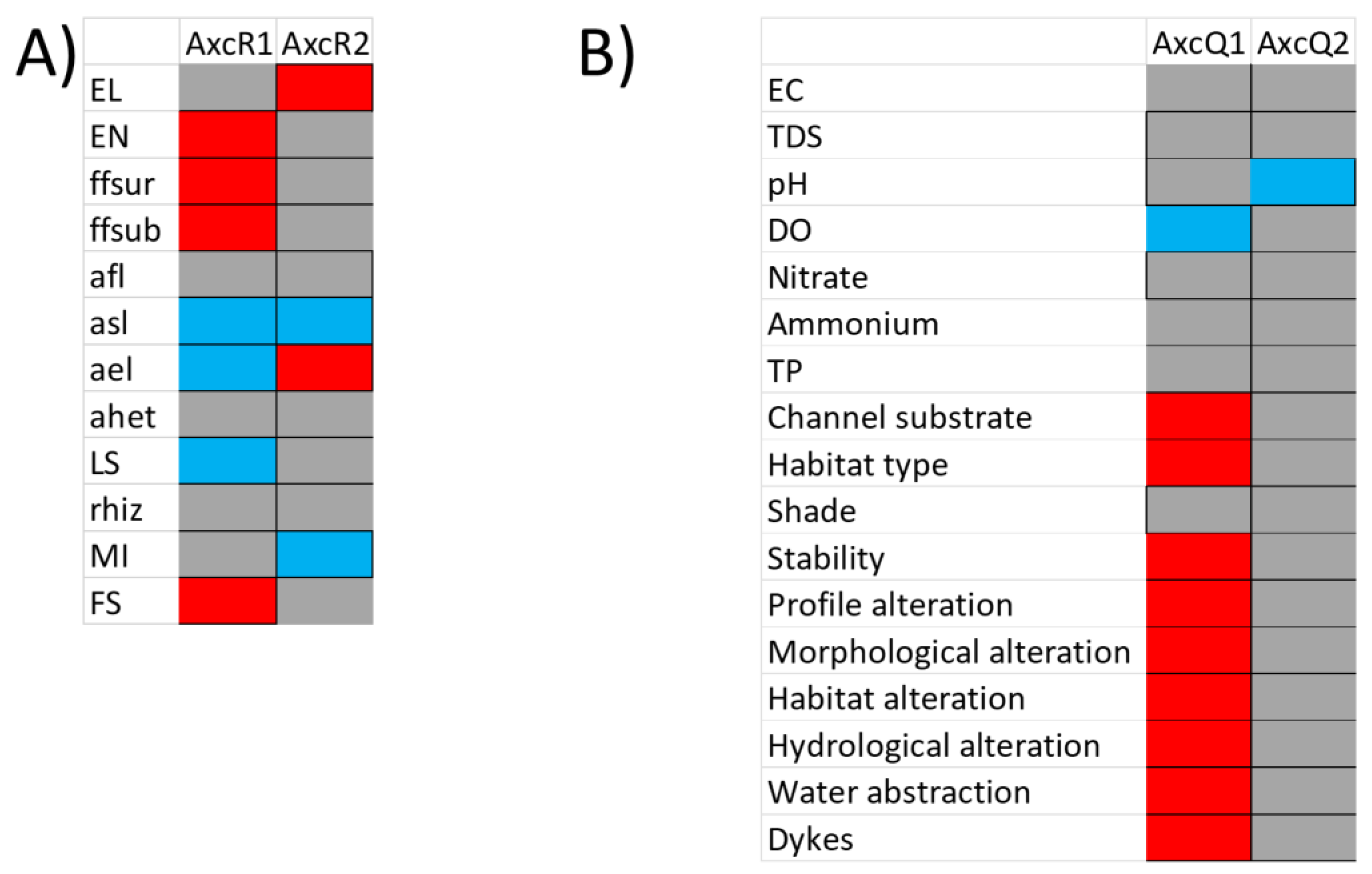
3.1. RLQ and Fourth Corner Analysis
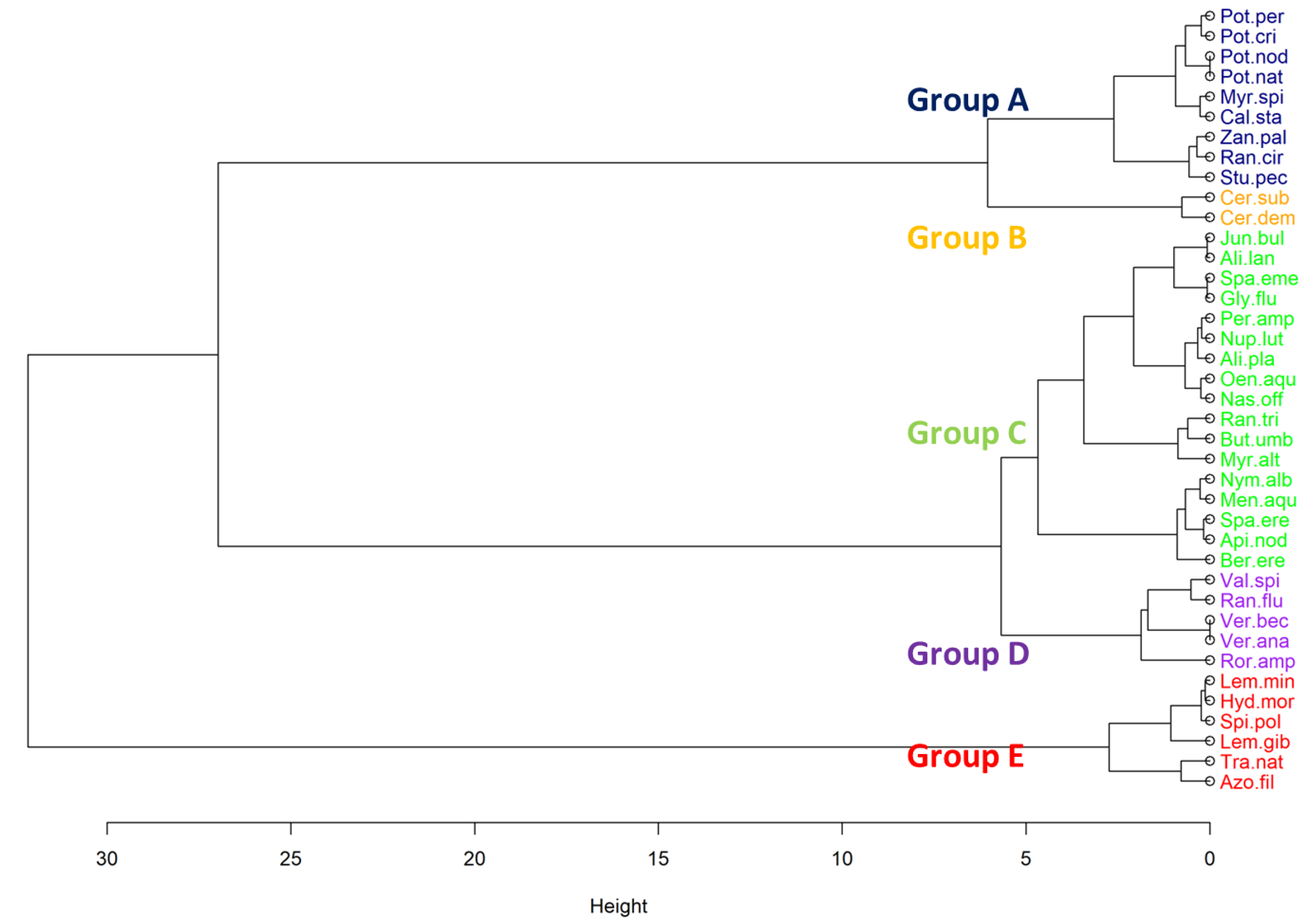
3.2. Macrophyte Functional Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Code | Name | Code | Name |
|---|---|---|---|
| Ali.lan | Alisma lanceolatum With. | Oen.aqu | Oenanthe aquatica L. |
| Ali.pla | Alisma plantago-aquatica L. | Per.amp | Persicaria amphibia (L.) Gray |
| Api.nod | Apium nodiflorum (L.) Lag. | Pot.cri | Potamogeton crispus L. |
| Azo.fil | Azolla filiculoides Lam. | Pot.nat | Potamogeton natans L. |
| Ber.ere | Berula erecta (Huds.) Coville | Pot.nod | Potamogeton nodosus Poir. |
| But.umb | Butomus umbellatus L. | Pot.per | Potamogeton perfoliatus L. |
| Cal.sta | Callitriche stagnalis Scop. | Ran.flu | Ranunculus fluitans Lam. |
| Cer.dem | Ceratophyllum demersum L. | Ran.tri | Ranunculus trichophyllus Chaix ex Vill. |
| Cer.sub | Ceratophyllum submersum L. | Ror.amp | Rorippa amphibia (L.) Besser |
| Gly.flu | Glyceria fluitans (L.) R.Br. | Sal.nat | Salvinia natans (L.) All |
| Hyd.mor | Hydrocharis morsus-ranae L. | Spa.eme | Sparganium emersum Rehmann |
| Jun.Bul | Juncus bulbosus L. | Spa.ere | Sparganium erectum L. |
| Lem.gib | Lemna gibba L. | Spi.pol | Spirodela polyrhiza (L.) Schleid. |
| Lem.min | Lemna minor L. | Stu.pec | Stuckenia pectinata (L.) Böerner |
| Men.aqu | Mentha aquatica L. | Tra.nat | Trapa natans L. |
| Myr.alt | Myriophyllum alterniflorum DC. | Val.spi | Vallisneria spiralis L. |
| Myr.spi | Myriophyllum spicatum L. | Ver.ana | Veronica anagalis-aquatica L. |
| Nas.off | Nasturtium officinale W.T.Aiton | Ver.bec | Veronica beccabunga L. |
| Nup.lut | Nuphar lutea (L.) Sm. | Zan.pal | Zannichellia palustris L. |
| Nym.alb | Nymphaea alba L. |
References
- Rodrigues, C.; Alves, P.; Bio, A.; Vieira, C.; Guimarães, L.; Pinheiro, C.; Vieira, N. Assessing the ecological status of small Mediterranean rivers using benthic macroinvertebrates and macrophytes as indicators. Environ. Monit. Assess. 2019, 191, 596. [Google Scholar] [CrossRef] [PubMed]
- Szoszkiewicz, K.; Jusik, S.; Pietruczuk, K.; Gebler, D. The Macrophyte Index for Rivers (MIR) as an Advantageous Approach to Running Water Assessment in Local Geographical Conditions. Water 2019, 12, 108. [Google Scholar] [CrossRef]
- Aguiar, F.C.; Segurado, P.; Urbanič, G.; Cambra, J.; Chauvin, C.; Ciadamidaro, S.; Dörflinger, G.; Ferreira, J.; Germ, M.; Manolaki, P.; et al. Comparability of river quality assessment using macrophytes: A multi-step procedure to overcome biogeographical differences. Sci. Total Environ. 2014, 476–477, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Hilt, S.; Alirangues Nuñez, M.M.; Bakker, E.S.; Blindow, I.; Davidson, T.A.; Gillefalk, M.; Hansson, L.