DNA Barcoding of Cold-Water Coral-Associated Ophiuroid Fauna from the North Atlantic
Abstract
1. Introduction
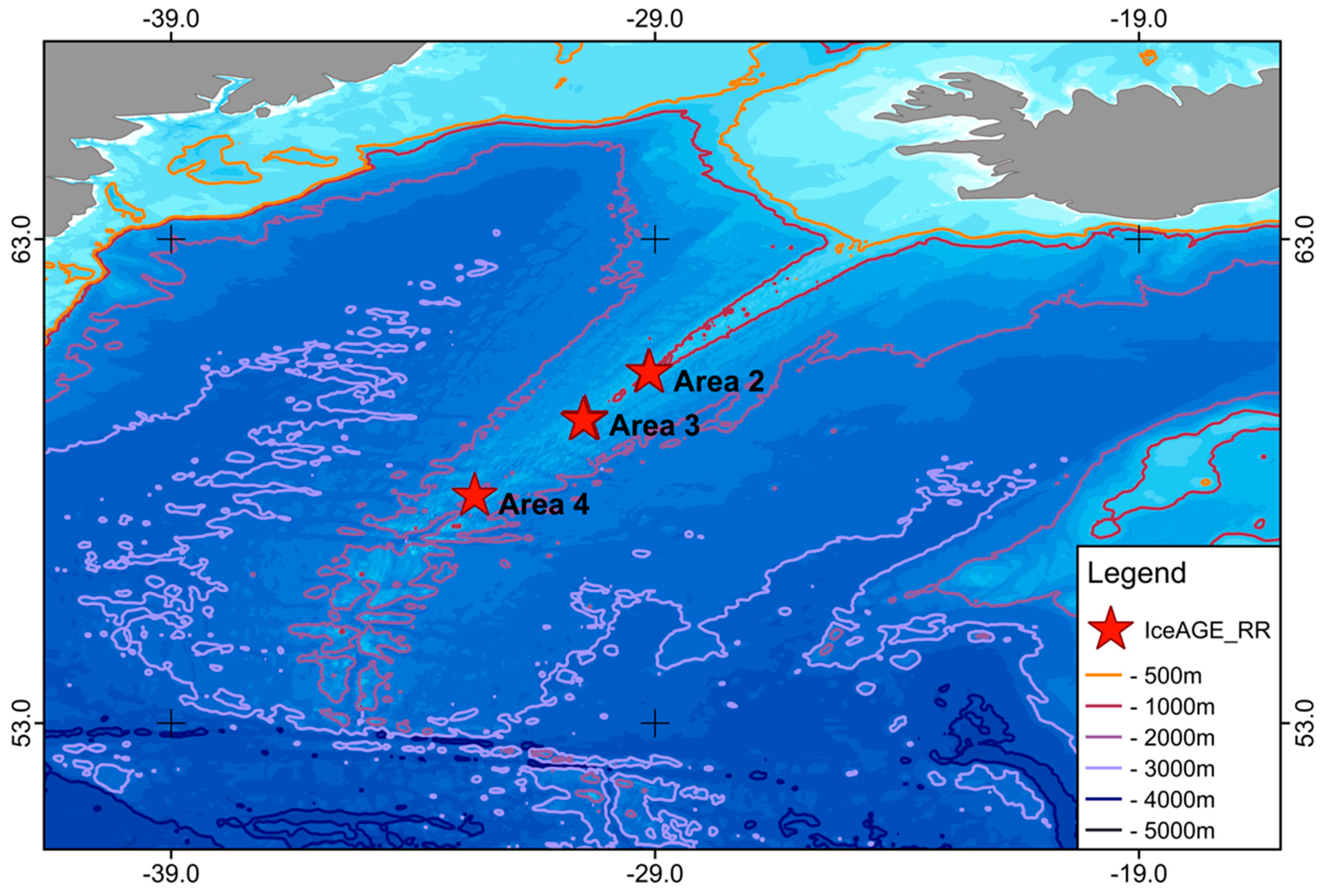
2. Materials and Methods
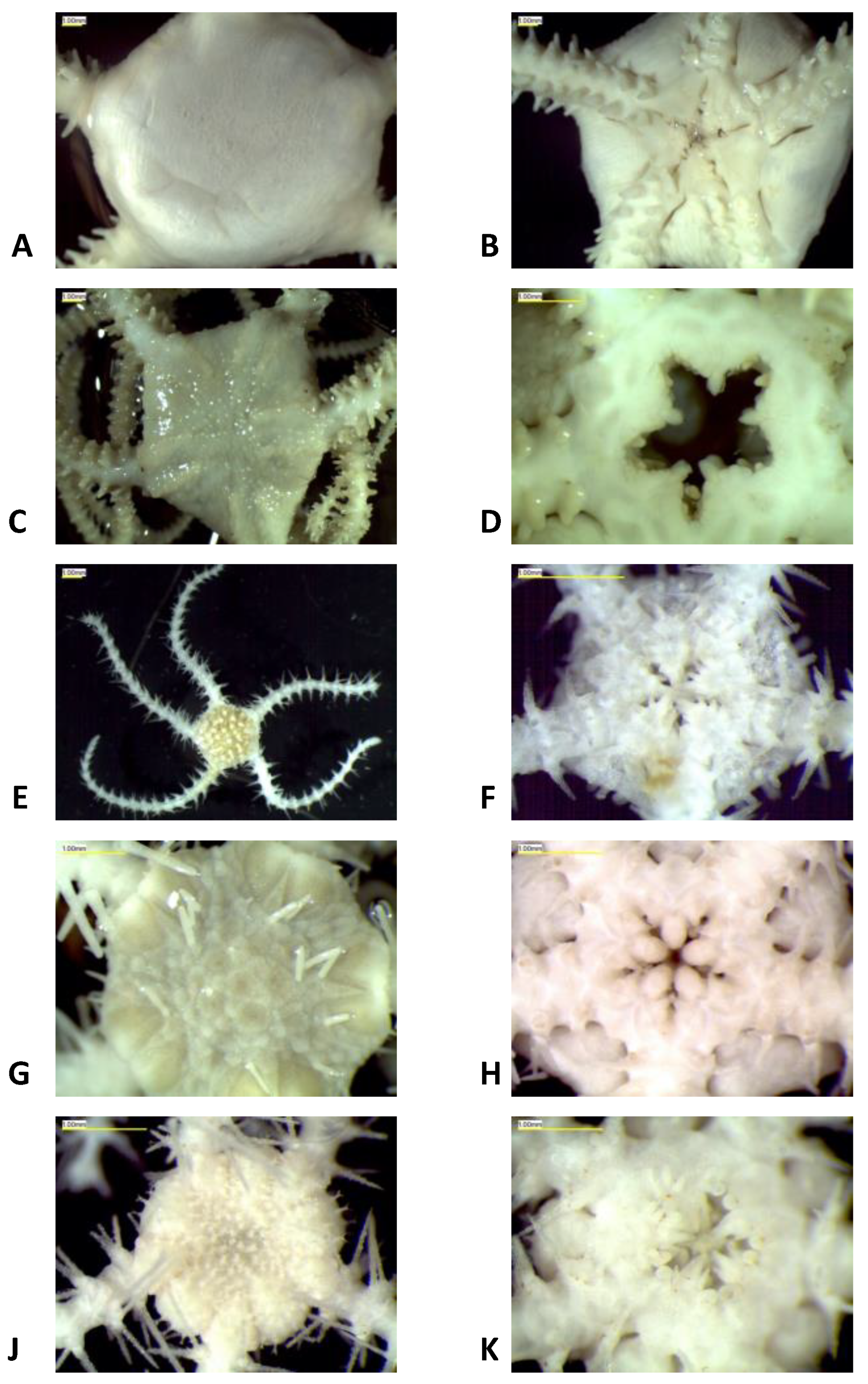
2.1. Morphological Identification
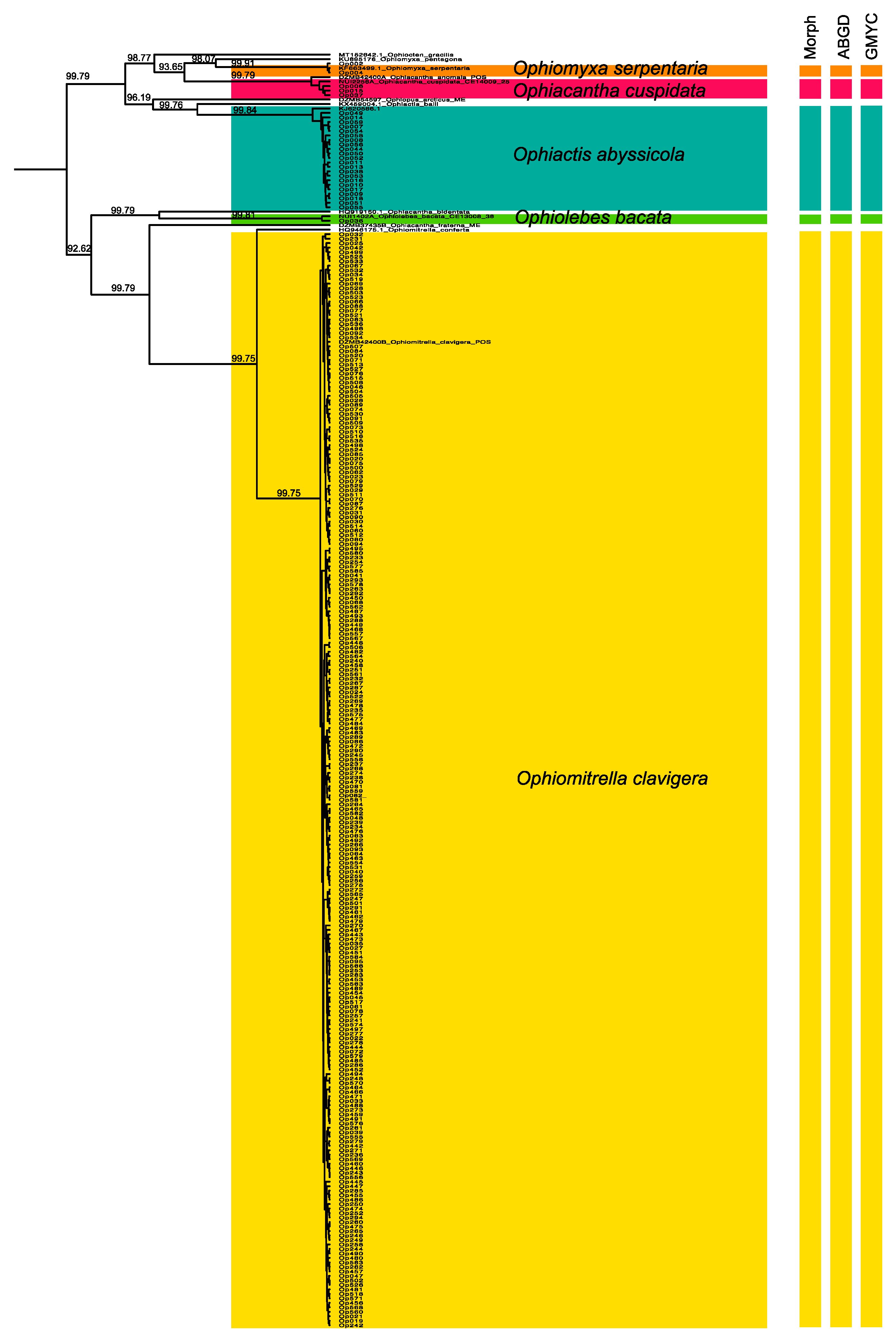
2.2. Molecular Species Delimitation
2.3. Image Data Analysis
3. Results
3.1. Morphological and Molecular Species Delimitation
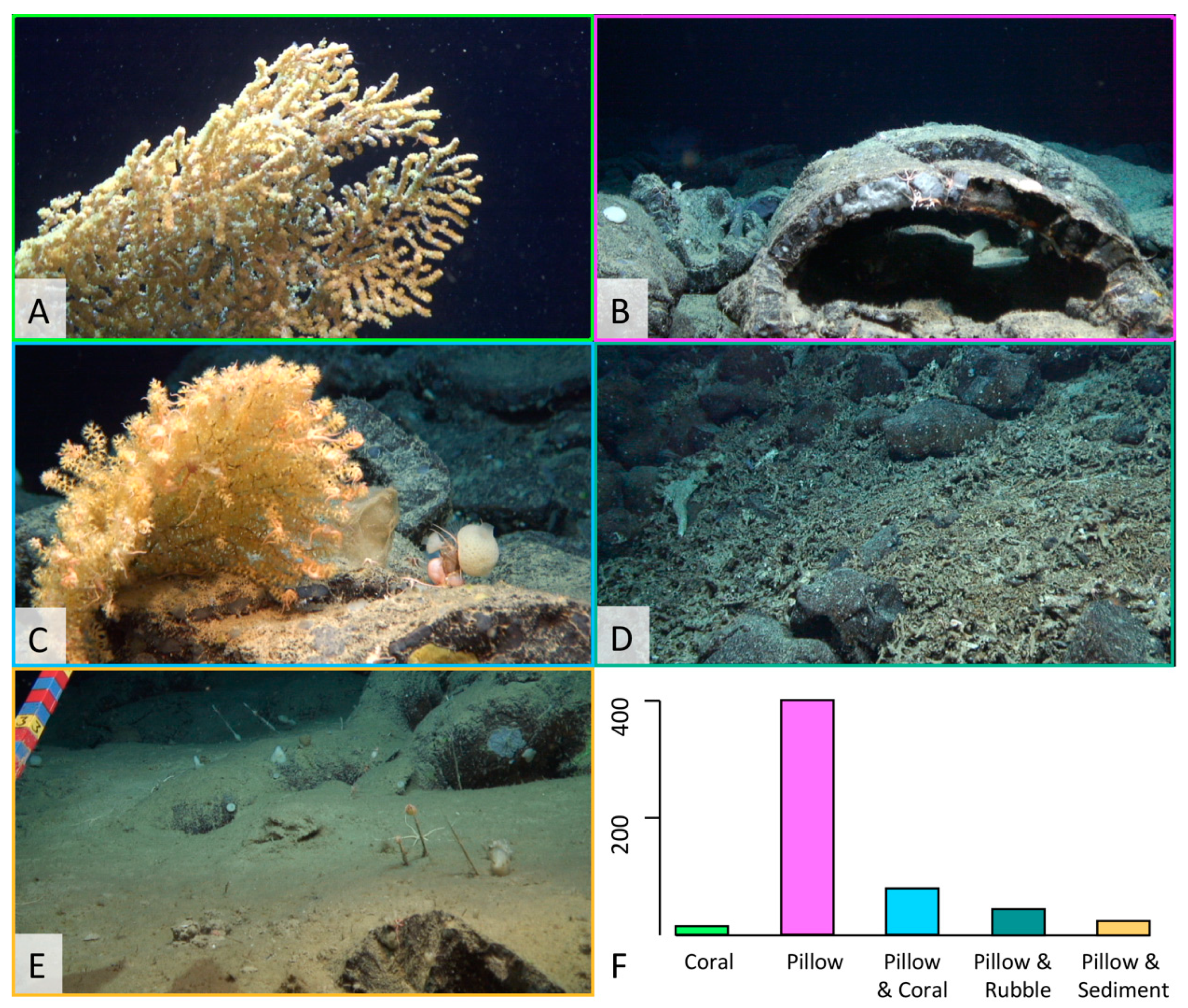
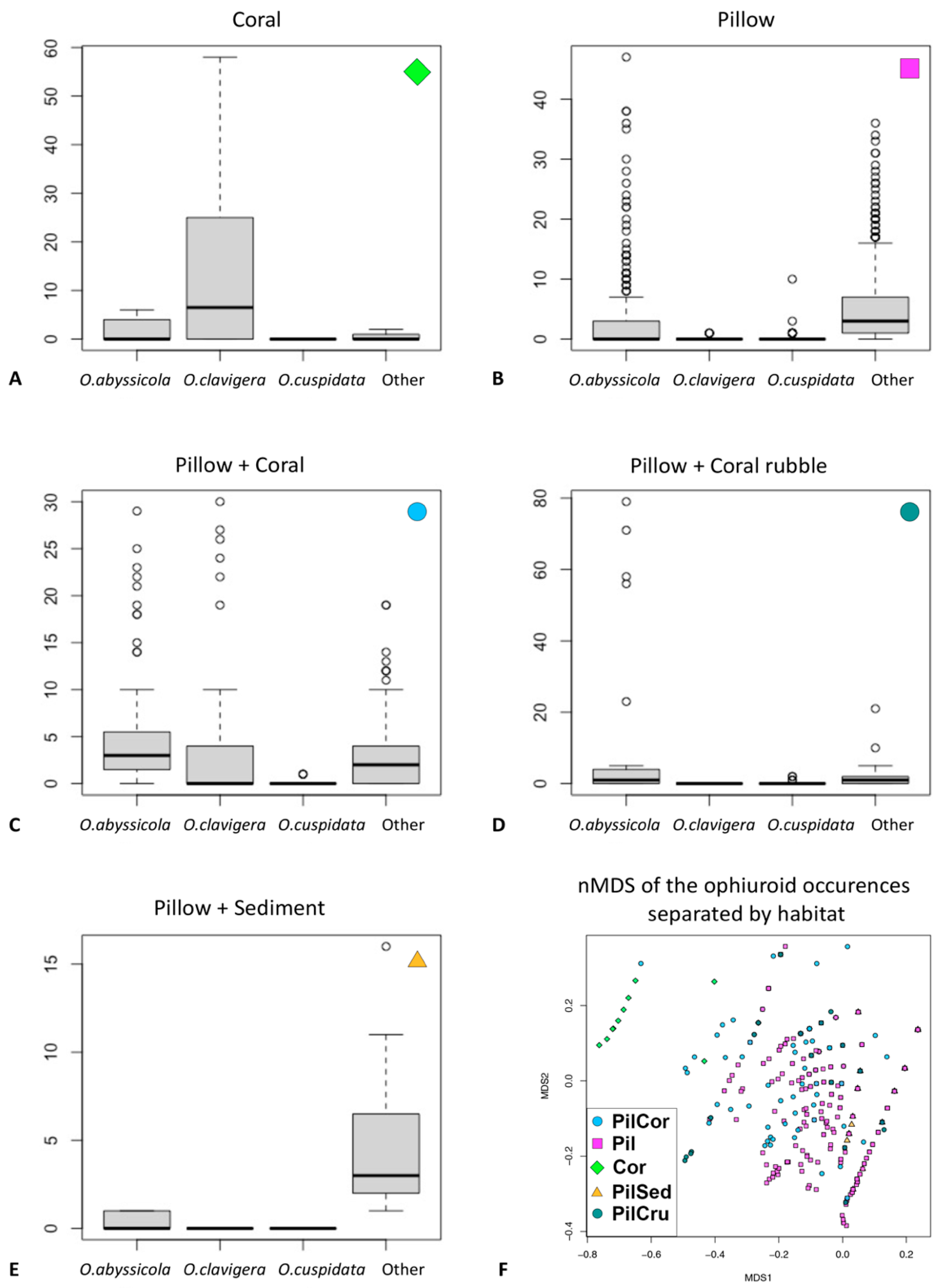
3.2. Ecological Analysis
4. Discussion
4.1. Ophiuroids Distribution According to Seafloor Heterogeneity
4.2. Deep Icelandic Ophiuroid Fauna
4.3. Species Delimitation and DNA Barcoding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brix, S.; Meißner, K.; Kaiser, S.; Svavarsson, J.; Yasuhara, M.; Martinez Arbizu, P. The IceAGE Project—A Follow up of BIOICE. Pol. Polar Res. 2014, 35, 141–150. [Google Scholar] [CrossRef]
- Copley, J.T.P.; Tyler, P.A.; Sheader, M.; Murton, B.J.; German, C.R. Megafauna from Sublittoral to Abyssal Depths along the Mid-Atlantic Ridge South of Iceland. Oceanol. Acta 1996, 19, 549–559. [Google Scholar]
- Devey, C.W.; Brix, S.; Barua, A.; Bodendorfer, M.; Cuno, P.; Frutos, I.; Huusmann, H.; Kurbjuhn, T.; Le Saout, M.; Linse, K.; et al. Detailed Mapping and Sampling of the Reykjanes Ridge, Cruise No. MSM75, 29 June 2018–8 August 2018, Reykjavik-Reykjavik; GEOMAR: Bonn, Germany, 2018. [Google Scholar] [CrossRef]
