Abstract
Many hemiparasites attach to a range of different host species, resulting in complex parasite–host interactions. Comprehensive molecular phylogenies allow the investigation of evolutionary relationships between these host plants. We surveyed the hosts of the laurel dodder (Cassytha filiformis, Lauraceae) in China, representing 184 species from 146 genera, 67 families, and spanning flowering plants, conifers, and ferns, using host phylogenetic relationships to investigate the susceptibility to attack by this hemiparasitic plant among the vascular plants. The process of produced well-formed haustoria by C. filiformis was also observed in detail for six different hosts. Our results show that C. filiformis grows mainly on trees and shrubs from phylogenetically divergent members of the rosid and asterid eudicot clades, often attacking multiple adjacent hosts simultaneously, and forming extensive colonies. However, whether and to what extent transitions between C. filiformis and host plants occur remain unclear. Physiological evidence for the complex parasite–host species interactions need to be studied in the future.
1. Introduction
Parasitic plants are a diverse group of 292 genera: approximately 4750 species (1% of all flowering plants) with parasitism evolving at least 12 times [1,2]. Parasitic plants negatively influence host growth and development [3]. They have had increased attention by researchers over the past three decades [1]. Parasitic plants can obtain water, mineral nutrients, and carbon from other plants using the haustorium [1,4]. Parasitic plants can either be photosynthetic hemiparasites or achlorophyllous holoparasites [5], but the majority are hemiparasites [2]. They occur in most major ecosystems across the world from subpolar to tropical latitudes, as well as agricultural ecosystems [6].
Generalist hemiparasitic plants as a group are morphologically diverse with a broad range of host interactions [2], often attacking multiple co-occurring plant species [7]. Parasite performance can be influenced by host characteristics, including biomass [8], carbon content [9], resistance [10], genetic constitution [11], rate of growth [12], host condition [13], secondary compounds [14], etc., complicating research into the evolution of host range [7]. Hemiparasite fitness has often been linked to host functional plant groups such as legumes, grasses, or forbs [15], but is influenced by life history strategies and resource-linked attributes [16]. However, some functional groups are monophyletic, while others have more complex evolutionary relationships and hemiparasite performance may sometimes be predicted better by host phylogenetic relationships [7]. Host identity may be an important factor in determining the success of the parasitic species.
The literature on parasitic plant host biology covers a range of taxa, including the root hemiparasites in Orobanchaceae [17] and Santalaceae [18], mistletoes of Loranthaceae [19] and Viscaceae [20], and the stem holoparasite Cuscuta L. (dodder, Convolvulaceae) [21] and hemiparasite Cassytha L. (laurel dodder, Lauraceae) [22]. Parasitic plants also interact with other plants, influencing competition, community biodiversity, and nutrient cycling [23,24,25]. Although molecular phylogenetic methods have been used to address several long-standing issues in parasitic plant taxonomy and evolutionary biology [1], few studies have examined the evolutionary relationships of host species.
There is a long tradition of the human use of parasitic plants for medicinal and cultural purposes worldwide [26]. Cassytha filiformis L. (laurel dodder) is a pantropical hemiparasite which has also been used variously for medicine, cosmetics, cushion, and rope-making in many regions [6,27], as well as being regarded as a serious weed in some tropical regions [7,28]. The species has been reported as having a very wide, possibly indiscriminate host range [28,29,30], although with some apparent host preferences [30,31]. Identifying its host spectrum and phylogenetic relationships may help with the use of this parasite to control invasive weeds by understanding their interactive biology, as has been suggested for C. pubescens R.Br. in Australia [32,33,34]. Parasitic plants develop haustoria for absorbing water and nutrients from the host plant, so understanding the seed germination to host selection and haustorial attachment process is critical [35].
Cassytha is the only parasitic genus Lauraceae, but it is also morphologically similar to Cuscuta L. (Convolvulaceae), with both being leafless, haustorial stem parasites [35]. Cassytha is hemiparasitic with white or light green flowers borne in spicate, capitate, or racemose inflorescences and 1-seeded drupes included in a dilated, fleshy, free perianth tube. In contrast, Cuscuta is holoparasitic, with white, orange, or purplish-red stems; white, yellow, or pink flowers in globular inflorescences; and two–four seeded capsules [36]. Cassytha parasitizes a wide range of mainly woody plants, including plants of agricultural and economic value, and although most species occur in Australia, C. filiformis occurs across the tropics worldwide [22,28]. According to Nickrent (2002), C. filiformis is indiscriminate in host choice, often attacking multiple co-occurring hosts simultaneously [29], and surveys by Li et al. (1992) in Nanning, Tianlin, Yulin, and other places in Guangxi recorded 137 host species from 113 genera and 58 families [37].
In China, C. filiformis causes serious damage to crop and forests in some regions, with Huang et al. (1957) finding that the severe parasitism of oil tea by C. filiformis caused a massive yield reduction in Shangsi and Luchuan, Guangxi, China [38]. Similarly, Zhang (1988) found that heavy infestation of Pinus massoniana Lamb by C. filiformis led to acute deciduous needle disease caused by Lophodermium pinastri Chev. in Fuzhou, Fujian [39]. These studies show the importance of investigating the host–parasite relationships of C. filiformis and their effects, but to date no systematic and comprehensive studies have been undertaken on host species occurring in China.
