Recovery Signals of Rhodoliths Beds since Bottom Trawling Ban in the SCI Menorca Channel (Western Mediterranean)
Abstract
:1. Introduction
2. Materials and Methods
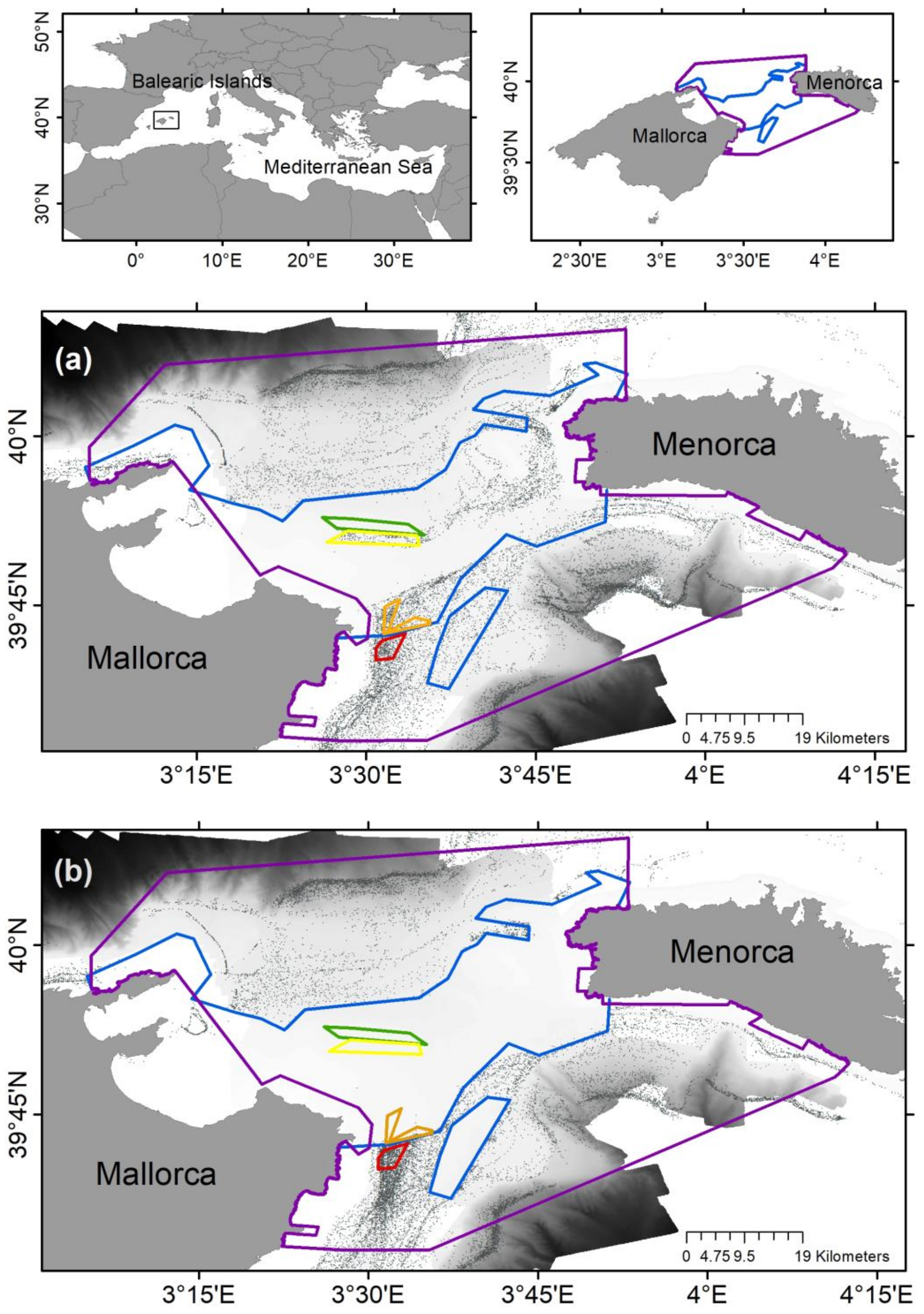
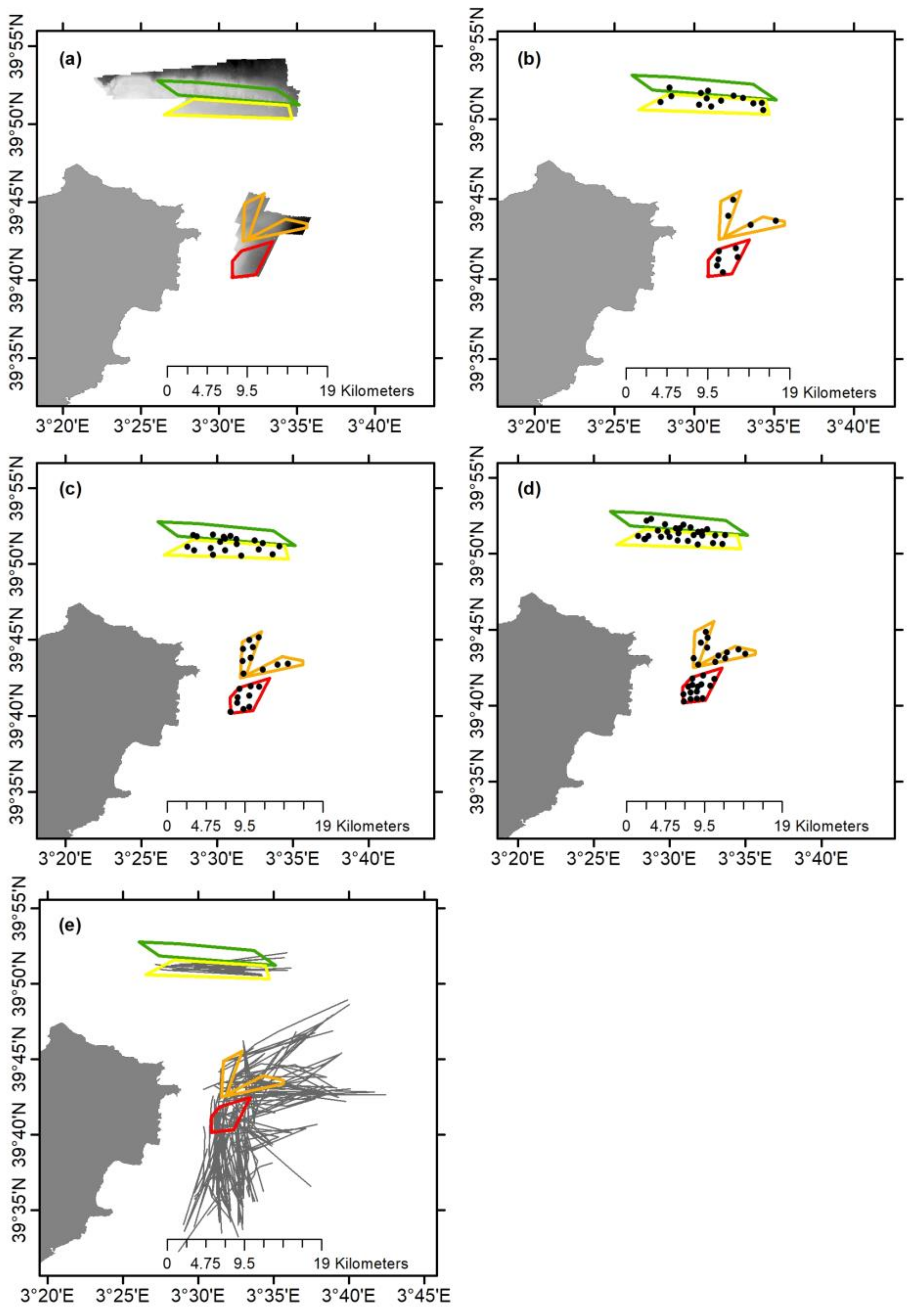
2.1. Sampling Strategy
2.2. Bathymetry and Sediment Characterization
2.3. Epibenthic Communities
2.4. Visual Observations
2.5. Hydrodynamic Conditions
2.6. Data Analysis
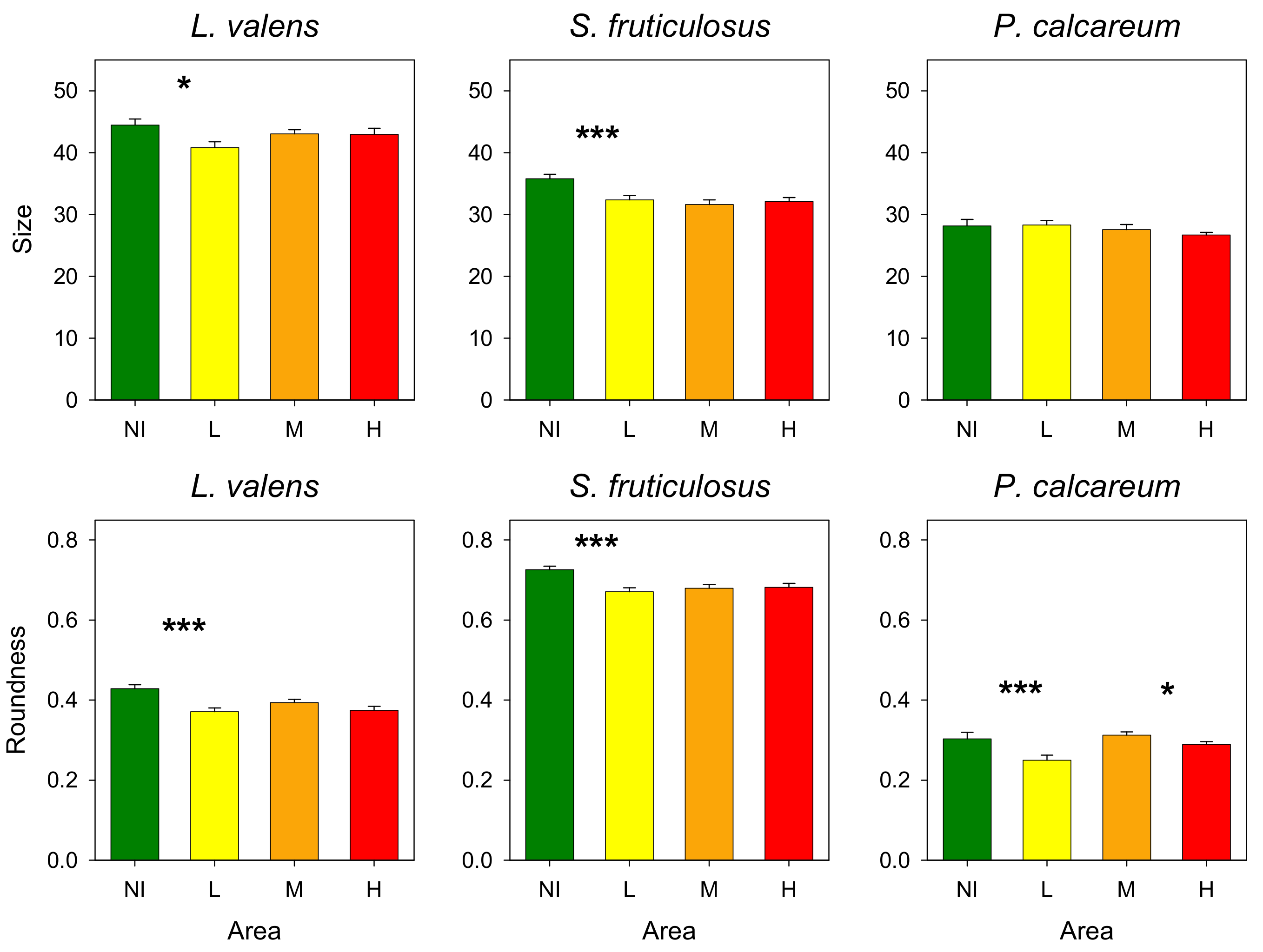
- Size: longest axis of each rhodolith.
- Roundness (R): used as a proxy of the sphericity of rhodoliths. Results measured range from 0 (elongated shape) to 1 (circle shape). It was calculated as follows:
2.7. Trawl Fishery
3. Results
3.1. Environmental Variables
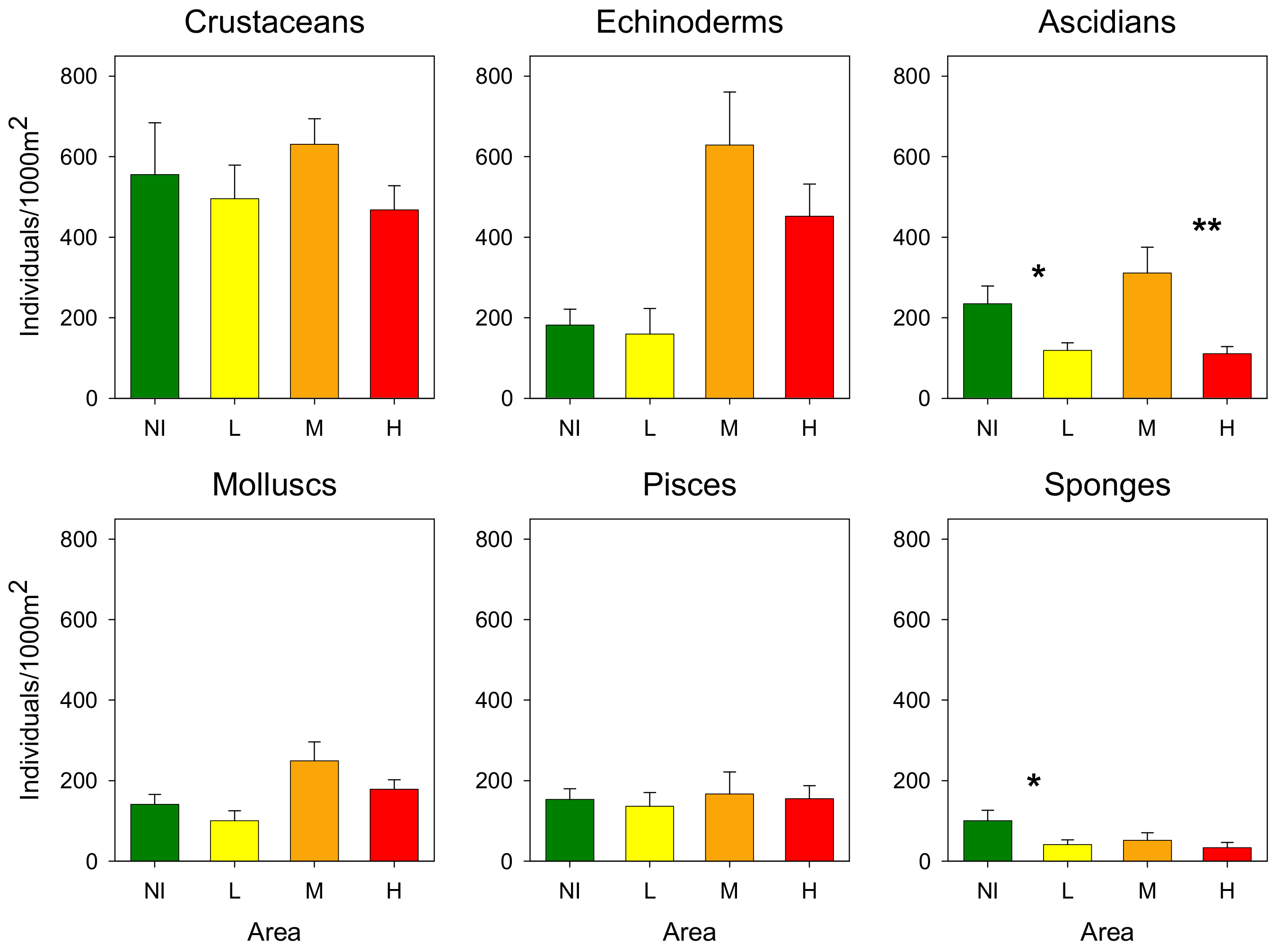
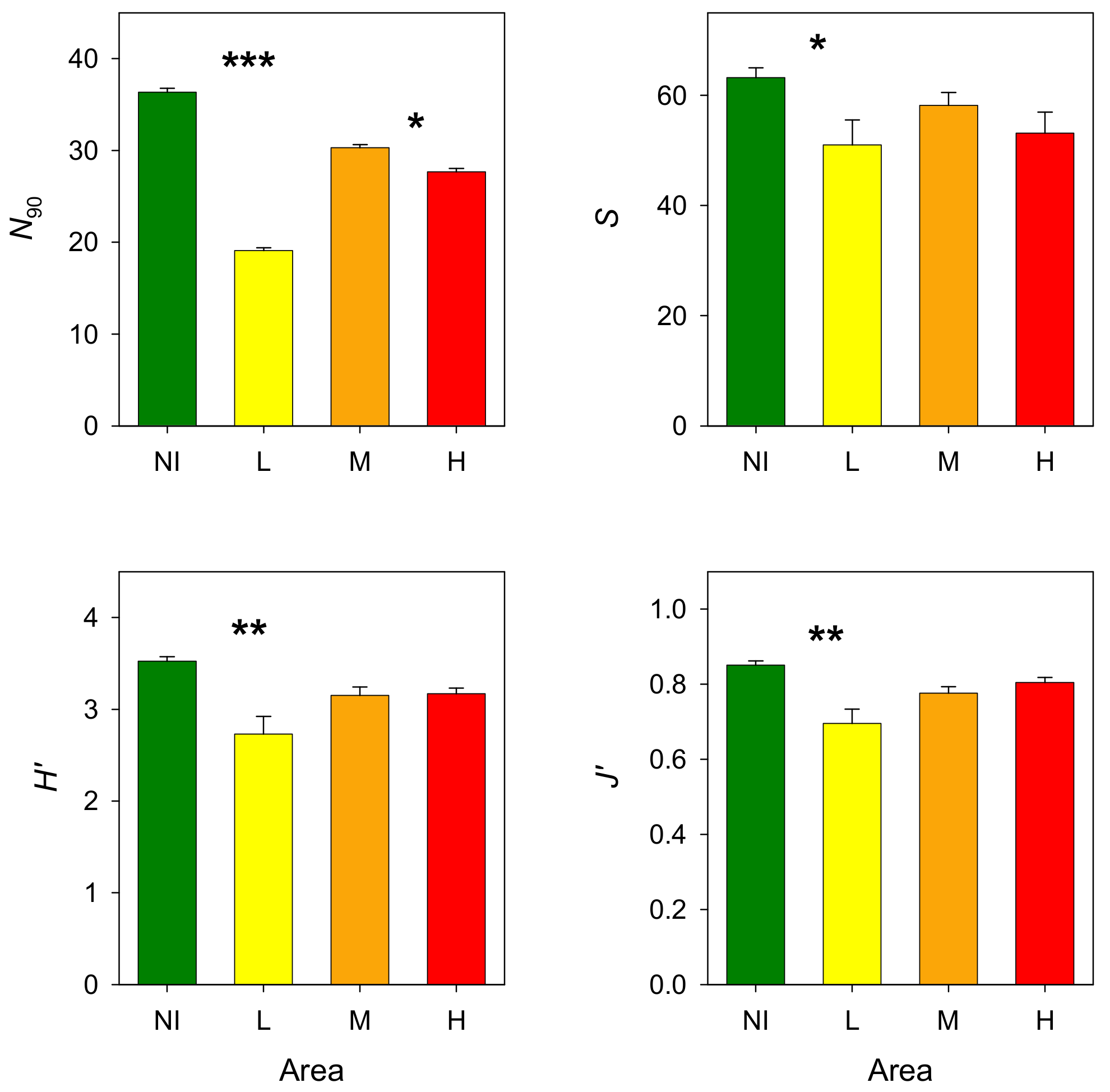
3.2. Epibenthic Communities
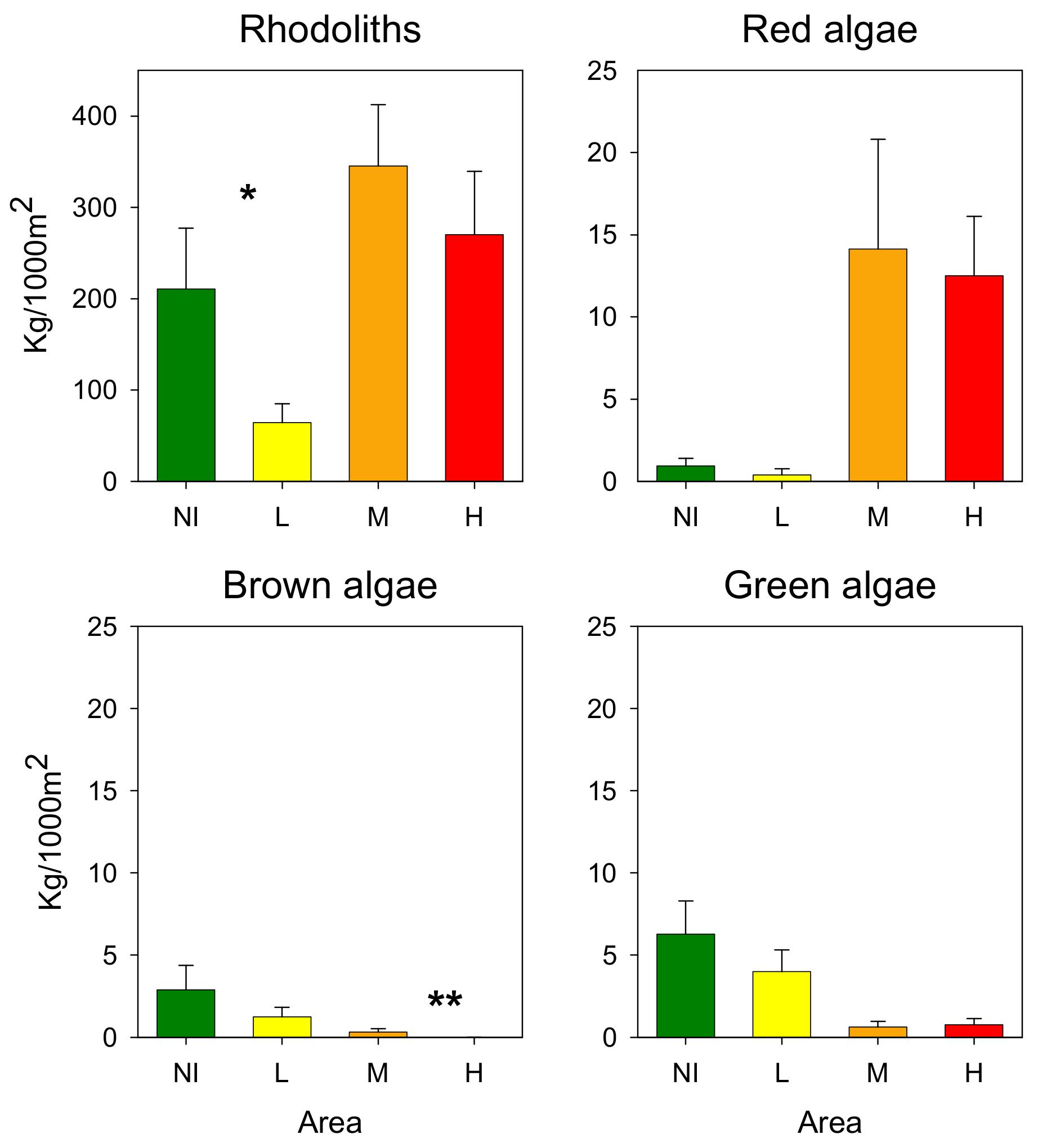
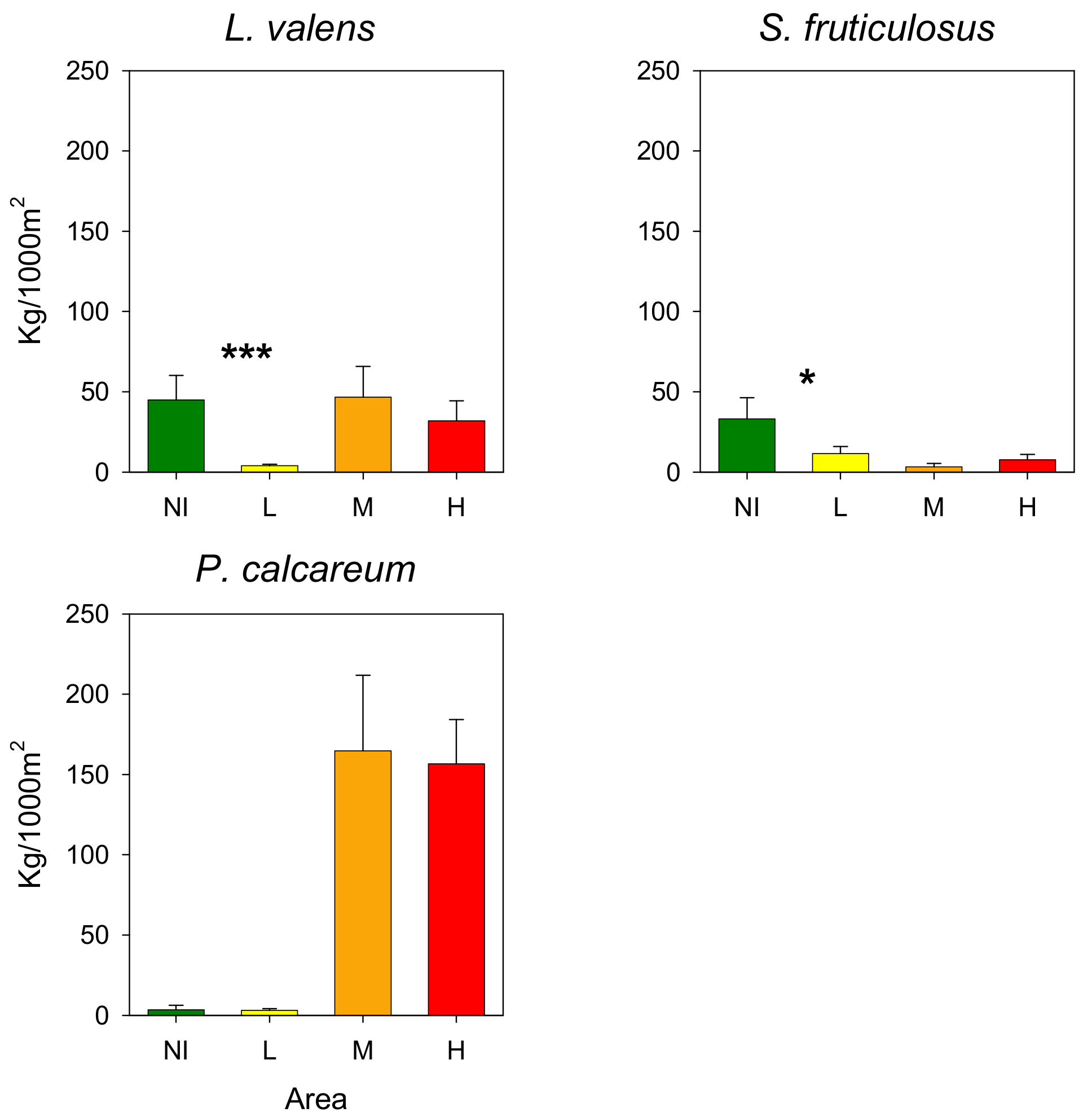
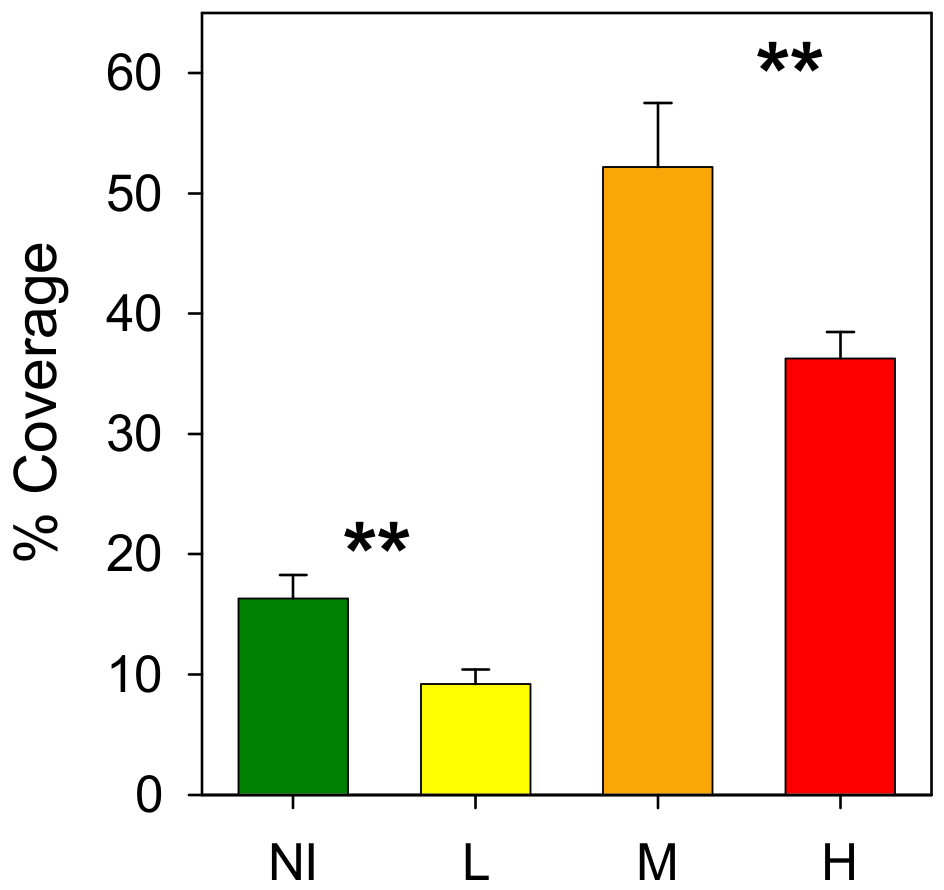
3.3. Rhodoliths
3.4. Bottom Trawl Captures
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basso, D.; Babbini, L.; Kaleb, S.; Bracchi, V.; Falace, A. Monitoring deep Mediterranean rhodoliths beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2015, 26, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Foster, M.S. Rhodoliths: Between rocks and soft places. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Basso, D.; Babbini, L.; Ramos-Espla, A.A.; Salomidi, M. Mediterranean Rhodolith Beds. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodriguez, R., Nelson, W., Aguirre, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 282–298. [Google Scholar]
- Littler, M.M.; Littler, D.S.; Dennis Hanisak, M. Deep-water rhodolith distribution, productivity, and growth history at sites of formation and subsequent degradation. J. Exp. Mar. Biol. Ecol. 1991, 150, 163–182. [Google Scholar] [CrossRef]
- Wilson, S.; Blake, C.; Berges, J.A.; Maggs, C.A. Environmental tolerances of free-living coralline algae (maerl): Implications for European marine conservation. Biol. Conserv. 2004, 120, 279–289. [Google Scholar] [CrossRef]
- Steller, D.L.; Hernández-Ayón, J.M.; Riosmena-Rodríguez, R.; Cabello-Pasini, A. Efecto de la temperatura sobre las tasas de fotosíntesis, crecimiento y calcificación del alga coralina de vida libre Lithophyllum margaritae. Ciencias Marinas 2007, 33, 441–456. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Steller, D.L.; Foster, M.S. Environmental factors influencing distribution and morphology of rhodoliths in Bahía Concepción, B.C.S., México. J. Exp. Mar. Biol. Ecol. 1995, 194, 201–212. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Moore, P.G. Scallop dredging has profound, long-term impacts on maerl habitats. ICES J. Mar. Sci. 2000, 57, 1407–1415. [Google Scholar] [CrossRef] [Green Version]
- Sciberras, M.; Rizzo, M.; Mifsud, J.R.; Camilleri, K.; Borg, J.A.; Lanfranco, E.; Schembri, P.J. Habitat structure and biological characteristics of a maerl bed off the Habitat structure and biological characteristics of a maerl bed off the northeastern coast of the Maltese Islands (central Mediterranean). Mar. Biodiver. 2009, 39, 251–264. [Google Scholar] [CrossRef]
