1. Introduction
Interest in conditions for restoring degraded landscapes is rising with increased recognition that global changes affect biodiversity and ecosystem function [
1]. Plantations affect the soil’s physical and chemical properties, modifying the number, diversity, and activity of the soil microbiota, including both free and symbiotic fungal populations [
2], enhancing biodiversity values [
3], soil seed banks [
4], and soil microbes [
5]. The study of arbuscular mycorrhizae (AM) as an index of restoration, in both undisturbed and degraded ecosystems, supplies relevant information for restoration programs, as AM fungi (AMF) can be the most important mediator of soil aggregation [
6]. Environmental changes can affect plant/soil-associated microorganisms either in number, diversity, or activity; however, restoration practices minimize soil disturbance, increase soil organic matter, or improve soil structure to maintain soil communities associated to native plant species.
Most studies use fast-growing, short-lived species as cover and later, the perennial plants establish themselves [
7]. However, those species may inhibit the subsequent establishment of perennial plants. Otherwise, restoring slow-growing species to facilitate colonization by other native species, thus increasing native species richness, can be a viable strategy [
8].
Initially, mycorrhizae were commonly used for restoration and increasing plant growth [
9]. Recently, more attention was focused on the non-nutritional effects of this symbiosis, such as reducing plant diseases and modifying water relationships, and its contribution to soil structure and erosion control due to its involvement in soil aggregate stabilization [
10,
11]. AMF reinforce soil structure in both a physical and a chemical way; thus, the extraradical hyphae of AMF involve soil and root particles and produce proteins such as glomalin, which stabilize soil aggregates [
12].
A need for better understanding of interactions between vegetation, fauna, soil microbiota, and soil properties has been emphasized. Thus, mycorrhizae [
13], livestock [
14], and nurse plants [
15,
16] are important in plant species establishment and persistence [
17]. Gelviz-Gelvez et al. [
17] showed suitable ecological attributes for restoration (plant cover, abundance, and mycorrhization), as well as tolerance to projected future climate changes, should be found in potential plant species for use in long-term ecological restoration projects.
More attention has been paid to mycorrhizae as essential symbiosis together with
Rhizobium, permitting plants to perform better under stressful and adverse conditions. The paradigm in ecological restoration was prevalently phytocentric, minimizing soil ecology [
7]. Moreover, restoration projects are mainly short-term, which is inadequate to evaluate plant vegetative growth, microbial changes, and characteristic soil processes [
18]. There is a need for more scientific information on forest restoration practices in tropical biomes [
19]. As existing practices are inadequate for preserving wild plant species with small populations [
19], effective strategies to preserve the regional biodiversity, ecosystem processes, and services [
20] are needed. The study of AM in both undisturbed and degraded ecosystems supplies relevant information for restoration programs, as undisturbed sites can indicate selected AMF for inoculation and management of indigenous AMF species, and degraded sites can indicate the need for improving specific microbial populations through inoculation.
Previous reports showed that riparian plant species were highly mycorrhizal-dependent [
21,
22]. Moreover, supplementing with P is generally required in the management strategy for revegetating riparian areas. Similarly, the application of compost and digestate can increase the soil aggregation, which was associated with the lability of soil organic carbon and variations in the abundance of eubacteria and glomalin concentration in soils [
22]. The riparian vegetation of Sabará and Velhas Rivers (São Francisco River basin) in Brazil, has been used for cattle and agriculture practices. The reintroduction of native plant species via transplants using AMF-inoculated seedlings of selected native tree species (
Centrolobium tomentosum Guill. ex Benth,
Anadenanthera colubrina (Vell.),
Erythrina speciosa Andrews,
Inga edulis Mart.,
Mimosa bimucronata (DC.) Kuntze,
Peltophorum dubium (Spreng.) Taub.,
Samanea inopinata Barneby & Grimes, and
Croton urucurana Baill.) resulted in a revegetated area rich in leguminous trees [
23]. Aiming to test if organic matter and phosphorus addition affect root colonization by AMF in riparian sites, we studied the ecosystem’s restoration, focusing on the benefits of AM symbiosis in Brazilian riparian sites, with a particular interest in the preserved area. We also proposed the hypothesis that arbuscular mycorrhizal and rhizobial inoculation for legumes promoted rapid establishment of the vegetation (native trees) cover.