Accordingly, we here investigate and summarize the hosts of C. filiformis in China, with a particular focus on its host phylogenetic relationships and host–parasite species interactions. Our aim is to understand the evolutionary relationships of the diverse range of host species in China and the potential growth responses of the hosts, specifically: (1) to determine the host range and responses to attack for C. filiformis in China and analyze the parasitic progress of C. filiformis; and (2) examine the evolutionary relationships among the hosts of C. filiformis.
2. Materials and Methods
2.1. Plant Materials
Collection sites in China were selected using data from the Chinese Virtual Herbarium (CVH, https://www.cvh.ac.cn, accessed on 5 May 2019), Plant Photo Bank of China (PPBC, http://ppbc.iplant.cn, accessed on 5 May 2019), and Global Biodiversity Information Facility (GBIF, https://www.gbif.org, accessed on 6 May 2019). We conducted a series of targeted field trips to the geographical range areas of particular interest in 2019–2021 (Figure S1). Efforts were made to ensure sampling from localities across most of the known geographical ranges of the species (144 sample sites; for details see Table S1 and Figure S1). Inflorescences and fruits of C. filiformis were gathered (with reproductive characters collected where possible) from Guangzhou (Guangdong), Lingshui (Hainan), and Xishuangbanna (Yunnan) (Figure 1), but the Guangzhou locality (Zhongkai University of Agriculture and Engineering) was the only one with ripe fruit, despite repeated site visits. Host plants were recorded for samples from six provinces: Fujian, Guangdong, Guangxi, Hainan, Yunnan, and Zhejiang (Figure S1; Table S1), with hosts defined as plants having direct haustorial connections to C. filiformis (Figure 2). Host identification was performed by experts at PE (Herbarium, Institute of Botany, CAS), Bing Liu, XTBG (Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences), Yunhong Tan and CATAS (Chinese Academy of Tropical Agricultural Sciences), and Shengzhuo Huang, using the reproductive or vegetative characters available on the vouchers. All vouchers were stored at the Herbarium of Xishuangbanna Tropical Botanical Garden (HITBC). In addition, we summarized the host plants of the published literature, together with data from our surveys (40 specimens by the previous published literature, 104 specimens by our surveys; details can be seen in Table S1). In total, the Chinese host range assessment was based on 184 specimens of C. filiformis.
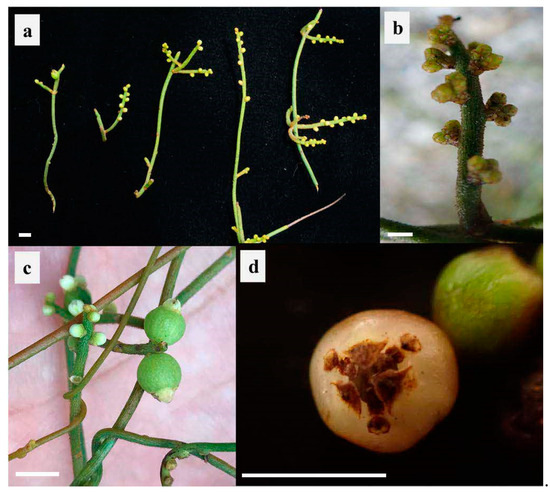
Figure 1.
Representative inflorescence and fruits of Cassytha filiformis. (a) Inflorescence collected in Guangzhou (Guangdong, longitude: 113°17′ E, latitude: 23°0′ N, altitude: 10 m). (b) Inflorescence collected in Xishuangbanna (Yunnan, longitude: 100°45′ E, latitude: 22°28′ N, altitude: 1129 m). (c) Inflorescence and unripe fruits collected in Lingshui (Hainan, longitude: 109°54′ E, latitude: 18°34′ N, altitude: 76 m). (d) Ripe fruit collected in Guangzhou (Guangdong, longitude: 113°17′ E, latitude: 23°0′ N, altitude: 10 m). Scale bars = 5 mm.

Figure 2.
The host Bridelia balansae and Cassytha filiformis with its haustoria. (a) Host and C. filiformis collected in Lingshui, Hainan (longitude: 109°55′ E, latitude: 18°33′ N, altitude: 42 m). (b) Vines and fruits. (c) The haustorium. Scale bars = 1 mm.
2.2. Growth Conditions of C. filiformis
The seeds of C. filiformis displayed physical dormancy [40], which was broken by soaking the seeds in warm water at 60 °C for 1 h. The treated seeds were then grown in the soils that collected in Guangzhou (Zhongkai University of Agriculture and Engineering, Guangdong, China) under controlled conditions in a PHCbi CO2 Incubator (PHC Holdings Corporation, Tokyo, Japan) with a 24–27 °C, 18 h light and 6 h dark cycle. Germination time was measured as the total number of days to seedling emergence after planting. Host seeds (Bidens pilosa L. (Asteraceae) herb; Chromolaena odorata (L.) R.M.King and H.Rob. (Asteraceae), herb; Cleome rutidosperma DC. (Cleomaceae), herb; Dimocarpus longan Lour. (Sapindaceae), tree; Mikania micrantha Kunth (Asteraceae), liana; and Scoparia dulcis L. (Plantaginaceae), herb) were also collected from the sites of C. filiformis seed collection, in Guangzhou (Zhongkai University of Agriculture and Engineering, Guangdong, China). These six species were confirmed as hosts of C. filiformis, based on field observations of haustorial attachment. We filled pots (18 cm diameter, 16 cm height) with soil collected from these field sites and sowed a minimum of five seeds of a single species per pot, with three replicate pots per host species for a total of eighteen pots. Hosts were observed at the same time each day (10 a.m.) in each pot once germination had commenced.
