Phylogeny of Serpulidae (Annelida, Polychaeta) Inferred from Morphology and DNA Sequences, with a New Classification
Abstract
1. Introduction
2. Materials and Methods
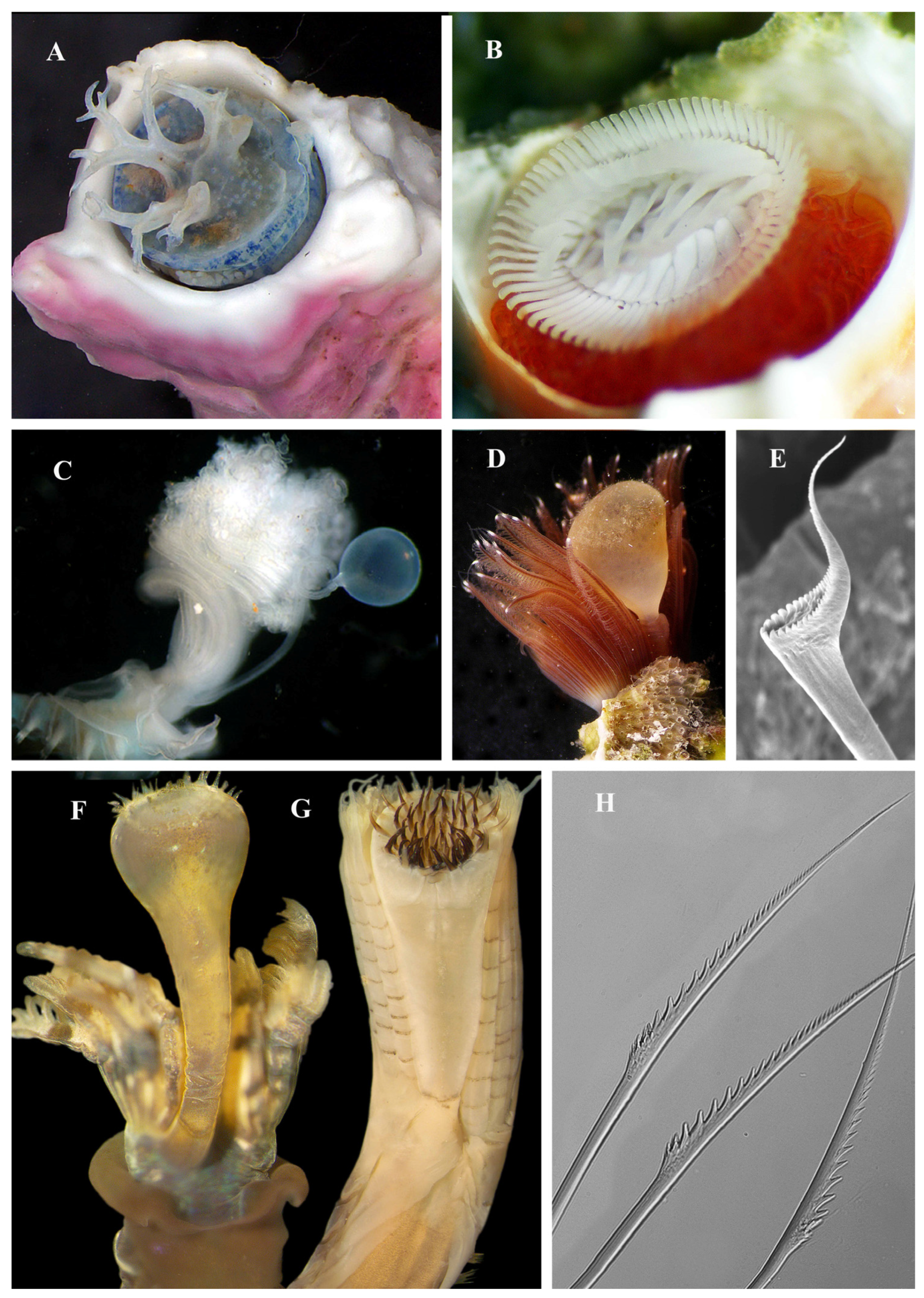
2.1. Taxa Used in This Study and Morphological Matrix
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Phylogenetic Analyses
2.3.1. Morphology Only Dataset
2.3.2. Molecular Dataset
2.3.3. Molecular + Morphology Dataset
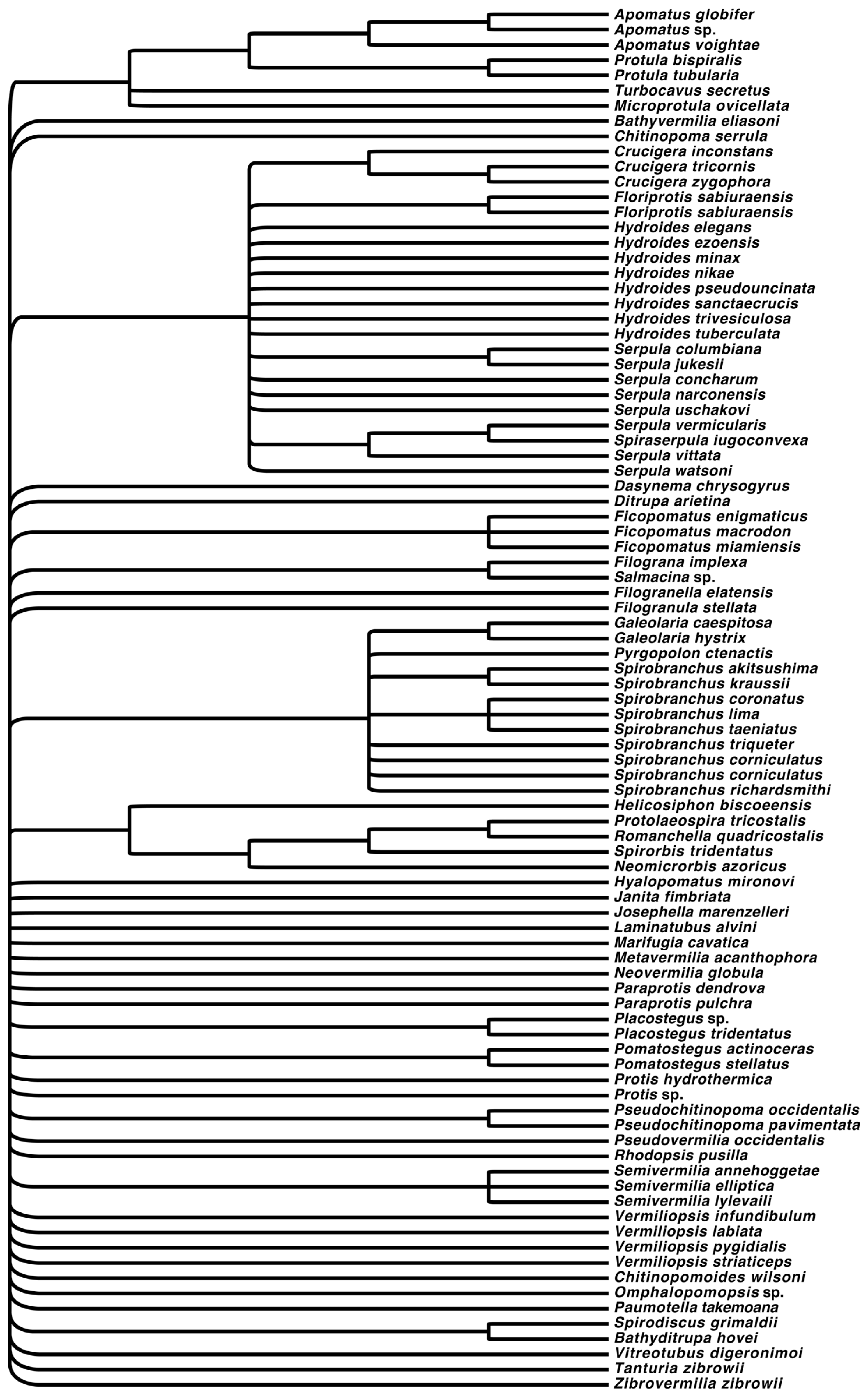
3. Results
3.1. Morphology Only Dataset
3.2. Molecular Only Dataset
3.3. Molecular + Morphology Dataset
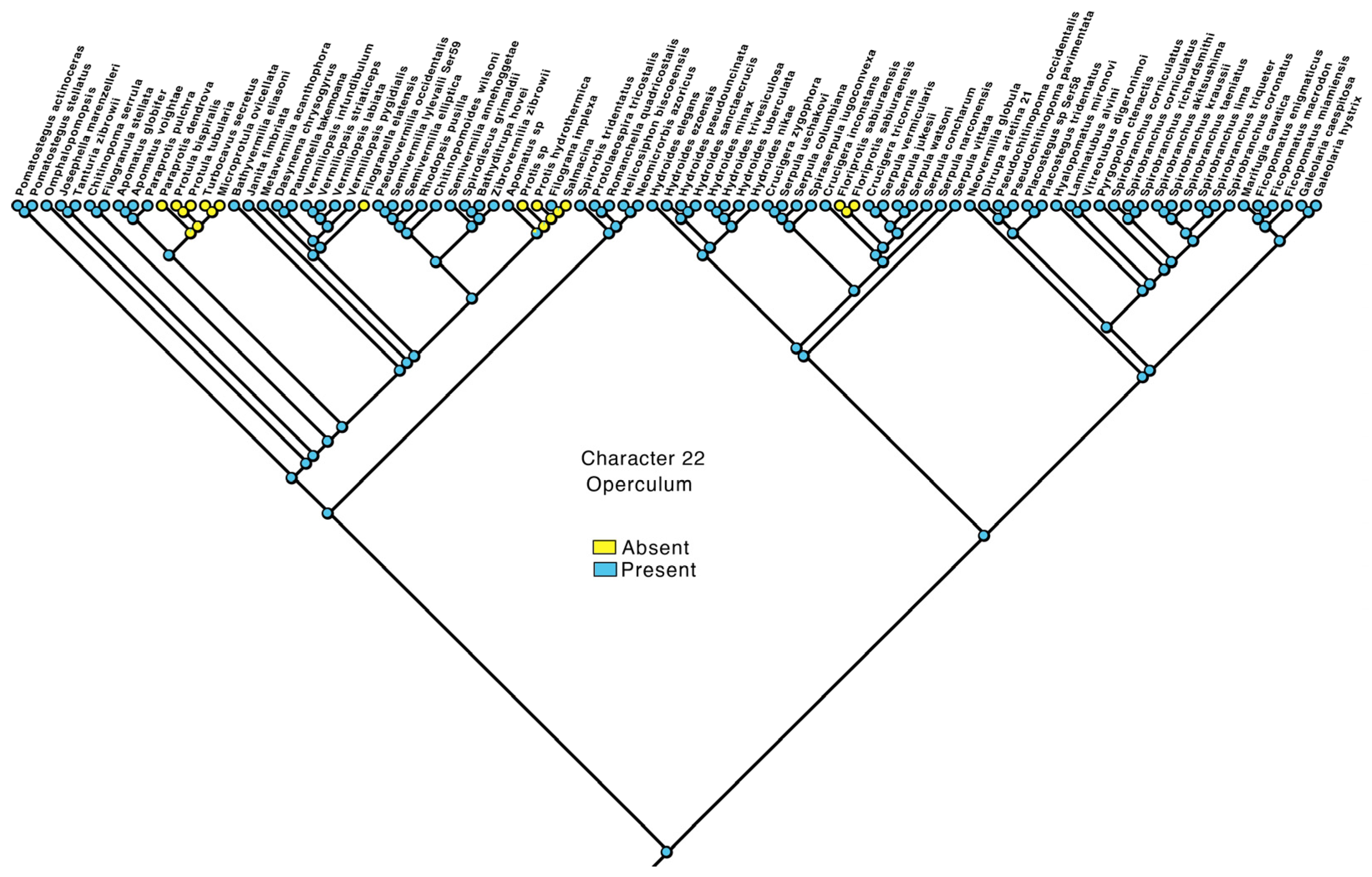
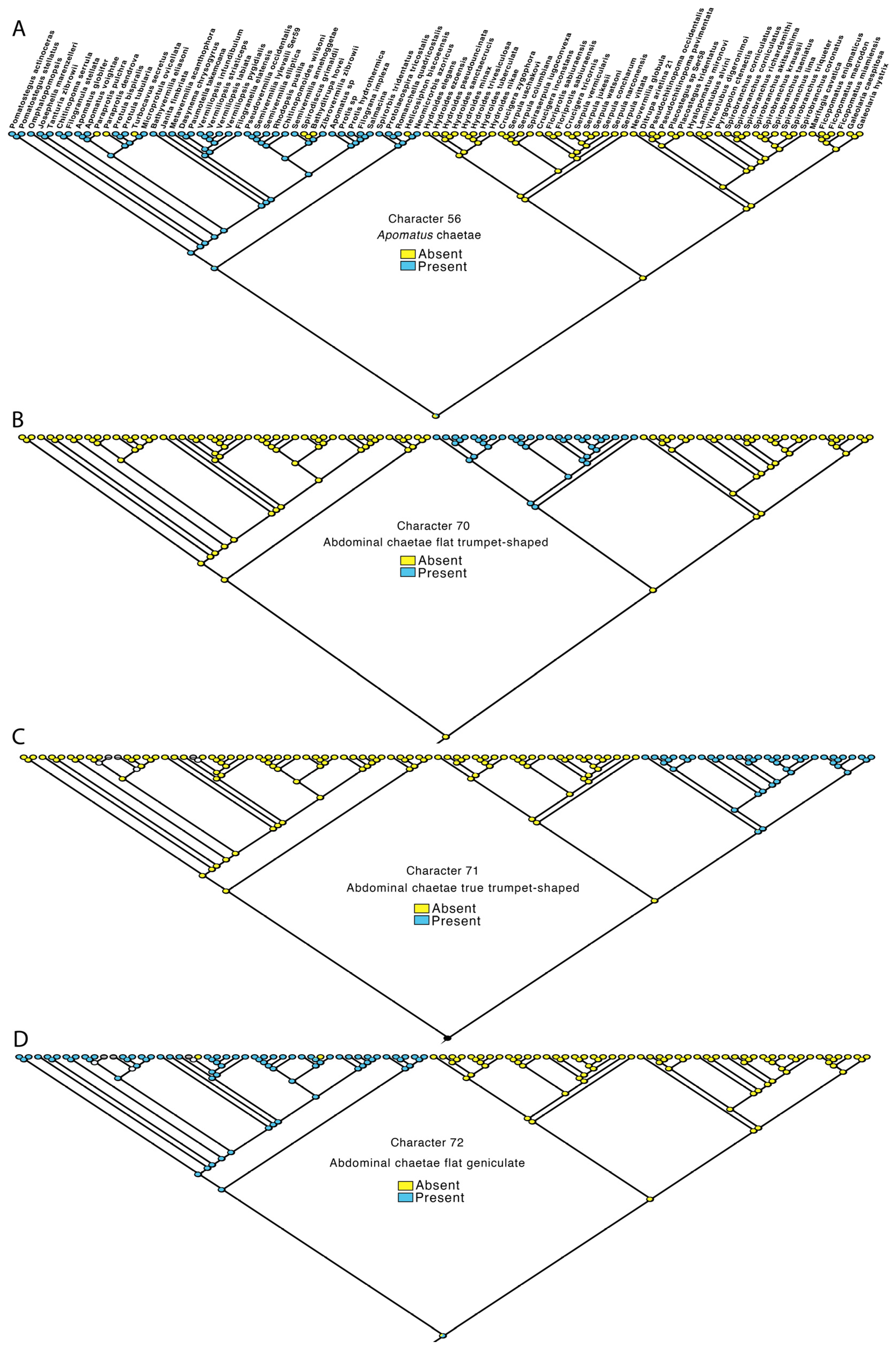
3.4. Transformations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Body symmetry: symmetrical—0, asymmetrical—1.
- Chaetal inversion: complete—0, incomplete—1.
- Radiolar lobes: fused—0, separate—1.
- Inter-radiolar membrane: absent—0, present —1.
- Radiolar eyespots: absent—0, present—1.
- Arrangements of radioles: in semi-circles—0, pectinately—1, in spiral—2.
- Radiolar stylodes: present—0, absent—1.
- Tube material: mucous—0, calcareous—1.
- Tube keels: absent—0, present—1.
- Tube (semi)circular in cross-section: no—0, yes—1.
- Tube triangular in cross-section: no—0, yes—1.
- Tube trapezoid in cross-section: no—0, yes—1.
- Tube quadrangular in cross-section: no—0, yes—1.