- Taylor, J.; Devey, C.; Le Saout, M.; Petersen, S.M.; Frutos, I.I.; Linse, K.; Loerz, A.-N.; Pałgan, D.; Tandberg, A.H.; Svavarsson, J.; et al. The Geological and Faunal Composition of Steinahóll Vent Sites, Reykjanes Ridge, Iceland. Front. Mar. Sci. 2021, 8, 520713. [Google Scholar] [CrossRef]
- German, C.R.; Briem, J.; Chin, C.; Danielsen, M.; Holland, S.; James, R.; Jónsdóttir, A.; Ludford, E.; Moser, C.; Ólafsson, J.; et al. Hydrothermal Activity on the Reykjanes Ridge: The Steinahóll Vent-Field at 63°06′ N. Earth Planet. Sci. Lett. 1994, 121, 647–654. [Google Scholar] [CrossRef]
- Malmberg, S.A.; Jónsson, S. Timing of Deep Convection in the Greenland and Iceland Seas. ICES J. Mar. Sci. 1997, 54, 300–309. [Google Scholar] [CrossRef]
- Meißner, K.; Fiorentino, D.; Schnurr, S.; Martinez Arbizu, P.; Huettmann, F.; Holst, S.; Brix, S.; Svavarsson, J. Distribution of Benthic Marine Invertebrates at Northern Latitudes—An Evaluation Applying Multi-Algorithm Species Distribution Models. J. Sea Res. 2014, 85, 241–254. [Google Scholar] [CrossRef]
- Ostmann, A.; Schnurr, S.; Martinez Arbizu, P. Marine Environment around Iceland: Hydrography, Sediments and First Predictive Models of Icelandic Deep—Sea Sediment Characteristics. Pol. Polar Res. 2014, 35, 151–176. [Google Scholar] [CrossRef]