2. Materials and Methods
2.1. Cultivation Conditions and Experimental Design
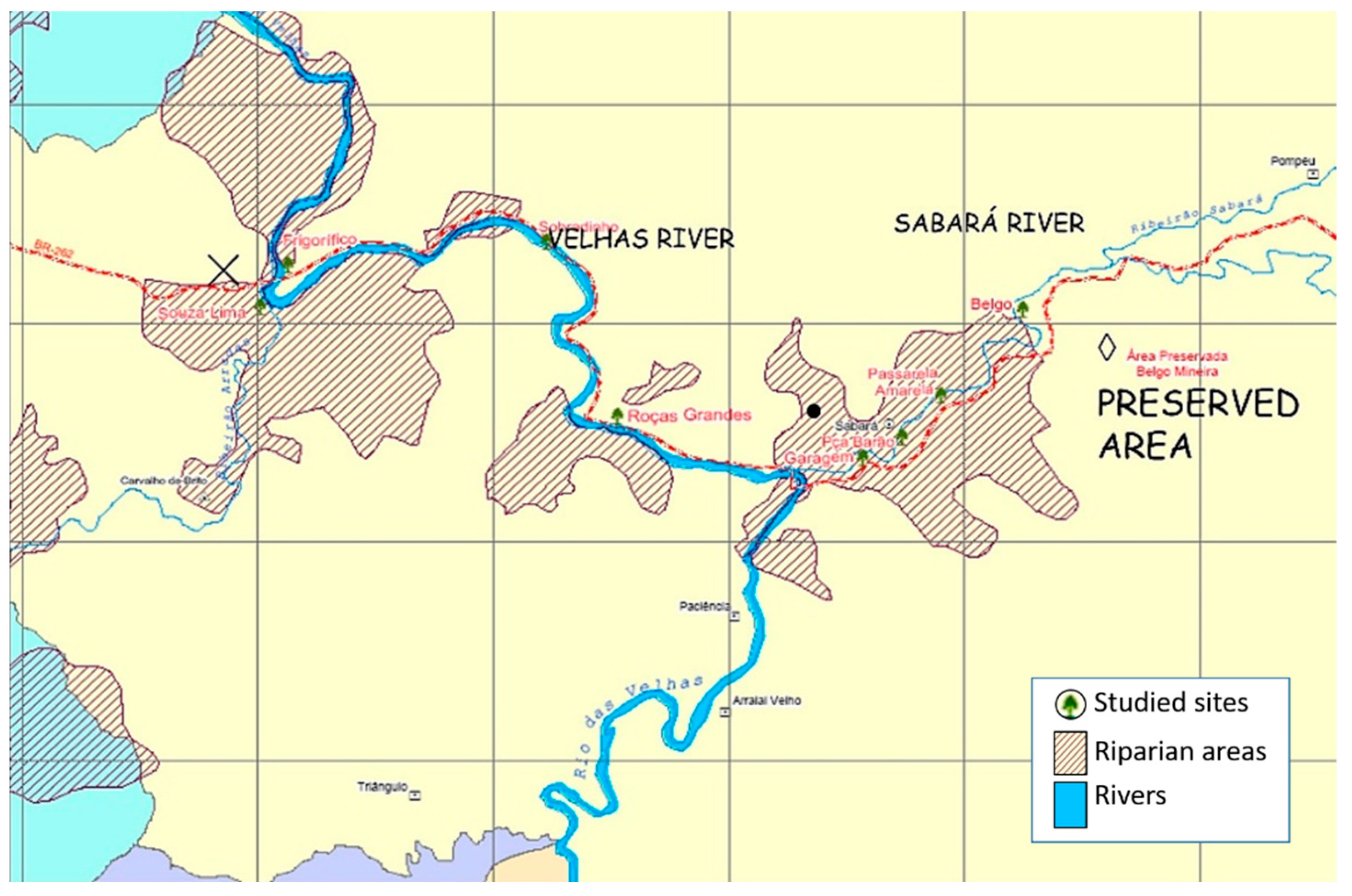
We compile results from studies carried out in 2007–2008, in Minas Gerais state, southeastern Brazil (19°53′00″ S Long: 043°58′00″ W Alt: 7869 m a.s.l.) (
Table 1,
Figure 1) [
22].
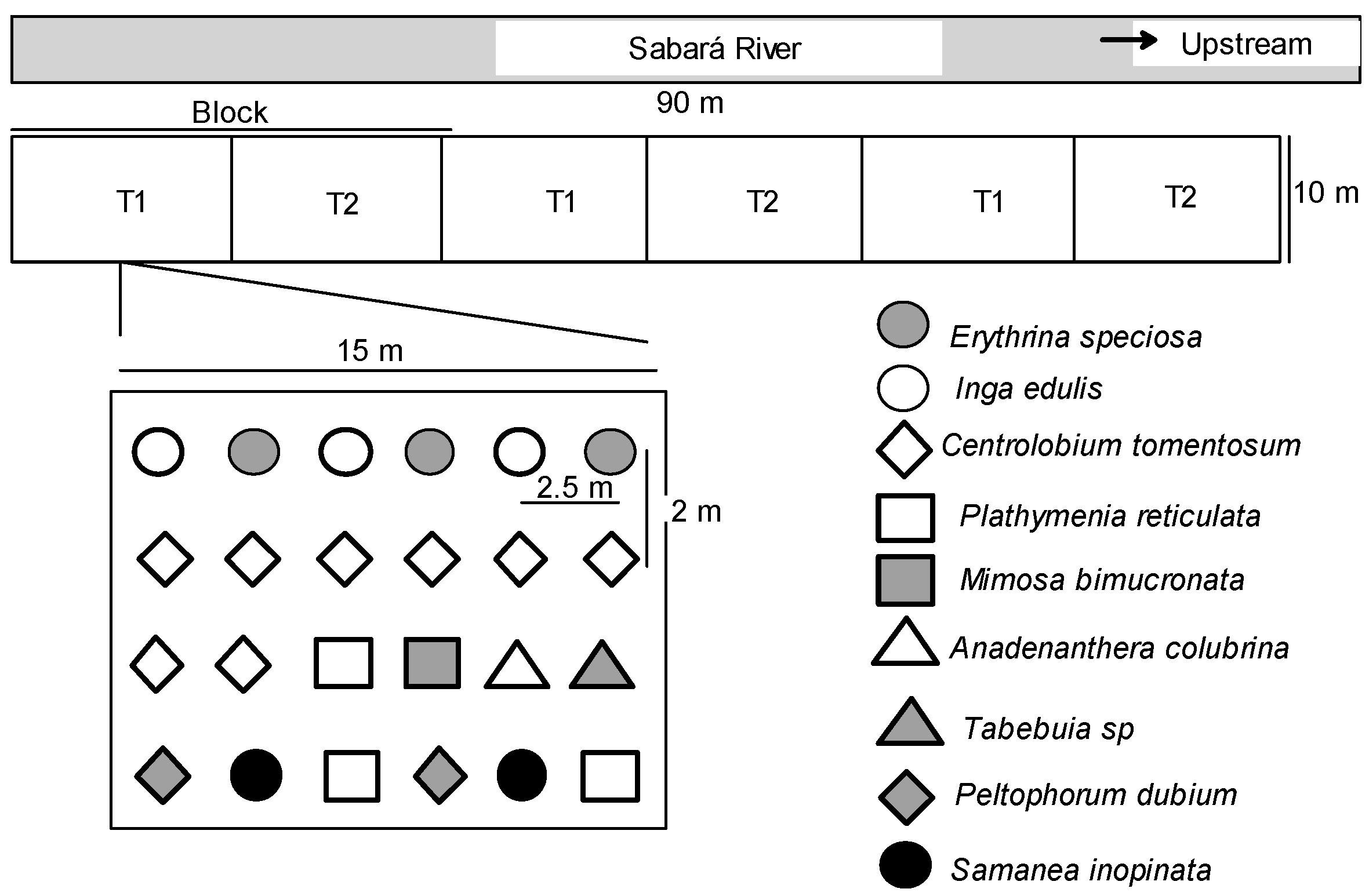
The climate of the region is tropical Aw with a dry winter (Köppen climate classification), with an annual mean temperature of 23 °C and annual mean precipitation of 1400 mm. Rainfall was unimodal-distributed, most coming between November and March (more than 400 mm monthly), followed by a prolonged dry season without or with few precipitations between May and August. The soil of the study site has sandy loam texture. The study was conducted in field sites previously used for cattle and agriculture practices. The field experiment consisted of a complete randomized block with three replications and each experimental plot was 150 m
2, with 2 × 2.5 m spacing (
Figure 2).
The riparian vegetation of Sabará and Velhas Rivers (São Francisco River basin), Brazil, have been used for cattle and agriculture practices. The reintroduction of native plant species via transplants using AMF-inoculated seedlings of native tree species belonging to Fabaceae (
Anadenanthera colubrina (Vell.) Brenan,
Centrolobium tomentosum Guill. ex Benth.,
Erythrina speciosa Andrews,
Inga edulis Mart.,
Mimosa bimucronata (DC.) Kuntze,
Peltophorum dubium (Spreng.) Taub.,
Plathymenia reticulata Benth., and
