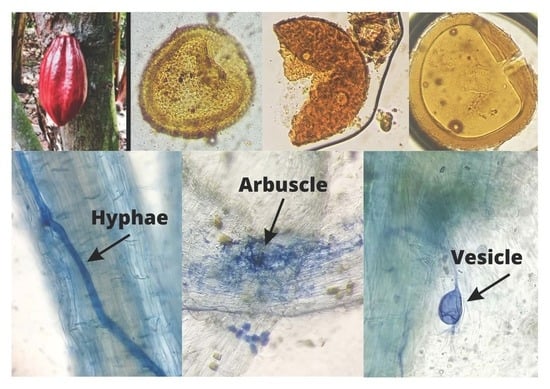
Discovering the Diversity of Arbuscular Mycorrhizal Fungi Associated with Two Cultivation Practices of Theobroma cacao
Abstract
1. Introduction
2. Materials and Methods
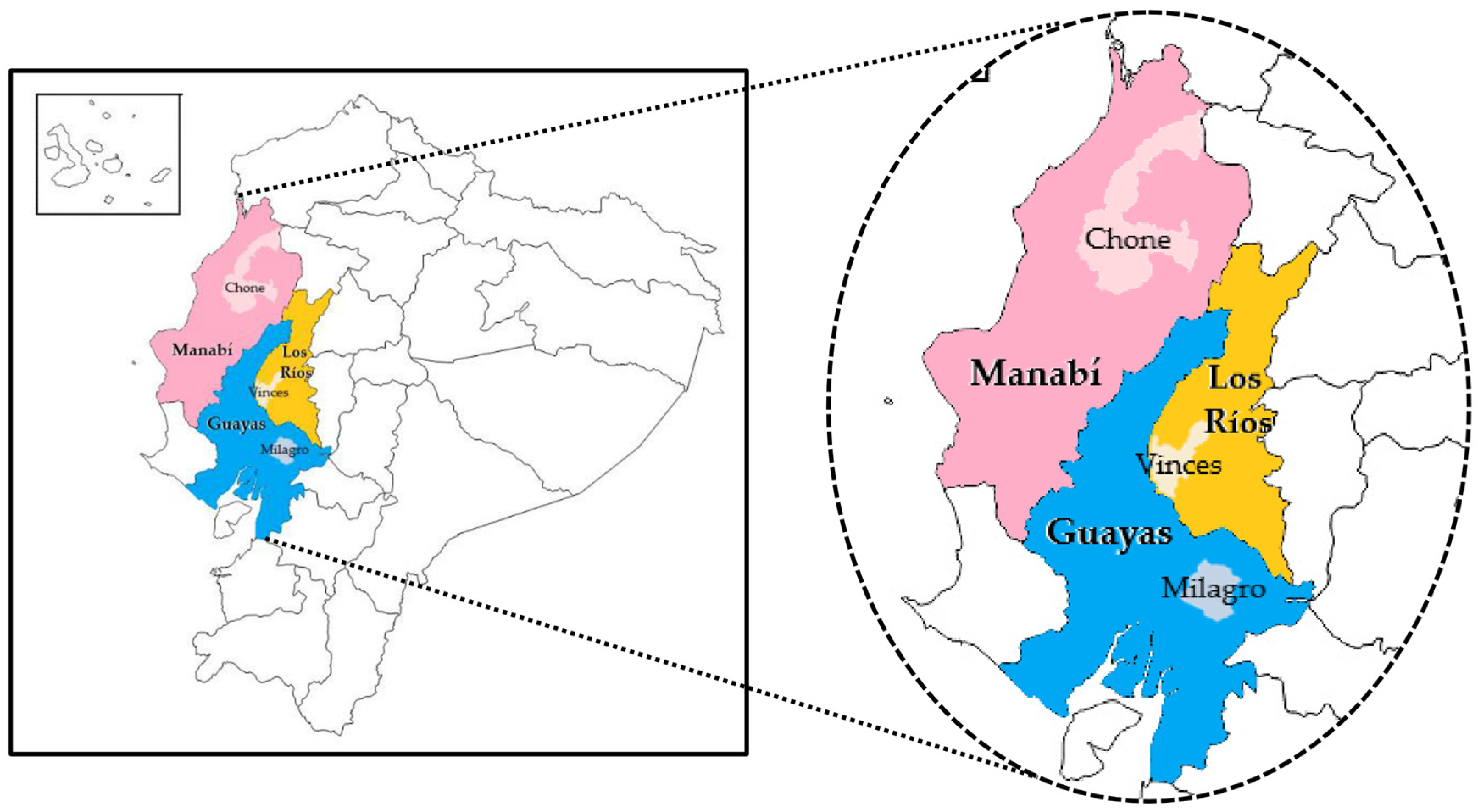
2.1. Location and Description of Cocoa Crops
2.2. Isolation of Spores and Identification of AMF
2.3. Staining of Colonized Roots
2.4. Molecular Analysis: DNA Extraction and Sequencing
Sequence Analysis
3. Results
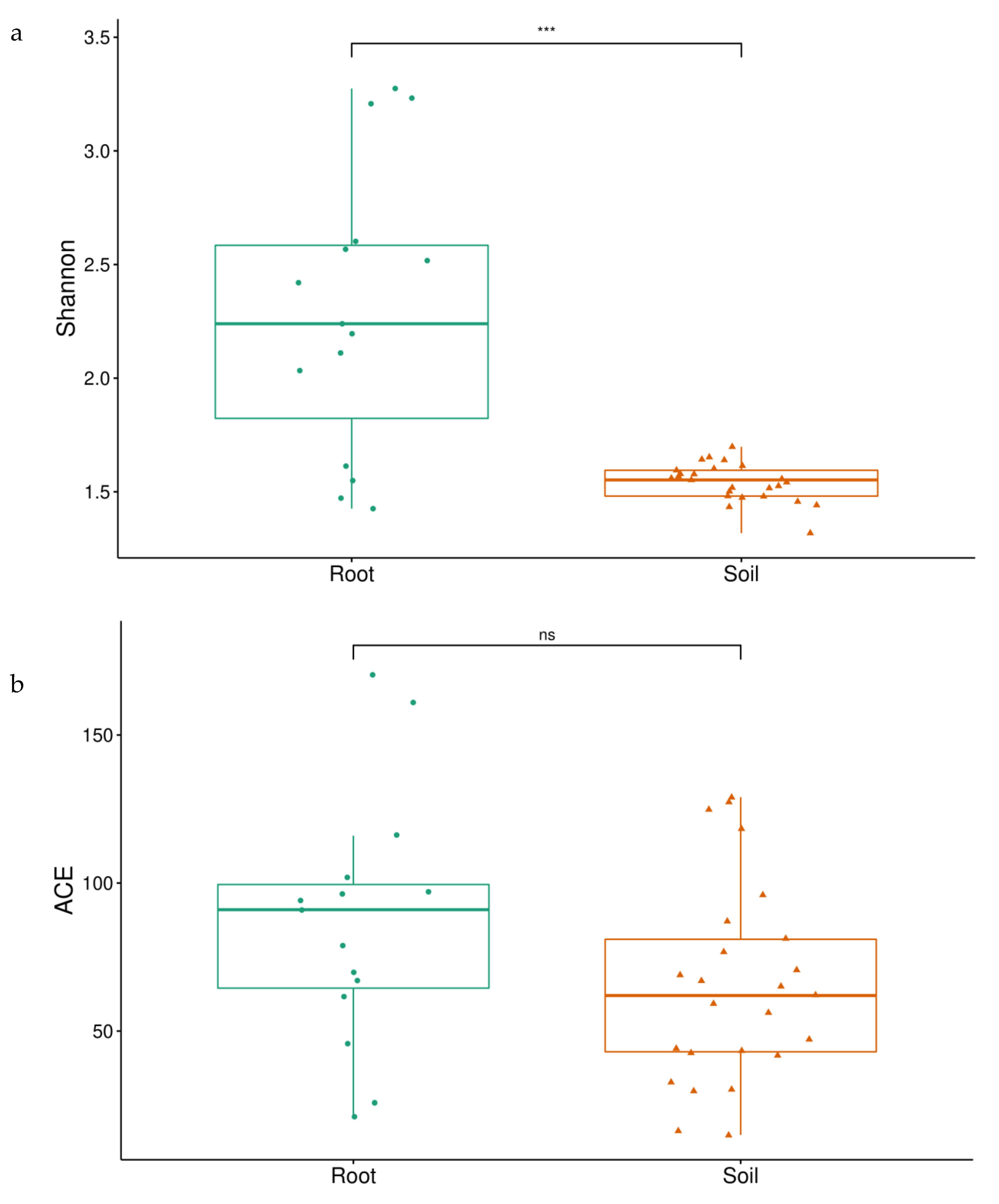
3.1. Isolation of Spores and Identification of AMF
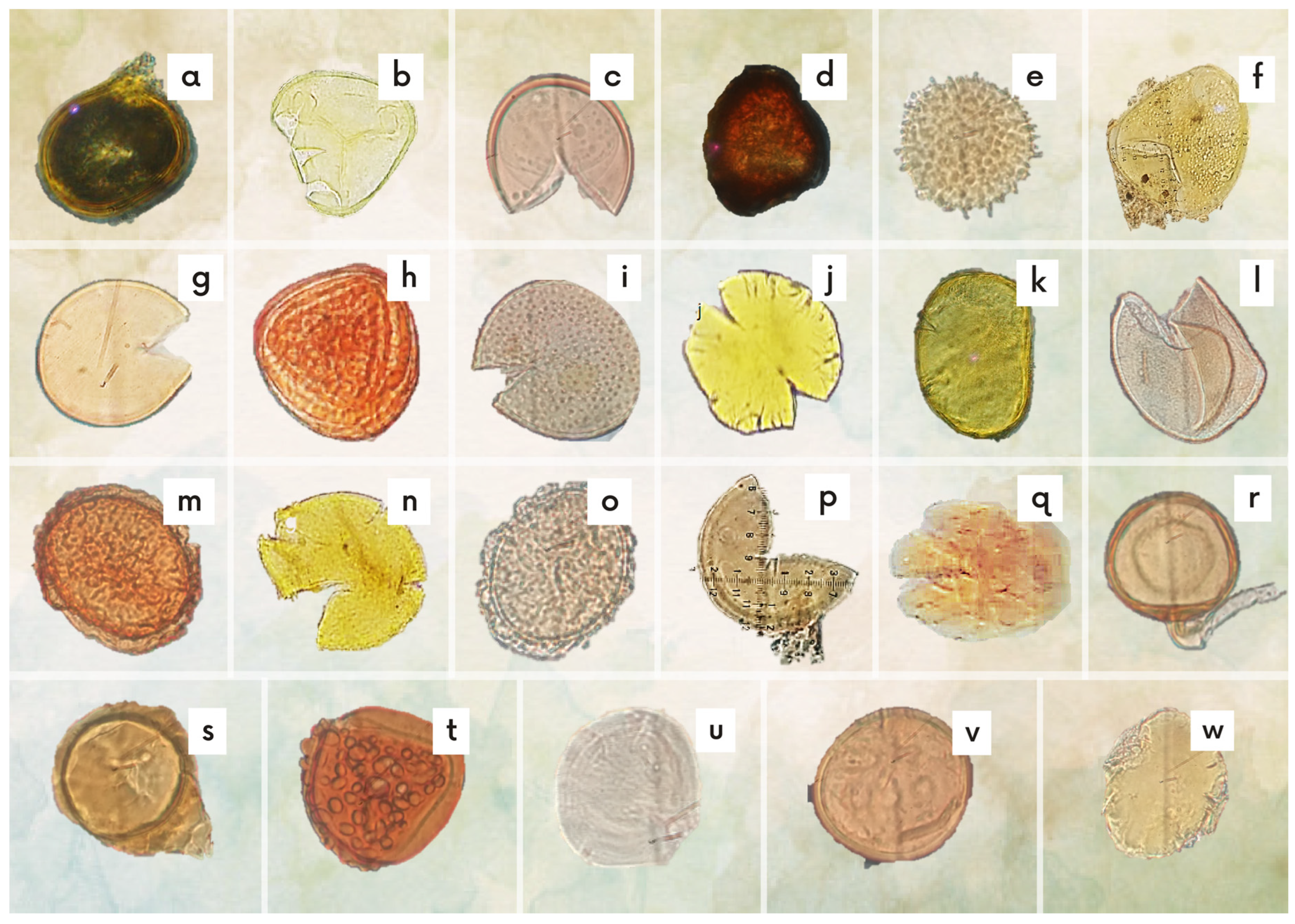
3.2. Morphological Analysis of Spores
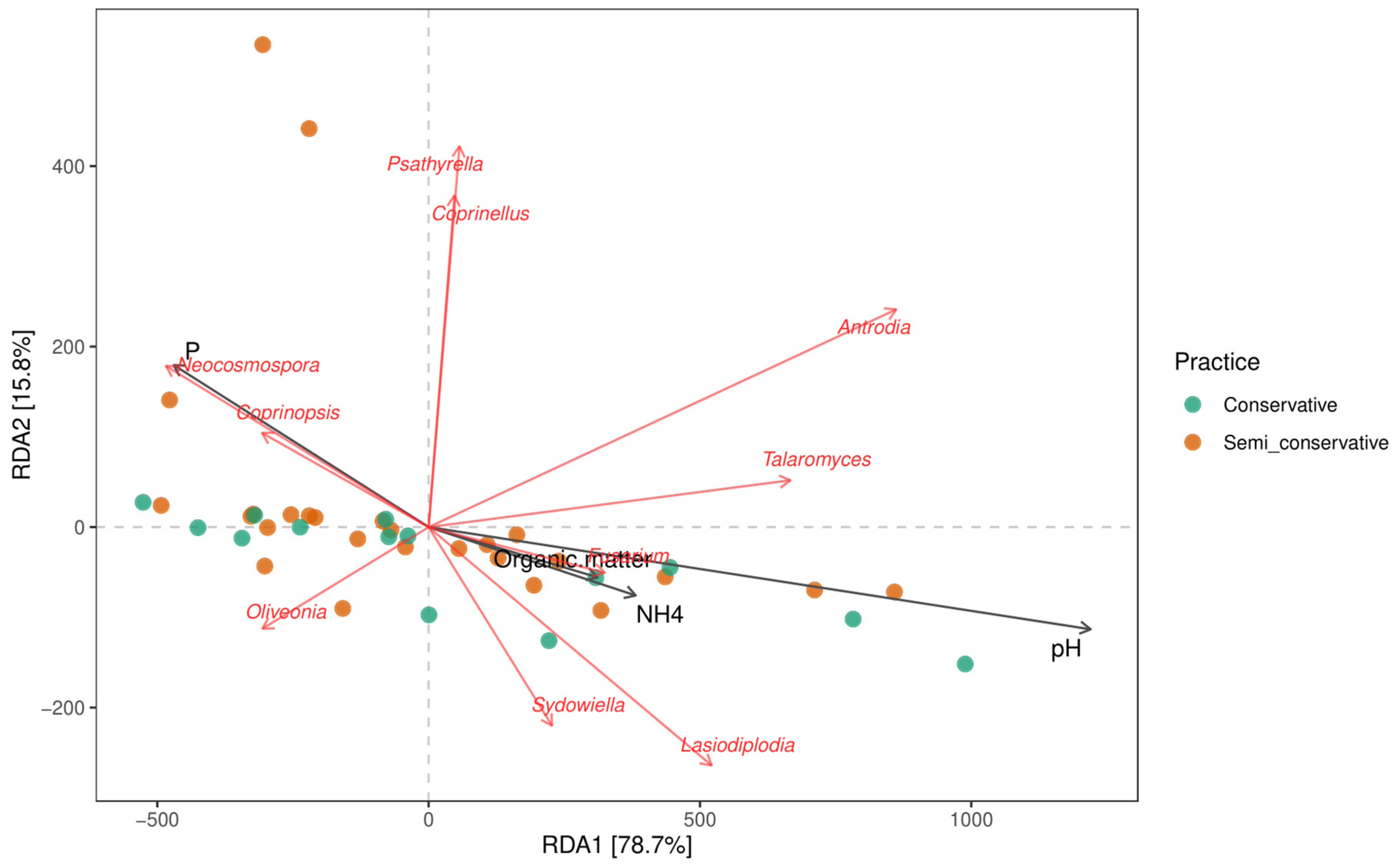
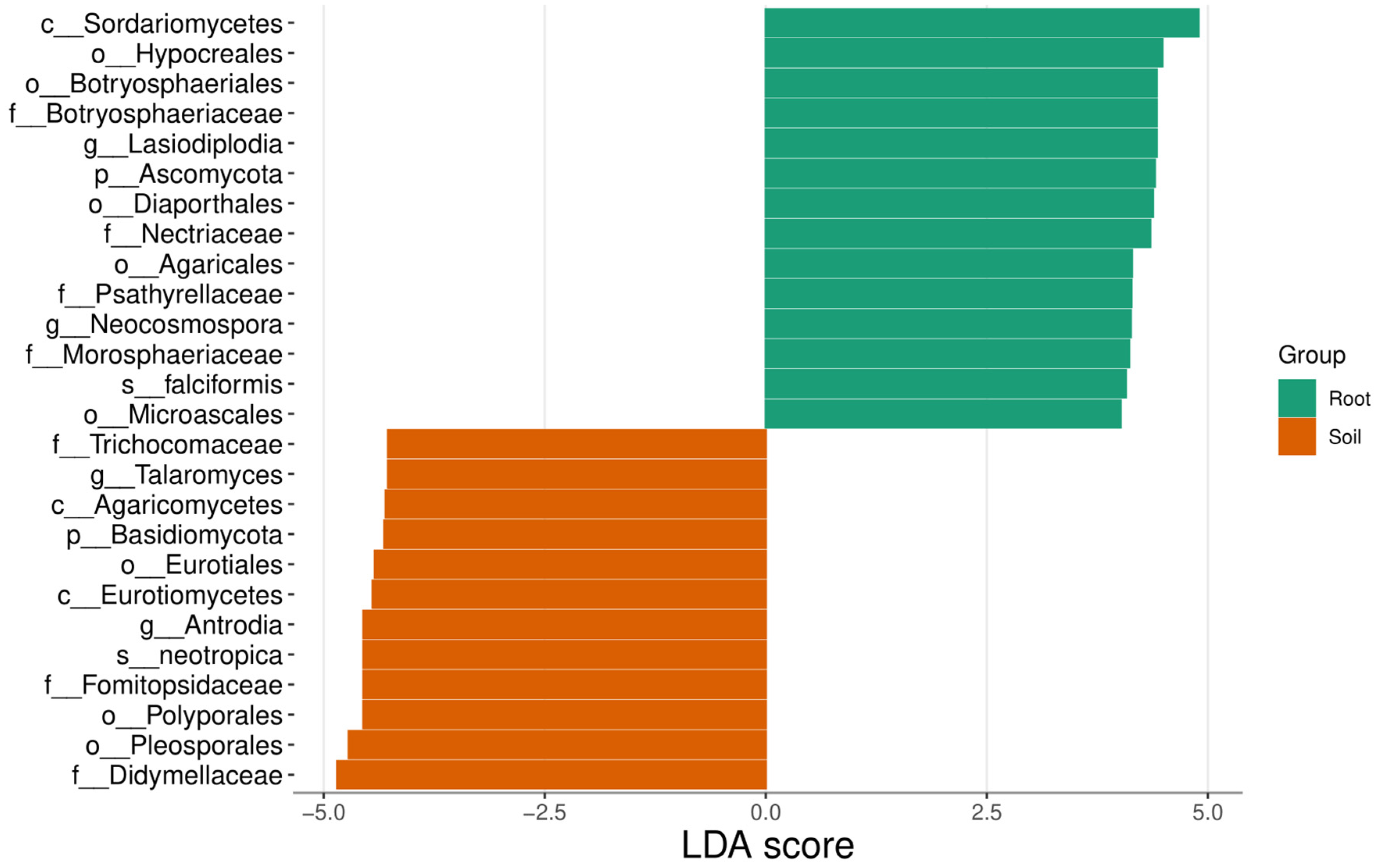
3.3. Molecular Analysis
4. Discussion
4.1. Mycorrhizal Morphotypes in Cocoa Cropping Systems
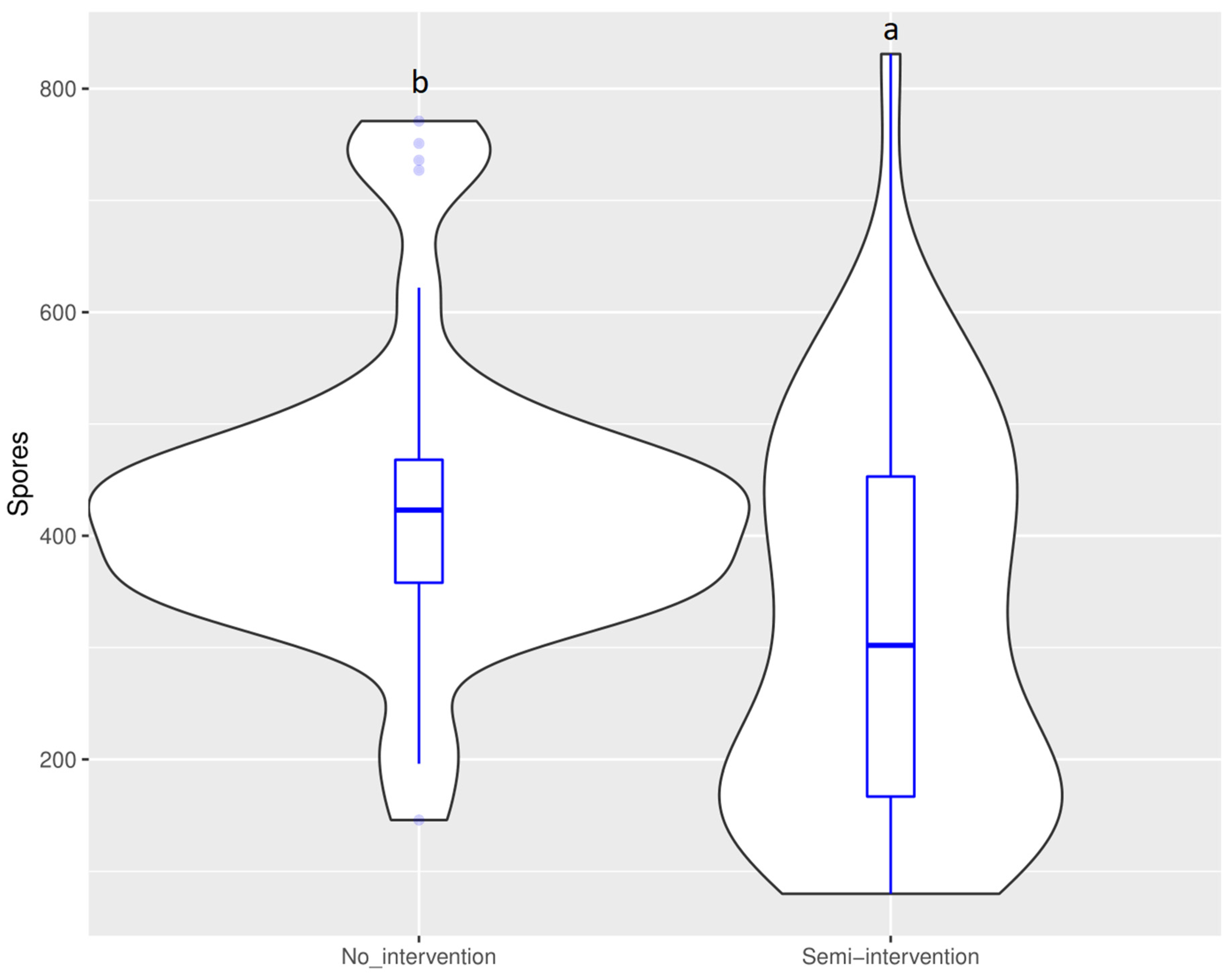
4.2. Arbuscular Mycorrhizal Bioindicators of Anthropogenic Intervention
4.3. Synergistic Contributions of AMF to Cocoa Crops
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Phylum: Glomeromycota | |||
|---|---|---|---|
| Class | Orden | Family | Genera |
| Glomeromycetes | Glomerales | Glomeraceae | Glomus |
| Funneliformis | |||
| Dominikia | |||
| Funneliglomus | |||
| Kamienskia | |||
| Septoglomus | |||
| Microdominikia | |||
| Microkamienskia | |||
| Nanoglomus | |||
| Oehlia | |||
| Halonatospora | |||
| Orientoglomus | |||
| Septoglomus | |||
| Simiglomus | |||
| Sclerocarpum | |||
| Silvaspora | |||
| Epigeocarpum | |||
| Rhizophagus | |||
| Sclerocystis | |||
| Gigasporales | Scutellosporaceae * | Bulbospora | |
| Scutellospora | |||
| Orbispora | |||
| Gigasporaceae * | Gigaspora | ||
| Dentiscutataceae * | Dentiscutata | ||
| Fuscutata | |||
| Quatunica | |||
| Intraornatosporaceae * | Intraornatospora | ||
| Paradentiscutata | |||
| Racocetraceae * | Racocetra | ||
| Cetraspora | |||
| Diversisporales | Acaulosporaceae | Acaulospora | |
| Kuklospora | |||
| Entrophosporaceae * | Entrophospora | ||
| Viscospora | |||
| Albahypha | |||
| Claroideoglomus * | |||
| Pacisporaceae | Pacispora | ||
| Sacculosporaceae | Sacculospora | ||
| Diversisporaceae | Corymbiglomus | ||
| Redeckera | |||
| Tricispora | |||
| Otospora | |||
| Sieverdingia | |||
| Desertispora | |||
| Diversispora | |||
| Paraglomeromycetes | Paraglomerales | Paraglomeraceae | Paraglomus |
| Innospora | |||
| Pervetustaceae | Pervetustus | ||
| Archaeosporomycetes | Archaeosporales | Geosiphonaceae | Geosiphon |
| Ambisporaceae | Ambispora | ||
| Polonosporaceae | Polonospora | ||
| Archaeosporaceae | Intraspora | ||
| Palaeospora | |||
| Archaeospora | |||
| Province | Canton | Farm | System | Geographic Coordinates |
|---|---|---|---|---|
| Los Ríos | Vinces | La Americana | Conservative | 79°48′14.86″ West Latitude and 1°38′23.84″ Longitude South |
| Edén | Semi-conservative | 79°49′8.02″ West latitude and 1°38′47.12″ longitude South | ||
| Manabí | Chone | San José de Olla Vieja | Conservative | 0°47′52.46″ Longitude South 80° 5′36.79″ West Latitude |
| Berto Zambrano | Semi-conservative | 0°47′52.04″ Longitude South 80° 8′3.70″ West Latitude | ||
| Guayas | Milagro | San José | Conservative | 2° 7′57.36″ Longitude South 79°29′4.35″ West Latitude |
| Virginia | Semi-conservative | 2°06′34.7″ Longitude South 79°29′47.5″ West Latitude |
