Detection and Monitoring of Riverine Dragonfly of Community Interest (Insecta: Odonata): Proposal for a Standardised Protocol Based on Exuviae Collection
Abstract
:1. Introduction
2. Materials and Methods
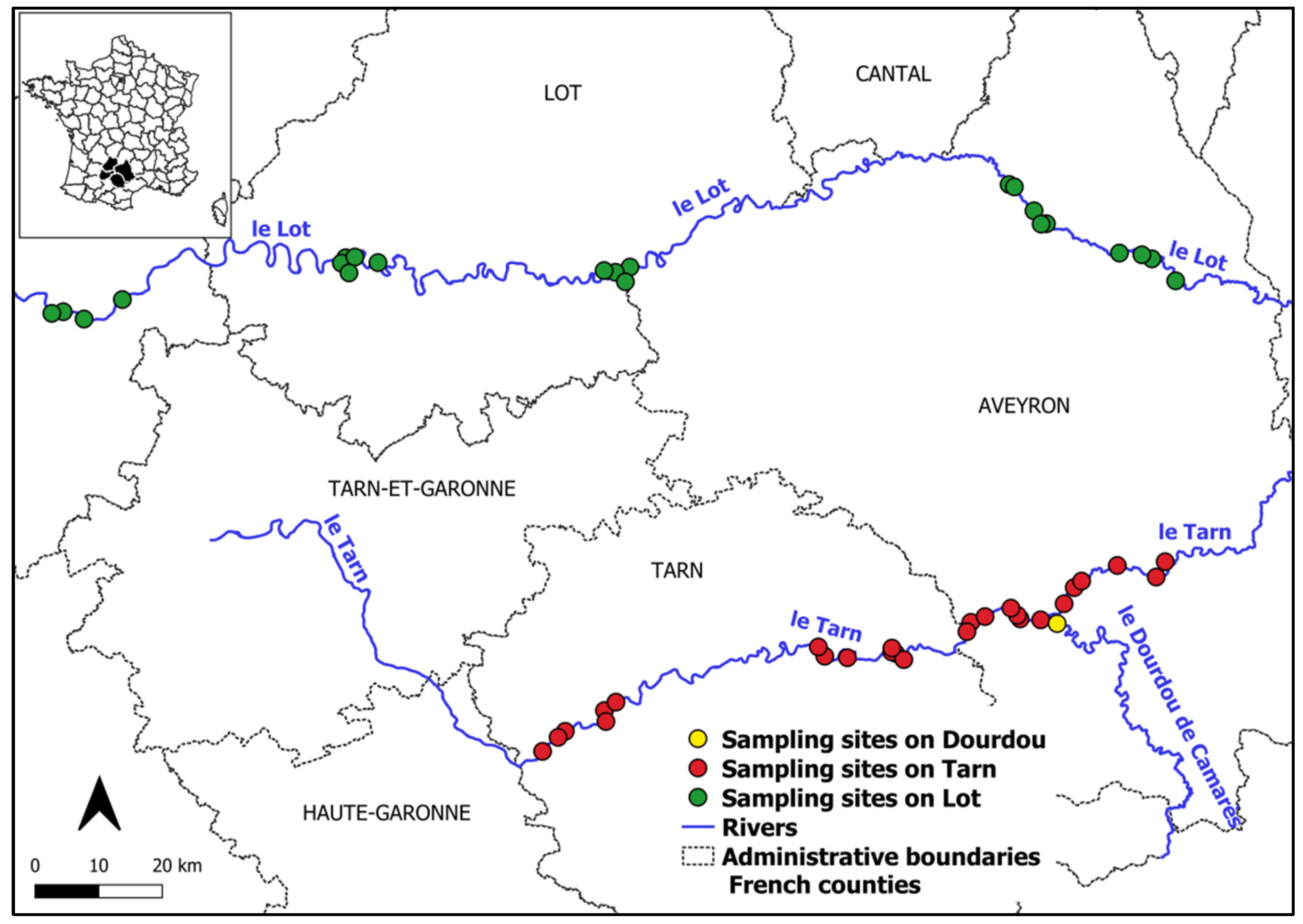
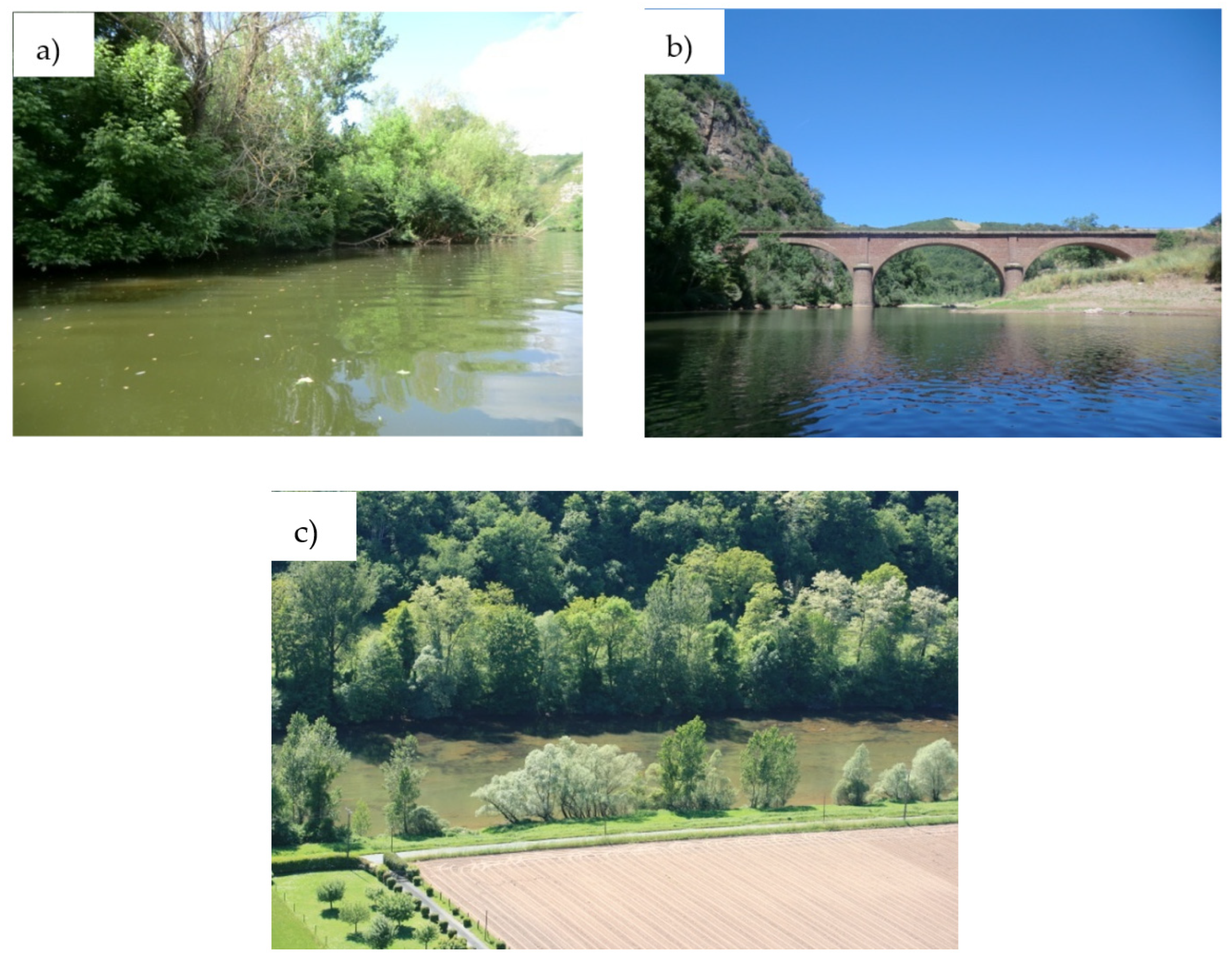
2.1. Monitoring Sites and Transects Location
2.2. Exuviae Collection
2.3. Practical Considerations: Time and Cost Required for Such a Protocol
2.4. Data Analysis
3. Results
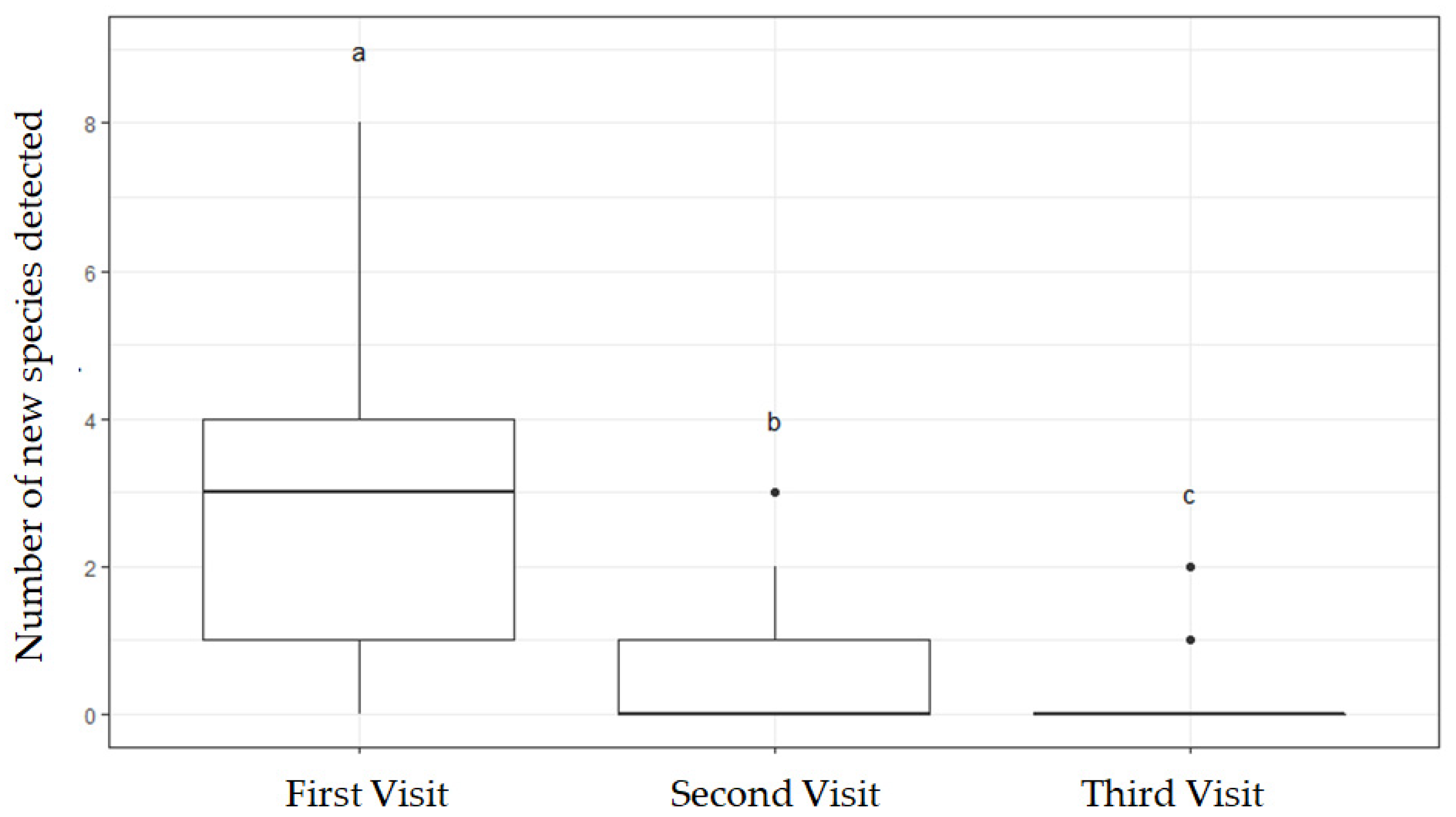
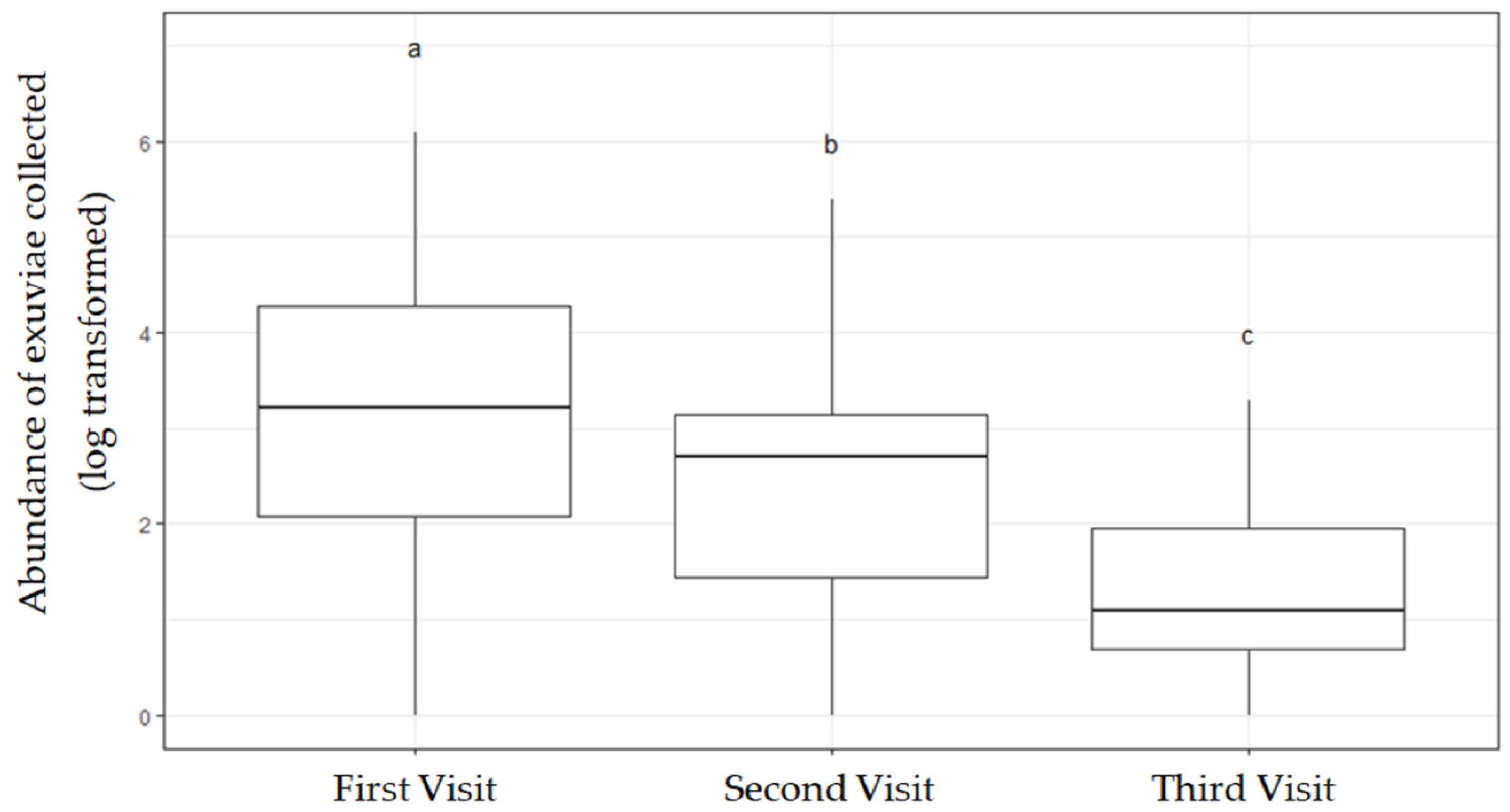
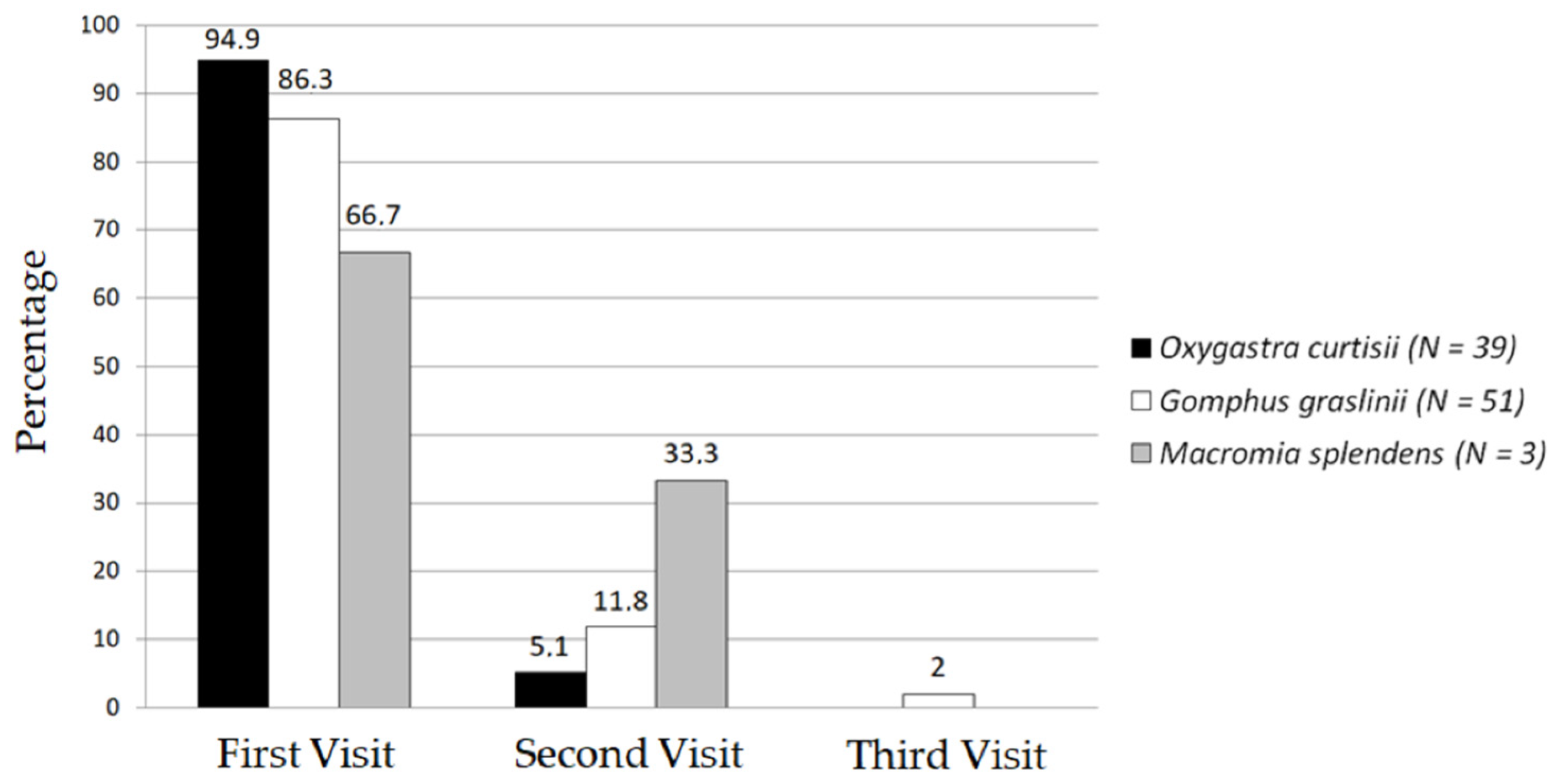
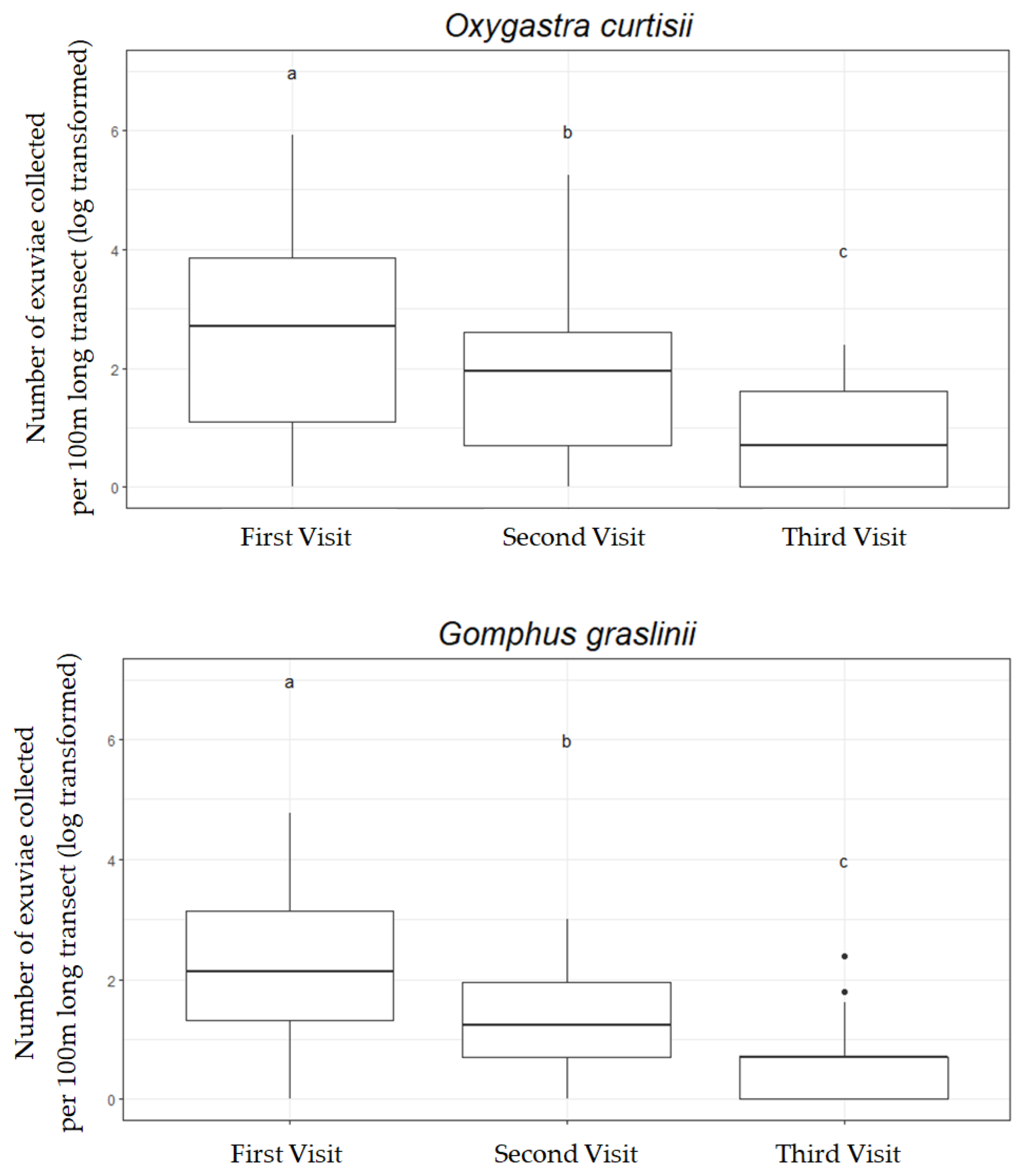
3.1. Number of Visits
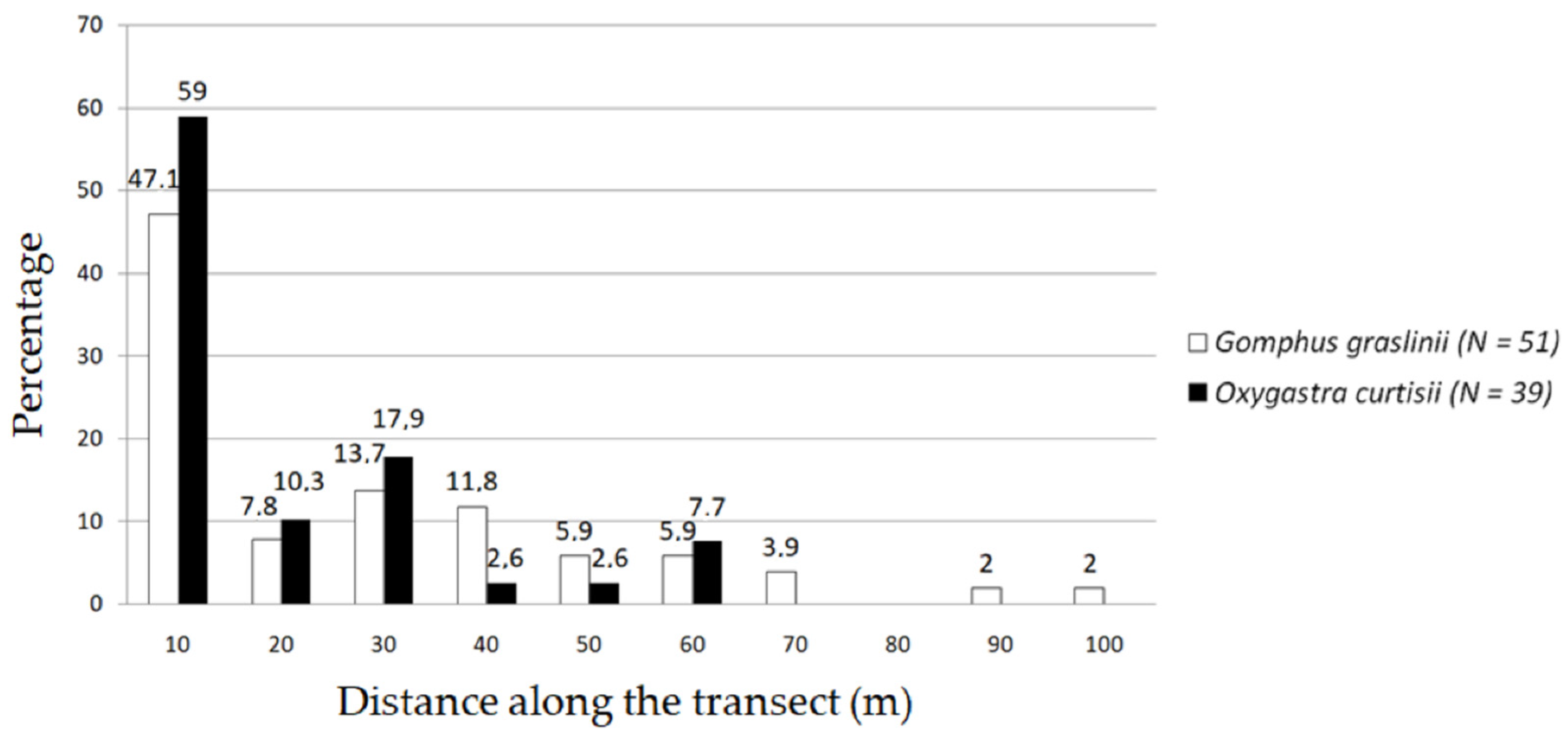
3.2. Transect Length
3.3. Practical Considerations: How Long Did It Take and How Much Did It Cost?
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Didham, R.K.; Barbero, F.; Collins, C.M.; Forister, M.L.; Hassall, C.; Leather, S.R.; Packer, L.; Saunders, M.E.; Stewart, A.J.A. Spotlight on Insects: Trends, Threats and Conservation Challenges. Insect Conserv. Divers. 2020, 13, 99–102. [Google Scholar] [CrossRef]
- Didham, R.K.; Basset, Y.; Collins, C.M.; Leather, S.R.; Littlewood, N.A.; Menz, M.H.M.; Müller, J.; Packer, L.; Saunders, M.E.; Schönrogge, K.; et al. Interpreting Insect Declines: Seven Challenges and a Way Forward. Insect Conserv. Divers. 2020, 13, 103–114. [Google Scholar] [CrossRef]
- Hallmann, C.A.; Sorg, M.; Jongejans, E.; Siepel, H.; Hofland, N.; Schwan, H.; Stenmans, W.; Müller, A.; Sumser, H.; Hörren, T.; et al. More than 75 Percent Decline over 27 Years in Total Flying Insect Biomass in Protected Areas. PLoS ONE 2017, 12, e0185809. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G. Where Have All the Insects Gone? Science 2017, 356, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Worldwide Decline of the Entomofauna: A Review of Its Drivers. Biol. Conserv. 2019, 232, 8–27. [Google Scholar] [CrossRef]
