Richness of Arbuscular Mycorrhizal Fungi in a Brazilian Tropical Shallow Lake: Assessing an Unexpected Assembly in the Aquatic-Terrestrial Gradient
Abstract
1. Introduction
2. Materials and Methods
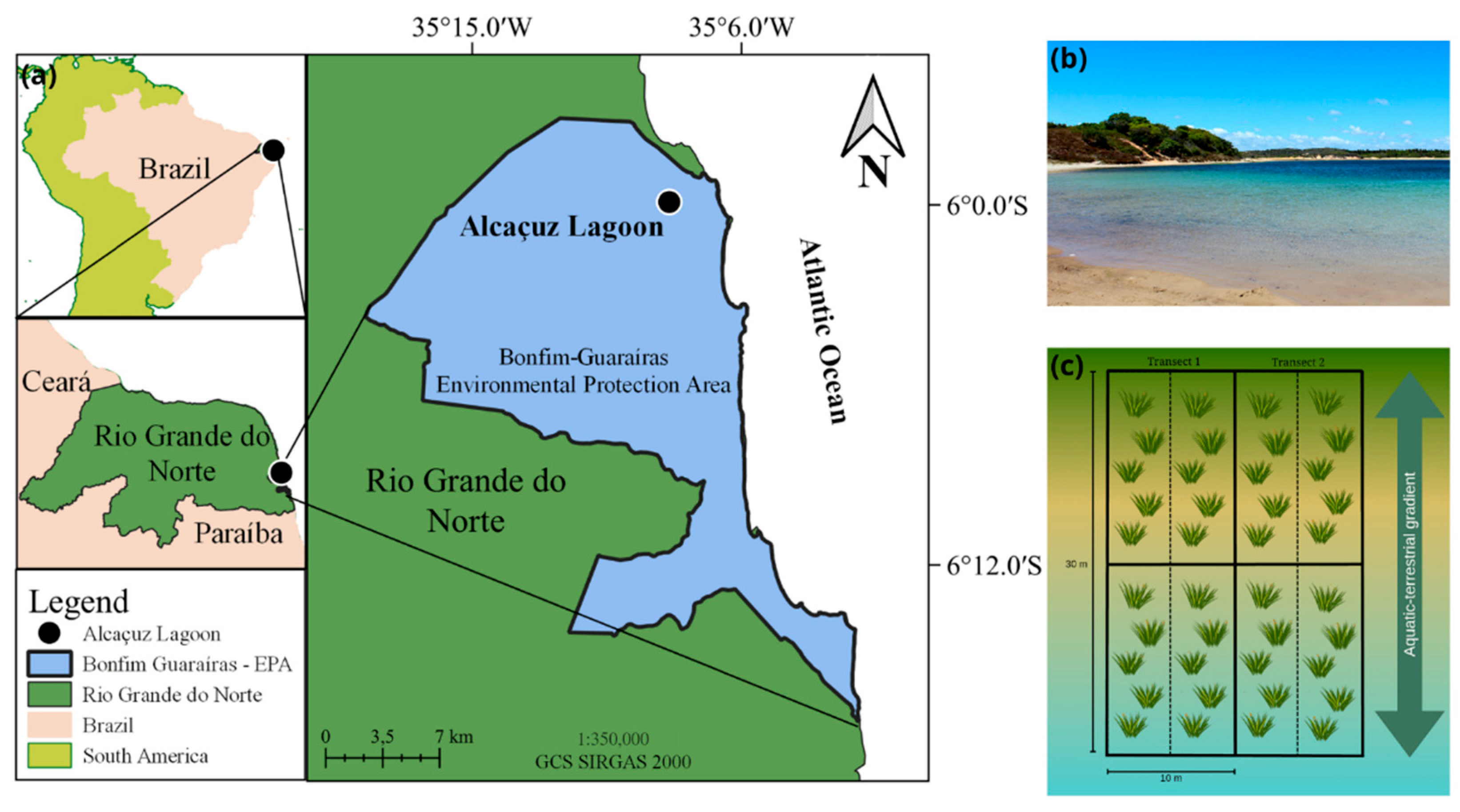
2.1. Study Area
2.2. Sediment Collection and Sampling
2.3. Extraction and Identification of Glomerospores
2.4. Ecological and Statistical Analysis of AMF Communities
3. Results
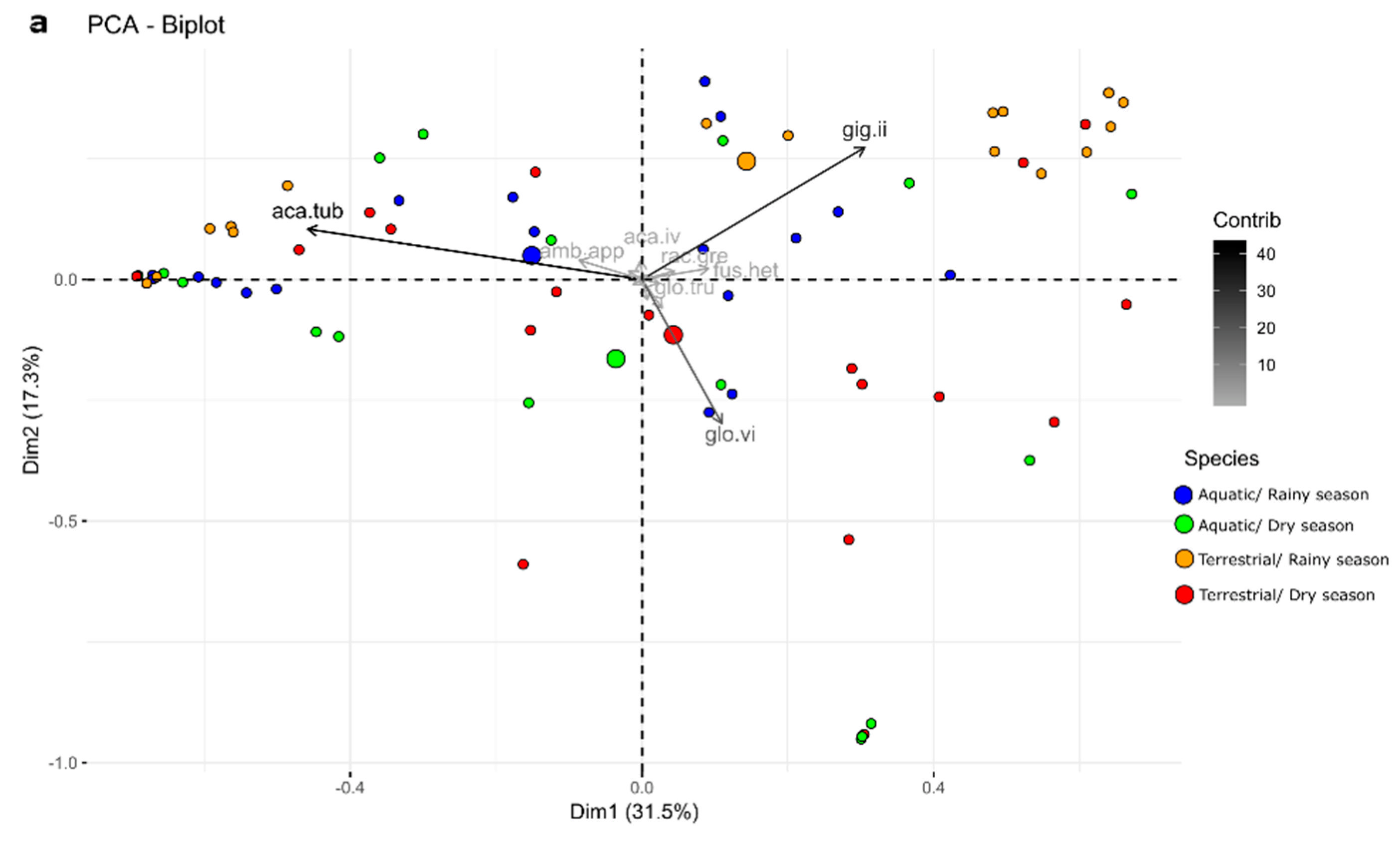
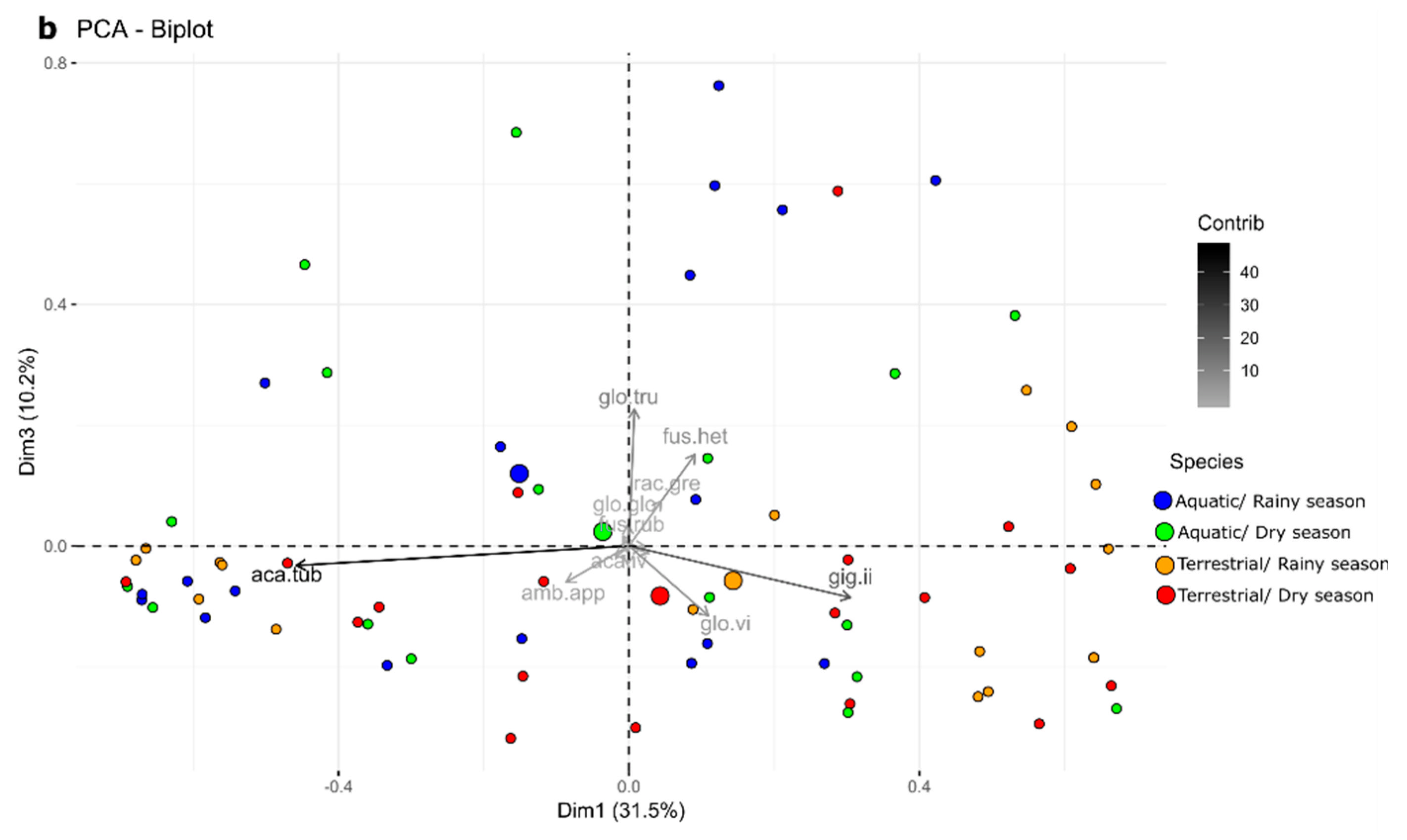
3.1. AMF Communities
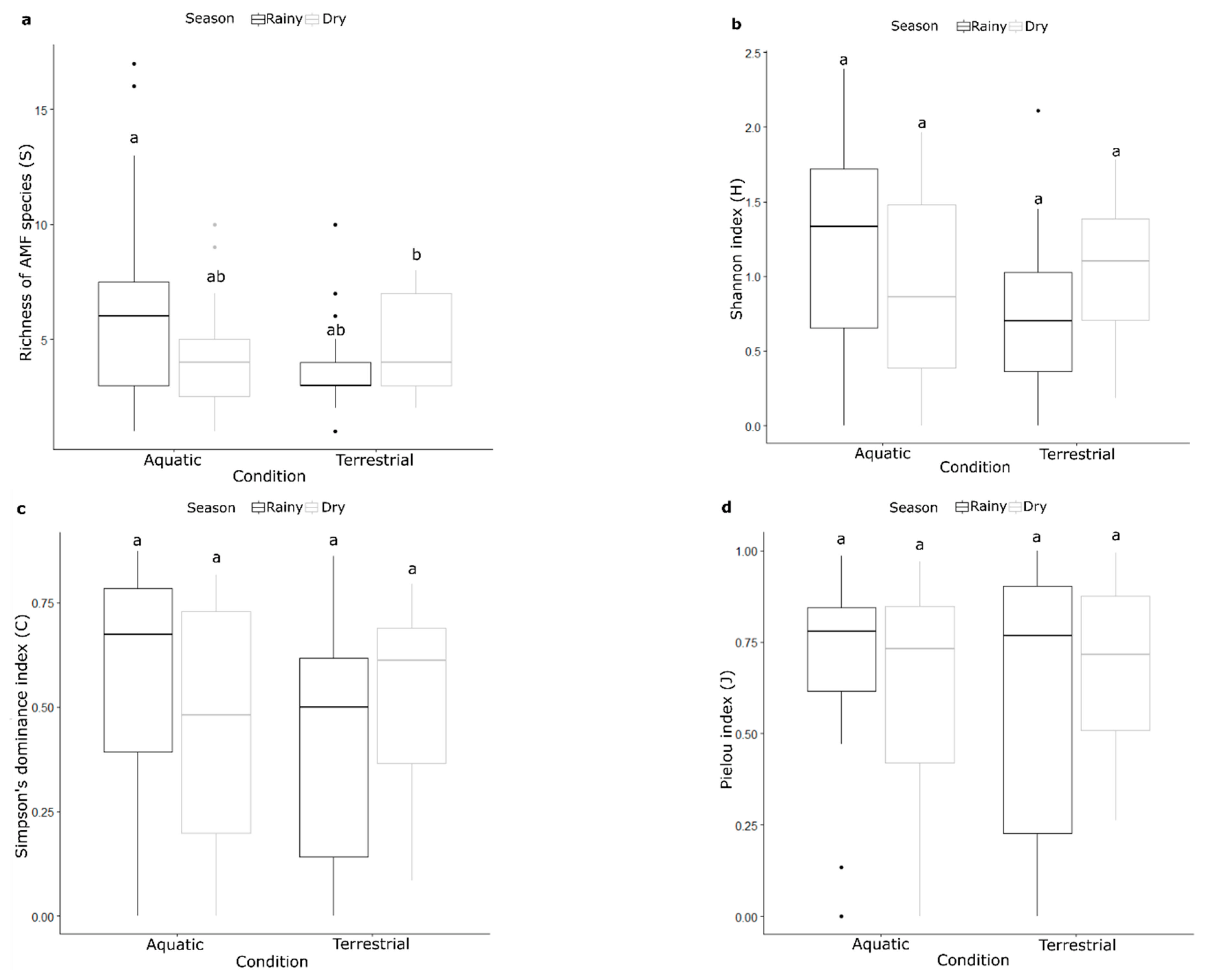
3.2. Ecological Indices
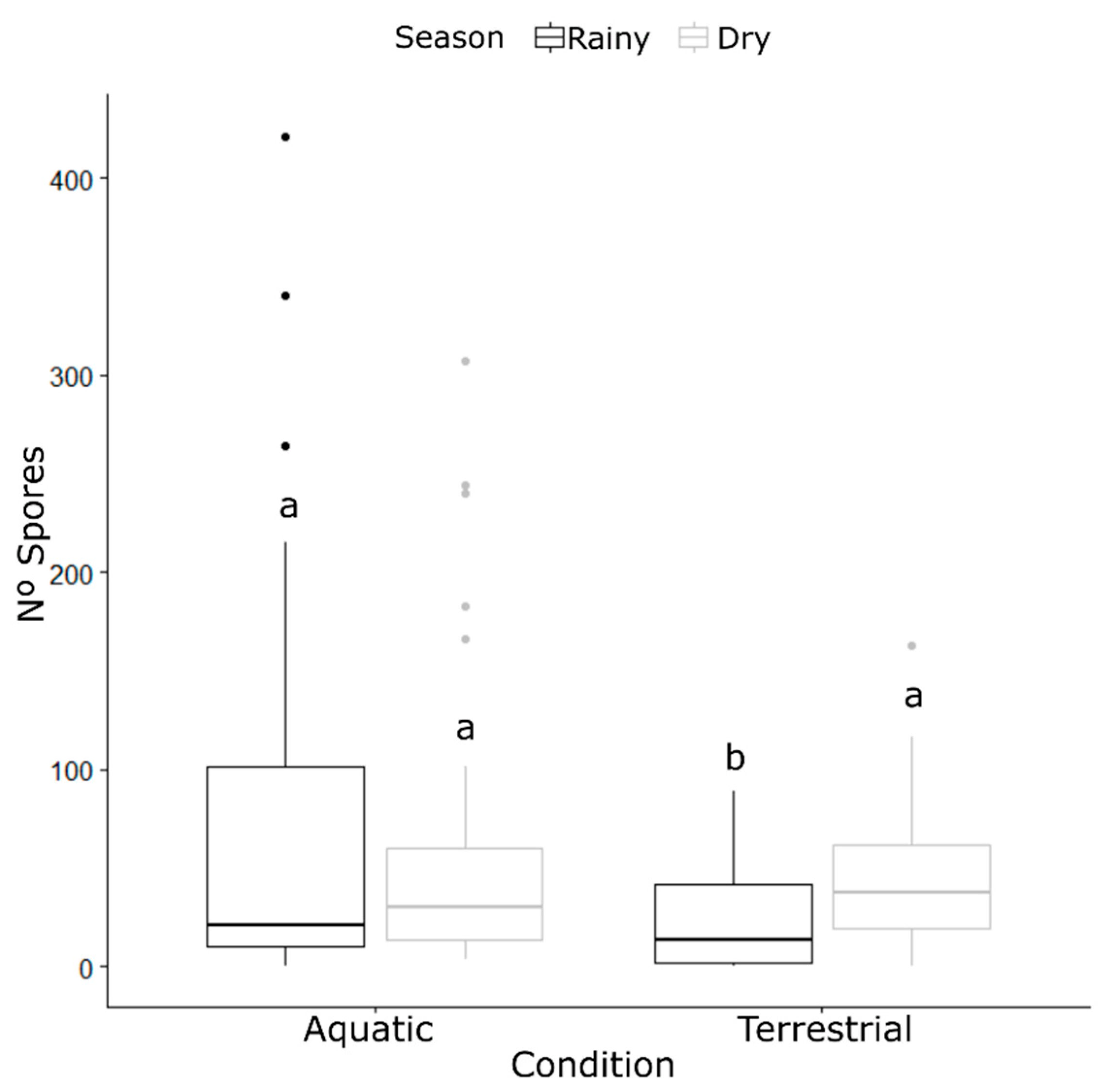
3.3. Glomerospore Number
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Döring, M.; Schigel, D.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Haelewaters, D.; Rajeshkumar, K.C.; Zhao, R.L.; Aptroot, A.; Leontyev, V.; Saxena, D.R.K.; et al. Outline of Fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa-2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Schüßler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- van Creij, J.; Wang, P.; Limpens, E. Arbuscular mycorrhiza, a fungal perspective. In Molecular Aspects of Plant Beneficial Microbes in Agriculture, 1st ed.; Sharma, V., Salwan, R., Tawfeeq Al-Ani, L.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 241–258. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Querejeta, J.I. Soil water retention and availability as influenced by mycorrhizal symbiosis: Consequences for individual plants, communities, and ecosystems. In Mycorrhizal Mediation of Soil; Johnson, N.C., Jansa, J., Gehring, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 299–317. [Google Scholar] [CrossRef]
- Ryszka, P.; Zarzyka-Ryszka, M.; Anielska, T.; Choczyński, M.; Turnau, K. Arbuscular mycorrhizal fungi from petroleum-impacted sites in the Polish Carpathians. Int. Biodeterior. Biodegrad. 2019, 138, 50–56. [Google Scholar] [CrossRef]
- Vilela, L.A.F.; Barbosa, M.V. Contribution of arbuscular mycorrhizal fungi in promoting cadmium tolerance in plants. In Cadmium Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Nahar, K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 553–586. [Google Scholar] [CrossRef]
- Goto, B.T.; Jobim, K. Laboratório de Biologia de Micorrizas. Available online: http://glomeromycota.wixsite.com/lbmicorrizas (accessed on 19 September 2022).
- Öpik, M.; Zobel, M.; Cantero, J.J.; Davison, J.; Facelli, J.M.; Hiiesalu, I.; Jairus, T.; Kalwij, J.M.; Koorem, K.; Leal, M.E.; et al. Global sampling of plant roots expands the described molecular diversity of arbuscular mycorrhizal fungi. Mycorrhiza 2013, 23, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Li, S.; Rosendahl, S. Ignored diversity of arbuscular mycorrhizal fungi in co-occurring mycotrophic and non-mycotrophic plants. Mycorrhiza 2021, 31, 93–102. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Zubek, S.; Meller, E.; Milczarski, P.; Malinowski, R.; Malicka, M.; Uszok, S.; Goto, B.T.; Bierza, W.; et al. Three new species of arbuscular mycorrhizal fungi of the genus Diversispora from maritime dunes of Poland. Mycologia 2022, 114, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Davison, J.; Moora, M.; Öpik, M.; Adholeya, A.; Ainsaar, L.; Bâ, A.; Burla, S.; Diedhiou, A.G.; Hiiesalu, I.; Jairus, T.; et al. Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 2015, 349, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, Q.; Koide, R.T.; Peng, Z.; Zhou, J.; Gu, X.; Yu, M. Arbuscular mycorrhizal fungal mediation of plant-plant interactions in a marshland plant community. Sci. World J. 2014, 2014, 923610. [Google Scholar] [CrossRef]
- Fusconi, A.; Mucciarelli, M. How important is arbuscular mycorrhizal colonization in wetland and aquatic habitats? Environ. Exp. Bot. 2018, 155, 128–141. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Jiang, Y.; Zhang, X.; Li, J.; Ban, Y. Arbuscular mycorrhizal fungi in two vertical-flow wetlands constructed for heavy metal-contaminated wastewater bioremediation. Environ. Sci. Pollut. Res. 2018, 25, 12830–12840. [Google Scholar] [CrossRef]
- Queiroz, M.B.D.; Jobim, K.; Vista, X.M.; Leroy, J.A.S.; Gomes, S.R.B.S.; Goto, B.T. Occurrence of Glomeromycota species in aquatic habitats: A global overview. Mycotaxon 2020, 135, 469. [Google Scholar] [CrossRef]
- Sudová, R.; Sýkorová, Z.; Rydlová, J.; Čtvrtlíková, M.; Oehl, F. Rhizoglomus melanum, a new arbuscular mycorrhizal fungal species associated with submerged plants in freshwater lake Avsjøen in Norway. Mycol. Prog. 2015, 14, 1–8. [Google Scholar] [CrossRef]
- Moora, M.; Öpik, M.; Davison, J.; Jairus, T.; Vasar, M.; Zobel, M.; Eckstein, R.L. AM fungal communities inhabiting the roots of submerged aquatic plant Lobelia dortmanna are diverse and include a high proportion of novel taxa. Mycorrhiza 2016, 26, 735–745. [Google Scholar] [CrossRef]
- Kjerfve, B. Coastal lagoons. In Elsevier Oceanography Series, 1st ed.; Kjerfve, B., Ed.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 60, pp. 1–8. [Google Scholar]
- Drake, D.C.; Kelly, D.; Schallenberg, M. Shallow coastal lakes in New Zealand: Current conditions, catchment-scale human disturbance, and determination of ecological integrity. Hydrobiologia 2010, 658, 87–101. [Google Scholar] [CrossRef]
- Ávila, I.G.; Tavares, M.H.; Chalegres, C.L.B.; Munar, A.M.; Fragoso, C.R.; da Motta-Marques, D.; Ruhoff, A. Southern coastal subtropical shallow lakes skin temperature driven by climatic and non-climatic factors. Environ. Monit. Assess. 2021, 193, 170. [Google Scholar] [CrossRef]
- Esteves, F.D.A.; Caliman, A.; Santangelo, J.M.; Guariento, R.D.; Farjalla, V.F.; Bozelli, R.L. Neotropical coastal lagoons: An appraisal of their biodiversity, functioning, threats and conservation management. Braz. J. Biol. 2008, 68, 967–981. [Google Scholar] [CrossRef] [PubMed]
- De Wit, R. Biodiversity of coastal lagoon ecosystems and their vulnerability to global change. In Ecosystems Biodiversity; Grillo, O., Venora, G., Eds.; InTech: Rijeka, Croatia, 2011; pp. 29–40. [Google Scholar] [CrossRef]
- Santos, W.A.A. Uso do Sensoriamento Remoto multiespectral para determinação da variação de lâminas d’água das lagoas no município de Nísia Floresta, Rio Grande do Norte, Brasil. Revista Brasileira Geografia Física 2013, 6, 1635–1647. [Google Scholar]