| Texture (%) | µg/mL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivation Practice | Farm | Sand | Silt | Clay | Organic Matter (%) | pH | NH4 | P | Ca | Zn | Cu | Fe |
| Conservative | Olla Vieja | 22 | 62 | 16 | 3.1 | 6.5 | 19 | 46 | 4938 | 3.8 | 4.1 | 50 |
| Conservative | San José | 16 | 56 | 28 | 5 | 6.4 | 30 | 20 | 4847 | 3.7 | 8.4 | 95 |
| Conservative | La Americana | 20 | 58 | 22 | 4.4 | 7.9 | 27 | 37 | 4632 | 9.1 | 6.3 | 109 |
| Semi-conservative | Virginia | 54 | 36 | 10 | 3.7 | 6.6 | 22 | 7 | 4314 | 3.3 | 8.8 | 117 |
| Semi-conservative | Berto Zambrano | 18 | 54 | 28 | 4.4 | 6.4 | 27 | 100 | 4456 | 5.5 | 6.3 | 42 |
| Semi-conservative | Edén | 38 | 42 | 20 | 2.7 | 6.1 | 16 | 105 | 2649 | 7.4 | 14.5 | 355 |
| Region | Primer | Nucleotide Sequence (5′ to 3′) | Amplicon | Reference |
|---|---|---|---|---|
| ITS | ITS86F | GTGAATCATCGAATCTTTGAA | 369 pb | [89] |
| ITS4 | TCCTCCGCTTATTGATATGC | |||
| ITS3 | GCATCGATGAAGAACGCAGC | 310 pb | [90] | |
| ITS4OF | GTACTAGGGGAATCCTTGTT |
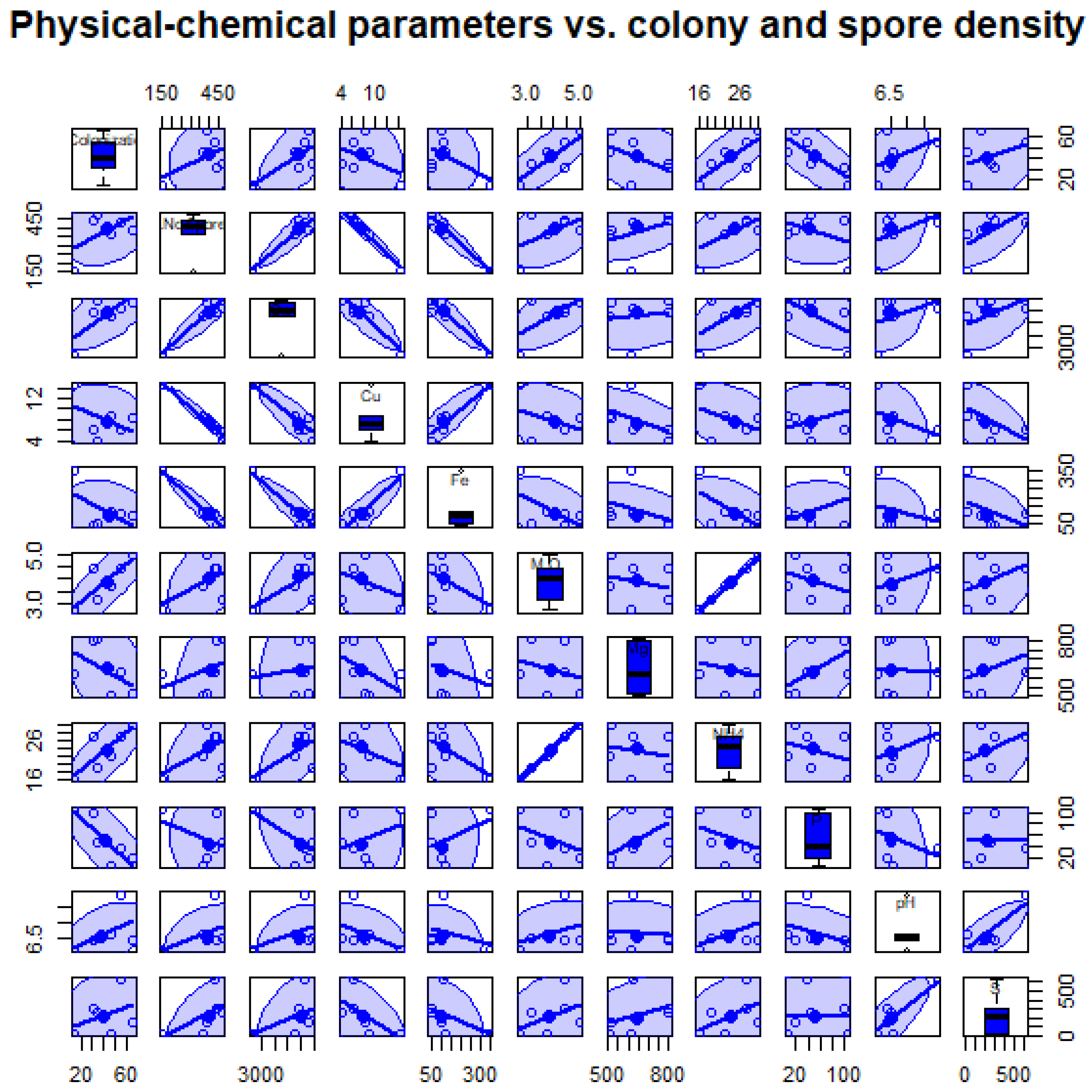
| Edaphic Factors | Correlation Coefficient | p | p Adjusted |
|---|---|---|---|
| Organic matter | −0.0320031423364575 | 0.634 | 0.722 |
| pH | 0.134693332544077 | 0.096 | 0.384 |
| NH4 | −0.0262467220468201 | 0.586 | 0.722 |
| P | −0.0227195060186735 | 0.722 | 0.722 |
| Ca | −0.0218924855036354 | 0.553 | 0.722 |
| Zn | 0.0897857088696029 | 0.08 | 0.384 |
| Cu | −0.0293286170633843 | 0.568 | 0.722 |
| Fe | −0.0547996422755585 | 0.711 | 0.722 |
References
- Lourdes, F.; Intriago, M.; Danilo, M.; Zenteno, C.; Ambrosio, J.; Neto, F.; Peña, M.M.; Roberto, W.; Caicedo, B.; Noemí, M.; et al. Cadena de comercialización del cacao nacional en la provincia de Los Ríos, Ecuador. Rev. Cienc. Tecnol. 2018, 11, 63–69. [Google Scholar]
- Saravia-Matus, S.L.; Rodríguez, A.G.; Saravia, J.A. Determinants of certified organic cocoa production: Evidence from the province of Guayas, Ecuador. Org. Agric. 2020, 10, 23–34. [Google Scholar] [CrossRef]
- Villacis, A.; Alwang, J.; Barrera, V. Cacao value chains and credence attributes: Lessons from Ecuador. J. Agribus. Dev. Emerg. Econ. 2022, 12, 549–566. [Google Scholar] [CrossRef]
- Apolo, B. Impact of Agricultural Exports on Agricultural Economic Growth in Ecuador: Case of Banana and Cocoa. J. Econ. Sustain. Dev. 2020, 11, 27–33. [Google Scholar] [CrossRef]
- Barro, O. Morfología y clasificación botánica del cacao. Man. Asist. Técn. 1981, 24, 25–42. [Google Scholar]
- van Vliet, J.A.; Giller, K.E. Mineral Nutrition of Cocoa: A Review, 1st ed.; Elsevier Inc.: Philadelphia, PA, USA, 2017; Volume 141. [Google Scholar]
- Wood, G.A.R.; Lass, R.A. Cocoa, 4th ed.; Longman: London, UK, 2001; pp. 172–174. [Google Scholar]
- Sessitsch, A.; Hardoim, P.; Döring, J.; Weilharter, A.; Krause, A.; Woyke, T.; Mitter, B.; Hauberg-Lotte, L.; Friedrich, F.; Rahalkar, M.; et al. Functional Characteristics of an Endophyte Community Colonizing Rice Roots as Revealed by Metagenomic Analysis. Mol. Plant-Microbe Interact. 2012, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An ecofriendly technology for nutrient recycling and environmental sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Oviatt, P.; Rillig, M.C. Mycorrhizal technologies for an agriculture of the middle. Plants People Planet 2021, 3, 454–461. [Google Scholar] [CrossRef]
