Characterization and Comparison of Eye Development and Phototransduction Genes in Deep- and Shallow-Water Shrimp Alvinocaris longirostris and Palaemon carinicauda
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Transcriptome Sequencing and Assembly
2.3. Gene Functional Annotation and Expression Analysis
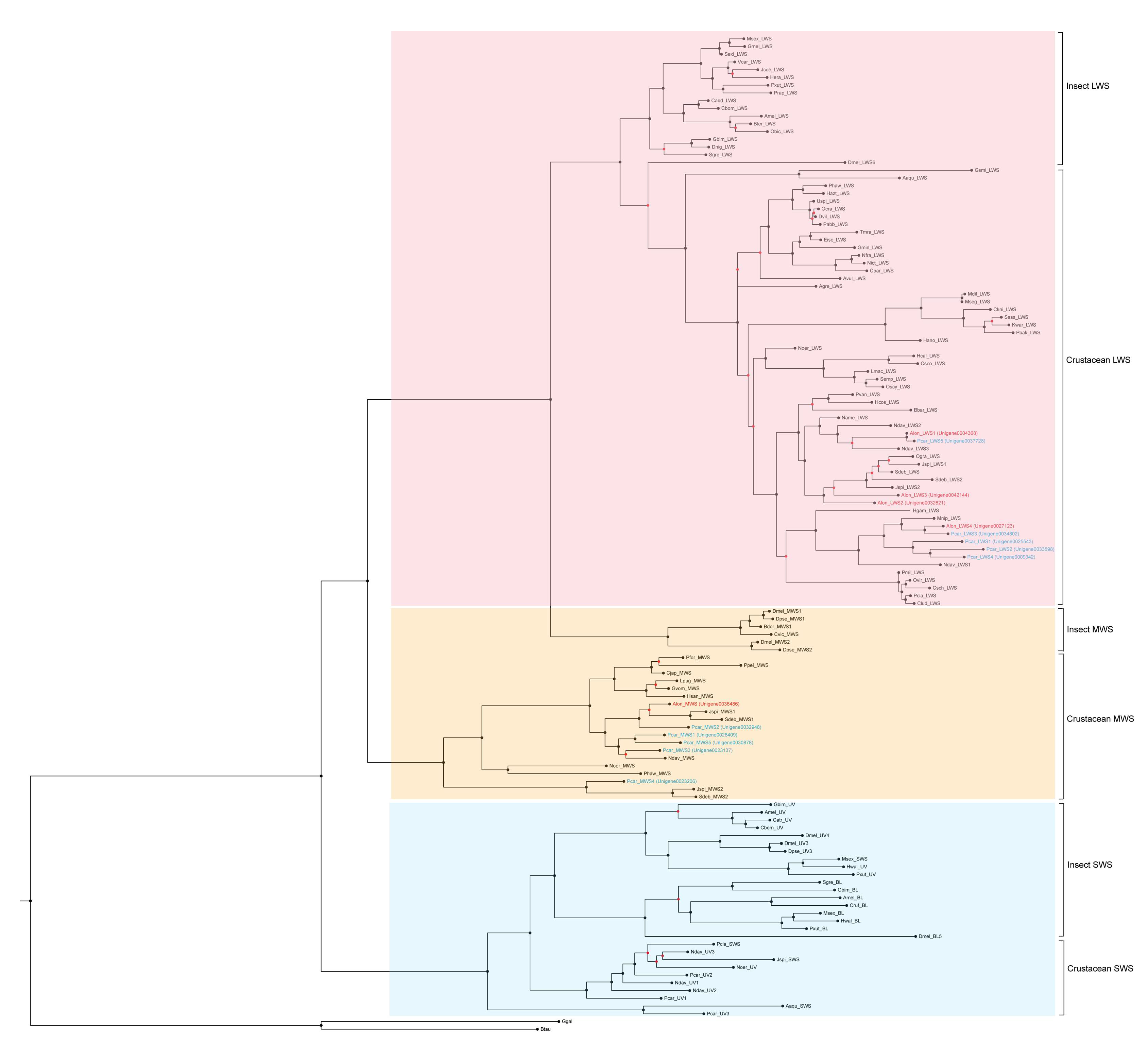
2.4. Phylogenetic and Evolutionary Analyses
2.5. Opsin Characterization Analysis
3. Results
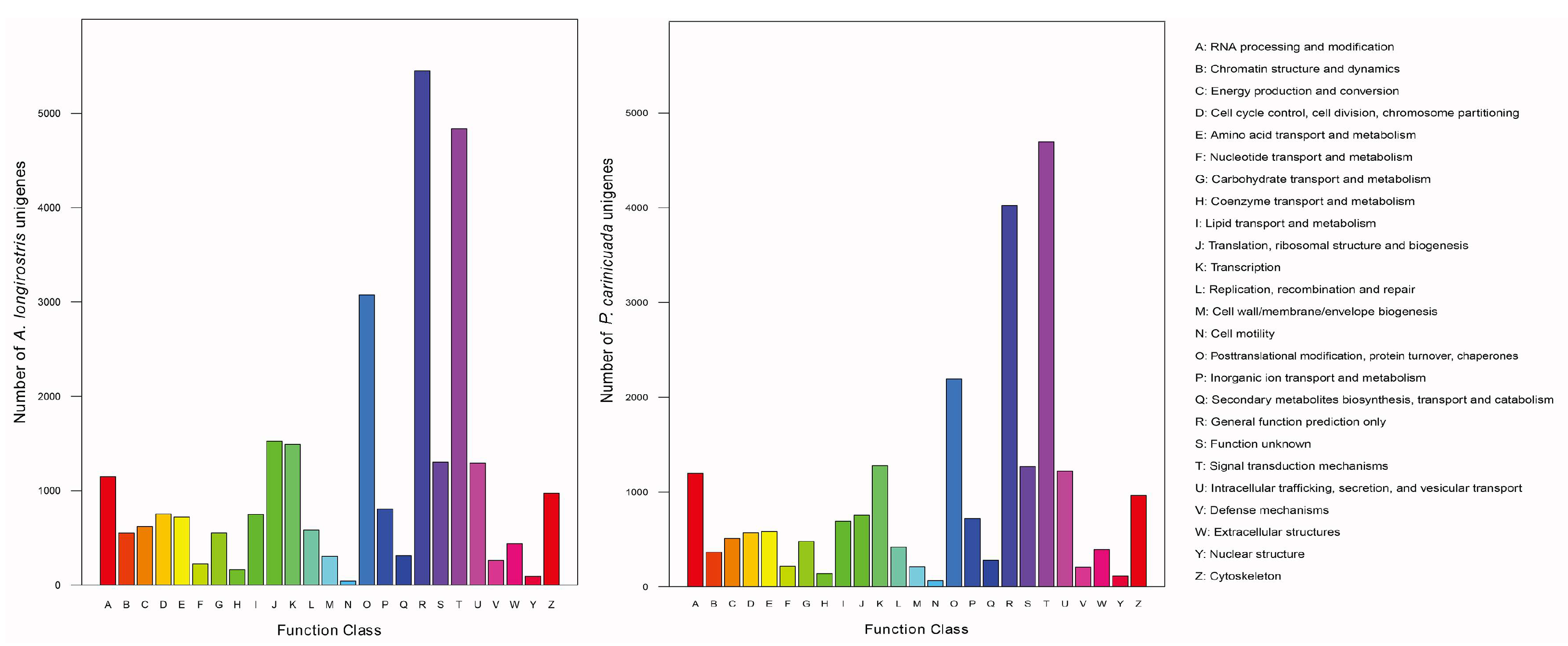
3.1. Transcriptome Assembly and Functional Annotation
3.2. Eye Development Related Genes
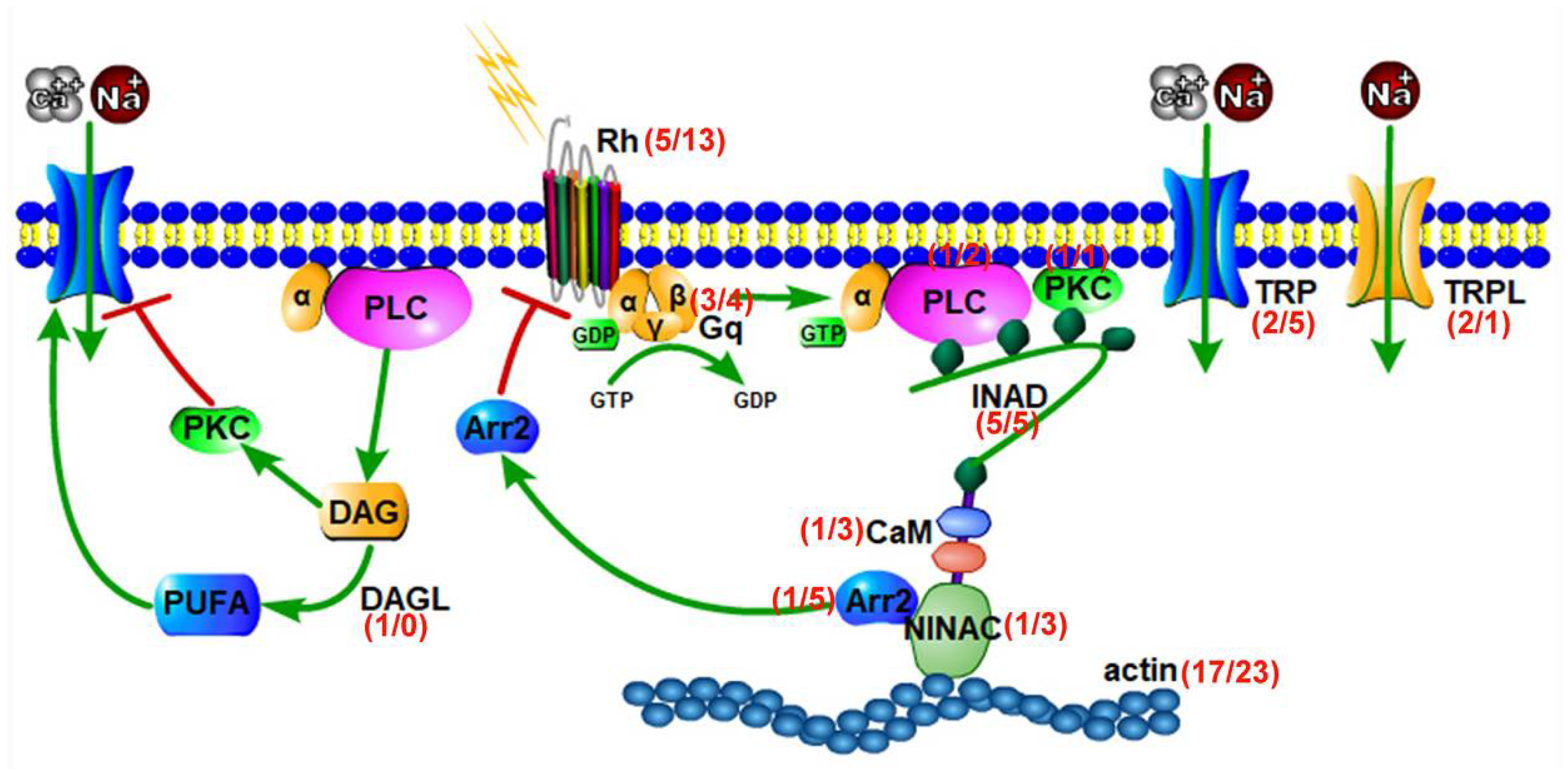
3.3. Genes Involved in the Phototransduction Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Dover, C.L. The Ecology of Deep-Sea Hydrothermal Vents; Princeton University Press: Princeton, NJ, USA, 2000. [Google Scholar]
- Levin, L.A. Ecology of cold seep sediments: Interactions of fauna with flow, chemistry and microbes. Oceanogr. Mar. Biol. 2005, 43, 11–56. [Google Scholar]
- White, S.N.; Chave, A.D.; Reynolds, G.T.; Gaidos, E.J.; Tyson, J.A.; Van Dover, C.L. Variations in ambient light emission from black smokers and flange pools on the Juan De Fuca Ridge. Geophys. Res. Lett. 2000, 27, 1151–1154. [Google Scholar] [CrossRef]
- Reynolds, G.T.; Lutz, R.A. Sources of light in the deep ocean. Rev. Geophys. 2001, 39, 123–136. [Google Scholar] [CrossRef]
- Widder, E.A. Bioluminescence in the ocean: Origins of biological, chemical, and ecological diversity. Science 2010, 328, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, S.; Frank, T.M.; Haddock, S.H.D.; Widder, E.A.; Messing, C.G. Light and vision in the deep-sea benthos: I. Bioluminescence at 500–1000 m depth in the Bahamian Islands. J. Exp. Biol. 2012, 215, 3335–3343. [Google Scholar] [CrossRef] [PubMed]
- Warrant, E.J.; Locket, N.A. Vision in the deep sea. Biol. Rev. 2004, 79, 671–712. [Google Scholar] [CrossRef]
- Hiller-Adams, P.; Case, J.F. Optical parameters of euphausiid eyes as a function of habitat depth. J. Comp. Physiol. A 1984, 154, 307–318. [Google Scholar] [CrossRef]
- Hiller-Adams, P.; Case, J.F. Eye size of pelagic crustaceans as a function of habitat depth and possession of photophores. Vision Res. 1988, 28, 667–680. [Google Scholar] [CrossRef]
- Wharton, D.N.; Jinks, R.N.; Herzog, E.D.; Battelle, B.A.; Kass, L.; Renninger, G.H.; Chamberlain, S.C. Morphology of the eye of the hydrothermal vent shrimp, Alvinocaris markensis. J. Mar. Biol. Assoc. UK 1997, 77, 1097–1108. [Google Scholar] [CrossRef]
- Elofsson, R.; Hallberg, E. Compound eyes of some deep-sea and Fiord Mysid crustaceans. Acta Zool. 1977, 58, 169–177. [Google Scholar] [CrossRef]
- Chamberlain, S.C.; Meyer-Rochow, V.B.; Dossert, W.P. Morphology of the eye of the giant deep-sea isopod Bathynomus giganteus. J. Morphol. 1986, 189, 145–156. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Nilsson, H.L. Compound eyes in polar regions, caves and the deep-sea. In Atlas of Arthropod Sensory Receptors; Eguchi, E., Tominaga, Y., Eds.; Springer: Berlin, Germany; Tokyo, Japan; New York, NY, USA, 1998; pp. 125–142. [Google Scholar]