- Nørrevang, A.; Brattegard, T.; Josefson, A.B.; Sneil, J.-A.; Tendal, O.S. List of BIOFAR Stations. Sarsia 1994, 79, 165–180. [Google Scholar] [CrossRef]
- Keeton, J.A.; Searle, R.C.; Parsons, B.; White, R.S.; Murton, B.J.; Parson, L.M.; Peirce, C.; Sinha, M.C. Bathymetry of the Reykjanes Ridge. Mar. Geophys. Res. 1997, 19, 55–64. [Google Scholar] [CrossRef]
- Hey, R.; Martinez, F.; Höskuldsson, Á.; Benediktsdóttir, Á. Propagating Rift Model for the V-Shaped Ridges South of Iceland. Geochem. Geophys. Geosyst. 2010, 11, 1–24. [Google Scholar] [CrossRef]
- Pałgan, D.; Devey, C.W.; Yeo, I.A. Volcanism and Hydrothermalism on a Hotspot–Influenced Ridge: Comparing Reykjanes Peninsula and Reykjanes Ridge, Iceland. J. Volcanol. Geotherm. Res. 2017, 348, 62–81. [Google Scholar] [CrossRef]
- Arnaud-Haond, S.; Van den Beld, I.M.J.; Becheler, R.; Orejas, C.; Menot, L.; Frank, N.; Grehan, A.; Bourillet, J.F. Two “Pillars” of Cold-Water Coral Reefs along Atlantic European Margins: Prevalent Association of Madrepora oculata with Lophelia pertusa, from Reef to Colony Scale. Deep Res. Part II Top. Stud. Oceanogr. 2017, 145, 110–119. [Google Scholar] [CrossRef]
- Frank, N.; Freiwald, A.; López Correa, M.; Wienberg, C.; Eisele, M.; Hebbeln, D.; Van Rooij, D.; Henriet, J.P.; Colin, C.; van Weering, T.; et al. Northeastern Atlantic Cold-Water Coral Reefs and Climate. Geology 2011, 39, 743–746. