Salinity as a Key Factor in Structuring Macrophyte Assemblages in Transitional Water Bodies: The Case of the Apulian Coastal Lagoons (Southern Italy)
Abstract
1. Introduction
2. Materials and Methods
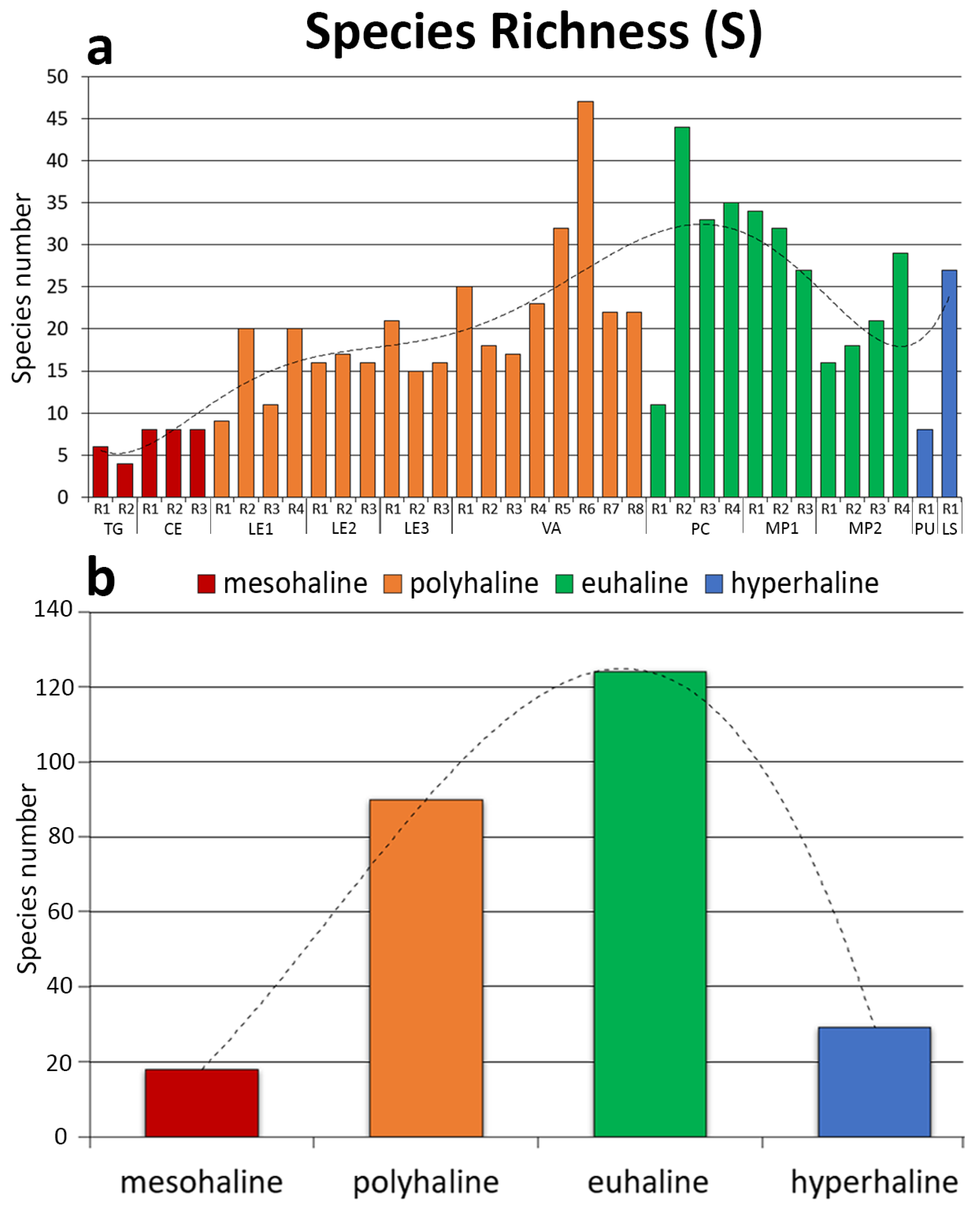
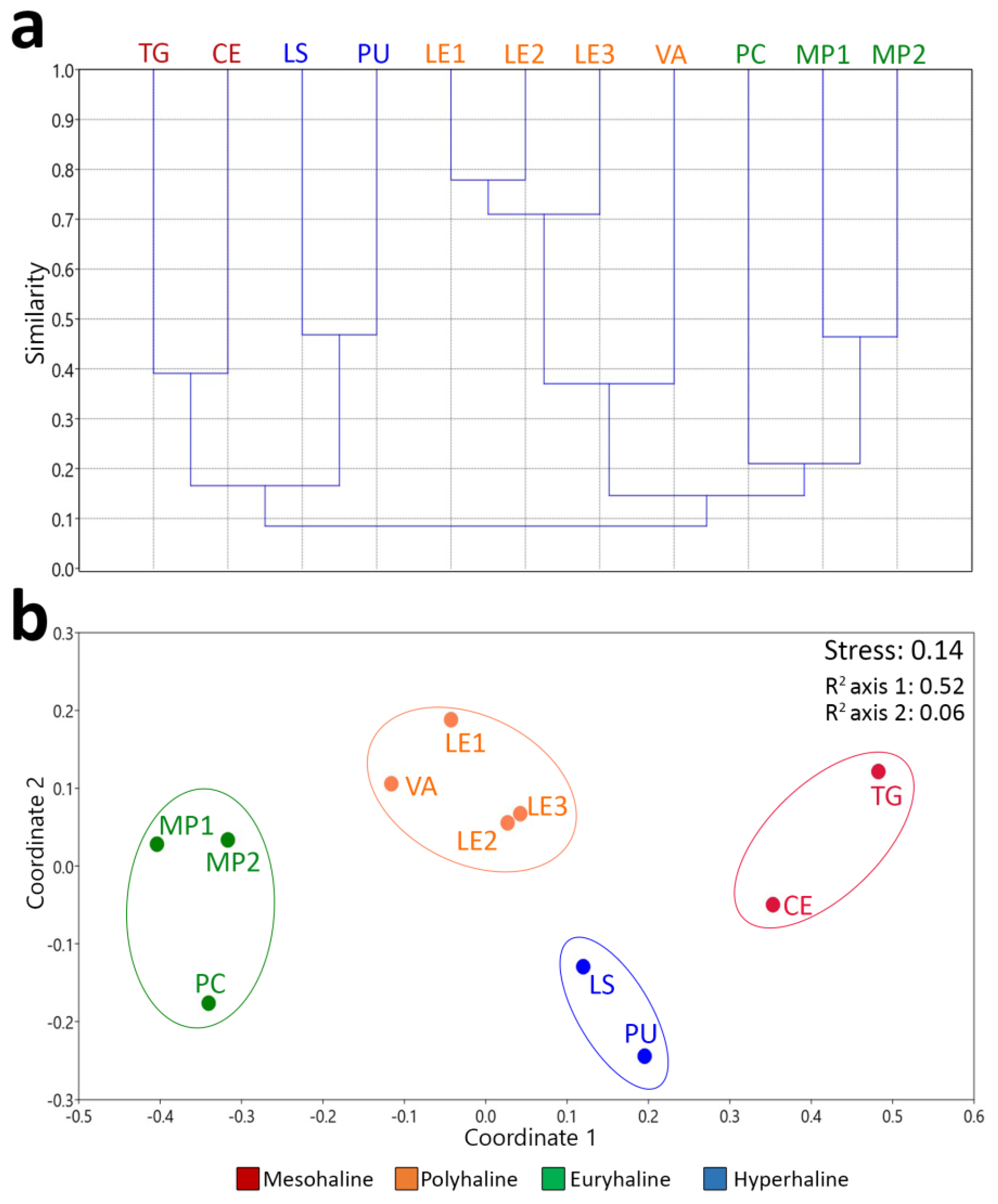
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Ruzafa, A.; Marcos, C.; Pérez-Ruzafa, I.M.; Pérez-Marcos, M. Coastal lagoons: “transitional ecosystems” between transitional and coastal waters. J. Coast. Conserv. 2011, 15, 369–392. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Pérez-Ruzafa, I.M.; Newton, A.; Marcos, C. Coastal lagoons: Environmental variability, ecosystem complexity and goods and services uniformity. In Coasts and Estuaries, the Future; Wolanski, E., Day, J., Elliott, M., Ramesh, R., Eds.; Elsevier: New York, NY, USA, 2019; pp. 253–276. [Google Scholar] [CrossRef]
- Tagliapietra, D.; Sigovini, M.; Ghirardini, A.V. A review of terms and definitions to categorize estuaries, lagoons and associated environments. Mar. Freshwater Res. 2009, 60, 497–509. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Koutsoubas, D.; Allen, I.; Dounas, C. Modelling the annual cycles of nutrients and phytoplankton in a Mediterranean lagoon (Gialova, Greece). Mar. Environ. Res. 1999, 48, 37–58. [Google Scholar] [CrossRef]
- Plus, M.; Chapelle, A.; Lazure, P.; Auby, I.; Levavasseur, G.; Verlaque, M.; Belsher, T.; Deslous-Paoli, J.-M.; Zaldívar, J.-M.; Murray, C.N. Modelling of oxygen and nitrogen cycling as a function of macrophyte community in the Thau lagoon. Cont. Shelf Res. 2003, 23, 1877–1898. [Google Scholar] [CrossRef]
- Pastres, R.; Solidoro, C.; Ciavatta, S.; Petrizzo, A.; Cossarini, G. Long-term changes of inorganic nutrients in the Lagoon of Venice (Italy). J. Marine Syst. 2004, 51, 179–189. [Google Scholar] [CrossRef]
- Le Fur, I.; Wit, R.D.; Plus, M.; Oheix, J.; Simier, M.; Ouisse, V. Submerged benthic macrophytes in Mediterranean lagoons: Distribution patterns in relation to water chemistry and depth. Hydrobiologia 2018, 808, 175–200. [Google Scholar] [CrossRef]
- Viaroli, P.; Bartoli, M.; Giordani, G.; Naldi, M.; Orfanidis, S.; Zaldivar, J.M. Community shifts, alternative stable states, biogeochemical controls and feedbacks in eutrophic coastal lagoons: A brief overview. Aquat. Conserv. 2008, 18, S105–S117. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C.; Ghetti, P.F. Rapid Quality Index (R-MaQI), based mainly on macrophyte associations, to assess the ecological status of Mediterranean transitional environments. Chem. Ecol. 2007, 23, 493–503. [Google Scholar] [CrossRef]
- Christia, C.; Tziortzis, I.; Fyttis, G.; Kashta, L.; Papastergiadou, E. A survey of the benthic aquatic flora in transitional water systems of Greece and Cyprus (Mediterranean Sea). Bot. Mar. 2011, 54, 169–178. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C.; Marcomini, A. Sedimentation rates and erosion processes in the lagoon of Venice. Environ. Int. 2005, 31, 983–992. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Agostini, S.; Marchand, B.; Pergent, G. Temporal and spatial changes of seagrass meadows in a Mediterranean coastal lagoon. Oceanol. Acta 2003, 25, 297–302. [Google Scholar] [CrossRef]
- EEC. Council Directive 92/43/EEC on the Conservation of Natural Habitats and of Wild Fauna and Flora. J. Eur. Commun. 1992, L 206, 7–50. [Google Scholar]
- Garrido, J.; Pérez-Bilbao, A.; Benetti, C.J. Biodiversity and Conservation of Coastal Lagoons. In Ecosystems Biodiversity; Grillo, O., Venora, G., Eds.; InTech: Vienna, Austria, 2011; pp. 1–28. [Google Scholar]