-A.; Janse, J.H.; Janssen, A.B.G.; Jeppesen, E.; et al. Response of Submerged Macrophyte Communities to External and Internal Restoration Measures in North Temperate Shallow Lakes. Front. Plant Sci. 2018, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Hilt, S. Regime shifts between macrophytes and phytoplankton-concepts beyond shallow lakes, unravelling stabilizing mechanisms and practical consequences. Limnetica 2015, 34, 467–480. [Google Scholar] [CrossRef]
- Gregg, W.W.; Rose, F.L. The effects of aquatic macrophytes on the stream microenvironment. Aquat. Bot. 1982, 14, 309–324. [Google Scholar] [CrossRef]
- Lind, L.; Hasselquist, E.M.; Laudon, H. Towards ecologically functional riparian zones: A meta-analysis to develop guidelines for protecting ecosystem functions and biodiversity in agricultural landscapes. J. Environ. Manag. 2019, 249, 109391. [Google Scholar] [CrossRef]
- Cole, L.J.; Stockan, J.; Helliwell, R. Managing riparian buffer strips to optimise ecosystem services: A review. Agric. Ecosyst. Environ. 2020, 296, 106891. [Google Scholar] [CrossRef]
- Pastor, A.; Holmboe, C.M.; Pereda, O.; Giménez-Grau, P.; Baattrup-Pedersen, A.; Riis, T. Macrophyte removal affects nutrient uptake and metabolism in lowland streams. Aquat. Bot. 2023, 189, 103694. [Google Scholar] [CrossRef]
- Weissteiner, C.J.; Bouraoui, F.; Aloe, A. Reduction of nitrogen and phosphorus loads to European rivers by riparian buffer zones. Knowl. Manag. Aquat. Ecosyst. 2013, 8, 2013044. [Google Scholar] [CrossRef]
- Walton, C.R.; Zak, D.; Audet, J.; Petersen, R.J.; Lange, J.; Oehmke, C.; Wichtmann, W.; Kreyling, J.; Grygoruk, M.; Jabłońska, E.; et al. Wetland buffer zones for nitrogen and phosphorus retention: Impacts of soil type, hydrology and vegetation. Sci. Total Environ. 2020, 727, 138709. [Google Scholar] [CrossRef] [PubMed]
- Valkama, E.; Usva, K.; Saarinen, M.; Uusi-Kämppä, J. A Meta-Analysis on Nitrogen Retention by Buffer Zones. J. Environ. Qual. 2019, 48, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gurnell, A. Plants as river system engineers: Further comments. Earth Surf. Process Landf. 2015, 40, 135–137. [Google Scholar] [CrossRef]
- Levi, P.S.; Riis, T.; Alnøe, A.B.; Peipoch, M.; Maetzke, K.; Bruus, C.; Baattrup-Pedersen, A. Macrophyte Complexity Controls Nutrient Uptake in Lowland Streams. Ecosystems 2015, 18, 914–931. [Google Scholar] [CrossRef]
- Preiner, S.; Dai, Y.; Pucher, M.; Reitsema, R.E.; Schoelynck, J.; Meire, P.; Hein, T. Effects of macrophytes on ecosystem metabolism and net nutrient uptake in a groundwater fed lowland river. Sci. Total Environ. 2020, 721, 137620. [Google Scholar] [CrossRef]
