Abstract
Phlebotomine sand flies are vectors of several diseases of importance for public health, including leishmaniosis, bartonellosis, and sand fly fevers. An entomological survey on blood-feeding Diptera was conducted in June–November 2020–2021 to know the diversity of insect vectors in Mallorca (Balearic Islands, Spain). Among the vectors collected, Phlebotomus (Larroussius) perfiliewi Parrot, 1930 was found being the first record of this species in Spain. Phlebotomus perfiliewi s.l. is one of the main vectors of Leishmania infantum in the Mediterranean Basin and Central Asia. The identification of this species was confirmed by both morphological features and DNA barcoding. Phylogenetic analyses showed that the specimens captured were Ph. perfiliewi s.s. (99.85–100% homologues from Italy and Algeria specimens), with a sequence divergence of 0.17%. The cytochrome c oxidase subunit I gene clearly separates the three species that make up the Ph. perfiliewi species complex. In addition, we also provide a brief discussion about their identification remarks, phylogenetic relationships, and vector status.
1. Introduction
Phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae) are blood-feeding insects of major medical–veterinary significance as they are vectors of a large number of pathogens to animals and humans, including protozoa, bacteria, and viruses [1]. The parasitic protozoan Leishmania sp. (Kinetoplastida: Trypanosomatidae) is responsible for two clinical major forms, cutaneous leishmaniosis and visceral leishmaniosis, which are endemic in several countries of the European Union, including the Iberian Peninsula, as well as the Balearic Islands [2]. In Europe, the geographic distribution of sand flies increased considerably in the last years, spreading into new areas [3] and causing progressively more autochthonous outbreaks of phlebotomine-borne diseases [4]. Therefore, studies on sand flies are increasing attention along most European countries.
Twenty-two species of sand flies were described in Europe [5], thirteen of them are present in Spain [6], and five in the Balearic Islands [7,8] [Phlebotomus perniciosus Newstead, 1911; Phlebotomus sergenti (Parrot, 1917); Phlebotomus papatasi (Scopoli, 1786); Sergentomyia minuta (Rondani, 1843); and Phlebotomus ariasi (Tonnoir, 1921)]. Based on their geographical distribution and abundance, Ph. perniciosus and S. minuta are the most widespread and abundant species in the Spanish territory [6]. Regarding their vector status, Ph. ariasi, Phlebotomus langeroni Nitzulescu, 1930, Phlebotomus mascittii Grassi, 1908, and primarily Ph. perniciosus are highlighted to be proven or suspected vectors of Leishmania infantum Nicolle, 1908 in Spain [6]. However, other sand fly species were also highlighted in other European countries to be involved in the transmission of L. infantum, such as Ph. tobbii Adler, Theodor and Lourie, 1930 and Ph. neglectus Tonnoir, 1921, as well as Phlebotomus papatasi (Scolopi, 1976) and Phlebotomus perfiliewi s.l. Parrot, 1930, which are also vectors of other Phlebovirus. The latter two species were pointed out to be changing their distribution range [3].
The taxonomic status of Ph. perfiliewi s.l. still remains undefined and some discrepancies were reported between morphological analysis and molecular markers [9]. Overall, at least three specific or subspecific value names are proposed into the Ph. perfiliewi species complex, depending on the authors: Phebotomus perfiliewi s.s. Parrot, 1930, Phlebotomus galilaeus Theodor, 1958, and Phlebotomus transcaucasicus Perfiliev, 1937, the former being the taxa that occurs in western Mediterranean regions.
The current study provides the finding of a new phlebotomine record from Spain, and in particular in Mallorca, the largest island of the Balearic Islands.
