High Diversity and Abundance of Foraminifera Associated with Mediterranean Benthic Red Algae Mats
Abstract
:1. Introduction
- What is the abundance and diversity of epiphytic foraminifera in P. crispa mats in relation to P. oceanica meadows?
- What is the composition of epiphytic foraminifera morphotypes in P. crispa mats?
2. Materials and Methods
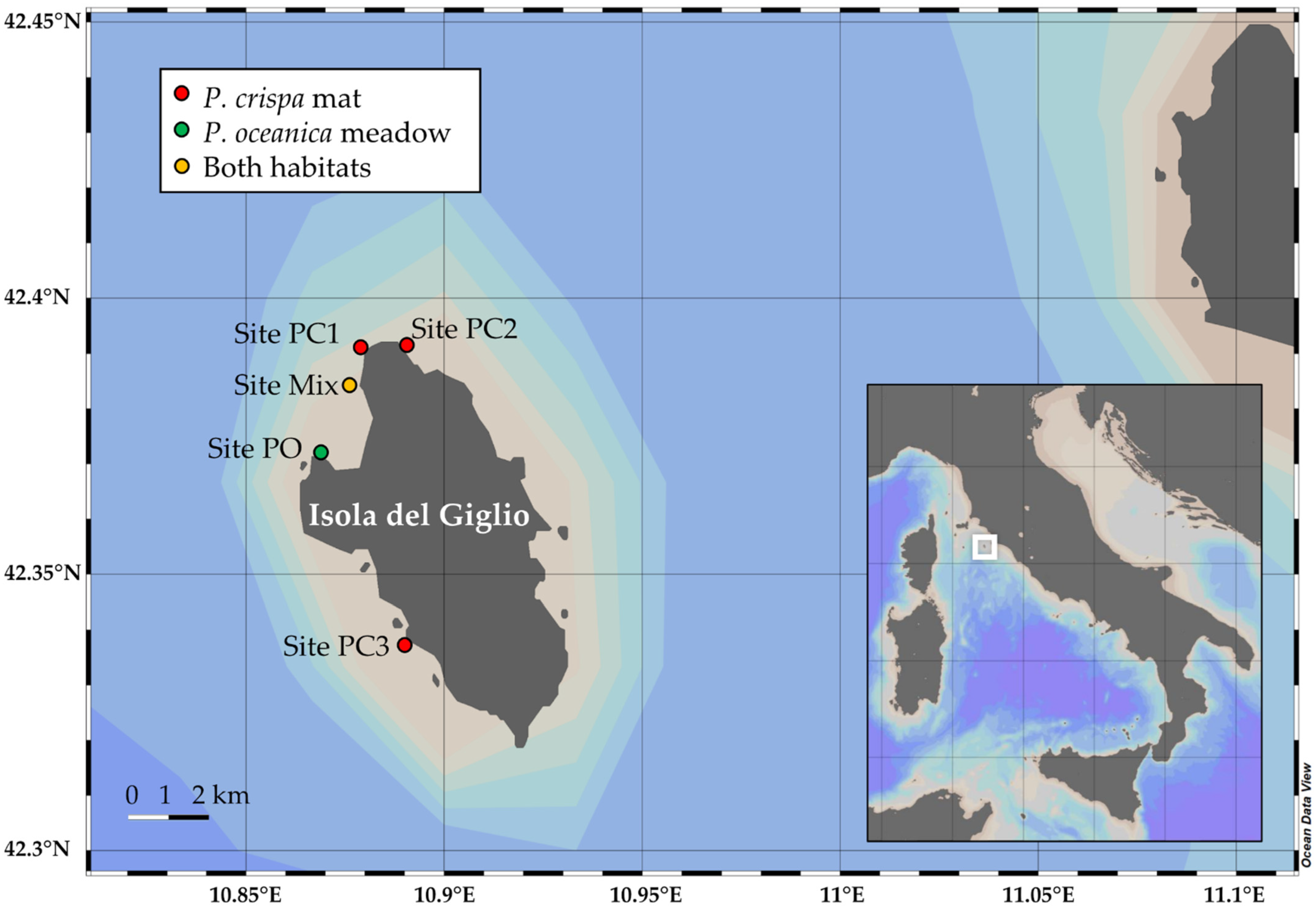
2.1. Sampling Activities
2.2. Species Identification
2.3. Diversity Descriptors
2.4. Statistical Analysis
3. Results
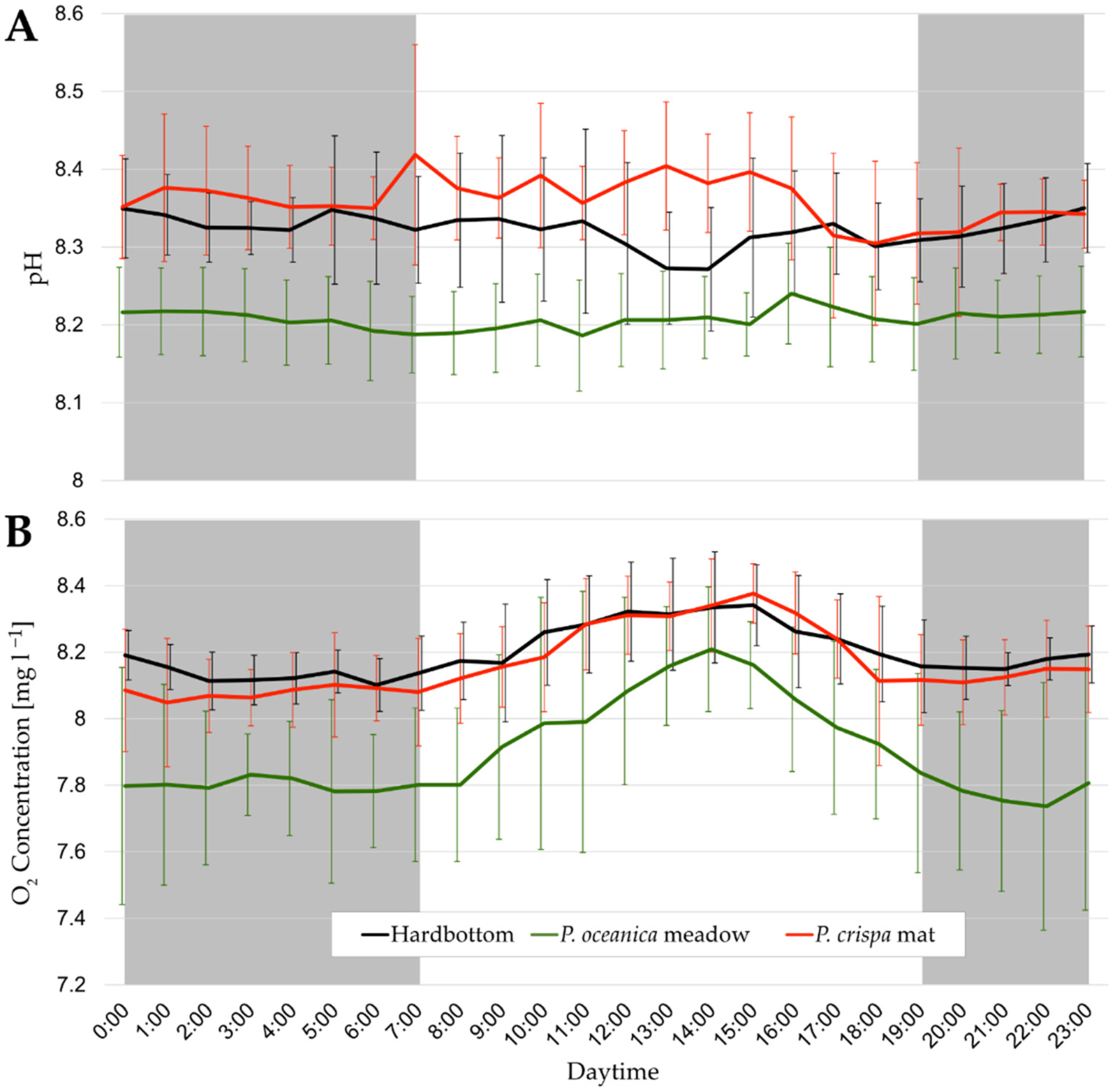
3.1. Daily Cycles of Water Parameters
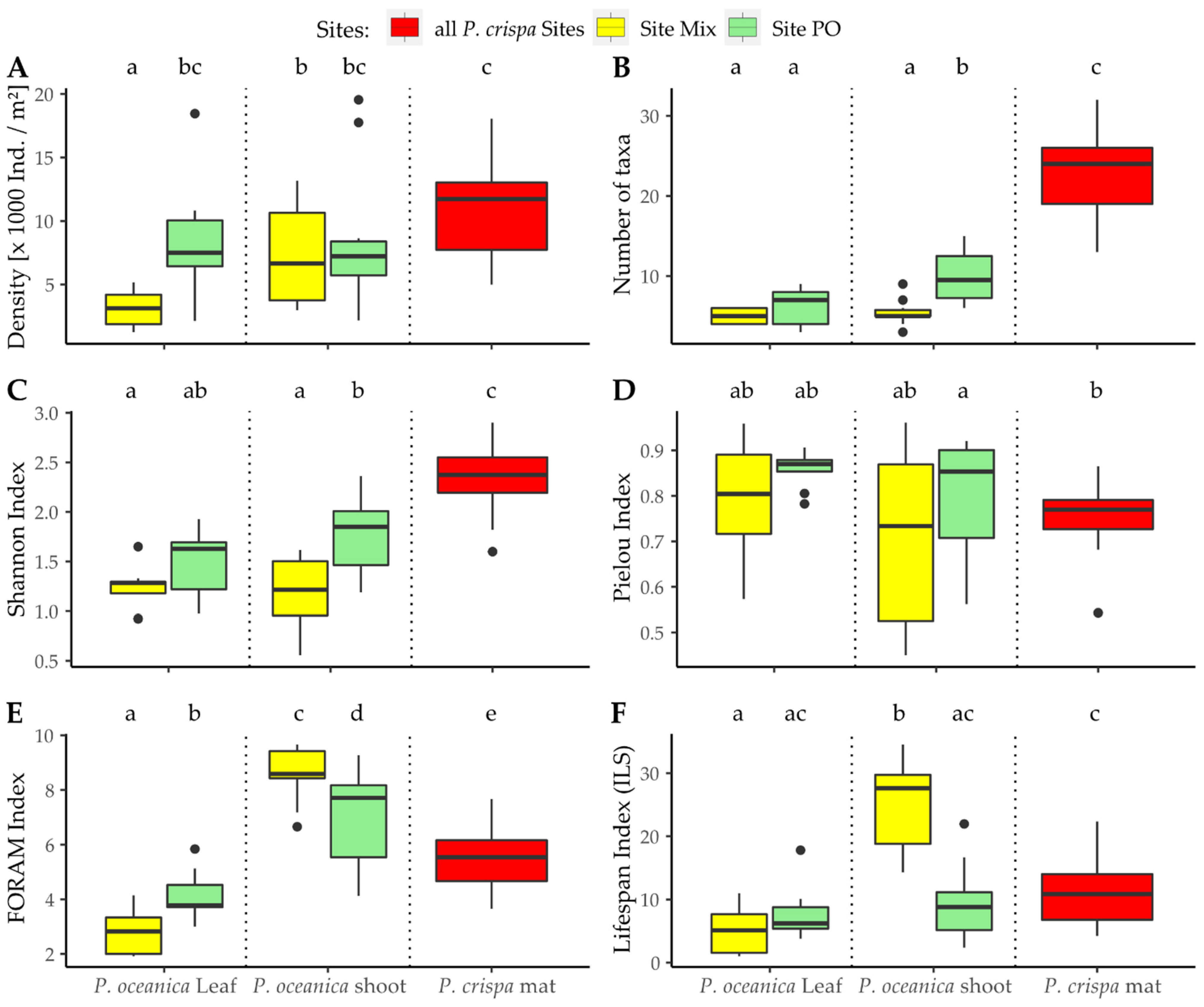
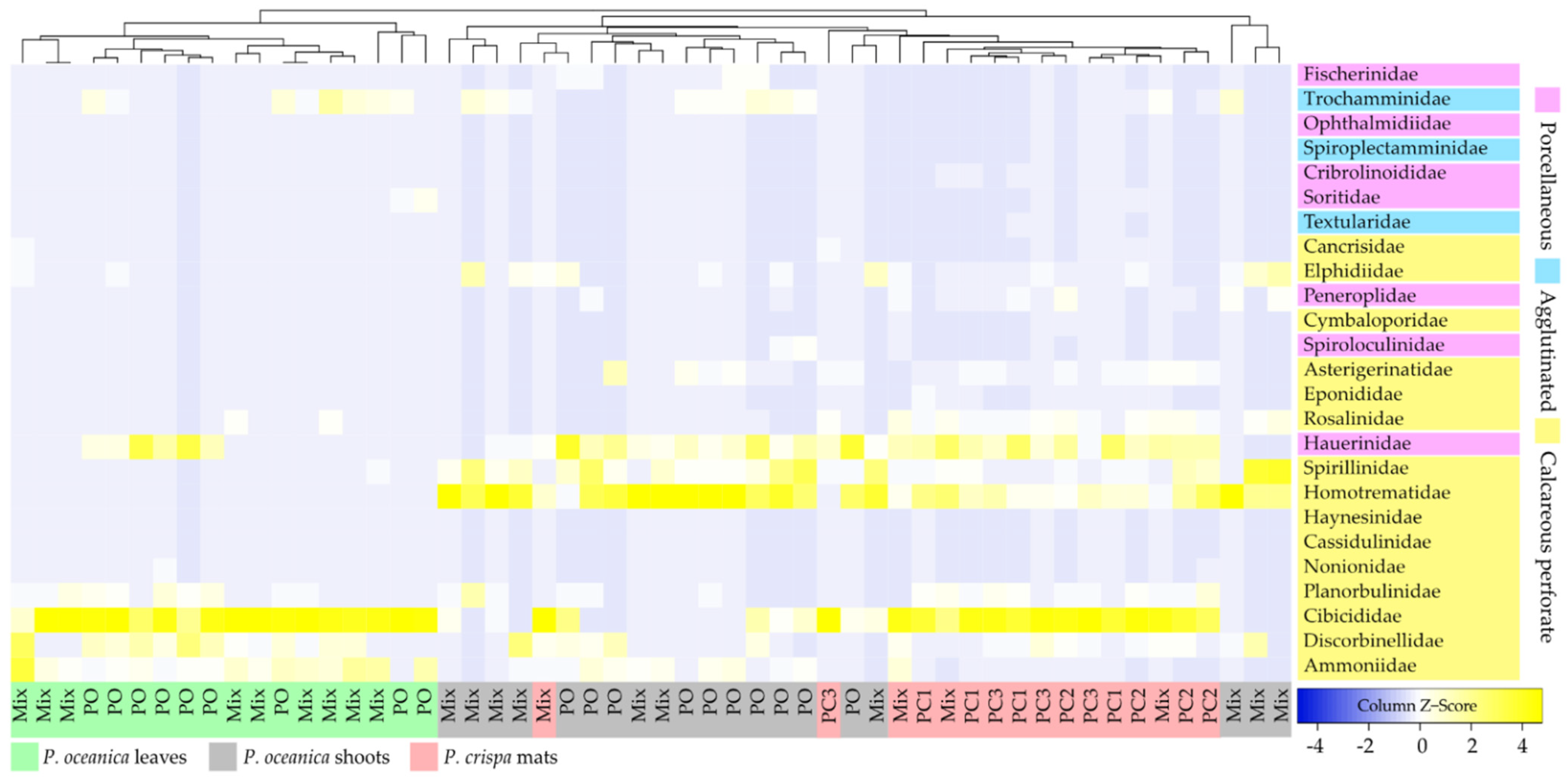
3.2. Diversity of the Foraminiferal Community
4. Discussion
4.1. Environmental Parameters inside P. crispa Mats
4.2. Abundance and Diversity of Epiphytic Foraminifera in P. crispa Mats
4.3. Composition of Epiphytic Foraminifera Morphotypes in P. crispa Mats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Leaves Site Mix | Leaves Site PO | Shoots Site Mix | Shoots Site PO | ||||
|---|---|---|---|---|---|---|---|---|
| AVG | STDEV | AVG | STDEV | AVG | STDEV | AVG | STDEV | |
| Vertebralina striata | 0 | 0 | 0 | 0 | 0 | 0 | 105 | 151 |
| Peneroplis pertusus | 0 | 0 | 0 | 0 | 92 | 177 | 41 | 82 |
| Sorites orbiculus | 0 | 0 | 73 | 160 | 0 | 0 | 0 | 0 |
| Species | Site PC1 | Site PC2 | Site PC3 | Site Mix | ||||
|---|---|---|---|---|---|---|---|---|
| AVG | STDEV | AVG | STDEV | AVG | STDEV | AVG | STDEV | |
| Peneroplis pertusus | 92 | 132 | 272 | 272 | 49 | 49 | 200 | 324 |
| Peneroplis planatus | 0 | 0 | 17 | 30 | 31 | 54 | 20 | 34 |