Samanea inopinata Barneby & Grimes) and Bignoniaceae (
Tabebuia sp.) resulted in a revegetated area (
Table 1) rich in leguminous trees.
We investigated a pristine site, the restored sites at Velhas and Sabará rivers, and a degraded surrounding area. In the restored plots at Sabará river, the treatments tested were: 1 = fertilized with organic matter (OM), 2 = fertilized with natural rock phosphate (P). For evaluation of root colonization, T1 was used as control (composed of reduced chemical fertilization and organic matter addition). Each treatment was replicated three times, each consisting of 24 plants.
Efficient strains were previously selected from culture collections (Federal University of Minas Gerais) against isolated nodules and used as rhizobial inocula specific for each native legume. Mycorrhizal inocula were multiplied using
Brachiaria decumbens. Among the inocula, selected AMF species were:
Acaulospora scrobiculata,
Gigaspora margarita,
Glomus brohultii, and
Scutellospora cerradensis [
23].
The degraded site, with a vegetation dominated by herbs (Asteraceae, Malvaceae, and Poaceae), and a preserved site up the river, were sampled. Roots and rhizospheric soil samples of tree legumes (C. tomentosum, I. edulis, and M. bimucronata) were collected in the field and analyzed in the laboratory.
Soil samples with three replicate plots per field site, obtained in 2007 at the different sites, were used for determination of chemical soil parameters and for AMF spore extraction.