- Barbera, C.; Bordehore, C.; Borg, J.A.; Glémarec, M.; Grall, J.; Hall-Spencer, J.M.; de la Huz, C.; Lanfranco, E.; Lastra, M.; Moore, P.G.; et al. Conservation and management of northeast Atlantic and Mediterranean maerl beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S65–S76. [Google Scholar] [CrossRef]
- Nelson, W.A. Calcified macroalgae: Critical to coastal ecosystems and vulnerable to change. Mar. Freshw. Res. 2009, 60, 787–801. [Google Scholar] [CrossRef]
- Teichert, S. Hollow rhodoliths increase Svalbard’ shelf biodiversity. Sci. Rep. 2014, 4, 6972. [Google Scholar] [CrossRef] [Green Version]
- Keegan, B. The macrofauna of maërl substrates on the west coast of Ireland. Cah. Biol. Mar 1974, 15, 513–530. [Google Scholar]
- Kamenos, N.A.; Moore, P.G.; Hall-Spencer, J.M. Small-scale distribution of juvenile gadoids in shallow inshore waters; what role does maerl play? ICES J. Mar. Sci. 2004, 61, 422–429. [Google Scholar] [CrossRef]
- Kamenos, N.A.; Moore, P.G.; Hall-Spencer, J.M. Nursery-area function of maërl grounds for juvenile queen scallops Aequipecten opercularis and other invertebrates. Mar. Ecol. Prog. Ser. 2004, 274, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Peña, V.; Bárbara, I.; Grall, J.; Maggs, C.A.; Hall-Spencer, J.M. The diversity of seaweeds on maerl in the NE Atlantic. Mar. Biodivers. 2014, 44, 533–551. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Moura, R.L.; Bastos, A.C.; Salgado, L.T.; Sumida, P.Y.; Guth, A.Z.; Francini-Filho, R.B.; Pereira-Filho, G.H.; Abrantes, D.P.; Brasileiro, P.S.; et al. Rhodolith beds are major CaCO3 bio-factories in the tropical South West Atlantic. PLoS ONE 2012, 7, e35171. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.S.; Harvey, R.M.; Merton, E. The distribution, significance and vulnerability of Australian rhodolith beds: A review. Mar. Freshw. Res. 2016, 68, 411–428. [Google Scholar] [CrossRef]
- Sanz-Lázaro, C.; Belando, M.D.; Marín-Guirao, L.; Navarrete-Mier, F.; Marín, A. Relationship between sedimentation rates and benthic impact on Maërl beds derived from fish farming in the Mediterranean. Mar. Environ. Res. 2011, 71, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Barberá, C.; Mallol, S.; Vergés, A.; Cabanellas-Reboredo, M.; Díaz, D.; Goñi, R. Maerl beds inside and outside a 25-year-old no-take area. Mar. Ecol. Prog. Ser. 2017, 572, 77–90. [Google Scholar] [CrossRef]
- Ordines, F.; Ramón, M.; Rivera, J.; Rodríguez-Prieto, C.; Farriols, M.T.; Guijarro, B.; Pasqual, C.; Massutí, E. Why long term trawled red algae beds off Balearic Islands (western Mediterranean) still persist? Reg. Stud. Mar. Sci. 2017, 15, 39–49. [Google Scholar] [CrossRef]
- Cabanellas-Reboredo, M.; Mallol, S.; Barberá, C.; Vergés, A.; Díaz, D.; Goñi, R. Morpho-demographic traits of two maërl-forming algae in beds with different depths and fishing histories. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 28, 133–145. [Google Scholar] [CrossRef]
- Carbonell, A.; Martín, P.; De Ranieri, S.; WEDIS team. Discards of the western Mediterranean trawl fleets. Rapp. Comm. Int. Pour l’Explor. Sci. Mer Méditerranée 1998, 35, 392–393. [Google Scholar]