- Van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-Analysis Reveals Declines in Terrestrial but Increases in Freshwater Insect Abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Denis, A.S. Impacts de l’anthropisation sur la Diversité Odonatologique au Sein des cours d’eau: Vers une Meilleure Prise en Compte des Espèces de la Directive Habitats Faune Flore. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2018. [Google Scholar]
- Dommanget, J.-L. Etude Faunistique et Bibliographique des Odonates de France; INRA-Muséum National d’Histoire Naturelle: Paris, France, 1987; p. 277. [Google Scholar]
- Dijkstra, K.-D.B. Guide Des Libellules de France et d’Europe; Guide Delachaux; Delachaux et Niestlé: Paris, France, 2015; ISBN 978-2-603-02153-8. [Google Scholar]
- de Las Heras, M.; Cordero-Rivera, A.; Cabana, M.; Romeo, A.; Rey-Muñiz, X.L.; Mezquita, I.; Gainzarain, J.A.; Salvador Vilariño, V.; Evangelio-Pinach, J.M.; Díaz, C.; et al. Distribución Ibérica de Gomphus Graslinii, Oxygastra Curtisii y Macromia Splendens (Insecta: Odonata), Especies Protegidas Por La Directiva Hábitats. Bol. Rola 2017, 9, 15–54. [Google Scholar]
- Houard, X. Plan National d’actions En Faveur Des Libellules-Agir Pour La Préservation Des Odonates Menacés et de Leurs Habitats 2020–2030; Office pour les Insectes et leur Environnement-DREAL Hauts-de-France-Ministère de la transition écologique: Toulouse, France, 2020; p. 66. [Google Scholar]
- European Union. Council Directive 92/43/EEC 1992 on the Conservation of Natural Habitats and of Wild Flora and Fauna. Off. J. Eur. Communities 1992, 206, 7–49. [Google Scholar]
- Costes, A.; Danflous, S.; Laurent, B.; Delpon, G. Premier retour d’expérience sur la prise en compte des odonates dans les DOCOB des sites Natura 2000 de Midi-Pyrénées. In Les Invertébrés dans la Conservation et la Gestion des Espaces Naturels; Muséum national d’Histoire naturelle: Toulouse, France, 2015; pp. 22–25. [Google Scholar]
