Dry Climate Filters Gymnosperms but Not Angiosperms through Seed Mass
Abstract
1. Introduction
2. Materials and Methods
2.1. Sources of Seed Mass and Species Distribution Data
2.2. Climatic Metrics
2.3. Methods
3. Results
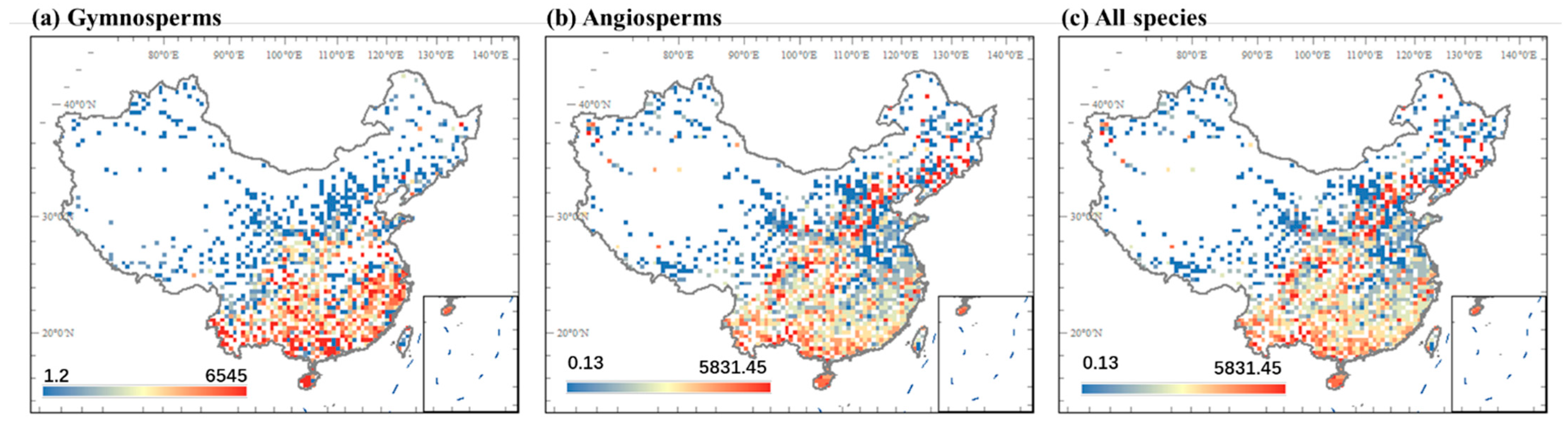
3.1. Geographic Patterns of Seed Mass
3.2. The Influence of Evolutionary History on Seed Mass
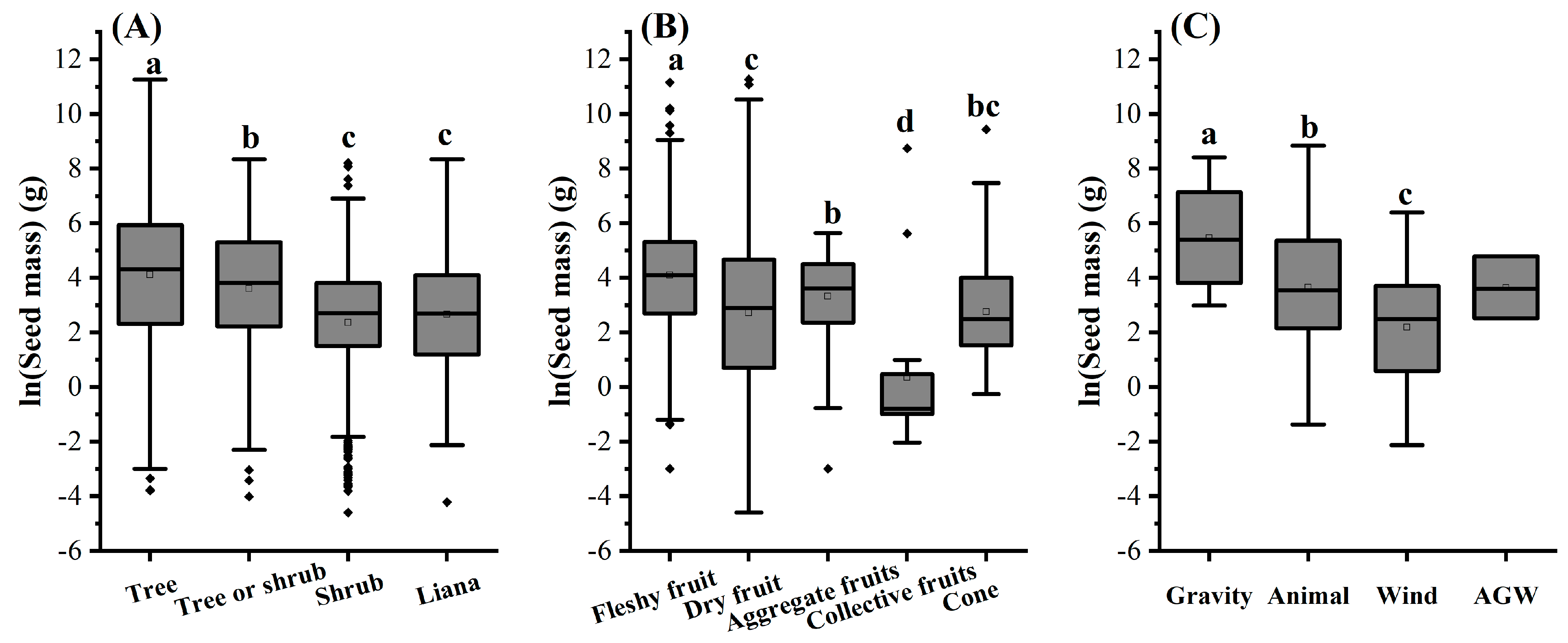
3.3. The Effects of Functional Traits on Seed Mass
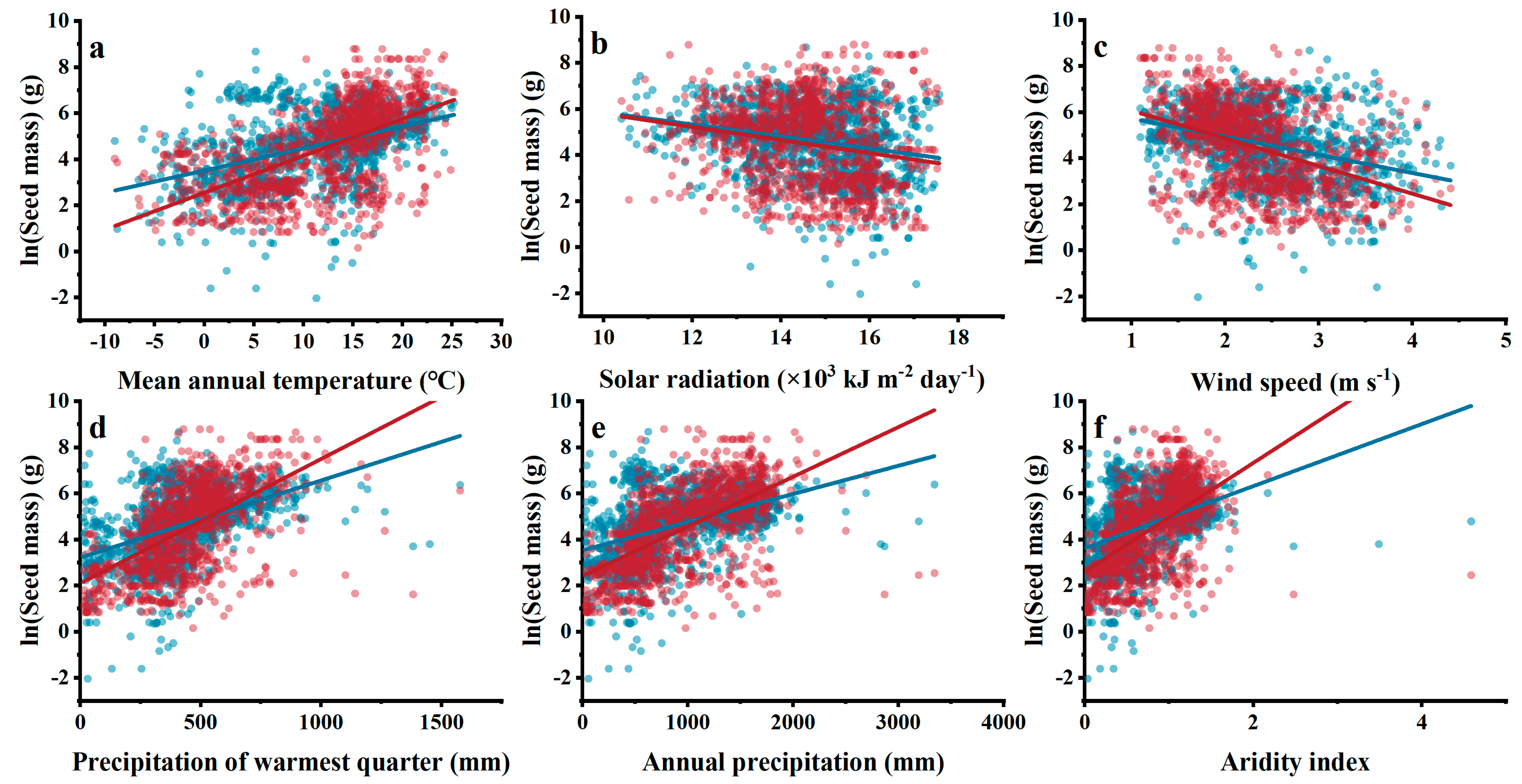
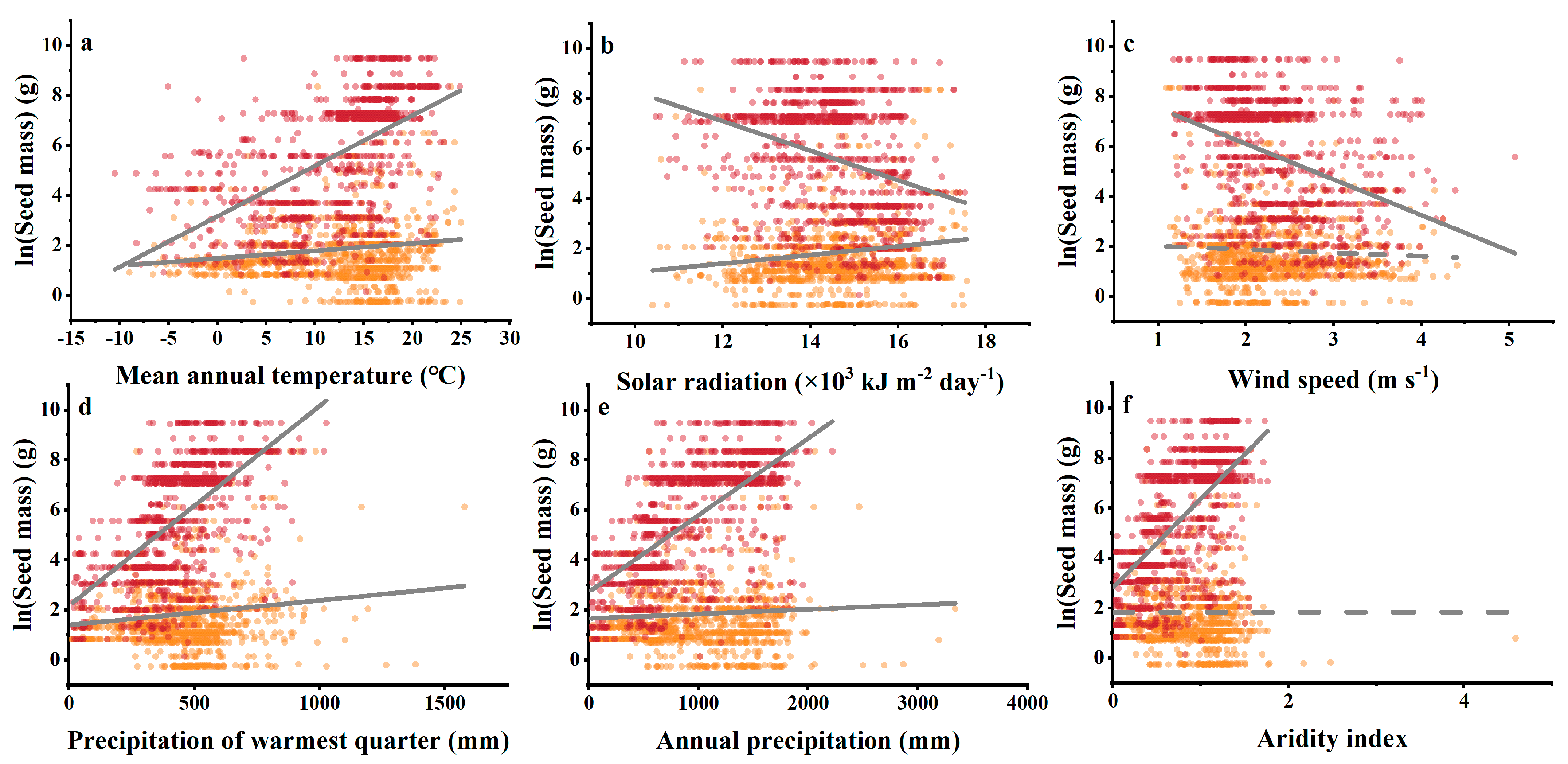
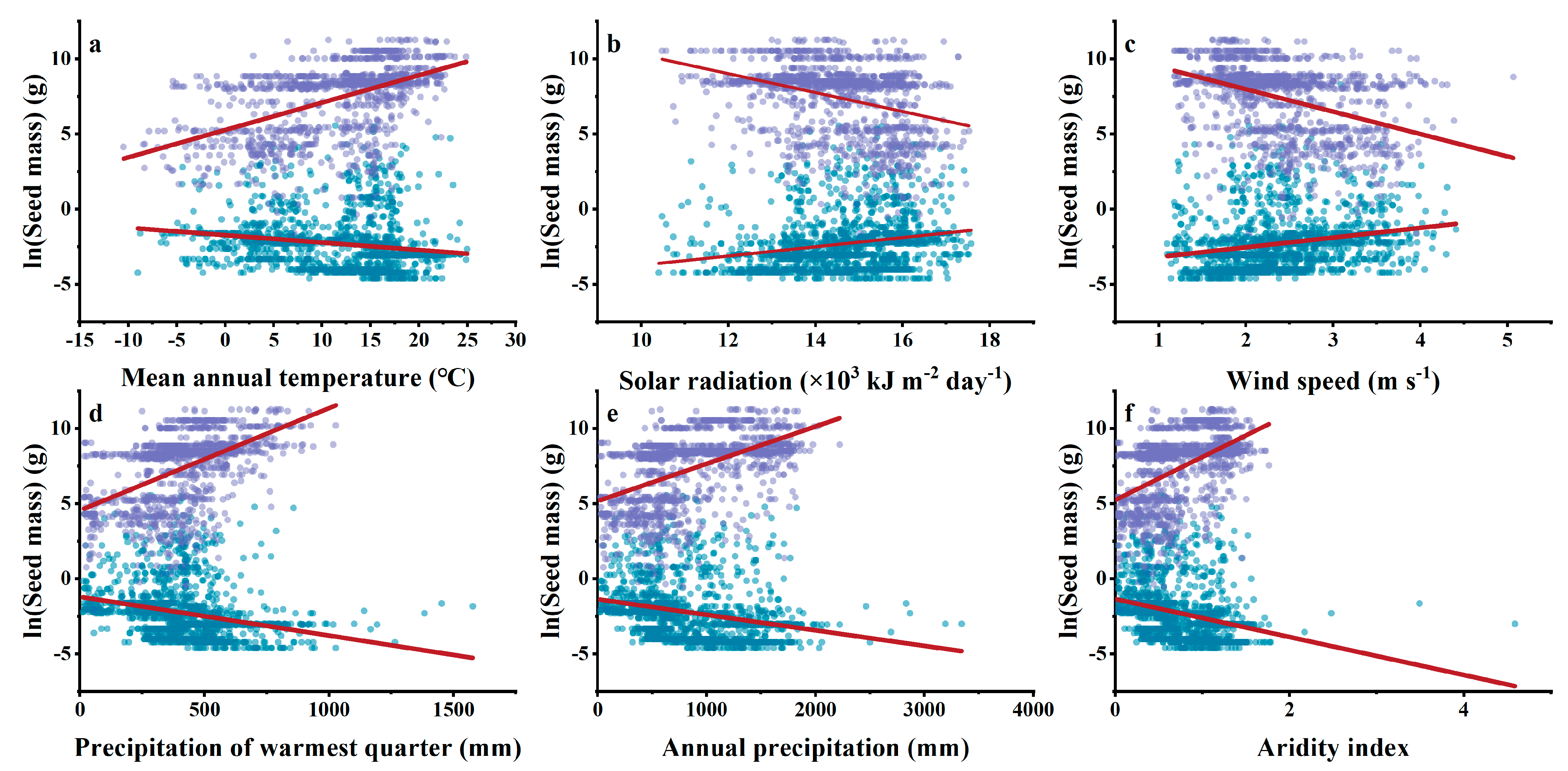
3.4. Relationships between Climatic Indices and Woody Plant Seed Mass
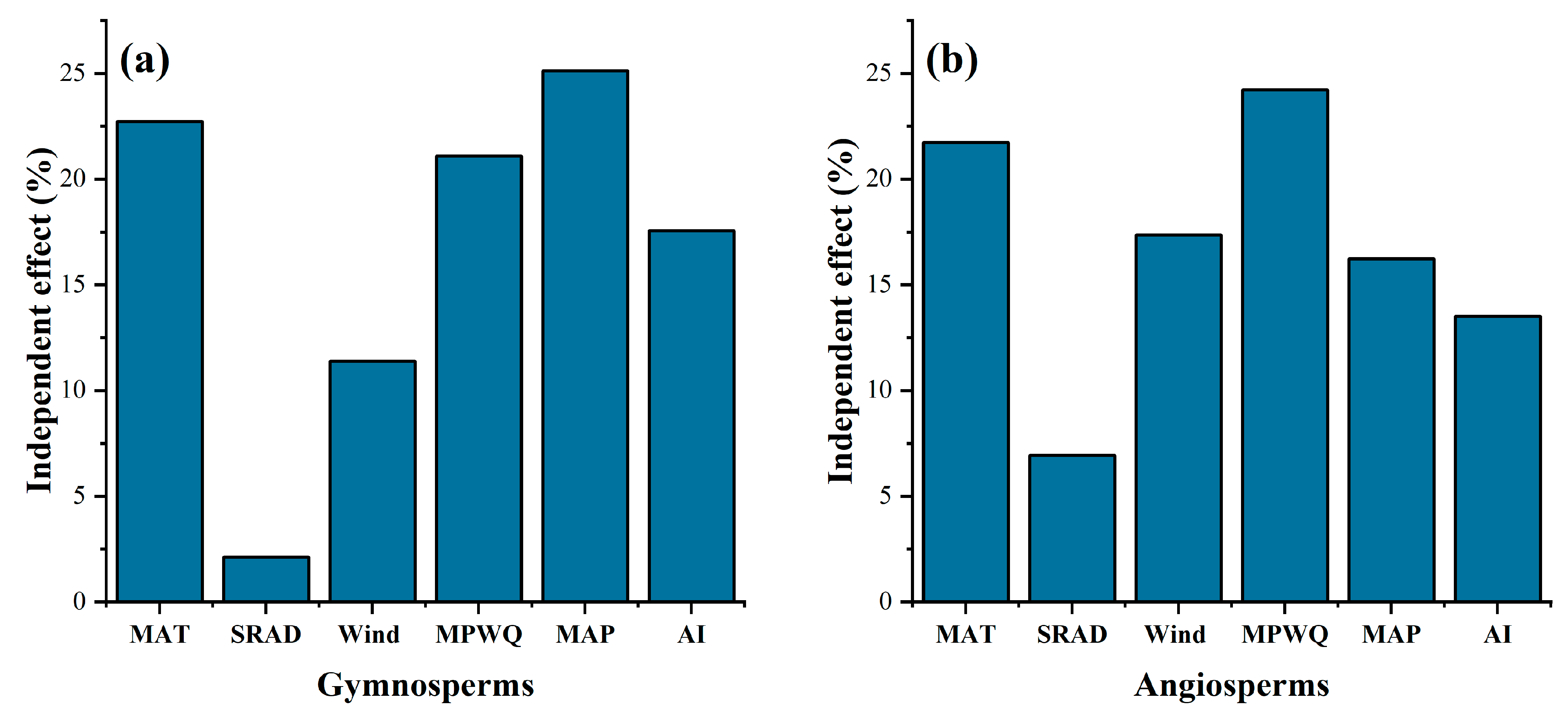
3.5. Contribution of Individual Environmental Factors to Seed Mass
4. Discussion
4.1. Distribution and Influencing Factors of Seed Mass
4.2. Differences in Effects of Environmental Factors on Gymnosperm and Angiosperm Seed Mass
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Willis, K.J.; McElwain, J.C. The Evolution of Plants; Oxford University Press: Oxford, UK, 2014. [Google Scholar]
- Leishman, M.R.; Wright, I.J.; Moles, A.T.; Westoby, M. The Evolutionary Ecology of Seed Size; Fenner, M., Ed.; CABI Publishing: Wallingford, UK, 2000; pp. 31–57. [Google Scholar]
- Saatkamp, A.; Cochrane, A.; Commander, L.; Guja, L.K.; Jimenez-Alfaro, B.; Larson, J.; Nicotra, A.; Poschlod, P.; Silveira, F.A.O.; Cross, A.T.; et al. A research agenda for seed-trait functional ecology. New Phytol. 2019, 221, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Leishman, M.R.; Westoby, M.; Jurado, E. Correlates of seed size variation: A comparison among five temperate floras. J. Ecol. 1995, 83, 517–529. [Google Scholar] [CrossRef]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Westoby, M. A brief history of seed size. Science 2005, 307, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.L.; Lovell, P.H.; Moore, K.G. The shapes and sizes of seeds. Annu. Rev. Ecol. Syst. 1970, 1, 327–356. [Google Scholar] [CrossRef]
