Molecular Phylogeny of Holarctic Aeshnidae with a Focus on the West Palaearctic and Some Remarks on Its Genera Worldwide (Aeshnidae, Odonata) †
Abstract
:1. Introduction



2. Materials and Methods
2.1. Materials
2.2. DNA Extraction and Sequencing
2.3. Reducing Artefacts
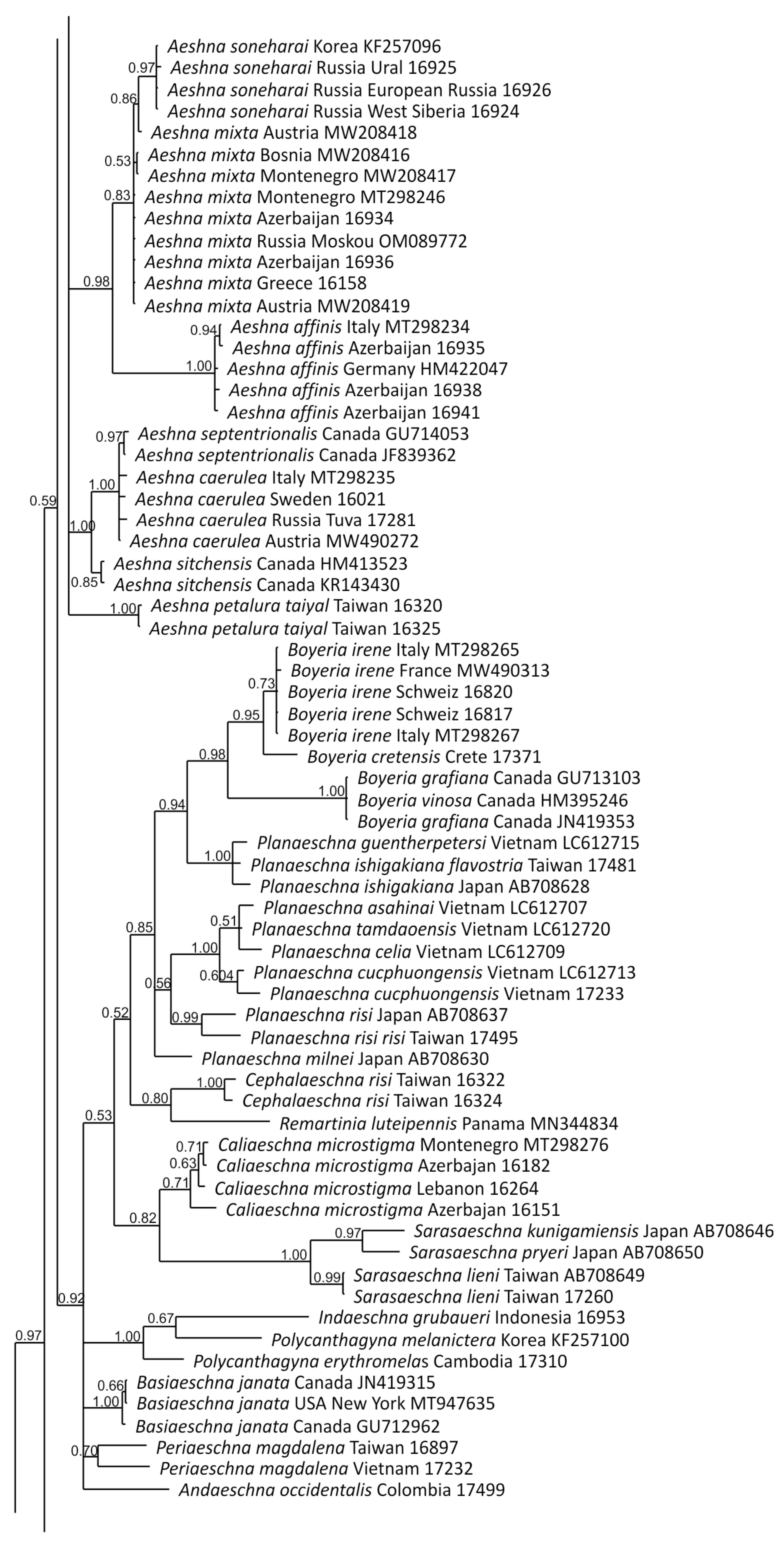
2.4. Phylogenetic Analysis
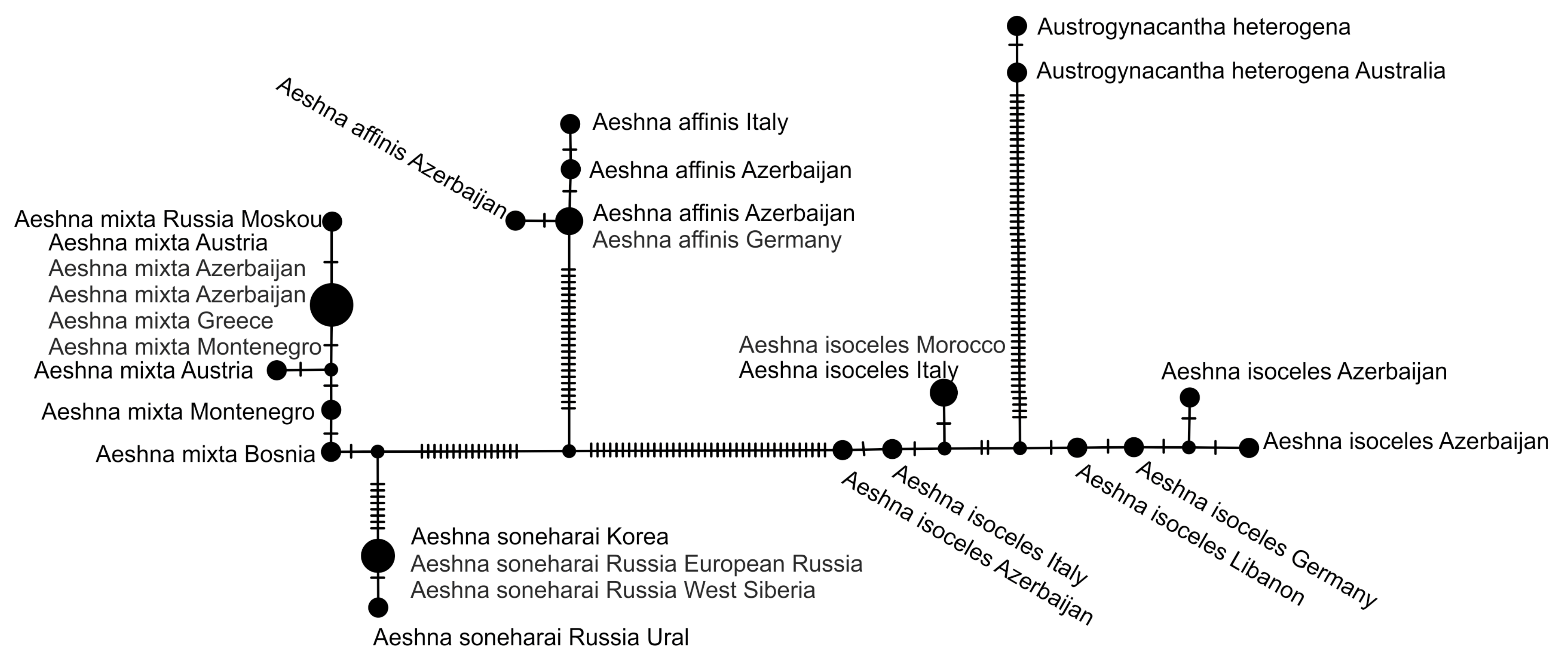
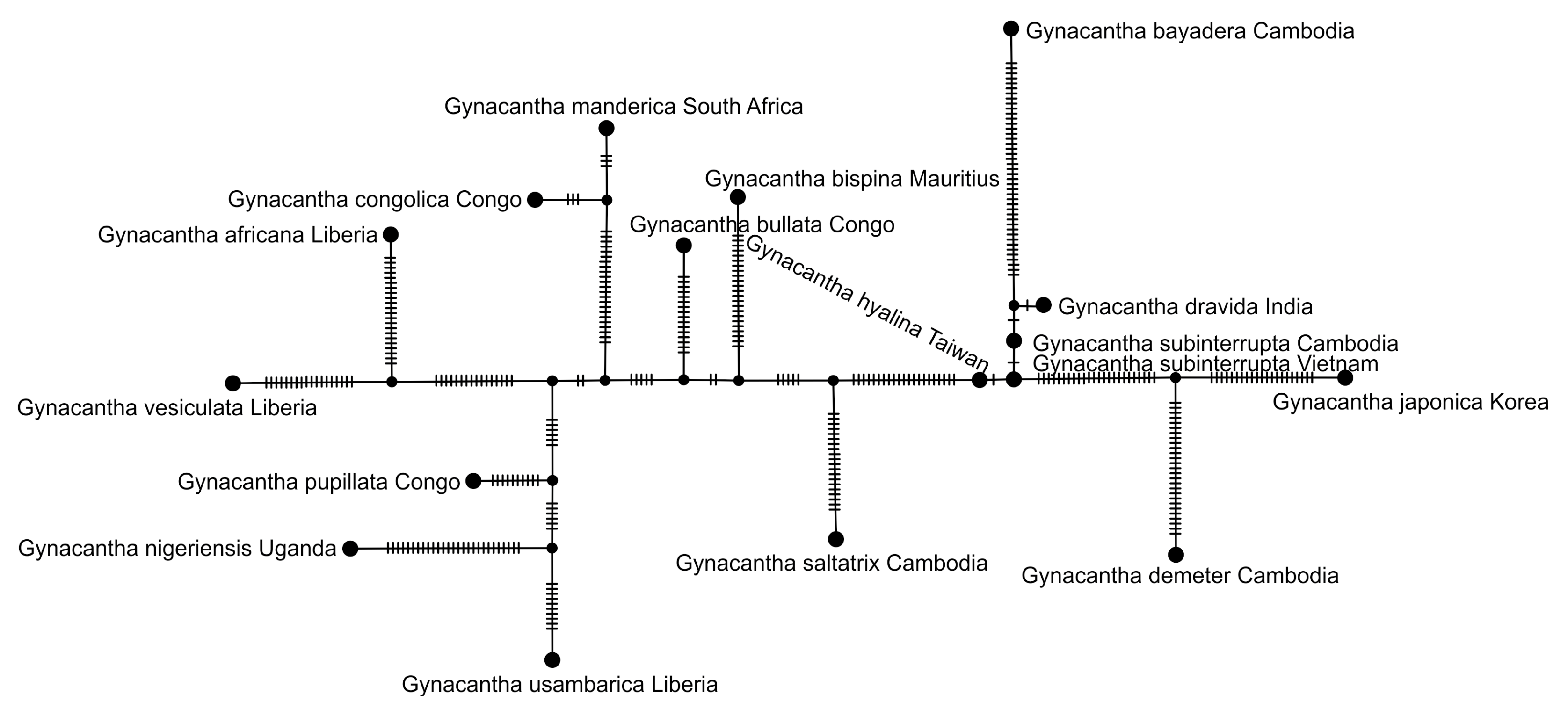
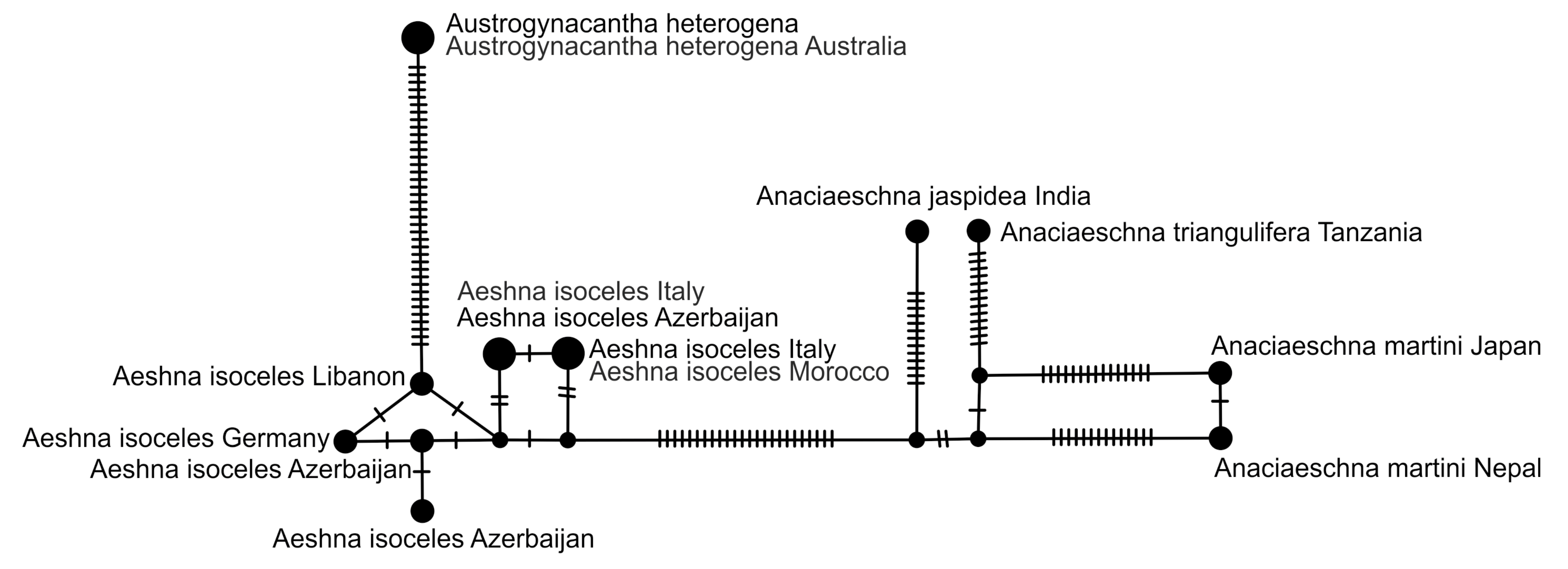
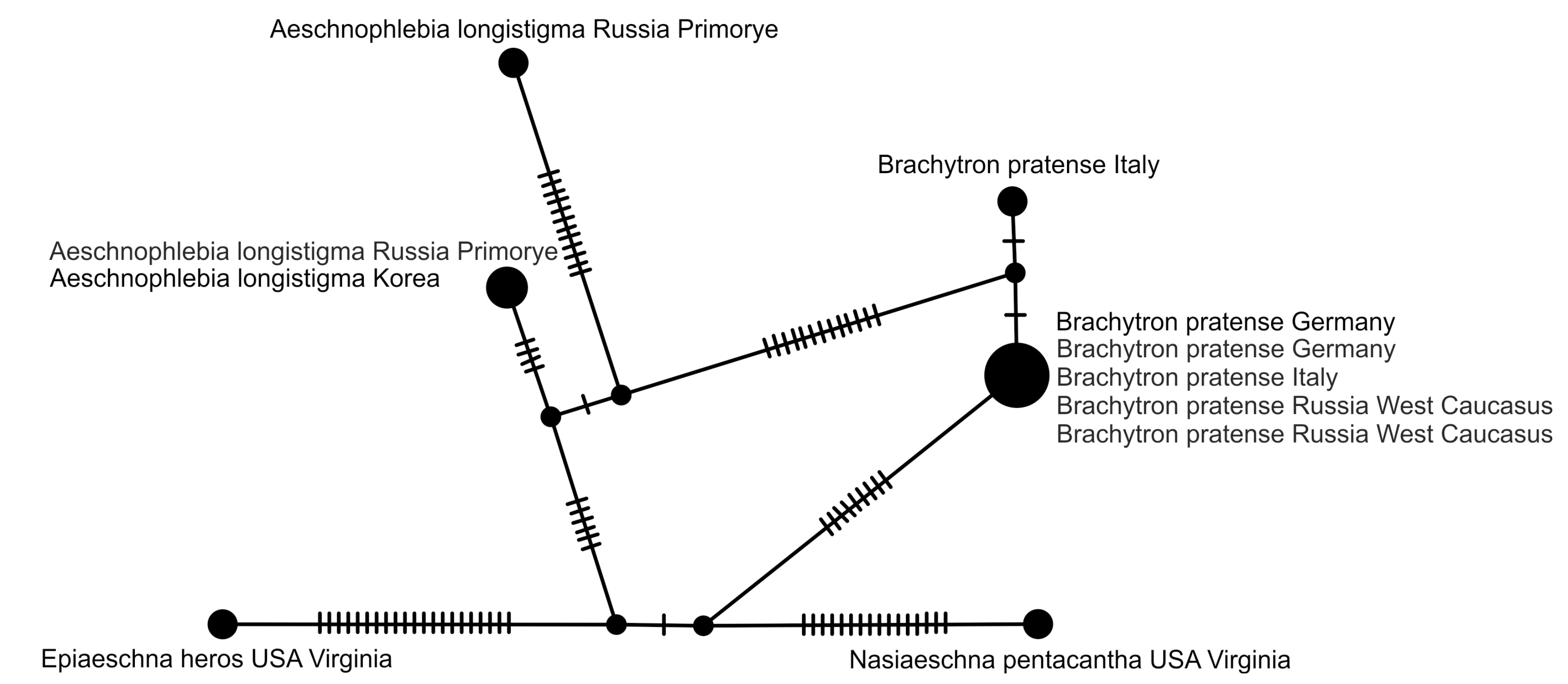
2.5. Haplotype Network Analysis
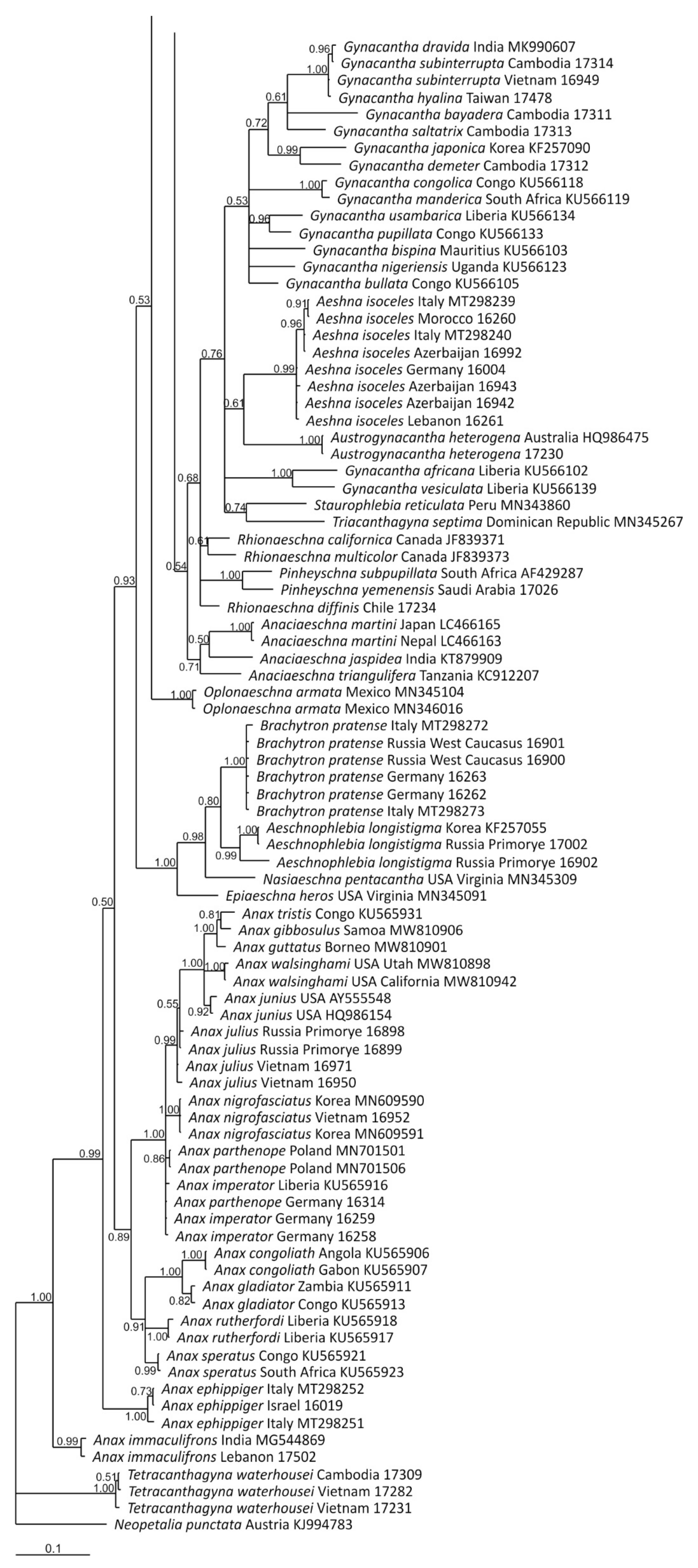
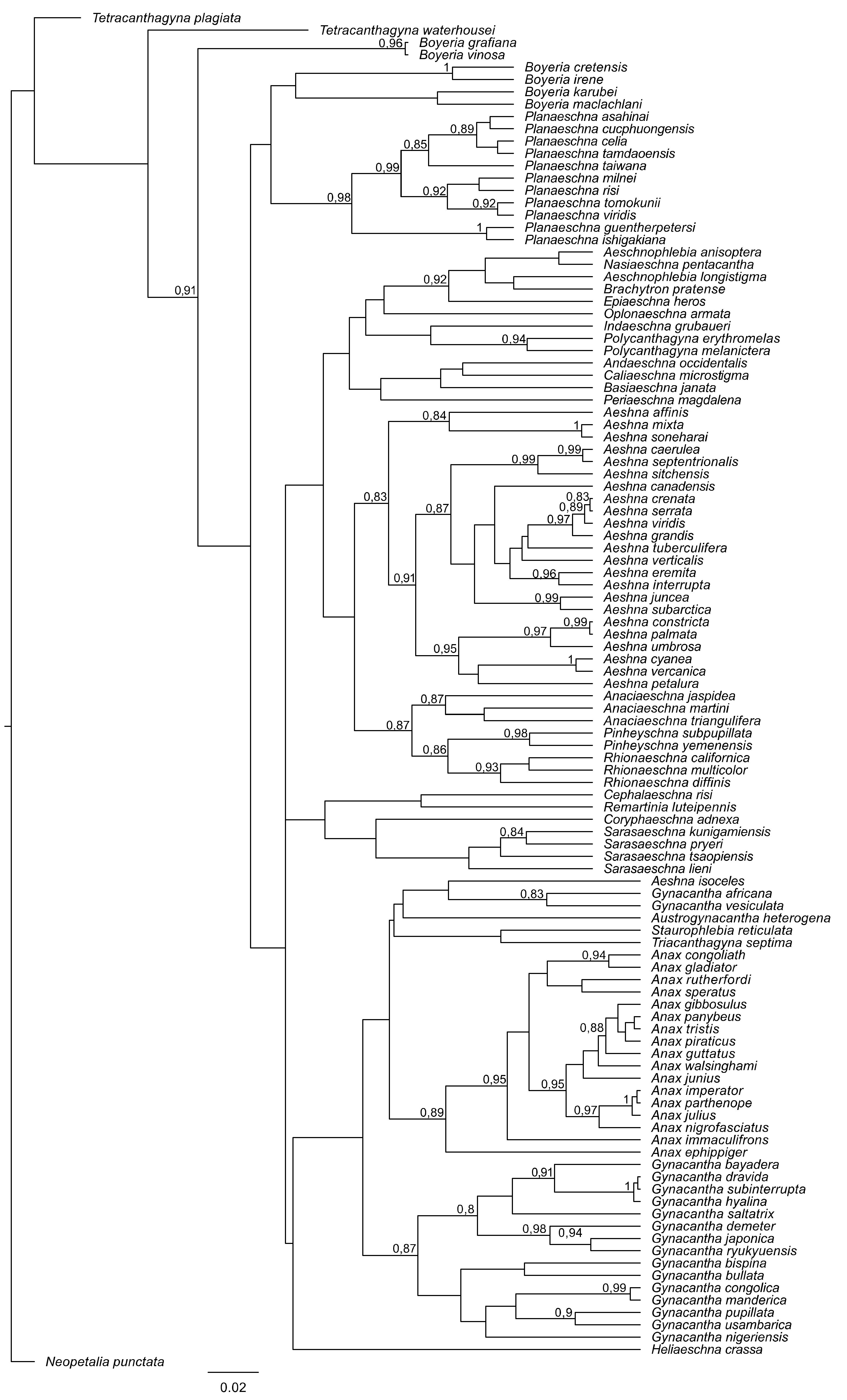
3. Results and Discussion
3.1. Analysis Based on the ITS Region
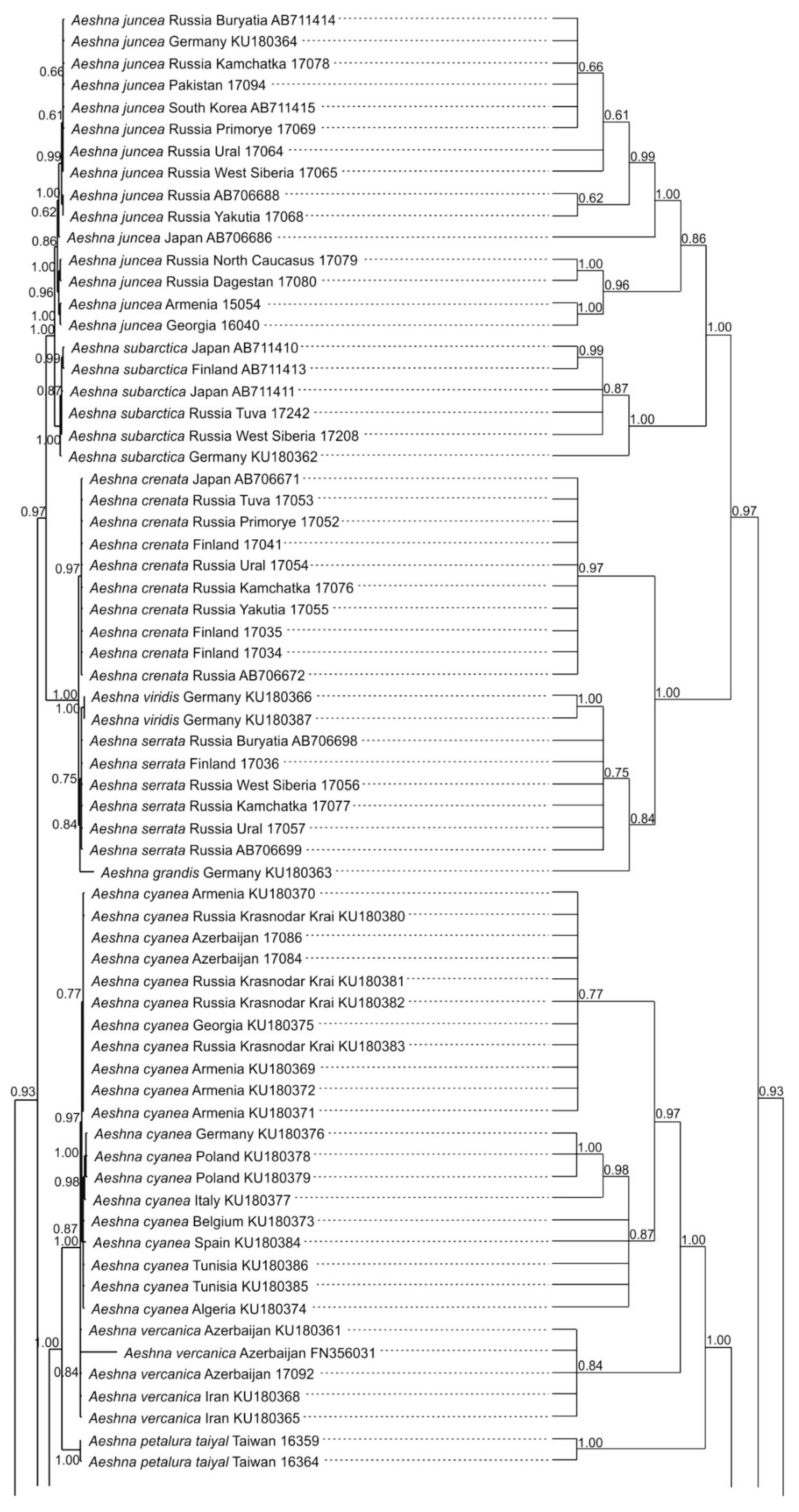
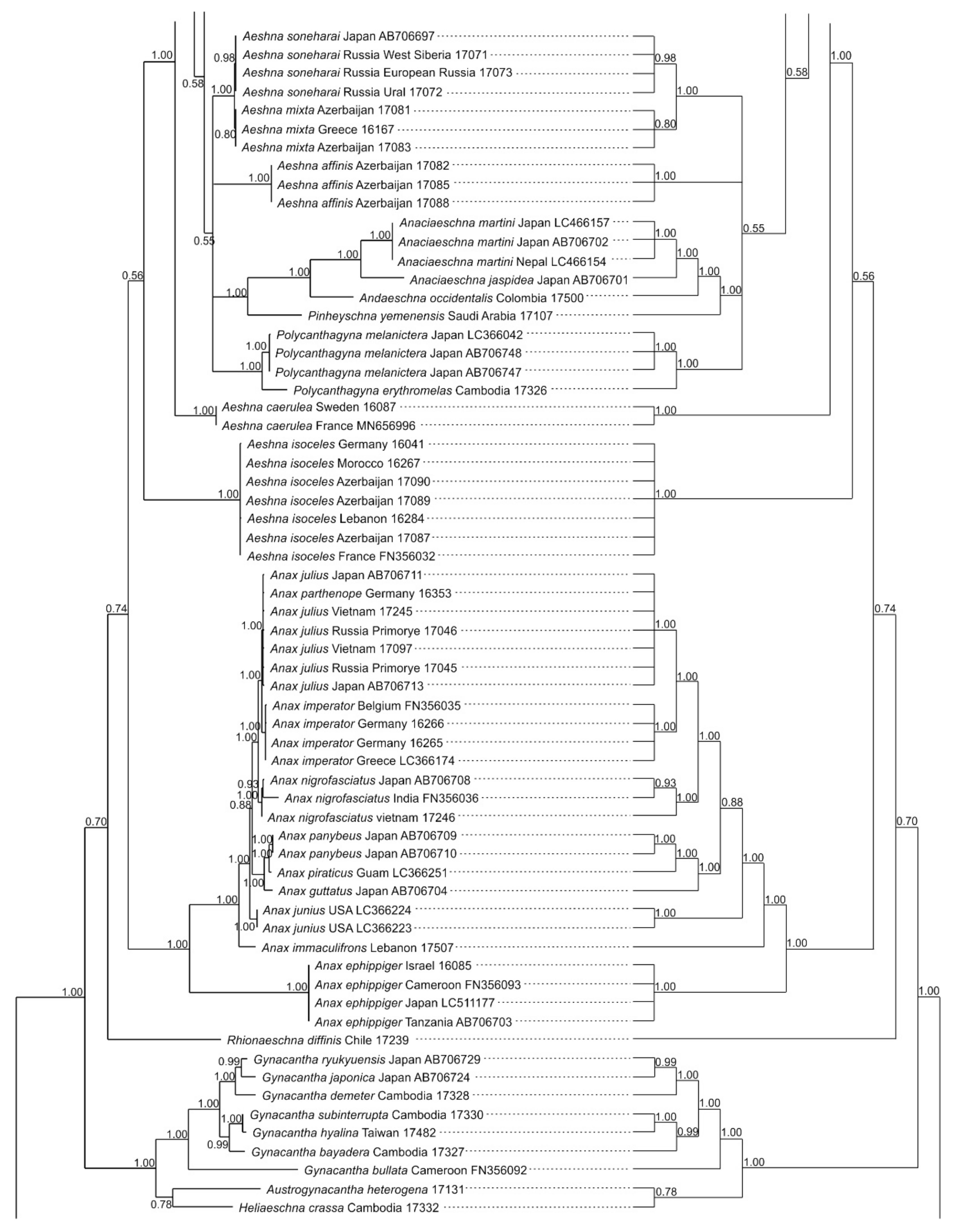
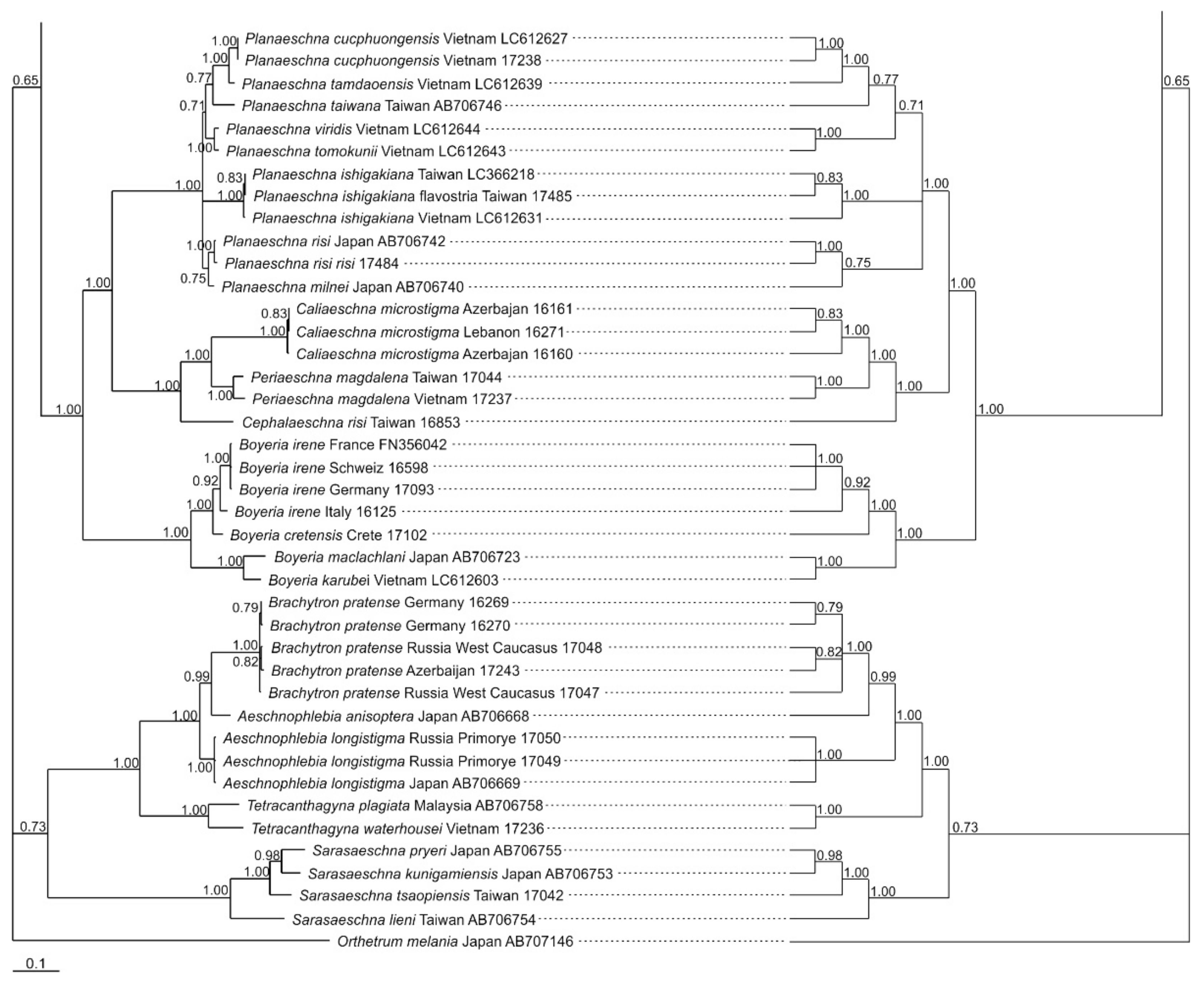
3.2. Analysis Based on the COI Gene
3.2.1. Analysis Based on Long COI Fragment
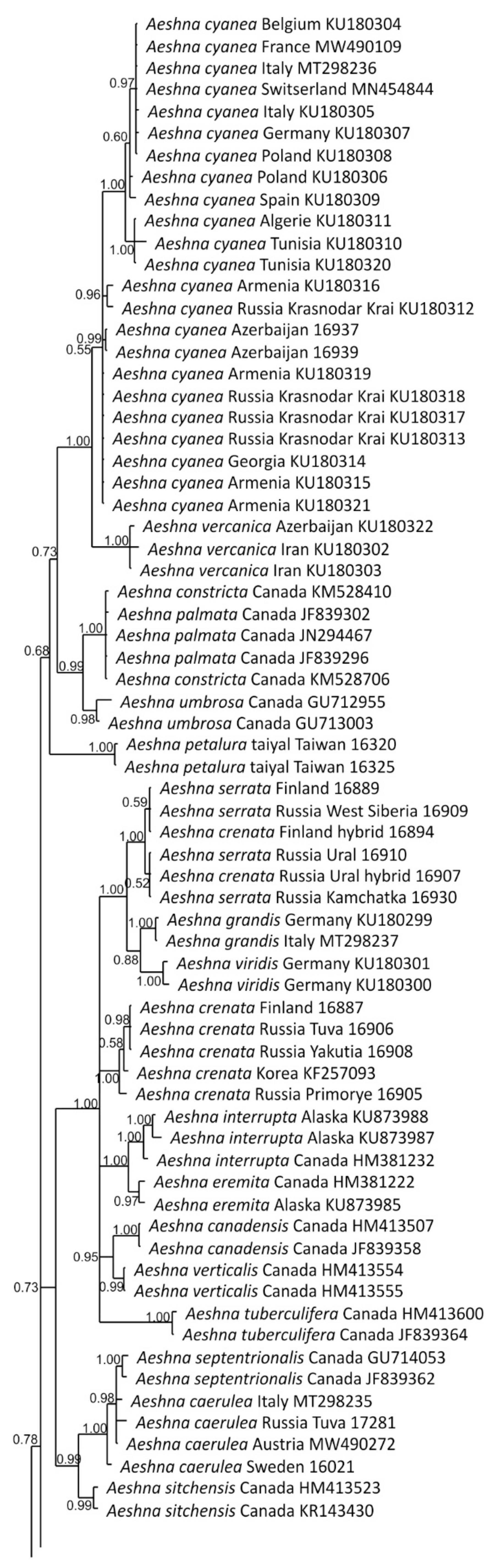

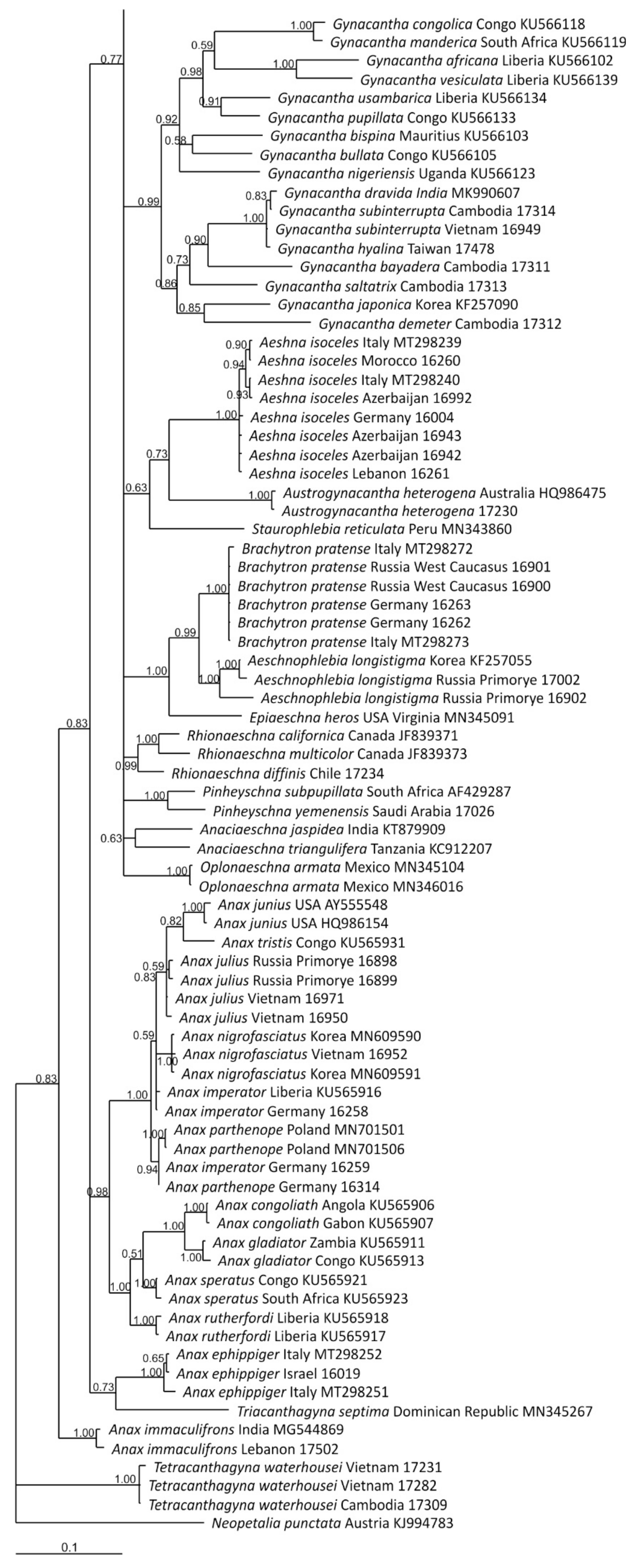
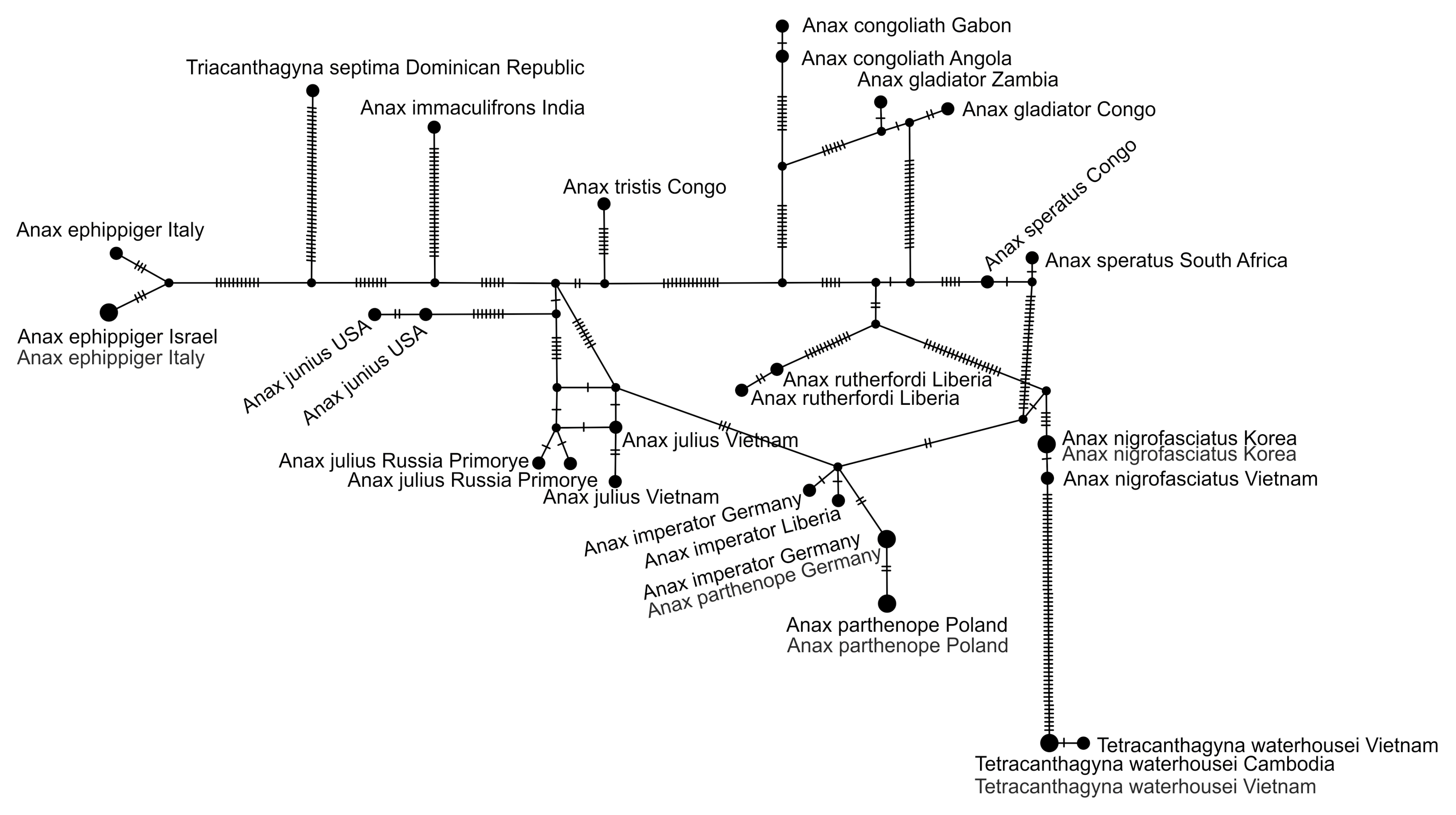
3.2.2. Analysis Based on a Short COI Fragment
3.3. StarBeast Analysis of COI and ITS Gene Fragments Together
4. Overall Discussion
5. Taxonomic Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Paulson, D.; Schorr, M.; Abbott, J.; Bota-Sierra, C.; Deliry, C.; Dijkstra, K.-D.; Lozano, F. (Coordinators). World Odonata List. OdonataCentral, University of Alabama. 2023. Available online: https://www.odonatacentral.org/app/#/wol/ (accessed on 22 July 2023).
- Walker, E.M. The North American Dragonflies of the Genus Aeshna; University of Toronto Library: Toronto, ON, Canada, 1912; Volume 11, pp. 1–213. [Google Scholar]
- Tillyard, R.J.; Fraser, F.C. A reclassification of the order Odonata based on some new interpretations of the venation of the dragonfly wing. Part III. Continuation and conclusion. Aust. Zool. 1940, 9, 359–396. [Google Scholar]
- Davies, D.A.L.; Tobin, P. The dragonflies of the world: A systematic list of the extant species of Odonata. 2. Anisoptera. Soc. Int. Odonatol. Rapid Commun. Suppl. 1985, 5, 1–151. [Google Scholar]
- Von Ellenrieder, N. A phylogenetic analysis of the extant Aeshnidae (Odonata: Anisoptera). Syst. Entomol. 2002, 27, 437–467. [Google Scholar] [CrossRef]
- Fleck, G.; Ullrich, B.; Brenk, M.; Wallnisch, C.; Orland, M.; Belidissel, S.; Misof, B. A phylogeny of anisopterous dragonflies (Insecta, Odonata) using mtRNA genes and mixed nucleotide⁄doublet models. J. Zool. Syst. Evol. Res. 2008, 46, 310–322. [Google Scholar] [CrossRef]
- Dijkstra, K.-D.B.; Kalkman, V.J. Phylogeny, classification and taxonomy of European dragonflies and damselflies (Odonata): A review. Org. Divers. Evol. 2012, 12, 209–227. [Google Scholar] [CrossRef]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef]
- Carle, F.; Kjer, K.; May, M. A molecular phylogeny and classification of Anisoptera (Odonata). Arthropod Syst. Phylogeny 2015, 73, 281–301. [Google Scholar] [CrossRef]
- Galimberti, A.; Assandri, G.; Maggioni, D.; Ramazzotti, F.; Baroni, D.; Bazzi, G.; Chiandetti, I.; Corso, A.; Ferri, V.; Galuppi, M.; et al. DNA barcoding and eDNA-based biomonitoring of Italian Odonata. Mol. Ecol. Resour. 2020, 21, 183–200. [Google Scholar] [CrossRef]
- Kohli, M.; Djernæs, M.; Sanchez Herrera, M.; Sahlen, G.; Pilgrim, E.; Simonsen, T.J.; Olsen, K.; Ware, J. Comparative phylogeography uncovers evolutionary past of Holarctic dragonflies. PeerJ 2021, 9, e11338. [Google Scholar] [CrossRef]
- Bybee, S.M.; Kalkman, V.J.; Erickson, R.J.; Frandsen, P.B.; Breinholt, J.W.; Suvorov, A.; Dijkstra, K.-D.B.; Cordero-Rivera, A.; Skevington, J.H.; Abbott, J.C.; et al. Phylogeny and classification of Odonata using targeted genomics. Mol. Phylogenetics Evol. 2021, 160, 107115. [Google Scholar] [CrossRef]
- Clement, R.A.; Saxton, N.A.; Standring, S.; Arnold, P.R.; Kaihileipihamekeola Johnson, K.; Bybee, D.R.; Bybee, S.M. Phylogeny, migration and geographic range size evolution of Anax dragonflies (Anisoptera: Aeshnidae). Zool. J. Linn. Soc. 2022, 194, 858–873. [Google Scholar] [CrossRef]
- Schneider, T.; Schneider, E.; Schneider, J.; Vierstraete, A.; Dumont, H.J. Aeshna vercanica spec. nov. from Iran (Anisoptera: Aeshnidae) and a new insight into the Aeshna-cyanea-group. Odonatologica 2015, 44, 81–106. [Google Scholar]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Koblmüller, S.; Assandri, G.; Chovanec, A.; Ekrem, T.; Fischer, I.; Galimberti, A.; Grabowski, M.; Haring, E.; Hausmann, A.; et al. Coverage and quality of DNA barcode references for Central and Northern European Odonata. PeerJ 2021, 9, e11192. [Google Scholar] [CrossRef] [PubMed]
- Haring, E.; Fischer, I.; Sittenthaler, M.; Wolf, P.; Chovanec, A.; Koblmueller, S.; Sattmann, H.; Beqiraj, S.; Pesic, V.; Zangl, L. Intraspecific genetic diversity in selected widespread dragonfly species (Insecta: Odonata). Acta Zoobot. Austria 2020, 157, 239–256. [Google Scholar]
- Kim, M.J.; Jung, K.S.; Park, N.S.; Wan, X.; Kim, K.-G.; Jun, J.; Yoon, T.J.; Bae, Y.J.; Lee, S.M.; Kim, I. Molecular phylogeny of the higher taxa of Odonata (Insecta) inferred from COI, 16S rRNA, 28S rRNA, and EF1-a sequences. Entomol. Res. 2014, 44, 65–79. [Google Scholar] [CrossRef]
- Futahashi, R. A revisional study of Japanese dragonflies based on DNA analysis (1). Tombo Acta Odonatol. Jpn. 2011, 53, 67–74. [Google Scholar]