- Granular overlay: absent—0, present—1.
- Tube wall transparency: completely opaque—0, with outer hyaline and inner opaque layer—1, completely hyaline—2.
- Tube coiling: straight or irregular—1, spirally coiled—2.
- Colonies due to asexual budding: absent—0, present—1.
- Adult tube attachment: attached—0, unattached—1.
- Internal tube structures: absent—0, present—1.
- Tabulae: absent—0, present—1.
- Colour of opaque tubes: white opaque—0, coloured—2.
- Operculum: absent—0, present—1.
- Pseudoperculum: absent—0, present—1.
- Pseudoperculum: borne on pinnulated radiole—0, borne on smooth short radiole—1.
- Opercular reinforcement: absent—0, present—1.
- Chitinous reinforcement: absent—0, present—1.
- Calcareous reinforcement: absent—0, present—1.
- Thickened cuticle: absent—0, present—1.
- Chitinous opercular reinforcement: without spines—0, with spines—1.
- Chitinous endplate: flat opercular plate or concave—0, elongated opercular cap—1, multi-tiered structure—2.
- Basal processes below operculum: absent—0, present—1.
- Serpula-type operculum: absent—0, present—1.
- Verticil on Serpula-type operculum: absent—0; present—1
- Type of calcareous opercular reinforcement: operculum infested with calcareous flakes—1, calcareous deposits forming distal plate—2, entirely calcified operculum—3.