- Dupont, P. Plan national d’actions en faveur des Odonates-Libellules et Demoiselles menacées 2011–2015; Office pour les Insectes et leur Environnement/Société Française d’Odonatologie-Ministère de l’Ecologie, de l’Energie et du Développement durable et de la Mer: Toulouse, France, 2010; p. 170. [Google Scholar]
- Kalkman, V.; Boudot, J.-P.; Bernard, R.; Conze, K.-J.; De Knijf, G.; Dyatlova, E.; Ferreira, S.; Jović, M.; Ott, J.; Riservato, E.; et al. European Red List of Dragonflies; Publications Office: Luxembourg, 2010; p. 28. [Google Scholar]
- UICN France, MNHN, OPIE & SFO. La Liste Rouge Des Espèces Menacées En France; Chapitre Libellules de France Métropolitaine; UICN France, MNHN, OPIE & SFO: Paris, France, 2016; p. 5. [Google Scholar]
- Charlot, B.; Danflous, S.; Louboutin, B.; Jaulin, S. Liste Rouge Des Odonates d’Occitanie-Rapport d’évaluation; CEN Midi-Pyrénées & OPIE: Toulouse, France, 2018; p. 102. [Google Scholar]
- Barneix, M.; Bailleux, G.; Soulet, D. Liste Rouge régionale des Odonates d’Aquitaine; Observatoire Aquitain de la Faune Sauvage (Coordination): Pessac, France, 2016; p. 40. [Google Scholar]
- Deliry, C. Le Groupe Sympetrum Liste Rouge des Odonates de la région Rhône-Alpes. In Histoires Naturelles; Flammarion: Paris, France, 2014; p. 35. [Google Scholar]
- Ministre de l’Ecologie et du Développement Durable Arrêté Du 23/04/07 Fixant Les Listes Des Insectes Protégés Sur l’ensemble Du Territoire et Les Modalités de Leur Protection. 2007. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000000465500/ (accessed on 25 June 2022).
- Foster, S.E.; Soluk, D.A. Evaluating Exuvia Collection as a Management Tool for the Federally Endangered Hine’s Emerald Dragonfly, Somatochlora Hineana Williamson (Odonata: Cordulidae). Biol. Conserv. 2004, 118, 15–20. [Google Scholar] [CrossRef]