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Vanreusel, A.; Gooday, A.J.; Levin, L.A.; Priede, I.G.; Buhl-Mortensen, P.; Gheerardyn, H.; King, N.J.; Raes, M. Biological Structures as a Source of Habitat Heterogeneity and Biodiversity on the Deep Ocean Margins. Mar. Ecol. 2010, 31, 21–50. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Brandt, A.; Danovaro, R.; De Mol, B.; Escobar, E.; German, C.R.; Levin, L.A.; Martinez Arbizu, P.; Menot, L.; Buhl-Mortensen, P.; et al. Deep, Diverse and Definitely Different: Unique Attributes of the World’s Largest Ecosystem. Biogeosciences 2010, 7, 2851–2899. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A. Reefs of the Deep: The Biology and Geology of Cold-Water Coral Ecosystems. Science 2006, 312, 543–547. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Fosså, J.H. Species Diversity and Spatial Distribution of Invertebrates on Deep–Water Lophelia Reefs in Norway. Proc. 10th Int. Coral Reef Symp. 2006, 1868, 1849–1868. [Google Scholar]
- Buhl-Mortensen, L.; Mortensen, P. Symbiosis in Deep-Water Corals. Symbiosis 2004, 37, 33–61. [Google Scholar]
- Davies, A.J.; Guinotte, J.M. Global Habitat Suitability for Framework-Forming Cold-Water Corals. PLoS ONE 2011, 6, e18483. [Google Scholar] [CrossRef]
