Study of Biodiversity of Algae and Cyanobacteria of Mutnovsky and Gorely Volcanoes Soils (Kamchatka Peninsula) Using a Polyphasic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collections
2.3. Cultivation of Strains and Morphological Identification
2.4. DNA Extraction, PCR
2.5. Phylogenetic Analysis
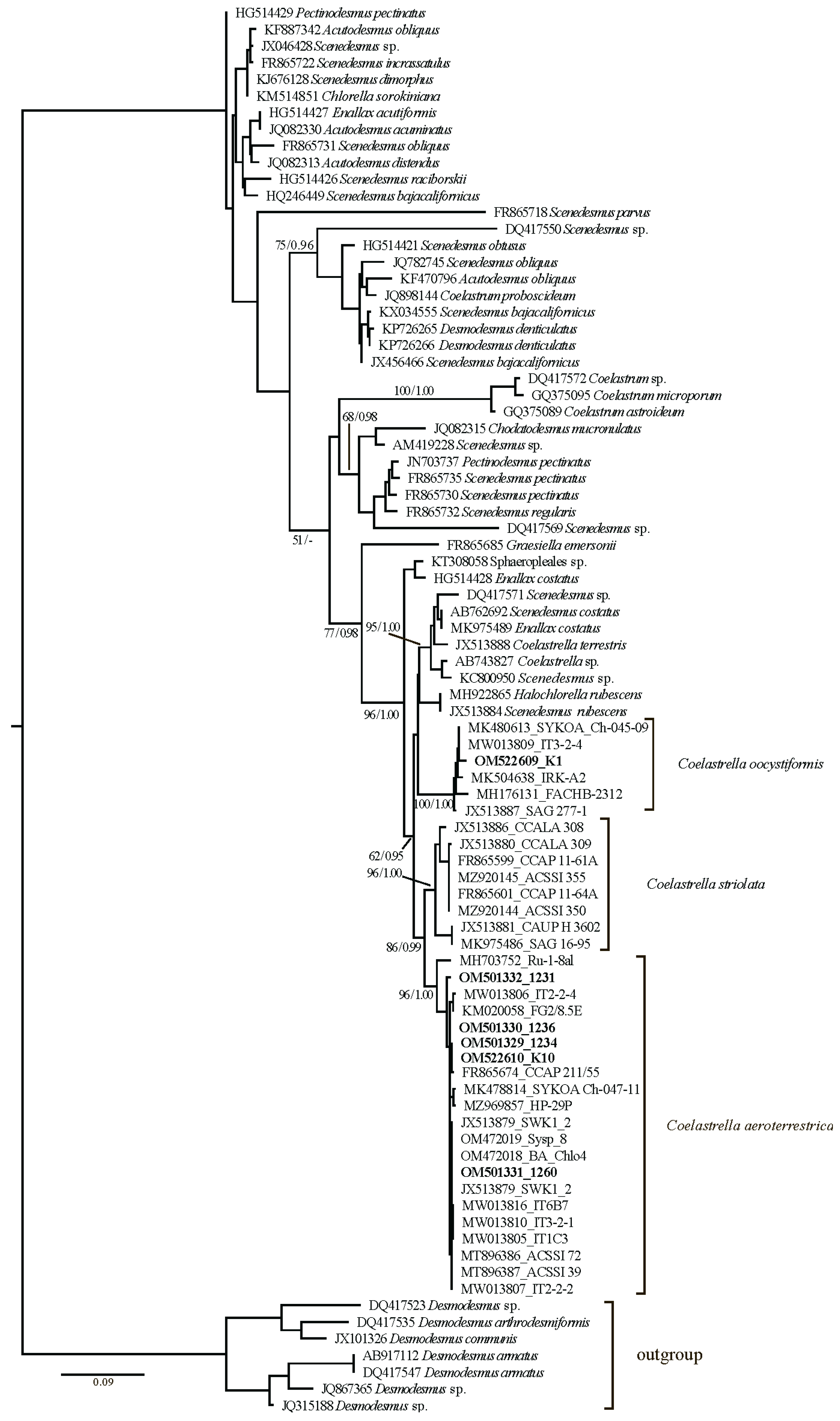
3. Results
| Taxa, Strain | Mutnovsky Volcano | Gorely Volcano | Genes, Percentage of Identity with Reference Strain, Accession Number of the Reference Strain | GenBank Accession Number, Publication Information | ||
|---|---|---|---|---|---|---|
| 2010 | 2020 | 2010 | 2020 | |||
| Cyanobacteria | ||||||
| Fischerella cf. major Gomont | K4 ** | |||||
| Leptolyngbya cf. foveolarum (Rabenhorst ex Gomont) Anagnostidis et Komárek | K4 | K8, K10 | ||||
| Microcoleus cf. calidus (Gomont ex Gomont) Strunecky, Komárek & J.R.Johansen *, strain 1267 | 103 | 16S, 16S-23S rRNA, 96.44% identity with Microcoleus sp. DAI, EF654029 | OM501356 | |||
| Phormidium cf. corium Gomont ex Gomont | 153 | |||||
| Roholtiella sp. *, strain K7 | K9 | 16-23S ITS rRNA, 97.71%, Roholtiella bashkiriorum RU9, KM268886 | ||||
| Stenomitos tremulus (J.R.Johansen & Casamatta) Miscoe & J.R.Johansen *, strain 1268 | 156 | 16S rRNA, 99.23% identity with Stenomitos tremulus UTCC 471, AF218371 | OM501358 | |||
| Stenomitos sp. *, strain 1317 | 99 | 16S-23S ITS rRNA, 96.69% identity with Stenomitos sp. WJT24NPBG20_P25, KF761557 | OM501357 | |||
| Synechocystis cf. salina Wislouch | 159 | |||||
| cf. Trichocoleus hospitus (Hansgirg ex Gomont) Anagnostidis | K1 | |||||
| Chlorophyta Chlorophyceae | ||||||
| Bracteacoccus bullatus Fučíková, Flechtner & Lewis *, strain 1366 | 182 | ITS rRNA, 98.00% identity with Bracteacoccus bullatus SAG 2032, JQ281848 | OM501335 | |||
| Bracteacoccus minor (Schmidle ex Chodat) Petrová *, strain 1228 | 99–101 * | ITS, 18 S rRNA, 99.41% identity with Bracteacoccus minor TTF-2-1-J, MT991535 | OM501328 | |||
| Bracteacoccus sp.1 | K2, K4, | K5, K10 | ||||
| Chlamydocapsa cf. lobata Broady | K4 | |||||
| Chlorococcum hypnosporum Starr *, strain 1269 | 102, 112 | 18S, ITS rRNA, 99.80% identity with authentic strain Chlorococcum hypnosporum SAG 213-6, JN904003 | OM501336 | |||
| Chlorococcum lobatum (Korshikov) F.E.Fritsch & R.P.John *, strain 1264 | 157 | 18S, ITS rRNA 99.20% identity with Chlorococcum lobatum SAG 12.84, AB936289 | OM501352 | |||
| Chlorolobion cf. lunulatum Hindák | 159 | 112, 154 | ||||
| Chlorosarcinopsis sp. | K4 | |||||
| Coelastrella aeroterrestrica Tschaikner, Gärtner & Kofler * | ||||||
| Strain C_aero K10 | K4 | ITS rRNA, 98.00% identity with authentic strain Coelastrella aeroterrestrica SWK1_2, JX513879 | OM522610 | |||
| Strain 1234 | 153 | ITS rRNA, 99.73% identity with authentic strain Coelastrella aeroterrestrica SWK1_2, JX513879 | OM501329 | |||
| Strain 1236 | 156 | ITS rRNA, 99.87% identity with authentic strain Coelastrella aeroterrestrica SWK1_2, JX513879 | OM501330 | |||
| Strain 1260 | 157 | ITS rRNA, 100% identity with authentic strain Coelastrella aeroterrestrica SWK1_2, JX513879 | OM501331 | |||
| Strain 1231 | 158 | ITS rRNA, 99.29% identity with authentic strain Coelastrella aeroterrestrica SWK1_2, JX513879 | OM501332 | |||
| Coelastrella oocystiformis (J.W.G.Lund) E.Hegewald & N.Hanagata *, strain K1 Coelast1 | K1 | ITS rRNA, 98.49% authentic strain identity with Coelastrella oocystiformis SAG 277-1, JX513887 | OM522609 | |||
| Coelastrella terrestris (Reisigl) Hegewald & N.Hanagata *, strain 1230 | 158 | ITS rRNA, 99.34% identity with Scotiellopsis terrestris (Reisigl) Punč. et Kalina SYKOA Ch-045-09, MK480613 | OM501333 | |||
| Neocystis mucosa Krienitz, C.Bock, Nozaki & M.Wolf * | ||||||
| Strain K2 N_muc | K2 | 18S rRNA, 99.50% identity with Neocystis mucosa, strain SAG 40.88 JQ920367 | OM522658 | |||
| Strain 1272 | 159 | ITS rRNA, 98.33% identity with Neocystis mucosa strain SAG 40.88, JQ920367 | OM501334 | |||
| Strain K1 N_muc. | K1 | 18S rRNA, 95.97%, identity with Neocystis mucosa strain SAG 40.88, JQ920367 | OM522657 | |||
| Trebouxiophyceae | ||||||
| Chlorella cf.chlorelloides (Naumann) C.Bock. Krienitz & Proeschold *, strain 1261 | 154 | 18S, ITS rRNA, 99.31% identity with Chlorella chlorelloides CB 2008/110, HQ111432 | OM501351 | |||
| Chlorella sp.2 | K7 | |||||
| Coccomyxa subellipsoidea E.Acton * | ||||||
| Strain 1249 | 155 | ITS rRNA, 99.80%, identity with Coccomyxa subellipsoidea P6065, MH753164 | OM501343 | |||
| Strain 1235 | 156 | ITS rRNA, 98.39%, identity with Coccomyxa subellipsoidea SAG 216-13, HG972978 | OM501344 | |||
| Strain 1271 | 159 | ITS rRNA, 99.25%, identity with Coccomyxa subellipsoidea P6065, MH753164 | OM501345 | |||