- Tiffney, B.H. Vertebrate dispersal of seed plants through time. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 1–29. [Google Scholar] [CrossRef]
- Wookey, P.A.; Robinson, C.H.; Parsons, A.N.; Welker, J.M.; Press, M.C.; Callaghan, T.V.; Lee, J.A. Environmental constraints on the growth, photosynthesis and reproductive development of Dryas octopetala at a high Arctic Polar Semi-Desert, Svalbard. Oecologia 1995, 102, 478–489. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Pitman, A.J.; Westoby, M. Factors that shape seed mass evolution. Proc. Natl. Acad. Sci. USA 2005, 102, 10540–10544. [Google Scholar] [CrossRef]
- Baker, H.G. Seed weight in relation to environmental conditions in California. Ecology 1972, 53, 997–1010. [Google Scholar] [CrossRef]
- Alqudah, A.M.; Samarah, N.H.; Mullen, R.E. Drought Stress Effect on Crop Pollination, Seed Set, Yield and Quality; Springer: Dordrecht, The Netherlands, 2010; Volume 6, pp. 193–213. [Google Scholar]
- Neuschulz, E.L.; Mueller, T.; Schleuning, M.; Böhning-Gaese, K. Pollination and seed dispersal are the most threatened processes of plant regeneration. Sci. Rep. 2016, 6, 29839. [Google Scholar] [CrossRef]
- Emery, N.J.; Offord, C.A. Environmental factors influencing fruit production and seed biology of the critically endangered Persoonia pauciflora (Proteaceae). Folia Geobot. 2019, 54, 99–113. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Dulamsuren, C.; Wommelsdorf, T.; Zhao, F.; Xue, Y.; Zhumadilov, B.Z.; Leuschner, C.; Hauck, M. Increased summer temperatures reduce the growth and regeneration of Larix sibirica in southern boreal forests of Eastern Kazakhstan. Ecosystems 2013, 16, 1536–1549. [Google Scholar] [CrossRef]