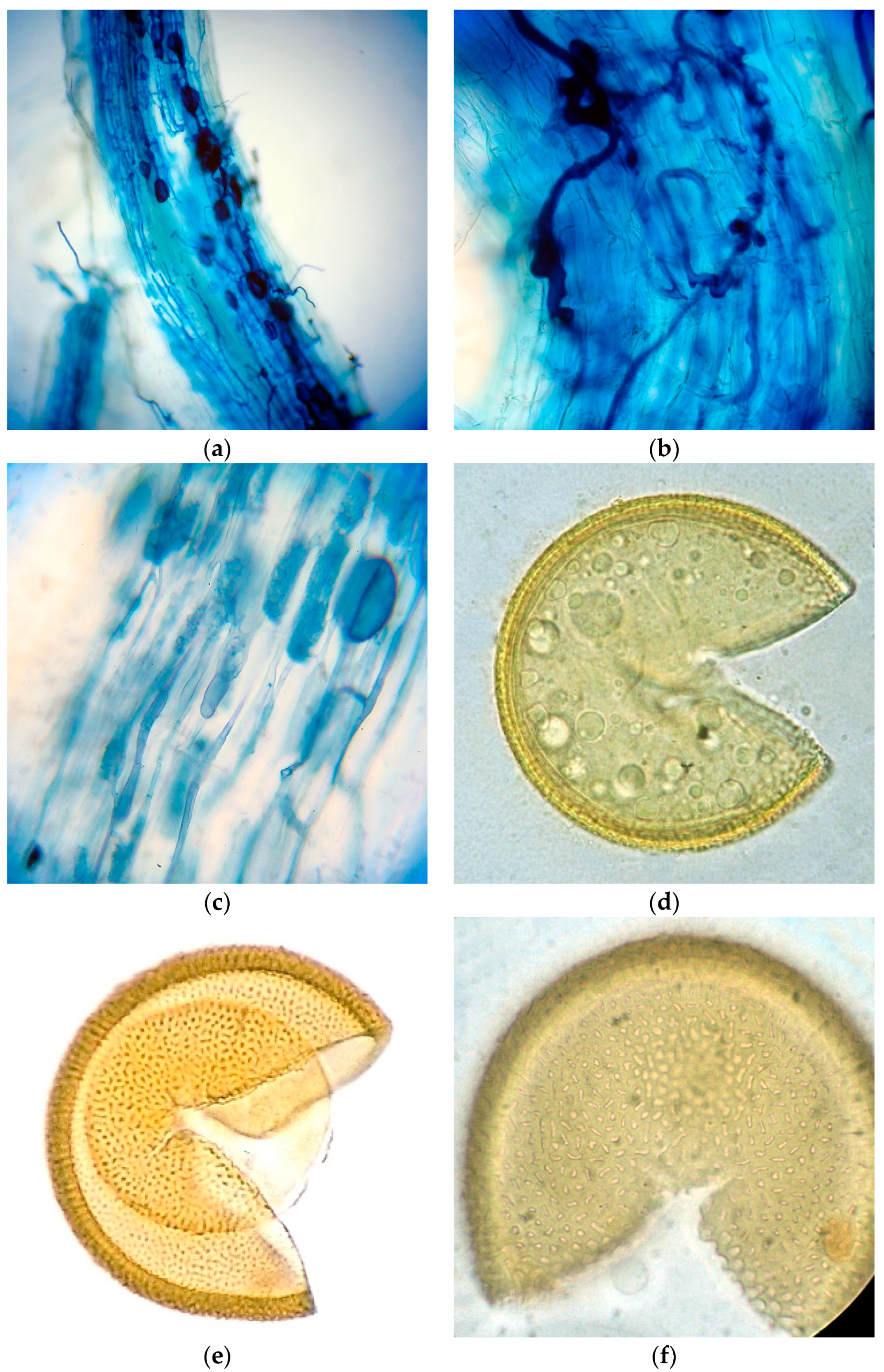
2.2. Root Staining
Samples of roots were collected from plant species only in the restored site and were fixed in a fixative solution. Roots were stained and assessed for mycorrhizal colonization as follows: fine roots were washed several times in tap water and bleached in 10% (
w/
v) KOH overnight and then heated to approximately 90 °C in a water bath for 1 h. The cooled root samples were washed and stained with 0.05% (
w/
v) Trypan blue water solution. Roots were cut into 1 cm segments and 30 of these 1 cm root fragments were examined per sample, under a compound microscope, for their AMF status. If at least one root segment was found to contain fungal mycelia, arbuscules, or vesicles, then the sample was considered as an AM plant, and recorded as “+”. Plants were recorded as non-mycorrhizal (“-”) when neither arbuscules/vesicles nor fungal mycelia were detected in their root cortical cells. Quantification of mycorrhizal colonization was according to [
24], and results were expressed as percentage of colonized segments (number of colonized segments/total analyzed segments). External hyphae (%) and auxiliary cells (AC) (%) were calculated as number of colonized segments by the structure/total analyzed segments.
Intensity of AMF colonization was also assessed (Equation (1) [
24]), in which %M indicates the intensity of mycorrhization according to an arbitrary scale of 1 to 5 (1 = trace of AMF colonization; 5 = >90% of the root cortex colonized). Then, %M is calculated as the proportion of root centimeters colonized by AMF, weighted by the intensity of the colonization: %M = (95
n5 + 70
n4 + 30
n3 + 5
n2 +
n1) ÷
N, where
n5,
n4,…
n1 indicate the number of root centimeters with an intensity 5, 4,…1, and
N is the number of fine root centimeters observed:
These data were arcsin (x/100)1/2 transformed. The data were subjected to one-way ANOVA using MINITAB version 13.2 and means were compared by Fisher’s least significant difference (LSD) test (p < 0.05). Photos were taken with a compound microscope using a camera.
2.3. Soil Aggregation
2.3.1. Aggregate Size Distribution
Dry aggregate size distribution was determined by the standard dry-sieving method [
25]. Briefly, 3 g of air-dried undisturbed sample was sieved through sieves with 2 mm and 0.5 mm openings. Two aggregate size classes were obtained (>2 mm and >0.5 mm).
2.3.2. Wet Aggregates
Size grades within the aggregate samples were separated by wet sieving; field-moist soil was used. The samples were wet sieved to acquire aggregate size fractions: >2000 μm, 250 to 2000 μm. These aggregates were separately classified as macroaggregates and microaggregates [
26].