- Bribiesca-Contreras, G.; O’Hara, T.D.; Verbruggen, H.; Hugall, A.F. Global Biogeographic Structuring of Tropical Shallow—Water Brittle Stars. J. Biogeogr. 2019, 46, 1287–1299. [Google Scholar] [CrossRef]
- Cho, W.; Shank, T.M. Incongruent Patterns of Genetic Connectivity among Four Ophiuroid Species with Differing Coral Host Specificity on North Atlantic Seamounts. Mar. Ecol. 2010, 31, 121–143. [Google Scholar] [CrossRef]
- Mosher, C.V.; Watling, L. Partners for Life: A Brittle Star and Its Octocoral Host. Mar. Ecol. Prog. Ser. 2009, 397, 81–88. [Google Scholar] [CrossRef]
- Starmer, J.A. An Annotated Checklist of Ophiuroids (Echinodermata) from Guam. Micronesica 2003, 35, 547–562. [Google Scholar]
- Bos, A.R.; Hoeksema, B.W. Mushroom Corals (Fungiidae) in the Davao Gulf, Philippines, with Records of Associated Fish and Other Cryptofauna. Raffles Bull. Zool. 2017, 65, 198–206. [Google Scholar]
- O’Hara, T.D.; Stöhr, S.; Hugall, A.F.; Thuy, B.; Martynov, A. Morphological Diagnoses of Higher Taxa in Ophiuroidea (Echinodermata) in Support of a New Classification. Eur. J. Taxon. 2018, 416, 1–35. [Google Scholar] [CrossRef]
- Stöhr, S.; O’Hara, T.D.; Thuy, B. Global Diversity of Brittle Stars (Echinodermata: Ophiuroidea). PloS ONE 2012, 7, e31940. [Google Scholar] [CrossRef]
- Stöhr, S.; O’Hara, T.; Thuy, B. (Eds.) World Ophiuroidea Database. 2022. Available online: https://www.marinespecies.org/ophiuroidea (accessed on 19 April 2022).