- Srivastava, S.; Johny, L.; Adholeya, A. Review of patents for agricultural use of arbuscular mycorrhizal fungi. Mycorrhiza 2021, 31, 127–136. [Google Scholar] [CrossRef]
- Báez-Pérez, A.; González-Chávez, M.C.Á.; Etchevers-Barra, J.D.; Prat, C.; Hidalgo-Moreno, C. Glomalina y secuestro de carbono en tepetates cultivados. Agrociencia 2010, 44, 517–529. [Google Scholar]
- Moina-Quimi, E.; Oviedo-Anchundia, R.; Nieto-Barciona, S.; Herrera-Samaniego, P.; Barcos-Arias, M. Evaluation of Arbuscular Mycorrhizal Fungi from humid tropical areas of Ecuador. Rev. Bionat. 2018, 3, 531–536. [Google Scholar] [CrossRef]
- Medina García, L.R. La agricultura, la salinidad y los hongos micorrízicos arbusculares꞉ Una necesidad, un problema y una alternativa. Cultiv. Trop. 2016, 37, 42–49. [Google Scholar] [CrossRef]
- Garzón, L.P. Importancia de las micorrizas arbusculares para un uso sostenible del suelo en la Amazonia colombiana. Luna Azul 2016, 42, 217–234. [Google Scholar] [CrossRef][Green Version]
- Lucía Camargo-Ricalde, S.; Montaño, N.M.; De La Rosa-Mera, C.J.; Adriana, S.; Arias, M. Micorrizas: Una Gran Unión Debajo Del Suelo. Rev. Digit. Univ. 2012, 13, 19. [Google Scholar]
- Nakano, A.; Takahashi, K.; Kimura, M. Effect of host shoot clipping on carbon and nitrogen sources for arbuscular mycorrhizal fungi. Mycorrhiza 2001, 10, 287–293. [Google Scholar] [CrossRef]
- Aguilera Gómez, L. Micorrizas arbusculares. Cienc. Ergo-Sum 2007, 14, 300–306. [Google Scholar]
- Gosling, P.; Ozaki, A.; Jones, J.; Turner, M.; Rayns, F.; Bending, G.D. Agriculture, ecosystems and environment organic management of tilled agricultural soils results in a rapid increase in colonisation potential and spore populations of arbuscular mycorrhizal fungi. Agric. Ecosyst. Environ. 2010, 139, 273–279. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; Van Der Heijden, M.; Sieverding, E. Soil biology and biochemistry soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Symanczik, S.; Gisler, M.; Thonar, C.; Schlaeppi, K. Application of mycorrhiza and soil from a permaculture system improved phosphorus acquisition in naranjilla. Front. Plant Sci. 2017, 8, 1263. [Google Scholar] [CrossRef]
- López, M.; López de Rojas, I.; España, M.; Izquierdo, A.; Herrera, L. Efecto de la fertilización inorgánica sobre la disponibilidad de nutrimentos en el suelo, nivel nutricional de la planta y hongos micorrícicos arburculares en plantaciones de Theobroma cacao. Agron. Trop. 2007, 57, 31–43. [Google Scholar]
- Buyer, J.S.; Baligar, V.C.; He, Z.; Arévalo-Gardini, E. Soil microbial communities under cacao agroforestry and cover crop systems in Peru. Appl. Soil Ecol. 2017, 120, 273–280. [Google Scholar] [CrossRef]
- Cué, J.L.; Torres García, A. Valoración agroecológica de las micorrizas vesículo arbúsculares. Science 2010, 327, 167–170. [Google Scholar] [CrossRef]
- Watkinson, S.; Boddy, L.; Money, N. The Fungi; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Furlan, V.; Bartschi, H.; Fortin, J.-A. Media for density gradient extraction of endomycorrhizal spores. Trans. Br. Mycol. Soc. 1980, 75, 336–338. [Google Scholar] [CrossRef]
- Guerra, B.; Chacon, M. Simbiosis micorrizica arbuscular y acumulación de aluminio en brachiaria decumbens y manihot esculenta. Biotecnol. Sect. Agropecu. Agroind. 2012, 10, 87–98. [Google Scholar]