- Worrall, J.J.; Rehfeldt, G.E.; Hamann, A.; Hogg, E.H.; Marchetti, S.B.; Michaelian, M.; Gray, L.K. Recent declines of Populus tremuloides in North America linked to climate. Forest Ecol. Manag. 2013, 299, 35–51. [Google Scholar] [CrossRef]
- Petrie, M.D.; Bradford, J.B.; Hubbard, R.M.; Lauenroth, W.K.; Andrews, C.M.; Schlaepfer, D.R. Climate change may restrict dryland forest regeneration in the 21st century. Ecology 2017, 98, 1548–1559. [Google Scholar] [CrossRef]
- Silva, C.S.; Puranik, S.; Round, A.; Brennich, M.; Jourdain, A.; Parcy, F.; Hugouvieux, V.; Zubieta, C. Evolution of the plant reproduction master regulators LFY and the MADS transcription factors: The role of protein structure in the evolutionary development of the flower. Front Plant Sci. 2016, 6, 1193. [Google Scholar] [CrossRef]
- Johnson, D.M.; McCulloh, K.A.; Woodruff, D.R.; Meinzer, F.C. Hydraulic safety margins and embolism reversal in stems and leaves: Why are conifers and angiosperms so different? Plant Sci. 2012, 195, 48–53. [Google Scholar] [CrossRef]
- Friedman, W.E. The meaning of Darwin’s “abominable mystery”. Am. J. Bot. 2009, 96, 5–21. [Google Scholar] [CrossRef]
- Onstein, R.E. Darwin’s second “abominable mystery”: Trait flexibility as the innovation leading to angiosperm diversity. New Phytol. 2020, 228, 1741–1747. [Google Scholar] [CrossRef]