- Van Dover, C.L.; Szuts, E.Z.; Chamberlain, S.C.; Cann, J.R. A novel eye in “eyeless” shrimp from hydrothermal vents of the Mid-Atlantic Ridge. Nature 1989, 337, 458–460. [Google Scholar] [CrossRef]
- Land, M.F. Vision: What is a naked retina good for? Nature 2002, 420, 30–31. [Google Scholar] [CrossRef]
- Tsachaki, M.; Sprecher, S.G. Genetic and developmental mechanisms underlying the formation of the Drosophila compound eye. Dev. Dynam. 2012, 241, 40–56. [Google Scholar] [CrossRef]
- Jacobsson, L.; Kronhamn, J.; Rasmuson-Lestander, A. The Drosophila Pax6 paralogs have different functions in head development but can partially substitute for each other. Mol. Genet. Genom. 2009, 282, 217–231. [Google Scholar] [CrossRef]
- Pignoni, F.; Hu, B.; Zavitz, K.H.; Xiao, J.; Garrity, P.A.; Zipursky, L. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 1997, 91, 881–891. [Google Scholar] [CrossRef]
- Pappu, K.S.; Chen, R.; Middlebrooks, B.W.; Woo, C.; Heberlein, U.; Mardon, G. Mechanism of hedgehog signaling during Drosophila eye development. Development 2003, 130, 3053–3062. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, J.; Mlodzik, M. Morphogenetic furrow initiation and progression during eye development in Drosophila: The roles of decapentaplegic, hedgehog and eyes absent. Development 2000, 127, 1325–1336. [Google Scholar] [CrossRef]
- Yang, X.; ZarinKamar, N.; Bao, R.; Friedrich, M. Probing the Drosophila retinal determination gene network in Tribolium (I): The early retinal genes dachshund, eyes absent and sine oculis. Dev. Biol. 2009, 333, 202–214. [Google Scholar] [CrossRef]
- Jeffery, W.R. Adaptive evolution of eye degeneration in the Mexican blind cavefish. J. Hered. 2005, 96, 185–196. [Google Scholar] [CrossRef]
- Jeffery, W.R. Evolution and development in the cavefish Astyanax. Curr. Top. Dev. Biol. 2009, 86, 191–221. [Google Scholar] [PubMed]
- Meng, F.; Braasch, I.; Phillips, J.B.; Lin, X.; Titus, T.; Zhang, C.; Postlethwait, J.H. Evolution of the eye transcriptome under constant darkness in Sinocyclocheilus cavefish. Mol. Biol. Evol. 2013, 30, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Carroll, S.B. Evolution at two levels: On genes and form. PLoS Biol. 2005, 3, e245. [Google Scholar] [CrossRef]
- Hardie, R.C. Phototransduction in Drosophila melanogaster. J. Exp. Biol. 2001, 204, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.L.; Blasic, J.R.; Bok, M.J.; Cameron, E.G.; Pringle, T.; Cronin, T.W.; Robinson, P.R. Shedding new light on opsin evolution. Proc. R. Soc. B 2012, 279, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, P.; Rollmann, S.M.; Cook, T.A.; Layne, J.E. Molecular evidence for color discrimination in the Atlantic sand fiddler crab, Uca pugilator. J. Exp. Biol. 2010, 213, 4240–4248. [Google Scholar] [CrossRef] [PubMed]