| cf. Coccomyxa viridis Chodat | 159,177 | |||||
| Coccomyxa sp. 1 | K4 | |||||
| Coccomyxa sp. 2. *, strain 1237 | 112 | 18S, ITS rRNA, 97.19% identity with Coccomyxa sp. Obi | OM501346 | |||
| Elliptochloris cf. reniformis Darienko & Pröschold *, strain 1291 | 156 | ITS rRNA, 97.73% identity with authentic strain Elliptochloris reniformis CAUP H7102, LT560354 | OM501339 | |||
| Elliptochloris cf. subsphaerica (Reisigl) Ettl & Gärtner *, strain 1245 | 112 | ITS rRNA, 96.30% identity with authentic strain Elliptochloris subsphaerica CAUP H7101, LT560348 | OM501340 | |||
| Eremochloris kamchatica Abdllin&Gontcharov * | (Abdullin et al., 2022) | |||||
| Strain 1238 | 154 | ITS rRNA, Eremochloris kamchatica Kk5-1 | OM501348 | |||
| Strain 1246 | 153 | ITS rRNA, Eremochloris kamchatica Kk5-1 | OM501347 | |||
| Strain 1247 | 156 | ITS rRNA, Eremochloris kamchatica Kk5-1 | OM501349 | |||
| Leptosira obovata Vischer *, strain K_10-5 | K4 | 18S rRNA, 99.55% identity with authentic strain Leptosira obovata SAG 445-1, Z68695 | OM522659 | |||
| Lobosphaera incisa (Reisigl) Karsten et al. * | ||||||
| Strain 1248 | 112 | ITS rRNA, 99.76% identity with Lobosphaera incisa chloroplast SAG 2468, LC366923 | OM501338 | |||
| Strain 1314 | 101 | ITS rRNA, 99.64% identity with Lobosphaera incisa chloroplast SAG 2468, LC366923 | OM501337 | |||
| Lobosphaera sp. *, strain K9 L_inc | K10 | 18S rRNA, 95% identity with Lobosphaera incisa chloroplast CAUP H 4301, LC366922 | ||||
| Micractinium sp. * | 103 | 18S, ITS rRNA, 99.91% identity with Micractinium sp. ACSSI 332 | OM501350 | |||
| Myrmecia sp.1 * | 159 | 99.84%, ITS rRNA, Myrmecia sp. Ru-s-3-3, MH703746 | OM501355 | |||
| Parietochloris pseudoalveolaris (T.R.Deason & Bold) Shin Watanabe & G.L.Floyd *, strain 1289 | 157* | + | ITS rRNA, 99.72% identity with Ettlia pseudoalveolaris NV-5, MT735204 | OM501353 | ||
| Parietochloris pseudoalveolaris (T.R.Deason & Bold) Shin Watanabe & G.L.Floyd *, strain 1306 | 158 | ITS, 99.14% identity with Ettlia pseudoalveolaris NV-5, MT735204 | OM501354 | |||
| Parietochloris sp. | K1 | |||||
| Pseudococcomyxa sp. | K5 | |||||
| Stichococcus sp. 1 | 100 | |||||
| Stichococcus sp. 2 *, strain 1286 | 102 | ITS rRNA, 88.44% identity with Stichococcus antarcticus A.Beck FiSo15/03cVI (M) M-0019691, MH670392 | OM501341 | |||
| Stichococcus sp. 3 *, strain 1270 | 155 | ITS rRNA, 89.86% identity with Stichococcus antarcticus A.Beck FiSo15/03cVI (M) M-0019691, MH670392 | OM501342 | |||
| Ochrophyta | ||||||
| Vischeria magna (J.B.Petersen) Kryvenda, Rybalka, Wolf & Friedl *, strain K10 V_magna | K4 | ITS rRNA, 97.44% identity with authentic strain Vischeria magna SAG 2554, MG596348 | OM522611 | |||
| Vischeria cf. stellata (Chodat) Pascher | 157 | |||||
| Vischeria sp. | 99–101 | |||||
| Charophyta | ||||||
| Klebsormidium nitens (Kützing) Lokhorst *, strain 1290 | 154 | ITS rRNA, 99.14% identity with Klebsormidium nitens SAG 335-1a, MN585749 | OM501327 | |||
| Klebsormidium sp1. | K1, K4 | |||||
| Klebsormidium sp.2. | 156,158 | |||||
| Mesotaenium sp.* | 182 | |||||
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuzyakina, T.I. Transformation of volcanic ash by microorganisms. In Volcanism and Associated Processes; Dalnauka: Petropavlovsk-Kamchatski, Russia, 1985; pp. 232–234. [Google Scholar]
- Treub, M. Notice sur la nouvelle flore de Krakatau. Ann. Jard. Bot. Buitenzorg 1888, 7, 213–223. [Google Scholar]
- Backer, C.A. The Problem of Krakatau as Seen by a Botanist; Springer Science & Business Media: The Hague, The Netherlands, 1929; p. 299. [Google Scholar]
- Whittaker, R.J.; Bush, M.B.; Richards, K. Plant recolonization and vegetation succession on the Krakatau Islands, Indonesia. Ecol. Monogr. 1989, 59, 59–123. [Google Scholar] [CrossRef]
- Gomez-Alvarez, V.; King, G.M.; Nusslein, K. Comparative bacterial diversity in recent Hawaiian volcanic deposits of different ages. FEMS Microbiol. Ecol. 2007, 60, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller-Dombois, D.; Boehmer, H.J. Origin of the Hawaiian rainforest and its transition states in long-term primary succession. Biogeosciences 2013, 10, 5171–5182. [Google Scholar] [CrossRef] [Green Version]
- Griggs, R.F. The eruption of Katmai. Nature 1918, 101, 497–499. [Google Scholar] [CrossRef] [Green Version]
- Maguire, B. The early development of freshwater biota on Surtsey. Surtsey Res. Progr. Rep. 1968, IV, 83–88. [Google Scholar]
- Van Eaton, A.R.; Harper, M.A.; Wilson, C.J.N. High-flying diatoms: Widespread dispersal of microorganisms in an explosive volcanic eruption. Geology 2013, 41, 1187–1190. [Google Scholar] [CrossRef]