2. Materials and Methods
As part of a project focused on the collection of blood-feeding arthropods, a multi-trapping entomological survey was conducted in eight farms (Formatges Burguera: 39.366579 and 3.02172891, 3 masl; Son Ajaume nou: 39.6448596 and 2.65217317, 89 masl; Can Cosme: 39.5222862 and 3.10583271, 80 masl; Son Simó: 39.8173189 and 3.05957789, 14 masl; Ranxo Ses Roques: 39.8331397 and 3.10518693, 3 masl; Centre Hipic Son Reus: 39.6377295 and 2.66639607, 76 masl; Sa Teulera: 39.5840583, 3.1387411, 69 masl; and Son Feliu: 39.5300347 and 3.0338470, 142 masl) in the island of Mallorca (Spain), between June and November 2020–2021. Two types of downdraft traps were located close to animal barns (composed mostly of domestic animals such as pigs, rabbits, cows, sheep, equines, and dogs) in each of the sampling sites. The first type was hand-made traps equipped with incandescent light (12 V, 0.3 amps) as attractant. The second type was mini CDC traps (6 V, model 512; Bioquip, Compton, CA, USA) with CO2 as bait. Both traps operated on a weekly basis with batteries and specimens were retained in collection cups with fine mesh in the bottom to prevent from escaping. A subsample of the total 520 sand fly female collections (ca. 35%) and all males (n = 841) were analyzed from the eight sampling sites. Head and terminal segments of the abdomen of each female and male terminalia were dissected and mounted on a microscope slide with Hoyer’s medium. The rest of the body was retained for molecular characterization. Morphological identification was based on features of the male and genitalia, and pharyngeal armature of females, following the available phlebotomine sand fly keys [10,11]. Diagnostic features of the specimens were photographed and measured (mean ± SD) under a compound microscope (Carl Zeiss 37081, Jena, Germany, 40× magnification) coupled with a camera (AxioCam ICc 1), and the images were processed by ZEN 2.3 lite software.
Four males and four females showing morphological features compatible with Ph. perfiliewi s.l. were selected for molecular characterization of the barcoding region. The DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) was used for genomic DNA extraction of sand flies following the manufacturer’s instructions. Reactions were performed using the universal DNA primers for polymerase chain reaction (PCR) amplification of a 658-bp fragment of the mitochondrial cytochrome c oxidase subunit I (COI) gene: LCO1490: 5′-GGTCAACAAATCATAAAGATATTGG-3′ and HCO2198: 5′-TAAACTTCAGGGTGACCAAAAAATCA-3′ [12] following thermocycling conditions, agarose gel electrophoresis, and PCR product purification methods described by Ruiz-Arrondo et al. [13]. In addition, the four Ph. perfiliewi s.l. female specimens previously selected were also screened for Leishmania sp. parasites following the procedures described elsewhere [14] with few modifications. Amplicons of COI were sequenced in both directions in the Genomics Platform of the CIBIR (La Rioja, Spain). The sequences were edited using Bioedit software 7.2 and compared with sequences previously deposited in GenBank.
Phylogenetic analyses were performed by constructing multiple alignments of nucleotide sequences including eight high-quality amplicon-length sequences with 658 pb, together with individuals of Ph. perfiliewi s.l. (n = 19) from other countries retrieved from GenBank. These countries were selected to hold the three members of the Ph. perfiliewi species complex: Ph. perfiliewi s.s., Ph. galilaleus, and Ph. transcaucasicus. In addition, one specimen of Ph. perniciosus collected in the present study was included as the outgroup. These analyses were constructed using MAFFT vs. 7 (https://mafft.cbrc.jp/alignment/server/, accessed on 15 January 2023) and subsequently edited with GBlocks (http://molevol.cmima.csic.es/castresana/Gblocks_server.html, accessed on 10 January 2023). The phylogenetic tree was built using the maximum likelihood (ML) method in IQ-tree v.2.2.0 (http://www.iqtree.org/, accessed on 10 January 2023). The best-fitting evolutionary model was TPM2u + F + I. Intraspecific and interspecific genetic divergences were calculated based on the Tamura–Nei model in MEGA X [15]. Sequence similarity searches were carried out through the Barcode of Life Data System (https://www.boldsystems.org/index.php/IDS_OpenIdEngine, accessed on 10 December 2022) and BLASTn (MegaBlast option; https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 10 December 2022). Detailed specimen records and sequence information of sand flies were submitted to the GenBank public database under the following accession numbers: OP824886-OP824894.
