Roholtiella volcanica sp. nov., a New Species of Cyanobacteria from Kamchatkan Volcanic Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sampling, Strain Isolation, and Cultivation
2.3. Phenotypic Analysis
2.4. DNA Extraction, PCR, and Sequencing
2.5. Phylogenetic Analysis
2.6. Analysis of 16S–23S ITS Region
2.7. Calculation of Percentage Similarity and p-Distance
3. Results
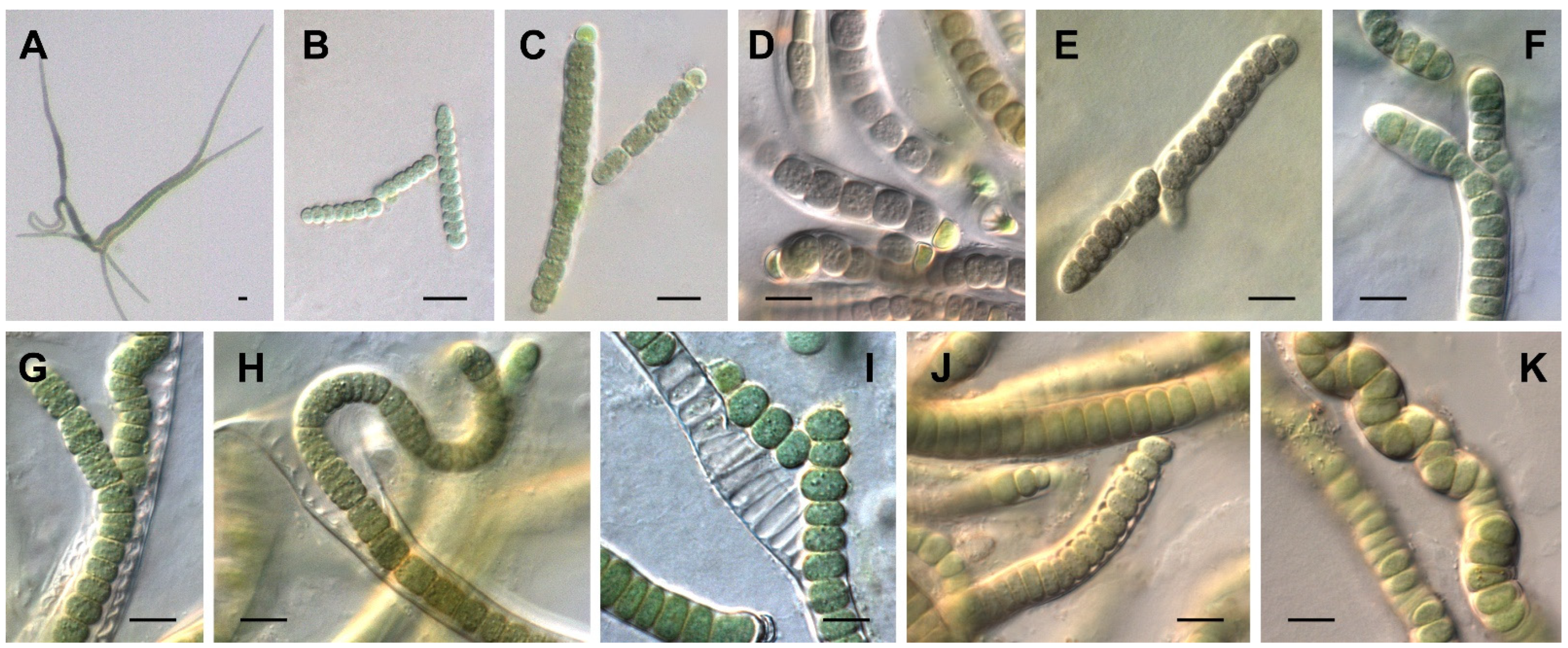
3.1. Morphological Characterization
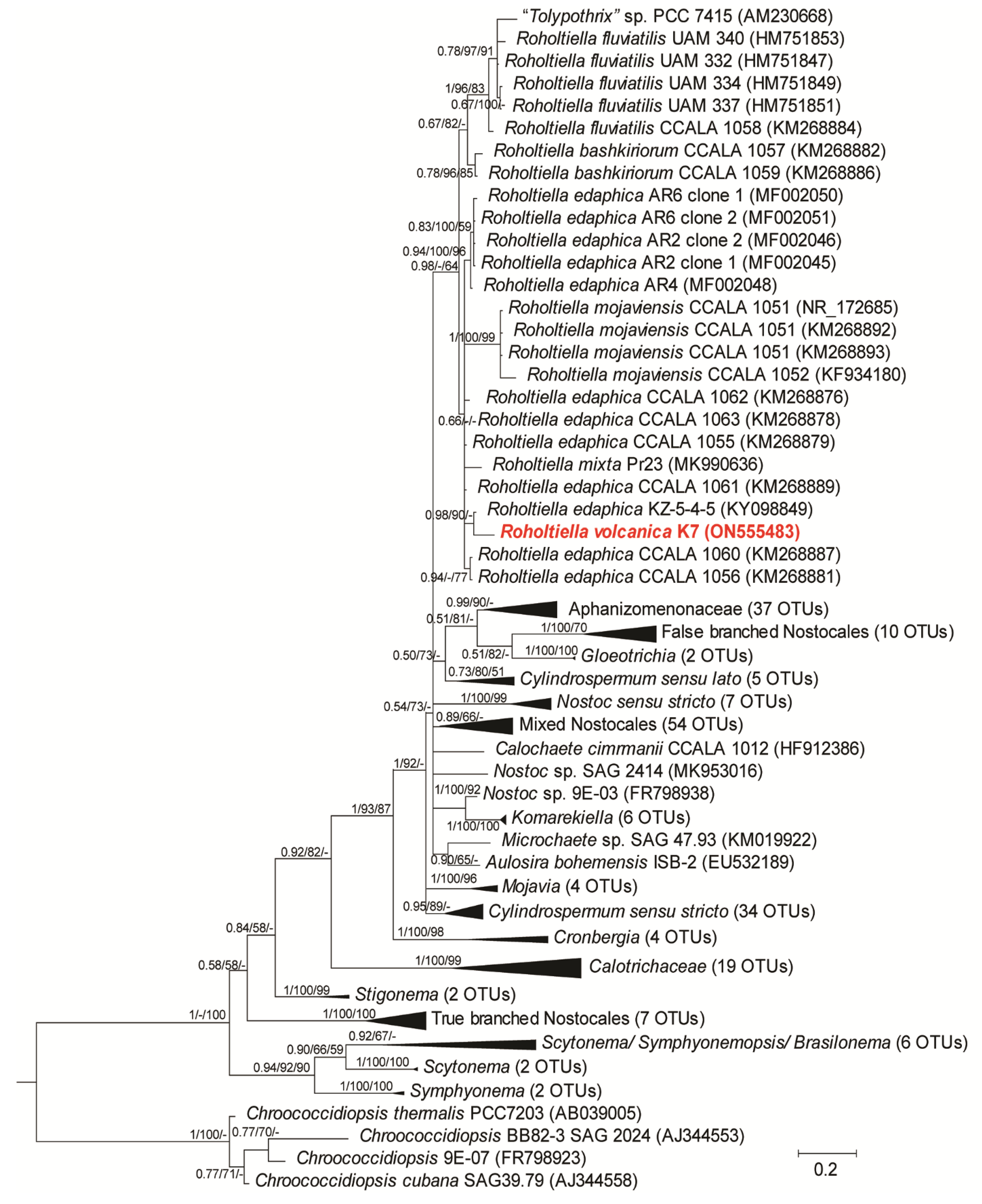
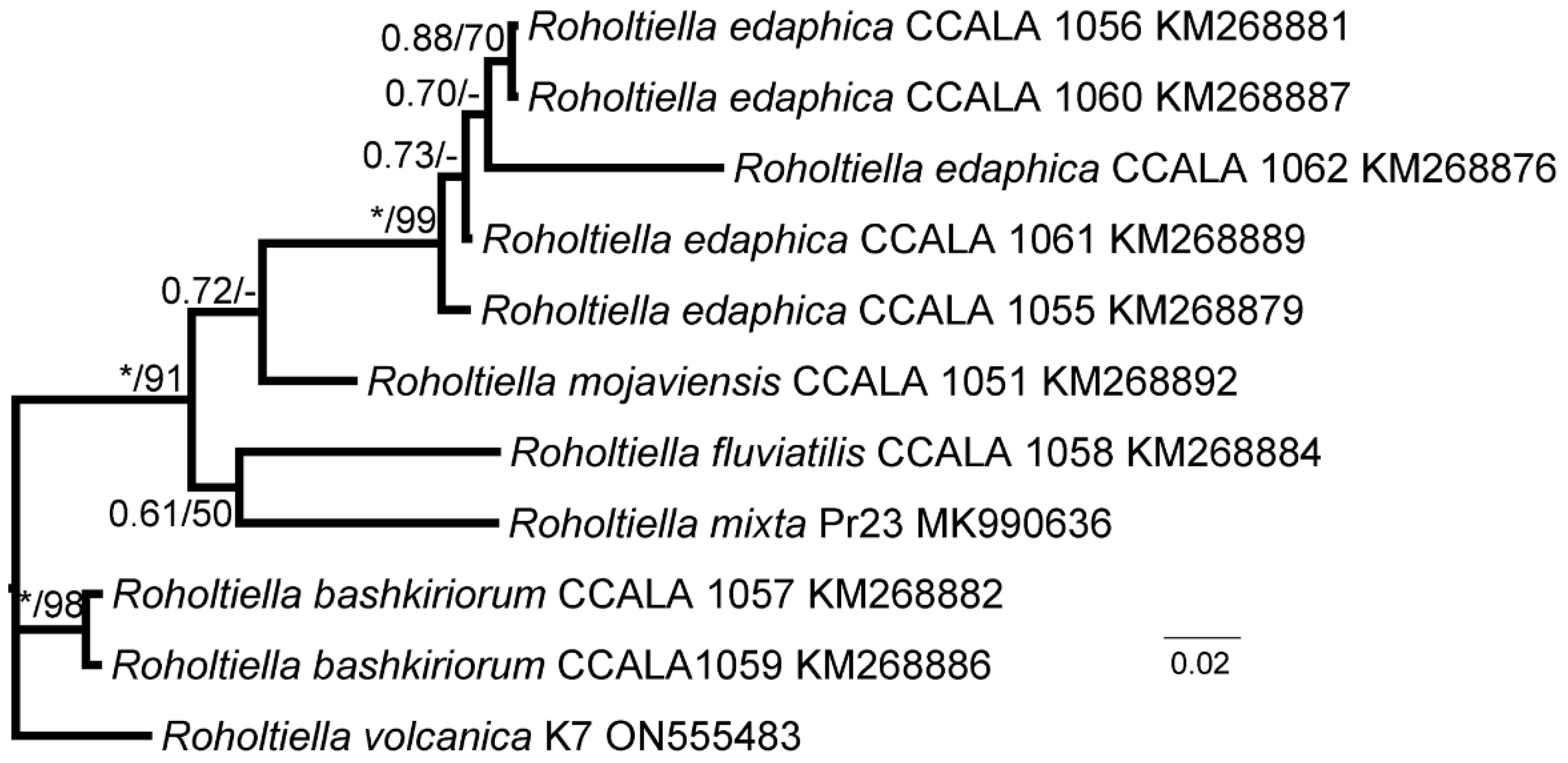
3.2. Phylogenetic Analysis
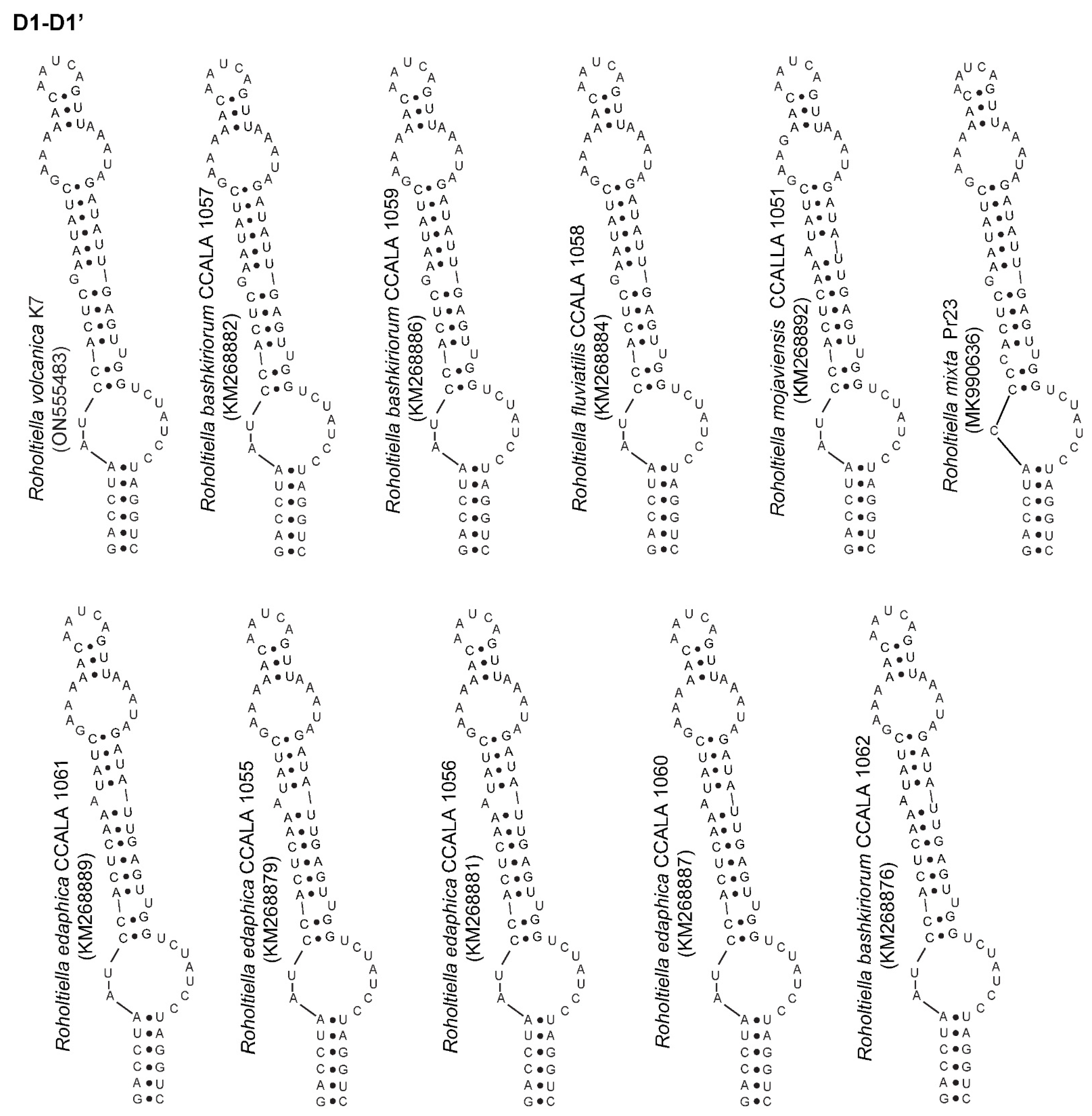
3.3. Analysis of the 16S–23S ITS Region
3.4. Taxonomic Description
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rejmánková, E.; Komárek, J.; Komárková, J. Cyanobacteria—A neglected component of biodiversity: Patterns of species diversity in inland marshes of northern Belize (Central America). Divers. Distrib. 2004, 10, 189–199. [Google Scholar] [CrossRef]
- Nabout, J.C.; da Silva Rocha, B.; Carneiro, F.M.; Sant’Anna, C.L. How many species of Cyanobacteria are there? Using a discovery curve to predict the species number. Biodivers. Conserv. 2013, 22, 2907–2918. [Google Scholar] [CrossRef]
- Engene, N.; Gunasekera, S.P.; Gerwick, W.H.; Paul, V.J. Phylogenetic inferences reveal large extent of novel biodiversityin chemically rich tropical marine cyanobacteria. Appl. Environ. Microbiol. 2013, 79, 1882–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patzelt, D.J.; Hodac, L.; Friedl, T.; Pietrasiak, N.; Johansen, J.R. Biodiversity of soil cyanobacteria in the hyper-arid Atacama Desert, Chile. J. Phycol. 2014, 50, 698–710. [Google Scholar] [CrossRef]
- Oren, A. Cyanobacteria in hypersaline environments: Biodiversity and physiological properties. Biodivers. Conserv. 2015, 24, 781–798. [Google Scholar] [CrossRef]
- Komárek, J. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef]
- Johansen, J.R.; González-Resendiz, L.; Escobar-Sánchez, V.; Segal-Kischinevzky, C.; Martínez-Yerena, J.; Hernández-Sánchez, J.; Hernández-Pérez, G.; León-Tejera, H. When will taxonomic saturation be achieved? A case study in Nunduva and Kyrtuthrix (Rivulariaceae, Cyanobacteria). J. Phycol. 2021, 57, 1699–1720. [Google Scholar] [CrossRef] [PubMed]