- Peña-Venegas, C.P.; Cardona, G.I.; Arguelles, J.H.; Arcos, A.L. Micorrizas arbusculares del sur de la Amazonia colombiana y su relación con algunos factores fisicoquímicos y biológicos del suelo. Acta Amaz. 2007, 37, 327–336. [Google Scholar] [CrossRef]
- De Paepe, A.E.; Sierpowska, J.; Garcia-Gorro, C.; Martinez-Horta, S.; Perez-Perez, J.; Kulisevsky, J.; Rodriguez-Dechicha, N.; Vaquer, I.; Subira, S.; Calopa, M.; et al. Catálogo de cepas de micorrizas arbusculares. J. Chem. Inf. Model. 2019, 53, 1689–1699. [Google Scholar]
- Peña, C.; Cardona, G.; Mazorra, A.; Arguellez, J.; Arcos, A. Micorrizas arbusculares de la Amazonia colombiana Catálogo ilustrado; Instituto Amazónico de Investigaciones Científicas SINCHI: Leticia, Colombia, 2006; p. 90. [Google Scholar]
- Méndez Cortes, H.; Olalde Portugal, V.; Marmolejo Monsivais, J. Manual para la Identificación de Hongos Micorrízico Arbusculares; Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional: Ciudad de México, Mexico, 2011; p. 503. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Garza, D.G. Tutorial básico en español de Qiime2. 2020; 1–21. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Mantel, N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967, 27, 12. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef] [PubMed]
- Thanni, B.; Merckx, R.; De Bauw, P.; Boeraeve, M.; Peeters, G.; Hauser, S.; Honnay, O. Spatial variability and environmental drivers of cassava—Arbuscular mycorrhiza fungi (AMF) associations across Southern Nigeria. Mycorrhiza 2022, 32, 1–13. [Google Scholar] [CrossRef]
- Tuesta-Pinedo, Á.L.; Trigozo-Bartra, E.; Cayotopa-Torres, J.J.; Arévalo-Gardini, E.; Arévalo-Hernández, C.O.; Zúñiga-Cernadez, L.B.; Leon-Ttacca, B. Optimización de la fertilización orgánica e inorgánica del cacao (Theobroma Cacao L.) con la inclusión de Trichoderma endófito y Micorrizas arbusculares. Rev. Tecnol. Marcha 2017, 30, 67. [Google Scholar] [CrossRef]
- Loján, P.; Senés-Guerrero, C.; Suárez, J.P.; Kromann, P.; Schüßler, A.; Declerck, S. Potato field-inoculation in Ecuador with Rhizophagus irregularis: No impact on growth performance and associated arbuscular mycorrhizal fungal communities. Symbiosis 2017, 73, 45–56. [Google Scholar] [CrossRef]
- Goldmann, K.; Boeddinghaus, R.S.; Klemmer, S.; Regan, K.M.; Heintz-Buschart, A.; Fischer, M.; Prati, D.; Piepho, H.P.; Berner, D.; Marhan, S.; et al. Unraveling spatiotemporal variability of arbuscular mycorrhizal fungi in a temperate grassland plot. Environ. Microbiol. 2020, 22, 873–888. [Google Scholar] [CrossRef]
- Oehl, F.; Silva, G.; Palenzuela, J. Dominikia bernensis, a new arbuscular mycorrhizal fungus from a Swiss no-till farming site, and D. aurea, D. compressa and D. indica, three new combinations in Dominikia. Nova Hedwig. 2014, 101, 65–76. [Google Scholar] [CrossRef]
- Vieira, L.C.; da Silva, D.K.A.; da Silva, I.R.; Gonçalves, C.M.; de Assis, D.M.A.; Oehl, F.; da Silva, G.A. Ecological aspects of arbuscular mycorrhizal fungal communities in different habitat types of a Brazilian mountainous area. Ecol. Res. 2019, 34, 182–192. [Google Scholar] [CrossRef]
- Yurkov, A.; Kryukov, A.; Mikhaylova, Y.; Zhurbenko, P. Illumina-based identification of arbuscular mycorrhizal fungi from Karachay-Cherkessia soils for development of bio-fertilizers. E3S Web Conf. 2021, 285, 03001. [Google Scholar] [CrossRef]