- Orfanidis, S.; Panayotidis, P.; Stamatis, N. An insight to the ecological evaluation index (EEI). Ecol. Indic. 2003, 3, 27–33. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Pérez-Ruzafa, I.M.; Marcos, C.; Pérez-Ruzafa, A. Suitability of benthic macrophyte indices (EEI, E-MaQI and BENTHOS) for detecting anthropogenic pressures in a Mediterranean coastal lagoon (Mar Menor, Spain). Ecol. Indic. 2012, 19, 48–60. [Google Scholar] [CrossRef]
- Orfanidis, S.; Dencheva, K.; Nakou, K.; Tsioli, S.; Papathanasiou, V.; Rosati, I. Benthic macrophyte metrics as bioindicators of water quality: Towards overcoming typological boundaries and methodological tradition in Mediterranean and Black Seas. Hydrobiologia 2014, 740, 61–78. [Google Scholar] [CrossRef]
- Tziortzis, I.; Kadis, K.; Papastegiadou, E. Use of Macrophyte assemblages for the ecological evaluation of two coastal lagoons of Greece according to WFD 2000/60/EC. J. Wetl. Biodivers. 2014, 4, 97–111. [Google Scholar]
- EEC. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 on establishing a framework for community action in the field of water policy. J. Eur. Commun. 2000, L327, 1–72. [Google Scholar]
- Sfriso, A.; Facca, C.; Ghetti, P.F. Validation of the Macrophyte Quality Index (MaQI) set up to assess the ecological status of Italian marine transitional environments. Hydrobiologia 2009, 617, 117–141. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C.; Bonometto, A.; Boscolo, R. Compliance of the macrophyte quality index (MaQI) with the WFD (2000/60/EC) and ecological status assessment in transitional areas: The Venice lagoon as study case. Ecol. Indic. 2014, 46, 536–547. [Google Scholar] [CrossRef]
- ISPRA. Implementazione della Direttiva 2000/60/CE—Linea Guida per L’applicazione Dell’indice MaQI (ISPRA, 2010. Implementation of the Directive 2000/60/CE—Guidelines for the Application of MaQI Index); ISPRA: Ispra, Italy, 2010. [Google Scholar]
- Wilkinson, M. Benthic estuarine algae and their environment: A review. In The Shore Environment: Methods and Ecosytems; Price, J.H., Irvine, D.E.G., Farnham, W.F., Eds.; Academic Press: London, UK, 1980; Volume 2, pp. 425–486. [Google Scholar]
- Russell, G. Salinity and seaweed vegetation. In The Physiological Vegetation of Amphibious and Intertidal Plants; Crawford, R.M., Ed.; Blackwell: Oxford, UK, 1987; pp. 32–35. [Google Scholar]
- Christia, C.; Giordani, G.; Papastergiadou, E. Environmental Variability and Macrophyte Assemblages in Coastal Lagoon Types of Western Greece (Mediterranean Sea). Water 2018, 10, 151. [Google Scholar] [CrossRef]
- Ferrarin, F.; Rapaglia, J.; Zaggia, L.; Umgiesser, G.; Zuppi, G.M. Coincident application of a mass balance of radium and a hydrodynamic model for the seasonal quantification of groundwater flux into the Venice Lagoon, Italy. Mar. Chem. 2008, 10, 179–188. [Google Scholar] [CrossRef]
- Krumgalz, B.S.; Hornung, H.; Oren, O.H. The study of a natural hypersaline lagoon in a desert area (the Bardawil Lagoon in Northern Sinai). Estuar. Coast. Mar. Sci. 1980, 10, 403–415. [Google Scholar] [CrossRef]
- Boutron, O.; Paugam, C.; Luna-Laurent, E.; Chauvelon, P.; Sous, D.; Rey, V.; Meulé, S.; Chérain, Y.; Cheiron, A.; Migne, E. Hydro-Saline Dynamics of a Shallow Mediterranean Coastal Lagoon: Complementary Information from Short and Long Term Monitoring. J. Mar. Sci. Eng. 2021, 9, 701. [Google Scholar] [CrossRef]
- Silva, E.I.L.; Katupotha, J.; Amarasinghe, O.; Manthrithilake, H.; Ariyaratna, R. Lagoons of Sri Lanka: From the Origins to the Present; International Water Management Institute: Colombo, Sri Lanka, 2013. [Google Scholar]