- Göthe, E.; Baattrup-Pedersen, A.; Wiberg-Larsen, P.; Graeber, D.; Kristensen, E.A.; Friberg, N. Environmental and spatial controls of taxonomic versus trait composition of stream biota. Freshw. Biol. 2017, 62, 397–413. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Garssen, A.; Göthe, E.; Hoffmann, C.C.; Oddershede, A.; Riis, T.; van Bodegom, P.M.; Larsen, S.E.; Soons, M. Structural and functional responses of plant communities to climate change-mediated alterations in the hydrology of riparian areas in temperate Europe. Ecol. Evol. 2018, 8, 4120–4135. [Google Scholar] [CrossRef]
- Bejarano, M.D.; Nilsson, C.; Aguiar, F.C. Riparian plant guilds become simpler and most likely fewer following flow regulation. J. Appl. Ecol. 2017, 55, 365–376. [Google Scholar] [CrossRef]
- Papastergiadou, E.; Stefanidis, K.; Dorflinger, G.; Giannouris, E.; Kostara, K.; Manolaki, P. Exploring biodiversity in riparian corridors of a Mediterranean island: Plant communities and environmental parameters in Cyprus rivers. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 150, 91–103. [Google Scholar] [CrossRef]
- Pan, Y.; García-Girón, J.; Iversen, L.L. Global change and plant-ecosystem functioning in freshwaters. Trends Plant Sci. 2023, 28, 646–660. [Google Scholar] [CrossRef]
- Tyrrell, C.D.; Chambers, P.A.; Culp, J.M. Harnessing aquatic plant growth forms to apply European nutrient-enrichment bioindicators to Canadian waters. Appl. Plant Sci. 2022, 10, e11487. [Google Scholar] [CrossRef]
- Toivonen, H.; Huttunen, P. Aquatic macrophytes and ecological gradients in 57 small lakes in southern Finland. Aquat. Bot. 1995, 51, 197–221. [Google Scholar] [CrossRef]
- Choudhury, M.I.; McKie, B.G.; Hallin, S.; Ecke, F. Mixtures of macrophyte growth forms promote nitrogen cycling in wetlands. Sci. Total Environ. 2018, 635, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.; Willby, N. Towards harmonization of ecological quality classification: Establishing common grounds in European macrophyte assessment for rivers. Hydrobiologia 2010, 652, 149–163. [Google Scholar] [CrossRef]
- Stefanidis, K.; Dimitrellos, G.; Sarika, M.; Tsoukalas, D.; Papastergiadou, E. Ecological Quality Assessment of Greek Lowland Rivers with Aquatic Macrophytes in Compliance with the EU Water Framework Directive. Water 2022, 14, 2771. [Google Scholar] [CrossRef]
- Stefanidis, K.; Oikonomou, A.; Papastergiadou, E. Responses of different facets of aquatic plant diversity along environmental gradients in Mediterranean streams: Results from rivers of Greece. J. Environ. Manag. 2021, 296, 113307. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, K.; Papastergiadou, E. Linkages between Macrophyte Functional Traits and Water Quality: Insights from a Study in Freshwater Lakes of Greece. Water 2019, 11, 1047. [Google Scholar] [CrossRef]