- Cronin, T.W.; Hariyama, T. Spectral sensitivity in crustacean eyes. In The Crustacean Nervous System; Wiese, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 499–511. [Google Scholar]
- Langecker, T.G.; Schmale, H.; Wilkens, H. Transcription of the opsin gene in degenerate eyes of cave-dwelling Astyanax fasciatus (Teleostei, Characidae) and of its conspecific epigean ancestor during early ontogeny. Cell Tissue Res. 1993, 273, 183–192. [Google Scholar] [CrossRef]
- Carlini, D.B.; Satish, S.; Fong, D.W. Parallel reduction in expression, but no loss of functional constraint, in two opsin paralogs within cave populations of Gammarus minus (Crustacea: Amphipoda). BMC Evol. Biol. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.B.; Furterer, A.; Carlson, B.M.; Stahl, B.A. An integrated transcriptome-wide analysis of cave and surface dwelling Astyanax mexicanus. PLoS ONE 2013, 8, e55659. [Google Scholar] [CrossRef]
- Hinaux, H.; Poulain, J.; da Silva, C.; Noirot, C.; Jeffery, W.R.; Casane, D.; Retaux, S. De novo sequencing of Astyanax mexicanus surface fish and pachon cavefish transcriptomes reveals enrichment of mutations in cavefish putative eye genes. PLoS ONE 2013, 8, e53553. [Google Scholar] [CrossRef]
- Stern, D.B.; Crandall, K.A. Phototransduction gene expression and evolution in cave and surface crayfishes. Integr. Comp. Biol. 2018, 58, 398–410. [Google Scholar] [CrossRef]
- Mejía-Ortíz, L.M.; Hartnoll, R.G. Modifications of eye structure and integumental pigment in two cave crayfish. J. Crustacean Biol. 2005, 25, 480–487. [Google Scholar] [CrossRef]
- Komai, T.; Segonzac, M. A revision of the genus Alvinocaris Williams and Chace (Crustacea: Decapoda: Caridea: Alvinocarididae), with descriptions of a new genus and a new species of Alvinocaris. J. Nat. Hist. 2005, 39, 1111–1175. [Google Scholar] [CrossRef]
- Sun, S.; Sha, Z.; Wang, Y. Phylogenetic position of Alvinocarididae (Crustacea: Decapoda: Caridea): New insights into the origin and evolutionary history of the hydrothermal vent alvinocarid shrimps. Deep-Sea Res. Part I 2018, 141, 93–105. [Google Scholar] [CrossRef]
- Xin, Q.; Hui, M.; Sha, Z. Eyes of differing colors in Alvinocaris longirostris from deep-sea chemosynthetic ecosystems: Genetic and molecular evidence of its formation mechanism. J. Oceanol. Limnol. 2021, 39, 282–296. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Simão, F.A.; Zdobnov, E.M. BUSCO update: Novel and streamlined workflows along with broader and deeper phylogenetic coverage for scoring of eukaryotic, prokaryotic, and viral genomes. Mol. Biol. Evol. 2021, 38, 4647–4654. [Google Scholar] [CrossRef] [PubMed]