- Galimberti, A.; Assandri, G.; Maggioni, D.; Ramazotti, F.; Baroni, D.; Bazzi, G.; Chiandetti, I.; Corso, A.; Ferri, V.; Galuppi, M.; et al. Italian odonates in the Pandora’s box: A comprehensive DNA barcoding inventory shows taxonomic warnings at the Holarctic scale. Mol. Ecol. Resour. 2021, 21, 183–200. [Google Scholar] [CrossRef]
- Sikes, D.S.; Bowser, M.; Morton, J.M.; Bickford, C.; Meierotto, S.; Hildebrandt, K. Building a DNA barcode library of Alaska’s non-marine arthropods. Genome 2017, 60, 248–259. [Google Scholar] [CrossRef]
- Hebert, P.D.; Ratnasingham, S.; Zakharov, E.V.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; de Waard, J.R. Counting animal species with DNA barcodes: Canadian nsects. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef]
- Onishko, V.V.; Kosterin, O.E.; Blinov, A.G.; Sukhikh, I.S.; Ogunleye, A.T.; Schröter, A. Aeshna soneharai Asahina, 1988, stat. rev., bona species—An overlooked member of European fauna? (Odonata: Aeshnidae). Odonatologica 2022, 5, 111–145. [Google Scholar] [CrossRef]
- Dumont, H.J.; Vierstraete, A.; Vanfleteren, J.R. A molecular phylogeny of the Odonata (Insecta). Syst. Entomol. 2010, 35, 6–18. [Google Scholar] [CrossRef]
- Conniff, K.; Sasamoto, A.; Futahashi, R.; Limbu, M.S. Revision of the status of Anaciaeschna donaldi and A. martini, with allied species, and distributional notes (Odonata: Aeshnidae). Odonatologica 2019, 48, 265–284. [Google Scholar]
- Bergmann, T.; Rach, J.; Damm, S.; Desalle, R.; Schierwater, B.; Hadrys, H. The potential of distance-based thresholds and character-based DNA barcoding for defining problematic taxonomic entities by CO1 and ND1. Mol. Ecol. Resour. 2013, 13, 1069–1081. [Google Scholar] [CrossRef]
- Dijkstra, K.-D.B.; Kipping, J.; Meziere, N. Sixty new dragonfly and damselfly species from Africa (Odonata). Odonatologica 2015, 44, 447–678. [Google Scholar]
- Futahashi, H.; Futahashi, M.; Futahashi, R. The first record of Anax ephippiger (Burmeister, 1839) from Toyama Prefecture, Honshu, Japan. Tombo Acta Odonatol. Jpn. 2020, 62, 131–132. [Google Scholar]
- Giribet, G.; Edgecombe, G.D.; Carpenter, J.M.; d’ Haese, C.; Wheeler, W.C. Is Ellipura monophyletic? A combined analysis of basal hexapod relationships with emphasis on the origin of insects. Org. Divers. Evol. 2004, 4, 319–340. [Google Scholar] [CrossRef]
- Futahashi, R. A revisional study of Japanese dragonflies based on DNA analysis (2). Tombo Acta Odonatol. Jpn. 2014, 56, 57–59. [Google Scholar]
- Rewicz, T.; Móra, A.; Szymczak, A.; Grabowski, M.; Calleja, E.J.; Pernecker, B.; Csabai, Z. First records raise questions: DNA barcoding of Odonata in the middle of the Mediterranean. Genome 2021, 64, 161–310. [Google Scholar] [CrossRef]
- Kompier, T.; Karub, H.; Futahashi, R.; Phan, Q.T. The genus Planaeschna McLachlan, 1895 and its subgroupings in Vietnam, with descriptions of three new species (Odonata: Aeshnidae). Zootaxa 2021, 5027, 1–35. [Google Scholar] [CrossRef]
- Schneider, T.; Vierstraete, A.; Müller, O.; van Pelt, G.J.; Casper, M.; Ikemeyer, D.; Snegovaya, N.; Dumont, H.J. Taxonomic revision of eastern part of Western Palaearctic Cordulegaster using molecular phylogeny and morphology, with the description of two new species (Odonata: Anisoptera: Cordulegastridae). Diversity 2021, 13, 667. [Google Scholar] [CrossRef]
- Schneider, T.; Vierstraete, A.; Müller, O.; van Pelt, G.J.; Caspers, M.; Ikemeyer, D.; Dumont, H.J. The Oracle of Delphi—A molecular phylogenetic approach to Greek Cordulegaster Leach in Brewster, 1815 (Odonata: Anisoptera: Cordulegastridae). Zootaxa 2022, 5125, 182–204. [Google Scholar] [CrossRef]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief Bioform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Douglas, J.; Jiménez-Silva, C.L.; Bouckaert, R. StarBeast3: Adaptive parallelised Bayesian pnference under the vultispecies coalescent. Syst. Biol. 2022, 71, 901–916. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Schmidt, E. Was ist Libellula isoceles O. F. Müller 1767? Entomol. Z. 1950, 60, 1–7, 13–14. [Google Scholar]
- Simonsen, T.J.; Kent, O.; Djernæs, M. The African-Iberian connection in Odonata: mtDNA and ncDNA based phylogeography of Aeshna cyanea (Müller, 1764) (Odonata: Aeshnidae) in Western Palaearctic. Arthropod Syst. Phylogeny 2020, 78, 309–320. [Google Scholar] [CrossRef]
- Von Ellenrieder, N. A synopsis of the Neotropical species of ‘Aeshna’ Fabricius: The genus Rhionaeschna Förster (Odonata: Aeshnidae). Tijdschr. Voor Entomol. 2003, 146, 67–207. [Google Scholar] [CrossRef]
- Borisov, S.N.; Kosterin, O.E.; Haritonov, A.Y. On the fauna of Odonata of Chukotka and other northern regions of the Holarctic. Evraziatskii Entomol. Zhurnal 2014, 13, 315–320. [Google Scholar]
- Whitehouse, F.C. British Columbia dragonflies (Odonata), with notes on distribution and habitats. Am. Midl. Nat. 1941, 26, 488–557. [Google Scholar] [CrossRef]
- Belyshev, B.F. The Dragonflies of Siberia (Odonata); Nauka: Novosibirsk, Russia, 1973; Volume I, pp. 337–620, (In Russian, English Title). [Google Scholar]
- Ferreira, S.; Boudot, J.-P.; Haissouti, M.L.; Alves, P.C.; Thompson, D.J.; Watts, P.C. Genetic distinctiveness of the damselfly Coenagrion puella in North Africa: An overlooked and endangered taxon. Conserv. Genet. 2016, 17, 985–991. [Google Scholar] [CrossRef]