- Manolaki, P.; Guo, K.; Vieira, C.; Papastergiadou, E.; Riis, T. Hydromorphology as a controlling factor of macrophytes assemblage structure and functional traits in the semi-arid European Mediterranean streams. Sci. Total Environ. 2020, 703, 1. [Google Scholar] [CrossRef]
- Tremp, H.; Kohler, A. The usefulness of macrophyte monitoring-systems, exemplified on eutrophication and acidification of running waters. Acta Bot. Gall. 1995, 142, 541–550. [Google Scholar] [CrossRef]
- Szoszkiewicz, K.; Ciecierska, H.; Kolada, A.; Schneider, S.C.; Szwabińska, M.; Ruszczyńska, J. Parameters structuring macrophyte communities in rivers and lakes–results from a case study in North-Central Poland. Knowl. Manag. Aquat. Ecosyst. 2014, 8, 2014034. [Google Scholar] [CrossRef]
- Elo, M.; Alahuhta, J.; Kanninen, A.; Meissner, K.K.; Seppälä, K.; Mönkkönen, M. Environmental Characteristics and Anthropogenic Impact Jointly Modify Aquatic Macrophyte Species Diversity. Front. Plant Sci. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Stefanidis, K.; Sarika, M.; Papastegiadou, E. Exploring environmental predictors of aquatic macrophytes in water-dependent Natura 2000 sites of high conservation value: Results from a long-term study of macrophytes in Greek lakes. Aquat. Conserv. 2018, 29, 1133–1148. [Google Scholar] [CrossRef]
- Schneider, B.; Cunha, E.R.; Marchese, M.; Thomaz, S.M. Explanatory variables associated with diversity and composition of aquatic macrophytes in a large subtropical river floodplain. Aquat. Bot. 2014, 121, 67–75. [Google Scholar] [CrossRef]
- Fu, H.; Zhong, J.; Yuan, G.; Ni, L.; Xie, P.; Cao, T. Functional traits composition predict macrophytes community productivity along a water depth gradient in a freshwater lake. Ecol. Evol. 2014, 4, 1516–1523. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin, F.S.; Cornelissen, J.H.C.; Díaz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.-L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Cavalli, G.; Baattrup-Pedersen, A.; Riis, T. The role of species functional traits in distributional patterns of lowland stream vegetation. Freshw. Sci. 2014, 33, 1074–1085. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Mouton, T.L.; Matheson, F.E.; Stephenson, F.; Champion, P.D.; Wadhwa, S.; Hamer, M.P.; Catlin, A.; Riis, T. Environmental filtering of native and non-native stream macrophyte assemblages by habitat disturbances in an agricultural landscape. Sci. Total Environ. 2018, 659, 1370–1381. [Google Scholar] [CrossRef]
- Baattrup-Pedersen, A.; Göthe, E.; Riis, T.; O’Hare, M.T. Functional trait composition of aquatic plants can serve to disentangle multiple interacting stressors in lowland streams. Sci. Total Environ. 2016, 543, 230–238. [Google Scholar] [CrossRef]
