Global Diversity, Distribution, and Genetic Studies of Stable Flies (Stomoxys sp.)
Abstract
1. Introduction
2. Diversity and Ecological Remarks
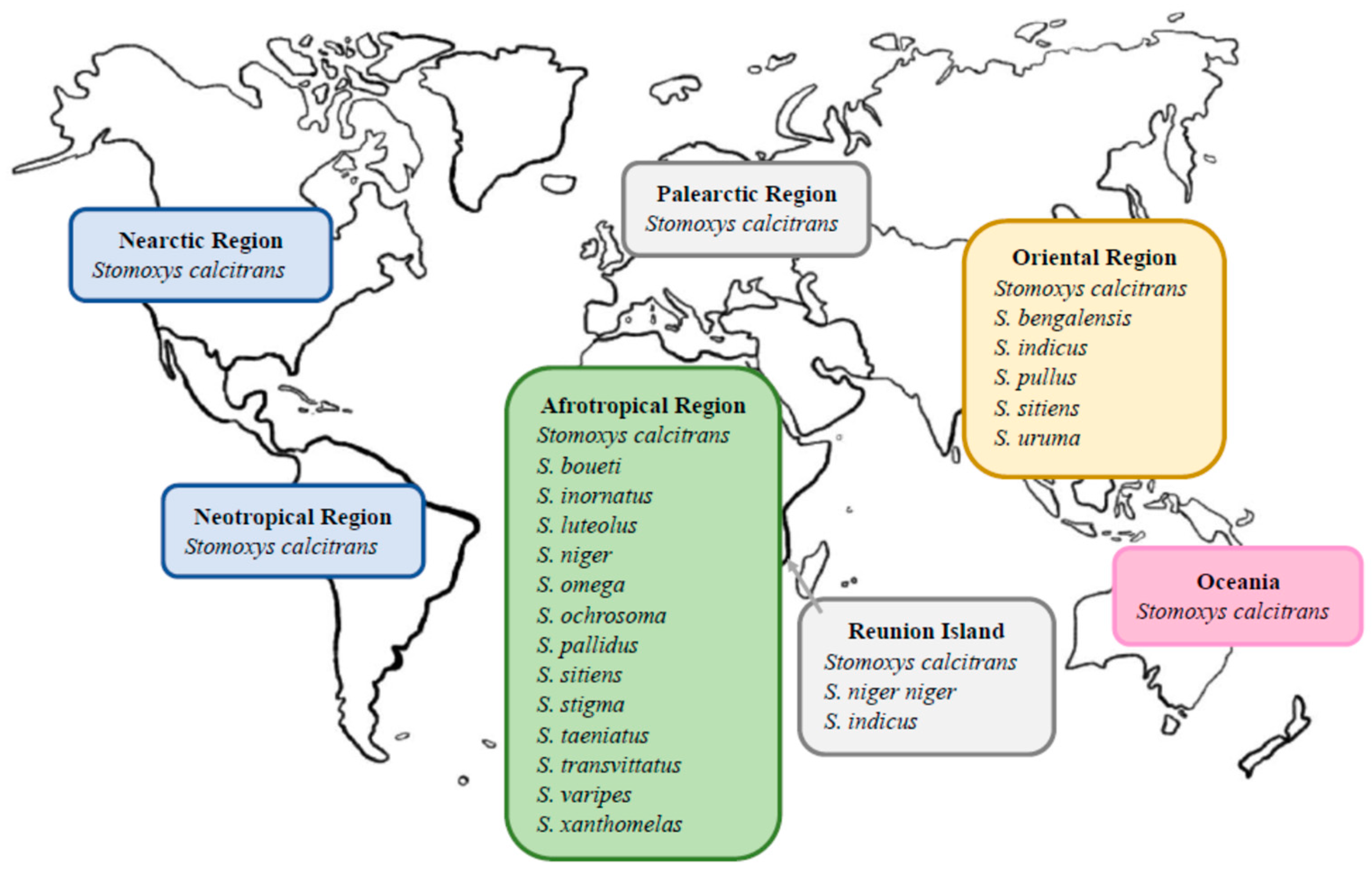
3. Distribution
4. Phylogeography of Stomoxys calcitrans
5. Phylogeny of Genus Stomoxys
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zumpt, F. The Stomoxyine Biting Flies of the World. Diptera: Muscidae. Taxonomy; Biology; Economic Importance and Control Measures; Gustav Fisher: Stuttgart, Germany, 1973; pp. 97–137. [Google Scholar]
- Foil, L.D.; Hogsette, J.A. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. l’Office Int. Epizoot. 1994, 13, 1125–1158. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; Milne, D.E.; Patterson, R.S.; Schreiber, E.T.; Milio, J.A. Nectar Feeding by Stomoxys calcitrans (Diptera, Muscidae)—Effects on Reproduction and Survival. Environ. Entomol. 1992, 21, 141–147. [Google Scholar] [CrossRef]
- Baldacchino, F.; Muenworn, V.; Desquesnes, M.; Desoli, F.; Charoenviriyaphap, T.; Duvallet, G. Transmission of pathogens by Stomoxys flies (Diptera; Muscidae): A review. Parasite 2013, 20, 26. [Google Scholar] [CrossRef]
- Taylor, D.B.; Moon, R.D.; Mark, D.R. Economic impact of stable flies (Diptera: Muscidae) on dairy and beef cattle production. J. Med. Entomol. 2012, 49, 198–209. [Google Scholar] [CrossRef]
- Blanc-Debrune, N. Impact Economique des Principales Espèces de Diptères Sur L’élevage Bovin Français et Méthodes de Luttes Associées. Ph.D. Thesis, Université Claude-Bernard-Lyon I, Villeurbanne, France, 2019; pp. 1–140. [Google Scholar]
- Rochon, K.; Hogsette, J.A.; Kaufman, P.E.; Olafson, P.U.; Swiger, S.L.; Taylor, D.B. Stable Fly (Diptera: Muscidae)—Biology, Management, and Research Needs. J. Integr. Pest Manag. 2021, 12, 38. [Google Scholar] [CrossRef]
- Salem, A.; Bouhsira, E.; Lienard, E.; Bousquet-Melou, A.; Jacquiet, P.; Franc, M. Susceptibility of two European strains of Stomoxys calcitrans (L.) to cypermethrin, deltamethrin, fenvalerate, lamda-cyhalothrin, permethrin and phoxim. Intern. J. Appl. Res. Vet. Med. 2012, 10, 249–257. [Google Scholar]
- Tainchum, K.; Shukri, S.; Duvallet, G.; Etienne, L.; Jacquiet, P. Phenotypic susceptibility to pyrethroids and organophosphate of wild Stomoxys calcitrans (Diptera: Muscidae) populations in southwestern France. Parasitol. Res. 2018, 117, 4027–4032. [Google Scholar] [CrossRef]
- Olafson, P.U.; Kaufman, P.E.; Duvallet, G.; Solórzano, J.A.; Taylor, D.B.; Trout Fryxell, R. Frequency of kdr and kdr-his Alleles in Stable Fly (Diptera: Muscidae) Populations from the United States, Costa Rica, France, and Thailand. J. Med. Entomol. 2019, 56, 1145–1149. [Google Scholar] [CrossRef]