3. Results and Discussion
Thirty-seven specimens of Ph. perfiliewi s.l. (12 females and 25 males) captured by suction traps, equipped either with light (n = 17) or with CO2 (n = 20), were recorded in two rural farms (Sa Teulera and Son Ajaume nou) of the island of Mallorca (Balearic Islands) from mid-June to mid-July 2021. The analysis showed that only 4.8% (n = 25) of the total sand fly male collections corresponded with Ph. perfiliewi s.l. from Sa Teulera (n = 24) and Son Ajaume nou (n = 1), respectively, and all the females of Ph. perfiliewi s.l. were derived from Sa Teulera (n = 12). So far, Ph. perfiliewi s.l. is distributed in the Mediterranean Basin (from coast of France and Corsica to eastwards of Turkey and Ukraine) and North Africa [16]. Therefore, the present finding represents the most westerly distribution of this species in Europe, increasing the number of known phlebotomine sand fly species in Spain to fourteen, and in the Balearic Archipelago to six.
Among the sand fly species cited in Europe, female Ph. perfiliewi s.l. can be misidentified with Ph. perniciosus, as both species share common morphological features. However, various characters were proposed to separate both species attending to morphology. For instance, females can be differentiated by the length and teeth of the pharyngeal armature and features of the spermatheca (number of body segments, shape of the neck, and size of bulges and ducts). In contrast, males are separated based on both the shape and colour of the terminalia of the aedeagus [10,11].
The morphological analysis of the sand fly females captured in our study indicated that the pharyngeal armature can be used as a reliable feature to discriminate both species (Ph. perniciosus and Ph. perfiliewi s.l.). In Ph. perniciosus the pharyngeal armature usually occupies more than a quarter length of pharynx and teeth are arranged disorderly (Figure 1A), whereas in Ph. perfiliewi s.l. the pharyngeal armature is smaller, and teeth end anteriorly in a clear line of demarcation (Figure 1B). We found that the number of segments of the body of the spermatheca is not a reliable morphological feature to separate both sand fly species, as there is an overlap ranging from 9 to 12 in Ph. perniciosus (n = 10 specimens examined from our study) and from 11 to 13 (n = 10 specimens examined) in Ph. perfiliewi s.l. (Figure 1C,D). This remark was also noted by other authors [11]. The neck of the spermatheca is longer (ca. 3/4 of the body of the spermatheca), more prominent, and thicker in Ph. perfiliewi s.l. (spermatheca body: 48.3 ± 3.4 µm; neck: ca. 37.8 ± 1.9 µm length and 3.0 ± 0.2 µm wide) than in Ph. perniciosus (spermatheca body: 40.6 ± 3.0 µm; neck: 24.6 ± 3.6 µm length and 2.1 ± 0.1 µm wide), which usually measures close to 2/4 the length of the spermatheca body. Bending next to the base of the neck is generally more apparent in Ph. perniciosus than in Ph. perfiliewi (Figure 1(E1,E2,F1,F2)). Ducts are not longer than three times (usually shorter) the length of a spermathecal body in Ph. perniciosus and about four times in Ph. perfiliewi s.l. Males can be easily separated by the terminalia of the aedeagus (Figure 2). The distal region of the aedeagus of Ph. perniciosus is bifid (fork-shaped, usually both ends are asymmetric) (Figure 2A–C), whereas in Ph. perfiliewi s.l., the aedeagus extremity is rounded (oar-shaped, usually having minuscules subapical denticles) (Figure 2B–D). The distal region of the aedeagus has a conspicuous colorless membrane (translucent) in Ph. perfiliewi s.l., whereas the aedeagus in Ph. perniciosus is completely dark or the subdistal region is lighter. The measures of the Ph. perfiliewi aedeagus (length: 141.0 ± 5.2 µm; thickness: 13.2 ± 1.4 µm; transparent part: 41.1 ± 3.4 µm) were similar to those reported by Depaquit [9], but overall, slightly higher.