To investigate the interaction between C. filiformis and hosts, we observed and recorded the growth and germination progress. Three replicates were used, each of which was pooled from at least five seeds. To obtain laurel dodder and hosts from host–laurel dodder parasitization systems, we put different host plants with five seeds of C. filiformis together to form the host–laurel dodder parasitization system. This system was cultivated under controlled conditions in PHCbi CO2 Incubator (18 h light, 6 h dark) at 24–27 °C. From germination to growth conditions, C. filiformis as well as hosts were observed at the same time each day (10 a.m.) in each pot.
2.3. Phylogenic Analysis
To examine the host–parasite relationship, a host frequency analysis was determined using two methods of classifying species: (1) life history (woody or herbaceous) and (2) taxonomic levels (family, genus, and species). We recorded the percentage of the number of instances C. filiformis was found on each of the hosts from the total host occurrences. The lists of family, genus, and species’ hosts were clustered using the R packages APE [41] and V.PhyloMaker2 [42], based on the botanical nomenclature of the World Plants (WP) Database for Pteridophytes and Gymnosperms (https://www.worldplants.de, accessed on 20 September 2022), the Angio-sperm Phylogeny Website (http://www.mobot.org/MOBOT/research/Apweb/, accessed on 20 September 2022), and the Angiosperm Phylogeny Group IV (APG IV) [43] classification for angiosperms. The percentage of different types was imported into Excel to summarize the hosts’ frequency.
3. Results
3.1. Biology of Laurel Dodder and Its Hosts
Cassytha filiformis and the host seeds germinated under the soil surface without light, with C. filiformis producing a rudimentary primary root about 0.5 mm long (Figure 3a). This root grows for 2–4 weeks, degenerating when the embryonic axis starts to grow and three–four adventitious roots 2–6 mm long and 0.2–0.4 mm thick develop at the base of the axis (Figure 3a). This free-living phase can last up to two months (Figure 3b,c), but unless a suitable host is found during this period, the seedling dies (Figure 3c).
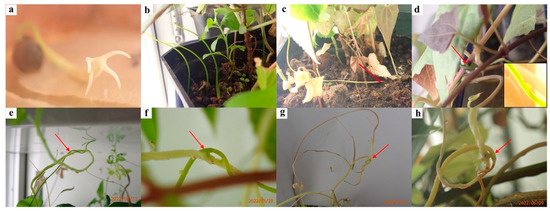
Figure 3.
Images of Cassytha filiformis growth and parasitic processes on host species in laboratory conditions. (a) Germinated seed with a rudimentary and short-lived root. (b) Independent growth stage of seedlings. (c) The dead C. filiformis seedlings unsuccessfully parasitized a viable host; the red arrow indicates the dead stem of C. filiformis. (d) The survived Cassytha filiformis seedlings successfully parasitized the viable host Mikania micrantha with the haustoria. (e–h) Cassytha filiformis–Cleome rutidosperma host associations via the haustoria. The thin stem is C. filiformis, and the thick stem is C. rutidosperma. The red arrows indicate infected parts.
For the laurel dodder as well as the hosts, the percent germination exceeded 80% (Table S2). The average time to germination of C. filiformis was 15 days (±10–20 SD) and for the host species: M. micrantha (3 ± 3–4), B. pilosa (5 ± 4–6), C. odorata (5 ± 4–6), C. rutidosperma (7 ± 6–8), S. dulcis (8 ± 7–9), and D. longan (21 ± 20–22). The host species belongs to the family Asteraceae germinated firstly (M. micrantha, three days), then B. pilosa and C. odorata (five days), followed by C. rutidosperma (Cleomaceae, seven days), S. dulcis (Scrophulariaceae, eight days), and D. longan (Sapindaceae, three weeks). It took eight weeks for parasitism to occur following germination of the host liana species M. micrantha (Figure 3d). Then, the same C. filiformis vine that was attacking M. micrantha (Asteraceae) was also connecting to herb species C. rutidosperma (Cleomaceae) a month later (Figure 3e–h). When the C. rutidosperma (Cleomaceae) plant died, some parts of the host in contact with the haustoria survived for a further two months (Figure 3f–h), apparently deriving water and nutrients from the parasite and even producing new shoots (Figure 3h). The signal transduction mechanism for infection is unclear yet. Except tree species D. longan (Sapindaceae), the other host species were all successfully parasitized. Under the controlled conditions, it seemed easier to infect herbs than woody plants.
Flowering and fruiting in wild Chinese C. filiformis occur from May to December, with considerable overlap (Figure 1a,c). The inflorescence is arranged into a spicate, capitate, or racemose (Figure 1a,b), and the ripe fruit is a drupe with a white translucent, fleshy pericarp (Figure 1d). The cultivated C. filiformis plants did not flower under the laboratory conditions used here, but this may be because at least several Australian Cassytha species take >5 years to flower from seed in the wild (J.G. Conran, pers. obs.).
