Abstract
Ericaceae are a group of plants with biotechnological and commercial importance. These plants establish symbiotic associations with a wide group of mycorrhizal fungi. National and global studies have focused on two of them: arbuscular endomycorrhizae and ectomycorrhizae. The most recent type of mycorrhiza recorded is the cavendishioid ectendomycorrhizae. The cavendishioid is one of the least-studied and understood mycorrhizae, along with monotropoid and arbutoid mycorrhizae. Among the potentialities of these ectendomycorrhizae are the alleviation of environmental stress, the facilitation of establishment, survival, the ability to form dual mycorrhizae, and their importance as a fundamental biological element of plant ecological successions. However, the factors that influence or correlate with the diversity of some of these fungal guilds, as well as the functional significance of their associations, are still unknown. The present review focuses on ericoid mycorrhiza from Ecuador in order to promote their study and thus take advantage of the benefits that they provide to endemic species and those of commercial interest. This material contributes significantly to reducing research gaps and channeling applied projects in the biological sciences.
1. Introduction
The Ericaceae are an important group of plants for the ecosystem, since some of them are dominant among most of the tropical vegetation [1]. They are one of the most abundant families in the genera and species of plants present in the Andes of Ecuador [2]. They form the main part of the undergrowth of low-altitude and mountain pine forests, as well as mixed pine and broadleaf forests, which also abound in low-altitude, submontane, and montane pluvial forests, and in cloud forests. Less commonly, they are found in riparian forests, low-lying serpentine thickets, and montane microphylls. [3]. It has been reported that in Ecuador, this group is distributed in greater quantity in the cloud forests with high humidity, located in remnants of forests in the southern zone [4]. These plant species are calcifugous, adapted to average temperatures between 6 to 24 °C, and grow around 1000–4000 m.a.s.l. [2,5,6,7]. Their presence in acid soils is remarkable. However, they have developed life forms in the diversity of habitats found ranging from terrestrial shrubs to hemiepiphytes and epiphytes [5]. The objective of this bibliographic review was to contextualize and synthesize the state of the art of mycorrhizae associated with ericaceous plants from Ecuador with a particular focus on the ericoid type to reduce research gaps in biological sciences.
For the present review, Google Scholar, PubMed, ScienceDirect, SciELO, and Springer databases were used to collect relevant articles on mycorrhizal symbiosis in ericaceous plants from Ecuador, neighboring countries, and hot spots for these plants. Key terms included “ericaceous of Ecuador”, “mycorrhizae in ericaceous”, “Monotropa”, “ericoid”, “arbutoid”, “ectomycorrhizae”, “cavendishioid”, and some genera of ericaceous, such as “Vaccinium”, “Gaultheria”, “Bejaria”, and “Cavendishia”. The phases of critical reviewing included reading-related titles, abstracts, full texts of articles, interpretations of results of inoculated mycorrhizae, reviews, and conclusions of the experiments in ericaceous. The collection was limited to research articles published in English, Spanish, and Portuguese from 1989 to 2021. The findings of the last three decades served for the elaboration of a table, graph, and discussion of them with a national approach for the use of mycorrhizae in native ericaceous with and without endemism.
2. Mycorrhizal Symbiosis of Ericaceous Plants
Ericaceae have a well-differentiated, long, thick main root and fine, fibrous, lignified secondary roots; according to the relationship between the primary and secondary roots, they belong to the pivoting type, since the primary root grows faster than the secondary roots [1]. Its roots grow in the first 30 cm of the soil surface. However, there are exceptions within the Vaccinium genus that do not have a taproot; instead, they have lateral roots that help absorb nutrients through a symbiotic relationship with mycorrhizae (Figure 1) [8].
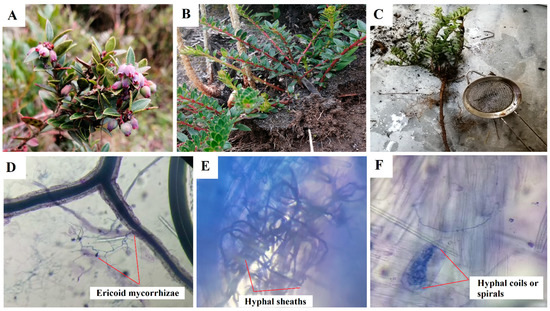
Figure 1.
Blueberry plants (Vaccinium floribundum); (A) immature fruits, (B) stems; and (C) mortiño roots; (D) hyphae extending out of the root; (E,F) intracellular structures of ericoid mycorrhizae in stained roots. Pictures: J. Naranjo-Morán and R. Moreira-Gómez.
In the rhizosphere of these plants, we find a considerable number of microorganisms. For instance, the fungi form endotrophic mycorrhizae, through which the plants acquire part of their nutrients from recalcitrant organic matter from the soil [9]. This type of fungi helps the roots to absorb organic nitrogen for good plant growth [1]. Other benefits include improved resistance to drought. In some associations, plant welfare is promoted by destroying toxic phenolics and increasing tolerance to salinity or heavy metals [10].
Some of the aspects that affect the mycorrhization of Ericaceae are locality, season, cultivar, plant age, phosphorus content, fertilization, pH, type of fungus, and its efficiency in the nutritional contribution to the consumption of photosynthates from the host, among others [11].