- de Lacerda, L.D.; De Araujo, D.S.D.; Maciel, N.C. Dry coastal ecosystems of the tropical Brazilian coast. In Ecosystems of the World 2B: Dry Coastal: Africa, America, Asia and Oceania, 1st ed.; van der Maarel, E., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; p. 477. [Google Scholar]
- Zickel, C.S.; Vicente, A.; Almeida, E.B., Jr.; Cantarelli, J.R.R.; Sacramento, A.C. Flora e vegetação das restingas no Nordeste Brasileiro. In Oceanografia: Um Cenário Tropical, 21st ed.; Eskinazi-Leça, E., Neumann-Leitão, S., Costa, M.F., Eds.; UFPE: Recife, Brasil, 2004; pp. 689–701. [Google Scholar]
- Silva, D.K.A.; Coutinho, F.P.; Escobar, I.E.C.; de Souza, R.G.; Oehl, F.; Silva, G.A.; Maia, L.C. The community of arbuscular mycorrhizal fungi in natural and revegetated coastal areas (Atlantic Forest) in northeastern Brazil. Biodivers. Conserv. 2015, 24, 2213–2226. [Google Scholar] [CrossRef]
- Rocha, R.M. A restinga como exemplo de ecossistema e a sua urbanização subsídios para possíveis intervenções. Paisagem Ambiente 1994, 6, 57–73. [Google Scholar] [CrossRef][Green Version]
- Scarano, F.R.; Duarte, H.M.; Ribeiro, K.T.; Rodrigues, P.J.F.P.; Barcellos, E.M.B.; Franco, A.C.; Lüttge, U. Four sites with contrasting environmental stress in southeastern Brazil: Relations of species, life form diversity, and geographic distribution to ecophysiological parameters. Bot. J. Linn. Soc. 2001, 136, 345–364. [Google Scholar] [CrossRef]
- Melo-Junior, J.C.F.; Boeguer, M.R.T. Riqueza, estrutura e interações edáficas em um gradiente de restinga do Parque Estadual do Acaraí, Estado de Santa Catarina, Brasil. Hoehnea 2015, 42, 207–232. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Melloni, R.; Caproni, A.L. Micorrizas arbusculares em dunas marítimas e em áreas de mineração. In Micorrizas 30 Anos de Pesquisa no Brasil; Siqueira, J.O., de Souza, F.A., Cardoso, E.J.B.N., Tsai, S.M., Eds.; Editora UFLA: Lavras, Brasil, 2010; pp. 341–360. [Google Scholar]
- Souza, R.G.; Goto, B.T.; Silva, D.K.; Silva, F.S.B.; Sampaio, E.V.S.B.; Maia, L.C. The role of arbuscular mycorrhizal fungi and cattle manure in the stablishment of Tocoyena selloana Schum. in mined dunes areas. Eur. J. Soil Biol. 2010, 46, 237–242. [Google Scholar] [CrossRef]
- Silva, D.K.A.; Pereira, C.M.R.; de Souza, R.G.; da Silva, G.A.; Oehl, F.; Maia, L.C. Diversity of arbuscular mycorrhizal fungi in restinga and dunes areas in Brazilian Northeast. Biodivers. Conserv. 2012, 21, 2361–2373. [Google Scholar] [CrossRef]
- Jobim, K.; Goto, B.T. Diversity of arbuscular mycorrhizal fungi (Glomeromycota) in maritime sand dunes of Brazilian northeast. Stud. Fungi 2016, 1, 43–55. [Google Scholar] [CrossRef]
- Silva, I.R.; da Silva, D.K.A.; de Souza, F.A.; Oehl, F.; Maia, L.C. Changes in arbuscular mycorrhizal fungal communities along a river delta island in northeastern Brazil. Acta Oecol. 2017, 79, 8–17. [Google Scholar] [CrossRef]
- da Silva, F.S.P.; Ignácio, I.G.; Saggin Júnior, O.J.; Patreze, C.M. Arbuscular Mycorrhizal fungal diversity in tropical sand dune and restinga at Peró Beach in Rio de Janeiro state, Brazil. Fungal Ecol. 2019, 40, 150–158. [Google Scholar] [CrossRef]