- Rěháková, K.; Johansen, J.R.; Casamatta, D.A.; Li, X.; Vincent, J. Morphological and molecular characterization of selected desert soil cyanobacteria: Three species new to science including Mojavia pulchra gen. et sp. nov. Phycologia 2007, 46, 481–502. [Google Scholar] [CrossRef]
- Thomazeau, S.; Houdan-Fourmont, A.; Coute, A.; Charlotte Duval, C. The contribution of sub-Saharan African strains to the phylogeny of cyanobacteria: Focusing on the Nostocaceae family (Nostocales, Cyanobacteria). J. Phycol. 2010, 46, 564–579. [Google Scholar] [CrossRef]
- Komárek, J., 3rd. Part: Heterocytous Genera. In Cyanoprokaryota; Springer: Berlin, Germany, 2013. [Google Scholar]
- Hentschke, G.S.; Johansen, J.R.; Pietrasiak, N.; Rigonato, J.; Fiore, M.F.; Santanna, C.L. Komarekiella atlantica gen. et sp. nov. (Nostocaceae, Cyanobacteria): A new subaerial taxon from the Atlantic Rainforest and Kauai, Hawaii. Fottea 2017, 17, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Li, X.; Yang, Y.; Jia, N.; Huo, D.; Li, R. Compactonostoc shennongjiaensis gen. & sp. nov. (Nostocales, Cyanobacteria) from a wet rocky wall in China. Phycologia 2019, 58, 200–210. [Google Scholar] [CrossRef]
- Cordeiro, R.; Luz, R.; Vasconcelos, V.; Gonçalves, V.; Fonseca, A. Cyanobacteria Phylogenetic Studies Reveal Evidence for Polyphyletic Genera from Thermal and Freshwater Habitats. Diversity 2020, 12, 298. [Google Scholar] [CrossRef]
- Bohunická, M.; Pietrasiak, N.; Johansen, J.R.; Gómez, E.B.; Hauer, T.; Gaysina, L.A.; Lukešová, A. Roholtiella, gen. nov. (Nostocales, Cyanobacteria)—A tapering and branching cyanobacteria of the family Nostocaceae. Phytotaxa 2015, 197, 84–103. [Google Scholar] [CrossRef] [Green Version]
- Mikhailyuk, T.I.; Vinogradova, O.N.; Glaser, K.; Karsten, U. New taxa for the flora of Ukraine, in the context of modern approaches to taxonomy of Cyanoprokaryota/Cyanobacteria. Int. J. Algae 2016, 18, 301–320. [Google Scholar] [CrossRef]