- Lukács, B.A.; Vojtkó, A.E.; Mesterházy, A.; Molnár, A.; Süveges, K.; Végvári, Z.; Brusa, G.; Cerabolini, B.E.L. Growth-form and spatiality driving the functional difference of native and alien aquatic plants in Europe. Ecol. Evol. 2017, 7, 950–963. [Google Scholar] [CrossRef]
- Skoulikidis, N.T.; Karaouzas, I.; Amaxidis, Y.; Lazaridou, M. Impact of EU Environmental Policy Implementation on the Quality and Status of Greek Rivers. Water 2021, 13, 1858. [Google Scholar] [CrossRef]
- Feio, M.J.; Aguiar, F.C.; Almeida, S.F.P.; Ferreira, J.; Ferreira, M.T.; Elias, C.; Serra, S.R.Q.; Buffagni, A.; Cambra, J.; Chauvin, C.; et al. Least Disturbed Condition for European Mediterranean rivers. Sci. Total Environ. 2014, 476–477, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Mair, P.; De Leeuw, J. A General Framework for Multivariate Analysis with Optimal Scaling: The R Package Aspect. J. Stat. Softw. 2010, 32, 1–32. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Auflage, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1991, 18, 1–248. [Google Scholar]
- Willby, N.J.; Abernethy, V.J.; Demars, B.O.L. Attribute-based classification of European hydrophytes and its relationship to habitat utilization. Freshw. Biol. 2000, 43, 43–74. [Google Scholar] [CrossRef]
- Tichý, L.; Axmanová, I.; Dengler, J.; Guarino, R.; Jansen, F.; Midolo, G.; Nobis, M.P.; Van Meerbeek, K.; Aćić, S.; Attorre, F.; et al. Ellenberg-type indicator values for European vascular plant species. J. Veg. Sci. 2023, 34, e13168. [Google Scholar] [CrossRef]
- Chevenet, F.; Dolédec, S.; Chessel, D. A Fuzzy Coding Approach for the Analysis of Long-Term Ecological Data. Freshw. Biol. 1994, 31, 295–309. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; ter Braak, C.J.F.; Champely, S. Matching species traits to environmental variables: A new three-table ordination method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Kleyer, M.; Dray, S.; Bello, F.; Lepš, J.; Pakeman, R.J.; Strauss, B.; Thuiller, W.; Lavorel, S. Assessing species and community functional responses to environmental gradients: Which multivariate methods? J. Veg. Sci. 2012, 23, 805–821. [Google Scholar] [CrossRef]
- Dray, S.; Legendre, P. Testing the species traits–environment relationships: The fourth-corner problem revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef]
- Kelley, L.A.; Gardner, S.P.; Sutcliffe, M.J. An Automated Approach for Clustering an Ensemble of NMR-Derived Protein Structures into Conformationally Related Subfamilies. Protein Eng. Des. Sel. 1996, 9, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Vukov, D.; Ilić, M.; Ćuk, M.; Igić, R. The Effect of Hydro-Morphology and Habitat Alterations on the Functional Diversity and Composition of Macrophyte Communities in the Large River. Front. Environ. Sci. 2022, 10, 863508. [Google Scholar] [CrossRef]