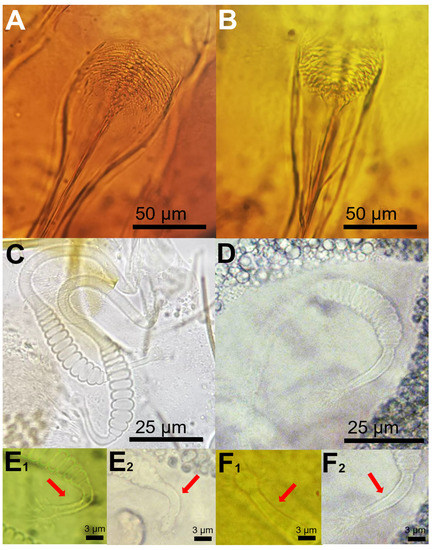
Figure 1.
Morphometric characters used to discriminate females of Ph. perniciosus (A,C,E) and Ph. perfiliewi s.l. (B,D,F). (A,B) = pharynx) and (C,D) = spermatheca and ((E1,E2) and (F1,F2)) = two different examples of spermatheca necks (arrows).
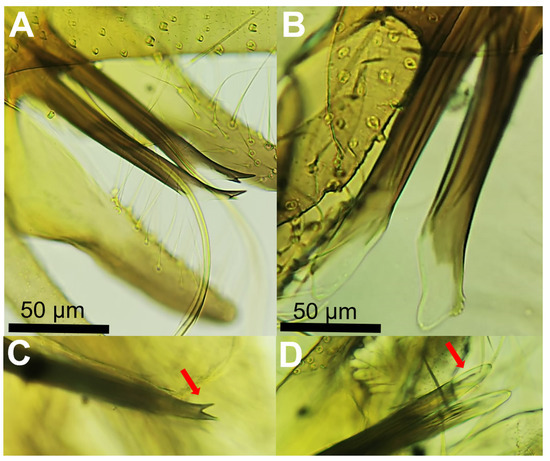
Figure 2.
Aedeagus (male genitalia) of Ph. perniciosus (A,C) and Ph. perfiliewi s.l. (B,D). Detail of the aedeagus tips (arrows).
The sand flies studied here were confirmed molecularly as Ph. perfiliewi s.l., showing 99.85–100% identity (query cover of 100%) with homologues from Italy (accession number: KY646194) and Algeria (KJ481177) (Figure 3). The collected Ph. perfiliewi s.l. specimens clustered together with the sequences of Ph. perfiliewi s.l. from Italy and Algeria and one individual from Jordan.

Figure 3.
Maximum likelihood phylogenetic tree based on COI sequences in Ph. perfiliewi s.l. At specific branches, the first and second values separated by “/” indicate the topological branch support for the ML analysis (aLRT/bootstrap), with values > 75% defining high stability. Red colour = cluster 1, Ph. galilaeus; yellow colour = cluster 2, Ph. transcaucasicus; and violet colour = cluster 3, Ph. perfiliewi s.s.
Individuals of Ph. perfiliewi s.l. from other countries (Turkey, Azerbaijan, Greece, Israel, and Jordan) were grouped in a different clade, which is further divided into two subclades (Figure 3). The ML tree showed that Ph. perfiliewi s.l. sequences grouped in three clades, each clade consisting of one of the three species forming the Ph. perfiliewi species complex. Our results are in agreement with those reported by other authors based on morphological features and both Cytochrome b (Cyt b) and internal transcribed ribosomal spacer 2 (ITS2) genes [9,17,18]. The sequences from Spain, Italy, Algeria, and one from Jordan correspond to Ph. perfiliewi s.s., which is a species well-distributed from North Africa to Crimea, including the western Mediterranean. Thus, phylogenetic analyses based on the COI gene showed congruent results on the known geographic distribution of the three species of the Ph. perfiliewi s.l. It is interesting to note that Depaquit et al. [9] observed that Ph. perfiliewi s.s. was closer to Ph. galilaeus than Ph. transcaucasicus based on the Cyt b gene, which slightly differs from our ML-tree results, where Ph. transcaucasicus was closer to Ph. galilaeus than Ph. perfiliewi s.s. In contrast, the above-mentioned authors obtained incongruent phylogenetic hypotheses based on the Cyt b and ITS2 genes [9]. A priori, the COI gene could offer a clearer resolution than the two aforementioned genetic markers, but a greater number of sequences from more diverse origins would be needed for further conclusions.