- Calcareous opercular spines: absent—0, non-movable—1, movable—2.
- Calcareous opercular talon: absent—0, short, embedded in opercular ampulla—1, long, continues into opercular peduncle—2.
- Opercular constriction: operculum gradually merges into peduncle without constriction—0, operculum separated from the peduncle by a constriction—1.
- Ontogeny of operculum: indirect—0, direct—1.
- The operculum-bearing radiole is not different from all other radioles—0, operculum-bearing radiole is modified into a thickened peduncle—1.
- Peduncle smooth, without pinnules—0, peduncle with pinnules—1.
- Distal peduncular wings: absent—0, present—1.
- Proximal peduncular wings: absent—0, present—1.
- Insertion of the opercular peduncle: as second dorsal radiole—0, as the first radiole—1, at the base of radiolar crown, median insertion covering several opercular radioles—2.
- Peduncle cross-section: circular—0, triangular—1, flattened—2.
- Peduncle width: as wide as normal radioles—0, wider than normal radioles—1, much wider than normal radioles—2.
- Peduncle surface texture: smooth—0, wrinkled—1.
- Collar: unlobed—0, trilobed—1.
- Collar tonguelets: absent—0, present—1.
- Chaetae on the collar segment (collar chaetae): absent—0, present—1.
- Special collar chaetae: absent—0, with basal modification—1, with distal modification—2.