3.2. Host Range of C. filiformis
After three years of field investigation, we found C. filiformis preferred to grow on trees and shrubs in well-lit, open, well-watered habitats, especially along roadsides. Based on our surveys and the published literature [37,44,45], we found that C. filiformis produced well-formed haustoria onto diverse vascular plants, including angiosperms, conifers, and ferns (Figure 4 and Figure 5; Table S1). The overall host range of Chinese C. filiformis included 184 species, which belonged to 146 genera, 67 families, and 32 orders (Table S1). In total, 80.80% of the host species in this study, defined by the presence of haustoria, were woody taxa, including trees (82/184, 44.57%), shrubs (58/184, 31.52%), and occasionally lianas (9/184, 4.91%), but herbaceous plants were also affected (35/184, 19.20%) (Figure S2). The most common hosts at order-, family-, genus-, and species levels are summarized in Table S1. Nine preferred orders had hosts with more than ten species (Asterales, Ericales, Fabales, Gentianales, Laurales, Malpighiales, Myrtales, Rosales, and Sapindales. All contain 30 families, 96 genera, and 126 species; Figure 5; Table S1 noted with different colors). To our knowledge, there is currently no comprehensive survey of the host range of laurel dodder for such a broad geographic range of China.
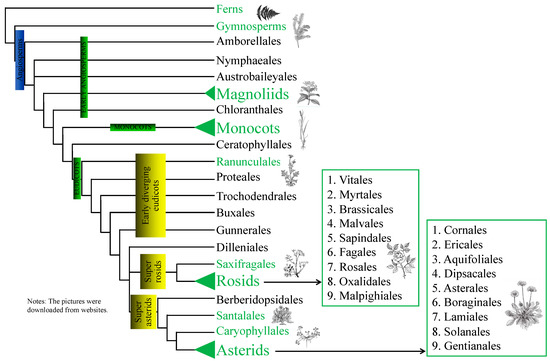
Figure 4.
A simplified phylogenetic tree showing major orders that include Cassytha filiformis hosts. The green labels indicate the host positions among the vascular phylogenetic relationships.
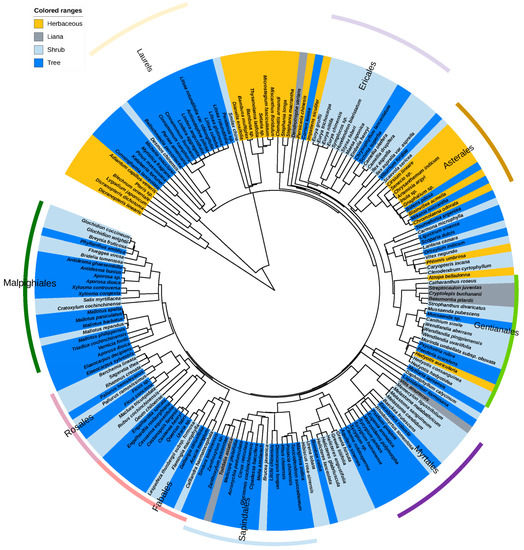
Figure 5.
The host plants’ phylogenetic tree for Cassytha filiformis in China. Yellow notes herbs. Grey notes lianas. Light blue notes shrubs; and darker blue notes trees. Hosts belonging to ferns, gymnosperms, and angiosperms are coded with different colors corresponding to Table S1.
The host range of C. filiformis spanned most of the vascular plants (Figure 4), with six ferns (Adiantum capillus-veneris L., Blechnopsis orientalis C. Presl, Dicranopteris pedata (Houttuyn) Nakaike, D linearis (Burm.) Underw., Lygodium japonicum (Thunb.) Sw., and Pteris sp. L.) and three conifers (Cunninghamia lanceolata (Lamb.) Hook., Keteleeria fortunei (Murr.) Carr., and Pinus massoniana Lamb.) parasitized by C. filiformis (Figure 5).
In the flowering plants, rosids were common hosts, with Malpighiales containing the largest number of host species (twenty-two species), and Sapindales containing the largest number of infected genera (fifteen genera), while the asterid order Ericales had the most host families (seven) (Figure 4 and Figure 5; Table S1). Euphorbiaceae was the largest single family with eight genera and thirteen species (Figure 5; Table S1). The order Gentianales represented the widest array of host plant growth forms, including the tree Psychotria rubra (Lour.) Poir., shrub Wendlandia aberrans Cowan, herb Hedyotis auricularia L., and liana Cryptolepis buchananii Roem. et Schult (Figure 5). Four angiosperm families had at least 10 host species (Table S1): Rubiaceae (12), Lauraceae (11), Phyllanthaceae (11), and Asteraceae (10). These results differ from the study by Zhang et al. [6], where they reported seven families at the world level with at least 10 host species: Anacardiaceae, Asteraceae, Euphorbiaceae, Fabaceae, Myrtaceae, Phyllanthaceae, and Rubiaceae. This confirms that C. filiformis is a generalist hemiparasite that is associated with many host species. These species are relatively common (Figure 4 and Figure 5), with no obvious evolutionary relationships, as observed by Zhang et al. [6].