- Gebler, D.; Szoszkiewicz, K. Response of Aquatic Plants to Extreme Alterations in River Morphology. Water 2022, 14, 3746. [Google Scholar] [CrossRef]
- Latsiou, A.; Kouvarda, T.; Stefanidis, K.; Papaioannou, G.; Gritzalis, K.; Dimitriou, E. Pressures and Status of the Riparian Vegetation in Greek Rivers: Overview and Preliminary Assessment. Hydrology 2021, 8, 55. [Google Scholar] [CrossRef]
- Horppila, J.; Nurminen, L. Effects of different macrophyte growth forms on sediment and P resuspension in a shallow lake. Hydrobiologia 2005, 545, 167–175. [Google Scholar] [CrossRef]
- Stefanidis, K.; Dimitriou, E. Differentiation in Aquatic Metabolism between Littoral Habitats with Floating-Leaved and Submerged Macrophyte Growth Forms in a Shallow Eutrophic Lake. Water 2019, 11, 287. [Google Scholar] [CrossRef]
- Goodwin, K.; Caraco, N.; Cole, J. Temporal dynamics of dissolved oxygen in a floatingleaved macrophyte bed. Freshw. Biol. 2008, 53, 1632–1641. [Google Scholar] [CrossRef]
- Demars, B.O.L.; Thompson, J.; Manson, J.R. Stream metabolism and the open diel oxygen method: Principles, practice, and perspectives. Limnol. Oceanogr. Methods 2015, 13, 356–374. [Google Scholar] [CrossRef]
- Caraco, N.F.; Cole, J.J. Contrasting Impacts of a Native and Alien Macrophyte on Dissolved Oxygen in a Large River. Ecol. Appl. 2002, 12, 1496–1509. [Google Scholar] [CrossRef]
- Caraco, N.; Cole, J.; Findlay, S.; Wigand, C. Vascular Plants as Engineers of Oxygen in Aquatic Systems. BioScience 2006, 56, 219–225. [Google Scholar] [CrossRef]
- Hornbach, D.J.; Schilling, E.G.; Kundel, H. Ecosystem Metabolism in Small Ponds: The Effects of Floating-Leaved Macrophytes. Water 2020, 12, 1458. [Google Scholar] [CrossRef]
- De Tezanos Pinto, P.; Allende, L.; O’Farrell, I. Influence of free-floating plants on the structure of a natural phytoplankton assemblage: An experimental approach. J. Plankton Res. 2007, 29, 47–56. [Google Scholar] [CrossRef]
- Ribaudo, C.; Tison-Rosebery, J.; Buquet, D.; Jan, G.; Jamoneau, A.; Abril, G.; Anschutz, P.; Bertrin, V. Invasive Aquatic Plants as Ecosystem Engineers in an Oligo-Mesotrophic Shallow Lake. Front. Plant Sci. 2018, 9, 1781. [Google Scholar] [CrossRef]
- Stefanidis, K.; Christopoulou, A.; Poulos, S.; Dassenakis, E.; Dimitriou, E. Nitrogen and Phosphorus Loads in Greek Rivers: Implications for Management in Compliance with the Water Framework Directive. Water 2020, 12, 1531. [Google Scholar] [CrossRef]