- Farriols, M.T.; Ordines, F.; Massutí, E. Discards reduction of non-commercial benthic species from a simple net modification. Fish. Res. 2021, 241, 105985. [Google Scholar] [CrossRef]
- Bordehore, C.; Ramos-Esplá, A.A.; Riosmena-Rodríguez, R. Comparative study of two maerl beds with different otter trawling history, southeast Iberian Peninsula. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S43–S54. [Google Scholar] [CrossRef]
- Grall, J.; Hall-Spencer, J.M. Problems facing maerl conservation in Brittany. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 55–64. [Google Scholar] [CrossRef]
- Ballesteros, E. Els fons rocosos profunds amb Osmundaria volubilis (Linné) R.E. Norris a les Balears. Boll. Soc. Hist. Natur. Illes Balears 1992, 35, 33–50. [Google Scholar]
- Ballesteros, E. The deep-water Peyssonnelia beds from the Balearic Islands (western Mediterranean). Mar. Ecol. 1994, 15, 233–253. [Google Scholar] [CrossRef]
- Ordines, F.; Massutí, E. Relationships between macro-epibenthic communities and fish on the shelf grounds of the western Mediterranean. Aquat. Conserv. Mar. Freshw. Ecosyst. 2009, 19, 370–383. [Google Scholar] [CrossRef]
- Farriols, M.T.; Ordines, F.; Somerfield, P.J.; Pasqual, C.; Hidalgo, M.; Guijarro, B.; Massutí, E. Bottom trawl impacts on Mediterranean demersal fish diversity: Not so obvious or are we too late? Cont. Shelf Res. 2017, 137, 84–102. [Google Scholar] [CrossRef] [Green Version]
- Acosta, A.; Canals, M.; López-Martínez, J.; Muñoz, A.; Herranz, P.; Urgeles, R.; Palomo, C.; Casamor, J.L. The Balearic Promontory geomorphology (western Mediterranean): Morphostructure and active processes. Geomorphology 2002, 49, 177–204. [Google Scholar] [CrossRef]
- Canals, M.; Ballesteros, E. Production of carbonate particles by phytobenthic communities on the Mallorca-Menorca shelf, northwestern Mediterranean Sea. Deep-Sea Res. II 1997, 44, 611–629. [Google Scholar] [CrossRef]
- Alonso, B.; Guillén, J.; Canals, M.; Serra, J.; Acosta, J.; Herranz, P.; Sanz, J.L.; Calafat, A.; Catafau, E. Los sedimentos de la plataforma balear. Acta Geol. Hisp. 1988, 23, 185–196. (In Spanish) [Google Scholar]
- Pinot, J.M.; Tintoré, J.; Gomis, D. Multivariate analysis of the surface circulation in the Balearic Sea. Progr. Oceanogr. 1995, 36, 343–376. [Google Scholar] [CrossRef]
- Pinot, J.-M.; Lopez-Jurado, J.L.; Riera, M. The CANALES experiment (1996–1998). Interannual, seasonal, and mesoscale variability of the circulation in the Balearic Channels. Progr. Oceanogr. 2002, 55, 335–370. [Google Scholar] [CrossRef]
- Barberá, C.; Moranta, J.; Ordines, F.; Ramón, M.; de Mesa, A.; Díaz-Valdés, M.; Grau, A.M.; Massutí, E. Biodiversity and habitat mapping of Menorca Channel (western Mediterranean): Implications for conservation. Biodivers. Conserv. 2012, 21, 701–728. [Google Scholar] [CrossRef]
- Grinyó, J.; Gori, A.; Greenacre, M.; Requena, S.; Canepa, A.; Iacono, C.L.; Ambroso, S.; Purroy, A.; Gili, J.M. Megabenthic assemblages in the continental shelf edge and upper slope of the Menorca Channel, Western Mediterranean Sea. Progr. Oceanogr. 2018, 162, 40–51. [Google Scholar] [CrossRef]