4. Discussion
4.1. Laurel Dodder Prefers Woody Hosts
Eighty-one percent of the hosts noted in this study were woody (Figure 5 and Figure S2), but numerous herbaceous plants were also affected, agreeing with the conclusions of previous studies such as those by Zhang et al. [6] and Parra-Tabla et al. [44]. However, the percentage of host life form preferences varies considerably between countries, with trees most widely affected in Tanzania (69.7%), China (44.6%), India (36.1%), and down to Japan (19.2%). Shrub hosts percentages were similar for China (36.9%) and India (36.1%), but lower for Japan and Tanzania (both ca. 20%) (Table 1). In contrast, herbaceous host ratios for the Japanese Ryukyu Islands were much higher than these other three reported regions (46.2%).

Table 1.
Host plants’ life forms for Cassytha filiformis infestation in four countries.
In addition to C. filiformis, the Australian species C. melantha and C. pubescens also seem to prefer woody hosts [46,47,48] and this preference for woody hosts may accord with the perennial life form and hemiparasitic nature of Cassytha. However, the networks created by a parasitic plant attached simultaneously to multiple hosts may trigger transferring systemic signals between the hosts [26]. Herbaceous species might also act as bridging hosts to allow Cassytha seedlings to survive long enough to grow onto nearby shrubs or trees [6]. It may also reflect the speciose nature of many woody host families, combined with the problem that as many herbaceous plants are annuals this could be problematic for their use by perennial Cassytha species [6]. The reasons why Cassytha seem to prefer certain plant families as hosts are unclear, though there is some evidence from Australia of a preference for nitrogen-fixing taxa [33,49]. However, the factors that might contribute to host susceptibility are currently unknown.
Although unrelated, Cuscuta and Cassytha are morphologically similar rootless stem parasites that spread by developing haustoria along their stems. Similarly, Cuscuta different species can vary in host specificity from a single species to hundreds of taxa covering diverse genera and families [35], with 237 species, 120 genera, and 32 families reported as hosts in one study [49]. Cassytha and Cuscuta mainly occupy different habitats and have different ecologies, which may indicate that the hemiparasitic Cassytha represent a less specialized parasite than the holoparasitic Cuscuta. It is not known what factors might contribute to the susceptibility of various hosts in Cassytha [50], and the location of hosts in Cuscuta occurs by a range of methods, including host chemistry [51,52]. Host choice in these two genera may also be influenced in part by the availability suitable host-derived resources, based on the degree to which the parasites can function independently [6,53]. Long-term host range divergence also helps to drive speciation in these two parasitic genera, but further study is needed.
4.2. The Host Phylogenetic Relationships of Laurel Dodder and Its Potential Application
The mechanisms by which C. filiformis selects the suitable hosts are complex. C. filiformis parasitizes plants throughout the vascular plant phylogeny. Vascular plants play a major role in global carbon cycling and are of fundamental importance to life on earth. Phylogenetic studies have led to tremendous progress in our understanding of the origin, phylogeny, and evolution of the plants [54]. The wide variability of the host cannot predict hemiparasite performance as the host species is scattered on vascular plant phylogenetic clades (Figure 4). Asterids (more than 80,000 species) and rosids (70,000 species) comprise more than half of the hosts (Figure 4 and Figure 5), as they are the two largest core eudicot clades (>50% of the total angiosperm species diversity) [55,56]. Some hosts’ clades do not comprise as many species as the above two clades, such as the ferns (about 10,500 to 15,000), gymnosperms (only 900 living species), and some angiosperm orders (Ranunculales, Saxifragales, and Santalales have about 2000 species, respectively) [43,56,57,58]. We found these host species have wide ranges and high population densities. So, the parasitism of the hosts seems likely related to the species’ richness and distributions. That is different from Parra-Tabla et al. who studied the host of C. filiformis distributed in the coastal dunes of Yucatan. They claimed the frequency of parasitized plants by C. filiformis was not dependent on host plant abundance [44].
Parasitic plants have been documented repeatedly to play the role of keystone species in the ecosystems [59]. Many parasitic plants also parasitize multiple hosts simultaneously; thus, they may serve as a common network connecting multiple individuals in a plant community [26]. Harmful effects on community dominants, including invasive species, may facilitate species coexistence and thus increase biodiversity [27]. In our experiment, we found C. filiformis may act as a trigger transferring systemic signal to connect M. micrantha (Asteraceae) and C. rutidosperma (Cleomaceae) (Figure 3). Haustorial connections very likely allow the flow or even exchange of various molecules between C. filiformis and hosts, and it has been long known that viruses [60] and phytoplasmas [61] can be transmitted between Cuscuta and its hosts. The study on Cassytha has been relatively neglected, leading to it being less well-characterized compared to its companion Cuscuta [62]. We would like to take this opportunity to encourage researchers to explore the detailed relations of C. filiformis–host associations because understanding the host associations will likely provide an insight into the transition between laurel dodder and their host plants.