- Rayburn, W.R.; Mack, R.N.; Metting, B. Conspicuous algal colonization of the ash from Mount St. Helens. J. Phycol. 1982, 18, 537–543. [Google Scholar] [CrossRef]
- Heal, O.W. Relation to vegetation and to other soil organisms. In Soil Biology; Burges, A., Row, F., Eds.; Academic Press: New York, NY, USA, 1967; pp. 95–149. [Google Scholar]
- Markhinin, E.K. Volcanoes and Life; Mysl: Moscow, Russia, 1980; p. 196. (In Russian) [Google Scholar]
- Henriksson, L.E.; Enekell, P.H.; Henriksson, E. Determination of the nitrogen-fixing capacity of algae in soil. Oikos 1972, 23, 420–423. [Google Scholar] [CrossRef]
- Schwabe, G.H. Nitrogen fixing blue-green algae as pioneer plants on Surtsey 1968–1973. Surtsey Res. Progr. Rep. 1974, 7, 22–25. [Google Scholar]
- Henriksson, L.; Henriksson, E. Studies in nitrogen cycle of Surtsey in 1972. Surtsey Res. Progr. Rep. 1974, 7, 36–44. [Google Scholar]
- Henriksson, L.E.; Rodgers, G.A. Further studies in the nitrogen cycle of Surtsey, 1974–1976. Surtsey Res. Progr. Rep. 1978, 8, 30–40. [Google Scholar]
- Broady, P.A. Green and yellow-green terrestrial algae from Surtsey (Iceland) in 1978. Surtsey Res. Progr. Rep. 1982, 9, 13–32. [Google Scholar]
- Henriksson, E.; Henriksson, L.E.; Skujins, J. Succesion of dinitrogen-foxing terrestrial cyanobacteria on the volcanic island Surtsey, Iceland. Phycologia 1989, 28, 9–17. [Google Scholar]
- Bölter, M.; Blume, H.P.; Kuhn, D. Soils and their microbiological properties from a transect from Cape Horn to the Antarctic Peninsula. Polar Biosci. 1999, 12, 54–67. [Google Scholar]
- Fermani, P.; Mataloni, G.; de Vijver, B.V. Soil microalgal communities on an Antarctic active volcano (Deception Island, South Shetlands). Polar Biol. 2007, 30, 1381–1393. [Google Scholar] [CrossRef]
- Costello, E.K.; Halloy, S.R.P.; Reed, S.C.; Sowell, P.; Schmidt, S.K. Fumarole-supported islands of biodiversity within a hyperarid, high-elevation landscape on Socompa volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 2009, 75, 735–747. [Google Scholar] [CrossRef] [Green Version]
- Karsten, U.; Friedl, T.; Schumann, R.; Hoyer, K.; Lembcke, S. Mycosporine-like amino acids and phylogenies in green algae: Prasiola and its relatives from the Trebouxiophyceae (Chlorophyta). J. Phycol. 2005, 41, 557–566. [Google Scholar] [CrossRef]
- Griggs, R.F. The colonization of the Katmai ash, a new and inorganic «soil». Amer. J. Bot. 1933, 20, 92–113. [Google Scholar] [CrossRef]
- Mayhew, L.E.; Geist, D.J.; Childers, S.E.; Pierson, J.D. Microbial Community Comparisons as a Function of the Physical and Geochemical Conditions of Galapagos Island Fumaroles. Geomicrobiol. J. 2007, 24, 615–625. [Google Scholar] [CrossRef]
- Hernández-Chavarría, F.; Sittenfeld, A. Research note: Preliminary report on the extreme endolithic microbial consortium of ‘Pailas Frías’, ‘Rincón de la Vieja’ Volcano, Costa Rica. Phycol. Res. 2006, 54, 104–107. [Google Scholar] [CrossRef]
- Kuzyakina, T.I. Microbiological studies of ashes collected during volcanic eruptions. Vulkanol. Seismol. 1983, 2, 92. [Google Scholar]
- Shtina, E.A.; Andreyeva, V.M.; Kuzyakina, T.I. Algae settlement of volcanic substrates. Bot. Zhurnal 1992, 8, 33–42. [Google Scholar]
- Kuzyakina, T.I. Ecology and Geochemical Activity of Microorganisms on Active Volcanoes and Hydrothermal Waters (Kunashir Island, Kuril Islands Kamchatka); Dalnauka: Vladivostok, Russia, 2004; pp. 1–251. [Google Scholar]
- Abdullin, S. Cyanobacteriae and algae of lava tubes in Kamchatka, Russia. Cave Karst Sci. 2013, 40, 141–144. [Google Scholar]
- Ilchibaeva, K.V.; Kunsbaeva, D.F.; Allaguvatova, R.Z.; Fazlutdinova, A.I.; Polokhin, O.V.; Sibirina, L.A.; Gontcharov, A.A.; Singh, P.; Gaysina, L.A. Preliminary data about algae and cyanobacteria of volcanic soils on Kuril islands. Theor. Appl. Ecol. 2018, 4, 119–126. [Google Scholar]
- Fazlutdinova, A.I.; Gabidullin, Y.Z.; Allaguvatova, R.Z.; Gaysina, L.A. Diatoms in Kamchatka’s Hot Spring Soils. Diversity 2020, 12, 435. [Google Scholar] [CrossRef]
- Allaguvatova, R.Z.; Nikulin, A.Y.; Nikulin, V.Y.; Bagmet, V.B.; Shokhrina, V.V.; Sterlyagova, A.S.; Gaysina, L.A.; Abdullin, S.R. New data on cyanobacteria and algae in the Russian Far East. Biota Environ. Nat. Areas 2021, 2, 3–14. [Google Scholar] [CrossRef]
- Fazlutdinova, A.; Gabidullin, Y.; Allaguvatova, R.; Gaysina, L. Diatoms in Volcanic Soils of Mutnovsky and Gorely Volcanoes (Kamchatka Peninsula, Russia). Microorganisms 2021, 9, 1851. [Google Scholar] [CrossRef]
- Gaysina, L.A.; Allaguvatova, R.Z.; Eliáš, M. The first record of genus Neocystis from Kamchatka volcano soils, confirmed by genetic data. IOP Conf. Ser. Earth Environ. Sci. 2021, 663, 012009. [Google Scholar] [CrossRef]