- Abdullin, S.R.; Nikulin, V.Y.; Nikulin, A.Y.; Manyakhin, A.Y.; Bagmet, V.B.; Suprun, A.R.; Gontcharov, A.A. Roholtiella mixta sp. nov. (Nostocales, Cyanobacteria): Morphology, molecular phylogeny, and carotenoid content. Phycologia 2021, 60, 73–82. [Google Scholar] [CrossRef]
- Jones, V.; Solomina, O. The geography of Kamchatka. Glob. Planet. Chang. 2015, 134, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Nazarova, L.; Bleibtreu, A.; Hoff, U.; Dirksen, V.; Diekmann, B. Changes in temperature and water depth of a small mountain lake during the past 3000 years in Central Kamchatka reflected by chironomid record. Quat. Int. 2017, 447, 46–58. [Google Scholar] [CrossRef]
- Ivanov, A. The Far East. In The Physical Geography of Northern Eurasia; Shahgedanova, M., Ed.; Oxford University Press: Oxford, UK, 2002; pp. 422–447. [Google Scholar]
- Neshataeva, V.Y. The Vegetation of Kamchatka Peninsula; KMK: Moscow, Russia, 2009; p. 537. (In Russian) [Google Scholar]
- Litvinenko, Y.S.; Zakharikhina, L.V. Zoning and geochemical characterization of volcanic soils on Kamchatka. Geochem. Int. 2009, 47, 463–475. [Google Scholar] [CrossRef]
- Zakharikhina, L.V.; Litvinenko, Y.S. Volcanism and Geochemistry of Soil and Vegetation Cover of Kamchatka. Communication 1. Geochemical features of volcanic surface ashes. Volcanol. Seismol. 2019, 2, 34–44. [Google Scholar]
- Zakharikhina, L.; Litvinenko, Y.S. Volcanism and Geochemistry of Soil and Vegetation Cover of Kamchatka. Communication 2. Specificity of forming the elemental composition of volcanic soil in cold and humid conditions. Volcanol. Seismol. 2019, 3, 25–33. [Google Scholar]
- Braitseva, O.A.; Ponomareva, V.V.; Sulerzhitsky, L.D.; Melekestsev, I.V.; Bailey, J. Holocene key-marker tephra layers in Kamchatka, Russia. Quat. Res. 1997, 47, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Gledhill, D. Kamchatka: A Journey and Guide to Russia’s Land of Ice and Fire; Odyssey Books and Guides: Hong Kong, China, 2007; p. 311. [Google Scholar]
