Delayed Signs of UV-C Damage to Chlorella sp. Observed through Fluorescent Staining
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Collection and Stocking
2.2. Induction of Cellular Apoptosis
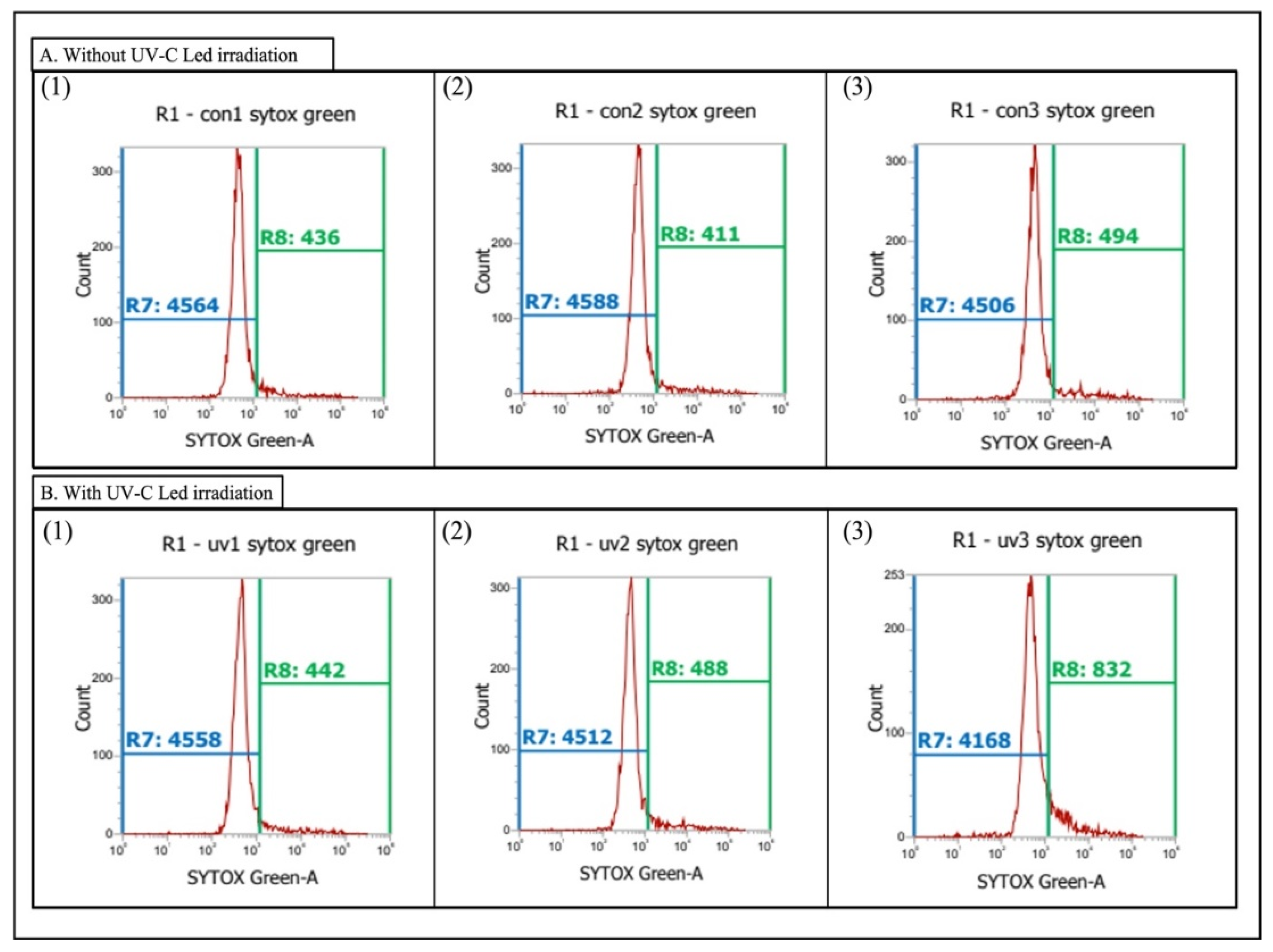
2.3. Plasma Membrane Damage on Chlorella sp.
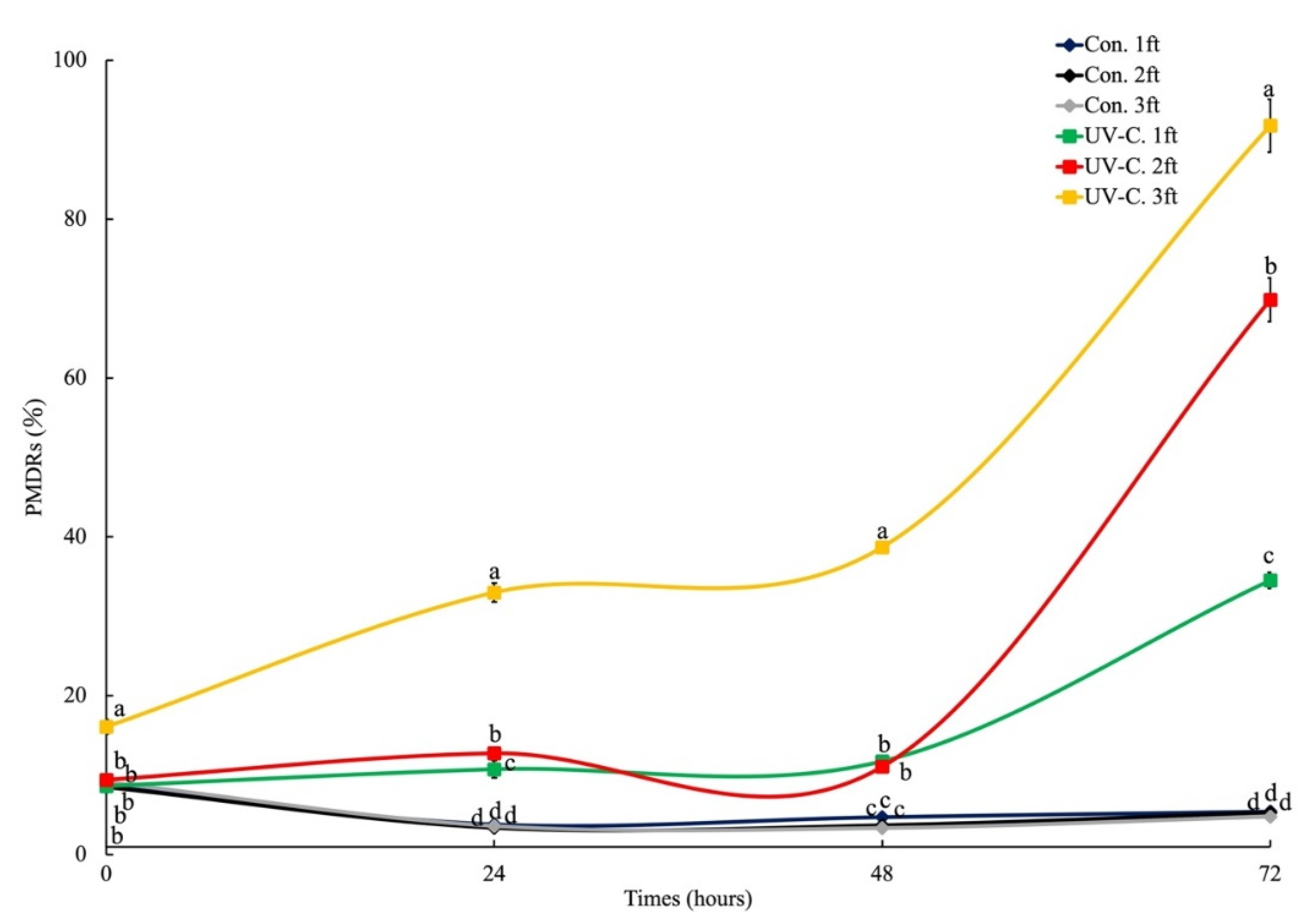
2.4. Phosphatidylserine Redistribution on Chlorella sp.
2.5. Statistical Analysis
3. Results
3.1. Plasma Membrane Damage on Chlorella sp.
3.2. Phosphatidylserine Redistribution on Chlorella sp.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maruyama, K. Classification of Chlorella strains by cell appearance and group sera. Bot. Mag. Tokyo 1977, 90, 57–66. [Google Scholar] [CrossRef]
- Halldal, P.; French, C.S. Algal growth in crossed gradients of light intensity and temperature. Plant Physiol. 1958, 33, 249–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teoh, M.L.; Chu, W.L.; Marchant, H.; Phang, S.M. Influence of culture temperature on the growth, biochemical composition and fatty acid profiles of six Antarctic microalgae. J. Appl. Phycol. 2004, 16, 421–430. [Google Scholar] [CrossRef]
- Kuhl, A.; Lorenzen, H. Chapter 10 Handling and Culturing of Chlorella. In Methods in Cell Biology; Prescott, D.M., Ed.; Academic Press: Cambridge, MA, USA, 1964; Volume 1, pp. 159–187. [Google Scholar]
- Scheffler, J. Underwater Habitats. Available online: https://illumin.usc.edu/underwater-habitats/ (accessed on 8 April 2022).