- Kyle, P.R.; Ponomareva, V.V.; Rourke Schluep, R. Geochemical characterization of marker tephra layers from major Holocene eruptions in Kamchatka, Russia. Int. Geol. Rev. 2011, 53, 1059–1097. [Google Scholar] [CrossRef]
- Castenholz, R.W. The occurrence of the thermophilic blue-green alga, Mastigocladus laminosus on Surtsey in 1970. Surtsey Res. Progr. Rep. 1972, 6, 14–19. [Google Scholar]
- Selyangin, O.B.; Ponomareva, V.V. Gorelovsky volcanic center, South Kamchatka: Structure and evolution. Volcanol. Seismol. 1999, 21, 163–194. [Google Scholar]
- Volynets, O.N.; Flerov, G.B.; Khrenov, A.P.; Ermakov, V.A. Volcanic petrology of the Great Tolbachik fissure eruption. Akad. Nauk SSSR. Doklady. Earth Sci. Sec. 1976, 238, 179–183. [Google Scholar]
- Neshataeva, V.Y. Vegetable cover of Kamchatka Peninsula and its geobotanical regionalization. Proc. Karelian Res. Cent. RAS 2011, 1, 3–22. [Google Scholar]
- Karpachevsky, L.O.; Alyabyina, I.O.; Zakharikhina, L.V.; Makeev, A.O.; Merechek, M.S.; Radyukin, A.Y.U.; Shoba, S.A. Kamchatka Soils; GEOS: Moscow, Russia, 2009; p. 224. (In Russian) [Google Scholar]
- Sokolov, I.A. Volcanic Activity and Soil Generation (in Kamchatka); Nauka: Moscow, Russia, 1973; p. 224. (In Russian) [Google Scholar]
- Sokolov, I.A. Features of the Geochemistry of Kamchatka Landscapes as a Result of Modern Volcanic Activity; Nauka: Moscow, Russia, 1967; pp. 72–95. (In Russian) [Google Scholar]
- Zonn, S.V.; Karpachevsky, L.O.; Stefin, V.V. Forest Soils of Kamchatka; Publishing house of the Academy of Sciences of the USSR: Moscow, Russia, 1963; pp. 182–198. (In Russian) [Google Scholar]
- Gollerbach, M.M.; Shtina, E.A. Soil Algae; Nauka: Leningrad, Russia, 1969; p. 228. (In Russian) [Google Scholar]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- Carmichael, W.W. Isolation, culture, and toxicity testing of toxic freshwater cyanobacteria (blue-green algae). In Fundamental Research in Homogenous Catalysis 3; Shilov, V., Ed.; Gordon & Breach: New York, NY, USA, 1968; pp. 1249–1262. [Google Scholar]
- McFaden, G.I.; Melkonian, M. Use of Hepes buffer for microalgal culture media and fixation for electron microscopy. Phycologia 1986, 25, 551–557. [Google Scholar] [CrossRef]
- Ettl, H.; Chlorophyta, I. Phytomonadina. In Süßwasserflora von Mitteleuropa; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Fischer Verlag: Jena, Germany, 1983; p. 808. [Google Scholar]
- Komárek, J.; Fott, B. Chlorophyceae (Grünalgen). Ordnung: Chlorococcales. Das Phytoplankton des Süsswassers. In Die Binnengewässer, Bd. 16., Teil 7., H. 1.; Schweizerbart Verlag: Stuttgart, Germany, 1983; p. 1044S. [Google Scholar]
- Ettl, H.; Gartner, G. Syllabus der Boden-, Luft- und Flechtenalgen, 2nd ed.; Springer: Berlin, Germany, 1995; p. 721. [Google Scholar]
- Andreeva, V.M. Soil and Aerophilic Green Algae (Chlorophyta: Tetrasporales, Chlorococcales, Chlorosarcinales (Chlorophyta: Tetrasporales, Chlorococcales, Chlorosarcinales); Nauka: Sankt-Peterburg, Russia, 1998; p. 352. (In Russian) [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Teil: Chroococcales. In Süßwasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Heynig, H., Mollenheuer, D., Eds.; Bd. 19/1; Spektrum Akademische Verlag GmbH: Heidelberg, Berlin, Germany, 1999; p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Oscillatoriales. In Süßwasserflora von Mitteleuropa; Springer Spektrum: Berlin, Germany, 2007. [Google Scholar]
- Komárek, J. 3rd Part: Heterocytous Genera. In Cyanoprokaryota; Springer: Berlin, Germany, 2013. [Google Scholar]
- Pröschold, T.; Darienko, T. The green puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New generic and species concept among this widely distributed genus. Phytotaxa 2020, 441, 113–142. [Google Scholar] [CrossRef]
- Shalygin, S.; Shalygina, R.R.; Redkina, V.V.; Gargas, C.B.; Johansen, J.R. Description of Stenomitos kolaenensis and S. hiloensis sp. nov. (Leptolyngbyaceae, Cyanobacteria) with an emendation of the genus. Phytotaxa 2020, 440, 108–128. [Google Scholar] [CrossRef]
- Abdullin, S.R.; Nikulin, A.Y.; Bagmet, V.B.; Nikulin, V.Y.; Gontcharov, A.A. New cyanobacterium Aliterella vladivostokensis sp. nov. (Aliterellaceae, Chroococcidiopsidales), isolated from temperate monsoon climate zone (Vladivostok, Russia). Phytotaxa 2021, 527, 221–233. [Google Scholar] [CrossRef]
- Nemcová, Y.; Eliáš, M.; Škaloud, P.; Hodac, L.; Neustupa, J. Jenufa, gen. nov.: A new genus of coccoid green algae (Chlorophyceae, incertae sedis) previously recorded by environmental sequencing. J. Phycol. 2011, 47, 928–938. [Google Scholar] [CrossRef]
- Gontcharov, A.A.; Nikulin, A.Y.; Nikulin, V.Y.; Bagmet, V.B.; Allaguvatova, R.Z.; Abdullin, S.R. New Species of Chloroidium (Trebouxiophyceae, Chlorophyta) from East Asia. Plants 2021, 10, 2560. [Google Scholar] [CrossRef]