- Christodoulou, M.; O’Hara, T.D.; Hugall, A.F.; Arbizu, P.M. Dark Ophiuroid Biodiversity in a Prospective Abyssal Mine Field. Curr. Biol. 2019, 29, 3909–3912. [Google Scholar] [CrossRef]
- Galaska, M.P.; Sands, C.J.; Santos, S.R.; Mahon, A.R.; Halanych, K.M. Geographic Structure in the Southern Ocean Circumpolar Brittle Star Ophionotus Victoriae (Ophiuridae) Revealed from MtDNA and Single-Nucleotide Polymorphism Data. Ecol. Evol. 2017, 7, 475–485. [Google Scholar] [CrossRef]
- O’Hara, T.D.; Hugall, A.F.; Woolley, S.N.C.; Bribiesca-contreras, G.; Bax, N.J. Contrasting processes drive ophiuroid phylodiversity across shallow and deep seafloors. Nature 2019, 565, 636–639. [Google Scholar] [CrossRef]
- Hugall, A.F.; O’Hara, T.D.; Hunjan, S.; Nilsen, R.; Moussalli, A. An Exon-Capture System for the Entire Class Ophiuroidea. Mol. Biol. Evol. 2015, 33, 281–294. [Google Scholar] [CrossRef]
- Jossart, Q.; Sands, C.J.; Sewell, M.A. Dwarf Brooder versus Giant Broadcaster: Combining Genetic and Reproductive Data to Unravel Cryptic Diversity in an Antarctic Brittle Star. Heredity 2019, 123, 622–633. [Google Scholar] [CrossRef]
- Khodami, S.; Martinez Arbizu, P.; Stöhr, S. Molecular Species Delimitation of Icelandic Brittle Stars (Echinodermata: Ophiuroidea). Pol. Polar Res. 2014, 35, 243–260. [Google Scholar] [CrossRef]
- O’Hara, T.D.; Hugall, A.F.; Thuy, B.; Moussalli, A. Phylogenomic Resolution of the Class Ophiuroidea Unlocks a Global Microfossil Record. Curr. Biol. 2014, 24, 1874–1879. [Google Scholar] [CrossRef]
- Woolley, S.N.C.; Foster, S.D.; O’Hara, T.D.; Wintle, B.A.; Dunstan, P.K. Characterising Uncertainty in Generalised Dissimilarity Models. Methods Ecol. Evol. 2017, 8, 985–995. [Google Scholar] [CrossRef]
- Ward, R.D.; Holmes, B.H.; O’Hara, T.D. DNA Barcoding Discriminates Echinoderm Species. Mol. Ecol. 2008, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Boissin, E.; Hoareau, T.B.; Paulay, G.; Bruggemann, J.H. DNA Barcoding of Reef Brittle Stars (Ophiuroidea, Echinodermata) from the Southwestern Indian Ocean Evolutionary Hot Spot of Biodiversity. Ecol. Evol. 2017, 7, 11197–11203. [Google Scholar] [CrossRef]
- McEdward, L.R.; Miner, B.G. Larval and Life-Cycle Patterns in Echinoderms. Can. J. Zool. 2001, 79, 1125–1170. [Google Scholar] [CrossRef]
- Schoener, A. Fecundity and Possible Mode of Development of Some Deep-Sea Ophiuroids. Limnol. Ocenaography 1972, 17, 193–199. [Google Scholar] [CrossRef]
- Tyler, P.A. Reproductive Patterns in Deep Sea Ophiuroids from the North East Atlantic. Proc. Th Eur. Colloq. Echinoderms 1979, 417–420. [Google Scholar]
- Stöhr, S. Who’s Who among Baby Brittle Stars (Echinodermata: Ophiuroidea): Postmetamorphic Development of Some North Atlantic Forms. Zool. J. Linn. Soc. 2005, 143, 543–576. [Google Scholar] [CrossRef]
- McClain, C.R.; Hardy, S.M. The Dynamics of Biogeographic Ranges in the Deep Sea. Proc. R. Soc. B Biol. Sci. 2010, 277, 3533–3546. [Google Scholar] [CrossRef]
- Schoener, A. Post-Larval Development of Five Deep-Sea Ophiuroids. Deep Res. 1967, 14, 645–660. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. B-Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Layton, K.K.S.; Corstorphine, E.A.; Hebert, P.D.N. Exploring Canadian Echinoderm Diversity through DNA Barcodes. PLoS ONE 2016, 11, e0166118. [Google Scholar] [CrossRef]
- Puillandre, N.; Modica, M.V.; Gustave, O.; Place, L.L.; West, C.P. Large-Scale Species Delimitation Method for Hyperdiverse Groups. Mol. Ecol. 2012, 21, 2671–2691. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for Primary Species Delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Meiklejohn, K.A.; Wallman, J.F.; Dowton, M. DNA Barcoding Identifies All Immature Life Stages of a Forensically Important Flesh Fly (Diptera: Sarcophagidae). J. Forensic Sci. 2013, 58, 184–187. [Google Scholar] [CrossRef]
- Bribiesca-Contreras, G.; Solis-Martin, F.A.; Laguarda-Figueras, A.; Zaldivar-Riveron, A. Identification of Echinoderms (Echinodermata) from an Anchialine Cave in Cozumel Island, Mexico, Using DNA Barcodes. Mol. Ecol. Resour. 2013, 13, 1137–1145. [Google Scholar] [CrossRef]
- Moreau, C.; Danis, B.; Jossart, Q.; Eléaume, M.; Sands, C.; Achaz, G.; Agüera, A.; Saucède, T. Is Reproductive Strategy a Key Factor in Understanding the Evolutionary History of Southern Ocean Asteroidea (Echinodermata)? Ecol. Evol. 2019, 9, 8465–8478. [Google Scholar] [CrossRef]
- Christodoulou, M.; O’Hara, T.D.; Hugall, A.; Khodami, S.; Rodrigues, C.F.; Hilario, A.; Vink, A.; Martinez Arbizu, P. Unexpected High Abyssal Ophiuroid Diversity in Polymetallic Nodule Fields of the Northeast Pacific Ocean, and Implications for Conservation. Biogeosci. Discuss. 2020, 17, 1845–1876. [Google Scholar] [CrossRef]