- Işik, O.; Sarihan, E.; Kuşvuran, E.; Gül, Ö.; Erbatur, O. Comparison of the fatty acid composition of the freshwater fish larvae Tilapia zillii, the rotifer Brachionus calyciflorus, and the microalgae Scenedesmus abundans, Monoraphidium minitum and Chlorella vulgaris in the algae-rotifer-fish larvae food chains. Aquaculture 1999, 174, 299–311. [Google Scholar] [CrossRef]
- Enyidi, U. Chlorella vulgaris as protein source in the diets of African catfish Clarias gariepinus. Fishes 2017, 2, 17. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017, 120, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; El-Hack, M.E.A.; Arif, M.; Khafaga, A.F.; Taha, A.E. The application of the microalgae Chlorella spp. as a supplement in broiler feed. World’s Poult. Sci. J. 2019, 75, 305–318. [Google Scholar] [CrossRef]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Yan, X.; Cheng, J.J.; Ruan, R. Screening of the dominant Chlorella pyrenoidosa for biofilm attached culture and feed production while treating swine wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Production of high-density Chlorella culture grown in fermenters. J. Appl. Phycol. 2011, 24, 35–43. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Ruano, M.V.; Borrás, L.; Barat, R.; Ferrer, J. Effect of ambient temperature variations on an indigenous microalgae-nitrifying bacteria culture dominated by Chlorella. Bioresour. Technol. 2019, 290, 121788. [Google Scholar] [CrossRef]
- Costa, S.S.; Peres, B.P.; Machado, B.R.; Costa, J.A.V.; Santos, L.O. Increased lipid synthesis in the culture of Chlorella homosphaera with magnetic fields application. Bioresour. Technol. 2020, 315, 123880. [Google Scholar] [CrossRef]
- Camargo, E.C.; Lonbardi, A.T. Effect of cement industry flue gas simulation on the physiology and photosynthetic performance of Chlorella sorokiniana. J. Appl. Phycol. 2017, 30, 861–871. [Google Scholar] [CrossRef]
- Candido, C.; Lombardi, A.T. The physiology of Chlorella vulgaris grown in conventional and biodigested treated vinasses. Algal Res. 2018, 30, 79–85. [Google Scholar] [CrossRef]
- Arora, N.; Philippidis, G.P. Insights into the physiology of Chlorella vulgaris cultivated in sweet sorghum bagasse hydrolysate for sustainable algal biomass and lipid production. Sci. Rep. 2021, 11, 6779. [Google Scholar] [CrossRef]
- Bai, M.D.; Hsu, H.J.; Wu, S.I.; Lu, W.C.; Wan, H.P.; Chen, J.C. Cell disruption of Chlorella vulgaris using active extracellular substances from Bacillus thuringiensis ITRI-G1 is a programmed cell death event. J. Appl. Phycol. 2017, 29, 1307–1315. [Google Scholar] [CrossRef]
- Leist, M.; Nicotera, P. The shape of cell death. Biochem. Biophys. Res. Commun. 1997, 236, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Maghsoudi, N.; Zakeri, Z.; Lockshin, R.A. Programmed cell death and apoptosis—where it came from and where it is going: From Elie Metchnikoff to the control of caspases. Exp. Oncol. 2012, 34, 146–152. [Google Scholar]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Bidle, K.D.; Falkowski, P.G. Cell death in planktonic photosynthetic microorganisms. Nat. Rev. Microbiol. 2004, 2, 643–655. [Google Scholar] [CrossRef]
- Bidle, K.D.; Bender, S.J. Iron starvation and culture age activate metacaspases and programmed cell death in the marine diatom Thalassiosira pseudonana. Eukaryot. Cell 2008, 7, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Kozik, C.; Young, E.B.; Sandgren, C.D.; Berges, J.A. Cell death in individual freshwater phytoplankton species: Relationships with population dynamics and environmental factors. Eur. J. Phycol. 2019, 54, 369–379. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Chen, Y.-C. The hormesis of the green macroalga Ulva fasciata with low-dose 60cobalt gamma radiation. J. Phycol. 2011, 47, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Chanley, M.H., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 26–60. [Google Scholar]
- Provasoli, L. Media and products for the cultivation of marine algae. In Culture and Collections of Algae; Watanabe, A., Hattori, A., Eds.; Japanese Society of Plant Physiology: Tokyo, Japan, 1968; pp. 63–75. [Google Scholar]