In our study, the sequence divergence was 0.17% for Spanish Ph. perfliewi s.s. and 2.9% for the other Ph. perfiliewi s.l. sequences retrieved from GenBank. When the sequences of the individuals were separated according to their geographical origin in the three species of the Ph. perfiliewi species s.l., the intraspecific divergence was 0.18% for Ph. perfiliewi s.s., 1.6% for Ph. galilaeus, and 1.4% for Ph. transcaucasicus (Table 1). The interspecific divergence varied between 3.7% and 4.5% among the three species of the Ph. perfiliewi s.l. and was higher than 7.0% when compared with Ph. perniciosus (Table 1).

Table 1.
Interspecific (between groups) pairwise Tamura–Nei model genetic divergence based on COI gene in the three Ph. perfiliewi species of the Perfiliewi complex and Ph. perniciosus.
In Europe, the main species involved in the transmission of leishmaniosis disease are Ph. perniciosus, Ph. ariasi, Ph. papatasi, and Ph. perfiliewi s.l. [19]. Phebotomus perfiliewi s.l. is considered a vector with an important role in the transmission dynamics of L. infantum in the Mediterranean Basin and in Central Asia [16]. Its vector role for L. infantum was recorded several times, particularly in Italy and Iran, but also as a proven or suspected vector of visceral leishmaniosis in Albania, Algeria, Croatia, Greece, Israel, Malta, Morocco, Palestine, Republic of Macedonia, Romania, Tunisia, Turkey, and Hungary [1,16,20,21,22,23,24]. In our study, the four specimens of Ph. perfiliewi s.l. individually analyzed for Leishmania parasites resulted negative, and therefore, its vector role in the Balearic Islands needs further research.
In countries such as Italy, Phlebotomus perfiliewi s.s. was overcome in abundance to Ph. perniciosus, particularly in rural areas along the central, south, and north Italy, where it plays a major role in the leishmaniosis sylvatic cycle, with wildlife acting as reservoirs rather than dogs [25,26,27]. In addition, Ph. perfiliewi s.s. was also related to the potential transmission of the Toscana virus (TOSV) and other phleboviruses [28,29], as well as Trypanosoma theileri Laveran, 1902 in 2016 in Italy [30]. Other species of the Perfiliewi complex, such as Ph. transcaucasicus, are also dominant in Iran, where it is a proven vector of L. infantum and Leishmania donovani Laveran and Mensil, 1903 [22,31,32].
In the Balearic Islands, leishmaniosis is considered a regionally endemic disease, with an overall rate of human leishmaniosis of ca. 0.7–3.5 cases per year/100,000 inhabitants, and the prevalence of canine leishmaniosis can reach 45% in some areas of the island [7,33]. The collections of Ph. perfiliewi s.s. obtained in the current study were in June–July, which is considered the peak of abundance in several Mediterranean countries [34].
4. Conclusions
The combination of identification by morphology and DNA barcoding is of great value in epidemiological studies, as it provides accurate species identification to separate Ph. perfiliewi s.l. from Ph. perniciosus, as well as to differentiates within the members of the Perfiliewi complex. Phlebotomus perfiliewi s.s. is cited here for the first time in Spain and the Balearic Islands, being the most westerly citation of this species in the Mediterranean Basin. This species was associated with farms and was captured either in light or in CO2 traps. Its epidemiological role remains unknown due to the low number of specimens analyzed in this study for the presence of L. infantum; however, based on its proven role in the transmission of Leishmania parasites and arboviruses in the Mediterranean Basin, the risk of leishmaniosis transmission exists all over the island.
