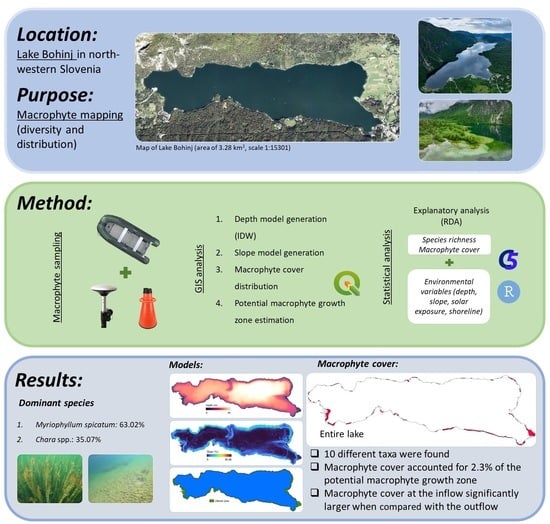
Distribution of Aquatic Macrophytes in the Littoral of Lake Bohinj (Slovenia)
Abstract
1. Introduction
2. Materials and Methods
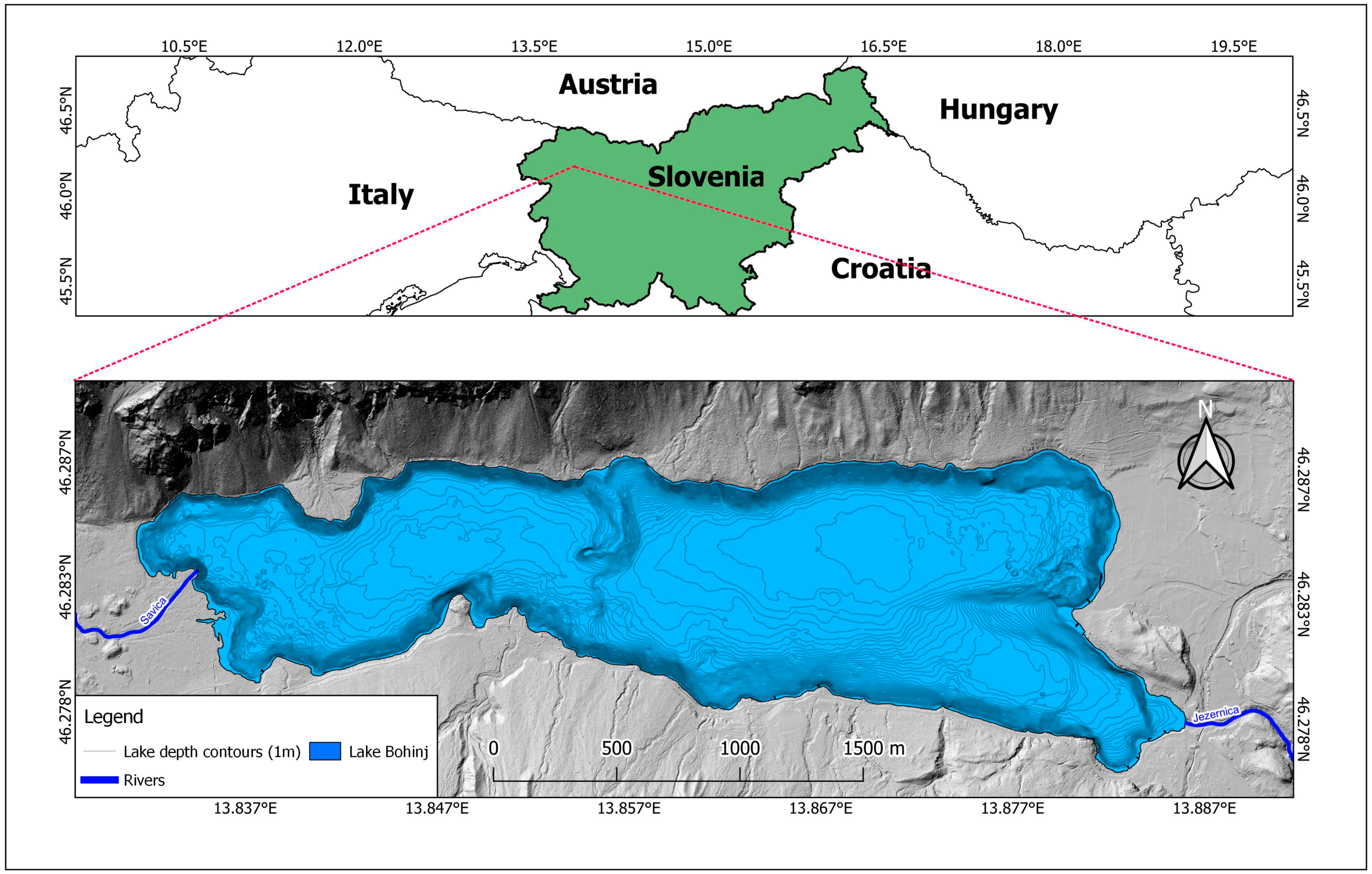
2.1. Study Site
2.2. Water Quality Parameters and Environmental Conditions
2.3. Sampling of Macrophytes
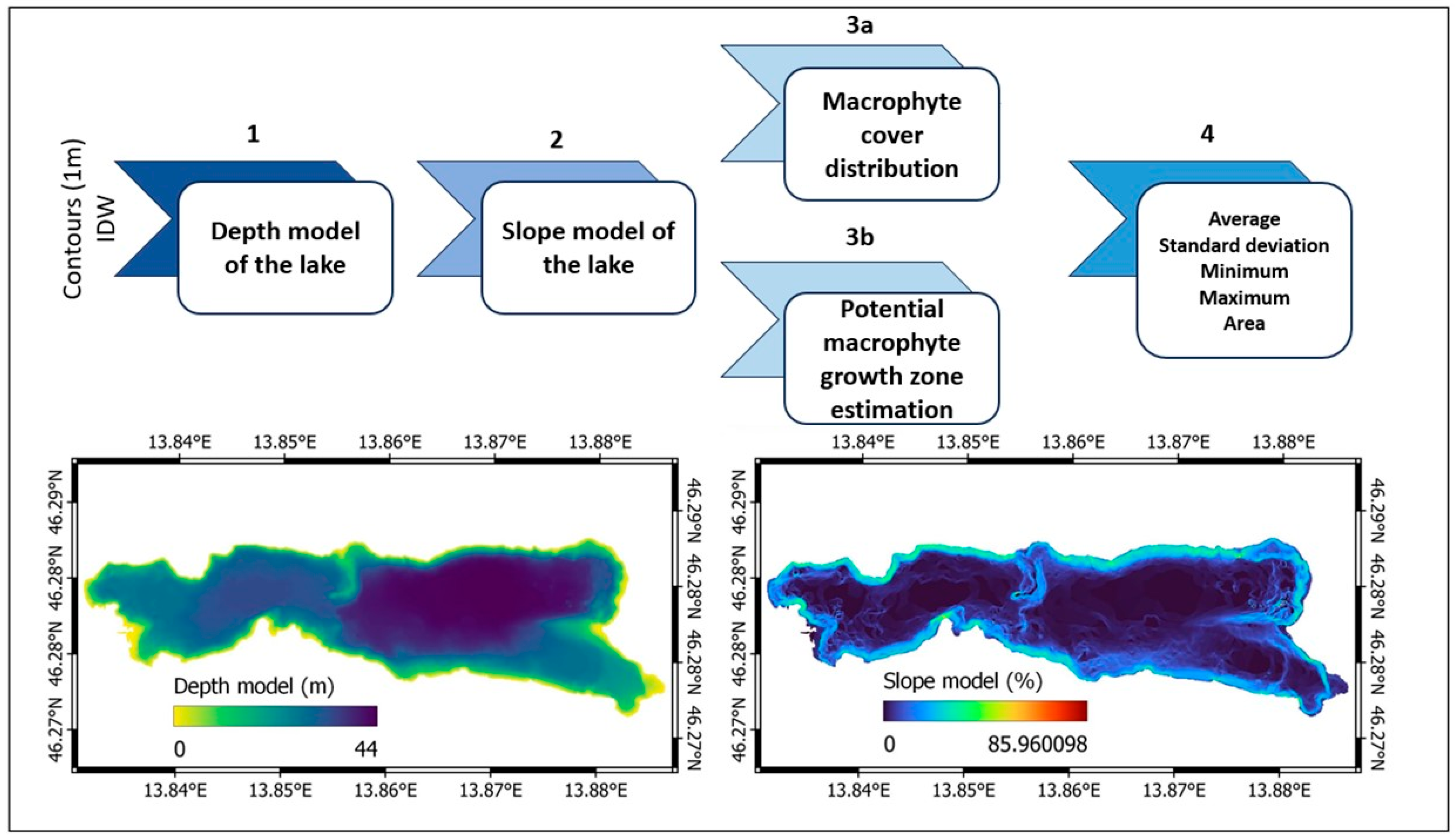
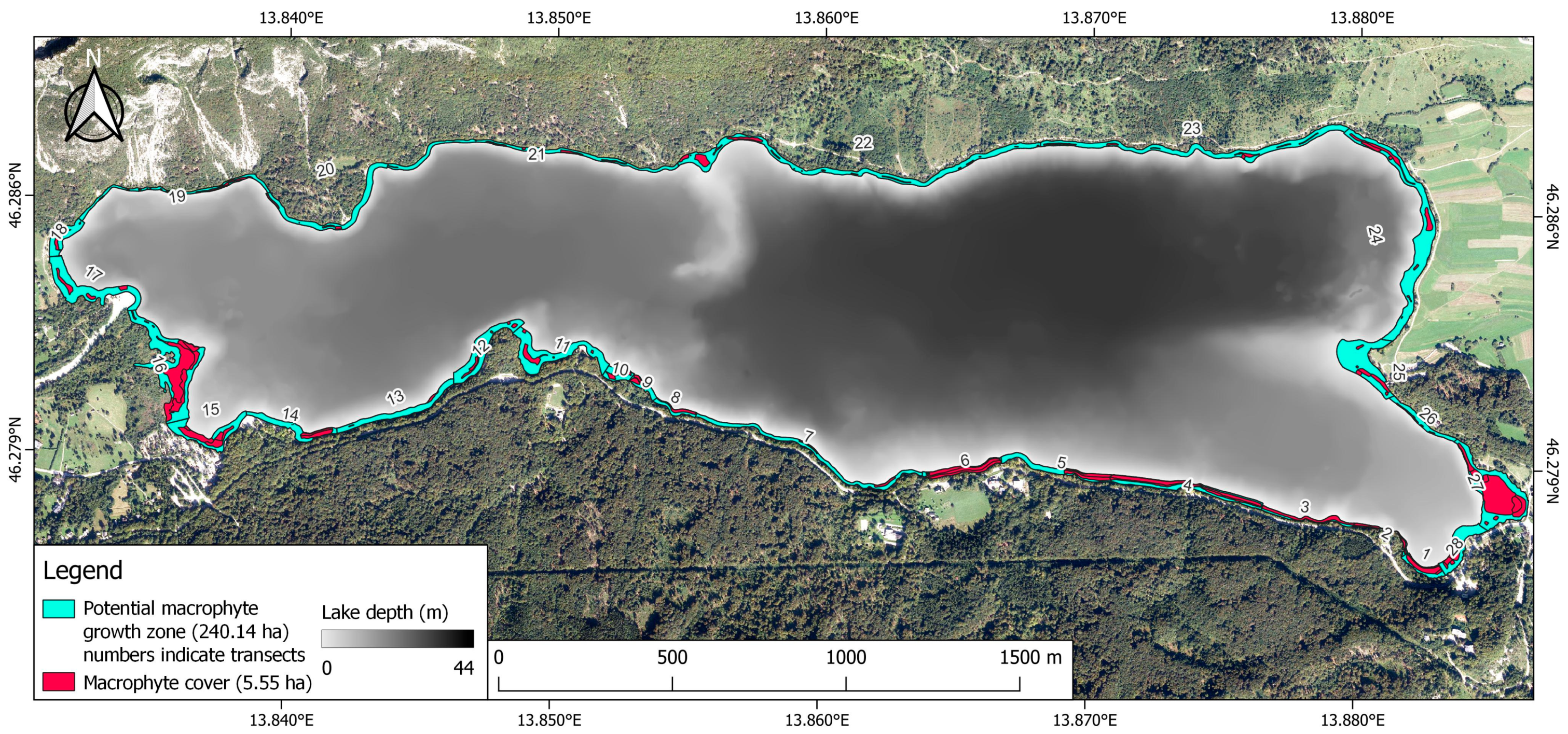
2.4. GIS Analysis
2.5. Statistical Analysis
3. Results
3.1. Water Quality Parameters
3.2. Macrophyte Species Composition and Distribution
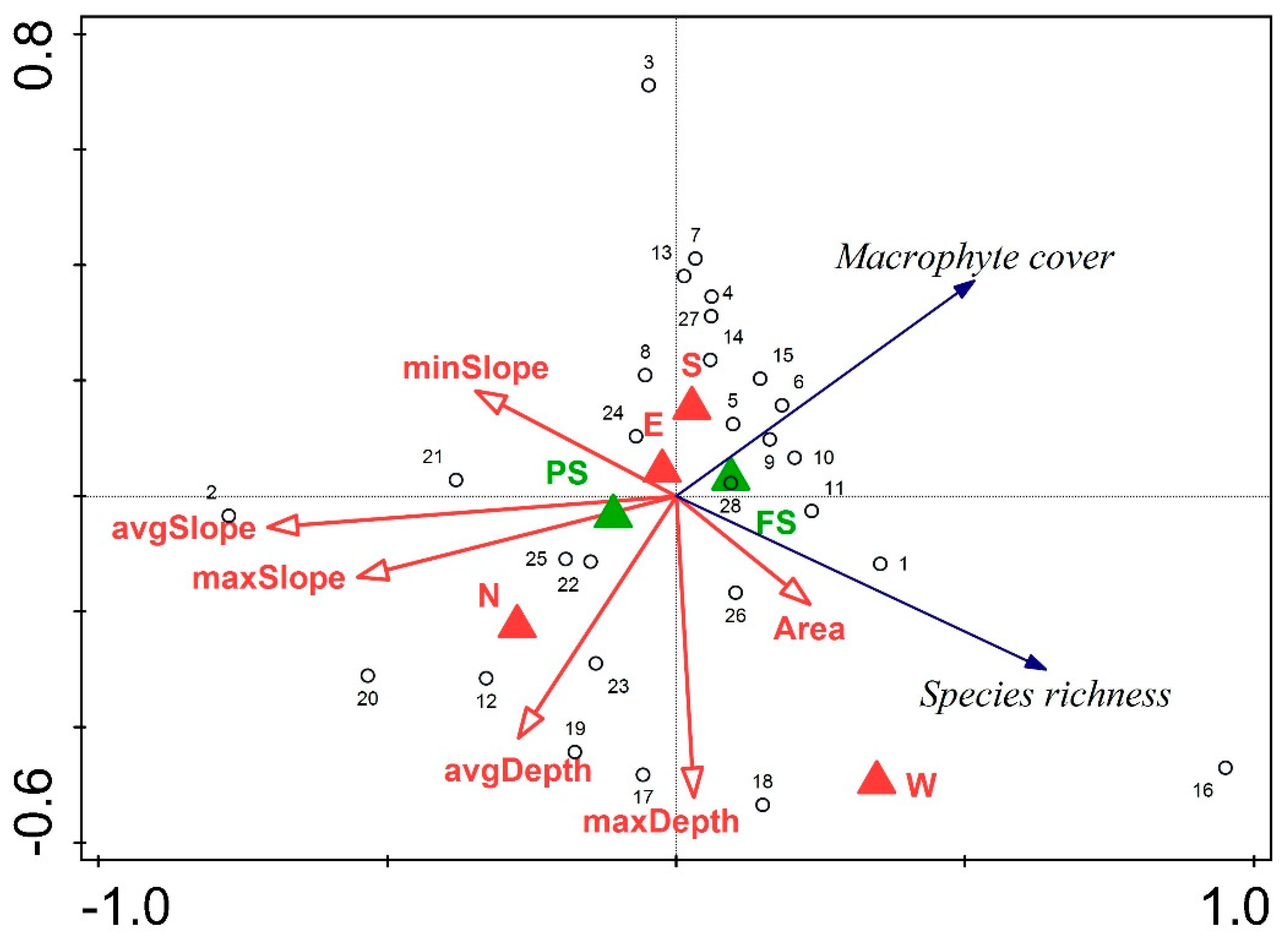
3.3. Relationship between Environmental Variables, Species Richness and Macrophyte Cover
4. Discussion
4.1. Local Catchment Area
4.2. Macrophytes—Species Found
4.3. Macrophyte Distribution and Cover
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wetzel, R.G. Limnology Lake and Reservoir Ecosystems; Academic Press: San Diego, CA, USA, 2001; ISBN 9780127447605. [Google Scholar]
- Scheffer, M.; Hosper, S.H.; Meijer, M.-L.; Moss, B.; Jeppesen, E. Alternative Equilibria in Shallow Lakes. Trends Ecol. Evol. 1993, 8, 275–279. [Google Scholar] [CrossRef]
- Pinero-Rodríguez, M.J.; Fernández-Zamudio, R.; Arribas, R.; Gomez-Mestre, I.; Díaz-Paniagua, C. The Invasive Aquatic Fern Azolla filiculoides Negatively Impacts Water Quality, Aquatic Vegetation and Amphibian Larvae in Mediterranean Environments. Biol. Invasions 2021, 23, 755–769. [Google Scholar] [CrossRef]
- Ehrlén, J.; Morris, W.F. Predicting Changes in the Distribution and Abundance of Species under Environmental Change. Ecol. Lett. 2015, 18, 303–314. [Google Scholar] [CrossRef]