- Oertli, B. The Use of Dragonflies in the Assessment and Monitoring of Aquatic Habitats. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Oxford Biology: New York, NY, USA, 2008; pp. 79–95. ISBN 978-0-19-923069-3. [Google Scholar]
- Hardersen, S. Dragonfly (Odonata) Communities at Three Lotic Sites with Different Hydrological Characteristics. Ital. J. Zool. 2008, 75, 271–283. [Google Scholar] [CrossRef]
- Giugliano, L.; Hardersen, S.; Santini, G. Odonata Communities in Retrodunal Ponds: A Comparison of Sampling Methods. Int. J. Odonatol. 2012, 15, 13–23. [Google Scholar] [CrossRef]
- Hardersen, S.; Corezzola, S.; Gheza, G.; Dell’Otto, A.; La Porta, G. Sampling and Comparing Odonate Assemblages by Means of Exuviae: Statistical and Methodological Aspects. J. Insect Conserv. 2017, 21, 207–218. [Google Scholar] [CrossRef]
- Raebel, E.M.; Merckx, T.; Riordan, P.; Macdonald, D.W.; Thompson, D.J. The Dragonfly Delusion: Why It Is Essential to Sample Exuviae to Avoid Biased Surveys. J. Insect Conserv. 2010, 14, 523–533. [Google Scholar] [CrossRef]
- Da Silva-Méndez, G.; Riso, S.; Lorenzo-Carballa, M.O.; Cordero-Rivera, A. Sampling Larvae, Exuviae or Adults of Odonata for Ecological Studies: A Test of Methods in Permanent Rivers in the Iberian Peninsula. Odonatologica 2022, 51, 63–81. [Google Scholar]
- Bried, J.T.; D’Amico, F.; Samways, M.J. A Critique of the Dragonfly Delusion Hypothesis: Why Sampling Exuviae Does Not Avoid Bias: Critiquing the Dragonfly Delusion. Insect Conserv. Divers. 2012, 5, 398–402. [Google Scholar] [CrossRef]
- Luque, P. Macromia Splendens i Gomphus Graslinii, Dues Noves Espècies d’odonats per a Catalunya. Butlletí Inst. Catalana Història Nat. 2006, 74, 113–116. [Google Scholar]
- Martínez-Martínez, D.; Olmo-Vidal, J.M.; Luque Pino, P.; Querol, J.M.; del Mai, M.J.M.; Casanova, C.B.; Pujol, A.G. Seguimiento de La Población de Macromia Splendens Mediante El Muestreo de Exuvias En La Terra Alta, Cataluña. In Proceedings of the Simposio Ibérico de Odonatología SIO-2015, Córdoba, Spain, 1–3 May 2015; Asociación de Educación Ambiental El Bosque Animado/Red de Observadores de Libélulas en Andalucía: Málaga, Spain, 2015. [Google Scholar]