Further entomological surveys should be conducted to identify the presence of this species in mainland Spain due to its potential role as a vector of diseases with interest for animal and public health. The authors suggest rechecking the material of sand flies previously collected through the Mediterranean coastline of Spain, as it might be possible that female specimens were misidentified with Ph. perniciosus due to their closely morphological features. In addition, it would be interesting to deepen whether this species only occurs in restricted areas located in rural areas of Mallorca, or perhaps it was recently introduced.
Author Contributions
Conceptualization, M.A.G., M.Á.M. and I.R.-A.; methodology and formal analysis, C.B. and R.G.-L.; writing—original draft, M.A.G. and I.R.-A.; writing—review, editing and visualization M.A.G., M.Á.M., I.R.-A., C.B. and R.G.-L.; project administration, funding acquisition and supervision, M.Á.M., R.G.-L. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Agriculture, Fisheries and Food council through the project num. BIA08/20-2 from the agricultural and fishing guarantee fund (FOGAIBA) of the Government of the Balearic Islands. Samplings were supported by own resources of ZAP-UIB and FOGAIBA. R.G.L. was initially supported by a Margalida Comas contract from the Government of the Balearic Islands and from the European Regional Development Fund (FEDER) (REF- PD/038/2019) and he is currently funded by a Juan de la Cierva 2019 Formación contract (FJC2019-041291-I) from the Ministry of Science and Innovation.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the farm owners for letting set the traps and the sampling procedures. We are also grateful to Marc Oliva Gadea, biology degree student from the Autonomous University of Madrid (UAM) for his support in mounting sand flies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of Public Health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Herrador, Z.; Gherasim, A.; Jimenez, B.C.; Granados, M.; San Martín, J.V.; Aparicio, P. Epidemiological changes in leishmaniasis in Spain according to hospitalization-based records, 1997–2011: Raising awareness towards leishmaniasis in non-HIV patients. PLoS Negl. Trop. Dis. 2015, 9, e0003594. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Hansford, K.M.; Van Bortel, W.; Zeller, H.; Alten, B. A Summary of the evidence for the change in European distribution of Phlebotomine sand flies (Diptera: Psychodidae) of Public Health importance. J. Vector Ecol. 2014, 39, 72–77. [Google Scholar] [CrossRef]
- González, E.; Jiménez, M.; Hernández, S.; Martín-Martín, I.; Molina, R. Phlebotomine sand fly survey in the focus of Leishmaniasis in Madrid, Spain (2012–2014): Seasonal dynamics, Leishmania Infantum infection rates and blood meal preferences. Parasites Vectors 2017, 10, 368. [Google Scholar] [CrossRef]
- Wagner, R.; Djong, H. Fauna Europaea: Psychodidae. In Fauna Europaea: Nematocera; De Jong, H., Ed.; version 1.3; Museum für Naturkunde Leibniz-Institut für Evolutions und Biodiversitätsforschung: Berlin, Germany, 2004; Available online: http://www.faunaeur.org (accessed on 15 November 2022).