- Special fin-and-blade collar chaetae: absent—0, present—1
- Special bayonet collar chaetae: absent—0, present—1.
- Special Spirobranchus collar chaetae: absent—0, present—1.
- Thoracic membranes: absent—0, present—1.
- Thoracic membranes end: short, second segment—0, mid-thorax—1, end of thorax—2, form apron—3.
- Thoracic Apomatus chaetae: absent—0, present—1.
- Thoracic uncini rasp-shaped: absent—0, present—1.
- Thoracic uncini saw-to-rasp: absent—0, present—1.
- Thoracic uncini saw-shaped: absent—0, present—1.
- Anterior tooth of thoracic uncini pointed: absent—0, present—1.
- Anterior tooth of thoracic uncini blunt elongated, with rows of teeth implanted over almost entire length of peg (Protula type): absent—0, present—1.
- Anterior tooth of thoracic uncini blunt rounded: absent—0, present—1.
- Anterior tooth of thoracic uncini blunt flattened, often gouged underneath: absent—0, present—1.
- Number of teeth in thoracic uncini in profile: < 8–0; 8–19–1; > 20–2.
- Number of uncinigerous thoracic segments: seven—0, six—1, five—2, four—3, three—4.
- Variable number of thoracic uncinigerous chaetigers: no—0, yes—1.
- Ventral arrangement of thoracic uncini: parallel, not forming triangular depression—0, converging posteriorly forming triangular depression—1, fused—2.
- Achaetous region in the beginning of abdomen: absent—0, present—1.
- Abdominal chaetae capillary: no—0, yes—1.
- Abdominal chaetae flat trumpet-shaped: no—0, yes—1.
- Abdominal chaetae true trumpet shaped: no—0, yes—1.
- Abdominal chaetae flat geniculate: no—0, yes—1.
- Abdominal chaetae acicular: no—0, yes—1.
- Posterior glandular pad: absent—0, present—1.
- Long capillary chaetae in posterior abdominal segments: absent—0, present—1.
References
- Rouse, G.W.; Pleijel, F.; Tilic, E. Annelida; Oxford University Press: London, UK; New York, NY, USA, 2022; pp. 1–418. [Google Scholar] [CrossRef]
- Capa, M.; Kupriyanova, E.K.; Nogueira, J.M.; Bick, A.; Tovar-Hernández, M.A. Fanworms: Yesterday, today and tomorrow. Diversity 2021, 13, 130. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; ten Hove, H.A.; Nishi, E. A taxonomic revision of the genus Pseudochitinopoma Zibrowius, 1969 (Serpulidae, Annelida) with descriptions of two new species. Zootaxa 2012, 3507, 57–78. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Ippolitov, A.P. Deep-sea serpulids (Annelida: Polychaeta) in tetragonal tubes: On a tube convergence path from Mesozoic to Recent. Zootaxa 2015, 4044, 151–200. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wong, E.; Ahyong, S.T.; Williamson, J.E.; Hutchings, P.A.; Kupriyanova, E.K. Barcoding and multi-locus phylogeography of the globally distributed calcareous tubeworm genus Hydroides Gunnerus, 1768 (Annelida, Polychaeta, Serpulidae). Mol. Phylogenet. Evol. 2018, 127, 732–745. [Google Scholar] [CrossRef]
- Rouse, G.W.; Kupriyanova, E.K. Laminatubus (Serpulidae, Annelida) from eastern Pacific hydrothermal vents and methane seeps, with description of two new species. Zootaxa 2021, 4915, 1–27. [Google Scholar]
- Kupriyanova, E.K.; Rzhavsky, A.V.; ten Hove, H.A. Family Serpulidae Rafinesque, 1815. In Handbook of Zoology. A Natural History of the Phyla of the Animal Kingdom; Schmidt–Rhaesa, A., Ed.; De Gruyter Publishers: Berlin, Germany, 2020; pp. 213–275. [Google Scholar]
- WoRMS. Serpulidae Rafinesque, 1815. 2022. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=988 (accessed on 23 December 2022).
- Pamungkas, J.; Glasby, C.J.; Read, G.B.; Wilson, S.P.; Costello, M.J. Progress and perspectives in the discovery of polychaete worms (Annelida) of the world. Helgol. Mar. Res. 2019, 73, 4. [Google Scholar] [CrossRef]
- Bastida-Zavala, J.R. Serpulids (Annelida: Polychaeta) from the Eastern Pacific, including a brief mention of Hawaiian serpulids. Zootaxa 2008, 1722, 1–61. [Google Scholar]
- Bastida-Zavala, R. Serpula and Spiraserpula (Polychaeta, Serpulidae) from the Tropical Western Atlantic and Gulf of Guinea. ZooKeys 2012, 198, 1–23. [Google Scholar] [CrossRef]
- Çinar, M.E. Alien polychaete species (Annelida: Polychaeta) on the Southern coast of Turkey (Levantine Sea, Eastern Mediterranean), with 13 new records for the Mediterranean Sea. J. Nat. Hist. 2009, 43, 2283–2328. [Google Scholar] [CrossRef]
- Nogueira, J.M.D.M.; Abbud, A. Three new serpulids (Polychaeta: Serpulidae) from the Brazilian Exclusive Economic Zone. Zoosymposia 2009, 2, 201–227. [Google Scholar]
- Tovar-Hernández, M.A.; Méndez, N.; Villalobos-Guerrero, T.F. Fouling polychaete worms from the Southern Gulf of California: Sabellidae and Serpulidae. Syst. Biodivers. 2009, 7, 319–336. [Google Scholar] [CrossRef]
- Pillai, T.G. A revision of the genera Galeolaria and Pyrgopolon (Polychaeta: Serpulidae), with discussions on opercular insertion as a character in their taxonomy and relationships, and their zoogeography. Zootaxa 2009, 2060, 47–58. [Google Scholar] [CrossRef]
- Sanfilippo, R. New species of Hyalopomatus Marenzeller, 1878 (Annelida, Polychaeta, Serpulidae) from Recent Mediterranean deep-water coral mounds and comments on some congeners. Zoosystema 2009, 31, 147–161. [Google Scholar] [CrossRef]
- Bailey-Brock, J.H.; Magalhães, W.F. A new species and record of Serpulidae (Annelida: Polychaeta) from Cross Seamount in the Hawaiian Chain. Zootaxa 2012, 3192, 49–58. [Google Scholar] [CrossRef]