- Bamber, R.N.; Batten, S.D.; Sheader, M.; Bridgwater, M.D. On the ecology of brackish water lagoons in Great Britain. Aquat. Conserv. Mar. Freshw. Ecosyst. 1992, 2, 65–94. [Google Scholar] [CrossRef]
- Kristiansen, A.; Pedersen, P.M.; Moseholm, L. Salinity-temperature effects on growth and reproduction of Scytosiphon lomentaria (Fucophyceae) along the salinity gradient in Danish waters. Phycologia 1994, 33, 444–454. [Google Scholar] [CrossRef]
- Düwel, L. Experimental Studies on Macroalgae along the Salinity Gradient in the Baltic Sea Area. Ph.D. Thesis, Botanical Institute, Faculty of Science, University of Copenhagen, Copenhagen, Denmark, 2001. [Google Scholar]
- Remane, A. Die Brackwasserfauna; Verhandlungen der Deutsche Zoologischen Gesellschaft: Greifswald, Germany, 1934; pp. 34–74. [Google Scholar]
- Adams, J.B.; Knoop, W.T.; Bate, G.C. The distribution of estuarine macrophytes in relation to freshwater. Bot. Mar. 1992, 35, 215–226. [Google Scholar] [CrossRef]
- Schubert, H.; Feuerpfeil, P.; Marquardt, R.; Telesh, I.; Skarlato, S. Macroalgal diversity along the Baltic Sea salinity gradient challenges Remane’s species-minimum concept. Mar. Pollut. 2011, 62, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- ISPRA. Guida alla Tipizzazione dei Corpi Idrici di Transizione ed alla Definizione delle Condizioni di Riferimento ai Sensi della Direttiva 2000/60/CE (ISPRA, 2007. Guide to the Typology of Transitional Water Bodies and to the Definition of Reference Conditions Following the Directive 2000/60/CE); ISPRA: Ispra, Italy, 2007. [Google Scholar]
- Battaglia, B. Final resolution of the symposium on the classification of brackish waters. Arch. Oceanogr. Limnol. 1959, 11, 243–248. [Google Scholar]
- Basset, A.; Sabetta, L.; Fonnesu, A.; Mouillot, D.; Do Chi, T.; Viaroli, P.; Giordani, G.; Reizopoulou, S.; Abbiati, M.; Carrada, G.C. Typology in Mediterranean transitional waters: New challenges and perspectives. Aquat. Conserv. 2006, 16, 441–455. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A.; Izzo, G.; Sfriso, A. Flora and Vegetation of the Italian Transitional Water Systems; CoRiLa, Multigraf: Spinea, Italy, 2009; 278p. [Google Scholar]
- Varvaglione, B.; Sabetta, L.; Basset, A. Tra Terra e Mare. Ecoguida alla Scoperta Delle Lagune e dei Laghi Costieri in Puglia (Between Earth and Sea. Eco-Guide to the Discovering of Lagoons and Coastal Lakes in the Apulian Region). Ph.D. Thesis, University of Lecce, Lecce, Italy, 2006. [Google Scholar]
- Apulian Region. Regional Water Protection Plan, Water Protection Service, General Report Area, 2009. Available online: https://www.sit.puglia.it (accessed on 6 March 2023). (In Italian).
- ISPRA. Protocols for Sampling and Determination of the Biological and the Physicochemical Quality in the Framework of the Transitional Water Monitoring Programs ex 2000/60/EC; ISPRA: Ispra, Italy, 2011. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2022; Available online: https://www.algaebase.org (accessed on 6 March 2023).
- Lucena-Moya, P.; Pardo, I.; Álvarez, M. Development of a typology for transitional waters in the Mediterranean ecoregion: The case of the islands. Estuar. Coast. Shelf Sci. 2009, 82, 61–72. [Google Scholar] [CrossRef]
- Carstens, M.; Claussen, U.; Bergemann, M.; Gaumert, T. Transitional waters in Germany: The Elbe estuary as an example. Aquat. Conserv. 2004, 14, 81–92. [Google Scholar] [CrossRef]
- Boix, D.; Gascón, S.; Sala, J.; Martinoy, M.; Gifre, J.; Quintana, X.D. A new index of water quality assessment in Mediterranean wetlands based on crustacean and insect assemblages: The case of Catalunya (NE Iberian peninsula). Aquat. Conserv. 2005, 15, 635–651. [Google Scholar] [CrossRef]