The classification of mycorrhizae by type of infection that has been described so far in Ericaceae includes ectomycorrhizae, ectendomycorrhizae, and endomycorrhizae. Ectendomycorrhizae can be of the arbutoid, monotropoid, and cavendishioid type; endomycorrhizae are of the ericoid and arbuscular type. Of these clades, the ectomycorrhizae and ectendomycorrhizae (arbutoids, monotropoids, and cavendishioids) form a multilayered mantle of hyphae around the root, while the ericoids, in contrast to the previous ones, develop their intra- and intercellular structures (Figure 2) [12].
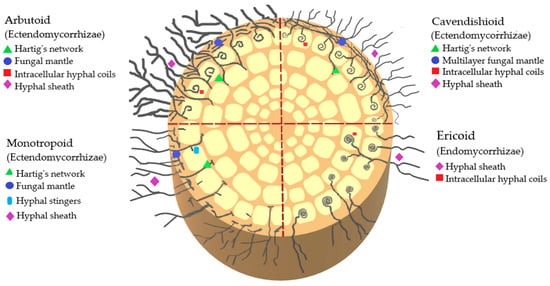
Figure 2.
Schematic representation of endomycorrhizae and ectendomycorrhizae that interact with ericaceous plants.
2.1. Ectomycorrhizae
Ectomycorrhizae are found in all subfamilies of Ericaceae, as well as in many other vascular plants, both conifers and angiosperms, and are characterized by a mantle or covering of fungal hyphae on the surface of the roots and by a Hartig network formed by fungal hyphae extending between the epidermal cells of the root [13]. Spore banks and other resistant ectomycorrhizal fungi propagules play a determining role in facilitating the regeneration of ecosystems after alterations, providing the environment the opportunity to recover. Resistant propagules of the genera Rhizopogon (Basidiomycetes), Tuber, and Wilcoxina (Ascomycetes) are abundant in the soil, and represent an important source of inoculant in the early stages of the ecosystem after a disturbance; some fungi from the same phyla colonize new roots from the extension of already-established mycelium, and others may arrive early in succession and persist in mature forests. The ability of some starter ectomycorrhizal fungi to germinate and occupy a given site depends partially on the absence of competitors given their characteristics of frequent fruiting, efficient dispersal, and the existence of long-lived spores [14].
2.2. Endomycorrhizae
Most ericaceas present an ericoid mycorrhizal infection (Figure 2). Its fundamental ecophysiological roles are the conditioning of the edaphic environment, the detoxification of the soil, and the acquisition of nitrogenous and phosphate nutrients [15]; the symbiosis between ericaceas and ericoid mycorrhizae may differ according to the following environmental conditions: pH, availability and concentration of heavy metals, and the concentration of mineral nutrients, among others [16].
This ericoid clade is contained in the rhizodermal cells of Ericaceae, and only their last rootlets are mycorrhized. They lack a mantle and Hartig’s network but do have hyphae [13]. They tend to form coils or spirals of hyphae on the outermost part of the root hairs of host cells where nutrient exchange occurs. The penetration of their hyphae and their coiling within the host’s epidermal cells increases the surface area through which nutrients can be transferred. Ericoid mycorrhizae are short-range from the surrounding soil and harbor a narrow taxonomic range within ascomycetes such as Rhizoscyphus ericae, Meliniomyces sp., Oidiodendron sp., and basidiomycetes of the Sebacinaceae family [9].
Plants with ericoid mycorrhizae are common in heathlands, tundra, and boreal and neotropical forests. They can coexist with trees that establish symbiosis with ectomycorrhizae [17]. This type of mycorrhiza does not show host specificity, and roots of ericoid plants are different in their coexisting genotype; they often harbor similar ericoid mycorrhizal communities [16]. Some ericaceous genera establish symbiosis with ericoid mycorrhizae in Zamora-Chinchipe, Ecuador, such as Vaccinium, Bejaria, and Gaultheria [18]. Some authors claim that the effectiveness of this mycorrhization decreases with altitude for low temperatures present and depends on local soil conditions [15]. The symbiosis of ericoid mycorrhizae with the Vaccinium genus promotes up to 20% development of stakes rooted at 90 days and approximately 40% at 120 days in non-natural growth sites [19].
Carrillo et al., (2015) proposed an effective mycorrhization option by mixing an inoculum of ericoid mycorrhizae collected from three different edaphoclimatic conditions in southern Chile with rootlets from Vaccinium corymbosum seedlings, micropropagated in the nursery for six months. Despite not detecting an effect on seedling growth of the three blueberry cultivars during this evaluation period, the use of ericoid inocula obtained from ericaceous plants was shown to be an effective mycorrhization option to improve the acclimatization and establishment of blueberry under different field conditions. In addition, Gonçalves et al., (2015) in their research with blueberry plants, concluded that the inoculation of ericoid fungi has a clear potential to stimulate plant growth, the absorption of nutrients such as Mn, Mo, K, Fe, and Cu, and its accumulation in roots and stem. However, the efficacy of these fungi appears to depend on the cultivar.