- Dow, R.A.; Butler, S.G.; Reels, G.T.; Steinhoff, O.M.; Stokvis, F.; Unggang, L. Previously unpublished Odonata records from Sarawak, Borneo, part IV: Bintulu Division including the Planted Forest Project and Similajau National Park. Faun. Stud. South-East. Pac. Isl. Odonata 2019, 27, 1–66. [Google Scholar]
- Dijkstra, K.-D.B.; Kalkman, V.J. Phylogeny and classification. In Atlas of the European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KKNV Publishing: Zeist, The Netherlands, 2015; pp. 15–25. [Google Scholar]
- Skvortsov, V.E.; Snegovaya, N.Y. A second addition to the knowledge of the Odonata fauna of Azerbaijan. Int. Dragonfly Fund Rep. 2015, 87, 1–38. [Google Scholar]
- Schröter, A.; Seehausen, M.; Kunz, B.; Günther, A.; Schneider, T.; Jödicke, R. Update of the Odonata fauna of Georgia, southern Caucasian ecoregion. Odonatologica 2015, 44, 279–342. [Google Scholar]
- Dijkstra, K.-D.B. Taxonomy and identification of the continental African Gynacantha and Heliaeschna species (Odonata: Aeshnidae). Int. J. Odonatol. 2005, 8, 1–168. [Google Scholar] [CrossRef]
- Kalkman, V.J.; Iversen, L.L.; Nielsen, E. Aeshna isoceles (Müller, 1767). In Atlas of the European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KKNV Publishing: Zeist, The Netherlands, 2015; pp. 157–158. [Google Scholar]
- Brauer, F. Dritter Bericht über die auf der Weltfahrt der kais. Fregatte Novara gesammelten Libellulinen. Verhandlungen Kais. Königlichen Zool. Bot. Ges. Wien 1865, 15, 501–512. [Google Scholar]
- Peters, G. Morphologische Differenzen zwischen nah verwandten Arten am Beispiel von Anax parthenope und A. julius (Odonata, Aeshnidae). Dtsch. Entomol. Z. 1986, 33, 11–19. [Google Scholar] [CrossRef]
- Martens, A.; Günther, A.; Suhling, F. Diversity in mate-guarding types within the genus Anax (Odonata: Aeshnidae). Libellula Suppl. 2012, 12, 113–122. [Google Scholar]
- Kalkman, V.J.; Proess, B. Anax parthenope (Selys, 1839). In Atlas of the European Dragonflies and Damselflies; Boudot, J.-P., Kalkman, V.J., Eds.; KKNV Publishing: Zeist, The Netherlands, 2015; pp. 177–179. [Google Scholar]
- Onishko, V.V.; Kosterin, O.E.; Voinov, I.O. Results of odonatological studies in southern Primorye, Russia, in 2011–2020. Int. Dragonfly Fund Rep. 2023, 177, 1–59. [Google Scholar]
- Ballard, J.W.; Whitlock, M.C. The incomplete natural history of mitochondria. Mol. Ecol. 2004, 13, 729–734. [Google Scholar] [CrossRef]
- Peters, G. Die Edellibellen Europas: Aeshnidae. Die neue Brehmbücherei 585; Ziemsen Verlag: Wittenberg Lutherstadt, Germany, 1987. [Google Scholar]
- Schmidt, E. Odonata in Die Tierwelt Mitteleuropas IV Band Insekten 1. Teil, Herausgegeben; von Brohmer, P., Ehrmann, P., Ulmer, G., Eds.; Verlag Quelle & Meyer: Leipzig, Germany, 1939; pp. IV1–IV66. [Google Scholar]
- Peters, G. Taxonomic and populatoin studies of British Columbia Aeshna species. Bull. Am. Odonatol. 1998, 5, 33–42. [Google Scholar]
- Bartenev, A.N. Contribution â l’odonatofauna des monts de la Caucasie. Bull. Musée Géorgie 1925, 2, 28–86, (In Russian, French Title). [Google Scholar]
- Bartenev, A.N. Neue Arten und Varietäten der Odonata des West Kaukasus. Zool. Anz. 1929, 85, 54–68. [Google Scholar]
- Bartenev, A.N. Über die Artengruppen Aeschna juncea und Aeschna clepsydra in dem paläarctischen Gebiete. Arb. Nord. Kaukasischen Assoc. Wiss. Inst. 1929, 54, 3–65, (In Russian, German Title). [Google Scholar]
- Kosterin, O.E. Reconsideration of three Odonata taxa described by A.N. Bartenev from the same place in West Caucasus. Odonatologica 2023, 52, 89–126. [Google Scholar] [CrossRef]
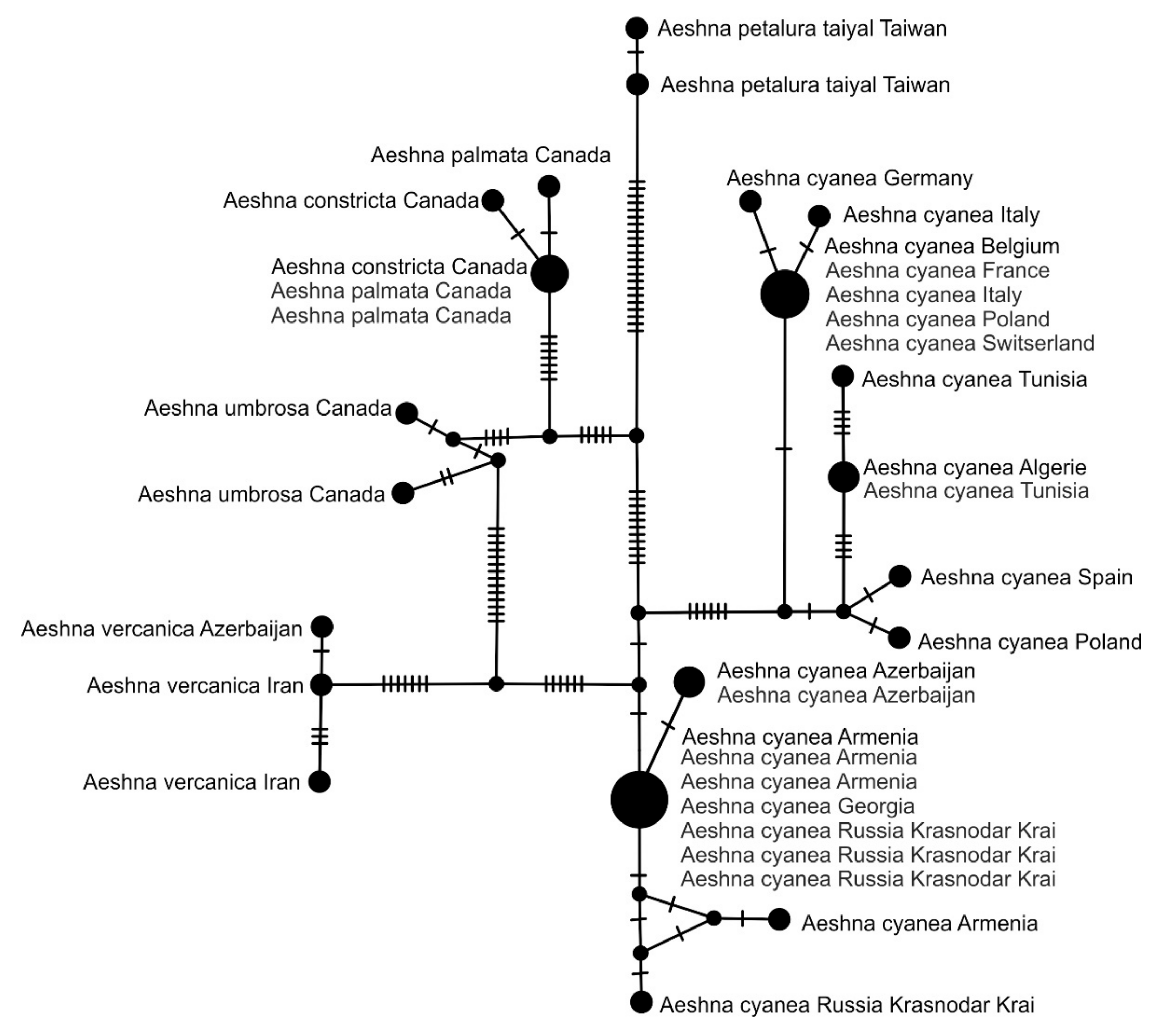

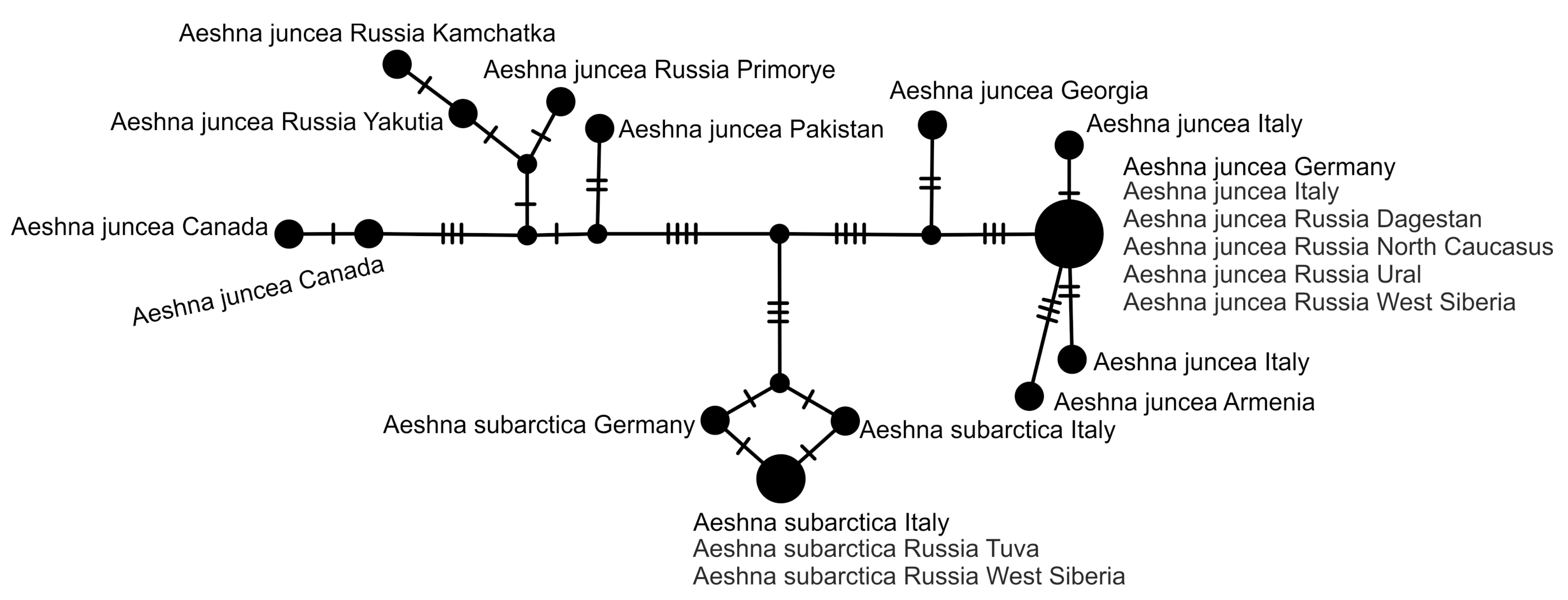


| Species | Latitude | Longitude | Country | Region | Collector/Reference | GenBank COI | GenBank ITS |
|---|---|---|---|---|---|---|---|
| Aeschnophlebia longistigma Selys, 1883 | Korea | [18] | KF257055 | no data | |||
| Aeschnophlebia longistigma | Japan | [19] | no data | AB706669 | |||
| Aeschnophlebia longistigma | 42.4700 | 130.6400 | Russia | Primorye, Lake Lotos | O. Kosterin leg. | OR130000 | OR133899 |
| Aeschnophlebia longistigma | 44.5100 | 132.7000 | Russia | Primorye, Khasan District, Prokhory Village | O. Kosterin leg. | OR130001 | OR133898 |
| Aeschnophlebia anisoptera Selys, 1883 | Japan | [19] | no data | AB706668 | |||
| Aeshna affinis Vander Linden, 1820 | Italy | [20] | MT298234 | no data | |||
| Aeshna affinis | Azerbaijan | Yardimli, Shefekli Village | N. Snegovaya leg. | OR130002 | OR133867 | ||
| Aeshna affinis | Azerbaijan | Shabran District | N. Snegovaya leg. | OR130003 | OR133869 | ||
| Aeshna affinis | Azerbaijan | Balaken, Gabagchol | N. Snegovaya leg. | OR130004 | OR133868 | ||
| Aeshna affinis | Germany | [15] | HM422047 | no data | |||
| Aeshna caerulea (Ström, 1783) | Italy | [20] | MT298235 | no data | |||
| Aeshna caerulea | Austria | [16] | MW490272 | no data | |||
| Aeshna caerulea | Sweden | Lapland | T. Schneider leg. | OR130006 | OR133866 | ||
| Aeshna caerulea | France | [15] | no data | MN656996 | |||
| Aeshna caerulea | 52.6400 | 96.8000 | Russia | Tuva Republic, Todzha District, Lake Ottug-Khol’ | O. Kosterin leg. | OR130005 | no data |
| Aeshna canadensis Walker, 1908 | Canada | [15] | HM413507 | no data | |||