Dry and wet aggregate (macro and microaggregates) contents from each site were compiled in order to analyze four replicates of each soil.
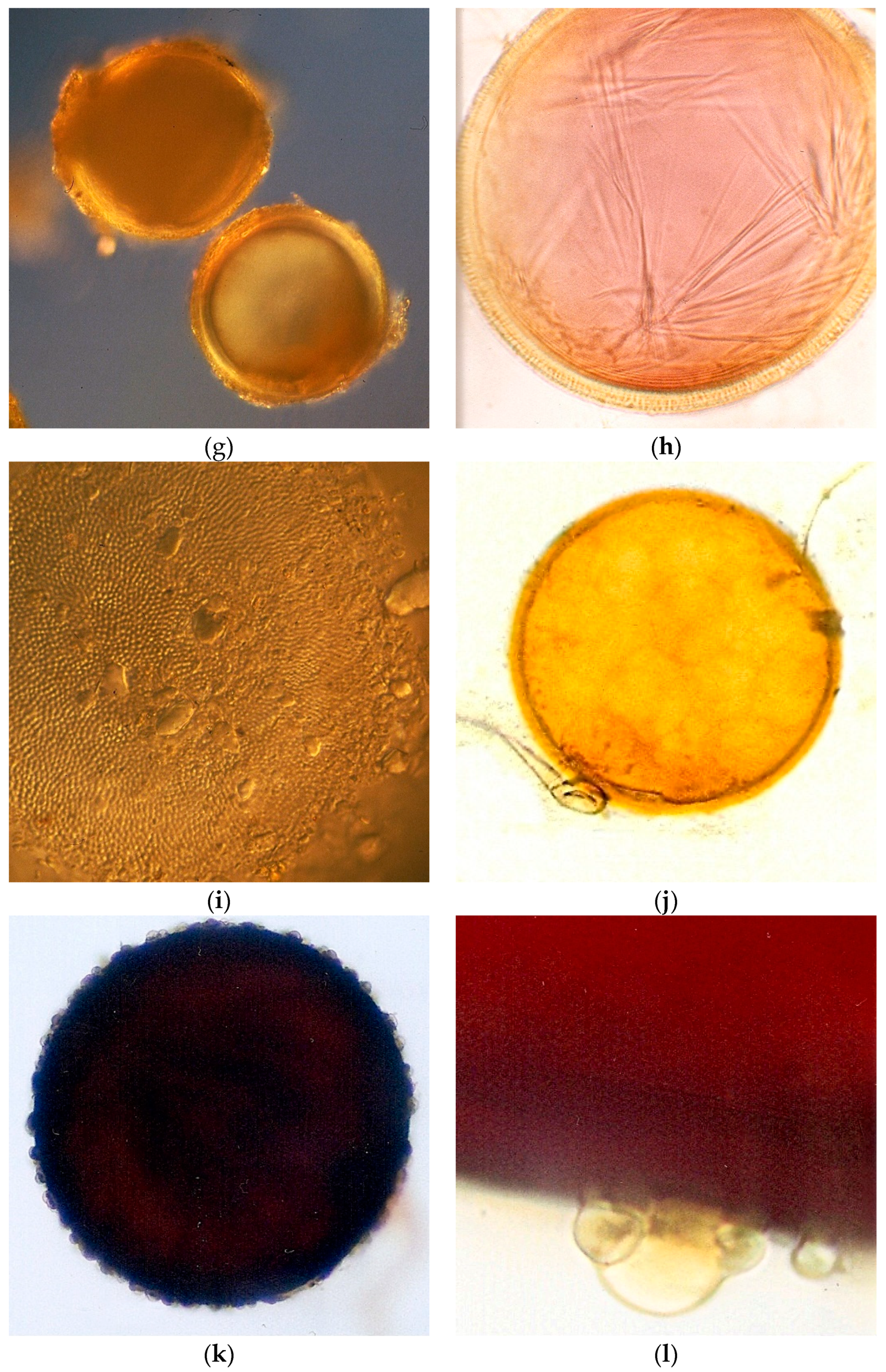
2.4. AMF Spore Isolation and Identification
AMF spores occurring in the soil samples (100 g soil dry weight) were extracted by wet sieving using sucrose density gradient centrifugation [
27] and the spore number was estimated. Spores were mounted onto slides using PVLG (polyvinyl alcohol-lactic acid-glycerol) both with and without a Melzer reagent. Morphological identification was carried out by microscopic observation of the spores and based on the species descriptions and the taxonomic classification of Glomeromycota, which were compared with the descriptions available at
http://www.amf-phylogeny.com/,
http://fungi.invam.wvu.edu/the-fungi/species-descriptions.html (accessed on 7 July 2021) and [
28].