| Sorites orbiculus | 0 | 0 | 41 | 72 | 0 | 0 | 10 | 17 |
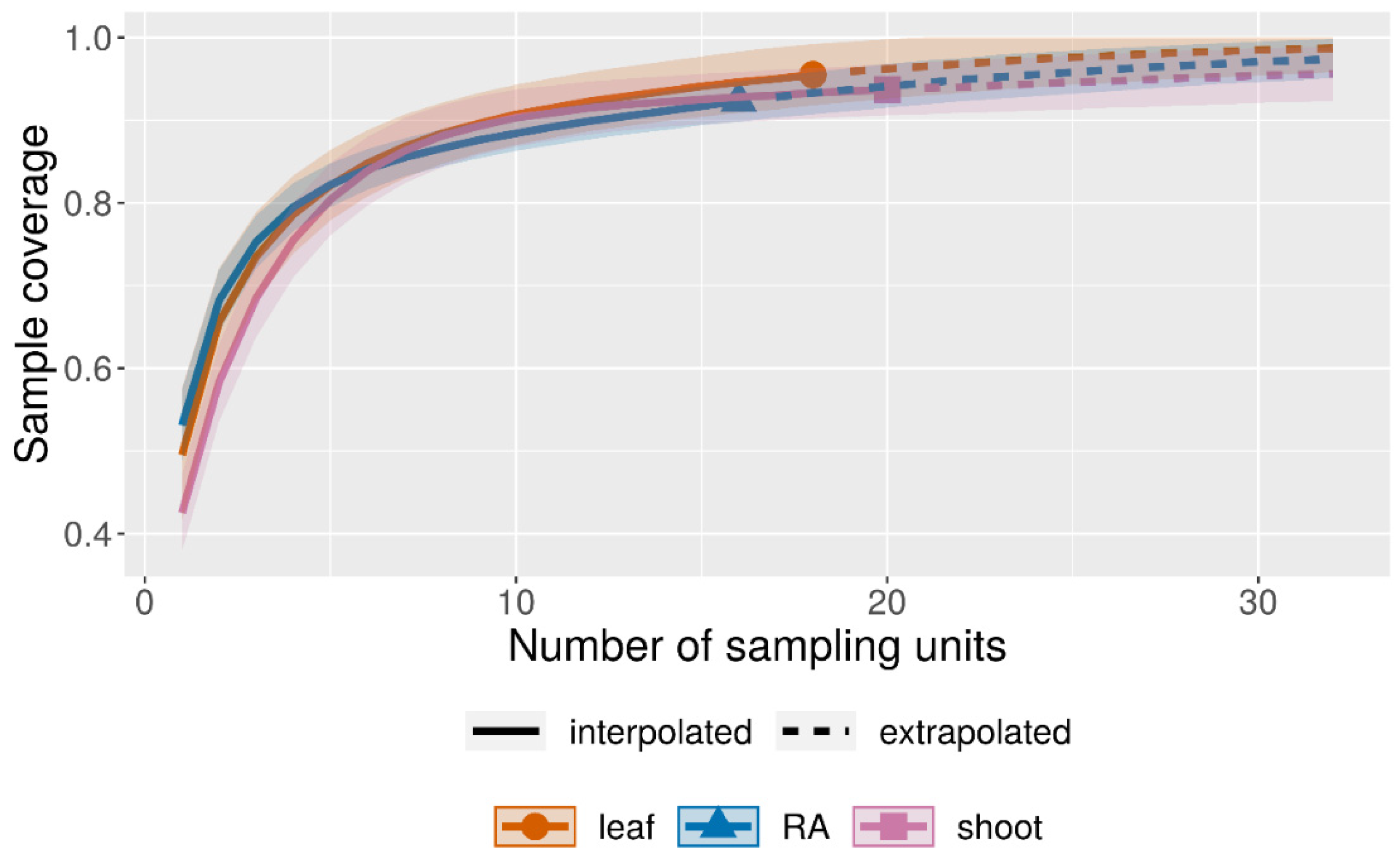

References
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defant, A. Physical Oceanography; Pergamon: New York, NY, USA, 1961; Volume 1. [Google Scholar]
- Medail, F.; Quezel, P. Biodiversity Hotspots in the Mediterranean Basin: Setting Global Conservation Priorities. Conserv. Biol. 1999, 13, 1510–1513. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Marine Biodiversity of the Mediterranean Sea: Situation, Problems and Prospects for Future. Mar. Pollut. Bull. 2016, 40, 367–376. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Charbonnel, E.; Diviacco, G.; Meinesz, A.; Pergent, G.; Pergent-Martini, C.; Ruitton, S.; Tunesi, L. Préservation et Conservation des Herbiers à Posidonia Oceanica; Ramoge: Tunis, Tunesia, 2006; ISBN 2905540303. [Google Scholar]
- Mazzella, L.; Buia, M.C.; Gambi, M.C.; Lorenti, M.; Russo, G.F.; Scipione, M.B.; Zupo, V. Plant-animal trophic relationships in the Posidonia oceanica ecosystem of the Mediterranean Sea: A review. Plant-Anim. Interact. Mar. Benthos 1992, 46, 165–187. [Google Scholar]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. In Oceanography and Marine Biology: An Annual Review; Gibson, R.N., Atkinson, R.J.A., Gordon, J.D.M., Eds.; Taylor & Francis: Abingdon, UK, 2006; pp. 123–195. [Google Scholar]
- Cocito, S. Bioconstruction and biodiversity: Their mutual influence. Sci. Mar. 2004, 68, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; Volume 79, ISBN 9780128151013. [Google Scholar]