| Aeshna canadensis | Canada | [15] | JF839358 | no data | |||
| Aeshna constricta Say, 1840 | Canada | [15] | KM528410 | no data | |||
| Aeshna constricta | Canada | [15] | KM528706 | no data | |||
| Aeshna crenata Hagen, 1856 | Finland | A. Schröter leg., Senckenberg Museum Frankfurt | OR130008 | OR133857 | |||
| Aeshna crenata | Finland | A. Schröter leg., Senckenberg Museum Frankfurt | no data | OR133853 | |||
| Aeshna crenata | 54.8200 | 69.7800 | Russia | S Ural, Bashkortostan, Uchaly District, at Muldashevo Village | O. Kosterin leg. | OR130011 | OR133856 |
| Aeshna crenata | 52.6000 | 96.8000 | Russia | Tuva, Todzha District, Lake Saylyg-Khol’ | O. Kosterin leg. | OR130010 | OR133859 |
| Aeshna crenata | 58.9900 | 126.2449 | Russia | Yakutia, Tommot Town env. | O. Kosterin leg. | OR130012 | OR133854 |
| Aeshna crenata | 45.0600 | 131.9900 | Russia | Primorye, Khanka District, Platono-Aleksandrovskaya Village env. | O. Kosterin leg. | OR130009 | OR133858 |
| Aeshna crenata | 53.3040 | 157.4730 | Russia | Kamchatka, Malki Village env. | O. Kosterin leg. | no data | OR133855 |
| Aeshna crenata | Korea | [18] | KF257093 | no data | |||
| Aeshna crenata | Japan | [19] | no data | AB706671 | |||
| Aeshna crenata | Russia | [19] | no data | AB706672 | |||
| Aeshna cyanea (Müller, 1764) | France | [16] | MW490109 | no data | |||
| Aeshna cyanea | Italy | [20] | MT298236 | no data | |||
| Aeshna cyanea | Azerbaijan | Siyazan, near Galaalty | N. Snegovaya leg. | OR130013 | OR133832 | ||
| Aeshna cyanea | Azerbaijan | Isailli, Garanokhur Lake | N. Snegovaya leg. | OR130014 | OR133833 | ||
| Aeshna cyanea | Switzerland | [15] | MN454844 | no data | |||
| Aeshna cyanea | Italy | [14] | KU180305 | KU180377 | |||
| Aeshna cyanea | Germany | [14] | KU180307 | KU180376 | |||
| Aeshna cyanea | Poland | [14] | KU180308 | KU180379 | |||
| Aeshna cyanea | Algerie | [14] | KU180311 | KU180374 | |||
| Aeshna cyanea | Tunisia | [14] | KU180310 | KU180385 | |||
| Aeshna cyanea | Tunisia | [14] | KU180320 | KU180386 | |||
| Aeshna cyanea | Poland | [14] | KU180306 | KU180378 | |||
| Aeshna cyanea | Spain | [14] | KU180309 | KU180384 | |||
| Aeshna cyanea | Armenia | [14] | KU180316 | KU180370 | |||
| Aeshna cyanea | Russia | Krasnodar Krai, Mostovskiy District, Psebay | [14] | KU180312 | KU180380 | ||
| Aeshna cyanea | Armenia | [14] | KU180319 | KU180371 | |||
| Aeshna cyanea | Russia | Krasnodar Krai, Mostovskiy District, Psebay | [14] | KU180318 | KU180383 | ||
| Aeshna cyanea | Russia | Krasnodar Krai, Mostovskiy District, Psebay | [14] | KU180317 | KU180382 | ||
| Aeshna cyanea | Russia | Krasnodar Krai, Mostovskiy District, Psebay | [14] | KU180313 | KU180381 | ||
| Aeshna cyanea | Georgia | [14] | KU180314 | KU180375 | |||
| Aeshna cyanea | Armenia | [14] | KU180315 | KU180369 | |||
| Aeshna cyanea | Armenia | [14] | KU180321 | KU180372 | |||
| Aeshna cyanea | Belgium | [14] | KU180304 | KU180373 | |||
| Aeshna eremita Scudder, 1866 | Canada | [15] | HM381222 | no data | |||
| Aeshna eremita | USA | Alaska | [21] | KU873985 | no data | ||
| Aeshna grandis (Linnaeus, 1758) | Germany | [14] | KU180299 | KU180363 | |||
| Aeshna grandis | Italy | [20] | MT298237 | no data | |||
| Aeshna interrupta Walker, 1908 | USA | Alaska | [21] | KU873988 | no data | ||
| Aeshna interrupta | USA | Alaska | [21] | KU873987 | no data | ||
| Aeshna interrupta | Canada | [15] | HM381232 | no data | |||
| Aeshna isoceles (Müller, 1767) | Italy | [20] | MT298239 | no data | |||
| Aeshna isoceles | Italy | [20] | MT298240 | no data | |||
| Aeshna isoceles | Lebanon | Bared Brook | T. Schneider leg. | OR130019 | OR133827 | ||
| Aeshna isoceles | Germany | Brandenburg | T. Schneider leg. | OR130018 | OR133825 | ||
| Aeshna isoceles | Morocco | Quiouane Middle Atlas | T. Schneider leg. | OR130020 | OR133830 | ||
| Aeshna isoceles | Azerbaijan | Zakatala, Geratap | N. Snegovaya leg. | OR130017 | OR133826 | ||
| Aeshna isoceles | Azerbaijan | Lenkoran, Azfilial Settlement | N. Snegovaya leg. | OR130016 | OR133829 | ||
| Aeshna isoceles | Azerbaijan | Agstafa, Poylu Village, Kura River | N. Snegovaya leg. | OR130015 | OR133828 | ||
| Aeshna isoceles | France | [15] | no data | FN356032 | |||
| Aeshna juncea (Linnaeus, 1758) | 58.5000 | 125.5067 | Russia | Yakutia, Aldan District, Lebedinyy Town | O. Kosterin leg. | OR130030 | OR133838 |
| Aeshna juncea | 56.4500 | 160.9500 | Russia | Kamchatka, at Klyuchi Village | O. Kosterin leg. | OR130025 | OR133843 |
| Aeshna juncea | 42.4600 | 130.6400 | Russia | Primorye, Lake Lotos | O. Kosterin leg. | OR130027 | OR133839 |
| Aeshna juncea | 43.3400 | 41.6740 | Russia | N Caucasus, Karachay-Cherkes Republic, Dombay env. | O. Kosterin leg. | OR130026 | OR133844 |
| Aeshna juncea | 53.3400 | 57.7900 | Russia | S Ural, Bashkortostan, Sargaya Village env. | O. Kosterin leg. | OR130028 | OR133840 |
| Aeshna juncea | 41.8000 | 47.4100 | Russia | Dagestan, Agul District, Lake Debrishara | O. Kosterin leg. | OR130024 | OR133845 |
| Aeshna juncea | 54.1500 | 83.6101 | Russia | W Siberia, Ordynskoe District, Spirino Village env. | O. Kosterin leg. | OR130029 | OR133841 |
| Aeshna juncea | Italy | [20] | MT298245 | no data | |||
| Aeshna juncea | Italy | [20] | MT298244 | no data | |||
| Aeshna juncea | Italy | [20] | MT298243 | no data | |||