- Amaral, R.; Fawley, K.P.; Němcová, Y.; Ševčíková, T.; Lukešová, A.; Fawley, M.W.; Santos, L.M.A.; Eliáš, M. Towards modern classification of eustigmatophytes: Neomonodaceae, fam. nov., with the description of three new genera. J. Phycol. 2020, 56, 630–648. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, J.; Smith, K.F.; Staden, R. A new DNA sequence assembly program. Nucleic Acids Res. 1995, 23, 4992–4999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galtier, N.; Gouy, M.; Gautier, C. SEAVIEW and PHYLO_WIN: Two graphic tools for sequence alignment and molecular phylogeny. Bioinformatics 1996, 12, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Casamatta, D.A.; Johansen, J.R.; Vis, M.L.; Broadwater, S.T. Molecular and morphological characterization of ten polar and near-polar strains within the Oscillatoriales (Cyanobacteria). J. Phycol. 2005, 41, 421–438. [Google Scholar] [CrossRef]
- Hanagata, N.; Karube, I.; Chihara, M. Bark-inhablting green algae in Japan (1). Scenedesmus komarekii and Coeastrella multistriata var. multistriata (Scotiellocystoideae, Chlorellaceae, Chlorophyceae). J. Jpn. Bot. 1996, 71, 87–97. [Google Scholar]
- Tschaikner, A.; Ingolić, E.; Stoyneva, M.P.; Gärtner, G. Autosporulation in the soil alga Coelastrella terrestris (Chlorophyta, Scenedesmaceae, Scenedesmoideae). Phytol. Balcanica 2007, 13, 29–34. [Google Scholar]
- Tschaikner, A.A.; Gärtner, G.; Kofler, W. Coelastrella aeroterrestrica sp. nov. (Chlorophyta, Scenedesmoideae)—A new obviously often overlooked aeroterrestrial species. Algol. Stud. 2008, 128, 11–20. [Google Scholar] [CrossRef]
- Uzunov, B.A.; Stoyneva, M.P.; Gärtner, G.; Kofler, W. First record of Coelastrella species (Chlorophyta: Scenedesmaceae) in Bulgaria. Ber. Des Nat. Med. Ver. Innsbr. 2008, 95, 27–34. [Google Scholar]
- Kaufnerová, V.; Eliáš, M. The demise of the genus Scotiellopsis Vinatzer (Chlorophyta). Nova Hedwig. 2013, 97, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Abdullin, S.R.; Bagmet, V.B.; Nikulin, A.Y.; Nikulin, V.Y.; Gorpenchenko, T.Y.; Grishin, S.Y.; Allaguvatova, R.Z.; Gontcharov, A.A. Emended description of the genus Eremochloris (Trebouxiophyceae, Chlorophyta), with Eremochloris kamchatica sp. nov. from Kamchatka, Russia. Phycologia 2022, 61, 175–183. [Google Scholar] [CrossRef]
- Rindi, F.; Allali, H.A.; Lam, D.W.; Lόpez-Bautista, J.M. An overview of the biodiversity and biogeography of terrestrial green algae. In Biodiversity Hotspots; Rescigno, V., Maletta, S., Eds.; Nova Science Publishers: New York, NY, USA, 2010; pp. 105–122. [Google Scholar]
- Flechtner, V.R.; Pietrasiak, N.; Lewis, L.A. Newly revealed diversity of green microalgae from wilderness areas of Joshua tree national park (JTNP). Monogr. West. N. Am. Nat. 2013, 6, 43–63. [Google Scholar] [CrossRef]
- Samolov, E.; Baumann, K.; Büdel, B.; Jung, P.; Leinweber, P.; Mikhailyuk, T.; Karsten, U.; Glaser, K. Biodiversity of Algae and Cyanobacteria in Biological Soil Crusts Collected Along a Climatic Gradient in Chile Using an Integrative Approach. Microorganisms 2020, 8, 1047. [Google Scholar] [CrossRef]
- Sommer, V.; Karsten, U.; Glaser, K. Halophilic Algal Communities in Biological Soil Crusts Isolated From Potash Tailings Pile Areas. Front. Ecol. Evol. 2020, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, G.H. Blue-green algae as pioneers on post-volcanic substrate (Surtsey/Iceland). In Taxonomy and Biology of Blue-Green Algae; Desikachary, T.V., Ed.; University of Madras: Chennai, India, 1972; pp. 419–424. [Google Scholar]
- Brock, T.D. Primary colonization of Surtsey, with special reference to the blue-green algae. Oikos 1973, 24, 239–243. [Google Scholar] [CrossRef]
- Fritsch, F.E. The Terrestrial Alga. J. Ecol. 1922, 10, 220–236. [Google Scholar] [CrossRef]
- Tarchevsky, V.V.; Shtina, E.A. The development of algae on industrial dumps. In Proceedings of the Interuniversity Conference Current State and Prospects for the Study of Soil Algae in the USSR, Kirov, Russia, 16 October 1967; pp. 146–150. (In Russian). [Google Scholar]
- Lukešová, A.; Hoffmann, L. Soil algae flora from acid rain impacted forest areas of the Krušne hory Mts. 1. Algal communities. Vegatatio 1996, 125, 123–136. [Google Scholar] [CrossRef]
- Lukešova, A. Soil algae in brown coal and lignite post-mining areas in Central Europe (Czech Republic and Germany). Restor. Ecol. 2001, 9, 341–350. [Google Scholar] [CrossRef]
- Khaybullina, L.S.; Gaysina, L.A.; Johansen, J.R.; Krautová, M. Examination of the terrestrial algae of the Great Smoky Mountains National Park, USA. Fottea 2010, 10, 201–215. [Google Scholar] [CrossRef] [Green Version]