- Bailey-Brock, J.H.; Magalhães, W.F.; Brock, R.E. Coral reef inhabiting tubeworms (Polychaeta: Serpulidae) from Enewetak, Kwajalein, Rongelap and Utirik Atolls, Marshall Islands. J. Mar. Biol. Assoc. UK 2012, 92, 967–988. [Google Scholar] [CrossRef]
- Prentiss, N.K.; Vasileiadou, K.; Faulwetter, S.; Arvanitidis, C.; ten Hove, H.A. A new genus and species of Serpulidae (Annelida, Polychaeta, Sabellida) from the Caribbean Sea. Zootaxa 2014, 3900, 204–222. [Google Scholar] [CrossRef]
- Pernet, B.; Barton, M.; Fitzhugh, K.; Harris, L.; Lizárraga, D.; Ohl, R.; Whitcraft, C. Establishment of the reef-forming tubeworm Ficopomatus enigmaticus (Fauvel, 1923) (Annelida: Serpulidae) in Southern California. Bioinvasions Rec. 2016, 5, 13–19. [Google Scholar] [CrossRef]
- Bastida-Zavala, J.R.; Buelna, A.S.R.; De León-González, J.A.; Camacho-Cruz, K.A.; Carmona, I. New records of sabellids and serpulids (Polychaeta: Sabellidae, Serpulidae) from the Tropical Eastern Pacific. Zootaxa 2016, 4184, 401–457. [Google Scholar] [CrossRef]
- Bastida-Zavala, J.R.; McCann, L.D.; Keppel, E.; Ruiz, G.M. The fouling serpulids (Polychaeta: Serpulidae) from United States coastal waters: An overview. Eur. J. Taxon. 2017, 344, 1–76. [Google Scholar] [CrossRef]
- Yee, A.; Mackie, J.; Pernet, B. The distribution and unexpected genetic diversity of the non-indigenous annelid Ficopomatus enigmaticus in California. Aquat. Invasions 2019, 14, 250–266. [Google Scholar] [CrossRef]
- Oliva, M.; De Marchi, L.; Sanches, M.V.; Pires, A.; Cuccaro, A.; Baratti, M.; Chiellini, F.; Morelli, A.; Freitas, R.; Pretti, C. Atlantic and Mediterranean populations of the widespread serpulid Ficopomatus enigmaticus: Developmental responses to carbon nanotubes. Mar. Pollut. Bull. 2020, 156, 111265. [Google Scholar] [CrossRef] [PubMed]
- Grosse, M.; Pérez, R.; Juan-Amengual, M.; Pons, J.; Capa, M. The elephant in the room: First record of invasive gregarious species of serpulids (calcareous tube annelids) in Majorca (Western Mediterranean). Sci. Mar. 2021, 81, 15–28. [Google Scholar] [CrossRef]
- Dales, R.P. The nature of the pigments in the crown of sabellid and serpulid polychaetes. J. Mar. Biol. Assoc. UK 1962, 42, 259–274. [Google Scholar] [CrossRef]
- Fauchald, K. The polychaete worms. Definitions and keys to the orders, families and genera. Nat. Hist. Mus. LA City. Sci. Ser. 1977, 28, 1–188. [Google Scholar]
- Pettibone, M.H. Annelida. In Synopsis and Classification of Living Organisms; Parker, S.P., Ed.; McGraw-Hill Book Co.: New York, NY, USA, 1982; Volume 2, pp. 1–43. [Google Scholar]
- Smith, R.S. Relationships within the order Sabellida (Polychaeta). Ophelia Suppl. 1991, 5, 249–260. [Google Scholar]
- Rousset, V.; Rouse, G.W.; Siddall, M.E.; Tillier, A.; Pleijel, F. The phylogenetic position of Siboglinidae (Annelida), inferred from 18S rRNA, 28S rRNA, and morphological data. Cladistics 2004, 20, 518–533. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Rouse, G.W. Yet another example of paraphyly in Annelida: Molecular evidence that Sabellidae contains Serpulidae. Mol. Phylogenet. Evol. 2008, 46, 1174–1181. [Google Scholar] [CrossRef]
- Tilic, E.; Sayyari, E.; Stiller, J.; Mirarab, S.; Rouse, G.W. More is needed—Thousands of loci are required to elucidate the relationships of the ‘Flowers of the Sea’ (Sabellida, Annelida). Mol. Phylogenet. Evol. 2020, 151, 106892. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; McDonald, T.A.; Rouse, G.W. Phylogenetic relationships within Serpulidae (Annelida: Polychaeta) inferred from molecular and morphological data. Zool. Scr. 2006, 35, 421–439. [Google Scholar] [CrossRef]
- Rafinesque, S.C. L’analyse de la Nature, Palermo, Italy, 1815; 224 p.
- Chamberlin, R.V. The Annelida Polychaeta. “Albatross” Expedition. Mem. Mus. Comp. Zool. Harv. Coll. 1919, 48, 1–518. [Google Scholar]
- Rioja, E. Estudio sistemático de las especies Ibéricas del Suborden Sabelliformia. Trab. Mus. Nac. Cien. Nat. Ser. Zool. 1923, 48, 1–144. [Google Scholar]
- Pillai, T.G. Some marine and brackish-water serpulid polychaetes from Ceylon, including new genera and species. Ceylon J. Sci. Biol. Sci. 1960, 3, 1–40. [Google Scholar]
- Uchida, H. Serpulid tube worms (Polychaeta, Sedentaria) from Japan with the systematic review of the group. Bull. Mar. Park Res. Stat. 1978, 2, 1–98. [Google Scholar]
- Pillai, T.G. Studies on a collection of spirorbids from Ceylon, together with a critical review and revision of spirorbid systematics and an account of their phylogeny and zoogeography. Ceylon J. Sci. Biol. Sci. 1970, 8, 100–172. [Google Scholar]
- Fitzhugh, K. A systematic revision of the Sabellidae-Caobangiidae-Sabellongidae complex (Annelida: Polychaeta). Bull. Am. Mus. Nat. Hist. 1989, 192, 1–104. [Google Scholar]
- Macdonald, T.A. Phylogenetic relations among spirorbid subgenera and the evolution of opercular brooding. Hydrobiologia 2003, 496, 125–143. [Google Scholar] [CrossRef]
- Lehrke, J.; ten Hove, H.A.; Macdonald, T.A.; Bartolomaeus, T.; Bleidorn, C. Phylogenetic relationships of Serpulidae (Annelida: Polychaeta) based on 18S rDNA sequence data, and implications for opercular evolution. Org. Divers. Evol. 2007, 7, 195–206. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; ten Hove, H.A.; Sket, B.; Zakšek, V.; Trontelj, P.; Rouse, G.W. Evolution of a unique freshwater cave-dwelling serpulid polychaete Marifugia cavatica Absolon and Hrabĕ, 1930. Syst. Biodivers 2009, 7, 389–401. [Google Scholar] [CrossRef]