- Moura-Júnior, E.G.D.; Paiva, R.M.S.D.; Ferreira, A.C.; Pacopahyba, L.D.; Tavares, A.S.; Ferreira, F.A.; Pott, A. Updated checklist of aquatic macrophytes from Northern Brazil. Acta Amazon. 2015, 45, 111–132. [Google Scholar] [CrossRef]
- Flora do Brasil. Jardim Botânico do Rio de Janeiro. Available online: http://floradobrasil.jbrj.gov.br/ (accessed on 10 September 2022).
- Marins, J.F.; Carrenho, R.; Thomaz, S.M. Occurrence and coexistence of arbuscular mycorrhizal fungi and dark septate fungi in aquatic macrophytes in a tropical river–floodplain system. Aquat. Bot. 2009, 91, 13–19. [Google Scholar] [CrossRef]
- Ortiz-Vera, M.P.; Olchanheski, L.R.; da Silva, E.G.; de Lima, F.R.; Martinez, L.R.D.P.R.; Sato, M.I.Z.; Araújo, W.L. Influence of water quality on diversity and composition of fungal communities in a tropical river. Sci. Rep. 2018, 8, 14799. [Google Scholar] [CrossRef]
- Queiroz, M.B.; Leroy, J.A.S.; Gomes, S.R.B.S.; Fiuza, P.O.; Goto, B.T. Arbuscular Mycorrhizal Fungi (Glomeromycota) species inhabiting sediments of lentic and lotic Brazilian ecosystems: Addition of new global records for aquatic condition. Nova Hedwigia 2022, 115, 227–251. [Google Scholar] [CrossRef]
- Ragupathy, S.; Mohankumar, V.; Mahadevan, A. Occurrence of vesicular—Arbuscular mycorrhizae in tropical hydrophytes. Aquat. Bot. 1990, 36, 287–291. [Google Scholar] [CrossRef]
- Kai, W.; Zhiwei, Z. Occurrence of arbuscular mycorrhizas and dark septate endophytes in hydrophytes from lakes and streams in southwest China. Int. Rev. Hydrobiol. 2006, 91, 29–37. [Google Scholar] [CrossRef]
- Baar, J.; Paradi, I.; Lucassen, E.C.; Hudson-Edwards, K.A.; Redecker, D.; Roelofs, J.G.; Smolders, A.J. Molecular analysis of AMF diversity in aquatic macrophytes: A comparison of oligotrophic and utra-oligotrophic lakes. Aquat. Bot. 2011, 94, 53–61. [Google Scholar] [CrossRef]
- da Costa, C.R.G.; Coelho Moura, D.; De Lima Marques, A.; Gomes Da Silva, M.; Maia Linhares, A.C.; De Sousa Silva, S.; Cavalcanti, M.I.P. Biological rhythms of Arbuscular Mycorrhizal Fungi under influence of seasonality in semiarid region in Brazil. Biol. Rhythm Res. 2019, 52, 1432–1437. [Google Scholar] [CrossRef]
- Maitra, P.; Zheng, Y.; Chen, L.; Wang, Y.L.; Ji, N.N.; Lü, P.P.; Guo, L.D. Effect of drought and season on arbuscular mycorrhizal fungi in a subtropical secondary forest. Fungal Ecol. 2019, 41, 107–115. [Google Scholar] [CrossRef]
- Vieira-Junior, W.G.; Moura, J.B.D.; Souza, R.F.D.; Braga, A.P.M.; Matos, D.J.D.C.; Brito, G.H.M.; Dutra e Silva, S. Seasonal variation in mycorrhizal community of different cerrado phytophysiomies. Front. Microbiol. 2020, 11, 576764. [Google Scholar] [CrossRef]
- Leroy, J.A.S. Checklist das Macrófitas Aquáticas do RN com Reforço Amostral e Florística do Grupo na APA Bonfim-Guaraíra. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2015. [Google Scholar]
- IDEMA. Instituto de Desenvolvimento Sustentável e do Meio Ambiente do Rio Grande do Norte. Área de Proteção Ambiental Bonfim-Guaraíra. Available online: http://idema.rn.gov.br (accessed on 19 September 2022).