- Kozlova, E.V.; Mazina, S.E.; Pešić, V. Phototrophs of illuminated entrance zones of caves in Montenegro. Ecol. Montenegrina 2019, 20, 24–39. [Google Scholar] [CrossRef]
- Kostikov, I.; Romanenko, P.; Demchenko, P.; Darienko, T.M.; Mikhayljuk, T.I.; Rybchinskiy, O.V.; Solonenko, A.M. Soil Algae of Ukraine; Phytosotsiologichniy Center: Kiev, Ukraine, 2001; p. 300. [Google Scholar]
- Smith, P.E. The Effects of Some Air Pollutants and Meteorological Conditions on Airborne Algae and Protozoa. J. Air Pollut. Control. Assoc. 1973, 23, 876–880. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, S.; Maltseva, I. Diversity of cyanobacteria and algae during primary succession in iron ore tailing dumps. Microb. Ecol. 2022, 83, 408–423. [Google Scholar] [CrossRef]
- Egorova, I.N.; Konovalov, M.S.; Dudareva, N.V. A peculiarities of algoflora composition, obtained in association with bryophytes of Sokhondo biosphere nature reserve (Zabaikalsky region, Russia). News of the Irkutsk State University. Ser. Biol. Ecol. 2009, 2, 8–11. [Google Scholar]
- Büdel, B.; Darienko, T.; Deutschewitz, K.; Dojani, S.; Friedl, T.; Mohr, K.I.; Salisch, M.; Reisser, W.; Weber, B. Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microb. Ecol. 2009, 57, 229–247. [Google Scholar] [CrossRef]
- Gomez, S.R.; Johansen, J.R.; Lowe, R.L. Epilithic aerial algae of Great Smoky Mountains National Park. Biol. Bratisl. 2003, 58, 603–615. [Google Scholar]
- Johansen, J.R.; Lowe, R.; Gomez, S.R.; Kociolek, J.P.; Makosky, S.A. New algal records for the Great Smoky Mountains National Park, USA, with an annotated checklist of all reported algal species for the park. Algol. Stud. 2004, 111, 17–44. [Google Scholar]
- Flechtner, V.R.; Johansen, J.R.; Belnap, J. The biological soil crusts of the San Nicolas Island: Enigmatic algae from a geographically isolated ecosystems. West. N. Am. Nat. 2008, 68, 405–436. [Google Scholar] [CrossRef] [Green Version]
- Bayramova, L.A. The main features of the algae flora of some soils of the Lankaran zone. In Academy of Sciences of the USSR. Biol. Sci. Ser. 1964, 2, 57. [Google Scholar]
- Shtina, E.L.; Bolyshev, N.I. Algae of solonetzes. Bot. Mag. 1960, 45, 1619–1829. [Google Scholar]
- Kabirov, R.R.; Safiulina, L.M. Peculiarities of ecology and distribution of unicellular soil alga Eustigmatos magnus (J.B. Petersen) Hibberd in Southern Ural (Russia). Int. J. Algae 2008, 10, 105–116. [Google Scholar]
- Bezdenezhnykh, K.A.; Kondakova, L.V.; Dabakh, E.V.; Ashikhmina, T.Y. Algological monitoring of soils in the vicinity of the plant “Maradykovskiy”. Theor. Appl. Ecol. 2021, 2, 81–88. [Google Scholar]
- Hegewald, E.; Bock, C.; Krienitz, L. A phylogenetic study on Scenedesmaceae with the description of a new species of Pectinodesmus and the new genera Verrucodesmus and Chodatodesmus (Chlorophyta, Chlorophyceae). Fottea 2013, 13, 14. [Google Scholar] [CrossRef]
- Borchhardt, N.; Baum, C.; Mikhailyuk, T.; Karsten, U. Biological soil crusts of Arctic Svalbard—water availability as potential controlling factor for microalgal biodiversity. Front. Microbiol. 2017, 8, 1485. [Google Scholar] [CrossRef]
- Chekanov, K.; Fedorenko, T.; Kublanovskaya, A.; Litvinov, D.; Lobakova, E. Diversity of carotenogenic microalgae in the White Sea polar region. FEMS Microbiol. Ecol. 2019, 96, fiz183. [Google Scholar] [CrossRef]
- Nikulin, A.Y.; Bagmet, V.B.; Nikulin, V.Y.; Abdullin, S.R. The study of the diversity of algae in soils under the vegetation of Sasa kurilensis on Iturup Island, Russia using molecular genetic approach. In Proceedings of the III All-Russian Scientific Conference with International Participation Modern Problems of Biochemistry, Genetics and Biotechnology, Ufa, Russia, 21–23 September 2021; pp. 147–152. [Google Scholar]
- Acton, E. Coccomyxa subellipsoidea, a new member of the Palmellaceae. Ann. Bot. 1909, 23, 573–578. [Google Scholar] [CrossRef]
- Blanc, G.; Agarkova, I.; Grimwood, J.; Kuo, A.; Brueggeman, A.; Dunigan, D.; Gurnon, J.; Ladunga, I.; Lindquist, E.; Lucas, S.; et al. The genome of the polar eukaryotic microalga Coccomyxa subellipsoidea reveals traits of cold adaptation. Genome Biol. 2012, 13, R39. [Google Scholar] [CrossRef] [Green Version]
- Pfaff, S.; Borchhardt, N.; Boy, J.; Karsten, U.; Gustavs, L. Desiccation tolerance and growth-temperature requirements of Coccomyxa (Trebouxiophyceae, Chlorophyta) strains from Antarctic biological soil crusts. Algol. Stud. 2016, 151, 3–19. [Google Scholar] [CrossRef]
- Jaag, O. Coccomyxa Schmidle. Monographie einer Algengattung. Beiträge Zur Kryptogamenflora Der Schweiz 1933, 8, 1–132. [Google Scholar]
- Masumoto, H.; Ohmura, Y.; Degawa, Y. Lichenomphalia meridionalis (Hygrophoraceae, lichenized Basidiomycota) new to Asia. Opusc. Philolichenum 2019, 18, 379–389. [Google Scholar]