- Su, H.; Chen, J.; Wu, Y.; Chen, J.; Guo, X.; Yan, Z.; Tian, D.; Fang, J.; Xie, P. Morphological traits of submerged macrophytes reveal specific positive feedbacks to water clarity in freshwater ecosystems. Sci. Total Environ. 2019, 684, 578–586. [Google Scholar] [CrossRef]
- Marshall, D.W.; Wade, K.; Unmuth, J.L. Nitrate pollution and expansion of free-floating plants in 3 lower Wisconsin River oxbow lakes. Lake Reserv. Manag. 2023, 39, 88–100. [Google Scholar] [CrossRef]
- Zhou, Y.; Stepanenko, A.; Kishchenko, O.; Xu, J.; Borisjuk, N. Duckweeds for Phytoremediation of Polluted Water. Plants 2023, 12, 589. [Google Scholar] [CrossRef]


| Category | Variable Name | Description |
|---|---|---|
| Physicochemical | EC | Electrical conductivity [μS/cm] |
| pH | Sorensen scale | |
| DO | Concentration of dissolved oxygen [mg/L] | |
| Nitrate | Nitrate concentration in the water [mg/L NO3−] | |
| Ammonium | Ammonia concentration in the water [mg/L NH4+] | |
| TP | Concentration of total phosphorus in the water [mg/L P] | |
| TDS | Concentration of total dissolved solids [mg/L] | |
| Hydromorphological | Channel substrate (Substrate) | Prevailing channel substrate, three levels: Fine (<2 mm), medium (2–64 mm), coarse (>64 mm) |
| Bed stability (Stability) | Stability of the riverbed using four levels: Solid (e.g., bedrock), stable, unstable, soft (e.g., mud) | |
| Shade | Channel shade using three levels: Absence of shade, semi-continuous shade, full shade | |
| Habitats | Type of river habitat: Pool, riffle, run, slack | |
| Channel profile alteration (Profile alt.) | Degree of channel profile modification present at the site/cross-section alteration | |
| Morphology alteration (Morphology alt.) | Degree of the morphological modification of the channel present at the site | |
| Habitat alteration (Habitat alt.) | Alteration of instream habitats | |
| Stream hydrology alteration (Hydrology) | Degree of the hydrological alteration present at the site | |
| Water abstraction (Abstraction) | Influence of water abstraction at the site | |
| Dykes | Influence of dykes at the site |
| Trait Code | Trait Name | Category | Values |
|---|---|---|---|
| EN | Ellenberg N—nitrogen preference | Ecological preference | 1: low nutrients, 5: intermediate levels of nutrients, 9: rich conditions of nutrients |
| EL | Ellenberg L—light preference | Ecological preference | 1: deep shade, 5: semi-shade, 9: full light |
| ffsur | Free-floating, surface | Life form | 0: no affinity to trait, 1: low affinity, 2: high affinity, 3: exclusive affinity to trait |
| ffsub | Free-floating submerged | Life form | |
| afl | Anchored floating leaves | Life form | |
| asl | Anchored submerged | Life form | |