- Schmidt, N.; El-khaled, Y.C.; Rossbach, F.I.; Wild, C. Fleshy red algae mats influence their environment in the Mediterranean Sea. Front. Mar. Sci. 2021, 8, 1626. [Google Scholar] [CrossRef]
- Lepoint, G.; Balancier, B.; Gobert, S. Seasonal and depth-related biodiversity of leaf epiphytic Cheilostome Bryozoa in a Mediterranean Posidonia oceanica meadow. Cah. Biol. Mar. 2014, 55, 57–67. [Google Scholar]
- Stachowicz, J.J. The Structure of Ecological Communities. Bioscience 2001, 51, 235–246. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Hay, M.E. Facultative mutualism between an herbivorous crab and a coralline alga: Advantages of eating noxious seaweeds. Oecologia 1996, 105, 377–387. [Google Scholar] [CrossRef]
- Duffy, J.E. Amphipods on seaweeds: Partners or pests? Oecologia 1990, 83, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Tomas, F.; Turon, X. Seasonal and small-scale spatial variability of herbivory pressure on the temperate seagrass Posidonia oceanica. Mar. Ecol. Prog. Ser. 2005, 301, 95–107. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Ceccherelli, G. Epiphyte assemblages of the Mediterranean seagrass Posidonia oceanica: An overview. Mar. Ecol. 2016, 37, 3–41. [Google Scholar] [CrossRef]
- Navone, A.; Bianchi, C.N.; Orru, P.; Ulzega, A. Saggio di cartografia geomorfologica e bionomica nel parco marino di Tavolara-Capo Coda di Cavallo (Sardegna nord-orientale). Oebalia 1992, XVII, 469–478. [Google Scholar]
- Bianchi, C.N.; Morri, C.; Navone, A. I popolamenti delle scogliere rocciose sommerse dell’Area Marina Protetta di Tavolara Punta Coda Cavallo (Sardegna nord-orientale). Sci. Rep. Port-Cros Natl. Park 2010, 24, 39–85. [Google Scholar]