- Ferreira, J.G.; Nobre, A.M.; Simas, T.C.; Silva, M.C.; Newton, A.; Bricker, S.B.; Wolff, W.J.; Stacey, P.E.; Sequeira, A. A methodology for defining homogeneous water bodies in estuaries e application to the transitional systems of the EU water Framework Directive. Estuar. Coast. Shelf Sci. 2006, 66, 468–482. [Google Scholar] [CrossRef]
- Kagalou, I.; Leonardos, I. Typology, classification and management issues of Greek lakes: Implication of the Water Framework Directive (2000/60/EC). Environ. Monit. Assess. 2009, 150, 469–484. [Google Scholar] [CrossRef]
- Por, F.D. A classification of hypersaline waters, based on trophic criteria. Mar. Ecol. 1980, 1, 121–131. [Google Scholar] [CrossRef]
- Wortmann, J.; Hearne, J.W.; Adams, J.B. Evaluating the effects of freshwater inflow on the distribution of estuarine macrophytes. Ecol. Modell. 1998, 106, 213–232. [Google Scholar] [CrossRef]
- Menéndez, M.; Hernandez, O.; Comin, F.A. Spatial distribution and ecophysiological characteristics of macrophytes in a Mediterranean coastal lagoon. Estuar. Coast. Shelf Sci. 2002, 55, 403–413. [Google Scholar] [CrossRef]
- Greve, T.M.; Binzer, T. Which Factors Regulate Seagrass Growth and Distribution? In European Seagrasses: An Introduction to Monitoring and Management; Borum, J., Duarte, C.M., Krause-Jensen, D., Greve, T.M., Eds.; The M&Ms Project: Hillerød, Denmark, 2004; pp. 19–23. [Google Scholar]
- Casagranda, C.; Boudouresque, C.F. Biomass of Ruppia cirrhosa and Potamogeton pectinatus in a Mediterranean brackish lagoon, Lake Ichkeul, Tunisia. Fundam. Appl. Limnol. 2007, 168, 243. [Google Scholar] [CrossRef]
- Christia, C.; Papastergiadou, E.S. Spatial and temporal variations of aquatic macrophytes and water quality in six coastal lagoons of western Greece. Belg. J. Bot. 2007, 140, 39–50. [Google Scholar]
- Obrador, B.; Moreno-Ostos, E.; Pretus, J.L. A Dynamic Model to Simulate Water Level and Salinity in a Mediterranean Coastal Lagoon. Estuaries Coasts 2008, 31, 1117–1129. [Google Scholar] [CrossRef]
- Antunes, C.; Correia, O.; da Silva, J.M.; Cruces, A.; da Conceição Freitas, M.; Branquinho, C. Factors involved in spatiotemporal dynamics of submerged macrophytes in a Portuguese coastal lagoon under Mediterranean climate. Estuar. Coast. Shelf Sci. 2012, 110, 93–100. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Fernández, A.I.; Marcos, C.; Gilabert, J.; Quispe, J.I.; García-Charton, J.A. Spatial and temporal variations of hydrological conditions, nutrients and chlorophyll in a Mediterranean coastal lagoon (Mar Menor, Spain). Hydrobiologia 2005, 550, 11–27. [Google Scholar] [CrossRef]
- Franco, A.; Franzoi, P.; Torricelli, P. Structure and functioning of Mediterranean lagoon fish assemblages: A key for the identification of water body types. Estuar. Coast. Shelf Sci. 2008, 79, 549–558. [Google Scholar] [CrossRef]
- Graham, L.; Graham, J.; Wilcox, L. Algae; Pearson Education, Inc.: San Francisco, CA, USA, 2009. [Google Scholar]
- Boegle, M.G.; Schneider, S.; Mannschreck, B.; Melzer, A. Differentiation of Chara intermedia and C. baltica compared to C. hispida based on morphology and amplified fragment length polymorphism. Hydrobiologia 2007, 586, 155–166. [Google Scholar] [CrossRef]
- den Hartog, C. The Seagrasses of the World; North Holland: Amsterdam, The Netherlands, 1970. [Google Scholar]
- Aiello, G.; Barra, D.; Coppa, M.G.; Valente, A.; Zeni, F. Recent infralittoral foraminiferida and ostracoda from the porto Cesareo Lagoon (Ionian Sea, Mediterranean). Boll. Soc. Paleontol. 2006, 45, 1. [Google Scholar]