- Manni, M.; Berkeley, M.R.; Seppey, M.; Zdobnov, E.M. BUSCO: Assessing genomic data quality and beyond. Curr. Protoc. 2021, 1, e323. [Google Scholar] [CrossRef]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Ye, J. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; William, B.A.; Mccue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Bracken-Grissom, H.D.; DeLeo, D.M.; Porter, M.L.; Iwanicki, T.; Sickles, J.; Frank, T.M. Light organ photosensitivity in deep-sea shrimp may suggest a novel role in counterillumination. Sci. Rep. 2020, 10, 4485. [Google Scholar] [CrossRef]
- DeLeo, D.M.; Bracken-Grissom, H.D. Illuminating the impact of diel vertical migration on visual gene expression in deep-sea shrimp. Mol. Ecol. 2020, 29, 3494–3510. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 2008, 9, 286–298. [Google Scholar] [CrossRef]
- Shigehiro, K.; Zmasek, C.M.; Osamu, N.; Kazutaka, K. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 2013, 41, W22–W28. [Google Scholar]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk Wim Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Anisimova, M.; Gil, M.; Dufayard, J.F.; Dessimoz, C.; Gascuel, O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.N.; Li, S.; Luan, Y.X. Pax6 in Collembola: Adaptive evolution of eye regression. Sci. Rep. 2016, 6, 20800. [Google Scholar] [CrossRef]
- Terakita, A.; Koyanagi, M.; Tsukamoto, H.; Yamashita, T.; Miyata, T.; Shichida, Y. Counterion displacement in the molecular evolution of the rhodopsin family. Nat. Struct. Mol. Biol. 2004, 11, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Glaser, T.; Walton, D.S.; Maas, R.L. Genomic structure, evolutionary conservation and aniridia mutations in the human PAX6 gene. Nat. Genet. 1992, 2, 232–239. [Google Scholar] [CrossRef]
- Jordan, T.; Hanson, I.; Zaletayev, D.; Hodgson, S.; Prosser, J.; Seawright, A.; Hastie, N.; van Heyningen, V. The human PAX6 gene is mutated in two patients with aniridia. Nat. Genet. 1992, 1, 328–332. [Google Scholar] [CrossRef]
- Quiring, R.; Walldorf, U.; Kloter, U.; Gehring, W.J. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 1994, 265, 785–789. [Google Scholar] [CrossRef]
- Kronhamn, J.; Frei, E.; Daube, M.; Jiao, R.; Rasmuson-Lestander, A. Headless flies produced by mutations in the paralogous Pax6 genes eyeless and twin of eyeless. Development 2002, 129, 1015–1026. [Google Scholar] [CrossRef]
- Czerny, T.; Halder, G.; Kloter, U.; Souabni, A.; Gehring, W.J.; Busslinger, M. Twin of eyeless, a second Pax-6 gene of Drosophila, acts upstream of eyeless in the control of eye development. Mol. Cell 1999, 3, 297–307. [Google Scholar] [CrossRef]
- Punzo, C.; Seimiya, M.; Flister, S.; Gehring, W.J.; Plaza, S. Differential interactions of eyeless and twin of eyeless with the sine oculis enhancer. Development 2002, 129, 625–634. [Google Scholar] [CrossRef]
- Gaten, E.; Herring, P.J.; Shelton, P.M.J.; Johnson, M.L. The development and evolution of the eyes of vent shrimps (Decapoda: Bresiliidae). Cah. Biol. Mar. 1998, 39, 287–290. [Google Scholar]