- Bravo-Barriga, D.; Ruiz-Arrondo, I.; Peña, R.E.; Lucientes, J.; Delacour-Estrella, S. Phlebotomine sand flies (Diptera, Psychodidae) from Spain: An updated checklist and extended distributions. Zookeys 2022, 1106, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Alcover, M.M.; Ballart, C.; Martín-Sánchez, J.; Serra, T.; Castillejo, S.; Portús, M.; Gállego, M. Factors influencing the presence of sand flies in Majorca (Balearic Islands, Spain) with special reference to Phlebotomus pernicious, vector of Leishmania Infantum. Parasites Vectors 2014, 7, 421. [Google Scholar] [CrossRef]
- Gil Collado, J.; Morillas-Marquez, F.; Sanchis Marin, M.C. Los flebotomos de España. Rev. Sanid. Hig. Publica 1989, 63, 15–34. [Google Scholar]
- Depaquit, J.Ô.; Bounamous, A.; Akhoundi, M.; Augot, D.; Sauvage, F.; Dvorak, V.; Chaibullinova, A.; Pesson, B.; Volf, P.; Léger, N. A Taxonomic study of Phlebotomus (Larroussius) perfiliewi s.L. Infect. Genet. Evol. 2013, 20, 500–508. [Google Scholar] [CrossRef]
- Gatt, P.; Williams, J.; Mifsud, D. New distributional data on sandflies from rubble walls in the Maltese Islands with an illustrated key to the Maltese species (Diptera: Phlebotominae). Bull. Entomol. Soc. Malta. 2009, 2, 95–110. [Google Scholar]
- Dantas-Torres, F.; Tarallo, V.D.; Otranto, D. Morphological keys for the identification of Italian phlebotomine sand flies (Diptera: Psychodidae: Phlebotominae). Parasites Vectors 2014, 7, 479. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for amplification of mitochondrial Cytochrome c Oxidase Subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Ruiz-Arrondo, I.; Hernández-Triana, L.M.; Ignjatović-Ćupina, A.; Nikolova, N.; Garza-Hernández, J.A.; Rodríguez-Pérez, M.A.; Oteo, J.A.; Fooks, A.R.; Lucientes Curdi, J. DNA barcoding of blackflies (Diptera: Simuliidae) as a tool for species identification and detection of hidden diversity in the eastern regions of Spain. Parasites Vectors 2018, 11, 463. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, L.; Prina, E.; Lang, T.; Milon, G. Real-Time PCR for detection and quantitation of Leishmania in mouse tissues. J. Clin. Microbiol. 2002, 40, 1666–1669. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- ECDC (European Centre for Disease Prevention and Control). Phlebotomus perfiliewi—Current Known Distribution. March 2022. Available online: https://www.ecdc.europa.eu/en/publications-data/phlebotomus-perfiliewi-current-known-distribution-march-2022 (accessed on 15 November 2022).
- Artemiev, M.M.; Neronov, V.M. Distribution and Ecology of Sandflies of the Old World (Genus Phlebotomus); Institut Ėvolyutsionnoĭ Morfologii i Ėkologii: Moscow, Russia, 1984; p. 207. [Google Scholar]
- Lewis, D.J. A Taxonomic Review of the Genus Phlebotomus (Diptera: Psychodidae); British Museum (Natural History): London, UK, 1982; pp. 121–209. [Google Scholar]
- García San Miguel, L.; Sierra, M.J.; Vazquez, A.; Fernandez-Martínez, B.; Molina, R.; Sanchez-Seco, M.P.; Lucientes, J.; Figuerola, J.; de Ory, F.; Monge, S.; et al. Phlebovirus-associated diseases transmitted by phlebotominae in Spain: Are we at risk? Enferm. Infecc. Microbiol. Clin. 2021, 39, 345–351. [Google Scholar] [CrossRef]
- Duport, M.; Lupaşcu, G.H.; Cristescu, A. Contribution à l’étude des phlébotomes des biotopes naturels de Roumanie. Arch. Roum. Path. Exp. Microbiol. 1971, 30, 387–398. [Google Scholar]
- Izri, M.A.; Belazzoug, S. Phlebotomus (Larroussius) perfiliewi naturally infected with dermotropic Leishmania infantum at Tenes, Algeria. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 399. [Google Scholar] [CrossRef]