- Howard, C.; Stephens, P.A.; Pearce-Higgins, J.W.; Gregory, R.D.; Willis, S.G. Improving Species Distribution Models: The Value of Data on Abundance. Methods Ecol. Evol. 2014, 5, 506–513. [Google Scholar] [CrossRef]
- Bowden, W.B.; Glime, J.M.; Riis, T. Macrophytes and Bryophytes. In Methods in Stream Ecology: Volume 1: Ecosystem Structure; Elsevier: Amsterdam, The Netherlands, 2017; pp. 243–271. [Google Scholar]
- Madsen, T.V.; Sand-Jensen, K. Photosynthetic Carbon Assimilation in Aquatic Macrophytes. Aquat. Bot. 1991, 41, 5–40. [Google Scholar] [CrossRef]
- Kolada, A. The Use of Aquatic Vegetation in Lake Assessment: Testing the Sensitivity of Macrophyte Metrics to Anthropogenic Pressures and Water Quality. Hydrobiologia 2010, 656, 133–147. [Google Scholar] [CrossRef]
- Søndergaard, M.; Phillips, G.; Hellsten, S.; Kolada, A.; Ecke, F.; Mäemets, H.; Mjelde, M.; Azzella, M.M.; Oggioni, A. Maximum Growing Depth of Submerged Macrophytes in European Lakes. Hydrobiologia 2013, 704, 165–177. [Google Scholar] [CrossRef]
- Grizzetti, B.; Pistocchi, A.; Liquete, C.; Udias, A.; Bouraoui, F.; van de Bund, W. Human Pressures and Ecological Status of European Rivers. Sci. Rep. 2017, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, A.; Vallinkoski, V.-M.; Leka, J.; Marjomäki, T.J.; Hellsten, S.; Hämäläinen, H. A Comparison of Two Methods for Surveying Aquatic Macrophyte Communities in Boreal Lakes: Implications for Bioassessment. Aquat. Bot. 2013, 104, 88–100. [Google Scholar] [CrossRef]
- Mjelde, M.; Thrane, J.; Demars, B.O.L. High Aquatic Macrophyte Diversity in Norwegian Lakes North of the Arctic Circle. Freshw. Biol. 2023, 68, 509–522. [Google Scholar] [CrossRef]