- Moranta, J.; Barberá, C.; Druet, M.; Zaragoza, N. Caracterización Ecológica de La Plataforma Continental (50–100 m) del Canal de Menorca, Informe Final LIFE+ INDEMARES (LIFE07/NAT/E/000732); Instituto de Ciencias del Mar–CSIC: Barcelona, Spain, 2014. (In Spanish) [Google Scholar]
- Folk, R.L. The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J. Geol. 1954, 62, 344–359. [Google Scholar] [CrossRef]
- Jennings, S.; Lancaster, J.; Woolmer, A.; Cotter, J. Distribution, diversity and abundance of epibenthic fauna in the North Sea. J. Mar. Biol. Assoc. 1999, 79, 385–399. [Google Scholar] [CrossRef]
- Reiss, H.; Kröncke, I.; Ehrich, S. Estimating the catching efficiency of a 2-m beam trawl for sampling epifauna by removal experiments. ICES J. Mar. Sci. 2006, 63, 1453–1464. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Shchepetkin, A.F.; McWilliams, J.C. The Regional Ocean Modeling System (ROMS): A split-explicit, free-surface, topography-following-coordinate oceanic model. Ocean Model. 2005, 9, 347–404. [Google Scholar] [CrossRef]
- Juza, M.; Mourre, B.; Renault, L.; Gómara, S.; Sebastián, K.; Lora, S.; Beltran, J.P.; Frontera, B.; Garau, B.; Troupin, C.; et al. SOCIB operational ocean forecasting system and multi-platform validation in the Western Mediterranean Sea. J. Oper. Oceanogr. 2016, 9, s155–s166. [Google Scholar] [CrossRef] [Green Version]
- Mourre, B.; Aguiar, E.; Juza, M.; Hernandez-Lasheras, J.; Reyes, E.; Heslop, E.; Escudier, R.; Cutolo, E.; Ruiz, S.; Mason, E.; et al. Assessment of high-resolution regional ocean prediction systems using muli-platform observations: Illustrations in the Western Mediterranean Sea. In New Frontiers in Operational Oceanography; Chassignet, E., Pascual, A., Tintoré, J., Verron, J., Eds.; GODAE Ocean View: Berlin, Germany, 2018; pp. 663–694. [Google Scholar]
- Hernandez-Lasheras, J.; Mourre, B. Dense CTD survey versus glider fleet sampling: Comparing data assimilation performance in a regional ocean model West of Sardinia. Ocean Sci. 2018, 14, 1069–1084. [Google Scholar] [CrossRef] [Green Version]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Farriols, M.T.; Ordines, F.; Massuti, E. N90, a Diversity Index Sensitive to Variations in Beta Diversity Components. Diversity 2021, 13, 489. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014; 260p. [Google Scholar]
- Martí, R.; Uriz, M.J.; Ballesteros, E.; Turon, X. Seasonal variation in structure of three algal communities under different Light conditions. Estuar. Coast. Shelf Sci. 2005, 64, 613–622. [Google Scholar] [CrossRef] [Green Version]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. In Oceanography and Marine Biology: An Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2006; Volume 44, pp. 123–195. [Google Scholar]
- Peterson, C.G.J. The animal communities of the sea bottom. Rep. Dan. Biol. Stn. 1913, 21, 1–44. [Google Scholar]
- Thorson, G. Bottom communities (sublittoral or shallow shelf). Geol. Soc. Am. Mem. 1957, 67, 461–534. [Google Scholar]
- Pérès, J.M.; Picard, J. Nouveau manuel de bionomie benthique de la mer Mediterranée. Rec. Trav. Stat. Mar. Endoume. 1964, 31, 1–137. (In French) [Google Scholar]
- Glemarec, M. The benthic communities of the European North Atlantic continental shelf. Oceanogr. Mar. Biol. 1973, 11, 263–289. [Google Scholar]