- Barros, A.T.M.; Rodrigues, V.D.; Cançado, P.H.D.; Domingues, L.N. Resistance of the stable fly, Stomoxys calcitrans (Diptera: Muscidae), to cypermethrin in outbreak areas in Midwestern Brazil. Rev. Bras. Parasitol. Veterinária 2019, 28, 5. [Google Scholar] [CrossRef]
- Reissert-Oppermann, S.; Bauer, B.; Steuber, S.; Clausen, P.-H. Insecticide resistance in stable flies (Stomoxys calcitrans) on dairy farms in Germany. Parasitol. Res. 2019, 118, 2499–2507. [Google Scholar] [CrossRef]
- Pont, A.C.; Mihok, S. A new species of Haematobosca Bezzi from Kenya (Diptera; Muscidae). Stud. Dipterol. 2000, 7, 25–32. [Google Scholar]
- Pont, A.C.; Dsouli, N. A new species of Haematobosca Bezzi from Gabon (Diptera; Muscidae). Stud. Dipterol. 2009, 15, 259–266. [Google Scholar]
- Pont, A.C.; Duvallet, G.; Changbunjong, T. A new species of Haematobosca Bezzi (Diptera: Muscidae) from Thailand. Zootaxa 2020, 4763, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Gamal-Eddin, F.M. Ecological studies on Stomoxys calcitrans L. and sitiens Rond. in Egypt; with suggestions on their control. Bull. Soc. Entom. Egypte 1959, 43, 245–283. [Google Scholar]
- Hafez, M.; Gamal-Eddin, F.M. On the feeding habits of Stomoxys calcitrans L. and sitiens Rond., with special reference to their biting cycle in nature. Bull. Soc. Entom. Egypte 1959, 43, 291–301. [Google Scholar]
- Gamal-Eddin, F.M. Experimental studies on the development stages of two blood-sucking flies (Stomoxys calcitrans Lin. and S. sitiens Rond.) in Egypt (Diptera: Stomoxydinae). J. Arab. Vet. Med. Assoc. 1963, 23, 309–338. [Google Scholar]
- Steyskal, G.C. The Gender of the Genus-Name Stomoxys Geoffroy, 1762 (Diptera; Muscidae); Entomological Society of Washington: Washington, DC, USA, 1975; p. 163. [Google Scholar]
- Mavoungou, J.F.; Nguema, R.M.; Acapovi, G.L.; Koumba, R.Z.; Mounioko, F.; Lendzele Sevidzem, S.; Kindzi Bakakas, I.; Gilles, J.; Duvallet, G.; M’Batchi, B.; et al. Breeding Sites of Stomoxys spp. (Diptera: Muscidae), a Preliminary Study in the Makokou Region (North-East-Gabon). Vector Biol. J. 2017, 2, 1. [Google Scholar] [CrossRef]
- Lendzele Sevidzem, S.; Mavoungou, J.F.; Zinga-Koumba, C.R.; Koumba, A.A.; Duvallet, G. Factors Influencing Seasonal and Daily Dynamics of the Genus Stomoxys Geoffroy; 1762 (Diptera: Muscidae) in the Adamawa Plateau, Cameroon. Int. J. Zool. 2019, 2019, 3636943. [Google Scholar] [CrossRef]
- Fiasson, R. Note sur les parasites animaux du haut-Apure {Venezuela). Rev. Sci. Médicales Pharm. Vétérinaires l’Afrique Française Libre 1943, 2, 125–151. [Google Scholar]