- Tolstykh, M.L.; Naumov, V.B.; Gavrilenko, M.G.; Ozerov, A.Y.; Kononkova, N.N. Chemical composition, volatile components, and trace elements in the melts of the Gorely volcanic center, southern Kamchatka: Evidence from inclusions in minerals. Geochem. Int. 2012, 50, 522–550. [Google Scholar] [CrossRef]
- Selyangin, O.B.; Ponomareva, V.V.; Gorelovsky Volcanic Center. Southern Kamchatka: Structure and Evolution. Volcanol. Seismol. 1999, 2, 3–23. (In Russian) [Google Scholar]
- Gaysina, L.A.; Bohunická, M.; Hazuková, V.; Johansen, J.R. Biodiversity of terrestrial cyanobacteria of the South Ural region. Cryptogam. Algol. 2018, 39, 167–198. [Google Scholar] [CrossRef] [Green Version]
- Kostikov, I.; Romanenko, P.; Demchenko, P.; Darienko, T.M.; Mikhayljuk, T.I.; Rybchinskiy, O.V.; Solonenko, A.M. Soil Algae of Ukraine; Phytosotsiologichniy Center: Kiev, Ukraine, 2001; p. 300. [Google Scholar]
- Carmichael, W.W. Isolation, culture, and toxicity testing of toxic freshwater cyanobacteria (blue-green algae). In Fundamental Research in Homogenous Catalysis 3; Shilov, V., Ed.; Gordon & Breach: New York, NY, USA, 1968; pp. 1249–1262. [Google Scholar]
- Andersen, R.A. Algal Culturing Techniques; Elsevier Academic Press: Burlington, MA, USA, 2005. [Google Scholar]
- McFadden, G.I.; Melkonian, M. Use of Hepes buffer for microalgal culture media and fixation for electron microscopy. Phycologia 1986, 25, 551–557. [Google Scholar] [CrossRef]
- Wilmotte, A.; Van Der Auwera, G.; De Wachter, R. Structure of the 16S ribosomal RNA of the thermophilic cyanobacterium Chlorogloeopsis HTF (‘Mastigocladus laminosus HTF’) strain PCC7518, and phylogenetic analysis. FEBS Lett. 1993, 317, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Nübel, U.; Garcia-Pichel, F.; Muyzer, G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 1997, 63, 3327–3332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Trifinopoulos, J.; Nguyen, L.T.; Von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, 232–235. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Nguyen, M.A.T.; Haeseler, A.V. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Zucker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acid Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Allaguvatova, R.Z.; Nikulin, A.Y.; Nikulin, V.Y.; Bagmet, V.B.; Gaysina, L.A. Study of Biodiversity of Algae and Cyanobacteria of Mutnovsky and Gorely Volcanoes Soils (Kamchatka Peninsula) Using a Polyphasic Approach. Diversity 2022, 14, 375. [Google Scholar] [CrossRef]
- Sokolov, I.A. Volcanic Activity and Soil Generation (in Kamchatka); Nauka: Moscow, Russia, 1973; p. 224. (In Russian) [Google Scholar]
- González-Resendiz, L.; Johansen, J.R.; León-Tejera, H.; Sanchéz, L.; Segal-Kischinevsky, C.; Escobar-Sánchez, V.; Morales, M. A bridge too far in naming species: A total evidence approach does not support recognition of four species in Desertifilum (Cyanobacteria). J. Phycol. 2019, 55, 898–911. [Google Scholar] [CrossRef] [PubMed]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawks-worth, D.L.; Herendeen, P.S.; Knapp, S. (Eds.) International Code of Nomenclature for algae, fungi, and plants. In Proceedings of the Nineteenth International Botanical Congress, Shenzhen, China, 23–29 July 2017; Regnum Vegetabile. Koeltz Botanical Books: Glashütten, Germany, 2018; p. 159. [Google Scholar]
- Erwin, P.M.; Thacker, R.W. Cryptic diversity of the symbiotic cyanobacterium Synechococcus spongiarum among sponge hosts. Mol. Ecol. 2008, 17, 2937–2947. [Google Scholar] [CrossRef]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.; Kovacik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef] [Green Version]
- Pietrasiak, N.; Mühlsteinová, R.; Siegesmund, M.A.; Johansen, J.R. Phylogenetic placement of Symplocastrum (Phormidiaceae) with a new combination S. californicum and two new species: S. flechtnerae and S. torsivum. Phycologia 2014, 53, 529–541. [Google Scholar] [CrossRef]
- González-Resendiz, L.; Johansen, J.R.; Escobar-Sánchez, V.; Segal-Kischinevzky, C.; Jiménez-García, L.F.; León-Tejera, H. Two new species of Phyllonema (Rivulariaceae, Cyanobacteria) with an emendation of the genus. J. Phycol. 2018, 54, 638–652. [Google Scholar] [CrossRef]