- Casoli, E.; Bonifazi, A.; Giandomanico, A.; Gravina, M.F.; Russo, G.F.; Sandulli, R.; Donnarumma, L. Comparative Analysis of Mollusc Assemblages from Different Hard Bottom Habitats in the Central Tyrrhenian Sea. Diversity 2019, 11, 74. [Google Scholar] [CrossRef] [Green Version]
- Bonifazi, A.; Ventura, D.; Gravina, M.F.; Lasinio, G.J.; Belluscio, A.; Ardizzone, G.D. Unusual algal turfs associated with the rhodophyta Phyllophora crispa: Benthic assemblages along a depth gradient in the Central Mediterranean Sea. Estuar. Coast. Shelf Sci. 2017, 185, 77–93. [Google Scholar] [CrossRef]
- Casoli, E.; Bonifazi, A.; Ardizzone, G.; Gravina, M.F. How algae influence sessile marine organisms: The tube worms case of study. Estuar. Coast. Shelf Sci. 2016, 178, 12–20. [Google Scholar] [CrossRef]
- Rossbach, F.I.; Casoli, E.; Beck, M.; Wild, C. Mediterranean Red Macro Algae Mats as Habitat for High Abundances of Serpulid Polychaetes. Diversity 2021, 13, 265. [Google Scholar] [CrossRef]
- Kostylev, E.F.; Tkachenko, F.P.; Tretiak, I.P. Establishment of Zernov’s Phyllophora field marine reserve: Protection and restoration of a unique ecosystem. Ocean Coast. Manag. 2010, 53, 203–208. [Google Scholar] [CrossRef]
- Koukousioura, O.; Dimiza, M.D.; Triantaphyllou, M.V.; Hallock, P. Living benthic foraminifera as an environmental proxy in coastal ecosystems: A case study from the Aegean Sea (Greece, NE Mediterranean). J. Mar. Syst. 2011, 88, 489–501. [Google Scholar] [CrossRef]