- Dudley, B.; Dunbar, M.; Penning, E.; Kolada, A.; Hellsten, S.; Oggioni, A.; Bertrin, V.; Ecke, F.; Søndergaard, M. Measurements of Uncertainty in Macrophyte Metrics Used to Assess European Lake Water Quality. Hydrobiologia 2013, 704, 179–191. [Google Scholar] [CrossRef]
- Heino, J.; García Girón, J.; Hämäläinen, H.; Hellsten, S.; Ilmonen, J.; Karjalainen, J.; Mäkinen, T.; Nyholm, K.; Ropponen, J.; Takolander, A.; et al. Assessing the Conservation Priority of Freshwater Lake Sites Based on Taxonomic, Functional and Environmental Uniqueness. Divers. Distrib. 2022, 28, 1966–1978. [Google Scholar] [CrossRef]
- Ciecierska, H.; Kolada, A. ESMI: A Macrophyte Index for Assessing the Ecological Status of Lakes. Environ. Monit. Assess. 2014, 186, 5501–5517. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, S.; Boissonnat, J.-B.; Lardeux, C.; Roberts, D.; Hanganu, J.; Billey, A.; Constantinescu, A.; Doroftei, M. Synergy of High-Resolution Radar and Optical Images Satellite for Identification and Mapping of Wetland Macrophytes on the Danube Delta. Remote Sens. 2020, 12, 2188. [Google Scholar] [CrossRef]
- Espel, D.; Courty, S.; Auda, Y.; Sheeren, D.; Elger, A. Submerged Macrophyte Assessment in Rivers: An Automatic Mapping Method Using Pléiades Imagery. Water Res. 2020, 186, 116353. [Google Scholar] [CrossRef]
- Novković, M.; Cvijanović, D.; Mesaroš, M.; Pavić, D.; Drešković, N.; Milošević, Đ.; Anđelković, A.; Damnjanović, B.; Radulović, S. Towards UAV Assisted Monitoring of Aquatic Vegetation Withing Large Rivers—The Middle Danube (Serbia). Carpathian J. Earth Environ. Sci. 2023, 18, 307–322. [Google Scholar] [CrossRef]
- Germ, M.; Remec-Rekar, Š.; Gaberščik, A. Weather Conditions and Chlorophyll Concentrations Determine Long-Term Macrophyte Community Dynamics of Lake Bohinj (Slovenia). Reg. Environ. Chang. 2019, 19, 339–348. [Google Scholar] [CrossRef]