- Mavoungou, J.F.; Picard, N.; Kohagne, L.T.; M’batchi, B.; Gilles, J.; Duvallet, G. Spatio-temporal variation of biting flies Stomoxys spp. (Diptera: Muscidae) along a man-made disturbance gradient, from primary forest to the city of Makokou (North-East; Gabon). Med. Vet. Entomol. 2013, 27, 339–345. [Google Scholar] [CrossRef]
- Guo, Y.; Hogsette, J.A.; Greene, G.L.; Jones, C.J. Survey report on pupal parasites of filth flies in livestock and poultry facilities in China. Chin. J. Biol. Control 1997, 13, 106–109. [Google Scholar]
- Tumrasvin, W.; Shinonaga, S. Studies on medically important flies in Thailand V. On 32 species belonging to the subfamilies Muscinae and Stomoxyinae including the taxonomic keys (Diptera: Muscidae). Bull. Tokyo Med. Dent. Univ. 1978, 25, 201–227. [Google Scholar] [PubMed]
- Masmeatathip, R.; Gilles, J.; Ketavan, C.; Duvallet, G. First survey of seasonal abundance and daily activity of Stomoxys spp. (Diptera: Muscidae) in Kamphaengsaen Campus, Nakornpathom Province, Thailand. Parasite 2006, 13, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Phasuk, J.; Prabaripai, A.; Chareonviriyaphap, T. A comparison of attractants for sampling Stomoxys calcitrans (Diptera: Muscidae) on dairy farms in Saraburi Province, Thailand. J. Econ. Entomol. 2016, 109, 942–946. [Google Scholar] [CrossRef]
- Masmeatathip, R.; Ketavan, C.; Duvallet, G. Morphological studies of Stomoxys spp. (Diptera: Muscidae) in Central Thailand. Kasetsart J. (Nat. Sci.) 2006, 40, 872–881. [Google Scholar]
- Keawrayup, S.; Duvallet, G.; Sukonthabhirom, S.; Chareonviriyaphap, T. Diversity of Stomoxys spp. (Diptera: Muscidae) and diurnal variations of activity of Stomoxys indicus and S. calcitrans in a farm, in Wang Nam Khiao District, Nakhon Ratchasima Province, Thailand. Parasite 2012, 19, 259–265. [Google Scholar] [CrossRef]
- Kano, R. Notes on the flies of medical importance in Japan. Jap. J. exp. Med. 1953, 23, 185–195. [Google Scholar]
- Muenworn, V.; Duvallet, G.; Tainchum, K.; Tuntakom, S.; Akratanakul, P.; Chareonviriyaphap, T. Geographic distribution of Stomoxys calcitrans in Thailand. J. Med. Entomol. 2010, 47, 791–797. [Google Scholar] [CrossRef]
- Changbunjong, T.; Weluwanarak, T.; Ratanakorn, P.; Maneeon, P.; Ganpanakngan, M.; Apiwathnasorn, C.; Sungvornyothin, S.; Sriwichai, P.; Sumruayphol, S.; Ruangsittichai, J. Distribution and abundance of Stomoxyini flies (Diptera: Muscidae) in Thailand. Southeast Asian J. Trop. Med. Public Health 2012, 43, 1400–1410. [Google Scholar]
- Reid, E.T.M. Notes on the distribution of Stomoxydinae (Dipt., Muscidae) in the southern Sudan. Entomologist’s Mon. Mag. 1956, 92, 343–346. [Google Scholar]