- Gray, J.S. Animal–sediment relationships. Oceanogr. Mar. Biol. 1974, 12, 223–261. [Google Scholar]
- Buchanan, J.B.; Sheader, M.; Kingston, P.F. Sources of variability in the benthic environment off the south Northumber- land Coast, 1971–1976. J. Mar. Biolog. Assoc. 1978, 58, 191–209. [Google Scholar] [CrossRef]
- Flint, R.W.; Holland, J.S. Benthic fauna variability on a transect in the Gulf of Mexico. Estuar. Coast. Shelf Sci. 1980, 10, 1–14. [Google Scholar] [CrossRef]
- Pérès, J.M. History of the Mediterranean biota and the colonization of the depths. In Western Mediterranean; Margalef, R., Ed.; Pergamon Press: London, UK, 1985; pp. 200–234. [Google Scholar]
- Otero-Ferrer, F.; Cosme, M.; Tuya, F.; Espino, F.; Haroun, R. Effect of depth and seasonality on the functioning of rhodolith seabeds. Estuar. Coast. Shelf Sci. 2020, 235, 106579. [Google Scholar] [CrossRef]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith bed diversity in the Gulf of California: The importance of rhodolith structure and consequences of disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S5–S20. [Google Scholar] [CrossRef]
- Dutertre, M.; Grall, J.; Ehrhold, A.; Hamon, D. Environmental factors affecting maerl bed structure in Brittany (France). Eur. J. Phycol. 2015, 50, 371–383. [Google Scholar] [CrossRef]
- Engel, J.; Kvitek, R. Effects of otter trawling on a benthic community in Monterey Bay National Marine Sanctuary. Conserv. Biol. 1998, 12, 1204–1214. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.J.; Papadopoulou, K.N.; Diliberto, S. Impact of otter trawling on an eastern Mediterranean commercial trawl fishing ground. ICES J. Mar. Sci. 2000, 57, 1340–1351. [Google Scholar] [CrossRef]
- Hiddink, J.G.; Jennings, S.; Kaiser, M.J.; Queirós, A.M.; Duplisea, D.E.; Piet, G.J. Cumulative impacts of seabed trawl disturbance on benthic biomass, production, and species richness in different habitats. Can. J. Fish. Aquat. Sci. 2006, 736, 721–736. [Google Scholar] [CrossRef]
- Hinz, H.; Prieto, V.; Kaiser, M.J. Trawl disturbance on benthic communities: Chronic effects and experimental predictions. Ecol. Appl. 2009, 19, 761–773. [Google Scholar] [CrossRef]
- Barberá, C.; Comalada, N.; Joher, S.; Valls, M.; Díaz-Valdés, M.; Moranta, J. Analysis of morphological characteristics of rhodoliths as indicator of habitat complexity and fishing pressure. XVII Iberian Symposium on Marine Biology Studies. Rev. Investigación Mar. AZTI-Tecnalia 2012, 19, 346. [Google Scholar]
- Arroyo, E.; Moya-Urbano, E.; García-Ruíz, C.; Esteban, A.; Ramos-Esplá, A.A. Ascidians (Chordata: Tunicata) from circalittoral and upper-bathyal soft bottoms sampled by experimental trawling in the Iberian Mediterranean Sea. Reg. Stu. Mar. Sci. 2021, 43, 101669. [Google Scholar] [CrossRef]
- Freese, J.L. Trawl-induced damage to sponges observed from a research submersible. Mar. Fish. Rev. 2003, 63, 7–13. [Google Scholar]
- Birkett, D.A.; Maggs, C.A.; Dring, M.J. Maerl (Volume 5). An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs; Scottish Association for Marine Science [UK Marine SACs Project]: Oban, UK, 1998; 116p. [Google Scholar]