| Aeshna juncea | Armenia | V. Ananian leg. | OR130021 | OR133846 | |||
| Aeshna juncea | Pakistan | Hindu Kush | T. Schneider leg. | OR130023 | OR133842 | ||
| Aeshna juncea | Georgia | Lesser Caucasus | T. Schneider leg. | OR130022 | OR133847 | ||
| Aeshna juncea | Germany | [14] | KU180297 | KU180364 | |||
| Aeshna juncea | Canada | [15] | JF839255 | no data | |||
| Aeshna juncea | Canada | [22] | KR143341 | no data | |||
| Aeshna juncea | Japan | [19] | no data | AB706686 | |||
| Aeshna juncea | Russia | [19] | no data | AB706688 | |||
| Aeshna juncea | Russia | Buryatia | [19] | no data | AB711414 | ||
| Aeshna juncea | South Korea | [19] | no data | AB711415 | |||
| Aeshna mixta Latreille, 1805 | Austria | [17] | MW208418 | no data | |||
| Aeshna mixta | Montenegro | [17] | MW208417 | no data | |||
| Aeshna mixta | Austria | [17] | MW208419 | no data | |||
| Aeshna mixta | Bosnia and Herzegovina | [17] | MW208416 | no data | |||
| Aeshna mixta | Montenegro | [20] | MT298246 | no data | |||
| Aeshna mixta | Azerbaijan | Kedabek, Novoivanovka Village | N. Snegovaya leg. | OR130031 | OR133863 | ||
| Aeshna mixta | Azerbaijan | Yardimli, Avash Village | N. Snegovaya leg. | OR130032 | OR133864 | ||
| Aeshna mixta | Greece | D.A.L. Davies leg. | OR130033 | OR133865 | |||
| Aeshna mixta | 55.6960 | 37.5200 | Russia | Moskow | [23] | OM089772 | no data |
| Aeshna palmata Hagen, 1856 | Canada | [15] | JF839302 | no data | |||
| Aeshna palmata | Canada | [15] | JN294467 | no data | |||
| Aeshna palmata | Canada | [15] | JF839296 | no data | |||
| Aeshna petalura Martin, 1908 | Taiwan | Yilan Co. SanXing Pond | Fang-Shuo Hu leg. | OR130034 | OR133834 | ||
| Aeshna petalura | Taiwan | Yilan Co. SanXing Pond | Fang-Shuo Hu leg. | OR130035 | OR133835 | ||
| Aeshna septentrionalis Burmeister, 1839 | Canada | [15] | GU714053 | no data | |||
| Aeshna septentrionalis | Canada | [15] | JF839362 | no data | |||
| Aeshna serrata Hagen, 1856 | Finland | A. Schröter leg., Senckenberg Museum Frankfurt | OR130036 | OR133851 | |||
| Aeshna serrata | 54.8600 | 83.0740 | Russia | W Siberia, Novosibirsk | O. Kosterin leg. | OR130039 | OR133850 |
| Aeshna serrata | 54.1400 | 61.2900 | Russia | S Ural, Chelyabinsk Province, Troitsk District | O. Kosterin leg. | OR130038 | OR133848 |
| Aeshna serrata | 56.4500 | 160.9500 | Russia | Kamchatka, at Klyuchi Village | O. Kosterin leg. | OR130037 | OR133849 |
| Aeshna serrata | Russia | [19] | no data | AB706698 | |||
| Aeshna serrata | Russia | [19] | no data | AB706699 | |||
| Aeshna sitchensis Hagen, 1861 | Canada | [15] | HM413523 | no data | |||
| Aeshna sitchensis | Canada | [22] | KR143430 | no data | |||
| Aeshna soneharai (Asahina, 1988) | 55.5400 | 60.5800 | Russia | S Ural, Lake Bol’shaya Akulya | O. Kosterin leg. | OR130041 | OR133860 |
| Aeshna soneharai | 55.7000 | 37.5200 | Russia | Moscow | V. Onishko leg. | OR130040 | OR133861 |
| Aeshna soneharai | 54.1500 | 83.6101 | Russia | W Siberia, Ordynskoe District, Spirino Village env. | O. Kosterin leg. | OR130042 | OR133862 |
| Aeshna soneharai | South Korea | [18] | KF257096 | no data | |||
| Aeshna soneharai | Japan | [19] | no data | AB706697 | |||
| Aeshna subarctica Walker, 1908 | Germany | [14] | KU180298 | KU180362 | |||
| Aeshna subarctica | 52.6400 | 96.8900 | Russia | Tuva, Lake Saylyg-Khol’ | O. Kosterin leg. | OR130043 | OR133837 |
| Aeshna subarctica | 54.8800 | 83.0484 | Russia | W Siberia, Novosibirsk | O. Kosterin leg. | OR130044 | OR133836 |
| Aeshna subarctica | Italy | [20] | MT298250 | no data | |||
| Aeshna subarctica | Italy | [20] | MT298249 | no data | |||
| Aeshna subarctica | Japan | [19] | no data | AB711410 | |||
| Aeshna subarctica | Finland | [19] | no data | AB711413 | |||
| Aeshna subarctica | Japan | [19] | no data | AB711411 | |||
| Aeshna tuberculifera Walker, 1908 | Canada | [15] | HM413600 | no data | |||
| Aeshna tuberculifera | Canada | [15] | JF839364 | no data | |||
| Aeshna umbrosa Walker, 1908 | Canada | [15] | GU712955 | no data | |||
| Aeshna umbrosa | Canada | [15] | GU713003 | no data | |||
| Aeshna vercanica Schneider et al. 2015 | Azerbaijan | [14] | KU180322 | KU180361 | |||
| Aeshna vercanica | Iran | [14] | KU180302 | KU180365 | |||
| Aeshna vercanica | Iran | [14] | KU180303 | KU180368 | |||
| Aeshna vercanica | Azerbaijan | Lenkoran, Azfilial Settlement | N. Snegovaya leg. | no data | OR133831 | ||
| Aeshna vercanica | Azerbaijan | [24] | no data | FN356031 | |||
| Aeshna verticalis Hagen, 1861 | Canada | [15] | HM413554 | no data | |||
| Aeshna verticalis | Canada | [15] | HM413555 | no data | |||
| Aeshna viridis Eversmann, 1836 | Germany | [14] | KU180301 | KU180366 | |||
| Aeshna viridis | Germany | [14] | KU180300 | KU180366 | |||
| Aeshna viridis | Germany | [14] | no data | KU180387 | |||
| Anaciaeschna jaspidea (Burmeister, 1839) | India | [15] | KT879909 | no data | |||
| Anaciaeschna jaspidea | Japan | [19] | no data | AB706701 | |||
| Anaciaeschna martini (Selys, 1897) | Japan | [25] | LC466165 | LC466157 | |||
| Anaciaeschna martini | Nepal | [25] | LC466163 | LC466154 | |||
| Anaciaeschna martini | Japan | [19] | no data | AB706702 | |||
| Anaciaeschna triangulifera McLachlan, 1896 | Tanzania | [26] | KC912207 | no data | |||
| Anax congoliath Fraser, 1953 | Angola | [27] | KU565906 | no data | |||