- Gaten, E.; Herring, P.J.; Shelton, P.M.J.; Johnson, M.L. Comparative morphology of the eyes of postlarval bresiliid shrimps from the region of hydrothermal vents. Biol. Bull. 1998, 194, 267–280. [Google Scholar] [CrossRef]
- Jinks, R.N.; Markley, T.L.; Taylor, E.E.; Perovich, G.; Dittel, A.I.; Epifanio, C.E.; Cronin, T.W. Adaptive visual metamorphosis in a deep-sea hydrothermal vent crab. Nature 2002, 420, 68–70. [Google Scholar] [CrossRef] [PubMed]
- Hardie, R.C.; Postma, M. Phototransduction in microvillar photoreceptors of Drosophila and other invertebrates. In The Senses: A Comprehensive Reference; Masland, R.H., Albright, T.D., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2008; Volume 1, pp. 77–130. [Google Scholar]
- Petrash, J.M.; Ruzycki, P.A.; Zablocki, G.J. Visionary genomics. Hum. Genom. 2011, 5, 519–521. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.L.; Speiser, D.I.; Zaharoff, A.K.; Caldwell, R.L.; Cronin, T.W.; Oakley, T.H. The evolution of complexity in the visual systems of stomatopods: Insights from transcriptomics. Integr. Comp. Biol. 2013, 53, 39–49. [Google Scholar] [CrossRef][Green Version]
- Marshall, J.; Carleton, K.L.; Cronin, T. Colour vision in marine organisms. Curr. Opin. Neurobiol. 2015, 34, 86–94. [Google Scholar] [CrossRef]
- Stieb, S.M.; Cortesi, F.; Sueess, L.; Carleton, K.L.; Salzburger, W.; Marshall, N.J. Why UV vision and red vision are important for damselfish (Pomacentridae): Structural and expression variation in opsin genes. Mol. Ecol. 2017, 26, 1323–1342. [Google Scholar] [CrossRef] [PubMed]
- White, S.N.; Chave, A.D. Investigations of ambient light emission at deep-sea hydrothermal vents. J. Geophys. Res. 2002, 107, 2001. [Google Scholar] [CrossRef]
- Herring, P.J. The spectral characteristics of luminous marine organisms. Proc. R. Soc. Lond. B 1983, 220, 183–217. [Google Scholar]
- Widder, E.A.; Latz, M.I.; Case, J.F. Marine bioluminescence spectra measured with an optical multichannel detection system. Biol. Bull. 1983, 165, 791–810. [Google Scholar] [CrossRef]
- Haddock, S.H.D.; Case, J.F. Bioluminescence spectra of shallow and deep-sea gelatinous zooplankton: Ctenophores, medusae, and siphonophores. Mar. Biol. 1999, 133, 571–582. [Google Scholar] [CrossRef]
- Oakley, T.H.; Huber, D.R. Differential expression of duplicated opsin genes in two eye types of ostracod crustaceans. J. Mol. Evol. 2004, 59, 239–249. [Google Scholar] [CrossRef]
- Porter, M.L.; Cronin, T.W.; McClellan, D.A.; Crandall, K.A. Molecular characterization of crustacean visual pigments and the evolution of pancrustacean opsins. Mol. Biol. Evol. 2007, 24, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.L.; Bok, M.J.; Robinson, P.R.; Cronin, T.W. Molecular diversity of visual pigments in Stomatopoda (Crustacea). Visual Neurosci. 2009, 26, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Denton, E.J. Light and vision at depths greater than 200 metres. In The Visual System of Fish; Douglas, R.H., Djamqoz, M.B.A., Eds.; Chapman & Hall: London, UK, 1990; pp. 127–148. [Google Scholar]