- Sousa, V.F. Levantamento Florístico e Potencial Ornamental de Plantas da Restinga do Rio Grande do Norte, Brasil: Subsídios para um Paisagismo Sustentável. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2016. [Google Scholar]
- Moreira, S.A.; da Silva, I.R.; Dutra, C.K.T.; Cunha, S.; dos Santos, G.C.; Sobrinho, B.F.N.; Bridi, G. Rota dos Nativos sob a perspectiva do turismo sustentável: Estudo em comunidades de Nísia Floresta (RN). RBEcotur 2021, 14, 254–281. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.D.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Gerdemann, J.W.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Jenkins, W.R.B. A rapid centrifugal-flotation technique for separating nematodes from soil. Plant Dis. Rep. 1964, 48, 692. [Google Scholar]
- Omar, M.B.; Boltland, L.; Heather, W.A. A Permanent mounting medium for fungi. Bull. Br. Mycol. Soc. 1979, 13, 31–32. [Google Scholar] [CrossRef]
- Medeiros, A.S.; Goto, B.T.; Ganade, G. Ecological restoration methods influence the structure of arbuscular mycorrhizal fungal communities in degraded drylands. Pedobiologia 2021, 84, 150690. [Google Scholar] [CrossRef]
- Błaszkowski, J. Glomeromycota; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2012; p. 303. [Google Scholar]
- Błaszkowski, J.; Kozłowska, A.; Niezgoda, P.; Goto, B.T.; Dalpé, Y. A new genus, Oehlia with Oehlia diaphana comb. nov. and an emended description of Rhizoglomus vesiculiferum comb. nov. in the Glomeromycotina. Nova Hedwigia 2018, 107, 501–518. [Google Scholar] [CrossRef]
- Schenck, N.C.; Pérez, Y. Manual for the Identification of VA Mycorrhizal Fungi, 3rd ed.; Synergistic publications: Gainesville, FL, USA, 1990; pp. 1–286. [Google Scholar]
- Oehl, F.; Alves a Silva, G.; Goto, B.T.; Costa Maia, L.; Sieverding, E. Glomeromycota: Two new classes and a new order. Mycotaxon 2011, 116, 365–379. [Google Scholar] [CrossRef]
- Oehl, F.; Silva, G.A.D.; Goto, B.T.; Sieverding, E. Glomeromycota: Three new genera and glomoid species reorganized. Mycotaxon 2011, 116, 75–120. [Google Scholar] [CrossRef]
- Goto, B.T.; Jardim, J.G.; Silva, G.A.D.; Furrazola, E.; Torres-Arias, Y.; Oehl, F. Glomus trufemii (Glomeromycetes), a new sporocarpic species from Brazilian sand dunes. Mycotaxon 2012, 120, 1–9. [Google Scholar] [CrossRef]
- Sieverding, E.; da Silva, G.A.; Berndt, R.; Oehl, F. Rhizoglomus, a new genus of the Glomeraceae. Mycotaxon 2014, 129, 373–386. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Goto, B.T.; Kozłowska, A. Halonatospora gen. nov. with H. pansihalos comb. nov. and Glomus bareae sp. nov.(Glomeromycota; Glomeraceae). Botany 2018, 96, 737–748. [Google Scholar] [CrossRef]
- Turrini, A.; Saran, M.; Giovannetti, M.; Oehl, F. Rhizoglomus venetianum, a new arbuscular mycorrhizal fungal species from a heavy metal-contaminated site, downtown Venice in Italy. Mycol. Prog. 2018, 17, 1213–1224. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Piątek, M.; Magurno, F.; Malicka, M.; Zubek, S.; Goto, B.T. Rhizoglomus dalpeae, R. maiae, and R. silesianum, new species. Mycologia 2019, 111, 965–980. [Google Scholar] [CrossRef]
- Shannon, C.; Weaver, W. The Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collection. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Oksanen, J. Vegan: Community Ecology Package. R Project. 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 5 November 2018).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://www.R-project.org/ (accessed on 16 May 2016).
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. Using car and effects Functions in Other Functions. Using Car Eff. Funct. Other Funct. 2020, 3, 1–5. [Google Scholar]
- Cao, L.J.; Chua, K.S.; Chong, W.K.; Lee, H.P.; Gu, Q.M. A comparison of PCA, KPCA and ICA for dimensionality reduction in support vector machine. Neurocomputing 2003, 55, 321–336. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Package ‘factoextra’: Extract and Visualize the Results of Multivariate Data Analyses. 2017. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 5 November 2018).
- Hervé, M.; Hervé, M.M. Package ‘RVAideMemoire’. 2020. Available online: https://CRAN.R-project.org/package=RVAideMemoire (accessed on 5 November 2018).
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S Software, 4th ed.; Springer: New York, NY, USA, 2002; pp. 1–501. [Google Scholar] [CrossRef]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.8. 2018. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 5 November 2018).