| Anax congoliath | Gabon | [27] | KU565907 | no data | |||
| Anax ephippiger (Burmeister, 1839) | Italy | [20] | MT298252 | no data | |||
| Anax ephippiger | Italy | [20] | MT298251 | no data | |||
| Anax ephippiger | Israel | Jordan Valley, near Had Nes | A. Leirich leg. coll. T. Schneider | OR130045 | OR133824 | ||
| Anax ephippiger | Tanzania | [19] | no data | AB706703 | |||
| Anax ephippiger | Japan | [28] | no data | LC511177 | |||
| Anax ephippiger | Cameroon | [24] | no data | FN356093 | |||
| Anax gibbosulus Rambur, 1842 | Samoa | [13] | MW810906 | no data | |||
| Anax gladiator Dijkstra & Kipping, 2015 | Zambia | [27] | KU565911 | no data | |||
| Anax gladiator | Congo | [27] | KU565913 | no data | |||
| Anax guttatus (Burmeister, 1839) | Japan | [19] | no data | AB706704 | |||
| Anax guttatus | Borneo | [13] | MW810901 | no data | |||
| Anax immaculifrons Rambur, 1842 | India | [15] | MG544869 | no data | |||
| Anax immaculifrons | Lebanon | T. Schneider leg. | OR346685 | OR350846 | |||
| Anax imperator Leach in Brewster, 1815 | Liberia | [27] | KU565916 | no data | |||
| Anax imperator | Germany | Brandenburg | T. Schneider leg. | OR130046 | OR133816 | ||
| Anax imperator | Germany | Brandenburg | T. Schneider leg. | OR130047 | OR133817 | ||
| Anax junius (Drury, 1773) | USA | [29] | AY555548 | no data | |||
| Anax junius | USA | [15] | HQ986154 | no data | |||
| Anax junius | USA | [30] | no data | LC366224 | |||
| Anax junius | USA | [30] | no data | LC366223 | |||
| Anax julius Brauer, 1865 | 45.2200 | 133.5130 | Russia | Primorye, Kirovskiy District, Gornye Klyuchi Village env. | O. Kosterin leg. | OR130048 | OR133818 |
| Anax julius | 45.0600 | 131.9900 | Russia | Primorye, Khanka District, Platono-Aleksandrovka Village env. | O. Kosterin leg. | OR130049 | OR133820 |
| Anax julius | Vietnam | Yen Bai | Dang leg. coll. T. Schneider | OR130051 | OR133821 | ||
| Anax julius | Vietnam | Yen Bai | Dang leg. coll. T. Schneider | OR130050 | OR133819 | ||
| Anax julius | Japan | [19] | no data | AB706711 | |||
| Anax julius | Japan | [19] | no data | AB706713 | |||
| Anax nigrofasciatus Oguma, 1915 | Korea | [15] | MN609590 | no data | |||
| Anax nigrofasciatus | Korea | [15] | MN609591 | no data | |||
| Anax nigrofasciatus | Vietnam | Sa Pa | Dang leg. coll. T. Schneider | OR130052 | OR133823 | ||
| Anax panybeus Hagen, 1867 | Japan | [19] | no data | AB706710 | |||
| Anax panybeus | Japan | [19] | no data | AB706709 | |||
| Anax parthenope (Selys, 1839) | Poland | [31] | MN701501 | no data | |||
| Anax parthenope | Poland | [31] | MN701506 | no data | |||
| Anax parthenope | Germany | Brandenburg | T. Schneider leg. | OR130053 | OR133822 | ||
| Anax piraticus Kennedy, 1934 | Guam | [30] | no data | LC366251 | |||
| Anax rutherfordi McLachlan, 1883 | Liberia | [27] | KU565918 | no data | |||
| Anax rutherfordi | Liberia | [27] | KU565917 | no data | |||
| Anax speratus Hagen, 1867 | Congo | [27] | KU565921 | no data | |||
| Anax speratus | South Africa | [27] | KU565923 | no data | |||
| Anax tristis Hagen, 1867 | Congo | [27] | KU565931 | no data | |||
| Anax walsinghami McLachlan, 1883 | USA | Utha | [13] | MW810898 | no data | ||
| Anax walsinghami | USA | California | [13] | MW810942 | no data | ||
| Andaeschna occidentalis Bota-Sierra, 2019 | Colombia | C.A.Bota-Sierra leg. | OR346684 | OR350845 | |||
| Austrogynacantha heterogena Tillyard, 1908 | −19.2828 | 146.8010 | Australia | Queensland | [15] | HQ986475 | no data |
| Austrogynacantha heterogena | Australia | Queensland | Australian Museum (D. Smith) | OR130054 | OR133872 | ||
| Basiaeschna janata (Say, 1840) | Canada | [15] | GU712962 | no data | |||
| Basiaeschna janata | 40.9100 | −73.7300 | USA | New York | [15] | MT947635 | no data |
| Basiaeschna janata | Canada | [15] | JN419315 | no data | |||
| Boyeria cretensis Peters, 1991 | Greece | Crete, Zakros | A. Martens leg. | OR130055 | OR133883 | ||
| Boyeria grafiana Williamson, 1907 | Canada | [15] | GU713103 | no data | |||
| Boyeria grafiana | Canada | [15] | JN419353 | no data | |||
| Boyeria irene (Fonscolombe, 1838) | Italy | [20] | MT298265 | no data | |||
| Boyeria irene | Italy | [20] | MT298267 | no data | |||
| Boyeria irene | France | [24] | no data | FN356042 | |||
| Boyeria irene | France | [16] | MW490313 | no data | |||
| Boyeria irene | Switzerland | Ennetburgen | S. Kohl leg. | OR130057 | OR133881 | ||
| Boyeria irene | Switzerland | Ennetburgen | S. Kohl leg. | OR130056 | no data | ||
| Boyeria irene | Germany | Lower Saxon | F. Suhling leg. coll. T. Schneider | no data | OR133880 | ||
| Boyeria irene | Italy | Calabria | T. Schneider leg. | no data | OR133882 | ||
| Boyeria karubei Yokoi, 2002 | Vietnam | [32] | no data | LC612603 | |||
| Boyeria maclachlani (Selys, 1883) | Japan | [19] | no data | AB706723 | |||
| Boyeria vinosa (Say, 1840) | Canada | [15] | HM395246 | no data | |||
| Brachytron pratense (Müller, 1764) | Italy | [20] | MT298272 | no data | |||
| Brachytron pratense | Italy | [20] | MT298273 | no data | |||
| Brachytron pratense | 44.7300 | 37.4600 | Russia | W Caucasus, Abrau Peninsula, Malyy Utrish Village env. | O. Kosterin leg. | OR130061 | OR133896 |
| Brachytron pratense | 44.7300 | 37.4600 | Russia | W Caucasus, Abrau Peninsula, Malyy Utrish Village env. | O. Kosterin leg. | OR130060 | OR133895 |