- Germ, M.; Golob, A.; Zelnik, I.; Klink, A.; Polechońska, L. Contents of Metals in Sediments and Macrophytes Differed between the Locations in an Alpine Lake Revealing Human Impacts—A Case Study of Lake Bohinj (Slovenia). Water 2023, 15, 1254. [Google Scholar] [CrossRef]
- Harpha Sea. Batimetrična Izmera Bohinjskega Jezera; Harpha Sea, Ltd.: Koper, Slovenia, 2016. [Google Scholar]
- Kamenik, C.; Schmidt, R.; Kum, G.; Psenner, R. The Influence of Catchment Characteristics on the Water Chemistry of Mountain Lakes. Arct. Antarct. Alp. Res. 2001, 33, 404–409. [Google Scholar] [CrossRef]
- James, M.R.; Weatherhead, M.; Stanger, C.; Graynoth, E. Macroinvertebrate Distribution in the Littoral Zone of Lake Coleridge, South Island, New Zealand—Effects of Habitat Stability, Wind Exposure, and Macrophytes. N. Z. J. Mar. Freshwater Res. 1998, 32, 287–305. [Google Scholar] [CrossRef]
- Sender, J. The Effect of Riparian Forest Shade on the Structural Characteristics of Macrophytes in a Mid-Forest Lake. Appl. Ecol. Environ. Res. 2016, 14, 249–261. [Google Scholar] [CrossRef]
- Cheruvelil, K.S.; Soranno, P.A. Relationships between Lake Macrophyte Cover and Lake and Landscape Features. Aquat. Bot. 2008, 88, 219–227. [Google Scholar] [CrossRef]
- Van Geest, G.J.; Roozen, F.C.J.M.; Coops, H.; Roijackers, R.M.M.; Buijse, A.D.; Peeters, E.T.H.M.; Scheffer, M. Vegetation Abundance in Lowland Flood Plan Lakes Determined by Surface Area, Age and Connectivity. Freshw. Biol. 2003, 48, 440–454. [Google Scholar] [CrossRef]