2.5. Statistical Analysis
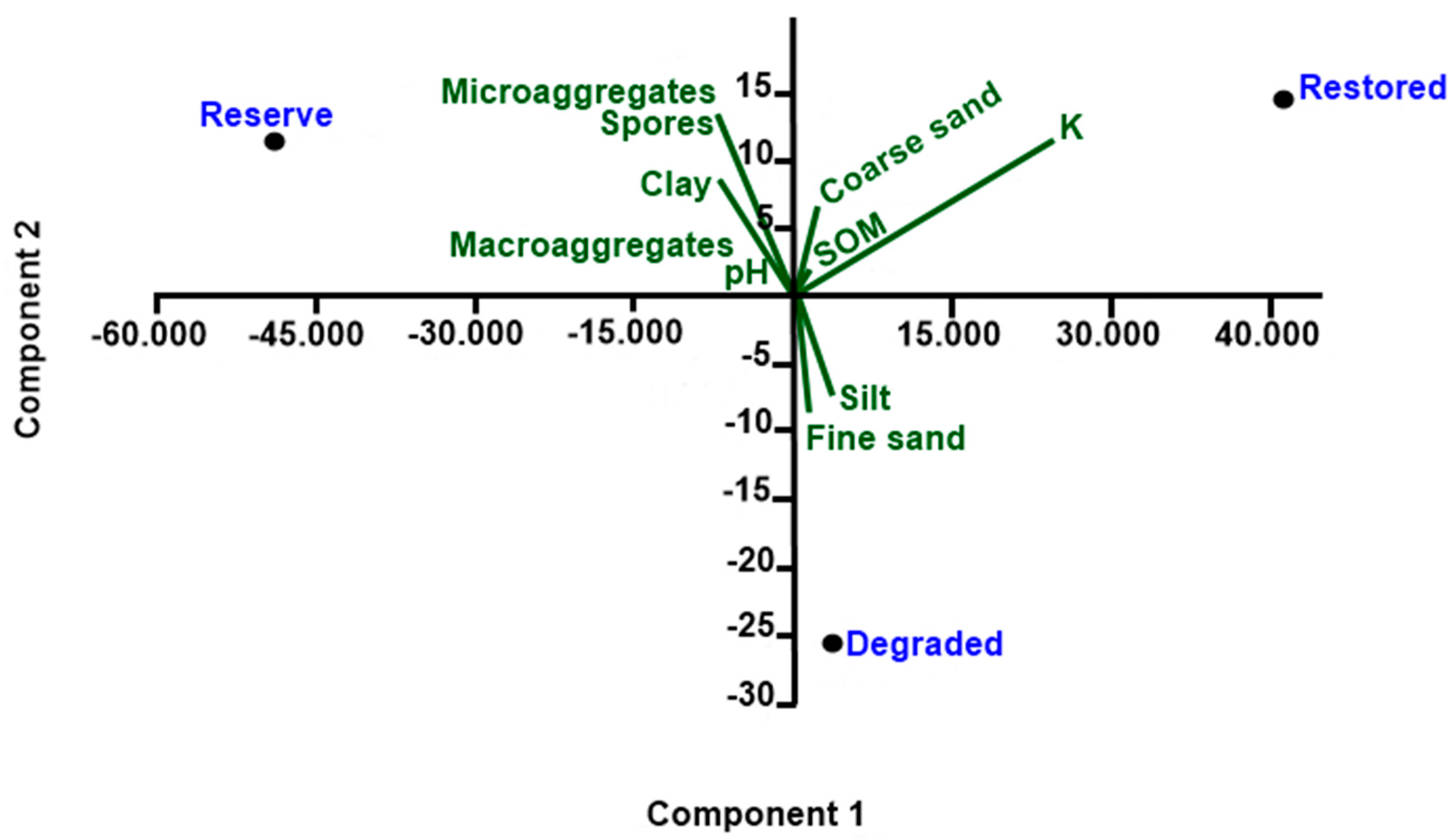
The significance of differences between field sites in spore abundance and species numbers was tested by using analysis of variance (ANOVA) one-way. Homoscedasticity and data normality were tested before performing analysis of variance and test of means. Tukey post hoc test was used to conduct pairwise tests for significant effects. Associated means for each treatment were reported using the software Minitab 17 (Minitab Inc., Pennsylvania State University, PA, USA, 2017). Principal component analysis (PCA) was performed with PAST (2021). PCA was used to visualize relationships among biological variables (spore counts) and associations with soil physical and chemical properties.
Differences in counts of stained intracellular AM fungal arbuscules between treatments were analyzed using analysis of variance (ANOVA) with post hoc t-testing. Statistical tests were carried out using the software Minitab 17 (Minitab Inc., 2017).
4. Discussion
The results showed that in the restored site of Brazil’s Velhas river and its tributaries, the root colonization by AMF was corroborated, as all the sampled tree species showed high AM colonization. Root colonization by AMF in the experimental site was high; vesicles were the most frequent AM structures present in the studied species in the revegetated site. In a review of the literature concerning riparian areas of Brazil [
29], authors were focused on the role that AMF play in relation to C input in ecosystems through the carbon sequestration process and AMF’s effects on plant nutrient uptake and biomass increase in pioneer woody species, and especially analyzed those species used in the revegetation of riparian forests. This bibliographic revision showed that AMF external hyphae have effects on soil structure, both physically (forming soil aggregates) and biochemically (by glomalin production). In addition, AMF spores and hyphae retain C-rich compound structures and, therefore, the restoration of riparian forests with woody mycorrhizal species could be proposed as an environmental service by means of C fixation using inoculated seedlings for riparian revegetation. Thus, previous results of research groups and this study have shown that AMF have positively affected soil aggregation and C sequestration by glomalin production [
23]; micro- and macroaggregates were shown to increase, in this work, by using the native and inoculated AM woody species in an ecofriendly revegetation strategy. Furthermore, the tree species used for reforestation were Fabaceae, associated also mostly with
Rhizobium N2-fixing bacteria. These results also agree with previous reports showing that legumes involved in double mutualistic symbioses with N2-fixing rhizobacteria-AMF are highly successful plants [
23], and, therefore, these host trees are better adapted for use in reforestation and restoration programs.