- Doucet, G. Clé de Détermination Des Exuvies Des Odonates de France, 3rd ed.; Société Française d’Odonatologie; Fondation Nature & Découvertes: Bois d’Arcy, France, 2016; ISBN 979-10-94955-00-0. [Google Scholar]
- Boudot, J.-P.; Grand, D.; Wildermuth, H.; Monnerat, C. Les Libellules de France, Belgique et Luxembourg, 2nd ed.; Parthenope; Biotope: Mèze, France, 2017; ISBN 978-2-36662-176-1. [Google Scholar]
- Ferreras-Romero, M.; Corbet, P.S. Seasonal Patterns of Emergence in Odonata of a Permanent Stream in Southwestern Europe. Aquat. Insects 1995, 17, 123–127. [Google Scholar] [CrossRef]
- Suhling, F. Temporal Patterns of Emergence of the Riverine Dragonfly Onychogomphus Uncatus (Odonata: Gomphidae). Hydrobiologia 1995, 302, 113–118. [Google Scholar] [CrossRef]
- Farkas, A.; Jakab, T.; Tóth, A.; Kalmár, A.F.; Dévai, G. Emergence Patterns of Riverine Dragonflies (Odonata: Gomphidae) in Hungary: Variations between Habitats and Years. Aquat. Insects 2012, 34, 77–89. [Google Scholar] [CrossRef]
- Zebsa, R.; Khelifa, R.; Kahalerras, A. Emergence Pattern, Microhabitat Choice, and Population Structure of the Maghribian Endemic Gomphus Lucasii Selys, 1849 (Odonata: Gomphidae) in Northeastern Algeria. Aquat. Insects 2014, 36, 245–255. [Google Scholar] [CrossRef]
- Maingeot, M.; Motte, G.; Goffart, P. Première Étude de l’émergence de La Cordulie à Corps Fin (Oxygastra Curtisii) Le Long de l’Ourthe. Nat. Belg. 2015, 96, 57–83. [Google Scholar]
- Golfieri, B.; Hardersen, S.; Maiolini, B.; Surian, N. Odonates as Indicators of the Ecological Integrity of the River Corridor: Development and Application of the Odonate River Index (ORI) in Northern Italy. Ecol. Indic. 2016, 61, 234–247. [Google Scholar] [CrossRef]