- Crepet, W.L.; Niklas, K.J. Darwin’s second “abominable mystery”: Why are there so many angiosperm species? Am. J. Bot. 2009, 96, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Brodribb, T.J.; McAdam, S.A.M.; Jordan, G.J.; Martins, S.C.V. Conifer species adapt to low-rainfall climates by following one of two divergent pathways. Proc. Natl. Acad. Sci. USA 2014, 111, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Station, S.N.F.F. Seeds of Woody Plants in China; China Forestry Publishing House: Beijing, China, 2001. [Google Scholar]
- Fang, J.; Wang, Z.; Tang, Z. Atlas of Woody Plants in China: Distribution and Climate; Springer: Cham, Switzerland, 2011. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zomer, R.J.; Xu, J.; Trabucco, A. Version 3 of the global aridity index and potential evapotranspiration database. Sci. Data 2022, 9, 409. [Google Scholar] [CrossRef]
- Jin, Y.; Qian, H.V. PhyloMaker: An R package that can generate very large phylogenies for vascular plants. Ecography 2019, 42, 1353–1359. [Google Scholar] [CrossRef]
- Smith, S.A.; Brown, J.W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 2018, 105, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, S.P.; Garland, T.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef]
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877–884. [Google Scholar] [CrossRef]
- Revell, L.J. Phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Olea, P.P.; Mateo-Tomás, P.; de Frutos, Á.; Plaistow, S. Estimating and modelling bias of the hierarchical partitioning public-domain software: Implications in environmental management and conservation. PLoS ONE 2010, 5, e11698. [Google Scholar] [CrossRef]