- Baltruschat, H.; Santos, V.M.; da Silva, D.K.A.; Schellenberg, I.; Deubel, A.; Sieverding, E.; Oehl, F. Unexpectedly high diversity of arbuscular mycorrhizal fungi in fertile Chernozem croplands in Central Europe. Catena 2019, 182, 104135. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Chwat, G.; Góralska, A.; Ryszka, P.; Kovács, G.M. Two new genera, Dominikia and Kamienskia, and D. disticha sp. nov. in Glomeromycota. Nov. Hedwig. 2015, 100, 225–238. [Google Scholar] [CrossRef]
- Chebaane, A.; Symanczik, S.; Oehl, F.; Azri, R.; Gargouri, M.; Mäder, P.; Mliki, A.; Fki, L. Arbuscular mycorrhizal fungi associated with Phoenix dactylifera L. grown in Tunisian Sahara oases of different salinity levels. Symbiosis 2020, 81, 173–186. [Google Scholar] [CrossRef]
- Wang, J.; Ma, S.; Wang, G.G.; Xu, L.; Fu, Z.; Song, J.; Zhang, J. Arbuscular mycorrhizal fungi communities associated with wild plants in a coastal ecosystem. J. For. Res. 2021, 32, 683–695. [Google Scholar] [CrossRef]
- Cofré, M.N.; Soteras, F.; Iglesias, M.d.R.; Velázquez, S.; Abarca, C.; Risio, L.; Ontivero, E.; Cabello, M.N.; Domínguez, L.S.; Lugo, M.A. Biodiversity of arbuscular mycorrhizal fungi in South America: A review. In Mycorrhizal Fungi in South America, 1st ed.; Pagano, M., Lugo, M., Eds.; Springer: New York, NY, USA, 2019; Volume 1, pp. 154–196. [Google Scholar]
- Hernández-Acosta, E.; Trejo-Aguilar, D.; Rivera-Fernández, A.; Ferrera-Cerrato, R. Arbuscular mycorrhiza as a biofertilizer in production of coffee. Terra Latinoam. 2020, 38, 613–628. [Google Scholar] [CrossRef]
- Coutinho, E.S.; Barbosa, M.; Beiroz, W.; Mescolotti, D.L.C.; Bonfim, J.A.; Louro Berbara, R.L.; Fernandes, G.W. Soil constraints for arbuscular mycorrhizal fungi spore community in degraded sites of rupestrian grassland: Implications for restoration. Eur. J. Soil Biol. 2019, 90, 51–57. [Google Scholar] [CrossRef]
- Assis, P.C.R.; Saggin Júnior, O.J.; Paulino, H.B.; Stürmer, S.L.; Siqueira, J.O.; Carneiro, M.A.C. Fungos micorrízicos arbusculares em campos de murundus após a conversão para sistemas agrícolas no cerrado. Rev. Bras. Ciênc. Do Solo 2014, 38, 1703–1711. [Google Scholar] [CrossRef]
- Channabasava, A.; Lakshman, H.C.; Muthukumar, T. Fly ash mycorrhizoremediation through Paspalum scrobiculatum L., inoculated with Rhizophagus fasciculatus. Comptes Rendus—Biol. 2015, 338, 29–39. [Google Scholar] [CrossRef]
- Danesh, Y.R.; Kariman, K.; Keskin, N.; Najafi, S. Characterization of arbuscular mycorrhizal fungal communities associated withvineyards in northwestern Iran. Turk. J. Agric. For. 2022, 46, 271–279. [Google Scholar] [CrossRef]
- Ortiz-Acevedo, A.; Medina-Sierra, M.; Echeverri-Gómez, J. Identificación de algunas cepas de hongos micorrícicos asociados al kikuyo (Cenchrus clandestinus (Hochst ex Chiov) Morrone) y su efecto en algunas variables agronómicas. Livest. Res. Rural Dev. 2017, 29, 88. [Google Scholar]
- Moreira, M.; Zucchi, M.I.; Gomes, J.E.; Tsai, S.M.; Alves-Pereira, A.; Cardoso, E.J.B.N. Araucaria angustifolia Aboveground Roots Presented High Arbuscular Mycorrhizal Fungal Colonization and Diversity in the Brazilian Atlantic Forest. Pedosphere 2016, 26, 561–566. [Google Scholar] [CrossRef]
- Álvarez Sánchez, J.; Sánchez Gallen, I.; Hernández Cuevas, L.; Hernández Oro, L.; Meli, P. Diversidad, abundancia y variación estacional en la comunidad de hongos micorrizógenos arbusculares en la selva Lacandona, Chiapas, México. Sci. Fungorum 2017, 45, 37–51. [Google Scholar] [CrossRef]