- Sciuto, F.; Sanfilippo, R.; Alongi, G.; Catra, M.; Serio, D.; Bejaoui, S.; Leonardi, R.; Viola, A.; Rosso, A. First data on ostracods and foraminifera living in Cystoseira communities in western Ionian Sea (southern Italy, Mediterranean Sea). Mediterr. Mar. Sci. 2017, 18, 64–76. [Google Scholar]
- Donnarumma, L.; Sandulli, R.; Appolloni, L.; Russo, G.F. Assessing molluscs functional diversity within different coastal habitats of Mediterranean marine protected areas. Ecol. Quest. 2018, 29, 35–51. [Google Scholar] [CrossRef] [Green Version]
- Buonocore, E.; Donnarumma, L.; Appolloni, L.; Miccio, A.; Russo, G.F.; Franzese, P.P. Marine natural capital and ecosystem services: An environmental accounting model. Ecol. Modell. 2020, 424, 109029. [Google Scholar] [CrossRef]
- Holzmann, M.; Habura, A.; Giles, H.; Bowser, S.S.; Pawlowski, J. Freshwater foraminiferans revealed by analysis of environmental DNA samples. J. Eukaryot. Microbiol. 2003, 50, 135–139. [Google Scholar] [CrossRef]
- Siemensma, F.; Apothéloz-Perret-Gentil, L.; Holzmann, M.; Clauss, S.; Völcker, E.; Pawlowski, J. Taxonomic revision of freshwater foraminifera with the description of two new agglutinated species and genera. Eur. J. Protistol. 2017, 60, 28–44. [Google Scholar] [CrossRef]
- Alve, E. Colonization of new habitats by benthic foraminifera: A review. Earth Sci. Rev. 1999, 46, 167–185. [Google Scholar] [CrossRef]
- Carnahan, E.A.; Hoare, A.M.; Hallock, P.; Lidz, B.H.; Reich, C.D. Foraminiferal assemblages in Biscayne Bay, Florida, USA: Responses to urban and agricultural influence in a subtropical estuary. Mar. Pollut. Bull. 2009, 59, 221–233. [Google Scholar] [CrossRef]