5. Conclusions
This study investigated the host range of C. filiformis over major areas of its geographic range in China and reported observations of the germination and parasitism of the selected host species. Cassytha filiformis was found to attach to many different host species, displaying complex parasite–host interactions. The results confirm that C. filiformis grows mainly on diverse woody species with divergent phylogenetic relationships. However, evidence for more complex parasite–host species interactions is unclear, and the physiological basis for these associations requires further study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d15040492/s1, Figure S1. The collection geographical range of Cassytha filiformis and its hosts in China; Figure S2. The percentage of different host life forms parasitized by Cassytha filiformis in China; Table S1. A summary of the hosts of Cassytha filiformis; Table S2. The hosts’ germination rates and parasitizing situation by laurel dodder.
Author Contributions
Conceived and designed the research: Z.-F.L., J.L. and X.-Q.C.; performed the sampling: Z.-F.L., S.-F.Z. and L.-N.D.; conducted the experiments, analyzed the data: Z.-F.L., X.-Y.Z. and X.Z.; wrote the manuscript: Z.-F.L.; contributed to the revision of the manuscript: J.G.C., J.L. and X.-Q.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by 1. the National Natural Science Foundation of China (31370245, 31770569, 31970222); 2. the Natural Science Foundation of Shandong (ZR2022QC214); 3. the Biodiversity Conservation Program of the Chinese Academy of Sciences (ZSSD-013); 4. the Science and Technology Basic Resources Investigation Program of China: Survey and Germplasm Conservation of Plant Species with Extremely Small Populations in South-West China (2017FY100102); 5. the 135 programs of the Chinese Academy of Sciences (2017XTBG-T03).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Nickrent, D.L. Parasitic angiosperms: How often and how many? Taxon 2020, 69, 5–27. [Google Scholar] [CrossRef]
- Twyford, A.D. Parasitic plants. Curr. Biol. 2018, 28, R857–R859. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.; Xie, J.; Zhuang, H.; Liu, H.; Shen, G.; Wu, J. Parasite dodder enables transfer of bidirectional systemic nitrogen signals between host plants. Plant Physiol. 2021, 185, 1395–1410. [Google Scholar] [CrossRef] [PubMed]
- Yoder, J.I.; Scholes, J.D. Host plant resistance to parasitic weeds; recent progress and bottlenecks. Curr. Opin. Plant Biol. 2010, 13, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Irving, L.J.; Cameron, D.D. You are what you eat: Interactions between root parasitic plants and their hosts. Adv. Bot. Res. 2009, 50, 87–138. [Google Scholar]
- Zhang, H.; Florentine, S.; Tennakoon, K.U. The angiosperm stem hemiparasitic genus Cassytha (Lauraceae) and its host interactions: A review. Front. Plant Sci. 2022, 13, 864110. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R.; Moore, P.G.P.; Twyford, A.D. Performance of generalist hemiparasitic Euphrasia across a phylogenetically diverse host spectrum. New Phytol. 2021, 232, 2165–2174. [Google Scholar] [CrossRef]
- Matthies, D. Interactions between a root hemiparasite and 27 different hosts: Growth, biomass allocation and plant architecture. Perspect. Plant Ecol. 2017, 24, 118–137. [Google Scholar] [CrossRef]
- Tesitel, J.; Tesitelova, T.; Fisher, J.P.; Leps, J.; Cameron, D.D. Integrating ecology and physiology of root-hemiparasitic interaction: Interactive effects of abiotic resources shape the interplay between parasitism and autotrophy. New Phytol. 2015, 205, 350–360. [Google Scholar] [CrossRef]
- Bize, P.; Jeanneret, C.; Klopfenstein, A.; Roulin, A. What makes a host profitable? Parasites balance host nutritive resources against immunity. Am. Nat. 2008, 171, 107–118. [Google Scholar] [CrossRef]
- Rowntree, J.K.; Cameron, D.D.; Preziosi, R.F. Genetic variation changes the interactions between the parasitic plant-ecosystem engineer Rhinanthus and its hosts. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Hautier, Y.; Hector, A.; Vojtech, E.; Purves, D.; Turnbull, L.A. Modelling the growth of parasitic plants. J. Ecol. 2010, 98, 857–866. [Google Scholar] [CrossRef]
- Bickford, C.P.; Kolb, T.E.; Geils, B.W. Host physiological condition regulates parasitic plant performance: Arceuthobium vaginatum subsp. cryptopodum on Pinus ponderosa. Oecologia 2005, 146, 179–189. [Google Scholar] [CrossRef]
- Adler, L.S. Alkaloid uptake increases fitness in a hemiparasitic plant via reduced herbivory and increased pollination. Am. Nat. 2000, 156, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Matthies, D. Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: Heterotrophic benefit and parasite-mediated competition. Oikos 1996, 75, 118–124. [Google Scholar] [CrossRef]