- Kupriyanova, E.K.; Nishi, E. Serpulidae (Annelida, Polychaeta) from Patton-Murray Seamount, Gulf of Alaska, North Pacific Ocean. Zootaxa 2010, 2665, 51–68. [Google Scholar] [CrossRef]
- Rzhavsky, A.V.; Kupriyanova, E.K.; Sikorski, A.V. Two new species of serpulid polychaetes from the Barents Sea. Fauna Norv. 2013, 32, 27–38. [Google Scholar] [CrossRef]
- Pillai, T.G. Studies on a collection of marine and brackish-water polychaete annelids of the family Serpulidae from Ceylon. Ceylon J. Sci. Biol. Sci. 1971, 9, 88–130. [Google Scholar]
- ten Hove, H.A.; Weerdenburg, J.C.A. A generic revision of the brackish-water serpulid Ficopomatus Southern 1921 (Polychaeta: Serpulinae), including Mercierella Fauvel 1923, Sphaeropomatus Treadwell 1934, Mercierellopsis Rioja 1945 and Neopomatus Pillai 1960. Biol. Bull. 1978, 154, 96–120. [Google Scholar] [CrossRef] [PubMed]
- Pillai, T.G. Ficopomatus talehsapensis, a new brackish-water species (Polychaeta: Serpulidae: Ficopomatinae) from Thailand, with discussions on the relationships of taxa constituting the subfamily, opercular insertion as a taxonomic character and their taxonomy, a key to its taxa, and their zoogeography. Zootaxa 2008, 1967, 36–52. [Google Scholar]
- Li, S.-C.; Wang, A.-T.; Deng, L. A new euryhaline species of the genus Ficopomatus Southern 1921 (Polychaeta: Serpulidae) from China. Zool. Stud. 2012, 51, 1165–1174. [Google Scholar]
- Styan, C.A.; McCluskey, C.F.; Sun, Y.; Kupriyanova, E.K. Cryptic sympatric species across the Australian range of the global estuarine invader Ficopomatus enigmaticus (Serpulidae, Annelida). Aquat. Invasions 2017, 12, 53–65. [Google Scholar] [CrossRef]
- Fauvel, P. Un nouveau serpulien d’eau saumâtre Mercierella n. g. enigmatica n. sp. Bull. Soc. Zool. France 1923, 47, 424–430. [Google Scholar]
- Absolon, K.; Hrabĕ, S. Über einen neuen Süsswasser–Polychaeten aus den Höhlengewässern der Herzegowina. Zool. Anz. 1930, 88, 249–264. [Google Scholar]
- Arteaga-Flórez, C.; Fernández-Rodríguez, V.; Londoño-Mesa, M.H. First record of the polychaete Ficopomatus uschakovi (Pillai, 1960) (Annelida, Serpulidae) in the Colombian Caribbean, South America. Zookeys 2014, 371, 1–11. [Google Scholar] [CrossRef]
- Caullery, M.; Mesnil, F. Note sur deux serpuliens nouveaux (Oriopsis metchnikowi n. g., n.sp. et Josephella marenzelleri n.g., n.sp.). Zool. Anz. 1896, 10, 482–486. [Google Scholar]
- Fauvel, P. Deuxième note préliminaire sur les Polychètes provenant des campagnes de l’Hirondelle et de la Princesse- Alice, ou déposées dans la Musée Océanographique de Monaco. Bull. Inst. Océanogr. 1909, 142, 1–76. [Google Scholar]
- Hartman, O. Catalogue of the Polychaetous Annelids of the World; University of Southern California Press: Los Angeles, CA, USA, 1959; Volume 23, pp. 1–628. [Google Scholar]
- ten Hove, H.A. Towards a phylogeny in serpulids (Annelida; Polychaeta). In Proceedings of the First International Polychaete Conference; Hutchings, P.A., Ed.; Linnean Society of New South Wales: Sydney, Australia, 1984; pp. 181–196. [Google Scholar]
- Kupriyanova, E.K. Life history evolution in Serpulimorph polychaetes: A phylogenetic analysis. Hydrobiologia 2003, 496, 105–114. [Google Scholar] [CrossRef]
- ten Hove, H.A.; Kupriyanova, E.K. Taxonomy of Serpulidae (Annelida, Polychaeta): The state of affairs. Zootaxa 2009, 2036, 1–126. [Google Scholar] [CrossRef]
- Rzhavsky, A.V.; Kupriyanova, E.K. Evolution of spirorbin brooding: A phylogenetic analysis and a test of an oxygen limitation hypothesis. Invertebr. Zool. 2019, 16, 409–430. [Google Scholar] [CrossRef]
- Pleijel, F. On character coding for phylogeny reconstruction. Cladistics 1995, 11, 309–315. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*. Phylogenetic Analysis Using Parsimony (and Other Methods); Version 4; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Nóren, M.; Jordelius, U. Phylogeny of Prolecithophora (Platyhelminthes) inferred from 18S rDNA sequences. Cladistics 1999, 15, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Osborn, K.J.; Rouse, G.W.; Goffredi, S.K.; Robison, B.H. Description and relationships of Chaetopterus pugaporcinus, an unusual pelagic polychaete (Annelida, Chaetopteridae). Biol. Bull. 2007, 212, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Rouse, G.W.; Hutchings, P.; Colgan, D. Assessing the usefulness of histone H3, U2 snRNA and 28S rDNA in analyses of polychaete relationships. Austr. J. Zool. 1999, 47, 499–516. [Google Scholar] [CrossRef]
- Burnette, A.B.; Struck, T.H.; Halanych, K.M. Holopelagic Poeobius meseres (“Poeobiidae,” Annelida) is derived from benthic flabelligerid worms. Biol. Bull. 2005, 208, 213–220. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Nixon, K.C. The Parsimony Ratchet, a new method for rapid parsimony analysis. Cladistics 1999, 15, 407–414. [Google Scholar] [CrossRef]
- Müller, K. PRAP—Computation of Bremer support for large data sets. Mol. Phylogenet. Evol. 2004, 31, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, G.; Lohman, D.J.; Meier, R. Sequence Matrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. RaxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Kozlov, A.M.; Diego, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Lewis, P.O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001, 50, 913–925. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.70. Available online: http://mesquiteproject.org (accessed on 12 December 2022).