- Douglas, R.H.; Hunt, D.M.; Bowmaker, J.K. Spectral sensitivity tuning in the deep-sea. In Sensory Processing in Aquatic Environments; Collin, S.P., Marshall, N.J., Eds.; Springer New York Inc.: New York, NY, USA, 2003; pp. 323–342. [Google Scholar]
- Hui, M.; Song, C.; Liu, Y.; Li, C.; Cui, Z. Exploring the molecular basis of adaptive evolution in hydrothermal vent crab Austinograea alayseae by transcriptome analysis. PLoS ONE 2017, 12, e0178417. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.T.; Tseng-Crank, J.; Kim, E.; Mahapatra, C.; Shino, S.; Zhou, Y.; An, L.; Doerge, R.W.; Pak, W.L. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron 2008, 58, 884–896. [Google Scholar] [CrossRef]
- Lev, S.; Katz, B.; Tzarfaty, V.; Minke, B. Signal-dependent hydrolysis of phosphatidylinositol 4,5-bisphosphate without activation of phospholipase C: Implications on gating of Drosophila TRPL (transient receptor potential-like) channel. J. Biol. Chem. 2012, 287, 1436–1447. [Google Scholar] [CrossRef]


| Index | Value (Percentage) |
|---|---|
| Numbers of unigenes | 46,709 |
| N50 length of unigenes | 1217 |
| Average length of unigenes (bp) | 718 |
| Annotated in NR | 16,866 (36.11%) |
| Annotated in Swiss-Prot | 12,431 (26.61%) |
| Annotated in KOG | 11,561 (24.75%) |
| Annotated in KEGG | 8674 (18.57%) |
| Annotated in GO | 2216 (13.14%) |
| Annotated in at least one database | 16,951 (36.29%) |
| Alvinocaris longirostris | Palaemon carinicauda | ||||
|---|---|---|---|---|---|
| Gene ID | RPKM | Annotation | GeneID | RPKM | Annotation |
| Opsin | |||||
| Unigene0004368 | 8.17 | rhodopsin [Penaeus vannamei] | Unigene0037728 | 90,886.59 | rhodopsin [Penaeus vannamei] |
| Unigene0032821 | 1.53 | rhodopsin-like [Penaeus vannamei] | Unigene0025543 | 63.29 | LWS opsin [Macrobrachium nipponense] |
| Unigene0042144 | 40.68 | rhodopsin-like [Penaeus vannamei] | Unigene0033598 | 180.53 | LWS opsin [Macrobrachium nipponense] |
| Unigene0027123 | 1.70 | LWS opsin [Macrobrachium nipponense] | Unigene0034802 | 15,756.87 | LWS opsin [Macrobrachium nipponense] |
| Unigene0036486 | 2.92 | opsin protein [Leptuca pugilator] | Unigene0009342 | 15.00 | LWS opsin [Macrobrachium nipponense] |
| Unigene0028409 | 20.80 | opsin protein [Leptuca pugilator] | |||
| Unigene0032948 | 1417.97 | opsin protein [Leptuca pugilator] | |||
| Unigene0023137 | 30.24 | opsin protein [Leptuca pugilator] | |||
| Unigene0023206 | 5.03 | opsin [Penaeus vannamei] | |||
| Unigene0030878 | 62.38 | opsin 1 [Gelasimus vomeris] | |||
| Unigene0027784 | 10.57 | UV2 opsin [Penaeus vannamei] | |||
| Unigene0032158 | 375.03 | UV2 opsin [Penaeus vannamei] | |||
| Unigene0032059 | 158.62 | opsin, UVS-like [Penaeus vannamei] | |||
| Gq | |||||