| Brachytron pratense | Germany | Potsdam | T. Schneider leg. | OR130059 | OR133894 | ||
| Brachytron pratense | Germany | Potsdam | T. Schneider leg. | OR130058 | OR133893 | ||
| Brachytron pratense | Azerbaijan | Lenkoran, Azfilial Settlement | N. Snegovaya leg. | no data | OR133897 | ||
| Caliaeschna microstigma (Schneider, 1845) | Montenegro | [20] | MT298276 | no data | |||
| Caliaeschna microstigma | Lebanon | Bared Brook | T. Schneider leg. | OR130064 | OR133889 | ||
| Caliaeschna microstigma | Azerbaijan | Balaken District, Djidjikhana | N. Snegovaya leg. coll. T. Schneider | OR130063 | OR133888 | ||
| Caliaeschna microstigma | Azerbaijan | Ordubad District, Agdere | N. Snegovaya leg. coll. T. Schneider | OR130062 | OR133887 | ||
| Cephalaeschna risi Asahina, 1981 | Taiwan | Qilan, Yilan Co. | Fang-Shuo Hu leg. | OR130065 | no data | ||
| Cephalaeschna risi | Taiwan | Fang-Shuo Hu leg. | OR130066 | OR133931 | |||
| Coryphaeschna adnexa (Hagen, 1861) | Bolivia | Nuflo de Chavez, San Julian | [15] | MN345399 | no data | ||
| Epiaeschna heros (Fabricius, 1798) | USA | Virginia | [15] | MN345091 | no data | ||
| Gynacantha africana (Palisot de Beauvois, 1807) | Liberia | Grand Bassa County | [27] | KU566102 | no data | ||
| Gynacantha bayadera Selys, 1892 | 13.6000 | 105.9300 | Cambodia | Stung Treng Province, Srae Ruessei Village env. | O. Kosterin leg. | OR130067 | OR133874 |
| Gynacantha bispina Rambur, 1842 | Mauritius | [27] | KU566103 | no data | |||
| Gynacantha bullata Karsch, 1891 | Democratic Republic Congo | Kisangani | [27] | KU566105 | no data | ||
| Gynacantha bullata | Cameroon | [24] | no data | FN356092 | |||
| Gynacantha congolica Dijkstra, 2015 | Democratic Republic Congo | Orientale | [27] | KU566118 | no data | ||
| Gynacantha demeter Ris, 1911 | 10.5240 | 103.7130 | Cambodia | Preah Sihanouk Province, Ream Peninsula | O. Kosterin leg. | OR130068 | OR133873 |
| Gynacantha dravida Lieftinck, 1960 | India | [15] | MK990607 | no data | |||
| Gynacantha hyalina Selys, 1882 | 25.2643 | 121.5840 | Taiwan | New Taipei, Aliban Ecological Farm | C. H. Ma & I. L. leg. Lee | OR130069 | OR133877 |
| Gynacantha japonica Bartenev, 1910 | Korea | [18] | KF257090 | no data | |||
| Gynacantha japonica | Japan | [19] | AB706724 | ||||
| Gynacantha manderica Grünberg, 1902 | South Africa | [27] | KU566119 | no data | |||
| Gynacantha nigeriensis (Gambles, 1956) | Uganda | [27] | KU566123 | no data | |||
| Gynacantha pupillata Dijkstra, 2015 | Democratic Republic Congo | Orientale | [27] | KU566133 | no data | ||
| Gynacantha ryukyuensis Asahina, 1962 | Japan | [19] | no data | AB706729 | |||
| Gynacantha saltatrix Martin, 1909 | 13.6000 | 105.9300 | Cambodia | Stung Treng Province, Srae Ruessei Village env. | O. Kosterin leg. | OR130070 | no data |
| Gynacantha subinterrupta Rambur, 1842 | 10.5010 | 193.7220 | Cambodia | Preah Sihanouk Province, Ream Peninsula | O. Kosterin leg. | OR130071 | OR133875 |
| Gynacantha subinterrupta | Vietnam | Bao Loc, Lam Dong | Dang leg. coll. T. Schneider | OR130072 | no data | ||
| Gynacantha usambarica Sjöstedt, 1909 | Liberia | [27] | KU566134 | no data | |||
| Gynacantha vesiculata Karsch, 1891 | Liberia | [27] | KU566139 | no data | |||
| Heliaeschna crassa Krüger, 1899 | 11.5810 | 103.1280 | Cambodia | Koh Kong Province, Tatai Commune | G. Chartier leg. | no data | OR133878 |
| Indaeschna grubaueri (Förster, 1904) | Indonesia | Kalimantan | coll. T. Schneider | OR130073 | no data | ||
| Nasiaeschna pentacantha (Rambur, 1842) | 37.8667 | −76.8000 | USA | Virginia | [15] | MN345309 | no data |
| Oplonaeschna armata (Hagen, 1861) | Mexico | [15] | MN345104 | no data | |||
| Oplonaeschna armata | Mexico | Oaxaca | [15] | MN346016 | no data | ||
| Periaeschna magdalena Martin, 1909 | Taiwan | New Taipei City | C. H. Ma leg. | OR130074 | OR133890 | ||
| Periaeschna magdalena | Vietnam | Dang leg. coll. T. Schneider | OR130075 | OR133891 | |||
| Pinheyschna subpupillata (McLachlan, 1896) | South Africa | [15] | AF429287 | no data | |||
| Pinheyschna yemenensis (Waterston, 1985) | Saudi Arabia | Soudah Waterfall | M. Waldhauser leg. | OR130076 | OR133871 | ||
| Planaeschna asahinai Karube, 2011 | Vietnam | [32] | LC612707 | no data | |||
| Planaeschna celia Wilson & Reels, 2001 | Vietnam | [32] | LC612709 | no data | |||
| Planaeschna cucphuongensis Karube, 1999 | Vietnam | [32] | no data | LC612627 | |||
| Planaeschna cucphuongensis | Vietnam | Dang leg. coll. T. Schneider | OR130077 | OR133885 | |||
| Planaeschna milnei (Selys, 1883) | Japan | [19] | AB708630 | AB706740 | |||
| Planaeschna risi Asahina, 1964 | Japan | [19] | AB708637 | AB706742 | |||
| Planaeschna risi | 24.8804 | 121.6645 | Taiwan | New Taipei, Jingualio | Fang-Shuo Hu leg. | OR130079 | OR133884 |
| Planaeschna taiwana Asahina, 1951 | Taiwan | [19] | no data | AB706746 | |||
| Planaeschna tamdaoensis Asahina, 1996 | Vietnam | [32] | LC612720 | LC612639 | |||
| Planaeschna tomokunii Asahina, 1996 | Vietnam | [32] | no data | LC612643 | |||
| Planaeschna ishigakiana Asahina, 1951 | Taiwan | [30] | no data | LC366218 | |||
| Planaeschna ishigakiana | Vietnam | [32] | no data | LC612631 | |||