- Hardersen, S.; Toni, I. Proposal for a Time-Based Standard Sampling Method for the Monitoring of Gomphus Flavipes (Charpentier, 1825) and Ophiogomphus Cecilia (Fourcroy, 1785) (Odonata: Gomphidae). Fragm. Entomol. 2019, 51, 55–62. [Google Scholar] [CrossRef]
- Dommanget, J.-L. Etude de Macromia splendens (Pictet, 1843) dans la vallée du Tarn (Tarn, Aveyron) et statut national de l’espèce (Odonata, Anisoptera, Macromiidae); Société Française d’Odonatologie-Ministère de l’Aménagement du Territoire et de l’Environnement Direction de la Nature et des Paysages: Paris, France, 2001; p. 134. [Google Scholar]
- Cordero-Rivera, A. Distribution, Habitat Requirements and Conservation of Macromia Splendens Pictet (Odonata: Corduliidae) in Galicia (Nw Spain). Int. J. Odonatol. 2000, 3, 73–83. [Google Scholar] [CrossRef]
- Lohr, M. Sur l’habitat et la répartition de Macromia splendens (Pictet, 1843) et Gomphus graslinii (Dale, 1834) dans la rivière de l’Hérault (département de l’Hérault); Société Française d’Odonatologie: Vallet, Loire-Atlantique, France, June 2005; pp. 115–124. [Google Scholar]
- Denis, A.S.; Payet, O.; Danflous, S.; Gouix, N.; Santoul, F.; Buisson, L.; Pelozuelo, L. Intraspecific Variability of the Phenology and Morphology of Three Protected Dragonflies between Natural and Artificial Habitats. J. Insect Conserv. 2018, 22, 419–431. [Google Scholar] [CrossRef]
- Danflous, S.; Norel, H.; Charlot, B.; Lim, M. Inventaire & Suivi Des Odonates d’intérêt Communautaire Sur Le Barrage Du Pinet–Résultats 2021; CEN Occitanie: Montpellier, France, 2021; p. 57. [Google Scholar]
- Cordero-Rivera, A.; Utzeri, C.; Santolamazza Carbone, S. Emergence and Adult Behaviour of Macromia Splendens (Pictet) in Galicia, Northwestern Spain (Anisoptera: Cordullidae). Odonatologica 1999, 28, 333–342. [Google Scholar]
- Lieftinck, M.A. Macromia Splendens (Pictet, 1843) in Europe with Notes on Its Habits, Larva, and Distibution (Odonata). Tijdschr. Voor Entomol. 1965, 108, 41–59. [Google Scholar]