- Hertel, D.; Therburg, A.; Villalba, R. Above- and below-ground response by Nothofagus pumilio to climatic conditions at the transition from the steppe–forest boundary to the alpine treeline in southern Patagonia, Argentina. Plant Ecol. Divers 2008, 1, 21–33. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Eriksson, O.; Friis, E.M.; Pedersen, K.R.; Crane, P.R. Seed size and dispersal systems of early Cretaceous angiosperms from Famalicão, Portugal. Int. J. Plant Sci. 2000, 161, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bellingham, P.J.; Sparrow, A.D. Resprouting as a life history strategy in woody plant communities. Oikos 2000, 89, 409–416. [Google Scholar] [CrossRef]
- Kruger, L.M.; Midgley, J.J.; Cowling, R.M. Resprouters vs. reseeders in South African forest trees; a model based on forest canopy height. Funct. Ecol. 1997, 11, 101–105. [Google Scholar] [CrossRef]
- Nzunda, E.F.; Lawes, M.J.; Lawes, M.J.; Clarke, P.J. Costs of resprouting are traded off against reproduction in subtropical coastal dune forest trees. Plant Ecol. 2011, 212, 1991–2001. [Google Scholar] [CrossRef]
- Bolmgren, K.; Eriksson, O. Seed mass and the evolution of fleshy fruits in angiosperms. Oikos 2010, 119, 707–718. [Google Scholar] [CrossRef]
- Bentos, T.V.; Mesquita, R.C.G.; Camargo, J.L.C.; Williamson, G.B. Seed and fruit tradeoffs–The economics of seed packaging in Amazon pioneers. Plant Ecol Divers 2014, 7, 371–382. [Google Scholar] [CrossRef]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Kingsford, R.T. Seed dispersal distance is more strongly correlated with plant height than with seed mass. J. Ecol. 2011, 99, 1299–1307. [Google Scholar] [CrossRef]
- Debain, S.; Curt, T.; Lepart, J. Seed mass, seed dispersal capacity, and seedling performance in a Pinus sylvestris population. Écoscience 2003, 10, 168–175. [Google Scholar] [CrossRef]