- Cardellicchio, N.; Annicchiarico, C.; Di Leo, A.; Giandomenico, S.; Spada, L. The Mar Piccolo of Taranto: An interesting marine ecosystem for the environmental problems studies. Environ. Sci. Pollut. Res. 2016, 23, 12495–12501. [Google Scholar] [CrossRef]
- Cecere, E.; Petrocelli, A.; Saracino, O.D. Biodiversity of phytobenthic communities in the marine reserve of Porto Cesareo. Biol. Mar. Medit. 2005, 12, 78–87. [Google Scholar]
- den Hartog, C.; Triest, L. A profound view and discourse on the typification and status of three confused taxa: Ruppia maritima, R. spiralis and R. cirrhosa. Bot. Mar. 2020, 63, 229–239. [Google Scholar] [CrossRef]
- Sfriso, A.; Curiel, D.; Rismondo, A. The Venice Lagoon. In Flora and Vegetation of the Italian Transitional Water Systems; Cecere, E., Petrocelli, A., Izzo, G., Sfriso, A., Eds.; CoRiLa, Multigraf: Spinea, Italy, 2009; pp. 17–80. [Google Scholar]
- Laugier, T.; Rigollet, V.; de Casabianca, M.L. Seasonal dynamics in mixed eelgrass beds, Zostera marina L. and Z. noltii Hornem., in a Mediterranean coastal lagoon (Thau lagoon, France). Aquat. Bot. 1999, 63, 51–69. [Google Scholar] [CrossRef]
- Bonometto, A.; Ponis, E.; Cacciatore, F.; Riccardi, E.; Pigozzi, S.; Parati, P.; Novello, M.; Ungaro, N.; Acquavita, A.; Manconi, P.; et al. A New Multi-Index Method for the Eutrophication Assessment in Transitional Waters: Large-Scale Implementation in Italian Lagoons. Environments 2022, 9, 41. [Google Scholar] [CrossRef]
- Sfriso, A.; Facca, C.; Bon, D.; Buosi, A. Macrophytes and ecological status assessment in the Po delta transitional systems, Adriatic Sea (Italy). Application of Macrophyte Quality Index (MaQI). Acta Adriat. 2016, 57, 209–225. [Google Scholar]
- Wallentinus, I. The Baltic Sea. Ecosys. World 1991, 24, 83–108. [Google Scholar]
- Sfriso, A.; Buosi, A.; Facca, C.; Sfriso, A. Role of environmental factors in affecting macrophyte dominance in transitional environments: The Italian Lagoons as a study case. Mar. Ecol. 2017, 38. [Google Scholar] [CrossRef]
- Akasaka, M.; Takamura, N. Hydrologic connection between ponds positively affects macrophyte α and γ diversity but negatively affects β diversity. Ecology 2012, 93, 967–973. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Gkafas, G.A.; Papathanasiou, V.; Orfanidis, S.; Küpper, F.C.; Arnaud-Haond, S.; Exadactylos, A. Biogeography pattern of the marine angiosperm Cymodocea nodosa in the eastern Mediterranean Sea related to the quaternary climatic changes. Ecol. Evol. 2022, 12. [Google Scholar] [CrossRef]
- Robinson, B.J.O.; Morley, S.A.; Rizouli, A.; Sarantopoulou, J.; Gkafas, G.A.; Exadactylos, A.; Küpper, F.C. New confirmed depth-limit of Antarctic macroalgae: Palmaria decipiens found at 100 m depth in the Southern Ocean. Polar. Biol. 2022, 45, 1459–1463. [Google Scholar] [CrossRef]
- Adams, J.B.; Bate, G.C.; O’Callaghan, M. Primary producers. In Estuaries of South Africa; Allanson, B.R., Baird, D., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 91–117. [Google Scholar]
- Hayakawa, Y.I.; Ogawa, T.; Yoshikawa, S.; Ohki, K.; Kamiya, M. Genetic and ecophysiological diversity of Cladophora (Cladophorales, Ulvophyceae) in various salinity regimes. Phycol. Res. 2012, 60, 86–97. [Google Scholar] [CrossRef]
- Rybak, A.S. Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol. Indic. 2018, 85, 253–261. [Google Scholar] [CrossRef]

| WB | Coordinates | Surface Area (km2) | Depth (m) | Salinity Class (PSU) * |
|---|---|---|---|---|
| Torre Guaceto (TG) | 40°42′51.34″ N–17°47′42.91″ E | 1.2 | 0.4–0.6 | Mesohaline (5–20) |
| Cesine (CE) | 40°21′33.46″ N–18°20′09.30″ E | 0.7 | 0.2–0.8 | Mesohaline (5–20) |
| Lesina (LE1) | 41°53′12.64″ N–15°21′15.65″ E | 18 | 0.7–2 | Polyhaline (20–30) |