- Butcher, R.W. Contributions to our knowledge of the smaller marine algae. J. Mar. Biol. Assoc. UK 1952, 3, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Kol, E. Kryobiologie. Biologie und Limnologie des Schnees und Eises. In I. Kryovegetation; Thienemann, A., Ed.; Die Binnengewässer: Stuttgart, Germany, 1968; Volume 24, pp. 1–216. [Google Scholar]
- Langhans, T.M.; Storm, C.; Schwabe, A. Community assembly of biological soil crusts of different successional stages in a temperate sand ecosystem, as assessed by direct determination and enrichment techniques. Microb. Ecol. 2009, 58, 394–407. [Google Scholar] [CrossRef] [PubMed]
- De Wever, A.; Leliaert, F.; Verleyen, E.; Vanormelingen, P.; Van der Gucht, K.; Hodgson, D.A.; Sabbe, K.; Vyverman, W. Hidden levels of phylodiversity in Antarctic green algae: Further evidence for the existence of glacial refugia. Proc. Biol. Sci. 2009, 276, 3591–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishnivetskaya, T.A. Viable Cyanobacteria and Green algae from the permafrost darkness. In Permafrost Soils; Margesin, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 73–84. [Google Scholar]
- Khan, N.; Tuffin, M.; Stafford, W.; Cary, C.; Lacap, D.C.; Pointing, S.B.; Cowan, D. Hypolithic microbial communities of quartz rocks from Miers Valley, McMurdo Dry Valleys, Antarctica. Polar. Biol. 2011, 34, 1657–1668. [Google Scholar] [CrossRef]
- Boyer, S.L.; Johansen, J.R.; Flechtner, V.R.; Howard, G.L. Phylogeny and genetic variance in terrestrial Microcoleus (Cyanophyceae) species based on sequence analysis of the 16S rRNA gene and associated 16S-23S ITS region. J. Phycol. 2002, 38, 1222–1235. [Google Scholar] [CrossRef]
- Rindi, F. Diversity, distribution and ecology of green algae and cyanobacteria in urban habitats. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 619–638. [Google Scholar]
- Strunecky, O.; Komarek, J.; Johansen, J.; Lukesova, A.; Elster, J. Molecular and morphological criteria for revision of the genus Microcoleus (Oscillatoriales, Cyanobacteria). J. Phycol. 2013, 49, 1167–1180. [Google Scholar] [CrossRef]
- Aguirre-Cavazos, D.E.; Moreno-Limón, S.; Salcedo-Martínez, S.M. Especies de algas de ríos de Nuevo León, México. Nuevos Regist. Para Estado Polibotánica 2018, 46, 1–25. [Google Scholar]
- Rindi, F.; Guiry, M.D.; López-Bautista, J.M. Distribution, morphology, and phylogeny of Klebsormidium (Klebsormidiales, Charophyceae) in urban environments in Europe. J. Phycol. 2008, 44, 1529–1540. [Google Scholar] [CrossRef]
- Rindi, F.; Mikhailyuk, T.I.; Sluiman, H.J.; Friedl, T.; López-Bautista, H. Phylogenetic relationships in Interfilum and Klebsormidium (Klebsormidiophyceae, Streptophyta). Mol. Phylogenet. Evol. 2011, 58, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Karsten, U.; Rindi, F. Ecophysiological performance of an urban strain of the aeroterrestrial green alga Klebsormidium sp. (Klebsormidiales, Klebsormidiophyceae). Eur. J. Phycol. 2010, 45, 426–435. [Google Scholar] [CrossRef] [Green Version]
- Mikhailyuk, T.; Glaser, K.; Holzinger, A.; Karsten, U. Biodiversity of Klebsormidium (Streptophyta) from alpine biological soil crusts (Alps, Tyrol, Austria, and Italy). J. Phycol. 2015, 51, 750–767. [Google Scholar] [CrossRef] [PubMed]
- Adlassnig, W.; Sassmann, S.; Lendl, T.; Wernitznig, S.; Hofhansl, F.; Lang, I.; Lichtscheidl, I.K. Metal contamination and retention of the former mining site Schwarzwand (Salzburg, Austria). Appl. Geochem. 2013, 35, 196–206. [Google Scholar] [CrossRef]
- Kunsbaeva, D.F.; Allaguvatova, R.Z.; Grishin, S.Y.; Abdullin, S.R.; Gaysina, L.A. Study of cyanobacteria and algae biodiversity from some volcanoes of Kamchatka. In Proceedings of the VI All Russian Conference with International Participation EcoBiotech 2019, Ufa, Russia, 1–4 October 2019; pp. 205–206. (In Russian). [Google Scholar]
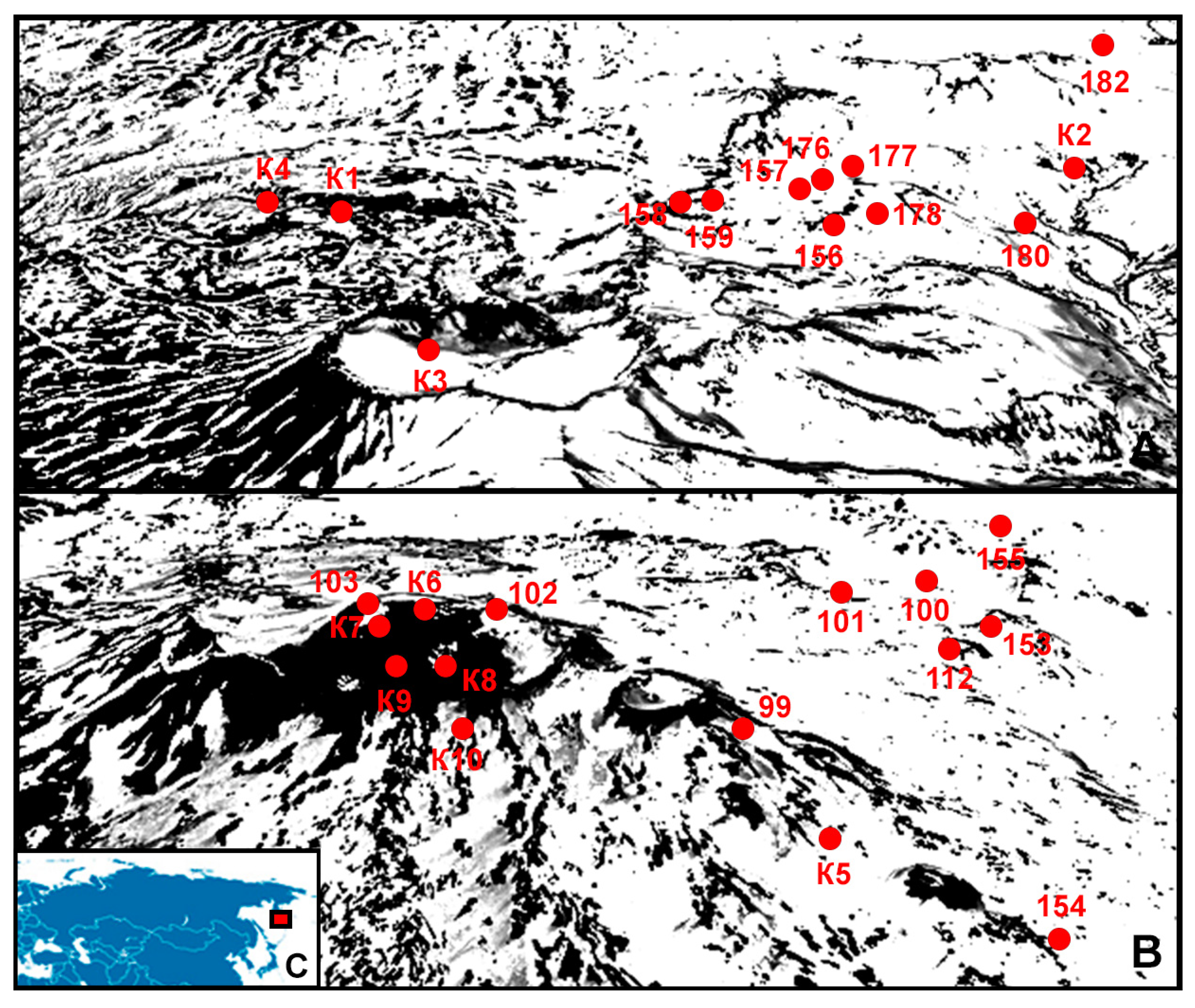


| Number | Description | Name | Year | GPS, Height above Sea Level | pH * | Soil Moisture, % * | Type of Soil * | Substrate Temperature |
|---|---|---|---|---|---|---|---|---|
| Mutnovsky volcano samples | ||||||||
| 1 | Canyon of the Vulkannaya river, under the bushes | K1 | 2010 | 52°28′29.4″ N 158°06′47.8″ E, 858 m | 5.45 | 50–68 | Mountain–tundra illuvial–humus soils | 11 |
| 2 | At the base of the volcano, not far from Dachnye springs, alder forest | K2 | 2010 | 52°31′54.6″ N 158°11′55.0″ E, 773 m | 6.25 | 75–85 | Humus–ocher soils | 14 |
| 3 | 300 m from the top of the volcano | K3 | 2010 | 52°27′26.4″ N 158°09′50.4″ E, 1627 m | − | − | Stone talus and placers, rocks | 13 |
| 4 | In the lower part of the Vulkannaya River canyon | K4 | 2010 | 52°28′17.3″ N 158°06′02.4″ E, 739 m | − | − | Rocks | 13 |