- Kupriyanova, E.K.; Bastida-Zavala, R.; Halt, M.N.; Lee, M.; Rouse, G.W. Phylogeny of the Serpula-Crucigera-Hydroides clade (Serpulidae; Annelida) using molecular and morphological data: Implications for operculum evolution. Invertebr. Syst. 2008, 22, 425–437. [Google Scholar] [CrossRef]
- Bastida-Zavala, J.R.; ten Hove, H.A. Revision of Hydroides Gunnerus, 1768 (Polychaeta: Serpulidae) from the Eastern Pacific Region and Hawaii. Beaufortia 2003, 53, 67–110. [Google Scholar]
- Bok, M.J.; Capa, M.; Nilsson, D.-E. Here, there and everywhere: The radiolar eyes of fan worms (Annelida, Sabellidae). Integr. Comp. Biol. 2016, 56, 784–795. [Google Scholar] [CrossRef]
- Vinn, O.; Kirsimiäe, K.; ten Hove, H.A. Tube ultrastructure of Pomatoceros americanus (Polychaeta, Annelida): Implications for the tube formation of serpulids. Est. J. Earth Sci. 2009, 58, 148–152. [Google Scholar] [CrossRef]
- Ippolitov, A.P.; Vinn, O.; Kupriyanova, E.K.; Jäger, M. Written in stone: History of serpulid polychaetes through time. Mem. Mus. Vict. 2014, 71, 123–160. [Google Scholar] [CrossRef]
- Simon, C.; van Niekerk, H.; Burghardt, I.; ten Hove, H.A.; Kupriyanova, E.K. Not out of Africa: Spirobranchus kraussii (Baird, 1865) is not a global fouling and invasive serpulid of Indo-Pacific origin. Aquat. Invasions 2019, 14, 221–249. [Google Scholar] [CrossRef]



| Species | Vouchers | 28S | 18S | Histone H3 | Cytochrome b |
|---|---|---|---|---|---|
| Apomatus globifer | ZMA V.Pol. 5250 | EU195362 | EU195378 | OQ397982 | OQ427448 |
| Apomatus sp. | FMNH 5201 | OQ389662 | OQ379428 | OQ397983 | OQ427449 |
| Apomatus voightae | FMNH 6217 | OQ389663 | GU441856 | - | OQ427450 |
| Bathyditrupa hovei | ZMA V.Pol 5325 | - | - | - | - |
| Bathyvermilia eliasoni | FMNH 6189 | - | GU441857 | - | - |
| Chitinopoma serrula | SAM E3524 | EU195350 | DQ317112 | OQ397984 | - |
| Chitinopomoides wilsoni | ZMA V.Pol. 3166 | - | - | - | - |
| Crucigera inconstans | SAM E3525 | EU184071 | DQ317113 | - | EU190464 |
| Crucigera tricornis | SAM E3587 | EU184067 | EU184056 | - | EU190474 |
| Crucigera zygophora | SAM E3503 | DQ242577 | DQ242543 | EF192929 | EU190470 |
| Dasynema chrysogyrus | AM W.45087 | OQ397664 | OQ379429 | - | - |
| Ditrupa arietina | SAM E3527 | EU195351 | DQ317114 | EF192933 | - |
| Ficopomatus enigmaticus | SAM E3356 | EU195373 | DQ317115 | OQ427487 | OQ427451 |
| Ficopomatus macrodon | SAM E3618 | EU167535 | EU167532 | OQ412612 | KP863778 |
| Ficopomatus miamiensis | SAM E3617 | EU167534 | EU167531 | OQ397989 | KP863779 |
| Filograna implexa | SAM E3528 | EU195347 | DQ317116 | - | OQ427452 |
| Filogranella elatensis | SAM E3661 | EU195370 | EU195385 | - | - |
| Filogranula stellata | SAM E3606 | EU195358 | EU195374 | OQ397985 | - |
| Floriprotis sabiuraensis | SAM E3659 | EU195371 | EU195386 | - | OQ427453 |
| Floriprotis sabiuraensis | SAM E7192 | OQ389665 | OQ379430 | - | OQ427454 |
| Galeolaria caespitosa | SAM E3529 | OQ389666 | OQ379431 | OQ412631 | EU184054 |
| Galeolaria hystrix | SAM E3526 | EU256550 | DQ314839 | OQ397988 | EU200441 |
| Helicosiphon biscoeensis | SIO-BIC A4000 | OQ392408 | OQ379432 | OQ412613 | - |
| Hyalopomatus mironovi | SAM E3728 | OQ651975 | GU063862 | MT468421 | MT468442 |
| Hydroides elegans | SAM E3616 | EU195369 | EU195384 | OQ412614 | OQ427455 |
| Hydroides ezoensis | SAM E3584 | EU184077 | EU184062 | - | OQ427456 |
| Hydroides nikae | SAM E3530 | EU184072 | DQ317117 | - | EU190466 |
| Hydroides minax | SAM E3597 | EU184074 | EU184063 | - | EU190475 |
| Hydroides pseudouncinata | ZMA V.Pol. 5240 | EU184075 | DQ140403 | - | EU190467 |
| Hydroides sanctaecrucis | SAM E3625 | EU184076 | EU184061 | - | - |
| Hydroides trivesiculosa | SAM E3601 | EU184073 | EU184060 | OQ397992 | EU190476 |
| Hydroides tuberculata | SAM E3596 | OQ389667 | EU184059 | - | EU190473 |
| Janita fimbriata | AM W.42388 | OQ389668 | OQ379433 | - | OQ427457 |
| Josephella marenzelleri | SAM E3620 | EU195359 | EU195375 | - | OQ427458 |
| Laminatubus alvini | SAM E3531 | EU195355 | DQ317118 | OQ412616 | OQ427459 |
| Marifugia cavatica | SAM E3612 | EU167533 | EU167530 | OQ397990 | OQ427460 |
| Metavermilia acanthophora | SAM E3533 | EU195352 | DQ317119 | - | OQ427461 |
| Microprotula ovicellata | ZMA V.Pol. 4046 | - | - | - | - |
| Neomicrorbis azoricus | ZMA V.Pol. 3905 | - | - | - | - |
| Neovermilia globula | SAM E3586 | EU195363 | EU195379 | - | - |
| Spirodiscus grimaldii | ZMA V.Pol. 3906 | - | - | - | - |
| Omphalopomopsis langerhansii | NHMWAN14552.2054 | - | - | - | - |
| Paraprotis dendrova | SAM E3591 | EU195361 | EU195377 | - | - |
| Paraprotis pulchra | SAM E3665 | OQ389669 | OQ379434 | OQ412629 | OQ427462 |