- Urbanc-Bercic, O. Aquatic Vegetation in Two Pre-Alpine Lakes of Different Trophic Levels (Lake Bled and Lake Bohinj): Vegetation Development from the Aspect of Bioindication. Acta Bot. Gall. 1995, 142, 563–570. [Google Scholar] [CrossRef][Green Version]
- Dong, B.; Zhou, Y.; Jeppesen, E.; Shi, K.; Qin, B. Response of Community Composition and Biomass of Submerged Macrophytes to Variation in Underwater Light, Wind and Trophic Status in a Large Eutrophic Shallow Lake. J. Environ. Sci. 2021, 103, 298–310. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing Pollutant Removal in Constructed Wetlands: Should We Pay More Attention to Macrophyte Species Selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef]
- Martinčič, A.; Wraber, T.; Jogan, N.; Podobnik, A.; Turk, B.; Vreš, B.; Ravnik, V.; Frajman, B.; Strgulc Krajšek, S.; Trčak, B.; et al. Mala Flora Slovenije: Ključ za Določanje Praprotnic in Semenk; Tehniška založba Slovenije: Ljubljana, Slovenia, 2007. [Google Scholar]
- Zelnik, I.; Kuhar, U.; Holcar, M.; Germ, M.; Gaberščik, A. Distribution of Vascular Plant Communities in Slovenian Watercourses. Water 2021, 13, 1071. [Google Scholar] [CrossRef]
- Aiken, S.G.; Newroth, P.R.; Wile, I. The Biology of Canadian Weeds.: 34. Myriophyllum spicatum L. Can. J. Plant Sci. 1979, 59, 201–215. [Google Scholar] [CrossRef]
- Nichols, S.A.; Shaw, B.H. Ecological Life Histories of the Three Aquatic Nuisance Plants, Myriophyllum spicatum, Potamogeton crispus and Elodea canadensis. Hydrobiologia 1986, 131, 3–21. [Google Scholar] [CrossRef]
- Grace, J.B.; Wetzel, R.G. The Production Biology of Eurasian Watermilfoil (Myriphyllum spicatum L.): A Review. J. Aquat. Plant Manag. 1978, 16, 1–11. [Google Scholar]
- Schwarz, A.-M.; de Winton, M.; Hawes, I. Species-Specific Depth Zonation in New Zealand Charophytes as a Function of Light Availability. Aquat. Bot. 2002, 72, 209–217. [Google Scholar] [CrossRef]