- Maia, L.C.; Passos, J.H.; Silva, J.A.; Oehl, F.; Assis, D.M.A. Species diversity of Glomeromycota in Brazilian biomes. Sydowia 2020, 72, 181–205. [Google Scholar] [CrossRef]
- da Silva, I.R.; de Souza, F.A.; da Silva, D.C.A.; Oehl, F.; Maia, L.C. Patterns of Arbuscular Mycorrhizal Fungal Distribution on Mainland and Island Sandy Coastal Plain Ecosystems in Brazil. Microb. Ecol. 2017, 74, 654–669. [Google Scholar] [CrossRef]
- De Carvalho, F.; de Souza, F.A.; Carrenho, R.; Moreira, F.M.S.; Jesus, E.C.; Fernandes, G.W. The mosaic of habitats in the high-altitude Brazilian rupestrian fields is a hotspot for arbuscular mycorrhizal fungi. Appl. Soil Ecol. 2012, 52, 9–19. [Google Scholar] [CrossRef]
- Pontes, J.S.; Oehl, F.; Marinho, F.; Coyne, D.; da Silva, D.K.A.; Yano-Melo, A.M.; Maia, L.C. Diversity of arbuscular mycorrhizal fungi in Brazil’s Caatinga and experimental agroecosystems. Biotropica 2017, 49, 413–427. [Google Scholar] [CrossRef]
- Koske, R.E.; Gemma, J.N. Arbuscular mycorrhizal fungi in Hawaiian sand dunes: Island of Kaua’i. Pac. Sci. 1996, 50, 36–45. [Google Scholar]
- Beena, K.R.; Raviraja, N.S.; Arun, A.B.; Sridhar, K.R. Diversity of arbuscular mycorrhizal fungi on the coastal sand dunes of the west coast of India. Curr. Sci. 2000, 79, 1459–1466. [Google Scholar]
- Camprubí, A.; Calvet, C.; Cabot, P.; Pitet, M.; Estaún, V. Arbuscular mycorrhizal fungi associated with psammophilic vegetation in Mediterranean coastal sand dunes. Span. J. Agric. Res. 2010, 8, 96–102. [Google Scholar] [CrossRef]
- Blaszkowski, J.; Czerniawska, B. Arbuscular mycorrhizal fungi (Glomeromycota) associated with roots of Ammophila arenaria growing in maritime dunes of Bornholm (Denmark). Acta Soc. Bot. Pol. 2011, 80, 63–76. [Google Scholar] [CrossRef]
- Hanlon, L.M. First Recorded Account of Arbuscular Mycorrhizal Fungi in Sand Dunes in South Eastern Australia: Biogeography and Species Richness. J. Coast. Res. 2021, 37, 280–290. [Google Scholar] [CrossRef]
- Johansen, R.B.; Vestberg, M.; Burns, B.R.; Park, D.; Hooker, J.E.; Johnston, P.R. A coastal sand dune in New Zealand reveals high arbuscular mycorrhizal fungal diversity. Symbiosis 2015, 66, 111–121. [Google Scholar] [CrossRef]
- Bever, J.D.; Morton, J.B.; Antonovics, J.; Schultz, P.A. Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland. J. Ecol. 1996, 84, 71–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, L.D.; Liu, R.J. Survey of arbuscular mycorrhizal fungi in deforested and natural forest land in the subtropical region of Dujiangyan, southwest China. Plant Soil 2004, 261, 257–263. [Google Scholar] [CrossRef]
- Powell, J.R.; Parrent, J.L.; Hart, M.M.; Klironomos, J.N.; Rillig, M.C.; Maherali, H. Phylogenetic trait conservatism and the evolution of functional trade-offs in arbuscular mycorrhizal fungi. Proc. R. Soc. B. 2009, 276, 4237–4245. [Google Scholar] [CrossRef]
- Schneider, J.; Stürmer, S.L.; Guilherme, L.R.G.; de Souza Moreira, F.M.; de Sousa Soares, C.R.F. Arbuscular mycorrhizal fungi in arsenic-contaminated areas in Brazil. J. Hazard. Mater. 2013, 262, 1105–1115. [Google Scholar] [CrossRef]
- Aguilera, P.; Cumming, J.; Oehl, F.; Cornejo, P.; Borie, F. Diversity of arbuscular mycorrhizal fungi in acidic soils and their contribution to aluminum phytotoxicity alleviation. In Aluminum Stress Adaptation in Plants; Panda, S.K., Baluska, F., Eds.; Springer: Cham, Switzerland, 2015; Volume 24, pp. 203–228. [Google Scholar] [CrossRef]
- Polo-Marcial, M.H.; Lara-Pérez, L.A.; Goto, B.T.; Margarito-Vista, X.; Andrade-Torres, A. Glomeromycota in Mexico: A country with very high richness. Sydowia 2021, 74, 33–63. [Google Scholar] [CrossRef]
- Freitas, R.O.; Buscardo, E.; Nagy, L.; Maciel, A.B.S.; Carrenho, R.; Luizão, R.C.C. Arbuscular mycorrhizal fungal communities along a pedo-hydrological gradient in a Central Amazonian terra firme forest. Mycorrhiza 2014, 24, 21–32. [Google Scholar] [CrossRef]
- Souza, T.A.F.D.; Rodriguez-Echeverría, S.; Andrade, L.A.D.; Freitas, H. Arbuscular mycorrhizal fungi in Mimosa tenuiflora (Willd.) Poir from Brazilian semi-arid. Braz. J. Microbiol. 2016, 47, 359–366. [Google Scholar] [CrossRef]
- D’Souza, J.; Rodrigues, B.F. Biodiversity of Arbuscular Mycorrhizal (AM) fungi in mangroves of Goa in West India. J. For. Res. 2013, 24, 515–523. [Google Scholar] [CrossRef]
- Duarte, L.M.; Bertini, S.C.B.; Stürmer, S.L.; Lambais, M.R.; Azevedo, L.C.B. Arbuscular mycorrhizal fungal communities in soils under three phytophysiognomies of the Brazilian Atlantic Forest. Acta Bot. Bras. 2018, 33, 50–60. [Google Scholar] [CrossRef]
- Silva, J.L.A.; Souza, A.F.; Jardim, J.G.; Goto, B.T. Community assembly in harsh environments: The prevalence of ecological drift in the heath vegetation of South America. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Yamato, M.; Yamada, H.; Maeda, T.; Yamamoto, K.; Kusakabe, R.; Orihara, T. Clonal spore populations in sporocarps of arbuscular mycorrhizal fungi. Mycorrhiza 2022, 32, 373–385. [Google Scholar] [CrossRef]
- Nielsen, K.B.; Kjøller, R.; Olsson, P.A.; Schweiger, P.F.; Andersen, F.Ø.; Rosendahl, S. Colonization and molecular diversity of arbuscular mycorrhizal fungi in the aquatic plants Littorella uniflora and Lobelia dortmanna in southern Sweden. Mycol. Res. 2004, 108, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Stürmer, S.L.; Oliveira, L.Z.; Morton, J.B. Gigasporaceae versus Glomeraceae (phylum Glomeromycota): A biogeographic tale of dominance in maritime sand dunes. Fungal Ecol. 2018, 32, 49–56. [Google Scholar] [CrossRef]
- de Souza, F.A.; Dalpé, Y.; Declerck, S.; Providencia, I.E.D.L.; Séjalon-Delmas, N. Life history strategies in Gigasporaceae: Insight from monoxenic culture. In In Vitro Culture of Mycorrhizas; Declerck, S., Fortin, J.A., Strullu, D.G., Eds.; Springer: Heidelberg, Germany, 2005; Volume 4, pp. 73–91. [Google Scholar] [CrossRef]
- Cordoba, A.S.; de Mendonça, M.M.; Stürmer, S.L.; Rygiewicz, P.T. Diversity of arbuscular mycorrhizal fungi along a sand dune stabilization gradient: A case study at Praia da Joaquina, Ilha de Santa Catarina, South Brazil. Mycoscience 2001, 42, 379–387. [Google Scholar] [CrossRef]