- Mandal, S.; Evelin, H.; Giri, B.; Singh, V.P.; Kapoor, R. Arbuscular mycorrhiza enhances the production of stevioside and rebaudioside-A in Stevia rebaudiana via nutritional and non-nutritional mechanisms. Appl. Soil Ecol. 2013, 72, 187–194. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.N.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Arbuscular Mycorrhizal Fungi (AMF) on Salt Stress Tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 1–8. [Google Scholar] [CrossRef]
- Essahibi, A.; Benhiba, L.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.). Trees—Struct. Funct. 2018, 32, 87–97. [Google Scholar] [CrossRef]
- Channabasava, A.; Lakshman, H.C.; Jorquera, M.A. Effect of fungicides on association of arbuscular mycorrhiza fungus Rhizophagus fasciculatus and growth of Proso millet (Panicum miliaceum L.). J. Soil Sci. Plant Nutr. 2015, 15, 35–45. [Google Scholar] [CrossRef]
- Chimal-Sánchez, E.; Montaño, N.M.; Camargo-Ricalde, S.L.; García-Sánchez, R.; Hernández-Cuevas, L.V. Nuevos registros de hongos micorrizógenos arbusculares para México. Rev. Mex. Biodivers. 2016, 87, 242–247. [Google Scholar] [CrossRef]
- Cervantes-Gámez, R.G.; Peñuelas-Rubio, O.; Araujo-Benard, N.; Fierro-Coronado, R.A.; Trejo-Aguilar, D.; Maldonado-Mendoza, I.E.; Cordero-Ramírez, J.D. Diversidad de hongos micorrízicos arbusculares asociados a plantas voluntarias de maíz en suelos de transición: Ecosistema natural—Uso agrícola. Sci. Fungorum 2021, 51, e1330. [Google Scholar] [CrossRef]
- Aranda, E.; Scervino, J.M.; Godoy, P.; Reina, R.; Ocampo, J.A.; Wittich, R.M.; García-Romera, I. Role of arbuscular mycorrhizal fungus Rhizophagus custos in the dissipation of PAHs under root-organ culture conditions. Environ. Pollut. 2013, 181, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.e.Z.; Cernava, T.; Liu, J.; Timm, C.M. Editorial: Novel Insights Into the Response of the Plant Microbiome to Abiotic Factors. Front. Plant Sci. 2021, 12, 607874. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Wagner, P.; Müller, H.; Matzer, R.; Unterfrauner, H.; Cernava, T.; Berg, G. Unraveling the Complexity of Soil Microbiomes in a Large-Scale Study Subjected to Different Agricultural Management in Styria. Front. Microbiol. 2020, 11, 1052. [Google Scholar] [CrossRef]
- Franke-Snyder, M.; Douds, D.D.; Galvez, L.; Phillips, J.G.; Wagoner, P.; Drinkwater, L.; Morton, J.B. Diversity of communities of arbuscular mycorrhizal (AM) fungi present in conventional versus low-input agricultural sites in eastern Pennsylvania, USA. Appl. Soil Ecol. 2001, 16, 35–48. [Google Scholar] [CrossRef]
- Bogoriense, H.; Division, B.; Centre, C.S.; Raya, J. The Distribution of Glomeromycota in Cacao Rhizosphere in Indonesia. Reinwardtia 2008, 12, 347–356. [Google Scholar]
- Boyetchko, S.M.; Tewari, J.P. Occurrence of vesicular-arbuscular mycorrhizal fungi in Alberta, Canada. Z. Für Naturforsch.—Sect. C J. Biosci. 1993, 48, 923–929. [Google Scholar] [CrossRef]
- Carreón-Abud, Y.; Beltrán-Nambo, M.A.; Martinez Trujillo, M. Efecto protector de los hongos micorrízicos arbusculares en plantas de jitomate (Solanum lycopersicum) expuestas a Cr (VI). Rev. Int. Bot. Exp. 2013, 82, 127–134. [Google Scholar]