- González-Resendiz, L.; Johansen, J.R.; Alba-Lois, L.; Segal-Kischinevzky, C.; Escobar-Sánchez, V.; Jimenez-Garcia, L.F.; Hauer, T.; León-Tejera, H. Nunduva, a new marine genus of Rivulariaceae (Nostocales, Cyanobacteria) from marine tropical rocky shores. Fottea 2018, 18, 86–105. [Google Scholar] [CrossRef] [Green Version]
- Fazlutdinova, A.; Gabidullin, Y.; Allaguvatova, R.; Gaysina, L. Diatoms in Volcanic Soils of Mutnovsky and Gorely Volcanoes (Kamchatka Peninsula, Russia). Microorganisms 2021, 9, 1851. [Google Scholar] [CrossRef]
- Johansen, J.R.; Casamatta, D.A. Recognizing cyanobacterial diversity through adoption of a new species paradigm. Algol. Stud. 2005, 116, 71–93. [Google Scholar] [CrossRef]
- Calabrese, S.; Scaglione, S.; D’Alessandro, W.; Brusca, L.; Bellamo, S.; Parello, F. A literature review and new data of trace metal fluxes from worldwide active volcanoes. In Proceedings of the Conferenza A. Rittman, Monti Rossi, Italy, 12–14 December 2012. Abstract, 2012, 41–42. [Google Scholar]
- Treub, M. Notice sur la nouvelle flore de Krakatau. Ann. Jard. Bot. Buitenzorg. 1888, 7, 213–223. [Google Scholar]
- Backer, C.A. The Problem of Krakatau as Seen by a Botanist; Springer Science & Business Media: The Hague, The Netherlands, 1929; p. 299. [Google Scholar]
- Whittaker, R.J.; Bush, M.B.; Richards, K. Plant recolonization and vegetation succession on the Krakatau Islands, Indonesia. Ecol. Monogr. 1989, 59, 59–123. [Google Scholar] [CrossRef]
- Gomez-Alvarez, V.; King, G.M.; Nusslein, K. Comparative bacterial diversity in recent Hawaiian volcanic deposits of different ages. FEMS Microbiol. Ecol. 2007, 60, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Mueller-Dombois, D.; Boehmer, H.J. Origin of the Hawaiian rainforest and its transition states in long-term primary succession. Biogeosciences 2013, 10, 5171–5182. [Google Scholar] [CrossRef] [Green Version]
- Rayburn, W.R.; Mack, R.N.; Metting, B. Conspicuous algal colonization of the ash from Mount St. Helens. J. Phycol. 1982, 18, 537–543. [Google Scholar] [CrossRef]