- Mortensen, T. Handbook of the Echinoderms of the British Isles; Backhuys: Rotterdam, The Netherlands, 1927; ISBN 9788578110796. [Google Scholar]
- Paterson, G.L.J.J. The Deep-Sea Ophiuroidea of the North Atlantic Ocean. Bull. Br. Mus. (Nat. Hist. Zool.) 1985, 49, 1–162. [Google Scholar]
- Estoup, A.; Largiader, C.R.; Perrot, E.; Chourrout, D. Rapid One-Tube DNA Extraction for Reliable PCR Detection of Fish Polymorphic Markers and Transgenes. Mol. Mar. Biol. Biotechnol. 1996, 5, 295–298. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Pons, J.; Barraclough, T.G.; Gomez-zurita, J.; Cardoso, A. Sequence-Based Species Delimitation for the DNA Taxonomy of Undescribed Insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef]
- Monaghan, M.T.; Wild, R.; Elliot, M.; Fujisawa, T.; Balke, M.; Inward, D.J.G.; Lees, D.C.; Ranaivosolo, R.; Eggleton, P.; Barraclough, T.G.; et al. Accelerated Species Inventory on Madagascar Using Coalescent-Based Models of Species Delineation. Syst. Biol. 2009, 58, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Durden, J.M.; Bett, B.J.; Schoening, T.; Morris, K.J.; Nattkemper, T.W.; Ruhl, H.A. Comparison of Image Annotation Data Generated by Multiple Investigators for Benthic Ecology. Mar. Ecol. Prog. Ser. 2016, 552, 61–70. [Google Scholar] [CrossRef]
- Langenkämper, D.; Zurowietz, M.; Schoening, T.; Nattkemper, T.W. BIIGLE 2.0—Browsing and Annotating Large Marine Image Collections. Front. Mar. Sci. 2017, 4, 83. [Google Scholar] [CrossRef]
- Smirnov, I.S.; Piepenburg, D.; Ahearn, C.; Juterzenka, K.V. Deep-Sea Fauna of European Seas: An Annotated Species Check-List of Benthic Invertebrates Living Deeper than 2000 m in the Seas Bordering Europe. Ophiuroidea. Invertebr. Zool. 2014, 11, 192–209. [Google Scholar] [CrossRef]
- Pearson, M.; Gage, J.D. Diets of Some Deep-Sea Brittle Stars in the Rockall Trough. Mar. Biol. 1984, 82, 247–258. [Google Scholar] [CrossRef]
- Tyler, P.A.; Emson, R.H.; Sumida, P.; Howell, K. Ophiuroid Distribution at Sublittoral and Bathyal Depths Round the Faroe Islands, NE Atlantic Ocean. Ann. Soc. Sci. Faroensis. Suppl. 2005, 41, 175–194. [Google Scholar]
- Amon, D.J.; Ziegler, A.F.; Dahlgren, T.G.; Glover, A.G.; Goineau, A.; Gooday, A.J.; Wiklund, H.; Smith, C.R. Insights into the Abundance and Diversity of Abyssal Megafauna in a Polymetallic-Nodule Region in the Eastern Clarion-Clipperton Zone. Sci. Rep. 2016, 6, 30492. [Google Scholar] [CrossRef]
- De Leo, F.C.; Smith, C.R.; Rowden, A.A.; Bowden, D.A.; Clark, M.R. Submarine Canyons: Hotspots of Benthic Biomass and Productivity in the Deep Sea. Proc. R. Soc. B Biol. Sci. 2010, 277, 2783–2792. [Google Scholar] [CrossRef]
- Hardy, S.M.; Smith, C.R.; Thurnherr, A.M. Can the Source-Sink Hypothesis Explain Macrofaunal Abundance Patterns in the Abyss? A Modelling Test. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150193. [Google Scholar] [CrossRef]
- Stöhr, S.; Segonzac, M. Deep-Sea Ophiuroids (Echinodermata) from Reducing and Non-Reducing Environments in the North Atlantic Ocean. J. Mar. Biol. Assoc. UK 2005, 85, 383–402. [Google Scholar] [CrossRef]
- Woolley, S.N.C.; Derek, P.; Dunstan, P.K.; Guillera-Arroita, G.; Lahoz_Monfort, J.J.; O’Hara, T.D. Deep-Sea Diversity Patterns Are Shaped by Energy Availability. Nature 2016, 533, 393–396. [Google Scholar] [CrossRef]
- Zeppilli, D.; Pusceddu, A.; Trincardi, F.; Danovaro, R. Seafloor Heterogeneity Influences the Biodiversity—Ecosystem Functioning Relationships in the Deep Sea. Nat. Publ. Gr. 2016, 6, 26352. [Google Scholar] [CrossRef]
- Okanishi, M.; Olbers, J.M.; Fujita, T. A Taxonomic Review of the Genus Asteromorpha Lütken (Echinodermata: Ophiuroidea: Euryalidae). Raffles Bull. Zool. 2013, 61, 461–480. [Google Scholar] [CrossRef]
- Schönberg, C.H.L.; Hosie, A.M.; Fromont, J.; Marsh, L.; O’Hara, T.D. Apartment-Style Living on a Kebab Sponge. Mar. Biodivers. 2016, 46, 331–332. [Google Scholar] [CrossRef]
- O’Hara, T.D.; Rowden, A.A.; Williams, A. Cold-Water Coral Habitats on Seamounts: Do They Have a Specialist Fauna? Divers. Distrib. 2008, 14, 925–934. [Google Scholar] [CrossRef]
- Martynov, A.V.; Litvinova, N.M. Deep-Water Ophiuroidea of the Northern Atlantic with Descriptions of Three New Species and Taxonomic Remarks on Certain Genera and Species. Mar. Biol. Res. 2008, 4, 76–111. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Paterson, G.L.J.; Cabrinovic, A.; Cunha, M.R. Deep-Sea Ophiuroids (Echinodermata: Ophiuroidea: Ophiurida) from the Gulf of Cadiz (NE Atlantic). Zootaxa 2011, 26, 1–26. [Google Scholar] [CrossRef]