| Unigene0018293 | 1.70 | Gαq [Litopenaeus vannamei] | Unigene0029827 | 306.06 | Gαq [Litopenaeus vannamei] |
| Unigene0018292 | 6.81 | Gαq [Panulirus argus] | Unigene0019099 | 1.37 | Gα [Anopheles gambiae] |
| Unigene0005837 | 20.00 | Gγ [Megachile rotundata] | Unigene0035586 | 88.09 | Gγ [Megachile rotundata] |
| Unigene0036996 | 147.32 | Gβ [Hyalella azteca] | |||
| PLC | |||||
| Unigene0047519 | 7.07 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase classes I and II isoform X2 [Cimex lectularius] | Unigene0032290 | 3.50 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase classes I and II isoform X1 [Cimex lectularius] |
| Unigene0007465 | 38.34 | phospholipid phospholipase C beta isoform [Homarus americanus] | |||
| PKC | |||||
| Unigene0035146 | 3.53 | PKC, brain isozyme [Trachymyrmex cornetzi] | Unigene0037047 | 12.51 | PKC, brain isozyme [Cimex lectularius] |
| TRP | |||||
| Unigene0031956 | 1.28 | TRP protein-like [Plutella xylostella] | Unigene0041701 | 4.31 | TRP protein-like [Tribolium castaneum] |
| Unigene0008099 | 1.87 | TRP protein-like [Hyalella azteca] | Unigene0025995 | 4.03 | TRP protein-like [Hyalella azteca] |
| Unigene0036355 | 138.36 | TRP protein-like [Hyalella azteca] | |||
| Unigene0025996 | 3.12 | TRP protein [Orchesella cincta] | |||
| Unigene0037466 | 63.95 | TRP channel [Danaus plexippus] | |||
| TRPL | |||||
| Unigene0002671 | 2.20 | TRPL protein [Hyalella azteca] | Unigene0036670 | 1807.23 | TRPL protein [Hyalella azteca] |
| Unigene0009926 | 1.04 | TRPL protein [Hyalella azteca] | |||
| CaM | |||||
| Unigene0041980 | 2.74 | calmodulin-alpha isoform [Papilio machaon] | Unigene0046282 | 1.20 | calmodulin-beta-like isoform [Aethina tumida] |
| Unigene0028022 | 1207.07 | calmodulin [Trichinella pseudospiralis] | |||
| Unigene0034208 | 21.68 | calmodulin-like protein [Zootermopsis nevadensis] | |||
| NINAC | |||||
| Unigene0013894 | 1.68 | myosin-IIIb-like [Hyalella azteca] | Unigene0036698 | 109.85 | myosin-IIIb-like [Hyalella azteca] |
| Unigene0036679 | 46.20 | myosin-IIIb-like [Orussus abietinus] | |||
| Unigene0036678 | 79.34 | NINAC-like isoform [Hyalella azteca] | |||
| Arrestin | |||||
| Unigene0037454 | 1.79 | arrestin homolog [Hyalella azteca] | Unigene0031031 | 135.94 | arrestin homolog [Hyalella azteca] |
| Unigene0031684 | 117.09 | arrestin homolog [Hyalella azteca] | |||
| Unigene0036855 | 33074.95 | arrestin homolog [Hyalella azteca] | |||
| Unigene0030229 | 6832.96 | arrestin homolog [Hyalella azteca] | |||
| Unigene0029989 | 609.90 | arrestin [Orchesella cincta] | |||
| DAGL | |||||
| Unigene0045000 | 2.47 | DAGL alpha-like [Hyalella azteca] | |||
| Actin | |||||
| Unigene0050853 | 29.43 | actin [Eulimnogammarus vittatus] | Unigene0038263 | 91.80 | actin [Eulimnogammarus cyaneus] |
| Unigene0014222 | 1.0 | actin [Chilodonella uncinata] | Unigene0034159 | 1824.31 | beta-actin [Macrobrachium nipponense] |