- Shtina, E.A.; Andreyeva, V.M.; Kuzyakina, T.I. Algae settlement of volcanic substrates. Bot. Zhurnal. 1992, 8, 33–42. [Google Scholar]
- Fermani, P.; Mataloni, G.; de Vijver, B.V. Soil microalgal communities on an Antarctic active volcano (Deception Island, South Shetlands). Polar Biol. 2007, 30, 1381–1393. [Google Scholar] [CrossRef]
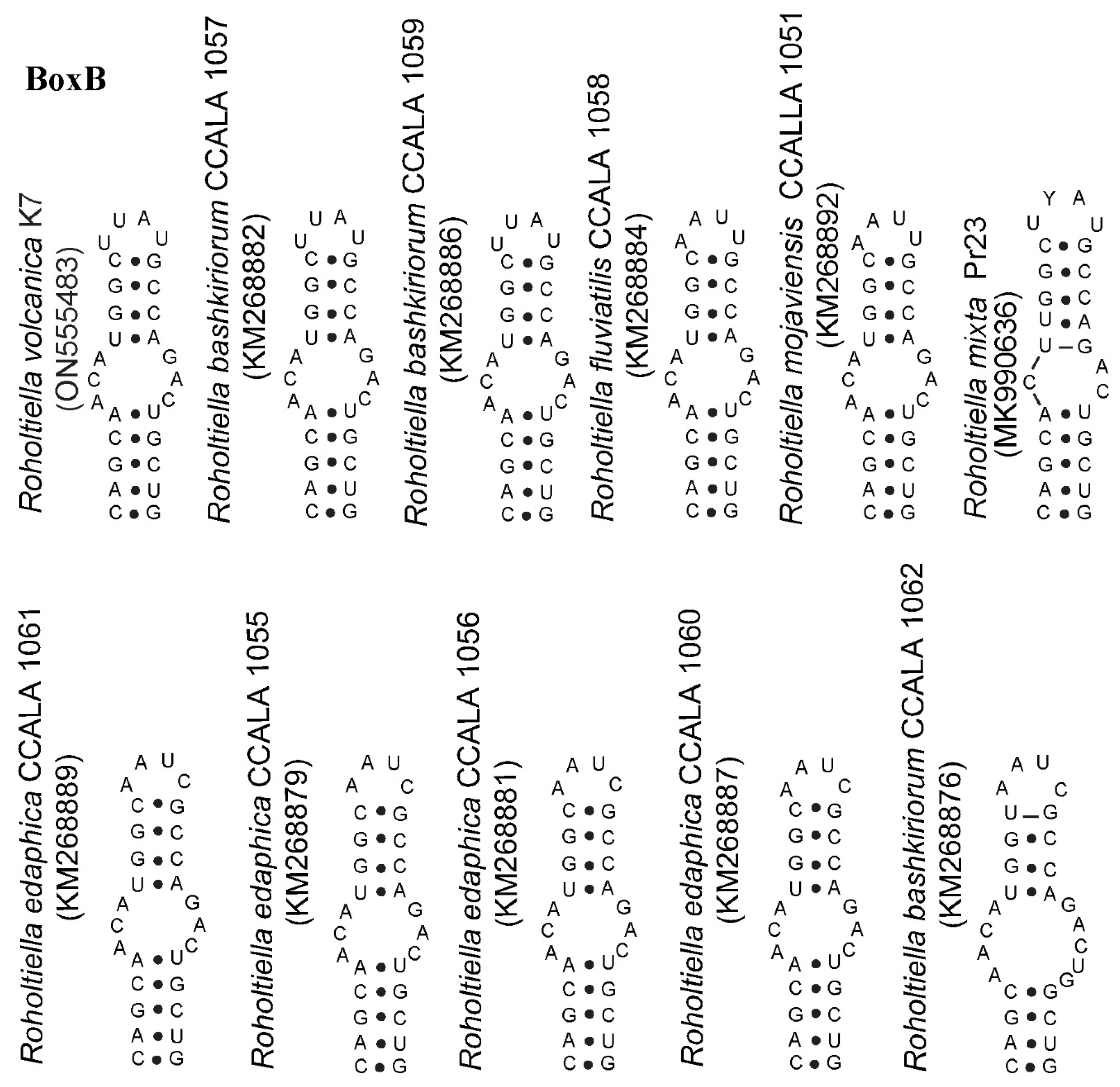
| Roholtiella volcanica K7 | AGAATCATCAAAACTACAGGGAATAGTACTT–BoxB–AGAA–TCCAGCCA |
| Roholtiella bashkiriorum CCALA 1057 | AGAATCATCAAAAGTACAGGGAATAGTACTT–BoxB–AGAA–TCCAGCCA |
| Roholtiella bashkiriorum CCALA 1059 | AGAATCATCAAAAGTACAGGGAATAGTACTT–BoxB–AGAA–TCCAGCCA |
| Roholtiella edaphica CCALA 1063 | AAAATCATAAAAACTACTGGGAATAGTG–TT–BoxB–TATTGTCCAGCCA |
| Roholtiella edaphica KZ_5_4_5 | ACAATCATAAAAACTATTGAGAATAGTG–TT–BoxB–TATTTTCCAGCCA |
| Roholtiella edaphica CCALA 1062 | AAAATGATAAAAACTACTGGGAATAGTG–TT–BoxB–TATTGTCCAGCCA |
| Roholtiella edaphica CCALA 1055-56; 1060-61 | AAAATCATAAAAACTACTGGGAATAGTG–TT–BoxB–TATTGTCCAGCCA |
| Roholtiella fluviatilis CCALA 1058 | ATAATCATTACAAGTATTGGAAATAGTAATT–BoxB–TTAT–TCCAGCCA |
| Roholtiella mojaviensis CCALA 1051-52 | ACAATCATARAAACTACTGGGAATAGTG–TT–BoxB–TAATATCCAGCCA |
| Roholtiella mixta Pr23 | ACAACCATTGAAACTATTGGAAATATAAATT–BoxB–TTAT–TCCAGCCA |
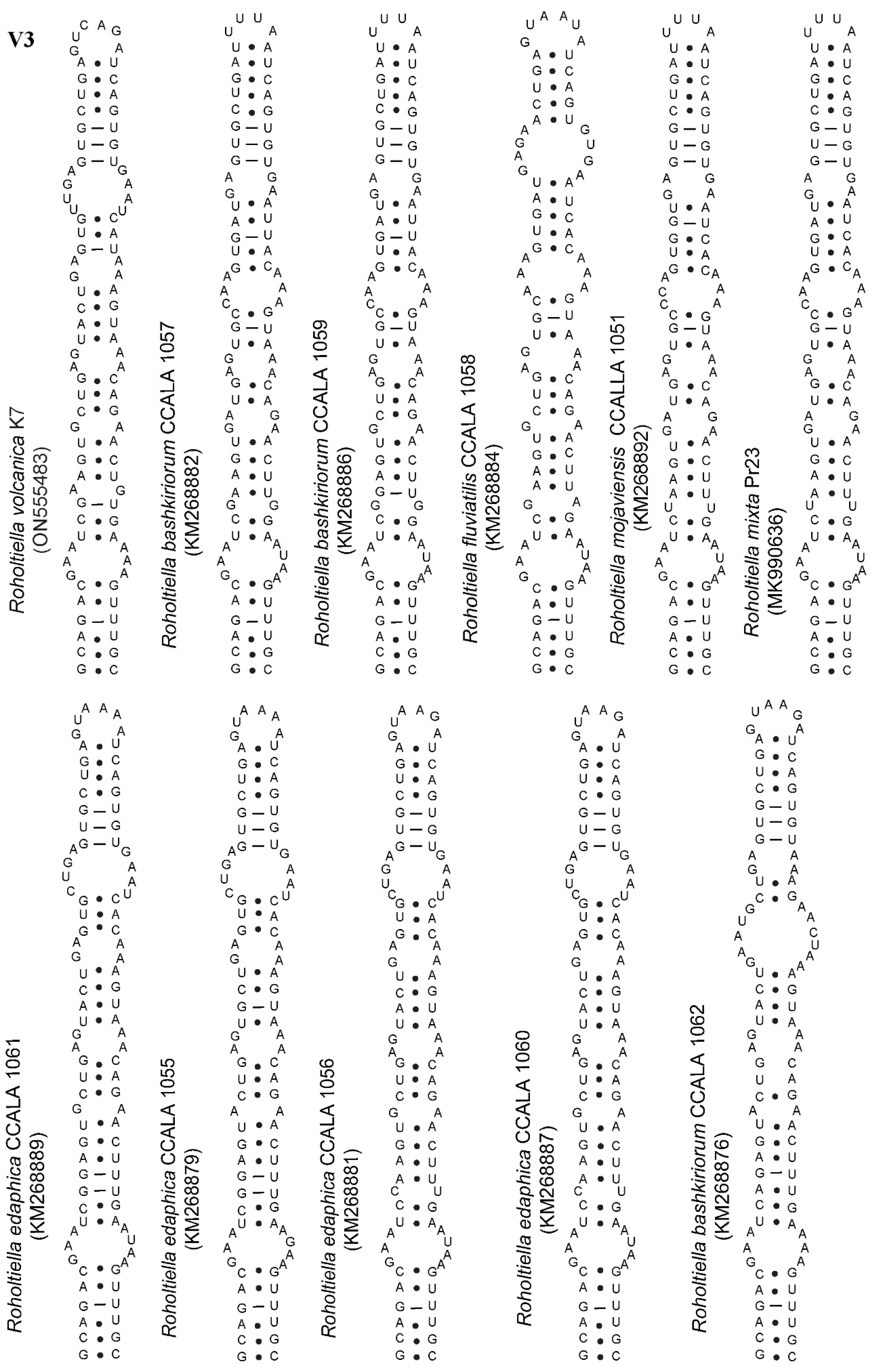
| Roholtiella volcanica K7 | GAAAAAAAGCAG–V3–AGTGGTGAACACCAAA–TGTATTGT |
| Roholtiella bashkiriorum CCALA 1057 | GAAAAAAAGCAG–V3–AGTGGTGAA–ACCAA––TGAATTGT |
| Roholtiella bashkiriorum CCALA 1059 | GAAAAAAAGCAG–V3–AGTGGTGAA–ACCAA––TGAATTGT |
| Roholtiella edaphica CCALA 1063 | GATTTAAAGCAG–V3–AGTGGTGAACACCAAA–TGTATTGT |
| Roholtiella edaphica KZ_5_4_5 | GA–AAAAAGCAG–V3–AGTGGTGAACACCAAA–TGTATAGT |
| Roholtiella edaphica CCALA 1062 | GATTTAAAGCAG–V3–AGTGGTGAACACCAAA–TGTATTGT |
| Roholtiella edaphica CCALA 1055-56; 1060-61 | GATTTAAAGCAG–V3–AGTGGTGAACACCAAA–TGTATTGT |
| Roholtiella fluviatilis CCALA 1058 | GA–AAAAAGCAG–V3–AGTGGTGAA–ACCAATTGTTTAAGT |
| Roholtiella mojaviensis CCALA 1051-52 | GA–AAAAAGCAG–V3–AGTGGTGAA–ACCAAT–TGTTTAGT |
| Roholtiella mixta Pr23 | GA–AAAAAGCAG–V3–AGTGGTGAACACCAA–TGTA–AGT |