In a comparative study, native mycorrhizae of Gaultheria sp. Were inoculated in plants of Vaccinium, showing a positive effect on their height, number of leaves, frequency, and intensity of root colonization; unlike other sources of mycorrhizae, the commercial product EndosporR did not have a representative effect on the growth and colonization variables [20].
Kottke et al., (2008) hypothesized that identical species of Sebacinales or Tulasnellales could link epiphytic orchids with ericaceous hemiepiphytes through mycorrhizal associations. Sequence analyses showed that the Sebacinales and Tulasnellales were shared only within the Ericaceae and the orchids, but not between them, i.e., common Basidiomycota guilds probably only exist between plants in the same family; their study also revealed that the sebacinaceous mycobionts of ericaceous plants from Ecuador and Canada showed different sequences.
2.3. Ectendomycorrhizae
The arbutoid clade resembles the ectomycorrhizae, both in its morphology and its fungal classification. Trees with ectomycorrhizae can establish dual mycorrhizae or double colonization with the arbutoid type, such as the roots of some ericaceous of the subfamily Arbutoideae and some members of the subfamily Pyroloideae; it has been reported that families of basidiomycetes such as Sebacinaceae, Clavulinaceae, Thelephoraceae, and some species of the genera Cortinarius, Inocybe, Russula, and Laccaria can form double mycorrhizal colonization in their hosts [18,21].
Furthermore, this type of arbutoid mycorrhizae involves the formation of a mycelium cover around the root. It has extensive intracellular colonization, a sheath of hyphae, and a Hartig network restricted to the outermost layer of cortical cells. They have also developed spirals of hyphae within the epidermal cells of the host to facilitate the transfer of nutrients between plants and fungi [21]. The arbutoid clade is present in Ericaceae that form thin roots or absorbing hairs, specifically the genera Arbutus and Arctostaphylos [18]. Arbutoid clade improves seedlings’ ability to resist drought and other negative climatic variations, favoring their establishment and facilitating the transfer of nutrients between the symbiosis [13]. Another of its benefits is its importance as a fundamental biological element of plant ecological successions, such as those responsible for directing the transition process to the climax stage of the adult forest [21].
The cavendishioid clade is like the arbutoid in its morphology, as it has a hyphal sheath, a Hartig network, intracellular hyphal coils, and forms multilayered hyphal mantles [5], but like the ericoid in their fungal association and plant affiliation, since it is formed in hemiepiphytes of Ericaceae associated with the Vaccinoideae subfamily. The Fungi that form cavendishioid mycorrhizae are the same as those that form ericoid mycorrhizae [18]. Cavendishioid-type mycorrhizae belong to the Neotropics and are present in plants of the Ericaceae family. For instance, in the San Francisco Biological Reserve, in Zamora-Chinchipe, Ecuador, this type of mycorrhizae has been found in the genera Cavendishia, Ceratostema, Diogenesia, Macleania, Orthaea, Psammisia, Sphyrospermum, and Thibaudia [22].
The monotropoid clade is distributed in temperate zones; its symbiosis is established between certain ascomycetes or basidiomycetes with ericaceous species of the genus Monotropa [12]. Ericaceae affiliated with the monotropoid clade are not photosynthetic; while they form a mantle of several capable hyphae around the root, their hyphae do not colonize cortical cells as occurs in arbutoid mycorrhizae, but instead establish a structure that grows within the cell wall, increasing its surface, but without penetrating the cell. In this case, the fungus does not receive nutrients from the plant, but rather feeds it with sugars from neighboring photosynthetically active trees that also form ectomycorrhizae. This fungus takes advantage of common mycorrhizal networks, allowing them to colonize shady forest habitats. Exogenous carbohydrates and phosphorus are transported from neighboring trees to the Ericaceae, for they are epiparasites. Since this is not a mutual symbiosis, it is not a mycorrhiza in the classical sense [23].
3. Associative Mechanism of Plant–Fungus Interaction
The recognition mechanism of an associated fungus for the formation of mycorrhizae involves many factors, such as the accumulation of phenolic compounds in the area of attempted penetration of a fungus, organic acids, volatile organic compounds, and phytohormones to initiate communication with plants, the chemotropic attraction between plants and fungi, recognition and subsequent adhesion of fungal infection structures to plant surfaces and their possible control by a balance of elicitors and suppressors, produced by the same fungi (Figure 3) [24]. For example, when the plant–fungus association is being established in ectomycorrhizae, the production and export of proteases, ligninases, and phenol-oxidase enzymes increase; there is also a slight increase in the production and export of enzymes in soil, which help break down soil waste and provide access to the nitrogen and carbon trapped within. Furthermore, the activities of these enzymes contribute to the formation of more hummus. Selected ectomycorrhizal fungi produce fatty acid esterase, an extra cellular enzyme, which degrade cuticle of plant litter thereby contributing to decomposition processes and to unmasking of nutrients [25]. Arbutoids associated with ericaceous plants may receive photosynthates from neighboring plants through shared mycorrhizal associations [24]. Likewise, ericoid and arbuscular mycorrhizae are known to mediate underground plant-to-plant signaling. In addition, arbuscular and ectomycorrhizal mycorrhizal fungi mediate the recognition of plant relatives [26]. The hyphae of ericoid mycorrhizae intercept the plasmodesma to obtain soluble carbohydrates, such as mannitol, trehalose, and hexoses, so that host invertases may not be necessary for the utilization of some sugars such as glucose and sucrose [27]. Carbohydrates such as glucose and mannose may play an important role in the adhesion process between the fungus and the plant [28].