- Moles, A.T.; Perkins, S.E.; Laffan, S.W.; Flores-Moreno, H.; Awasthy, M.; Tindall, M.L.; Sack, L.; Pitman, A.; Kattge, J.; Aarssen, L.W.; et al. Which is a better predictor of plant traits: Temperature or precipitation? J. Veg. Sci. 2014, 25, 1167–1180. [Google Scholar] [CrossRef]
- Ramírez Valiente, J.A.; Solé Medina, A.; Pyhäjärvi, T.; Savolainen, O.; Cervantes, S.; Kesälahti, R.; Kujala, S.T.; Kumpula, T.; Heer, K.; Opgenoorth, L.; et al. Selection patterns on early-life phenotypic traits in Pinus sylvestris are associated with precipitation and temperature along a climatic gradient in Europe. New Phytol. 2021, 229, 3009–3025. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, H.; Xu, C.; Wu, X.; Liang, B.; Cao, J.; Chen, D. Modeling vegetation greenness and its climate sensitivity with deep-learning technology. Ecol. Evol. 2021, 11, 7335–7345. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.; Turnbull, T.L.; Adams, M.A. Disentangling respiratory acclimation and adaptation to growth temperature by Eucalyptus. New Phytol. 2012, 195, 149–163. [Google Scholar] [CrossRef]
- Murray, B.R.; Brown, A.; Dickman, C.R.; Crowther, M.S. Geographical gradients in seed mass in relation to climate. J. Biogeogr. 2004, 31, 379–388. [Google Scholar] [CrossRef]
- Simpson, A.H.; Richardson, S.J.; Laughlin, D.C. Soil–climate interactions explain variation in foliar, stem, root and reproductive traits across temperate forests. Global Ecol. Biogeogr. 2016, 25, 964–978. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Z.; Zhao, L.; Piao, S. Spatial patterns of climatological temperature lapse rate in mainland China: A multi-time scale investigation. J. Geophys. Res. Atmos. 2015, 120, 2661–2675. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, Q.; Singh, V.P.; Liu, L.; Shi, P. Spatiotemporal patterns of annual and seasonal precipitation extreme distributions across China and potential impact of tropical cyclones. Int. J. Climatol. 2017, 37, 3949–3962. [Google Scholar] [CrossRef]