| Roholtiella volcanica K7 | Roholtiella bashkiriorum CCALA 1057 | Roholtiella bashkiriorum CCALA 1059 | Roholtiella fluviatilis CCALA 1058 | Roholtiella mojaviensis CCALA 1051 | Roholtiella mixta Pr23 | Roholtiella edaphica CCALA 1061 | Roholtiella edaphica CCALA 1055 | Roholtiella edaphica CCALA 1056 | |
|---|---|---|---|---|---|---|---|---|---|
| Roholtiella volcanica K7 | |||||||||
| Roholtiella bashkiriorum CCALA 1057 | 5.0 | ||||||||
| Roholtiella bashkiriorum CCALA 1059 | 5.0 | 0.6 | |||||||
| Roholtiella fluviatilis CCALA 1058 | 12.0 | 10.2 | 10.2 | ||||||
| Roholtiella mojaviensis CCALA 1051 | 10.0 | 7.0 | 7.6 | 8.5 | |||||
| Roholtiella mixta Pr23 | 12.1 | 9. | 9.8 | 10.3 | 7.4 | ||||
| Roholtiella edaphica CCALA 1061 | 7.9 | 9.3 | 8.7 | 10.5 | 6.7 | 10.6 | |||
| Roholtiella edaphica CCALA 1055 | 8.7 | 9.9 | 9.3 | 10.5 | 6.7 | 10.6 | 1.29 | ||
| Roholtiella edaphica CCALA 1056 | 7.6 | 9.6 | 9.6 | 10.5 | 6.7 | 10.6 | 0.9 | 2.0 | |
| Roholtiella edaphica CCALA 1060 | 7.6 | 9.6 | 9.6 | 10.5 | 6.7 | 10.6 | 0.9 | 2.0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaysina, L.A.; Johansen, J.R.; Saraf, A.; Allaguvatova, R.Z.; Pal, S.; Singh, P. Roholtiella volcanica sp. nov., a New Species of Cyanobacteria from Kamchatkan Volcanic Soils. Diversity 2022, 14, 620. https://doi.org/10.3390/d14080620
Gaysina LA, Johansen JR, Saraf A, Allaguvatova RZ, Pal S, Singh P. Roholtiella volcanica sp. nov., a New Species of Cyanobacteria from Kamchatkan Volcanic Soils. Diversity. 2022; 14(8):620. https://doi.org/10.3390/d14080620
Chicago/Turabian StyleGaysina, Lira A., Jeffrey R. Johansen, Aniket Saraf, Rezeda Z. Allaguvatova, Sagarika Pal, and Prashant Singh. 2022. "Roholtiella volcanica sp. nov., a New Species of Cyanobacteria from Kamchatkan Volcanic Soils" Diversity 14, no. 8: 620. https://doi.org/10.3390/d14080620
APA StyleGaysina, L. A., Johansen, J. R., Saraf, A., Allaguvatova, R. Z., Pal, S., & Singh, P. (2022). Roholtiella volcanica sp. nov., a New Species of Cyanobacteria from Kamchatkan Volcanic Soils. Diversity, 14(8), 620. https://doi.org/10.3390/d14080620