- Potin, P.; Floc’h, J.Y.; Augris, C.; Cabioch, J. Annual growth rate of the calcareous red alga Lithothamnion corallioides (Corallinales, Rhodophyta) in the Bay of Brest, France. Hydrobiologia 1990, 204, 263–267. [Google Scholar] [CrossRef]
- Hiddink, J.G.; Jennings, S.; Kaiser, M.J. Assessing and predicting the relative ecological impacts of disturbance on habitats with different sensitivities. J. App. Ecol. 2007, 44, 405–413. [Google Scholar] [CrossRef]
- Lambert, G.I.; Jennings, S.; Kaiser, M.J.; Davies, T.W.; Hiddink, J.G. Quantifying recovery rates and resilience of seabed habitats impacted by bottom fishing. J. Appl. Ecol. 2014, 51, 1326–1336. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, M.J. Significance of bottom-fishing disturbance. Conserv. Biol. 1998, 12, 1230–1235. [Google Scholar] [CrossRef]
- Guijarro, B.; Ordines, F.; Massutí, E. Improving the ecological efficiency of the bottom trawl fishery in the Western Mediterranean: It’s about time! Marine Policy 2017, 83, 204–214. [Google Scholar] [CrossRef]
- Grinyó, J.; Garriga, A.; Soler-Membrives, A.; Santín, A.; Ambroso, S.; López-González, P.J.; Díaz, D. Soft corals assemblages in deep environments of the Menorca Channel (Western Mediterranean Sea). Progr. Oceanogr. 2020, 188, 102435. [Google Scholar] [CrossRef]
- Quetglas, A.; Merino, G.; Ordines, F.; Guijarro, B.; Garau, A.; Grau, A.M.; Oliver, P.; Massutí, E. Management Assessment and management of western Mediterranean small-scale fisheries. Ocean Coast. Manag. 2016, 133, 95–104. [Google Scholar] [CrossRef]
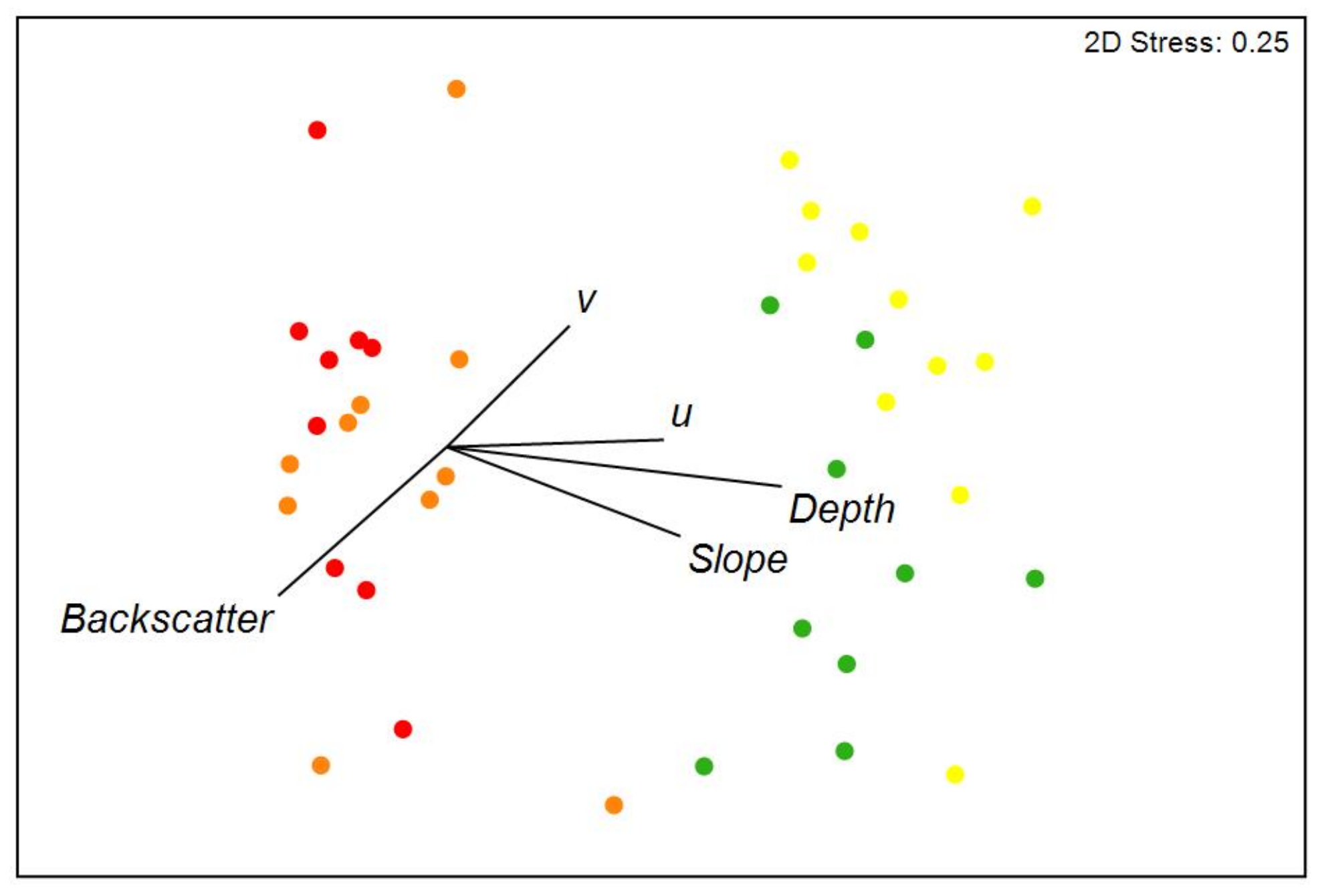
| Variables | Non-Impact | Low Effort History | Medium Effort History | High Effort | |
|---|---|---|---|---|---|
| Seafloor bathymetry | Depth (m) | 70.3 ± 2.1 | 70.4 ± 2.9 | 59.4 ± 2.5 | 58.1 ± 2.1 |
| Slope (°) | 0.6 ± 0.2 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | |
| Backscatter (dB) | −20.8 ± 2.7 | −23.1 ± 2.8 | −18.9 ± 2.5 | −18.9 ± 1.8 | |
| Orientation (°) | 138.1 ± 36.6 | 145.0 ± 27.8 | 157.2 ± 45.7 | 125.9 ± 19.2 | |
| Sediments | Gravel (%) | 2.3 ± 1.9 | 2.8 ± 1.6 | 6.4 ± 5.0 | 9.9 ± 11.2 |
| Sand (%) | 97.0 ± 2.2 | 96.7 ± 1.5 | 92.8 ± 5.4 | 89.5 ± 11.4 | |
| Mud (%) | 0.7 ± 0.3 | 0.5 ± 0.2 | 0.8 ± 0.4 | 0.6 ± 0.2 | |
| Bottom currents | u (cm/s) | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.8 ± 0.3 | −0.2 ± 0.3 |
| v (cm/s) | −2.4 ± 0.4 | −2.4 ± 0.6 | −3.6 ± 0.2 | −2.5 ± 0.4 | |
| |v| (cm/s) | 3.1 ± 0.3 | 3.1 ± 0.4 | 3.7 ± 0.2 | 2.4 ± 0.5 | |
| Species/Taxa | Non-Impacted | Species/Taxa | Low Effort History | ||
|---|---|---|---|---|---|
| A | %C | A | %C | ||
| Inachus dorsettensis | 113.1 | 12.3 | Pagurus prideaux | 267.0 | 35.4 |
| Buenia massutii | 99.2 | 11.2 | Buenia massutii | 118.3 | 12.8 |
| Pagurus prideaux | 74.9 | 7.4 | Inachus dorsettensis | 67.3 | 10.7 |
| Ascidia mentula | 64.7 | 6.7 | Anapagurus laevis | 39.3 | 3.9 |
| Anapagurus laevis | 73.2 | 5.3 | Ophiura ophiura | 27.0 | 3.7 |
| Liocarcinus spp. | 45.8 | 4.2 | Pagurus forbesii | 19.4 | 2.8 |
| Aplidium nordmanni | 57.1 | 4.2 | Ascidia mentula | 14.9 | 2.3 |
| Ophiura albida | 48.8 | 4.0 | Aplidium nordmanni | 25.6 | 2.0 |
| Ciona spp. | 28.0 | 2.6 | Dardanus arrosor | 16.3 | 1.9 |
| Pilumnus spinifer | 19.0 | 2.2 | Spatangus purpureus | 84.5 | 1.9 |