| Lesina (LE2) | 41°53′01.23″ N–15°27′20.15″ E | 17 | 0.7–2 | Polyhaline (20–30) |
| Lesina (LE3) | 41°53′57.20″ N–15°31′00.45″ E | 16 | 0.7–2 | Polyhaline (20–30) |
| Varano (VA) | 41°52′43.65″ N–15°44′35.42″ E | 60.5 | 0.5–5 | Polyhaline (20–30) |
| Porto Cesareo (PC) | 40°14′31.80″ N–17°54′32.82″ E | 2 | 0.3–5 | Euhaline (30–40) |
| Mar Piccolo (MP1) | 40°29′19.68″ N–17°15′29.51″ E | 9.7 | 0.5–12 | Euhaline (30–40) |
| Mar Piccolo (MP2) | 40°29′22.92″ N–17°18′29.18″ E | 11 | 0.5–12 | Euhaline (30–40) |
| Margherita di Savoia (LS) | 41°25′27.34″ N–15°59′53.29″ E | 8.5 | 0.4–0.6 | Hyperhaline (>40) |
| Punta della Contessa (PU) | 40°35′42.31″ N–18°02′30.05″ E | 2 | 0.4–1 | Hyperhaline (>40) |
| HH | EH | PH | MH | |
|---|---|---|---|---|
| MAGNOLIOPHYTA | ||||
| Alismatales | ||||
| Cymodocea nodosa | +++ | + | ||
| Ruppia spiralis | ++++ | ++ | ++ | |
| Zannichellia palustris | + | |||
| Zostera marina | + | |||
| Zostera noltei | ++++ | |||
| Saxifragales | ||||
| Myriophyllum spicatum | + | |||
| CHAROPHYTA | ||||
| Charales | ||||
| Chara baltica | +++ | |||
| Chara contraria | + | |||
| Lamprothamnium papulosum | + | |||
| Lamprothamnium succinctum | + | |||
| Zygnematales | ||||
| Spirogyra sp. | ++++ | |||
| Zygnema sp. | + | |||
| CHLOROPHYTA | ||||
| Bryopsidales | ||||
| Bryopsis cupressina | + | |||
| Bryopsis hypnoides | + | |||
| Bryopsis secunda | + | |||
| Caulerpa cylindracea | + | |||
| Caulerpa prolifera | + | |||
| Codium bursa | + | |||
| Derbesia tenuissima | + | + | + | |
| Halimeda tuna | + | |||
| Pedobesia simplex | + | |||
| Cladophorales | ||||
| Aegagropila linnaei | + | + | + | |
| Anadyomene stellata | ++ | |||
| Chaetomorpha aerea | ++ | + | ||
| Chaetomorpha ligustica | + | |||
| Chaetomorpha linum | + | ++ | + | |
| Chaetomorpha tortuosa | + | + | + | + |
| Cladophora albida | + | + | ||
| Cladophora coelothrix | + | |||
| Cladophora dalmatica | + | + | + | |
| Cladophora fracta | + | + | + | |
| Cladophora glomerata | + | + | + | |
| Cladophora hutchinsiae | + | |||
| Cladophora laetevirens | + | |||
| Cladophora lehmanniana | + | + | ||
| Cladophora liniformis | + | + | ||
| Cladophora prolifera | + | + | ||
| Cladophora rupestris | + | + | ||
| Cladophora sericea | ++ | + | ||
| Cladophora vadorum | + | + | + | ++ |
| Cladophora vagabunda | ++ | + | + | |
| Cladophoropsis membranacea | + | |||
| Lychaete echinus | + | + | ||
| Rhizoclonium riparium | + | |||
| Valonia macrophysa | + | |||
| Valonia utricularis | ++ | |||
| Dasycladales | ||||
| Acetabularia acetabulum | + | |||
| Dasycladus vermicularis | + | |||
| Ulotrichales | ||||
| Ulothrix flacca | + | |||
| Ulothrix implexa | + | + | + | |
| Urospora penicilliformis | ++ | + | + | |
| Ulvales | ||||
| Blidingia marginata | + | |||
| Blidingia minima | + | |||
| Ulva australis | + | + | + | |
| Ulva clathrata | + | + | ||
| Ulva compressa | + | + | + | |
| Ulva curvata | + | + | ||
| Ulva flexuosa | + | + | + | |
| Ulva intestinalis | +++ | + | + | + |
| Ulva kylinii | + | |||
| Ulva linza | + | |||
| Ulva prolifera | ++ | + | ||
| Ulva prolifera subsp. blidingiana | + | |||
| Ulva pseudorotundata | + | + | ||
| Ulva rigida | + | + | ||
| Ulvaria obscura | + | |||
| Ulvella lens | + | |||
| Ulvella viridis | + | + | ||
| Umbraulva dangeardii | + | |||
| OCHROPHYTA-Phaeophyceae | ||||
| Dictyotales | ||||
| Dictyota dichotoma | ++ | |||
| Dictyota dichotoma var. intricata | + | |||
| Dictyota implexa | + | |||
| Dictyota mediterranea | + | |||
| Padina ditristromatica | + | |||
| Padina pavonica | + | |||
| Ectocarpales | ||||
| Ectocarpus fasciculatus | + | |||
| Ectocarpus siliculosus | + | |||
| Pylaiella littoralis | + | |||