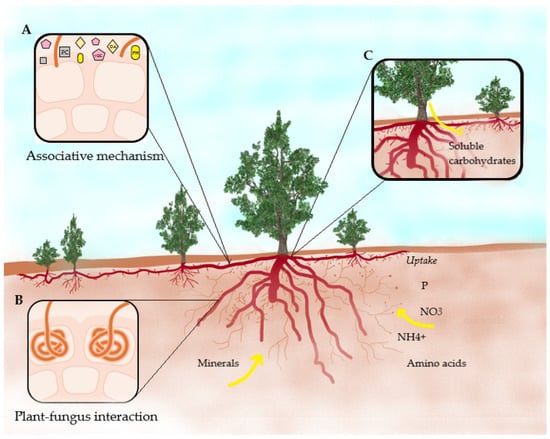
Figure 3.
Schematic diagram of ericoid mycorrhizal interaction between plants and fungi according to their mechanisms. (A) Recognition mechanism of an associated fungus for the formation of mycorrhizae; (B) establishment of the plant–fungi association; and (C) transfer of soluble carbohydrates from plant to fungus. PC: phenolic compounds, VOC: volatile organic compounds, OA: organic acids, PH: phytohormones.
Plant mycorrhizal types differ somewhat in their main dispersal vectors. Ericoids are dispersed by animals or by the wind. Ectomycorrhizae and arbuscular exhibit multiple dispersal strategies, such as the dispersal of fungal spores through animal droppings [29], dispersal of airborne propagules (spores, hyphae, sclerotia), physical soil movement, codispersion of seeds and fungal propagules by biotic dispersal mechanisms such as endozoochory by rodents [14], or secondary abiotic dispersal by wind and water after being deposited in excrement [30].
Codispersal of mycorrhizal fungal spores attached to seeds has rarely been reported. For example, the spores of some pteridophytes and gametophytes can be mycoheterotrophic, thus requiring specific fungal partners. The presence of fungi adapted to the destination site, or the joint dispersal of partners becomes a prerequisite for the maintenance and establishment of this type of plant population [31]. Even though fungi produce microscopic diaspores, they can also have limited dispersal; the location of ectomycorrhizal plants and the limited dispersion of their associated fungi make it difficult for them to establish in plant matrices dominated by arbuscular or ericoid species. Most ectomycorrhizal fungi have limited distribution ranges determined by their hosts in different ecological niches [32].
4. Considerations before the Investigation of Ericoid Mycorrhizae
For sampling, it is pertinent to consider that mycorrhizal colonization does not tend to follow a linear pattern of development in root tissues under experimental conditions and could be adjusted to a negative binomial distribution. This means that the infection begins at one or more points in the radicular system and spreads radially towards the rest of the roots, giving an infection pattern where there are simultaneous points with a high intensity of infection, as well as others with a mild or no infection [16]. Therefore, an efficient evaluation of mycorrhizal infection will depend on sampling procedures considering whether the infection has reached a dynamic equilibrium in the host root system [33].
According to Narcisa Urgiles et al. (2016), for the observation of mycorrhizal structures, fine last-order roots of 1.5 to 2.5 cm long must be rinsed in 10% KOH at 65 °C in a water bath. The incubation time can vary from 2 h to 3 days, depending on the clarification or removal of tannins. If the roots remain dark after KOH, they need to be incubated with 15% H2O2 for 15 min at room temperature. After the clarification step, the roots should be rinsed in triplicate in water before being acidified to white with 10% HCl for 2 min. Then, they are transferred to a solution of 0.05% methyl blue or aniline blue in 90% lactic acid. The roots are stained between 2 to 4 h at 65 °C in a water bath. Finally, the stained roots are placed on a microscope slide in a few drops of lactic acid for observation under light microscopy. Due to the delicacy of the ericoid and cavendishioid associations, it is sufficient to rinse the roots with KOH, and treatments with H2O2 can be avoided.
The sexual structures of fungi or sporomes can grow above ground or underground and are responsible for dispersing spores of many species of mycorrhizal fungi. The natural production of sporomes can be restricted to certain times of the year and depends on specific environmental conditions. Therefore, their study in ecosystems has limitations since it constitutes a phenophase of the life cycle of fungi [34]. Note that the absence of sporomes does not reflect the loss of those species in communities, such as colonies of mycelia or mycorrhizae on plant roots. This must be considered before proposing species of mycorrhizal fungi as threatened or endangered [17].
5. Distribution of Ericaceous Plants in Ecuador
In the herbaceous páramo of the Chibuleo people, Tungurahua province, the Ericaceae occupy a relative diversity of 2.56%. In the Quero Canton, the Páramo de la Ciénega and the Páramo del Igualata are habitats for Disterigma empetrifolium (Kanth) and Vaccinium floribundum Kunth. The Pillaro canton, Baquerizo Moreno parish, is the habitat of Macleania rupestris and Guaultheria glomerata. In the Leonan de Llucud forest, province of Chimborazo, the species Maclenia cordifolium was found [2]. From the moors of Carchi to Loja, the mortiño is distributed, a wild “superfruit” native to the Ecuadorian moor, adapted from 1000 to 4500 m above sea level. Currently used in juices, sweets, and jams, it is of great interest due to its content of antioxidants, vitamins, minerals, and sugars [35]. Two more species of this genus have been recorded in the provinces of Azuay and Loja: Vaccinium distichum and Vaccinium crenatum [36].