- Beaune, D.; Sellier, Y.; Lambert, É.; Grandjean, F. The Use of Chara Spp. (Charales: Characeae) as a Bioindicator of Physico-Chemical Habitat Suitability for an Endangered Crayfish Austropotamobius pallipes in Lentic Waters. Aquat. Conserv. 2018, 28, 506–511. [Google Scholar] [CrossRef]
- Preston, C.D. Pondweeds of Great Britain and Ireland, 1st ed.; Botanical Society of Britain and Ireland: London, UK, 1995; Volume 8, p. 352. [Google Scholar]
- Germ, M.; Golob, A.; Zelnik, I.; Klink, A.; Polechońska, L. Diversity of Macrophytes and Differences in the Contents of Metals between Macrophyte Species in Alpine Lake Bohinj (Slovenia). In Tackling Present and Future Environmental Challenges of a European Riverscape; IAD Proceedings: Krems, Austria, 2023. [Google Scholar] [CrossRef]
- Wraber, T.; Skoberne, P.; Seliškar, A.; Vreš, B.; Babij, V.; Čarni, A.; Čušin, B.; Dakskobler, I.; Surina, B.; Šilc, U.; et al. Rdeči seznam praprotnic in semenk (Pteridophyta & Spermatophyta). In Odredba o Uvrstitvi Ogroženih Rastlinskih in Živalskih Vrst v Rdeče Sezname, Odredba o Uvrstitvi Ogroženih Rastlinskih in Živalskih Vrst v Rdeče Sezname; Ministry of Environment: Ljubljana, Slovenia, 2002; pp. 5–20. [Google Scholar]
- Schutten, J.; Dainty, J.; Davy, A.J. Root Anchorage and Its Significance for Submerged Plants in Shallow Lakes. J. Ecol. 2005, 93, 556–571. [Google Scholar] [CrossRef]
- Lacoul, P.; Freedman, B. Environmental Influences on Aquatic Plants in Freshwater Ecosystems. Environ. Rev. 2006, 14, 89–136. [Google Scholar] [CrossRef]