Currently, the paradigm of the inhibition of AMF colonization with the addition of P in soil, which was based mainly on small-scale and laboratory experiments, is being tested in large-scale field work. Thus, in field studies, addition of P is required to stimulate root colonization [
30]. In the studied riparian sites, the plants were fertilized with what is considered a minimal amount of P that will be diluted with time. Additional organic matter (OM) and P favored colonization, especially of
C. tomentosum (mainly by vesicles and extraradical hyphae); however, colonization intensity was the same among treatments. In the studied riparian areas, OM and P addition did not affect root colonization by AMF; further, in the control treatment, values of root colonization also remained high. The number of AMF species have been increasing in the preserved site. The positive effect on AMF sporulation, hyphal growth, and plant growth for restoration purposes of OM addition was previously reported in Brazil [
31] and in agronomic ecosystems worldwide [
32]. Our results also agree with on-farm AMF production to inoculate native woody species using different P concentrations in Brazil [
33]. These authors found high colonization by
Rhizophagus clarus and
Claroideoglomus etunicatum of inoculated trees with one exception,
Garcinia gardneriana; furthermore, inoculation of
Luehea divaricata,
Centrolobium robustum, and
Cedrella fissilis increased plant growth in height, stem diameter, and shoot biomass while P addition resulted in the improvement of shoot phosphorus content for the most tree species [
33]. Moreover, AMF and the chemical manner in which P is incorporated in riparian ecosystems have an added value that lies in the ability of AMF and riparian environments to act as a buffer and prevent leaching of excess P, functioning as biological filters of the P excess that pollutes the neighboring water bodies [
34]. Thus, our results reinforce the use of AMF inoculation, together with OM and P addition, as a successful strategy of colonized tree seedling production to be applied for revegetation of degraded riparian ecosystems and to avoid the runoff of inorganic P into rivers.
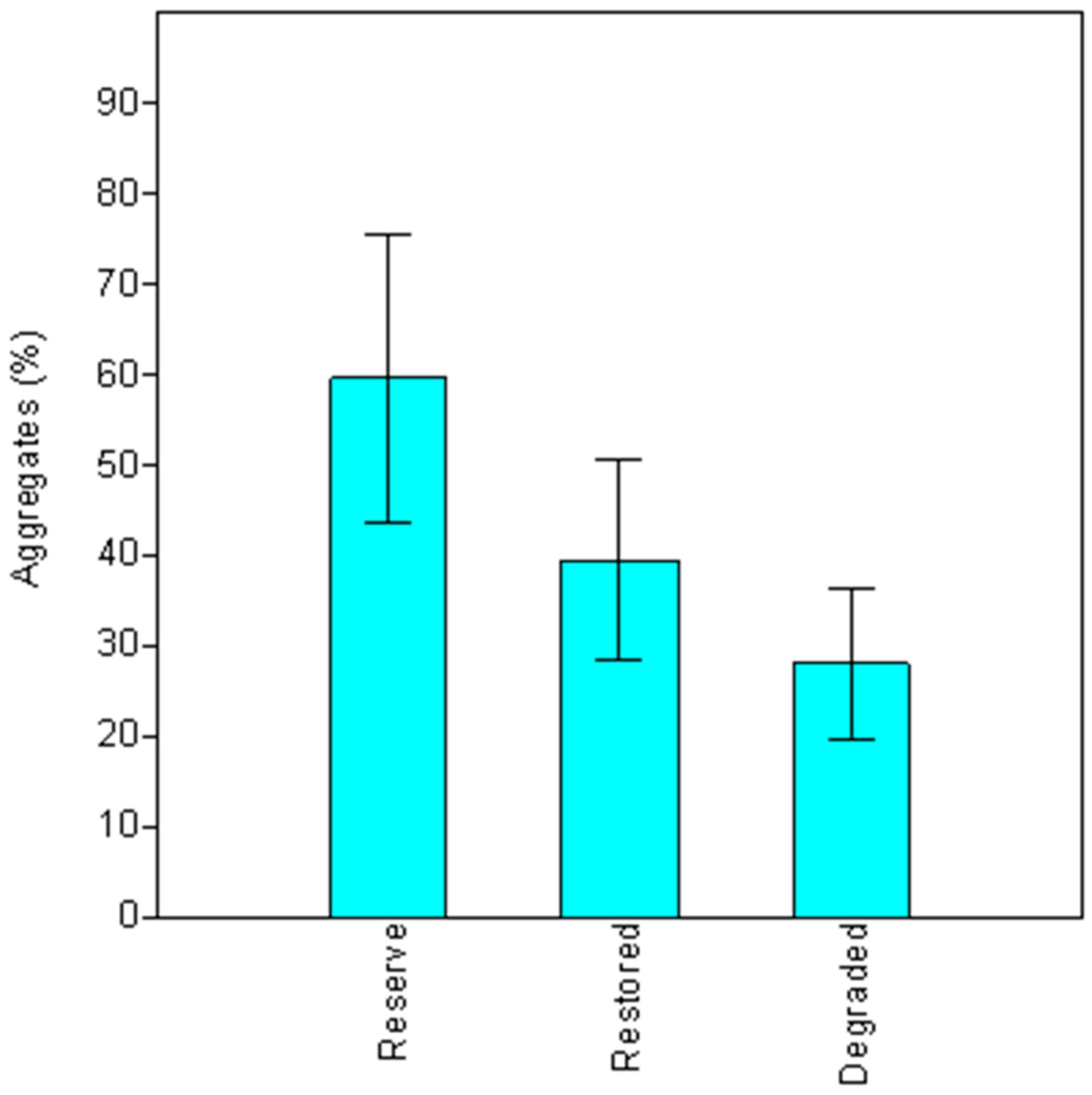
Among the riparian areas studied, soil aggregates had their maximum values in the pristine site, were intermediate in restored areas, and reached the lowest values in degraded sites. AMF mycelia in soil are involved in building the aggregate by means of a chemical (through a glycoprotein glomalin production) and physical (by hyphae interconnecting soil particles) binding that help to stablish a better soil structure [
35]. The soil macroaggregation is needed for efficient water infiltration, to reduce surface runoff, control soil erosion, reduce nutrients and organic matter losses, increase gas exchange, and, thus, to improve plant growth. Furthermore, the dead mycelia preserve soil structure and contribute to the stocks of organic matter and physical binder involved in soil aggregation, with the consequent reduction of soil compaction and improvement soil fertility [
34] and references therein. Therefore, in Velhas river, glomalin’s beneficial effect on restoration of these riparian sites was demonstrated before by this research group [
23], and the new soil aggregation results showed the crucial and relevant involvement of AMF in the woody plant management for this Brazilian ecosystem.
The AMF effect on soil aggregation found in the studied riparian areas could be explained by the fact that the preserved and experimental areas also presented higher AMF spore number and species richness than the degraded area that include spores of
Glomus in the Sabará sites,
Acaulospora and
Scutellospora in the Sabará and Velhas sites, and