- Stöhr, S.; O’Hara, T.D. Deep-Sea Ophiuroidea (Echinodermata) from the Danish Galathea II Expedition, 1950–1952, with Taxonomic Revisions. Zootaxa 2021, 4963, 505–529. [Google Scholar] [CrossRef]
- Hunter, R.L.; Halanych, K.M. Evaluating Connectivity in the Brooding Brittle Star Astrotoma agassizii across the Drake Passage in the Southern Ocean. J. Hered. 2008, 99, 137–148. [Google Scholar] [CrossRef]
- Galaska, M.P.; Sands, C.J.; Santos, S.R.; Mahon, A.R.; Halanych, K.M. Crossing the Divide: Admixture across the Antarctic Polar Front Revealed by the Brittle Star Astrotoma agassizii. Biol. Bull. 2017, 232, 198–211. [Google Scholar] [CrossRef]
- Thurber, A.R.; Sweetman, A.K.; Narayanaswamy, B.E.; Jones, D.O.B.; Ingels, J.; Hansman, R.L. Ecosystem Function and Services Provided by the Deep Sea. Biogeosciences 2014, 11, 3941–3963. [Google Scholar] [CrossRef]
- Pérez-Portela, R.; Arranz, V.; Rius, M.; Turon, X. Cryptic Speciation or Global Spread? The Case of a Cosmopolitan Marine Invertebrate with Limited Dispersal Capabilities. Sci. Rep. 2013, 3, 3197. [Google Scholar] [CrossRef]
- Schwentner, M.; Lörz, A.N. Population Genetics of Cold-Water Coral Associated Pleustidae (Crustacea, Amphipoda) Reveals Cryptic Diversity and Recent Expansion off Iceland. Mar. Ecol. 2021, 42, e12625. [Google Scholar] [CrossRef]
- Sponer, R.; Deheyn, D.; Roy, M.S. Large Genetic Distances within a Population of Amphipholis squamata (Echinodermata; Ophiuroidea) Do Not Support Colour Varieties as Sibling Species. Mar. Ecol. Prog. Ser. 2001, 219, 169–175. [Google Scholar] [CrossRef][Green Version]
- Laming, S.R.; Christodoulou, M.; Martinez Arbizu, P.; Hilário, A. Comparative Reproductive Biology of Deep-Sea Ophiuroids Inhabiting Polymetallic-Nodule Fields in the Clarion-Clipperton Fracture Zone. Front. Mar. Sci. 2021, 8, 663798. [Google Scholar] [CrossRef]
- Gebruk, A.V.; Kremenetskaia, A.; Rouse, G.W. A Group of Species “Psychropotes longicauda” (Psychropotidae, Elasipodida, Holothuroidea) from the Kuril-Kamchatka Trench Area (North-West Pacific). Prog. Oceanogr. 2020, 180, 102222. [Google Scholar] [CrossRef]
- Gooday, A.J.; Holzmann, M.; Goineau, A.; Pearce, R.B.; Voltski, I.; Weber, A.A.T.; Pawlowski, J. Five New Species and Two New Genera of Xenophyophores (Foraminifera: Rhizaria) from Part of the Abyssal Equatorial Pacific Licensed for Polymetallic Nodule Exploration. Zool. J. Linn. Soc. 2018, 183, 723–748. [Google Scholar] [CrossRef]
- Lim, S.C.; Wiklund, H.; Glover, A.G.; Dahlgren, T.G.; Tan, K.S. A New Genus and Species of Abyssal Sponge Commonly Encrusting Polymetallic Nodules in the Clarion-Clipperton Zone, East Pacific Ocean. Syst. Biodivers. 2017, 15, 507–519. [Google Scholar] [CrossRef]
- Taboada, S.; Riesgo, A.; Wiklund, H.; Paterson, G.L.J.; Koutsouveli, V.; Santodomingo, N.; Dale, A.C.; Smith, C.R.; Jones, D.O.B.; Dahlgren, T.G.; et al. Implications of Population Connectivity Studies for the Design of Marine Protected Areas in the Deep Sea: An Example of a Demosponge from the Clarion-Clipperton Zone. Mol. Ecol. 2018, 27, 4657–4679. [Google Scholar] [CrossRef]
- Halanych, K.M.; Mahon, A.R. Challenging Dogma Concerning Biogeographic Patterns of Antarctica and the Southern Ocean. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 355–378. [Google Scholar] [CrossRef]
- Hajibabaei, M.; Singer, G.A.C.; Hebert, P.D.N.; Hickey, D.A. DNA Barcoding: How It Complements Taxonomy, Molecular Phylogenetics and Population Genetics. Trends Genet. 2007, 23, 167–172. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eichsteller, A.; Taylor, J.; Stöhr, S.; Brix, S.; Martìnez Arbizu, P. DNA Barcoding of Cold-Water Coral-Associated Ophiuroid Fauna from the North Atlantic. Diversity 2022, 14, 358. https://doi.org/10.3390/d14050358
Eichsteller A, Taylor J, Stöhr S, Brix S, Martìnez Arbizu P. DNA Barcoding of Cold-Water Coral-Associated Ophiuroid Fauna from the North Atlantic. Diversity. 2022; 14(5):358. https://doi.org/10.3390/d14050358
Chicago/Turabian StyleEichsteller, Angelina, James Taylor, Sabine Stöhr, Saskia Brix, and Pedro Martìnez Arbizu. 2022. "DNA Barcoding of Cold-Water Coral-Associated Ophiuroid Fauna from the North Atlantic" Diversity 14, no. 5: 358. https://doi.org/10.3390/d14050358
APA StyleEichsteller, A., Taylor, J., Stöhr, S., Brix, S., & Martìnez Arbizu, P. (2022). DNA Barcoding of Cold-Water Coral-Associated Ophiuroid Fauna from the North Atlantic. Diversity, 14(5), 358. https://doi.org/10.3390/d14050358