| Spatangus purpureus | 46.5 | 2.2 | Molgula appendiculata | 21.9 | 1.9 |
| Dardanus arrosor | 18.1 | 2.0 | Eurynome aspera | 15.1 | 1.9 |
| Pagurus forbesii | 18.8 | 2.0 | Calyptraea chinensis | 16.3 | 1.8 |
| Palliolum spp. | 18.3 | 1.9 | Fusinus pulchellus | 13.9 | 1.6 |
| Mimachlamys varia | 26.0 | 1.7 | Turritella turbona | 10.2 | 1.4 |
| Mycale (Aegogropila) contarenni | 16.2 | 1.7 | Pilumnus spinifer | 10.5 | 1.2 |
| Turritella turbona | 20.9 | 1.3 | Cystodytes dellechiajei | 12.0 | 1.1 |
| Macropodia linaresi | 14.4 | 1.2 | Ophiura albida | 8.7 | 1.1 |
| Ebalia tuberosa | 12.8 | 1.1 | Echinaster sepositus | 5.7 | 0.8 |
| Eurynome aspera | 18.0 | 1.1 | |||
| Parthenopoides massena | 20.1 | 1.1 | |||
| Inachus thoracicus | 10.4 | 1.0 | |||
| Molgula appendiculata | 10.1 | 1.0 | |||
| Ophiura ophiura | 19.5 | 1.0 | |||
| Pisa armata | 9.0 | 1.0 | |||
| Polycarpa mamillaris | 10.9 | 0.9 | |||
| Calcarea sp. | 15.6 | 0.8 | |||
| Macropodia rostrata | 7.7 | 0.8 | |||
| Laevicardium oblongum | 8.1 | 0.8 | |||
| Cystodytes dellechiajei | 11.7 | 0.8 | |||
| Arnoglossus thori | 6.8 | 0.8 | |||
| Vanneaugobius dollfusi | 18.2 | 0.7 | |||
| Calyptraea chinensis | 9.2 | 0.7 | |||
| Diazona violacea | 6.5 | 0.7 | |||
| Clausinella fasciata | 6.7 | 0.7 | |||
| Siphonochalina sp. | 10.7 | 0.7 | |||
| Echinaster sepositus | 9.8 | 0.6 | |||
| Species/Taxa | Medium Effort History | Species/Taxa | High Effort | ||
|---|---|---|---|---|---|
| A | %C | A | %C | ||
| Ophioconis forbesi | 307.1 | 16.3 | Ophioconis forbesi | 250.8 | 23.4 |
| Pagurus prideaux | 138.4 | 11.7 | Anapagurus laevis | 88.2 | 10.3 |
| Anapagurus laevis | 106.5 | 6.8 | Pagurus prideaux | 99.4 | 8.7 |
| Aplidium nordmanni | 121.6 | 6.6 | Pagurus forbesii | 48.1 | 6.4 |
| Spatangus purpureus | 114.8 | 5.2 | Gibbula fanulum | 47.1 | 4.4 |
| Molgula appendiculata | 111.1 | 4.3 | Dardanus arrosor | 41.4 | 3.7 |
| Dardanus arrosor | 49.5 | 3.8 | Turritella turbona | 29.9 | 2.7 |
| Cestopagurus timidus | 39.4 | 3.4 | Echinaster sepositus | 26.4 | 2.6 |
| Pagurus forbesii | 37.8 | 3.2 | Molgula appendiculata | 32.7 | 2.5 |
| Turritella turbona | 48.0 | 2.9 | Paguristes eremita | 31.2 | 2.4 |
| Echinaster sepositus | 33.0 | 2.4 | Cestopagurus timidus | 30.2 | 2.1 |
| Calyptraea chinensis | 36.0 | 2.2 | Ophiopsila aranea | 55.5 | 2.0 |
| Inachus dorsettensis | 32.3 | 2.1 | Ebalia tuberosa | 21.9 | 1.9 |
| Ebalia tuberosa | 23.5 | 2.1 | Buenia affinis | 28.3 | 1.7 |
| Gibbula fanulum | 28.8 | 2.0 | Clausinella fasciata | 13.4 | 1.7 |
| Liocarcinus spp. | 29.9 | 1.9 | Calyptraea chinensis | 18.1 | 1.7 |
| Buenia affinis | 71.7 | 1.6 | Aplidium nordmanni | 29.3 | 1.6 |
| Ophiopsila aranea | 76.0 | 1.5 | Inachus dorsettensis | 15.9 | 1.3 |
| Galathea intermedia | 22.8 | 1.1 | Ophiura albida | 15.1 | 1.2 |
| Inachus thoracicus | 15.0 | 1.0 | Parthenopoides massena | 16.4 | 1.1 |
| Suberites domuncula | 13.8 | 0.9 | Buenia massutii | 24.9 | 1.1 |
| Buenia massutii | 20.9 | 0.9 | Serranus hepatus | 35.7 | 1.0 |
| Ascidia mentula | 11.1 | 0.9 | Lanice conchilega | 8.9 | 1.0 |
| Polycarpa mamillaris | 14.5 | 0.8 | Diplecogaster bimaculata bimaculata | 9.4 | 0.9 |
| Mimachlamys varia | 20.3 | 0.7 | Ascidia mentula | 7.9 | 0.8 |