- de Assis, D.M.A.; Oehl, F.; Goncalves, C.M.; da Silva, D.K.A.; da Silva, G.A. Community structure of arbuscular mycorrhizal fungi in fluvial and maritime dunes of Brazilian Northeast. Appl. Soil Ecol. 2016, 108, 136–146. [Google Scholar] [CrossRef]
- Hart, M.M.; Reader, R.J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol. 2002, 153, 335–344. [Google Scholar] [CrossRef]
- Souza, R.G.; Silva, D.K.A.; Oliveira, J.R.G.; Goto, B.T.; Silva, F.S.B.; Sampaio, E.V.S.B.; Maia, L.C. Use of mycorrhizal seedlings on recovery of mined dunes in northeastern Brazil. Pedobiologia 2012, 55, 303–309. [Google Scholar] [CrossRef]
- Souza, R.G.; Silva, D.K.A.; Mello, C.M.A.; Goto, B.T.; Silva, F.S.B.; Sampaio, E.V.S.B.; Maia, L.C. Arbuscular Mycorrhizal Fungi in revegetated mined dunes. Land Degrad. Dev. 2013, 24, 147–155. [Google Scholar] [CrossRef]
- Andersen, F.Ø.; Andersen, T. Effects of arbuscular mycorrhizae on biomass and nutrients in the aquatic plant Littorella uniflora. Freshw. Biol. 2006, 51, 1623–1633. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Bao, X.; Björn, L.O.; Li, S.; Olsson, P.A. Response differences of arbuscular mycorrhizal fungi communities in the roots of an aquatic and a semiaquatic species to various flooding regimes. Plant Soil 2016, 403, 361–373. [Google Scholar] [CrossRef]
- Xu, Z.; Lv, Y.; Fang, M.; Liu, J.; Zeng, H.; Ban, Y. Diverse and abundant arbuscular mycorrhizal fungi in ecological floating beds used to treat eutrophic water. Appl. Microbiol. Biotechnol. 2021, 105, 6959–6975. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.N.D.; Oliveira, L.A.D. Seasonal dynamics of arbuscular mycorrhizal fungi in plants of Theobroma grandiflorum Schum and Paullinia cupana Mart. of an agroforestry system in Central Amazonia, Amazonas state, Brazil. Braz. J. Microbiol. 2005, 36, 262–270. [Google Scholar] [CrossRef]
- Guadarrama, P.; Castillo, S.; Ramos-Zapata, J.A.; Hernández-Cuevas, L.V.; Camargo-Ricalde, S.L. Arbuscular mycorrhizal fungal communities in changing environments: The effects of seasonality and anthropogenic disturbance in a seasonal dry forest. Pedobiologia 2014, 57, 87–95. [Google Scholar] [CrossRef]
- Cuenca, G.; Lovera, M. Seasonal variation and distribution at different soil depths of arbuscular mycorrhizal fungi spores in a tropical sclerophyllous shrubland. Botany 2010, 88, 54–64. [Google Scholar] [CrossRef]
- Bouamri, R.; Dalpé, Y.; Serrhini, M.N. Effect of seasonal variation on arbuscular mycorrhizal fungi associated with date palm. Emir. J. Food Agric. 2014, 26, 977–986. [Google Scholar] [CrossRef]
- Bytyçi, P.; Shala-Abazi, A.; Zhushi-Etemi, F.; Bonifazi, G.; Hyseni-Spahiu, M.; Fetoshi, O.; Çadraku, H.; Feka, F.; Millaku, F. The Macrophyte Indices for Rivers to Assess the Ecological Conditions in the Klina River in the Republic of Kosovo. Plants 2022, 11, 1469. [Google Scholar] [CrossRef] [PubMed]
- Beck-Nielsen, D.; Vindbak Madsen, T. Occurrence of vesicular-arbuscular mycorrhiza in aquatic macrophytes from lakes and streams. Aquat. Bot. 2001, 71, 141–148. [Google Scholar] [CrossRef]
- Rickerl, D.H.; Sancho, F.O.; Ananth, S. Vesicular-arbuscular endomycorrhizal colonization of wetland plants. J. Environ. Qual. 1994, 23, 913–916. [Google Scholar] [CrossRef]
- Bohrer, K.E.; Friese, C.F.; Amon, J.P. Seasonal dynamics of arbuscular mycorrhizal fungi in differing wetland habitats. Mycorrhiza 2004, 14, 329–337. [Google Scholar] [CrossRef]
- Dolinar, N.; Šraj, N.; Pongrac, P.; Regvar, M.; Gaberščik, A. The presence of mycorrhiza in different habitats of an intermittent aquatic ecosystem. In Water and Nutrient Management in Natural and Constructed Wetlands; Vymazal, J., Ed.; Springer: Dordrecht, The Netherlands, 2010; Volume 1, pp. 299–308. [Google Scholar] [CrossRef]
- Fabián, D.; Guadarrama, P.; Hernadez-Cuevas, L.; Ramos-Zapata, J.A. Arbuscular mycorrhizal fungi in a coastal wetland in Yucatan, Mexico. Bot. Sci. 2018, 96, 24–34. [Google Scholar] [CrossRef]
- Sidhoum, W.; Bahi, K.; Fortas, Z. The effect of salinity gradient and heavy metal pollution on arbuscular mycorrhizal fungal community structure in some Algerian wetlands. Acta Bot. Croat. 2020, 79, 3–14. [Google Scholar] [CrossRef]
- Wirsel, S.G.S. Homogenous stands of a wetland grass harbour diverse consortia of arbuscular mycorrhizal fungi. FEMS Microbiol. Ecol. 2004, 48, 129–138. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Meller, E.; Milczarski, P.; Zubek, S.; Malicka, M.; Uszok, S.; Casieri, L.; Goto, B.T.; Magurno, F. New taxa in Glomeromycota: Polonosporaceae fam. nov., Polonospora gen. nov., and P. polonica comb. nov. Mycol. Progress 2021, 20, 941–951. [Google Scholar] [CrossRef]

| Families/Species | RA (A/R) | RA (A/D) | RA (T/R) | RA (T/D) | FO (A/R) | FO (A/D) | FO (T/R) | FO (T/D) |
|---|---|---|---|---|---|---|---|---|
| Ambisporaceae | ||||||||
| Ambispora appendicula (Spain, Sieverd., N.C. Schenck) C. Walker | 6.86 | 3.52 | 2.21 | 14.97 | 65 | 21.05 | 40 | 45 |
| Ambispora gerdemannii (S.L. Rose, B.A. Daniels & Trappe) C. Walker, Vestberg & A. Schüssler | 0.71 | - | 0.4 | - | 15 | - | 5 | - |
| Ambispora sp. | 1.78 | 0.23 | - | 0.94 | 25 | 5.26 | - | 10 |
| Acaulosporaceae | ||||||||
| Acaulospora denticulata Sieverd. & S. Toro | 0.12 | - | - | - | 5 | - | - | - |
| Acaulospora foveata Trappe & Janos | 0.24 | 0.23 | - | - | 5 | 5.26 | - | - |
| Acaulospora herrerae Furrazola, B.T. Goto, G.A. Silva, Sieverd. & Oehl | - | - | - | 0.13 | - | - | - | 5 |
| Acaulospora ignota Błaszk., Góralska, Chwat & B.T. Goto | - | 0.23 | - | - | - | 5.26 | - | - |
| Acaulospora morrowiae Spain & N.C. Schenck | 1.07 | 0.23 | - | - | 45 | 5.26 | - | - |
| Acaulospora spinosa C. Walker & Trappe | 0.24 | - | - | - | 10 | - | - | - |
| Acaulospora spinulifera Oehl, V.M. Santos, J.S. Pontes & G.A. Silva | - | 0.23 | - | - | - | 5.26 | - | - |
| Acaulospora tuberculata Janos & Trappe | 17.63 | 30.05 | 67.67 | 17.51 | 75 | 63.16 | 40 | 55 |
| Acaulospora cf. colossica | - | 0.47 | 0.2 | - | - | 10.53 | 5 | - |
| Acaulospora cf. cavernata | 0.59 | 0.23 | - | 4.14 | 15 | 5.26 | - | 25 |
| Acaulospora cf. herrerae | - | - | 0.2 | - | - | - | 5 | - |
| Acaulospora cf. morrowiae | 3.91 | 1.64 | 1.41 | 1.20 | 15 | 21.05 | 20 | 5 |
| Acaulospora sp. | 0.59 | 0.7 | 1 | 0.27 | 5 | 15.79 | 15 | 10 |
| Dentiscutataceae | ||||||||
| Dentiscutata cf. cerradensis | - | - | - | 0.13 | - | - | - | 5 |