Gigaspora only in the Gaia stream of the Sabará site. Considering the life strategies of AMF, the species of Glomeraceae are ruderals. They are characterized mainly by their high growth rates and intraradical colonization, early spore production, and low densities of hyphae in the soil. The Acaulosporaceae species are stress-tolerant, due to their reduced growth rates, long mycelium life, and resistance to acidity and low temperature. The species of families with large spores, such as Gigasporaceae, Racocetraceae, and Scutellosporaceae, are considered competitive and they present high densities of hyphae in the soil and late production of spores during the growing season [
36]. Therefore, the diversity of genera found in the Velhas river and riparian sites studied could also explain the high root colonization percentages due to the presence of
Glomus species capable of a quick and efficient colonization of host roots. In addition,
Gigaspora and
Scutellospora species could be responsible for the production of significant amounts of hyphae in the soil, with the consequent increase in the formation of soil aggregates. Furthermore,
Acaulospora species were present in the three sites, probably due to their stress-tolerant conditions. In addition, some AMF species found in Velhas river and Sabará riparian sites were also reported for other riparian ecosystems of Brazil, such as
Acaulospora foveata,
A. spinosa,
A. scrobiculata, and
Gigaspora sp. However,
Racocetra gregaria,
Scutellospora aurigloba,
S. reticulata, and
Septoglomus constrictum have been newly reported in Brazilian riparian areas [
23,
37].
5. Conclusions
The present study quantified the occurrence of AMF symbiosis in native plantations installed for restoration of degraded riparian ecosystems. In the restored fields, a higher soil aggregation was found, together with a high root colonization and AM spore number in the plant rhizospheres. The results revealed the high root colonization and a diverse community of AMF associated with native tree species in the planted riparian forests. However, the undisturbed site presented prominent values. Moreover, the addition of OM or P did not improve root colonization by AMF; nevertheless, it was a guarantee for a successful restoration.
This study showed that AMF are important components in riparian areas, and brings information for inoculant production in ecological restoration using mixed plantations. Thus, these findings can be used to maintain the sustainable management of riparian ecosystems. Undisturbed systems with diversified native trees can promote important soil environmental services in addition to their fundamental role in the wild environment, which must be preserved for future studies.
We demonstrated that inoculated plantations affect the soil’s physical attributes and the associated microbiota and, thus, the ecological restoration of tropical riparian sites. Meta-analysis of field restoration studies [
21] has also showed that the addition of mycorrhizal fungi facilitates a rapid revegetation, especially in N-fixing woody plants, grasses with a C
4 photosynthetic pathway, and plants that grow in low available P soils. Moreover, these authors [
21] found that the positive effect of mycorrhizal inoculation on hosts involved in restoration experiments was context dependent. Therefore, in Velhas river and its riparian tributaries studied, the benefits of seedling inoculation of legume trees applied to their restoration were also probed in a context of OM and P addition, successfully reinforcing a context for the application of this methodology to restore these areas.
Finally, this study allows us to affirm that AMF are important components in riparian areas. In addition, the study provides information regarding inoculant production for restoration using mixed plantations, with a particular focus on preserved areas. As roots and AMF mediate soil stabilization, restoration with inoculated plants is a successful strategy. It was concluded that mycorrhization is a crucial ecological attribute present in the tested plant species, and that the best approach to inoculate a restoration site with propagules of native AMF spores is via inoculated seedling transplants.