| Paumotella takemoana | USNM 19432 | - | - | - | - |
| Placostegus sp. | SAM E3589 | OQ397665 | OQ379435 | OQ412628 | - |
| Placostegus tridentatus | SAM E3585 | EU195364 | OQ379436 | OQ412622 | - |
| Pomatostegus actinoceras | AM W.42378 | OQ389670 | OQ379437 | - | - |
| Pomatostegus stellatus | SAM E3607 | EU195367 | EU195382 | - | - |
| Protis hydrothermica | SAM E3541 | EU195356 | DQ317122 | - | - |
| Protis sp. | SAM E3727 | OQ389671 | OQ379438 | - | OQ427463 |
| Protula bispiralis | SAM E3657 | OQ389672 | OQ379439 | OQ412609 | OQ427464 |
| Protula tubularia | SAM E3542 | EU195349 | DQ317123 | EF192934 | OQ427465 |
| Pseudochitinopoma occidentalis | SAM E3501 | DQ242575 | DQ242542 | OQ412626 | OQ427466 |
| Pseudochitinopoma pavimentata | SAM E3660 | OQ397666 | OQ379440 | OQ412627 | OQ427467 |
| Pseudovermilia occidentalis | SAM E3613 | EU195368 | EU195383 | - | OQ427468 |
| Pyrgopolon ctenactis | SIO-BIC A25451 | OQ389673 | OQ379441 | OQ412625 | - |
| Rhodopsis pusilla | SAM E3621 | EU195360 | EU195376 | OQ397987 | OQ427469 |
| Salmacina sp. | SAM E3499 | EU256545 | DQ317126 | - | OQ427470 |
| Semivermilia annehoggettae | SAM E3628 | OQ389674 | OQ379442 | - | OQ427471 |
| Semivermilia elliptica | SAM E3664 | EU195372 | EU195387 | OQ397986 | OQ427472 |
| Semivermilia lylevaili | SAM E3629 | OQ397667 | OQ389601 | - | - |
| Serpula columbiana | SAM E3505 | DQ242576 | DQ317127 | - | EU190469 |
| Serpula concharum | ZMA V.Pol. 5245 | EU184066 | DQ140408 | - | EU190468 |
| Serpula jukesii | SAM E3536 | EU184069 | DQ317129 | - | EU190465 |
| Serpula narconensis | SIO-BIC A3469 | OQ389676 | OQ379443 | OQ397991 | - |
| Serpula uschakovi | SAM E3593 | EU184078 | EU184065 | - | EU190477 |
| Serpula vermicularis | SAM E3537 | EU184070 | DQ317128 | - | EU190479 |
| Serpula vittata | SAM E3594 | EU184079 | EU184064 | - | EU190471 |
| Serpula watsoni | SAM E3595 | EU184068 | EU184057 | - | EU190472 |
| Spiraserpula iugoconvexa | AM W.42093 | OQ389680 | OQ379444 | - | OQ427473 |
| Spirobranchus akitsushima | ZMA V.Pol. 3201 | EU195365 | EU195380 | - | OQ427474 |
| Spirobranchus corniculatus | ZMA V.Pol. 5247 | OQ389677 | OQ379446 | - | OQ427475 |
| Spirobranchus corniculatus | SAM E3608 | EU195366 | EU195381 | - | OQ427476 |
| Spirobranchus coronatus | SAM E3609 | OQ389678 | OQ379445 | OQ412624 | OQ427477 |
| Spirobranchus kraussii | AM W.49977 | OQ397668 | MK308673 | OQ412619 | MK308658 |
| Spirobranchus lima | SAM E3538 | EU256547 | DQ317130 | EF192930 | OQ427478 |
| Spirobranchus richardsmithi | SAM E3610 | OQ389679 | OQ379447 | - | OQ427479 |
| Spirobranchus taeniatus | SAM E3532 | EU195353 | DQ317120 | OQ412618 | OQ427480 |
| Spirobranchus triqueter | SAM E3534 | EU195348 | DQ317121 | EF192932 | OQ427481 |
| Tanturia zibrowii | ZMA V.Pol. 4668 | - | - | - | - |
| Turbocavus secretus | USNM 251863 | - | OQ379448 | OQ412611 | OQ427483 |
| Vermiliopsis infundibulum | ZMA V.Pol. 5248 | OQ389681 | DQ140411 | - | OQ427484 |
| Vermiliopsis labiata | SAM E3543 | EU256549 | DQ317131 | - | OQ427485 |
| Vermiliopsis pygidialis | SAM E3544 | EU256546 | DQ317132 | - | - |
| Vermiliopsis striaticeps | SAM E3545 | EU256548 | DQ317133 | EF192931 | OQ427486 |
| Vitreotubus digeronimoi | ZMA V.Pol.3907 | - | - | - | - |
| Zibrovermilia zibrowii | AM W.46387 | - | - | - | - |
| Protolaeospira tricostalis | SAM E3487 | DQ242606 | DQ318587 | EF192936 | - |
| Romanchella quadricostalis | SAM E3491 | DQ242608 | DQ242559 | EF192935 | - |
| Spirorbis tridentatus | SAM E3477 | DQ242602 | DQ242573 | OQ412623 | - |
| Manayunkia athalassia | SAM E3518 | DQ209245 | EF116202 | EF192917 | - |
| Schizobranchia insignis | GenBank | AY732225 | AY732222 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupriyanova, E.; ten Hove, H.A.; Rouse, G.W. Phylogeny of Serpulidae (Annelida, Polychaeta) Inferred from Morphology and DNA Sequences, with a New Classification. Diversity 2023, 15, 398. https://doi.org/10.3390/d15030398
Kupriyanova E, ten Hove HA, Rouse GW. Phylogeny of Serpulidae (Annelida, Polychaeta) Inferred from Morphology and DNA Sequences, with a New Classification. Diversity. 2023; 15(3):398. https://doi.org/10.3390/d15030398
Chicago/Turabian StyleKupriyanova, Elena, Harry A. ten Hove, and Greg W. Rouse. 2023. "Phylogeny of Serpulidae (Annelida, Polychaeta) Inferred from Morphology and DNA Sequences, with a New Classification" Diversity 15, no. 3: 398. https://doi.org/10.3390/d15030398
APA StyleKupriyanova, E., ten Hove, H. A., & Rouse, G. W. (2023). Phylogeny of Serpulidae (Annelida, Polychaeta) Inferred from Morphology and DNA Sequences, with a New Classification. Diversity, 15(3), 398. https://doi.org/10.3390/d15030398