- Garnier, E. Growth analysis of congeneric annual and perennial grass species. J. Ecol. 1992, 80, 665–675. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Press, M.C. Linking physiological traits to impacts on community structure and function: The role of root hemiparasitic Orobanchaceae (ex-Scrophulariaceae). J. Ecol. 2005, 93, 67–78. [Google Scholar] [CrossRef]
- Tennakoon, K.U.; Pate, J.S.; Arthur, D. Ecophysiological aspects of the woody root hemiparasite Santalum acuminatum (R Br) A DC and its common hosts in south western Australia. Ann. Bot. 1997, 80, 245–256. [Google Scholar] [CrossRef]
- Le, Q.V.; Tennakoon, K.U.; Metali, F.; Lim, L.B.L.; Bolin, J.F. Ecophysiological responses of mistletoe Dendrophthoe curvata (Loranthaceae) to varying environmental parameters. J. Trop. For. Sci. 2016, 28, 59–67. [Google Scholar]
- Glatzel, G.; Geils, B.W. Mistletoe ecophysiology: Host–parasite interactions. Botany 2009, 87, 10–15. [Google Scholar] [CrossRef]
- Furuhashi, T.; Furuhashi, K.; Weckwerth, W. The parasitic mechanism of the holostemparasitic plant Cuscuta. J. Plant Interact. 2011, 6, 207–219. [Google Scholar] [CrossRef]
- Weber, J. A taxonomic revision of Cassytha (Lauraceae) in Australia. J. Adel. Bot. Gard. 1981, 3, 187–262. [Google Scholar]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Smith, R.S.; Shiel, R.S.; Peacock, S.; Simkin, J.M.; Quirk, H.; Hobbs, P.J. Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature 2006, 439, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, M.J.; Dick, J.T.A.; Dunn, A.M. Diverse effects of parasites in ecosystems: Linking interdependent processes. Front. Ecol. Environ. 2012, 10, 186–194. [Google Scholar] [CrossRef]
- Tesitel, J.; Li, A.R.; Knotkova, K.; McLellan, R.; Bandaranayake, P.C.G.; Watson, D.M. The bright side of parasitic plants: What are they good for? Plant Physiol. 2021, 185, 1309–1324. [Google Scholar] [CrossRef]
- Adamu, A.A.; Garba, F.N.; Ahmed, T.M.; Abubakar, A. Pharmacognostic studies and elemental analysis of Cassytha filiformis Linn. J. Pharmacogn. Phytother. 2017, 9, 131–137. [Google Scholar] [CrossRef]
- Nelson, S.C. Cassytha filiformis. In Plant Disease PD-42; University of Hawaii: Honolulu, HI, USA, 2008; pp. 1–10. [Google Scholar]
- Nickrent, D.L. Plantas parásitas en el mundo. Capitulo 2. In Plantas Parásitas de la Península Ibérica e Islas Baleares, López-Sáez, J.A., Catalán, P., Sáez, L., Eds.; Mundi-Prensa Libros, S.A.: Madrid, Spain, 2002; pp. 7–27. [Google Scholar]
- Werth, C.R.; Pusateri, W.P.; Eshbaugh, W.H.; Wilson, T.K. Field observations on the natural history of Cassytha filiformis L. (Lauraceae) in the Bahamas. In The 2nd International Symposium on Parasitic Weeds; Musselman, L.J., Worsham, A.D., Eplee, R.E., Eds.; North Carolina State University College: Raleigh, NC, USA, 1979; pp. 94–102. [Google Scholar]
- Thorogood, C.J.; Rumsey, F.J.; Hiscock, S.J. Host-specific races in the holoparasitic angiosperm Orobanche minor: Implications for speciation in parasitic plants. Ann. Bot. 2009, 103, 1005–1014. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Facelli, J.M.; Watling, J.R. High water availability increases the negative impact of a native hemiparasite on its non-native host. J. Exp. Bot. 2016, 67, 1567–1575. [Google Scholar] [CrossRef]
- Cirocco, R.M.; Facelli, J.M.; Watling, J.R. Does nitrogen affect the interaction between a native hemiparasite and its native or introduced leguminous hosts? New Phytol. 2017, 213, 812–821. [Google Scholar] [CrossRef]
- Prider, J.; Watling, J.; Facelli, J.M. Impacts of a native parasitic plant on an introduced and a native host species: Implications for the control of an invasive weed. Ann. Bot. 2009, 103, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, K.; Iwase, K.; Furuhashi, T. Role of light and plant hormones in stem parasitic plant (Cuscuta and Cassytha) twining and haustoria induction. Photochem. Photobiol. 2021, 97, 1054–1062. [Google Scholar] [CrossRef]
- Li, H.W.; Li, J.; Huang, P.H.; Wei, F.N.; Cui, H.B.; van der Werff, H. Lauraceae. In Flora of China, Calycanthaceae–Schisandraceae; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MI, USA, 2008; Volume 7, pp. 102–254. [Google Scholar]
- Li, Q.; Yao, D.; Cai, J.; Huang, L. A field survey of Cassytha filiformis host range. Guangxi Plant Prot. 1992, 4, 21–24. [Google Scholar]
- Huang, Z.J. Some observations on the host of Cassytha filiformis and the exploration of its harmfulness. J. Sci. Res. Repert. Guangxi Agric. Coll. 1957, 54–60. [Google Scholar]
- Shao, S.Z. Records of the detriment of Cassytha filiformis on Pinus massoniana. For. Pest Commun. 1988, 4, 3. [Google Scholar]