- Milker, Y.; Schmiedl, G. A taxonomic guide to modern benthic shelf foraminifera of the western Mediterranean sea. Palaeontol. Electron. 2012, 15, 1–134. [Google Scholar] [CrossRef]
- Casieri, S.; Frezza, V.; Mancini, S.; Carboni, M.G. Living sessile epiphytic foraminifera from Posidonia oceanica meadows of Ischia and Ponza Islands. Giornate Paleontol. 1993, 8, 9–13. [Google Scholar]
- Semeniuk, T.A. Spatial variability in epiphytic foraminifera from micro-to regional scale. J. Foraminifer. Res. 2000, 30, 99–109. [Google Scholar] [CrossRef]
- Mateu-Vicens, G.; Khokhlova, A.; Sebastian-Pastor, T. Epiphytic foraminiferal indices as bioindicators in Mediterranean seagrass meadows. J. Foraminifer. Res. 2014, 44, 325–339. [Google Scholar] [CrossRef]
- Cimerman, F.; Langer, M.R. Mediterranean Foraminifera; Slovenska Akademija Znanosti in Umetnosti: Ljubljana, Slovenia, 1991; ISBN 9789401054805. [Google Scholar]
- Langer, M.R. Epiphytic foraminifera. Mar. Micropaleontol. 1993, 20, 235–265. [Google Scholar] [CrossRef]
- Langer, M. Recent epiphytic foraminifera from Vulcano (Mediterranean Sea). Rev. Paléobiologie 1988, 2, 827–832. [Google Scholar]
- Mateu-Vicens, G.; Box, A.; Deudero, S.; Rodríguez, B. Comparative analysis of epiphytic foraminifera in sediments colonized by seagrass Posidonia oceanica and invasive macroalgae Caulerpa spp. J. Foraminifer. Res. 2010, 40, 134–147. [Google Scholar] [CrossRef]
- Lee, J.J. Algal symbiosis in larger foraminifera. Symbiosis 2006, 42, 63–75. [Google Scholar]
- Ross, C.A. Biology and Ecology of Marginopora vertebralis (Foraminiferida), Great Barrier Reef. J. Protozool. 1972, 19, 181–193. [Google Scholar] [CrossRef]