| Serranus hepatus | 26.5 | 0.7 | Laetmonice hystrix | 12.5 | 0.7 |
| Parthenopoides massena | 10.2 | 0.7 | Pagurus cuanensis | 7.1 | 0.7 |
| Paguristes eremita | 12.4 | 0.7 | Inachus thoracicus | 10.2 | 0.7 |
| Macropodia rostrata | 9.1 | 0.7 | |||
| Odondebuenia balearica | 10.6 | 0.7 | |||
| Eurynome aspera | 10.0 | 0.6 | |||
| Low Effort History | n/30′ | se |
| Ophiura ophiura | 5.7 | 2.9 |
| Paguridae | 5.2 | 4.6 |
| Echinaster sepositus | 2.8 | 1.4 |
| Ascidia mentula | 2.3 | 1.1 |
| Dardanus arrosor | 2.3 | 1.6 |
| Pagurus prideauxi | 1.7 | 1.7 |
| Parastichopus regalis | 1.3 | 0.6 |
| Aplidium spp. | 1.0 | 0.5 |
| Suberites domuncula | 0.7 | 0.3 |
| Aequipecten opercularis | 0.7 | 0.4 |
| Molgula appendiculata | 0.7 | 0.7 |
| Inachus thoracicus | 0.7 | 0.4 |
| Marthasterias glacialis | 0.5 | 0.3 |
| Phallusia mammillata | 0.5 | 0.5 |
| Pleurobranchia meckeli | 0.5 | 0.5 |
| Botryllus schlosseri | 0.5 | 0.5 |
| Medium effort history and High effort | n/30′ | se |
| Dardanus arrosor | 14.8 | 2.7 |
| Spatangus purpureus | 9.9 | 3.1 |
| Sphaerechinus granularis | 8.3 | 4.3 |
| Suberites domuncula | 8.3 | 2.3 |
| Pagurus prideaux | 5.7 | 4.8 |
| Echinaster sepositus | 2.8 | 0.6 |
| Ascidia mentula | 2.4 | 1.1 |
| Paguridae | 1.9 | 0.8 |
| Inachus spp. | 1.5 | 0.9 |
| Ascidia virginia | 1.0 | 0.7 |
| Aequipecten opercularis | 0.9 | 0.4 |
| Phallusia mammillata | 0.8 | 0.4 |
| Polycarpa mamillaris | 0.8 | 0.5 |
| Ophiura ophiura | 0.7 | 0.3 |
| Parastichopus regalis | 0.6 | 0.4 |
| Diazona violacea | 0.5 | 0.3 |
| Aplidium spp. | 0.5 | 0.2 |
| Ophiura spp. | 0.5 | 0.3 |
| Luidia ciliaris | 0.5 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farriols, M.T.; Irlinger, C.; Ordines, F.; Palomino, D.; Marco-Herrero, E.; Soto-Navarro, J.; Jordà, G.; Mallol, S.; Díaz, D.; Martínez-Carreño, N.; et al. Recovery Signals of Rhodoliths Beds since Bottom Trawling Ban in the SCI Menorca Channel (Western Mediterranean). Diversity 2022, 14, 20. https://doi.org/10.3390/d14010020
Farriols MT, Irlinger C, Ordines F, Palomino D, Marco-Herrero E, Soto-Navarro J, Jordà G, Mallol S, Díaz D, Martínez-Carreño N, et al. Recovery Signals of Rhodoliths Beds since Bottom Trawling Ban in the SCI Menorca Channel (Western Mediterranean). Diversity. 2022; 14(1):20. https://doi.org/10.3390/d14010020
Chicago/Turabian StyleFarriols, Maria Teresa, Camille Irlinger, Francesc Ordines, Desirée Palomino, Elena Marco-Herrero, Javier Soto-Navarro, Gabriel Jordà, Sandra Mallol, David Díaz, Natalia Martínez-Carreño, and et al. 2022. "Recovery Signals of Rhodoliths Beds since Bottom Trawling Ban in the SCI Menorca Channel (Western Mediterranean)" Diversity 14, no. 1: 20. https://doi.org/10.3390/d14010020
APA StyleFarriols, M. T., Irlinger, C., Ordines, F., Palomino, D., Marco-Herrero, E., Soto-Navarro, J., Jordà, G., Mallol, S., Díaz, D., Martínez-Carreño, N., Díaz, J. A., Fernandez-Arcaya, U., Joher, S., Ramírez-Amaro, S., R. de la Ballina, N., Vázquez, J.-T., & Massutí, E. (2022). Recovery Signals of Rhodoliths Beds since Bottom Trawling Ban in the SCI Menorca Channel (Western Mediterranean). Diversity, 14(1), 20. https://doi.org/10.3390/d14010020