- Mahadevan, N.; Jayasuriya, K. Water-impermeable fruits of the parasitic angiosperm Cassytha filiformis (Lauraceae): Confirmation of physical dormancy in Magnoliidae and evolutionary considerations. Aust. J. Bot. 2013, 61, 322–329. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qian, H.V. PhyloMaker2: An updated and enlarged R package that can generate very large phylogenies for vascular plants. Plant Divers. 2022, 44, 335–339. [Google Scholar] [CrossRef]
- The Angiosperm Phylogeny Group. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 12385. [Google Scholar] [CrossRef]
- Parra-Tabla, V.; Tun-Garrido, J.; García-Franco, J.; Martínez, M. The recent expansion of the invasive hemiparasitic plant Cassytha filiformis and the reciprocal effect with its main hosts. Res. Square 2023. [Google Scholar] [CrossRef]
- Buriyo, A.S.; Kasuga, L.; Moshi, H.N.; Nene, W.A. Ecological distribution and abundance of the parasitic weed, Cassytha filiformis L. (Lauraceae) in major cashew, Anacardium occidentale L. growing regions in Tanzania. Int. J. Basic Appl. Sci. 2015, 5, 109–116. [Google Scholar]
- Zhang, C.; Ma, H.; Sanchez-Puerta, M.V.; Li, L.; Xiao, J.; Liu, Z.; Ci, X.; Li, J. Horizontal gene transfer has impacted cox1 gene evolution in Cassytha filiformis. J. Mol. Evol. 2020, 88, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Rajanna, L.; Shivamurthy, G.R. Occurrence of graniferous tracheary elements in the haustorium of Cassytha filiformis Linn., a stem parasite of Lauraceae. Taiwania 2001, 46, 40–48. [Google Scholar]
- Kokubugata, G.; Nakamura, K.; Forster, P.I.; Wilson, G.W.; Holland, A.E.; Hirayama, Y.; Yokota, M. Cassytha pubescens and C. glabella (Lauraceae) are not disjunctly distributed between Australia and the Ryukyu Archipelago of Japan—Evidence from morphological and molecular data. Aust. Syst. Bot. 2012, 25, 364–373. [Google Scholar] [CrossRef]
- Costea, M.; ElMiari, H.; Farag, R.; Fleet, C.; Stefanović, S. Cuscuta sect. Californicae (Convolvulaceae) revisited:‘cryptic’ speciation and host range differentiation. Syst. Bot. 2020, 45, 638–651. [Google Scholar] [CrossRef]
- Facelli, E.; Wynn, N.; Tsang, H.T.; Watling, J.R.; Facelli, J.M. Defence responses of native and invasive plants to the native generalist vine parasite Cassytha pubescens—Anatomical and functional studies. Aust. J. Bot. 2020, 68, 300. [Google Scholar] [CrossRef]
- Kelly, C.K. Resource choice in Cuscuta europaea. Proc. Natl. Acad. Sci. USA 1992, 89, 12194–12197. [Google Scholar] [CrossRef]
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Plant defenses against parasitic plants show similarities to those induced by herbivores and pathogens. Plant Signal. Behav. 2010, 5, 929–931. [Google Scholar] [CrossRef]
- Machado, M.A.; Zetsche, K. A structural, functional and molecular analysis of plastids of the holoparasites Cuscuta reflexa and Cuscuta europaea. Planta 1990, 181, 91–96. [Google Scholar] [CrossRef]
- Liu, Z.-F.; Ma, H.; Ci, X.-Q.; Li, L.; Song, Y.; Liu, B.; Li, H.-W.; Wang, S.-L.; Qu, X.-J.; Hu, J.-L.; et al. Can plastid genome sequencing be used for species identification in Lauraceae? Bot. J. Linn. Soc. 2021, 197, 1–14. [Google Scholar] [CrossRef]
- Schonenberger, J.; von Balthazar, M. Asterids. Bot. J. Linn. Soc. 2013, 173, 321–324. [Google Scholar] [CrossRef]
- Fay, M.F. Rosids. Bot. J. Linn. Soc. 2013, 172, 399–403. [Google Scholar] [CrossRef]
- Soltis, D.E.; Mort, M.E.; Latvis, M.; Mavrodiev, E.V.; O’Meara, B.C.; Soltis, P.S.; Burleigh, J.G.; Rubio de Casas, R. Phylogenetic relationships and character evolution analysis of Saxifragales using a supermatrix approach. Am. J. Bot. 2013, 100, 916–929. [Google Scholar] [CrossRef]
- Su, H.-J.; Hu, J.-M.; Anderson, F.E.; Der, J.P.; Nickrent, D.L. Phylogenetic relationships of Santalales with insights into the origins of holoparasitic Balanophoraceae. Taxon 2015, 64, 491–506. [Google Scholar] [CrossRef]
- Tesitel, J.; Fibich, P.; de Bello, F.; Chytry, M.; Leps, J. Habitats and ecological niches of root-hemiparasitic plants: An assessment based on a large database of vegetation plots. Preslia 2015, 87, 87–108. [Google Scholar]
- Birschwilks, M.; Haupt, S.; Hofius, D.; Neumann, S. Transfer of phloem-mobile substances from the host plants to the holoparasite Cuscuta sp. J. Exp. Bot. 2006, 57, 911–921. [Google Scholar] [CrossRef]
- Kaminska, M.; Korbin, M. Graft and dodder transmission of phytoplasma affecting lily to experimental hosts. Acta Physiol. Plant. 1999, 21, 21–26. [Google Scholar] [CrossRef]
- Lanini, W.T.; Kogan, M. Biology and management of Cuscuta in crops. Cienc. Investig. Agrar. 2005, 32, 127–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).