- Oshaghi, M.A.; Ravasan, N.M.; Hide, M.; Javadian, E.A.; Rassi, Y.; Sadraei, J.; Mohebali, M.; Mehdi Sedaghat, M.; Hajjaran, H.; Zarei, Z.; et al. Phlebotomus perfiliewi transcaucasicus is circulating both Leishmania donovani and L. infantum in northwest Iran. Exp. Parasitol. 2009, 123, 218–225. [Google Scholar] [CrossRef]
- Zivkovic, V. Recherches récentes sur les phlébotomes (Diptera, Psychodidae) dans un foyer endémique de leishmaniose viscérale en Serbie (Yougoslavie). Acta Parasitol. Lugoslavica 1975, 6, 37–43. [Google Scholar]
- Farkas, R.; Tánczos, B.; Bongiorno, G.; Maroli, M.; Dereure, J.; Ready, P.D. First surveys to investigate the presence of canine leishmaniasis and its phlebotomine vectors in Hungary. Vector-Borne Zoonotic Dis. 2011, 11, 823–834. [Google Scholar] [CrossRef]
- Calzolari, M.; Carra, E.; Rugna, G.; Bonilauri, P.; Bergamini, F.; Bellini, R.; Varani, S.; Dottori, M. Isolation and molecular typing of Leishmania Infantum from Phlebotomus perfiliewi in a reemerging focus of leishmaniasis, Northeastern Italy. Microorganisms 2019, 7, 644. [Google Scholar] [CrossRef]
- Millán, J.; Ferroglio, E.; Solano-Gallego, L. Role of wildlife in the epidemiology of Leishmania infantum infection in Europe. Parasitol. Res. 2014, 113, 2005–2014. [Google Scholar] [CrossRef]
- Maresca, C.; Scoccia, E.; Barizzone, F.; Catalano, A.; Mancini, S.; Pagliacci, T.; Porrini, M.; Principato, M.; Venditti, G.; Grelloni, V. A survey on canine leishmaniasis and phlebotomine sand flies in Central Italy. Res. Vet. Sci. 2009, 87, 36–38. [Google Scholar] [CrossRef]
- Calzolari, M.; Angelini, P.; Finarelli, A.C.; Cagarelli, R.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Tamba, M.; Dottori, M.; et al. Human and entomological surveillance of Toscana virus in the Emilia-Romagna region, Italy, 2010 to 2012. Eurosurveillance 2014, 19, 20978. [Google Scholar] [CrossRef]
- Calzolari, M.; Chiapponi, C.; Bellini, R.; Bonilauri, P.; Lelli, D.; Moreno, A.; Barbieri, I.; Pongolini, S.; Lavazza, A.; Dottori, M. Isolation of three novel reassortant phleboviruses, Ponticelli I, II, III, and of Toscana virus from field-collected sand flies in Italy. Parasites Vectors 2018, 11, 84. [Google Scholar] [CrossRef]
- Calzolari, M.; Rugna, G.; Clementi, E.; Carra, E.; Pinna, M.; Bergamini, F.; Fabbi, M.; Dottori, M.; Sacchi, L.; Votýpka, J. Isolation of a trypanosome related to Trypanosoma theileri (Kinetoplastea: Trypanosomatidae) from Phlebotomus perfiliewi (Diptera: Psychodidae). Biomed Res. Int. 2018, 2018, 2597074. [Google Scholar] [CrossRef]
- Rassi, Y.; Javadian, E.; Nadim, A.; Rafizadeh, S.; Zahraii, A.; Azizi, K.; Mohebali, M. Phlebotomus perfiliewi transcaucasicus, a vector of Leishmania infantum in Northwestern Iran. J. Med. Entomol. 2009, 46, 1094–1098. [Google Scholar] [CrossRef]
- Rassi, Y.; Sanei Dehkordi, A.; Oshaghi, M.A.; Abai, M.R.; Mohtarami, F.; Enayati, A.; Zarei, Z.; Javadian, E. First Report on Natural Infection of the Phlebotomus tobbi by Leishmania infantum in Northwestern Iran. Exp. Parasitol. 2012, 131, 344–349. [Google Scholar] [CrossRef]
- Epidemiología. Informes y Estadísticas de La Red de Vigilancia Epidemiológica de Les Illes Balears. February 2022. Available online: https://www.caib.es/sites/epidemiologia/es/informes_anuales-11681/ (accessed on 10 December 2022).
- Cazan, C.D.; Păstrav, I.R.; Györke, A.; Oguz, G.; Alten, B.; Mihalca, A.D. Seasonal dynamics of a population of Phlebotomus (Larroussius) perfiliewi Parrot, 1930 (Diptera: Psychodidae) in North-Eastern Romania. Parasitol. Res. 2019, 118, 1371–1384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).