| 5 | Clump of sedge on the crumbling southern slope | 156 | 2020 | 52°31′20.7″ N 158°09′91.1″ E, 1053 m | 4.34 | 55 | Mountain–tundra sod frozen | 11 |
| 6 | Alpine meadow | 157 | 2020 | 52°30′74.4″ N 158°09′90.2″ E, 1039 m | 4.74 | 75 | Mountain–tundra illuvial–humus soils | 10 |
| 7 | Willow curtain | 158 | 2020 | 52°30′08.7″ N 158°09′50.7″ E, 1145 m | 4.23 | 65 | Mountain–tundra sod frozen | 11 |
| 8 | Alpine meadow dominated by willow and legumes | 159 | 2020 | 52°29′95.2″ N 158°09′29.4″ E, 1193 m | 4.52 | 68 | Mountain–tundra illuvial–humus soils | 10 |
| 9 | Alpine meadow | 176 | 2020 | 52°31′12.8″ N 158°09′81.2″ E, 1065 m | 5.62 | 70 | Mountain–tundra illuvial–humus soils | 11 |
| 10 | Alder Dwarf Curtain | 177 | 2020 | 52°31′03.9″ N 158°09′74.5″ E, 1067 m | 5.15 | 78 | Mountain–tundra illuvial–humus soils | 13 |
| 11 | Forbs with the dominance of wormwood, willow on the slope of the stream | 178 | 2020 | 52°30′93.4″ N 158°10′34.0″ E, 945 m | 5.05 | 65 | Tundra volcanic illuvial–humus soils | 11 |
| 12 | Alpine forb meadow on a volcanic plateau near the Mutnovskaya geothermal station | 182 | 2020 | 52°33′87.0″ N 158°11′22.0″ E, 885 m | 5.36 | 73 | Mountain–tundra illuvial–humus soils | 13 |
| Gorely volcano samples | ||||||||
| 1 | Slope, flat area among sedges | K5 | 2010 | 52°32′46.2″ N 158°02′39.9″ E, 1501 m | 4.35 | 55 | Illuvial–humus volcanic destructive soils | 12 |
| 2 | The trail along the edge of the crater, green layer on the surface of the ground | K6 | 2010 | 52°33′26.4″ N 158°02′09.2″ E, 1758 m | 5.68 | 45 | Volcanic ash, sand | 18 |
| 3 | Down the east slope | K7 | 2010 | 52°33′19.1″ N 158°01′57.4″ E, 1690 m | 5.15 | 55 | Tundra volcanic illuvial–humus soils | 14 |
| 4 | At the edge of a crater with a lake | K8 | 2010 | 52°33′12.8″ N 158°02′20.7″ E, 1675 m | 5.25 | 45 | Sulfur deposits around the crater | 16 |
| 5 | Down the east slope | K9 | 2010 | 52°33′10.8″ N 158°02′06.0″ E, 1645 m | 4.65 | 55 | Tundra volcanic illuvial–humus soils | 12 |
| 6 | Down the east slope | K10 | 2010 | 52°32′53.7″ N 158°02′21.6″ E, 1555 m | 4.55 | 55 | Tundra volcanic illuvial–humus soils | 12 |
| 10 | Eastern slope under the snowfield | 99 | 2020 | 52°34′26.7″ N 158°04′92.9″E, 1060 m | 4.35 | 65 | Mountain–tundra sod frozen | 10 |
| 11 | Solidified lava flow on the eastern slope | 100 | 2020 | 52°34′07.9″ N 158°04′92.9″ E, 1192 m | 4.48 | 60 | Mountain–tundra sod frozen | 11 |
| 12 | Dead bean clump | 101 | 2020 | 52°33′91.3″ N 158°04′35.9″ E, 1310 m | 4.72 | 57 | Mountain–tundra sod frozen | 12 |
| 13 | Scree of soil under the rock | 102 | 2020 | 52°33′53.1″ N 158°02′32.5″ E, 1784 m | − | − | Rocks | 14 |
| 14 | Thermal vapor outlet along the edge of the caldera | 103 | 2020 | 52°33′30.6″ N 158°01′74.2″ E, 1805 m | − | − | Rocks | 32 |
| 15 | Dead clump of sedge on a lava terrace | 112 | 2020 | 52°33′67.4″ N 158°05′14.1″ E, 1226 m | 4.47 | 45 | Mountain–tundra sod frozen | 10 |
| 16 | The vent of a small side crater filled with lava chips | 153 | 2020 | 52°33′75.4″ N 158°05′33.7″ E, 1207 m | 4.82 | 56 | Mountain–tundra sod frozen | 11 |
| 17 | Alpine meadow at the foot of the volcano | 154 | 2020 | 52°34′27.6″ N 158°05′46.1″ E, 1064 m | 4.76 | 75 | Mountain–tundra illuvial–humus soils | 10 |
| 18 | Old overgrown alluvial cone overgrown with sedges | 155 | 2020 | 52°34′65.0″ N 158°05′30.6″ E, 1002 m | 4.35 | 65 | Mountain–tundra sod frozen | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allaguvatova, R.Z.; Nikulin, A.Y.; Nikulin, V.Y.; Bagmet, V.B.; Gaysina, L.A. Study of Biodiversity of Algae and Cyanobacteria of Mutnovsky and Gorely Volcanoes Soils (Kamchatka Peninsula) Using a Polyphasic Approach. Diversity 2022, 14, 375. https://doi.org/10.3390/d14050375
Allaguvatova RZ, Nikulin AY, Nikulin VY, Bagmet VB, Gaysina LA. Study of Biodiversity of Algae and Cyanobacteria of Mutnovsky and Gorely Volcanoes Soils (Kamchatka Peninsula) Using a Polyphasic Approach. Diversity. 2022; 14(5):375. https://doi.org/10.3390/d14050375
Chicago/Turabian StyleAllaguvatova, Rezeda Z., Arthur Yu. Nikulin, Vyacheslav Yu. Nikulin, Veronika B. Bagmet, and Lira A. Gaysina. 2022. "Study of Biodiversity of Algae and Cyanobacteria of Mutnovsky and Gorely Volcanoes Soils (Kamchatka Peninsula) Using a Polyphasic Approach" Diversity 14, no. 5: 375. https://doi.org/10.3390/d14050375
APA StyleAllaguvatova, R. Z., Nikulin, A. Y., Nikulin, V. Y., Bagmet, V. B., & Gaysina, L. A. (2022). Study of Biodiversity of Algae and Cyanobacteria of Mutnovsky and Gorely Volcanoes Soils (Kamchatka Peninsula) Using a Polyphasic Approach. Diversity, 14(5), 375. https://doi.org/10.3390/d14050375