| Unigene0002593 | 8.28 | actin [Portunus trituberculatus] | Unigene0035399 | 2.07 | actin 1 [Procambarus clarkii] |
| Unigene0046083 | 14.64 | actin 1 [Procambarus clarkii] | Unigene0038246 | 9.52 | actin-2 [Penaeus vannamei] |
| Unigene0046852 | 2532.89 | beta-actin [Penaeus monodon] | Unigene0035402 | 13.00 | actin 1 [Penaeus vannamei] |
| Unigene0039765 | 128.24 | actin 6 [Pandalus platyceros] | Unigene0035632 | 15.64 | actin 6 [Pandalus platyceros] |
| Unigene0014452 | 1.69 | actin [Armadillidium vulgare] | Unigene0038264 | 1.67 | actin [Penaeus vannamei] |
| Unigene0012144 | 686.58 | skeletal muscle actin 6 [Rimicaris exoculata] | Unigene0035395 | 5.76 | skeletal muscle actin 6 [Rimicaris exoculata] |
| Unigene0034554 | 16.89 | actin-like [Penaeus vannamei] | Unigene0038262 | 39.92 | skeletal muscle actin 6 [Palaemon varians] |
| Unigene0009093 | 12.08 | actin-2 [Penaeus vannamei] | Unigene0034152 | 80.94 | actin-like [Penaeus vannamei] |
| Unigene0038252 | 111.22 | skeletal muscle actin 8 [Homarus americanus] | |||
| Unigene0034150 | 65.39 | skeletal muscle alpha actin [Pandalus platyceros] | |||
| Unigene0035634 | 25.35 | actin 2 [Penaeus vannamei] | |||
| INAD PDZ | |||||
| Unigene0046429 | 4.50 | multiple PDZ domain protein [Portunus trituberculatus] | Unigene0011764 | 1.83 | PDZ domain-containing protein 2 [Portunus trituberculatus] |
| Unigene0000247 | 1.57 | multiple PDZ domain protein-like [Zootermopsis nevadensis] | Unigene0020200 | 1.52 | PDZ domain-containing protein 2 [Penaeus vannamei] |
| Unigene0042843 | 3.33 | PDZ domain-containing protein 2 [Penaeus vannamei] | Unigene0034891 | 38.85 | multiple PDZ domain protein-like isoform X5 [Penaeus vannamei] |
| Unigene0026310 | 3.19 | PDZ domain-containing protein 2 [Penaeus vannamei] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, M.; Xin, Q.; Cheng, J.; Sha, Z. Characterization and Comparison of Eye Development and Phototransduction Genes in Deep- and Shallow-Water Shrimp Alvinocaris longirostris and Palaemon carinicauda. Diversity 2022, 14, 653. https://doi.org/10.3390/d14080653
Hui M, Xin Q, Cheng J, Sha Z. Characterization and Comparison of Eye Development and Phototransduction Genes in Deep- and Shallow-Water Shrimp Alvinocaris longirostris and Palaemon carinicauda. Diversity. 2022; 14(8):653. https://doi.org/10.3390/d14080653
Chicago/Turabian StyleHui, Min, Qian Xin, Jiao Cheng, and Zhongli Sha. 2022. "Characterization and Comparison of Eye Development and Phototransduction Genes in Deep- and Shallow-Water Shrimp Alvinocaris longirostris and Palaemon carinicauda" Diversity 14, no. 8: 653. https://doi.org/10.3390/d14080653
APA StyleHui, M., Xin, Q., Cheng, J., & Sha, Z. (2022). Characterization and Comparison of Eye Development and Phototransduction Genes in Deep- and Shallow-Water Shrimp Alvinocaris longirostris and Palaemon carinicauda. Diversity, 14(8), 653. https://doi.org/10.3390/d14080653