- Leutenegger, S. Symbiosis in benthic foraminifera: Specificity and host adaptations. J. Foraminifer. Res. 1984, 14, 16–35. [Google Scholar] [CrossRef]
- Langer, M.R. Assessing the contribution of foraminiferan protists to global ocean carbonate production. J. Eukaryot. Microbiol. 2008, 55, 163–169. [Google Scholar] [CrossRef]
- Uthicke, S.; Thompson, A.; Schaffelke, B. Effectiveness of benthic foraminiferal and coral assemblages as water quality indicators on inshore reefs of the Great Barrier Reef, Australia. Coral Reefs 2010, 29, 209–225. [Google Scholar] [CrossRef] [Green Version]
- Uthicke, S.; Nobes, K. Benthic Foraminifera as ecological indicators for water quality on the Great Barrier Reef. Estuar. Coast. Shelf Sci. 2008, 78, 763–773. [Google Scholar] [CrossRef]
- Hallock, P.; Lidz, B.H.; Cockey-Burkhard, E.M.; Donnelly, K.B. Foraminifera as bioindicators in coral reef assessment and monitoring: The foram index. Environ. Monit. Assess. 2003, 81, 221–238. [Google Scholar] [CrossRef]
- Pawlowski, J.; Esling, P.; Lejzerowicz, F.; Cedhagen, T.; Wilding, T.A. Environmental monitoring through protist next-generation sequencing metabarcoding: Assessing the impact of fish farming on benthic foraminifera communities. Mol. Ecol. Resour. 2014, 14, 1129–1140. [Google Scholar] [CrossRef] [Green Version]
- Prazeres, M.; Roberts, T.E.; Pandolfi, J.M. Variation in sensitivity of large benthic Foraminifera to the combined effects of ocean warming and local impacts. Sci. Rep. 2017, 7, 45227. [Google Scholar] [CrossRef] [Green Version]