- Bin Alam, M.D.Z.; Otaki, M.; Furumai, H.; Ohgaki, S. Direct and indirect inactivation of Microcystis aeruginosa by UV-radiation. Water Res. 2001, 35, 1008–1014. [Google Scholar] [CrossRef]
- Sakai, H.; Oguma, K.; Katayama, H.; Ohgaki, S. Effects of low-or medium-pressure ultraviolet lamp irradiation on Microcystis aeruginosa and Anabaena variabilis. Water Res. 2007, 41, 11–18. [Google Scholar] [CrossRef]
- Sakai, H.; Oguma, K.; Katayama, H.; Ohgaki, S. Effects of low or medium-pressure UV irradiation on the release of intracellular microcystin. Water Res. 2007, 41, 3458–3464. [Google Scholar] [CrossRef]
- Sakai, H.; Katayama, H.; Oguma, K.; Ohgaki, S. Kinetics of Microcystis aeruginosa growth and intracellular microcystins release after UV irradiation. Environ. Sci. Technol. 2009, 43, 896–901. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, X.; Au, D.W.T.; Mao, X.; Yuan, K. The effects of sub-lethal UV-C irradiation on growth and cell integrity of cyanobacteria and green algae. Chemosphere 2010, 78, 541–547. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Zhan, X.M.; Dao, G.H.; Hu, H.Y. UV-C irradiation for harmful algal blooms control: A literature review on effectiveness, mechanisms, influencing factors and facilities. Sci. Total Environ. 2020, 723, 137986. [Google Scholar] [CrossRef]
- Copia, J.; Gaete, H.; Zuniga, G.; Hidalgo, M.; Cabrera, E. Effect of ultraviolet B radiation on the production of polyphenols in the marine microalga Chlorella sp. Lat. Am. J. Aquat. Res. 2012, 40, 113–123. [Google Scholar] [CrossRef]
- Shih, M.F.; Cherng, J.Y. Protective effects of Chlorella-derived peptide against UVC-induced cytotoxicity through inhibition of caspase-3 activity and reduction of the expression of phosphorylated FADD and cleaved PARP-1 in skin fibroblasts. Molecules 2012, 17, 9116–9128. [Google Scholar] [CrossRef] [Green Version]
- Pfendler, S.; Alaoui-Sosse, B.; Alaoui-Sosse, L.; Bousta, F.; Aleya, L. Effects of UV-C radiation on Chlorella vulgaris, a biofilm-forming alga. J. Appl. Phycol. 2018, 30, 1607–1616. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, Y.; Wei, J.; Zhang, X.; Zhang, L.; Yang, Z.; Huang, Y. Ultraviolet-B radiation stress alters the competitive outcome of algae: Based on analyzing population dynamics and photosynthesis. Chemosphere 2021, 272, 129645. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V. Development of a short-term assay based on the evaluation of the plasma membrane integrity of the alga Pseudokirchneriella subcapitata. Appl. Microbiol. Biotechnol. 2012, 95, 1035–1042. [Google Scholar] [CrossRef] [Green Version]




| Stock | Composition | Concentration (g L−1 ddH2O) | Usage |
|---|---|---|---|
| Stock A | NaNO3 | 75.0 | 1 mL L−1 sterilized water |
| NaH2PO4·2H2O | 5.6 | ||
| NH4Cl | 26.8 | ||
| Na2EDTA | 4.36 | ||
| FeCl3·6H2O | 3.15 | ||
| Stock B | MnCl2·4H2O | 0.18 | 1 mL L−1 sterilized water |
| ZnSO4·7H2O | 0.023 | ||
| CoCl2·6H2O | 0.01 | ||
| CuSO4·5H2O | 0.01 | ||
| Na2MoO4·2H2O | 0.006 | ||
| Vitamin solution stock | Vitamin B1 (Thiamine) | 0.2 g | 0.5 mL L−1 sterilized water |
| Vitamin B7 (Biotin) | 0.001 g | ||
| Vitamin B12 (Cyanocobalamin) | 0.001 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lung, W.Q.C.; Yeh, H.-Y.; Yang, S.-J.; Huang, C.-Y.; Nan, F.-H.; Lee, M.-C. Delayed Signs of UV-C Damage to Chlorella sp. Observed through Fluorescent Staining. Diversity 2022, 14, 376. https://doi.org/10.3390/d14050376
Lung WQC, Yeh H-Y, Yang S-J, Huang C-Y, Nan F-H, Lee M-C. Delayed Signs of UV-C Damage to Chlorella sp. Observed through Fluorescent Staining. Diversity. 2022; 14(5):376. https://doi.org/10.3390/d14050376
Chicago/Turabian StyleLung, Wei Qing Chloe, Han-Yang Yeh, Sheng-Jie Yang, Chin-Yi Huang, Fan-Hua Nan, and Meng-Chou Lee. 2022. "Delayed Signs of UV-C Damage to Chlorella sp. Observed through Fluorescent Staining" Diversity 14, no. 5: 376. https://doi.org/10.3390/d14050376
APA StyleLung, W. Q. C., Yeh, H.-Y., Yang, S.-J., Huang, C.-Y., Nan, F.-H., & Lee, M.-C. (2022). Delayed Signs of UV-C Damage to Chlorella sp. Observed through Fluorescent Staining. Diversity, 14(5), 376. https://doi.org/10.3390/d14050376