- Bilek, A. Ergänzende Beobachtungen Zur Lebensweise von Macromia Splendens (Pictet 1843) Und Einingen Anderen in Der Guyenne Vorkommenden Odonata-Arten. Entomol. Z. 1969, 79, 117–124. [Google Scholar]
- Pelozuelo, L.; Costes, A.; Delpon, G.; Calvignac, R.; Alquier, D.; Haber, E.; Polisset, P. Macromia Splendens en Midi Pyrénées: Enfin des Nouvelles en 2012! OPIE Midi-Pyrénées & LPO Tarn: Labruguière, France, 2012; pp. 1–15. [Google Scholar]
- Danflous, S.; Norel, H.; Charlot, B.; Lim, M. Inventaire & Suivi Des Odonates d’intérêt Communautaire Sur le Barrage Du Pinet; CEN Occitanie: Montpellier, France, 2020; p. 53. [Google Scholar]
- Chovanec, A.; Waringer, J. Ecological Integrity of River-Floodplain Systems-Assessment by Dragonfly Surveys (Insecta: Odonata). Regul. Rivers Res. Manag. 2001, 17, 493–507. [Google Scholar] [CrossRef]
- Corbet, P.S. Are Odonata Useful as Bioindicators? Libellula 1993, 12, 91–102. [Google Scholar]
- Baeta, R.; Bard, D.; Chantereau, M.; Fritsch, B.; Herbrecht, F.; Hudin, S.; Itrac-Bruneau, R.; Multeau, D.; Paillat, R.; Rambourdin, M.; et al. Protocole de Suivi Diachronique Des Populations Ligériennes de Gomphus Flavipes et d’Ophiogomphus Cecilia. 2015. 6p + annexes. Available online: http://odonates.pnaopie.fr/wp-content/uploads/2015/02/GomphesdeLoire_Protocole_avril2015.pdf (accessed on 25 June 2022).
- DuBois, R.B. Detection Probabilities and Sampling Rates for Anisoptera Exuviae along River Banks: Influences of Bank Vegetation Type, Prior Precipitation, and Exuviae Size. Int. J. Odonatol. 2015, 18, 205–215. [Google Scholar] [CrossRef]
- Baeta, R. Suivi Diachronique Des Populations Ligériennes de Gomphus Flavipes et d’Ophiogomphus Cecilia En Région Centre Val-de-Loire (Saison 2016-Deuxième Année de Suivi à l’échelle Régionale). 2017, p. 13. Available online: http://www.anepe-caudalis.fr/wa_files/GomphesLoire_IndreetLoire_Saison2016_VF.pdf (accessed on 25 June 2022).