In the herbaceous and shrubby moors of Loja, there is a wild genetic diversity where the following stand out: Macleania rupestris, Bejaria subssesiles, Cavendishia bracteata, and Gaultheria erecta, whose fruits are useful for feeding fauna, and in the case of Macleania rupestris, also humans [37]. In addition, in the Podocarpus National Park in Loja, Urgiles et al. (2018) found a relative density of Ericaceae of 12.1%, with Vaccinium floribundum being one of the eight species with the highest record in the sampling area, with an importance value index of 71% of the most representative species recorded in the bush moor. This is due to the dispersal pattern of the species, the number of individuals, and their mycorrhization status [38]. The mycorrhization status greatly influences their competitiveness and integration or disintegration in the forestry community. While the arbuscular mycorrhizal associations promote very diverse communities, associations with basidiomycetes or ascomycetes with ectomycorrhizal fungi promote monodominant forests [39]. 90% of endemic Ericaceae are threatened as a result of deforestation, habitat fragmentation, colonization, expansion of the agricultural frontier, and to a lesser degree, grazing and fire caused by man [40].
6. Ericaceae Studied in Ecuador
In the critical review of scientific articles, documents have been identified that report that approximately 230 species of Ericaceae in Ecuador [41,42], representing 22 genera, and 98 endemic species have been recorded to date [22]. Ericaceae fruits feed insects such as ants, cockroaches, and bees [43], birds such as hummingbirds, and mammals, which act as pollination vectors or dispersing agents of seeds and propagules of mycorrhizal-forming fungi. [31]. The tropical ericaceous flowers of America are fetching. Their bracts have contrasting colors such as red, violet, and orange, attractive to birds. Their leaves and fruits may contain tannins with astringent and antirheumatic use [1].
Some species may have different ethnobotanical uses; they can be medicinal and edible, as is the case of the blueberry (Vaccinium floribundum) [9] and other species with ornamental characteristics. Within the Macleania genus, the species M. ecuadoriensis, M. laurina, M. rupestris, and M. popenoei have also been described as edible berries with similar characteristics [36]; M. rupestris is used for the preparation of beverages and handicrafts. The Cavendishia genus is characterized by being a pioneer in the repopulation of habitats affected by natural factors such as landslides and volcanic activity, and anthropological factors such as road construction; its growth in branches allows the accumulation of considerable amounts of humidity, resulting in a barrier against fires, even among species that form pyrophilic thickets; in addition, they play an inducing and promoting role in regeneration and ecological restoration, facilitating the incursion of other species less suitable for initial conditions, that is, they stimulate the rise of the high Andean forest over areas of secondary “subpáramos” [1]. Other known uses are the care and rehabilitation of gorges, the recovery and stabilization of unevenness, and sources of erosion or landslides.
Information is still limited, since little research has been conducted (Table 1) on wild species, and even more so in the Andean region, which presents climatic variants of ecological importance. Most of the national studies focus on six genera of Ericaceae of the 22 identified, predominantly Vaccinium, Bejaria, Gaultheria, Cavendishia, Macleania, and Psammisia. The themes that concern them revolve around their ethnobotany and the diversity of plants and fungi in tropical mountain forests. Studies of mycorrhizae in Ericaceae focus on the identification of associated fungi, their classification, and determining whether there is colonizing duality in the roots of Ericaceae with the different types of associations that may exist in the rhizosphere of their habitats.

Table 1.
Research associated with Ericaceae from Ecuador and their mycorrhizal associations.
7. Future Research Perspectives
The ericaceous plants of Ecuador contain a diversity of endemic and cosmopolitan microorganisms. At present, information remains limited given the research approaches around its ethnobotany, plant diversity, and fungi diversity in tropical montane forests. The wild ericaceous species mentioned in this review lie in a region with ecologically important climatic variants. Ericaceae experts have seen the necessity of collecting theoretical backgrounds and methods for any reason, such as to promote new research considering their potential uses; their ecological and nutritional importance within local communities and sites of concentration of biodiversity, due to the conservation status of certain Ericaceae; their increasing vulnerability and the current critical state of some Ericaceae; added to the specialization that merits the study of ericaceous mycorrhizae and their fundamental role in the conservation of this plant family.
The synergy and the role that mycorrhizae have in the rhizosphere of Ericaceae is a key factor. Future research should consider the evaluation of native inoculum for bioprospecting and the conservation of ericaceous species in a state of threat as a consequence of the anthropic disturbance that causes the loss of endemic plants in the montane forests of Ecuador. Such is the case of the mortiño, a wild ericaceous plant, whose fruits are used without the existence of programs or strategies for the regeneration of its natural populations. The semidomestication of this plant continues to involve an arduous process of biotechnological research on the microorganisms of the rhizosphere (bacteria and filamentous and mycorrhizal fungi). We suggest using mixtures of mycorrhizal consortia associated with various regional points of natural development for the timely acclimatization of plants with commercial and biotechnological interest, such as the mortiño.