| Dentiscutata cf. scutata | - | - | - | 0.27 | - | - | - | 5 |
| Dentiscutata sp. | 0.12 | 0.23 | - | - | 5 | 5.26 | - | - |
| Fuscutata aurea Oehl, C.M. Mello & G.A. Silva | 0.47 | - | 0.6 | 1.34 | 10 | - | 10 | 20 |
| Fuscutata heterogama Oehl, F.A. de Souza, L.C. Maia & Sieverd. | 5.33 | 2.35 | 4.02 | 1.87 | 35 | 10.53 | 30 | 25 |
| Fuscutata rubra (Stürmer & J.B. Morton) Oehl, F.A. de Souza & Sieverd. | 0.12 | - | - | 18.45 | 5 | - | - | 25 |
| Fuscutata cf. aurea | 0.47 | 0.23 | - | - | 5 | 5.26 | - | - |
| Fuscutata cf. rubra | 1.07 | - | - | - | 5 | - | - | - |
| Fuscutata sp. | 0.47 | 0.23 | - | - | 10 | 5.26 | - | - |
| Diversisporaceae | ||||||||
| Redeckera fulva (Berk. & Broome) C. Walker & A. Schüssler | 0.24 | 0.47 | - | - | 10 | 5.26 | - | - |
| Entrophosporaceae | ||||||||
| Claroideoglomus etunicatum (W.N. Becker & Gerd.) C. Walker & A. Schüssler | - | - | - | 0.27 | - | - | - | 5 |
| Claroideoglomus cf. etunicatum | 0.12 | - | - | - | 5 | - | - | - |
| Gigasporaceae | ||||||||
| Gigaspora cf. gigantea | 0.12 | - | 0.4 | - | 5 | - | 5 | - |
| Gigaspora sp. | 7.93 | 11.5 | 15.46 | 15.68 | 55 | 68.42 | 80 | 65 |
| Glomeraceae | ||||||||
| Glomus glomerulatum Sieverd. | 2.49 | 6.34 | 0.2 | 0.53 | 10 | 10.53 | 5 | 5 |
| Glomus spinuliferum Sieverd. & Oehl | 0.12 | - | - | 0.40 | 5 | - | - | 5 |
| Glomus trufemii B.T. Goto, G.A. Silva & Oehl | 34.67 | 5.4 | - | - | 35 | 36.84 | - | - |
| Glomus cf. ambisporum | - | - | - | 0.13 | - | - | - | 5 |
| Glomus cf. badium | - | 0.47 | - | 0.13 | - | 5.26 | - | 5 |
| Glomus cf. brohultii | - | - | 0.8 | 0.13 | - | - | 15 | 5 |
| Glomus cf. glomerulatum | 0.12 | - | - | - | 5 | - | - | - |
| Glomus cf. trufemii 1 | 4.73 | 1.64 | 0.4 | 4.81 | 25 | 15.79 | 5 | 10 |
| Glomus cf. trufemii 2 | - | 22.77 | - | 9.63 | - | 31.58 | - | 35 |
| Glomus sp. 1 | 0.95 | 0.23 | - | - | 20 | 5.26 | - | - |
| Glomus sp. 2 | - | - | - | 0.13 | - | - | - | 5 |
| Oehlia diaphana (J.B. Morton & C. Walker) Błaszk., Kozłowska & Dalpé | - | - | 0.2 | - | - | - | 5 | - |
| Rhizoglomus clarum (T.H. Nicolson & N.C. Schenck) Sieverd., G.A. Silva & Oehl | - | 1.17 | 0.4 | 0.53 | - | 10.3 | 10 | 15 |
| Rhizoglomus manihotis (R.H. Howeler, Sieverd. & N.C. Schenck) Sieverd., G.A. Silva & Oehl | 0.71 | 1.88 | - | - | 15 | 21.05 | - | - |
| Rhizoglomus microaggregatum (Koske, Gemma & P.D. Olexia) Sieverd., G.A. Silva & Oehl | 0.12 | - | - | 0.13 | 5 | - | - | 5 |
| Rhizoglomus cf. aggregatum | 0.47 | - | - | - | 10 | - | - | - |
| Rhizoglomus cf. clarum | 0.83 | 0.23 | - | 1.47 | 10 | 5.26 | - | 10 |
| Rhizoglomus cf. intraradices | 0.47 | 0.47 | - | - | 5 | 5.26 | - | - |
| Rhizoglomus cf. invermaium | 0.59 | 0.7 | - | 2.27 | 5 | 5.26 | - | 10 |
| Sclerocystis sinuosa Gerd. & B.K. Bakshi | 0.12 | - | - | - | 5 | - | - | - |
| Septoglomus cf. titan | 0.12 | - | - | - | 5 | - | - | - |
| Septoglomus sp. | 0.12 | - | 1.2 | - | 5 | - | 30 | - |
| Simiglomus sp. | - | - | 0.4 | - | - | - | 5 | - |
| Paraglomeraceae | ||||||||
| Paraglomus occultum (C. Walker) J.B. Morton & D. Redecker | - | 0.47 | - | - | - | 5.26 | - | - |
| Racocetraceae | ||||||||
| Cetraspora gilmorei (Trappe & Gerd.) Oehl, F.A. de Souza & Sieverd. | - | 0.47 | - | - | - | 5.26 | - | - |
| Racocetra gregaria (N.C. Schenck & T.H. Nicolson) Oehl, F.A. de Souza & Sieverd. | 0.83 | 3.05 | 2.61 | 2.41 | 20 | 31.58 | 30 | 30 |
| Racocetra cf. tropicana | 0.47 | - | - | - | 5 | - | - | - |
| Racocetra sp. | 0.36 | 0.23 | - | 0.13 | 10 | 5.26 | - | 5 |
| Scutellosporaceae | ||||||||
| Scutellospora sp. 1 | 0.24 | - | - | - | 5 | - | - | - |
| Scutellospora sp. 2 | 1.3 | 1.64 | 0.2 | - | 10 | 5.26 | 5 | - |
| Response | Sum Sq | Df | F Values | Pr (>F) |
|---|---|---|---|---|
| S | ||||
| Condition | 8.286 | 1 | 7.7657 | 0.006825 ** |
| Season | 4.735 | 1 | 4.4377 | 0.038690 * |
| Condition: Season | 5.654 | 1 | 5.2989 | 0.024275 * |
| Residuals | 75.753 | 71 | - | - |
| H | ||||
| (Intercept) | 29.6915 | 1 | 82.9543 | 1.316 × 10−13 *** |
| Condition | 0.5620 | 1 | 1.5701 | 0.2143 |
| Season | 0.0006 | 1 | 0.0016 | 0.9677 |
| Residuals | 25.7707 | 72 | - | - |
| C | ||||
| (Intercept) | 5.9945 | 1 | 87.1474 | 5.655 × 10−14 *** |
| Condition | 0.2200 | 1 | 3.1984 | 0.07798 |
| Season | 0.0709 | 1 | 1.0312 | 0.31333 |
| Condition: Season | 0.2107 | 1 | 3.0632 | 0.08440 |
| Residuals | 4.8838 | 71 | - | - |
| J | ||||
| (Intercept) | 10.8885 | 1 | 132.4999 | <2 × 10−16 *** |
| Condition | 0.0059 | 1 | 0.0714 | 0.7900 |
| Season | 0.0030 | 1 | 0.0361 | 0.8498 |
| Residuals | 5.9168 | 72 | - | - |
| NG | Df | Chisq | Pr (>Chisq) |
|---|---|---|---|
| (Intercept) | 1 | 816.9725 | <2.2 × 10−16 *** |
| Condition | 1 | 22.5457 | 2.052 × 10−6 *** |
| Season | 1 | 1.0576 | 0.303753 |
| Condition: Season | 1 | 8.3396 | 0.003879 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, S.R.B.S.; de Queiroz, M.B.; Leroy, J.A.S.; de Lima, J.L.R.; Freire, F.A.d.M.; Jobim, K.; de Souza, F.A.; Goto, B.T. Richness of Arbuscular Mycorrhizal Fungi in a Brazilian Tropical Shallow Lake: Assessing an Unexpected Assembly in the Aquatic-Terrestrial Gradient. Diversity 2022, 14, 1046. https://doi.org/10.3390/d14121046
Gomes SRBS, de Queiroz MB, Leroy JAS, de Lima JLR, Freire FAdM, Jobim K, de Souza FA, Goto BT. Richness of Arbuscular Mycorrhizal Fungi in a Brazilian Tropical Shallow Lake: Assessing an Unexpected Assembly in the Aquatic-Terrestrial Gradient. Diversity. 2022; 14(12):1046. https://doi.org/10.3390/d14121046
Chicago/Turabian StyleGomes, Stephania Ruth Basilio Silva, Mariana Bessa de Queiroz, Juliana Aparecida Souza Leroy, Juliana Luiza Rocha de Lima, Fúlvio Aurélio de Morais Freire, Khadija Jobim, Francisco Adriano de Souza, and Bruno Tomio Goto. 2022. "Richness of Arbuscular Mycorrhizal Fungi in a Brazilian Tropical Shallow Lake: Assessing an Unexpected Assembly in the Aquatic-Terrestrial Gradient" Diversity 14, no. 12: 1046. https://doi.org/10.3390/d14121046
APA StyleGomes, S. R. B. S., de Queiroz, M. B., Leroy, J. A. S., de Lima, J. L. R., Freire, F. A. d. M., Jobim, K., de Souza, F. A., & Goto, B. T. (2022). Richness of Arbuscular Mycorrhizal Fungi in a Brazilian Tropical Shallow Lake: Assessing an Unexpected Assembly in the Aquatic-Terrestrial Gradient. Diversity, 14(12), 1046. https://doi.org/10.3390/d14121046