| ael | Anchored emergent | Life form | |
| ahet | Anchored, heterophylly | Life form | |
| LS | Leaf size | Morphology | 1: <1 cm2, 2: 1–20 cm2, 3: 20–100 cm2, 4: >100 cm2 |
| FS | Fruit size | Morphology | 1: <1 mm, 2: 1–3 mm, 3: > 3 mm |
| MI | Morphology Index | Morphology | 1: low, 5: high |
| rhiz | Reproduction by rhizome | Dispersal | 0: absence, 1: presence |
| Axis 1 | Axis 2 | |
|---|---|---|
| Eigenvalues decomposition | 0.71 | 0.10 |
| % of total co-inertia | 76.86 | 10.39 |
| Inertia and co-inertia R (env) | 3.79 | 5.33 |
| Inertia and co-inertia Q (trait) | 2.55 | 4.99 |
| Correlation L (sp) | 0.27 | 0.15 |
| Environmental Variable | Contribution to Total Inertia (%) | Macrophyte Trait | Contribution to Total Inertia (%) |
|---|---|---|---|
| Water abstraction | 13.84 | Anchored emergent leaves | 20.34 |
| Channel substrate | 12.78 | Free-floating, surface | 20.23 |
| Hydrological alteration | 11.04 | Anchored submerged | 14.96 |
| Dissolved oxygen | 9.57 | Ellenberg N | 8.02 |
| Habitat type | 7.41 | Free-floating submerged | 7.33 |
| Habitat alteration | 7.16 | Fruit size | 6.92 |
| pH | 6.30 | Leaf size | 5.86 |
| Nitrate | 5.32 | Morphology index | 4.82 |
| Channel profile alteration | 5.11 | Ellenberg Light | 4.71 |
| Dykes influence | 4.27 | Anchored floating leaves | 2.52 |
| Channel morphological alteration | 4.04 | Anchored, heterophylly | 2.22 |
| Stability | 3.48 | Rhizome | 2.03 |
| Total phosphorus | 3.18 | ||
| Ammonium | 1.91 | ||
| Channel shade | 1.65 | ||
| Conductivity | 1.56 | ||
| Total dissolved solids | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanidis, K.; Oikonomou, A.; Dimitrellos, G.; Tsoukalas, D.; Papastergiadou, E. Relationships between Environmental Factors and Functional Traits of Macrophyte Assemblages in Running Waters of Greece. Diversity 2023, 15, 949. https://doi.org/10.3390/d15090949
Stefanidis K, Oikonomou A, Dimitrellos G, Tsoukalas D, Papastergiadou E. Relationships between Environmental Factors and Functional Traits of Macrophyte Assemblages in Running Waters of Greece. Diversity. 2023; 15(9):949. https://doi.org/10.3390/d15090949
Chicago/Turabian StyleStefanidis, Konstantinos, Anthi Oikonomou, Georgios Dimitrellos, Dionysios Tsoukalas, and Eva Papastergiadou. 2023. "Relationships between Environmental Factors and Functional Traits of Macrophyte Assemblages in Running Waters of Greece" Diversity 15, no. 9: 949. https://doi.org/10.3390/d15090949
APA StyleStefanidis, K., Oikonomou, A., Dimitrellos, G., Tsoukalas, D., & Papastergiadou, E. (2023). Relationships between Environmental Factors and Functional Traits of Macrophyte Assemblages in Running Waters of Greece. Diversity, 15(9), 949. https://doi.org/10.3390/d15090949