- Mavoungou, J.F.; Gilles, J.; Duvallet, G. Stomoxys xanthomelas Roubaud; 1937: Une espèce de la canopée en Afrique équatoriale. Bull. Société Entomol. Bull. Société Entomol. Fr. 2007, 112, 481–483. [Google Scholar] [CrossRef]
- Mavoungou, J.F.; Simo, G.; Gilles, J.; De Stordeur, E.; Duvallet, G. Écologie des stomoxes (Diptera: Muscidae) au Gabon. II- Origine des repas de sang et conséquences épidémiologiques. Parasite 2008, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, W.H. Observations on Stomoxys ochrosoma Speiser (Diptera Muscidae) as an associate of army ants (Dorylinae) in Est Africa. Proc. R. Entomol. Soc. Lond. (A) 1942, 17, 38–42. [Google Scholar]
- Mavoungou, J.F.; Jay-Robert, P.; Gilles, J.; Atsame, E.; Duvallet, G. Ecology of Stomoxys flies (Diptera: Muscidae) in Gabon. I- First survey in different ecological areas. Parasite 2008, 15, 27–34. [Google Scholar] [CrossRef]
- Bitome Essono, P.Y.; Dechaume-Moncharmont, F.-X.; Mavoungou, J.; Obiang Mba, R.; Duvallet, G.; Bretagnolle, F. Distribution and abundance of hematophagous flies (Glossinidae, Stomoxys, and Tabanidae) in two national parks of Gabon. Parasite 2015, 22, 23. [Google Scholar] [CrossRef]
- Hogsette, J.A.; Ruff, J.P. Stable Fly (Diptera: Muscidae) Migration in Northwest Florida. Environ. Entomol. 1985, 14, 170–175. [Google Scholar] [CrossRef]
- Jones, C.J.; Hogsette, J.A.; Patterson, R.S.; Milne, D.E.; Propp, G.D.; Milio, J.F.; Rickard, L.G.; Ruff, J.P. Origin of Stable Flies (Diptera; Muscidae) on West Florida Beaches—Electrophoretic Analysis of Dispersal. J. Med. Entomol. 1991, 28, 787–795. [Google Scholar] [CrossRef]
- Gilles, J.; David, J.-F.; Duvallet, G.; de La Roque, S.; Tillard, E. Efficiency of traps for Stomoxys calcitrans and Stomoxys niger niger on Reunion Island. Med. Vet. Entomol. 2007, 21, 65–69. [Google Scholar] [CrossRef]
- Szalanski, A.L.; Taylor, D.B.; Peterson, R.D. Population genetics and gene variation of stable fly populations (Diptera: Muscidae) in Nebraska. J. Med. Entomol. 1996, 33, 413–420. [Google Scholar] [CrossRef]
- Kneeland, K.M.; Skoda, S.R.; Foster, J.E. Amplified fragment length polymorphism used to investigate genetic variability of the stable fly (Diptera: Muscidae) across North America. J. Med. Entomol. 2013, 50, 1025–1030. [Google Scholar]