| Planaeschna ishigakiana | 24.7499 | 121.5585 | Taiwan | New Taipei, Fushan | Fang-Shuo Hu leg. | OR130078 | OR133886 |
| Planaeschna viridis Karube, 2004 | Vietnam | [32] | no data | LC612644 | |||
| Polycanthagyna erythromelas (McLachlan, 1896) | 12.3860 | 103.0550 | Cambodia | Pursat Province, Phnom Tumpor Mt | O. Kosterin leg. | OR130080 | OR133870 |
| Polycanthagyna melanictera (Selys, 1883) | Korea | [18] | KF257100 | no data | |||
| Polycanthagyna melanictera | Japan | [30] | no data | LC366042 | |||
| Polycanthagyna melanictera | Japan | [19] | no data | AB706747 | |||
| Polycanthagyna melanictera | Japan | [19] | no data | AB706748 | |||
| Remartinia luteipennis (Burmeister, 1839) | Panama | [15] | MN344834 | no data | |||
| Rhionaeschna californica (Calvert, 1895) | Canada | [15] | JF839371 | no data | |||
| Rhioaeschna diffinis (Rambur, 1842) | Chile | Parc National Le Campenie, Cajan Grande | Ch. Pineda leg. coll. T. Schneider | OR130081 | OR133879 | ||
| Rhionaeschna multicolor (Hagen, 1861) | Canada | [15] | JF839373 | no data | |||
| Sarasaeschna kunigamiensis (Ishida, 1972) | Japan | [19] | no data | AB706753 | |||
| Sarasaeschna kunigamiensis | Japan | [19] | AB708646 | no data | |||
| Sarasaeschna lieni (Yeh & Chen, 2000) | Taiwan | [19] | AB708649 | no data | |||
| Sarasaeschna lieni | Taiwan | Yilan Co., Yunshan Township | Fang-Shuo Hu leg. | OR130082 | no data | ||
| Sarasaeschna lieni | Taiwan | [19] | no data | AB706754 | |||
| Sarasaeschna pryeri (Martin, 1909) | Japan | [19] | no data | AB706755 | |||
| Sarasaeschna pryeri | Japan | [19] | AB708650 | no data | |||
| Sarasaeschna tsaopiensis (Yeh & Chen, 2000) | Taiwan | Yilan Co., Yunshan Township | Fang-Shuo Hu leg. | no data | OR133900 | ||
| Staurophlebia reticulata (Burmeister, 1839) | −12.8833 | −71.2333 | Peru | Madre de Dios | [15] | MN343860 | no data |
| Tetracanthagyna plagiata (Waterhouse, 1877) | Malaysia | [15] | no data | AB706758 | |||
| Tetracanthagyna waterhousei McLachlan, 1898 | 12.5700 | 107.4150 | Cambodia | Mondulkiri Province, at Buu Sraa Waterfalls | O. Kosterin leg. | OR130083 | no data |
| Tetracanthagyna waterhousei | Vietnam | Bao Loc, Lam Dong | Dang leg. coll. T. Schneider | OR130085 | no data | ||
| Tetracanthagyna waterhousei | Vietnam | Bao Loc, Lam Dong | Dang leg. coll. T. Schneider | OR130084 | OR133892 | ||
| Triacanthagyna septima (Selys in Sagra, 1857) | Dominican Republic | Santiago | [15] | MN345267 | no data |
| Currently Used Names | Valid Name According to This Paper |
|---|---|
| New genus | |
| - | Isoaeschna gen. nov. |
| Synonymizations at the generic rank | |
| Aeschnophlebia Selys, 1883 syn. nov. | Brachytron Evans, 1845 |
| Epiaeschna Hagen in Selys, 1883 syn. nov. | Brachytron Evans, 1845 |
| Nasiaeschna Selys in Förster, 1900 syn. nov. | Brachytron Evans, 1845 |
| Polycanthagyna Fraser, 1933 syn. nov. | Indaeschna Fraser, 1926 |
| Synonymization at species rank | |
| Gynacantha hyalina Selys, 1882 syn. nov. | Gynacantha subinterrupta Rambur, 1842 |
| Synonymization at subspecies rank | |
| Aeshna juncea atshischgho Bartenef, 1929 | Aeshna juncea crenatoides Bartenef, 1925 |
| Species to subspecies level | |
| Aeshna septentrionalis Burmeister, 1839 | Aeshna caerulea septentrionalis Burmeister, 1839 |
| New combinations | |
| Aeshna isoceles (Müller, 1767) | Isoaeschna isoceles (Müller, 1767) comb. nov. |
| Aeschnophlebia anisoptera Selys, 1883 | Brachytron anisoptera (Selys, 1883) comb. nov. |
| Aeschnophlebia longistigma Selys, 1883 | Brachytron longistigma (Selys, 1883) comb. nov. |
| Epiaeschna heros (Fabricius, 1798) | Brachytron heros (Fabricius, 1798) comb. nov. |
| Nasiaeschna pentacantha (Rambur, 1842) | Brachytron pentacantha comb. nov. |
| Polycanthagyna erythromelas (McLachlan, 1896) | Indaeschna erythromelas (McLachlan, 1896) comb. nov. |
| Polycanthagyna melanictera (Selys, 1883) | Indaeschna melanictera (Selys, 1883) comb. nov. |
| Polycanthagyna ornithocephala (McLachlan, 1896) | Indaeschna ornithocephala (McLachlan, 1896) comb. nov. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, T.; Vierstraete, A.; Kosterin, O.E.; Ikemeyer, D.; Hu, F.-S.; Snegovaya, N.; Dumont, H.J. Molecular Phylogeny of Holarctic Aeshnidae with a Focus on the West Palaearctic and Some Remarks on Its Genera Worldwide (Aeshnidae, Odonata). Diversity 2023, 15, 950. https://doi.org/10.3390/d15090950
Schneider T, Vierstraete A, Kosterin OE, Ikemeyer D, Hu F-S, Snegovaya N, Dumont HJ. Molecular Phylogeny of Holarctic Aeshnidae with a Focus on the West Palaearctic and Some Remarks on Its Genera Worldwide (Aeshnidae, Odonata). Diversity. 2023; 15(9):950. https://doi.org/10.3390/d15090950
Chicago/Turabian StyleSchneider, Thomas, Andy Vierstraete, Oleg E. Kosterin, Dietmar Ikemeyer, Fang-Shuo Hu, Nataly Snegovaya, and Henri J. Dumont. 2023. "Molecular Phylogeny of Holarctic Aeshnidae with a Focus on the West Palaearctic and Some Remarks on Its Genera Worldwide (Aeshnidae, Odonata)" Diversity 15, no. 9: 950. https://doi.org/10.3390/d15090950
APA StyleSchneider, T., Vierstraete, A., Kosterin, O. E., Ikemeyer, D., Hu, F.-S., Snegovaya, N., & Dumont, H. J. (2023). Molecular Phylogeny of Holarctic Aeshnidae with a Focus on the West Palaearctic and Some Remarks on Its Genera Worldwide (Aeshnidae, Odonata). Diversity, 15(9), 950. https://doi.org/10.3390/d15090950