Given this scenario, research opportunities are open to counteract the following questions: What is the influence of the anthropogenic impact on the establishment and functioning of ericoid mycorrhizae associated with ericaceous? What is the level of biological and ecological complexity of their interactions in the changing environmental context of Ecuador? What is the importance of phylogeographic analyses in Ericaceae of the genus Vaccinium? In addition, there are topics of interest to our country, for example, the sustainable use of edible Ericaceae by local communities and the importance of symbiotic interactions for their conservation and management.
8. Conclusions
The present review of ericaceous mycorrhizae’s state of the art allows us to understand the specific interactions of the symbiotic exchange of soil–plant–fungus nutrients and part of the associative mechanisms of Ericaceae in Ecuador.
The information provides significant details of the biodiversity of mycorrhizae in Ericaceae with a focus on ericoid mycorrhizal fungi, in their interaction with native and endemic species of the genus Vaccinium, Gaultheria, and Macleania, among others, which allows for a reduction in the research gaps in the development of biotechnological strategies for the conservation, preservation, and restoration of natural resources.
Author Contributions
Conceptualization, methodology, investigation, and writing—original draft preparation, A.P.F.d.V.; writing—review and editing, M.B.-A., J.N.-M. and D.P.T.; visualization, A.P.F.d.V., J.N.-M. and R.M.-G.; supervision and funding acquisition, M.B.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Botero, D.O.; Bustamante, M.J.; Cerón, M.A.; Guevara, J.D. Anatomía y morfología de Cavendishia cordifolia (Ericaceae). In Morfoanatomía Reproductiva De Plantas Vasculares: Teoría y Estudio de Casos; Universidad Nacional de Colombia: Bogotá, Colombia, 2010; pp. 62–73. ISBN 9789587017953. [Google Scholar]
- Caranqui, J.; Ortíz, M. Diversity and Floristic Composition in the Analogous Vegetation of Indiviso, Baquerizo Moreno, Tungurahua. ESPOCH Congr. Ecuad. J. STEAM 2021, 1, 1120–1128. [Google Scholar] [CrossRef]
- Iturralde, R.B. Diversidad y distribución de Ericaceae en las Antillas Mayores. Rev. Del Jardín Botánico Nac. 2006, 27, 65–73. [Google Scholar]
- Urgiles, N.; Cofre, D.; Loján, P.; Maita, J.; Albarez, P.; Báez, S.; Tamargo, E.; Eguiguren, P.; Ojeda, T.; Aguirre, N. Plant diversity, community structure, and aereal biomass in a paramo ecosystem of Southern Ecuador. Bosques Latid. Cero 2018, 8, 13. [Google Scholar]
- Setaro, S.; Weiß, M.; Oberwinkler, F.; Kottke, I. Sebacinales form ectendomycorrhizas with Cavendishia nobilis, a member of the Andean clade of Ericaceae, in the mountain rain forest of southern Ecuador. New Phytol. 2006, 169, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Castro Zaruma, E.; Moreno Lalama, C. Variación de Caracteres Funcionales de Morfos de Flores de Bejaria resinosa con Relación a la Comunidad de Visitantes Florales. Bachelor’s Thesis, Universidad del Azuay, Cuenca, Ecuador, 2020. [Google Scholar]
- Meléndez-Jácome, M.R.; Flor-Romero, L.E.; Sandoval-Pacheco, M.E.; Vasquez-Castillo, W.A.; Racines-Oliva, M.A. Vaccinium spp.: Karyotypic and phylogenetic characteristics, nutritional composition, edaphoclimatic conditions, biotic factors and beneficial microorganisms in the rhizosphere. Sci. Agropecu. 2021, 12, 109–120. [Google Scholar] [CrossRef]
- Gui, L.X.; Lu, S.S.; Chen, Q.; Yang, L.; Xiao, J.X. iTRAQ-based proteomic analysis reveals positive impacts of arbuscular mycorrhizal fungi inoculation on photosynthesis and drought tolerance in blueberry. Trees—Struct. Funct. 2021, 35, 81–92. [Google Scholar] [CrossRef]
- Vohník, M.; Sadowsky, J.J.; Kohout, P.; Lhotáková, Z.; Nestby, R.; Kolařík, M. Novel root-fungus symbiosis in Ericaceae: Sheathed ericoid mycorrhiza formed by a hitherto undescribed basidiomycete with affinities to trechisporales. PLoS ONE 2012, 7, e39524. [Google Scholar] [CrossRef]
- Bermudes, D.; Benzing, D.H. Fungi in neotropical epiphyte roots. BioSystems 1989, 23, 65–73. [Google Scholar] [CrossRef]
- Vega, A.M.; Muñoz, C.S. Presencia de micorrizas en Ericaceas en Chile. Aricultura Tec. 1994, 54, 332–339. [Google Scholar]