- Dsouli-Aymes, N.; Michaux, J.; De Stordeur, E.; Couloux, A.; Veuille, M.; Duvallet, G. Global population structure of the stable fly (Stomoxys calcitrans) inferred by mitochondrial and nuclear sequence data. Infect. Genet. Evol. 2011, 11, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-L.; Lu, C.-N.; Tzeng, H.-Y.; Krafsur, E.S.; Tu, W.-C.; Yeh, W.-B. Global population genetic structure and lineage differentiation of the stable fly, Stomoxys calcitrans. Med. Vet. Entomol. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Changbunjong, T.; Weluwanarak, T.; Samung, Y.; Ruangsittichai, J. Molecular identification and genetic variation of hematophagous flies (Diptera: Muscidae: Stomoxyinae) in Thailand based on cox1 barcodes. J. Asia-Pac. Entomol. 2016, 19, 1117–1123. [Google Scholar] [CrossRef]
- Kneeland, K.M.; Skoda, S.R.; Foster, J.E. Genetic variability of the stable fly assessed on a global scale using amplified fragment length polymorphism. Insect Sci. 2016, 23, 695–703. [Google Scholar] [CrossRef]
- Olafson, P.U.; Aksoy, S.; Attardo, G.M.; Buckmeier, G.; Chen, X.; Coates, C.J.; Davis, M.; Dykema, J.; Emrich, S.J.; Friedrich, M.; et al. The genome of the stable fly, Stomoxys calcitrans, reveals potential mechanisms underlying reproduction, host interactions, and novel targets for pest control. BMC Biol. 2021, 19, 41. [Google Scholar] [CrossRef]
- Dsouli, N.; Delsuc, F.; Michaux, J.; De Stordeur, E.; Couloux, A.; Veuille, M.; Duvallet, G. Phylogenetic analyses of mitochondrial and nuclear data in haematophagous flies support the paraphyly of the genus Stomoxys (Diptera: Muscidae). Infect. Genet. Evol. 2011, 11, 663–670. [Google Scholar] [CrossRef]

| Genera | Number of Species | Number of Taxa * |
|---|---|---|
| Rhinomusca Malloch (1932) | 2 | 2 |
| Neivamyia Pinto & Fonseca (1930) | 5 | 5 |
| Bruceomyia Malloch (1932) | 1 | 1 |
| Parastomoxys Zumpt (1973) | 1 | 1 |
| Prostomoxys Zumpt (1973) | 1 | 1 |
| Stygeromyia Austen (1907) | 2 | 2 |
| Haematobosca Bezzi (1907) | 15 | 15 |
| Haematobia Lepeletier & Serville (1828) | 6 | 9 |
| Haematostoma Malloch (1932) | 1 | 1 |
| Stomoxys Geoffroy (1762) | 18 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duvallet, G.; Hogsette, J.A. Global Diversity, Distribution, and Genetic Studies of Stable Flies (Stomoxys sp.). Diversity 2023, 15, 600. https://doi.org/10.3390/d15050600
Duvallet G, Hogsette JA. Global Diversity, Distribution, and Genetic Studies of Stable Flies (Stomoxys sp.). Diversity. 2023; 15(5):600. https://doi.org/10.3390/d15050600
Chicago/Turabian StyleDuvallet, Gérard, and Jerome A. Hogsette. 2023. "Global Diversity, Distribution, and Genetic Studies of Stable Flies (Stomoxys sp.)" Diversity 15, no. 5: 600. https://doi.org/10.3390/d15050600
APA StyleDuvallet, G., & Hogsette, J. A. (2023). Global Diversity, Distribution, and Genetic Studies of Stable Flies (Stomoxys sp.). Diversity, 15(5), 600. https://doi.org/10.3390/d15050600