- Bornette, G.; Puijalon, S. Response of Aquatic Plants to Abiotic Factors: A Review. Aquat. Sci. 2011, 73, 1–14. [Google Scholar] [CrossRef]
- Toman, M.J.; Urbanič, G.; Tavzes, B. Macroinvertebrate Assemblages of the Inflow, the Outflow and the Upper Littoral Zone of the Prealpine Lake Bohinj, Slovenia. In Proceedings of the 11th World Lakes Conference, Nairobi, Kenya, 31 October–4 November 2005; Odada, E.O., Olago, D.O., Ochola, W., Ntiba, M., Wandiga, S., Gichuki, N., Oyieke, H., Eds.; Ministry of Water and Irrigation Internation Lake Environment Committee, 2005; pp. 282–285. [Google Scholar]
- Barko, J.W.; Hardin, D.G.; Matthews, M.S. Growth and Morphology of Submersed Freshwater Macrophytes in Relation to Light and Temperature. Canad. J. Bot. 1982, 60, 877–887. [Google Scholar] [CrossRef]
- Barko, J.W.; Adams, M.S.; Clesceri, N.L. Environmental Factors and Their Consideration in the Management of Submersed Aquatic Vegetation: A Review. J. Aquat. Plant Manag. 1986, 24, 1–10. [Google Scholar]
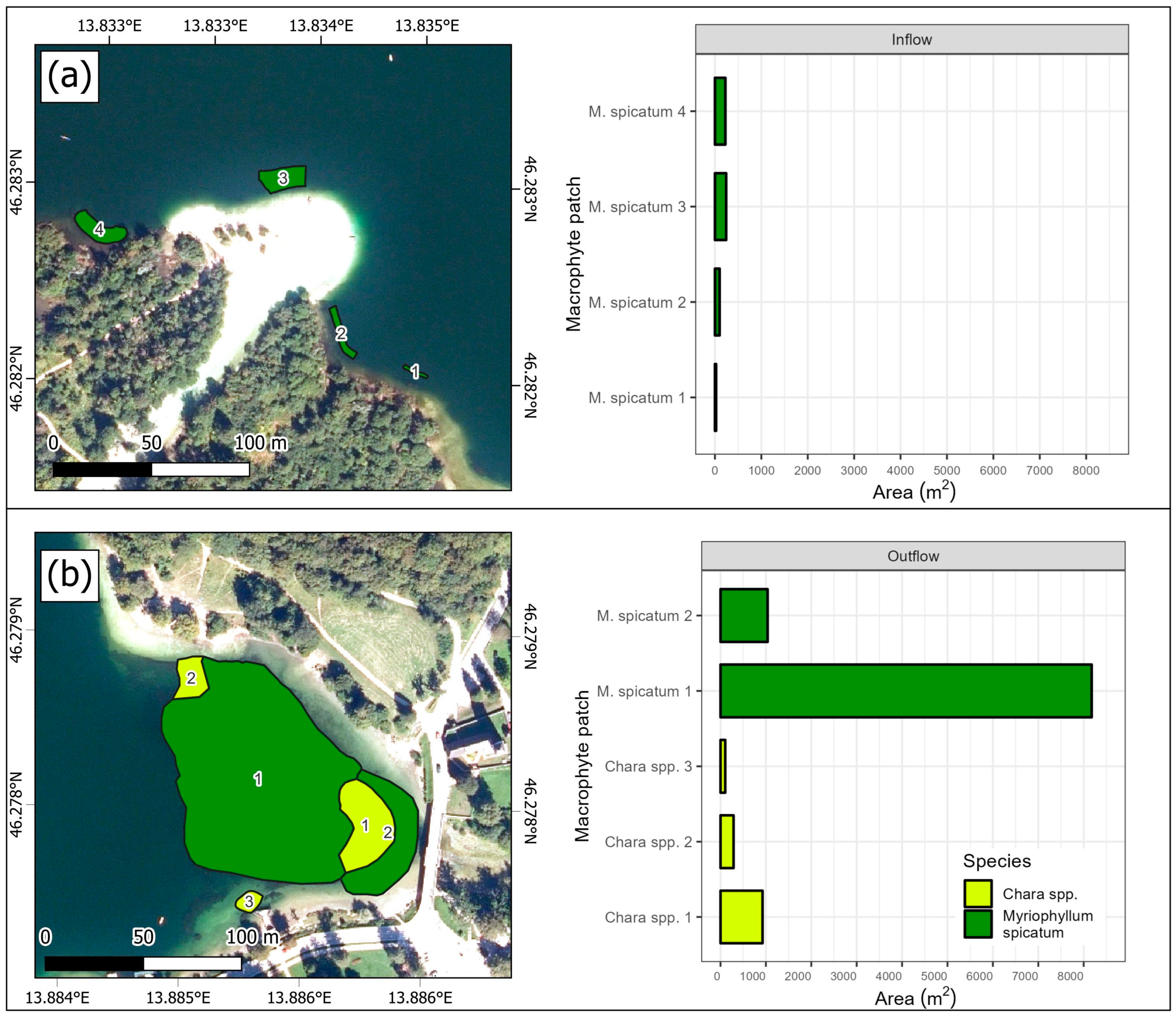
| Parameter | 1 m | 10 m |
|---|---|---|
| Temperature (°C) | 14.5 | 8.3 |
| pH | 8.5 | 8.5 |
| Electric conductivity (µS/cm) | 165 | 166 |
| Oxygen concentration (mg/L) | 11.7 | 12.6 |
| Species | Avg Slope (%) | Min Slope (%) | Max Slope (%) | Avg Depth (m) | Min Depth (m) | Max Depth (m) | Area (m2) | Percentage (%) |
|---|---|---|---|---|---|---|---|---|
| Chara spp. | 19.12 ± 7.42 | 0 | 48.12 | 3.62 ± 1.2 | 0 | 12.99 | 19,465.46 | 35.07 |
| M. spicatum | 20.47 ± 7.67 | 0 | 62.4 | 2.65 ± 0.74 | 0 | 10.04 | 34,979.81 | 63.02 |
| P. australis | 2.82 | 0 | 3.94 | 0.3 | 0 | 1.14 | 985.76 | 1.77 |
| P. alpinus | 11.8 ± 4.5 | 2.19 | 38.22 | 2.78 ± 1.53 | 0.62 | 5.01 | 46.46 | <1 |
| P. crispus | 3.09 | 3.01 | 3.28 | 1.26 | 1.1 | 1.41 | 18.88 | <1 |