| Scytosiphon lomentaria | + | + | ||
| Fucales | ||||
| Cystoseira aurantia | + | |||
| Cystoseira compressa | + | |||
| Gongolaria barbata | +++ | + | ||
| RHODOPHYTA | ||||
| Acrochaetiales | ||||
| Acrochaetium secundatum | + | |||
| Bangiales | ||||
| Neoyropia leucosticta | + | + | ||
| Bonnemaisoniales | ||||
| Asparagopsis taxiformis | ++ | |||
| Ceramiales | ||||
| Acrosorium ciliolatum | + | |||
| Aglaothamnion feldmanniae | + | |||
| Aglaothamnion tenuissimum | + | |||
| Aglaothamnion tripinnatum | + | |||
| Alsidium corallinum | + | + | ||
| Alsidium helminthochorton | + | |||
| Anotrichium furcellatum | + | |||
| Antithamnion cruciatum | + | |||
| Carradoriella denudata | + | + | ||
| Carradoriella elongata | + | |||
| Ceramium ciliatum | + | |||
| Ceramium cimbricum | + | ++ | ||
| Ceramium cimbricum f. flaccidum | + | |||
| Ceramium codii | + | |||
| Ceramium deslongchampsii | + | + | ||
| Ceramium diaphanum | + | ++ | ||
| Ceramium echionotum | + | + | ||
| Ceramium siliquosum | + | + | ||
| Ceramium tenerrimum | + | + | ||
| Chondria capillaris | + | + | +++ | |
| Chondria coerulescens | + | |||
| Chondria dasyphylla | + | + | ||
| Chondria polyrhiza | + | |||
| Dasya pedicellata | + | +++ | ||
| Dasya ocellata | + | |||
| Gayliella mazoyerae | + | + | ||
| Griffithsia opuntioides | + | |||
| Griffithsia schousboei | + | |||
| Herposiphonia secunda | + | + | ||
| Heterosiphonia crispella | + | |||
| Hypoglossum hypoglossoides | + | |||
| Laurencia obtusa | + | |||
| Leptosiphonia fibrillosa | + | + | + | |
| Lophosiphonia cristata | + | |||
| Lophosiphonia obscura | + | + | + | + |
| Melanothamnus harveyi | + | + | ||
| Osmundea oederi | + | |||
| Palisada perforata | + | |||
| Palisada thuyoides | + | |||
| Polysiphonia atlantica | + | + | ||
| Polysiphonia opaca | + | |||
| Polysiphonia stricta | ++ | + | ||
| Pterothamnion crispum | + | |||
| Rytiphlaea tinctoria | + | |||
| Spyridia filamentosa | ++ | + | ||
| Vertebrata fruticulosa | + | |||
| Vertebrata fucoides | + | |||
| Vertebrata furcellata | + | + | + | |
| Vertebrata reptabunda | + | |||
| Wrangelia penicillata | + | |||
| Colaconematales | ||||
| Colaconema caespitosum | + | |||
| Colaconema corymbiferum | + | |||
| Colaconema daviesii | + | + | ||
| Corallinales | ||||
| Amphiroa rigida | + | |||
| Corallina officinalis | + | |||
| Ellisolandia elongata | + | |||
| Hydrolithon cruciatum | + | + | ||
| Hydrolithon farinosum | + | + | ||
| Jania rubens | + | |||
| Jania virgata | + | |||
| Melobesia membranacea | + | + | ||
| Pneophyllum fragile | + | |||
| Titanoderma pustulatum | + | |||
| Erythropeltales | ||||
| Erythrotrichia carnea | + | + | ||
| Gelidiales | ||||
| Gelidium crinale | + | + | ||
| Pterocladiella capillacea | + | |||
| Gigartinales | ||||
| Caulacanthus ustulatus | + | |||
| Chondracanthus acicularis | + | |||
| Chondracanthus teedei | + | |||
| Feldmannophycus rayssiae | + | |||
| Gymnogongrus griffithsiae | + | + | ||
| Hypnea corona | + | |||
| Hypnea musciformis | + | |||
| Hypnea spinella | + | |||
| Schmitziella endophloea | + | |||
| Wurdemannia miniata | + | + | ||
| Gracilariales | ||||
| Gracilaria bursa-pastoris | + | |||
| Gracilaria gracilis | ++ | ++ | ||
| Gracilaria longa | + | |||
| Halymeniales | ||||
| Grateloupia filicina | + | + | ||
| Rhodymeniales | ||||
| Champia parvula | + | + | ||
| Chylocladia verticillata | + | |||
| Lomentaria articulata | + | |||
| Rhodymenia ardissonei | + | |||
| Rhodymenia sp. | + | |||
| Stylonematales | ||||
| Stylonema alsidii | + | |||
| Pairwise ANOSIM | R-Values | p-Values |
|---|---|---|
| hyperhaline vs. euhaline | 1 | 0.1019 |