- Baeta, R.; Herbrecht, F.; Fierimonte, B. Stylurus flavipes et Ophiogomphus cecilia deux mystérieuses libellules. Loire Terroirs 2020, 105, 10–17. [Google Scholar]
- Aliberti Lubertazzi, M.A.; Ginsberg, H.S. Persistence of Dragonfly Exuviae on Vegetation and Rock Substrates. Northeast. Nat. 2009, 16, 141–147. [Google Scholar] [CrossRef]
- Rach, J.; DeSalle, R.; Sarkar, I.N.; Schierwater, B.; Hadrys, H. Character-Based DNA Barcoding Allows Discrimination of Genera, Species and Populations in Odonata. Proc. R. Soc. B Biol. Sci. 2008, 275, 237–247. [Google Scholar] [CrossRef]
- Damm, S.; Schierwater, B.; Hadrys, H. An Integrative Approach to Species Discovery in Odonates: From Character-Based DNA Barcoding to Ecology. Mol. Ecol. 2010, 19, 3881–3893. [Google Scholar] [CrossRef]
- Schmidt, K.J.; Soluk, D.A.; Maestas, S.E.M.; Britten, H.B. Persistence and Accumulation of Environmental DNA from an Endangered Dragonfly. Sci. Rep. 2021, 11, 18987. [Google Scholar] [CrossRef]



| Species | Conservation Status | French Region 3 | Legal Status | ||
|---|---|---|---|---|---|
| Europe 1 | France 2 | Europe 4 | France 5 | ||
| M. splendens | VU | VU | VU (Occitanie) | Annexes II et IV | Art. 2 |
| EN (Aquitaine) | |||||
| VU (Rhônes-Alpes) | |||||
| G. graslinii | NT | LC | NT (Occitanie) | Annexes II et IV | Art. 2 |
| LC (Aquitaine) | |||||
| VU (Rhônes-Alpes) | |||||
| O. curtisii | NT | LC | LC (Occitanie) | Annexes II et IV | Art. 2 |
| LC (Aquitaine) | |||||
| LC (Rhônes-Alpes) | |||||
| Odonata Species | Rivers | ||
|---|---|---|---|
| Lot (n = 44) | Tarn (n = 52) | Dourdou de Camarès (n = 2) | |
| *O. curtisii | 22 (50) | 16 (30.8) | 1 (50) |
| *M. splendens | 1 (2.3) | 2 (3.8) | - |
| *G. graslinii | 27 (61.4) | 24 (46.2) | - |
| *G. vulgatissimus | 23 (52.3) | 31 (59.6) | 2 (100) |
| *O. forcipatus | 34 (72.3) | 45 (86.5) | 2 (100) |
| *B. irene | 26 (59.1) | 27 (51.9) | 1 (50) |
| S. metallica | 12 (27.3) | 6 (11.5) | - |
| O. cancellatum | 16 (36.4) | 1 (1.9) | - |
| G. pulchellus | 5 (11.4) | 6 (11.5) | - |
| A. imperator | 4 (9.1) | 2 (3.8) | - |
| A. parthenope | 4 (9.1) | 2 (3.8) | - |
| Odonata Species | Rivers | ||
|---|---|---|---|
| Lot (n = 44) | Tarn (n = 52) | Dourdou de Camarès (n = 2) | |
| *O. curtisii | 29.4 (91.5) | 11.7 (26.7) | 3 (4.2) |
| *M. splendens | 0.02 (0.2) | 0.1 (0.3) | - |
| *G. graslinii | 8.8 (13.8) | 10.7 (24.5) | - |
| *G. vulgatissimus | 6.3 (18.1) | 5.9 (11) | 8.5 (6.4) |
| *O. forcipatus | 10.6 (14.9) | 17.6 (17.2) | 94.5 (36.1) |
| *B. irene | 5.2 (13.3) | 2.2 (3.2) | 2.5 (0.7) |
| S. metallica | 0.7 (1.4) | 0.2 (0.6) | - |
| O. cancellatum | 1.8 (5.4) | 0.02 (0.1) | - |
| G. pulchellus | 0.3 (1.1) | 0.2 (0.6) | - |
| A. imperator | 0.05 (0.2) | 0.04 (0.2) | - |
| A. parthenope | 0.02 (0.2) | 0.1 (0.3) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arguel, L.; Denis, A.S.; Danflous, S.; Gouix, N.; Santoul, F.; Buisson, L.; Pelozuelo, L. Detection and Monitoring of Riverine Dragonfly of Community Interest (Insecta: Odonata): Proposal for a Standardised Protocol Based on Exuviae Collection. Diversity 2022, 14, 728. https://doi.org/10.3390/d14090728
Arguel L, Denis AS, Danflous S, Gouix N, Santoul F, Buisson L, Pelozuelo L. Detection and Monitoring of Riverine Dragonfly of Community Interest (Insecta: Odonata): Proposal for a Standardised Protocol Based on Exuviae Collection. Diversity. 2022; 14(9):728. https://doi.org/10.3390/d14090728
Chicago/Turabian StyleArguel, Loan, Alice S. Denis, Samuel Danflous, Nicolas Gouix, Frédéric Santoul, Laëtitia Buisson, and Laurent Pelozuelo. 2022. "Detection and Monitoring of Riverine Dragonfly of Community Interest (Insecta: Odonata): Proposal for a Standardised Protocol Based on Exuviae Collection" Diversity 14, no. 9: 728. https://doi.org/10.3390/d14090728
APA StyleArguel, L., Denis, A. S., Danflous, S., Gouix, N., Santoul, F., Buisson, L., & Pelozuelo, L. (2022). Detection and Monitoring of Riverine Dragonfly of Community Interest (Insecta: Odonata): Proposal for a Standardised Protocol Based on Exuviae Collection. Diversity, 14(9), 728. https://doi.org/10.3390/d14090728