- Sandel, B.; Goldstein, L.J.; Kraft, N.J.B.; Okie, J.G.; Shuldman, M.I.; Ackerly, D.D.; Cleland, E.E.; Suding, K.N. Contrasting trait responses in plant communities to experimental and geographic variation in precipitation. New Phytol. 2010, 188, 565–575. [Google Scholar] [CrossRef]
- Coomes, D.A.; Grubb, P.J. Colonization, tolerance, competition and seed-size variation within functional groups. Trends Ecol. Evol. 2003, 18, 283–291. [Google Scholar] [CrossRef]
- Pakeman, R.J.; Garnier, E.; Lavorel, S.; Ansquer, P.; Castro, H.; Cruz, P.; Doležal, J.; Eriksson, O.; Freitas, H.; Golodets, C.; et al. Impact of abundance weighting on the response of seed traits to climate and land use. J. Ecol. 2008, 96, 355–366. [Google Scholar] [CrossRef]
- Link, T.E.; Unsworth, M.; Marks, D. The dynamics of rainfall interception by a seasonal temperate rainforest. Agr. Forest Meteorol. 2004, 124, 171–191. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Valladares, F. Tolerance to shade, drought, and waterlogging of temperate northern hemisphere trees and shrubs. Ecol. Monogr. 2006, 76, 521–547. [Google Scholar] [CrossRef]
- Li, X.; Piao, S.; Wang, K.; Wang, X.; Wang, T.; Ciais, P.; Chen, A.; Lian, X.; Peng, S.; Peñuelas, J. Temporal trade-off between gymnosperm resistance and resilience increases forest sensitivity to extreme drought. Nat. Ecol. Evol. 2020, 4, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Hartmann, H.; Ciais, P.; Kimball, J.S.; Schwalm, C.R.; Camarero, J.J.; Chen, A.; Gentine, P.; Yang, Y.; et al. Timing and order of extreme drought and wetness determine bioclimatic sensitivity of tree growth. Earth’s Future 2022, 10, e2021E–e2530E. [Google Scholar] [CrossRef]