| hyperhaline vs. polyhaline | 1 | 0.0649 |
| hyperhaline vs. mesohaline | 1 | 0.3290 |
| euhaline vs. polyhaline | 0.9630 | 0.0278 * |
| euhaline vs. mesohaline | 1 | 0.0986 |
| polyhaline vs. mesohaline | 1 | 0.0659 |
| Global ANOSIM | R-values | p-values |
| 0.9545 | 0.0002 *** |
| TAXON | Av. Abund. | Av. Sim. | Contrib. % | Cum. % | Group Av. Sim. % | |
|---|---|---|---|---|---|---|
| MESO HALINE | Spirogyra sp. | 80.92 | 22.76 | 58.20 | 58.20 | 39.11 |
| Cladophora vadorum | 40.83 | 12.41 | 31.75 | 89.95 | ||
| Chara baltica | 56.33 | 3.93 | 10.05 | 100.00 | ||
| POLYHALINE | Zostera noltei | 76.66 | 12.49 | 22.65 | 22.65 | 55.15 |
| Chondria capillaris | 63.03 | 10.92 | 19.79 | 42.45 | ||
| Dasya pedicellata | 56.25 | 8.60 | 15.60 | 58.05 | ||
| Ceramium cimbricum | 32.04 | 4.31 | 7.82 | 65.86 | ||
| Ceramium diaphanum | 30.61 | 3.82 | 6.93 | 72.79 | ||
| Ceramium deslongchampsii | 23.91 | 3.73 | 6.77 | 79.57 | ||
| Ruppia spiralis | 37.50 | 2.50 | 4.53 | 84.10 | ||
| Gracilaria gracilis | 26.35 | 1.63 | 2.96 | 87.06 | ||
| Cladophora vadorum | 7.81 | 1.40 | 2.53 | 89.59 | ||
| EUHALINE | Gongolaria barbata | 50.06 | 3.23 | 10.97 | 10.97 | 29.49 |
| Cymodocea nodosa | 51.19 | 3.18 | 10.79 | 21.76 | ||
| Valonia utricularis | 37.50 | 3.00 | 10.16 | 31.93 | ||
| Chaetomorpha aerea | 44.58 | 2.74 | 9.29 | 41.22 | ||
| Ceramium deslongchampsii | 23.75 | 2.67 | 9.06 | 50.28 | ||
| Dictyota dichotoma | 31.81 | 2.34 | 7.93 | 58.21 | ||
| Spyridia filamentosa | 32.08 | 2.16 | 7.31 | 65.52 | ||
| Gracilaria gracilis | 26.94 | 1.55 | 5.27 | 70.80 | ||
| Cladophora prolifera | 19.03 | 1.11 | 3.76 | 74.55 | ||
| Chaetomorpha linum | 17.94 | 0.92 | 3.13 | 77.69 | ||
| Hypnea corona | 13.47 | 0.78 | 2.64 | 80.32 | ||
| Rhodymenia ardissonei | 16.03 | 0.64 | 2.17 | 82.50 | ||
| Cladophora vagabunda | 4.58 | 0.52 | 1.77 | 84.26 | ||
| Cladophora laetevirens | 6.11 | 0.49 | 1.65 | 85.92 | ||
| Ceramium diaphanum | 4.72 | 0.41 | 1.40 | 87.32 | ||
| Gayliella mazoyerae | 3.89 | 0.34 | 1.17 | 88.48 | ||
| Carradoriella denudata | 2.78 | 0.32 | 1.07 | 89.55 | ||
| HYPERHALINE | Ruppia spiralis | 100.00 | 20.02 | 42.74 | 42.74 | 46.85 |
| Ulva intestinalis | 57.50 | 10.01 | 21.37 | 64.10 | ||
| Ulva prolifera | 42.00 | 5.81 | 12.39 | 76.50 | ||
| Urospora penicilliformis | 28.00 | 5.61 | 11.97 | 88.46 | ||
| Polysiphonia stricta | 27.00 | 5.41 | 11.54 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tursi, A.; Lisco, A.; Chimienti, G.; Mastrototaro, F.; Ungaro, N.; Bottalico, A. Salinity as a Key Factor in Structuring Macrophyte Assemblages in Transitional Water Bodies: The Case of the Apulian Coastal Lagoons (Southern Italy). Diversity 2023, 15, 615. https://doi.org/10.3390/d15050615
Tursi A, Lisco A, Chimienti G, Mastrototaro F, Ungaro N, Bottalico A. Salinity as a Key Factor in Structuring Macrophyte Assemblages in Transitional Water Bodies: The Case of the Apulian Coastal Lagoons (Southern Italy). Diversity. 2023; 15(5):615. https://doi.org/10.3390/d15050615
Chicago/Turabian StyleTursi, Andrea, Anna Lisco, Giovanni Chimienti, Francesco Mastrototaro, Nicola Ungaro, and Antonella Bottalico. 2023. "Salinity as a Key Factor in Structuring Macrophyte Assemblages in Transitional Water Bodies: The Case of the Apulian Coastal Lagoons (Southern Italy)" Diversity 15, no. 5: 615. https://doi.org/10.3390/d15050615
APA StyleTursi, A., Lisco, A., Chimienti, G., Mastrototaro, F., Ungaro, N., & Bottalico, A. (2023). Salinity as a Key Factor in Structuring Macrophyte Assemblages in Transitional Water Bodies: The Case of the Apulian Coastal Lagoons (Southern Italy). Diversity, 15(5), 615. https://doi.org/10.3390/d15050615