- Camargo-Ricalde, S.; Montaño, N.; De La Rosa-Mera, C.; Montaño-Arias, A. Micorrizas: Una gran unión debajo del suelo. Rev. Digit. Univ. 2012, 13, 19. [Google Scholar]
- Cullings, K.W. Single phylogenetic origin of ericoid mycorrhizae within the Ericaceae. Can. J. Bot. 1996, 74, 1896–1909. [Google Scholar] [CrossRef]
- Buscardo, E.; Rodríguez-Echeverría, S.; Angelis, P.; Freitas, H. Comunidades de hongos ectomicorrícicos en ambientes propensos al fuego: Compañeros esenciales para el reestablecimiento de pinares mediterráneos. Ecosistemas 2009, 18, 55–63. [Google Scholar] [CrossRef]
- Cáceres, Y.; Rada, F. ¿Cómo responde la especie leñosa Vaccinum meridionale a la temperatura en su límite altitudinal de distribución en los Andes tropicales? Ecotrópicos 2011, 24, 80–91. [Google Scholar]
- Vohník, M. Ericoid mycorrhizal symbiosis: Theoretical background and methods for its comprehensive investigation. Mycorrhiza 2020, 30, 671–695. [Google Scholar] [CrossRef] [PubMed]
- Luna., C.N.; Ruiz, L.V. Simbiosis micorrícica: Un análisis. In Interacciones Ecológicas; Universidad de Guadalajara: Guadalajara, Mexico, 2014; Volume 1, pp. 37–61. [Google Scholar] [CrossRef]
- Urgiles, N.; Haug, I.; Setaro, S.; Aguirre, N. Introduction to Mycorrhizas in the Tropics with Emphasis on the Montane Forest in Southern Ecuador. EDILOJA: Loja, Ecuador, 2016; Volume 348977215, ISBN 9789978355329. [Google Scholar]
- Noboa Silva, V. Efecto de seis tipos de sustratos y tres dosis de ácido a naftalenacético en la propagación vegetativa de Mortiño (Vaccinium floribundum Kunth). Eur. Sci. J. ESJ 2019, 15, 359. [Google Scholar] [CrossRef]
- Bautista, J.M.; Posadas, L.; Urbina, J.; Larsen, J.; Segura, S. Colonization by mycorrhizae in the production of cranberry seedlings in nursery (Vaccinium spp.) cv Biloxi. Rev. Mex. Cienc. Agrícolas 2017, 8, 695–703. [Google Scholar] [CrossRef]
- Català, S.; Garrido, I.; Tejedor, F. Dna barcoding de especies mediterráneas críticas (i): Primeros datos moleculares de gomphidius tyrrhenicus d. Antonini & m. Antonini y sus implicaciones evolutivas en el ambiente mediterráneo. Butlletí Soc. Micológica Valencia 2011, 16, 239. [Google Scholar]
- Pedraza-Peñalosa, P.; Valencia, R.; Montúfar, R.; Santiana, J.; Tye, A. Ericaceae. In Libro Rojo de Las Plantas Endémicas del Ecuador; Pontificia Universidad Católica del Ecuador: Quito, Ecuador, 2017. [Google Scholar]
- Camarena-Gutierrez, G. Interaccion planta-hongos micorrizicos arbusculares. Rev. Chapingo Ser. Cienc. For. Del Ambiente 2012, 18, 409–421. [Google Scholar] [CrossRef]
- Cullings, K. Molecular phylogeny of the Monotropoideae (Ericaceae) with a note on the placement of the Pyroloideae. J. Evol. Biol. 1994, 7, 501–516. [Google Scholar] [CrossRef]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef]
- Figueiredo, A.F.; Boy, J.; Guggenberger, G. Common mycorrhizae network: A review of the theories and mechanisms behind underground interactions. Front. Fungal Biol. 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Hughes, E.; Mitchell, D.T. Utilization of sucrose by Hymenoscyphus ericae (an ericoid endomycorrhizal fungus) and ectomycorrhizal fungi. Mycol. Res. 1995, 99, 1233–1238. [Google Scholar] [CrossRef]
- Bonfante-Fasolo, P.; Perotto, S. Visualization of surface sugar residues in mycorrhizal ericoid fungi by fluorescein conjugated lectins. Symbiosis 1986, 1, 269–288. [Google Scholar]
- Correia, M.; Heleno, R.; da Silva, L.; Costa, J.; Rodríguez-Echeverría, S. First evidence for the joint dispersal of mycorrhizal fungi and plant diaspores by birds. New Phytol. 2019, 222, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.; Heleno, R.; Vargas, P.; Rodríguez-Echeverría, S. Should I stay or should I go? Mycorrhizal plants are more likely to invest in long-distance seed dispersal than non-mycorrhizal plants. Ecol. Lett. 2018, 21, 683–691. [Google Scholar] [CrossRef]
- Naranjo-Morán, J.; Vera-Morales, M.; Barcos-Arias, M.; Oviedo-Anchundia, R.; Sánchez-Rendón, V.; Pino-Acosta, A. Dispersión y transporte de propágulos micorrícicos en el bosque seco tropical. Ecosistemas 2021, 30, 2062. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zobel, M. How mycorrhizal associations drive plant population and community biology. Science 2020, 367, eaba1223. [Google Scholar] [CrossRef]