- Gañan, L.; Benavides, M.M.B.; Asakawa, N. Efecto de la micorrización sobre el crecimiento de plántulas de plátano en sustrato con y sin la presencia de nematodos fitoparásitos. Acta Agron. 2011, 60, 297–305. [Google Scholar]
- Oehl, F.; Sieverding, E. Pacispora, a new vesicular arbuscular mycorrhizal fungal genus in the glomeromycetes. J. Appl. Bot. 2004, 78, 72–82. [Google Scholar]
- Rokni, N.; Goltapeh, M.E. Alizadeh Some new recorded species of arbuscular mycorrhizal fungi associated with sugarcane crop in Iran. J. Agric. Technol. 2010, 6, 67–78. [Google Scholar]
- Belay, Z.; Vestberg, M.; Assefa, F. Diversity and abundance of arbuscular mycorrhizal fungi across different land use types in a humid low land área of Ethiopia. Afr. J. Microbiol. Res. 2015, 18, 47–69. [Google Scholar]
- Khadija, B.; Yolande, D.; Khadija, B.; Najib, S.; Mostafa, K. Impact of crop rotation on mycorrhizal fungi in irrigated soils of the Doukkala (Morocco). Int. J. Environ. Agric. Res. 2015, 1, 48–55. [Google Scholar]
- Silva, P.; Aguilar, A.; Dibán, M.; Mujica, M. Mycorrhizas in the South American Mediterranean-type ecosystem: Chilean matorral. In Mycorrhizal Fungi in South America, 1st ed.; Pagano, M., Lugo, M., Eds.; Springer: New York, NY, USA, 2019; Volume 1, pp. 193–202. [Google Scholar]
- Kozjek, K.; Kundel, D.; Kushwaha, S.K.; Olsson, P.A.; Ahrén, D.; Fliessbach, A.; Birkhofer, K.; Hedlund, K. Long-term agricultural management impacts arbuscular mycorrhizal fungi more than short-term experimental drought. Appl. Soil Ecol. 2021, 168, 104140. [Google Scholar] [CrossRef]
- Guarnaccia, V.; Van Niekerk, J.; Crous, P.W.; Sandoval-Denis, M. Neocosmospora spp. associated with dry root rot of citrus in South Africa. Phytopathol. Mediterr. 2021, 60, 79–100. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Lombard, L.; Crous, P.W. Back to the Roots: A Reappraisal of Neocosmospora; Naturalis Biodiversity Center: Leiden, The Netherlands, 2019; Volume 43, ISBN 2006743457. [Google Scholar]
- Zheng, F.; Xu, G.; Zheng, F.Q.; Ding, X.F.; Xie, C.P. Neocosmospora rubicola causing stem rot of Pitaya (Hylocereus costaricensis) in China. Plant Dis. 2018, 102, 2653. [Google Scholar] [CrossRef]
- Khuseib Hamed Al-Rashdi, F.; Al-Sadi, A.M.; Al-Riyamy, B.Z.; Maharachchikumbura, S.S.N.; Khalfan Al-Ruqaishi, H.; Velazhahan, R. Alternaria alternata and Neocosmospora sp. from the medicinal plant Euphorbia larica exhibit antagonistic activity against Fusarium sp., a plant pathogenic fungus. All Life 2020, 13, 223–232. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites of lasiodiplodia theobromae: Distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins 2020, 12, 457. [Google Scholar] [CrossRef]
- Coutinho, I.B.L.; Freire, F.C.O.; Lima, C.S.; Lima, J.S.; Gonçalves, F.J.T.; Machado, A.R.; Silva, A.M.S.; Cardoso, J.E. Diversity of genus Lasiodiplodia associated with perennial tropical fruit plants in northeastern Brazil. Plant Pathol. 2017, 66, 90–104. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Meller, E.; Milczarski, P.; Zubek, S.; Malicka, M.; Uszok, S.; Casieri, L.; Goto, B.T.; Magurno, F. New taxa in Glomeromycota: Polonosporaceae fam. nov., Polonospora gen. nov., and P. polonica comb. nov. Mycol. Prog. 2021, 20, 941–951. [Google Scholar] [CrossRef]