- DeSoto, L.; Cailleret, M.; Sterck, F.; Jansen, S.; Kramer, K.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Bigler, C.; Camarero, J.J.; et al. Low growth resilience to drought is related to future mortality risk in trees. Nat. Commun. 2020, 11, 545. [Google Scholar] [CrossRef]
- Stahl, U.; Kattge, J.; Reu, B.; Voigt, W.; Ogle, K.; Dickie, J.; Wirth, C. Whole-plant trait spectra of North American woody plant species reflect fundamental ecological strategies. Ecosphere 2013, 4, t128. [Google Scholar] [CrossRef]

| n | Blomberg’s K | Pagel’s λ | |||
|---|---|---|---|---|---|
| Seed Mass | K | P | λ | P | |
| 1580 | 0.150 | 0.001 | 0.777 | 0.000 | |
| Skewness | Kurtosis | |||
|---|---|---|---|---|
| Value | Standard Error | Value | Standard Error | |
| Gymnosperms | 0.919 | 0.182 | 0.474 | 0.361 |
| Angiosperms | −0.152 | 0.048 | 0.441 | 0.096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Liu, H.; Xu, C.; Dai, J.; Han, B. Dry Climate Filters Gymnosperms but Not Angiosperms through Seed Mass. Diversity 2023, 15, 401. https://doi.org/10.3390/d15030401
Qi Y, Liu H, Xu C, Dai J, Han B. Dry Climate Filters Gymnosperms but Not Angiosperms through Seed Mass. Diversity. 2023; 15(3):401. https://doi.org/10.3390/d15030401
Chicago/Turabian StyleQi, Yang, Hongyan Liu, Chongyang Xu, Jingyu Dai, and Biao Han. 2023. "Dry Climate Filters Gymnosperms but Not Angiosperms through Seed Mass" Diversity 15, no. 3: 401. https://doi.org/10.3390/d15030401
APA StyleQi, Y., Liu, H., Xu, C., Dai, J., & Han, B. (2023). Dry Climate Filters Gymnosperms but Not Angiosperms through Seed Mass. Diversity, 15(3), 401. https://doi.org/10.3390/d15030401