- Vega, A.R.; Garciga, M.; Rodriguez, A.; Prat, L.; Mella, J. Blueberries mycorrhizal symbiosis outside of the boundaries of natural dispersion for ericaceous plants in Chile. Acta Hortic. 2009, 810, 665–672. [Google Scholar] [CrossRef]
- Garibay-Orijel, R.; Martínez-Ramos, M.; Cifuentes, J. Disponibilidad de esporomas de hongos comestibles en los bosques de pino-encino de Ixtlán de Juárez, Oaxaca. Rev. Mex. Biodivers. 2009, 80, 521–534. [Google Scholar] [CrossRef]
- Llivisaca-Contreras, S.A.; Manzano-Santana, P.; Ruales, J.; Naranjo-Morán, J.; Serrano-Mena, L.; Chica-Mart, E.; Cevallos-Cevallos, J.M. Mortiño (Vaccinium floribundum Kunth): An Underutilized superplant from the Andes. Horticulturae 2022, 8, 358. [Google Scholar] [CrossRef]
- Coba Santamaría, P.; Coronel, D.; Verdugo, K.; Paredes, M.; Yugsi, E.; Huachi, L. Estudio etnobotánico del mortiño (Vaccinium floribundum) como alimento ancestral y potencial alimento funcional. Granja 2012, 16, 5. [Google Scholar] [CrossRef]
- Aguirre Mendoza, Z.; Aguirre Mendoza, N.; Muñoz Ch, J. Biodiversidad de la provincia de Loja, Ecuador. Arnaldoa 2017, 24, 523–542. [Google Scholar] [CrossRef]
- Mostacedo, B.; Fredericjsen, T. Manual de Métodos Básicos de Muestreo y Análisis en Ecología Vegetal; Proyecto de Manejo Froestal Sostenible (BOLFOR): Santa Cruz de la Sierra, Bolivia, 2000. [Google Scholar]
- Kottke, I.; Haug, I. The significance of mycorrhizal diversity of trees in the tropical mountain forest of southern Ecuador. Iyonia 2004, 7, 49–56. [Google Scholar]
- Kottke, I.; Haug, I.; Setaro, S.; Suárez, J.P.; Weiß, M.; Preußing, M.; Nebel, M.; Oberwinkler, F. Guilds of mycorrhizal fungi and their relation to trees, ericads, orchids and liverworts in a neotropical mountain rain forest. Basic Appl. Ecol. 2008, 9, 13–23. [Google Scholar] [CrossRef]
- Luteyn, J.L. Ericacea del Ecuador. New York Botanical Garden; Institute of Systematic Botany: New York, NY, USA, 2007; pp. 1–20. [Google Scholar]
- Luteyn, J. The plant family Ericaceae (“Blueberries”) in Ecuador: Ecology, diversity, economic importance, and conservation. Rev. Ecuat. Med. Cienc. Biol. 2021, 42, 79–98. [Google Scholar] [CrossRef]
- Uehara, Y.; Sugiura, N. Cockroach-mediated seed dispersal in Monotropastrum humile (Ericaceae): A new mutualistic mechanism. Bot. J. Linn. Soc. 2017, 185, 113–118. [Google Scholar] [CrossRef]
- Luteyn, J.L. Diversity, adaptation, and endemism in neotropical ericaceae: Biogeographical patterns in the vaccinieae. Bot. Rev. 2002, 68, 55–87. [Google Scholar] [CrossRef]
- Setaro, S.; Kottke, I.; Oberwinkler, F. Anatomy and ultrastructure of mycorrhizal associations of neotropical Ericaceae. Mycol. Prog. 2006, 5, 243–254. [Google Scholar] [CrossRef]
- Selosse, M.; Setaro, S.; Glatard, F.; Richard, F.; Urcelay, C.; Weiß, M. Sebacinales are common mycorrhizal associates of Ericaceae. New Phytol. 2007, 174, 864–878. [Google Scholar] [CrossRef]
- Setaro, S.D.; Kron, K. Neotropical and north american vaccinioideae (ericaceae) share their mycorrhizal sebacinales-an indication for concerted migration? PLoS Curr. 2011, 3, RRN1227. [Google Scholar] [CrossRef]
- Setaro, S.D.; Garnica, S.; Herrera, P.I.; Suárez, J.P.; Göker, M. A clustering optimization strategy to estimate species richness of Sebacinales in the tropical Andes based on molecular sequences from distinct DNA regions. Biodivers. Conserv. 2012, 21, 2269–2285. [Google Scholar] [CrossRef]
- Setaro, S.; Suárez, J.P.; Herrera, P.; Cruz, D.; Kottke, I. Distinct but closely related Sebacinales form mycorrhizae with coexisting Ericaceae and Orchidaceae in a Neotropical Mountain Area. In Piriformospora Indica; Springer: Berlin/Heidelberg, Germany, 2013; pp. 81–105. [Google Scholar] [CrossRef]
- Kottke, I.; Setaro, S.; Haug, I.; Herrera, P. Mycorrhiza networks promote biodiversity and stabilize the tropical mountain rain forest ecosystem: Perspectives for understanding complex communities. In Ecosystem Services, Biodiversity and Environmental Change in a Tropical Mountain Ecosystem of South Ecuador; Springer: Berlin/Heidelberg, Germany, 2013; pp. 187–203. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