- Anand, K.; Pandey, G.K.; Kaur, T.; Pericak, O.; Olson, C.; Mohan, R.; Yadav, A.; Devi, R.; Kour, D.; Rai, A.K.; et al. Arbuscular mycorrhizal fungi as a potential biofertilizers for agricultural sustainability. J. Appl. Biol. Biotechnol. 2022, 10, 90–107. [Google Scholar] [CrossRef]
- Sieverding, E.; Da Silva, G.A.; Berndt, R.; Oehl, F. Rhizoglomus, a new genus of the Glomeraceae. Mycotaxon 2014, 129, 373–386. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Jobim, K.; Niezgoda, P.; Meller, E.; Malinowski, R.; Milczarski, P.; Zubek, S.; Magurno, F.; Casieri, L.; Bierza, W.; et al. New Glomeromycotan Taxa, Dominikia glomerocarpica sp. nov. and Epigeocarpum crypticum gen. nov. et sp. nov. From Brazil, and Silvaspora gen. nov. From New Caledonia. Front. Microbiol. 2021, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhang, W.; Zhang, S.B.; Wang, J.H. Similar mycorrhizal fungal communities associated with epiphytic and lithophytic orchids of Coelogyne corymbosa. Plant Divers. 2020, 42, 362–369. [Google Scholar] [CrossRef]
- Esposito, F.; Jacquemyn, H.; Waud, M.; Tyteca, D. Mycorrhizal fungal diversity and community composition in two closely related Platanthera (orchidaceae) species. PLoS ONE 2016, 11, e0164108. [Google Scholar] [CrossRef]



| System | Classification | ||
|---|---|---|---|
| Semi-Conservative | Conservative | Taxonomy | |
| 0 | 3 | Phylum | Glomeromycota |
| 11 | 15 | Class | Glomeromycetes |
| 0 | 1 | Order | Diversisporales |
| 2 | 0 | Order | Glomerales |
| 29 | 125 | Family | Glomeraceae |
| 15 | 0 | Genus | Archaeospora spp. |
| 2 | 0 | Genus | Diversispora spp. |
| 3 | 0 | Genus | Dominikia spp. |
| 41 | 0 | Genus | Glomus sp. |
| 0 | 3 | Genus | Septoglomus spp. |
| 1 | 0 | Species | Dominikia bernensis |
| 8 | 0 | Species | Dominikia disticha |
| 2 | 2 | Species | Glomus aggregatum |
| 0 | 8 | Species | Kamienskia perpusilla |
| 0 | 10 | Species | Pacispora scintillans |
| 0 | 76 | Species | Rhizophagus custos |
| 0 | 2 | Species | Rhizophagus diaphanus |
| 0 | 103 | Species | Rhizophagus fasciculatus |
| 114 | 348 | Total | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco Flores de Valgaz, A.; Naranjo-Morán, J.; Reyes Román, G.; Oviedo-Anchundia, J.; Ratti Torres, M.; Barcos-Arias, M. Discovering the Diversity of Arbuscular Mycorrhizal Fungi Associated with Two Cultivation Practices of Theobroma cacao. Diversity 2022, 14, 651. https://doi.org/10.3390/d14080651
Pacheco Flores de Valgaz A, Naranjo-Morán J, Reyes Román G, Oviedo-Anchundia J, Ratti Torres M, Barcos-Arias M. Discovering the Diversity of Arbuscular Mycorrhizal Fungi Associated with Two Cultivation Practices of Theobroma cacao. Diversity. 2022; 14(8):651. https://doi.org/10.3390/d14080651
Chicago/Turabian StylePacheco Flores de Valgaz, Angela, Jaime Naranjo-Morán, Guillermo Reyes Román, Javier Oviedo-Anchundia, Maria Ratti Torres, and Milton Barcos-Arias. 2022. "Discovering the Diversity of Arbuscular Mycorrhizal Fungi Associated with Two Cultivation Practices of Theobroma cacao" Diversity 14, no. 8: 651. https://doi.org/10.3390/d14080651
APA StylePacheco Flores de Valgaz, A., Naranjo-Morán, J., Reyes Román, G., Oviedo-Anchundia, J., Ratti Torres, M., & Barcos-Arias, M. (2022). Discovering the Diversity of Arbuscular Mycorrhizal Fungi Associated with Two Cultivation Practices of Theobroma cacao. Diversity, 14(8), 651. https://doi.org/10.3390/d14080651