| P. perfoliatus | 7.54 ± 3.16 | 0.69 | 9.78 | 1.39 ± 0.61 | 0.54 | 1.92 | 6.92 | <1 |
| R. circinatus/B. foeniculaceum | 7.66 | 7.65 | 7.67 | 1.26 | 1.22 | 1.3 | 0.32 | <1 |
| Comparison | Test | p-Value | Average Macrophyte Cover (%) |
|---|---|---|---|
| South shore vs. north shore | Welch’s t-test | 0.02859 | N shore 0.97 |
| S shore 2.56 | |||
| Fully sunlit vs. partly shaded | Welch’s t-test | 0.2725 | Sunlit 2.58 |
| Shaded 1.84 |
| Group Variables | Variable | % of Explained Variance |
|---|---|---|
| Slope | avgSlope | 16.9 |
| maxSlope | 1.2 | |
| minSlope | 1.1 | |
| Depth | avgDepth | 6.4 |
| maxDepth | 5.1 | |
| Shallow littoral shading | Fully sunlit | 0.7 |
| Partly sunlit | 0.7 | |
| Shoreline | N | 1.3 |
| S | 4.1 | |
| E | 1.3 | |
| W | 6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojdanič, N.; Germ, M.; Andlovic, M.; Černela, D.; Zelnik, I. Distribution of Aquatic Macrophytes in the Littoral of Lake Bohinj (Slovenia). Diversity 2023, 15, 1115. https://doi.org/10.3390/d15111115
Ojdanič N, Germ M, Andlovic M, Černela D, Zelnik I. Distribution of Aquatic Macrophytes in the Littoral of Lake Bohinj (Slovenia). Diversity. 2023; 15(11):1115. https://doi.org/10.3390/d15111115
Chicago/Turabian StyleOjdanič, Nik, Mateja Germ, Maša Andlovic, Dorotej Černela, and Igor Zelnik. 2023. "Distribution of Aquatic Macrophytes in the Littoral of Lake Bohinj (Slovenia)" Diversity 15, no. 11: 1115. https://doi.org/10.3390/d15111115
APA StyleOjdanič, N., Germ, M., Andlovic, M., Černela, D., & Zelnik, I. (2023). Distribution of Aquatic Macrophytes in the Littoral of Lake Bohinj (Slovenia). Diversity, 15(11), 1115. https://doi.org/10.3390/d15111115