- Prazeres, M.; Ainsworth, T.; Roberts, T.E.; Pandolfi, J.M.; Leggat, W. Symbiosis and microbiome flexibility in calcifying benthic foraminifera of the great Barrier Reef. Microbiome 2017, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schlitzer, R. Ocean Data View 2016. Available online: http://odv.awi.de (accessed on 21 December 2021).
- Kikuchi, T.; Pérès, J.M. Animal communities in seagrass beds: A review. In Seagrass Ecosystems: A Scientific Perspective; McRoy, C.P., Helfferich, C., Eds.; Marcel Dekker: New York, NY, USA, 1967; pp. 147–193. [Google Scholar]
- Kikuchi, T. Handbook of Seagrass Biology: An Ecosystem Perspective; Phillips, R.C., McRoy, C.P., Eds.; Garland STPM Press: New York, NY, USA, 1980. [Google Scholar]
- Chao, A.; Chiu, C.-H. Nonparametric Estimation and Comparison of Species Richness. eLS 2016, 1–11. [Google Scholar] [CrossRef]
- Murray, J.W. British nearshore foraminiferids. In Synopses of the British Fauna (New Series); Kermack, D.M., Barnes, R.S.K., Eds.; Academic Press: London, UK, 1979; p. 68. ISBN 012 511850 3. [Google Scholar]
- Holbourn, A.; Henderson, A.S.; Macleod, N. Atlas of Benthic Foraminifera; Wiley-Blackwell: Hoboken, NJ, USA, 2013; ISBN 978-1-118-38980-5. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Novak, R. Spatial and seasonal distribution of the meiofauna in the seagrass Posidonia oceanica. Neth. J. Sea Res. 1982, 16, 380–388. [Google Scholar] [CrossRef]
- Lee, J.J.; Anderson, O.R. Symbiosis in foraminifera. In Biology of Foraminifera; Academic Press: London, UK, 1991; pp. 157–220. [Google Scholar]
- Glas, M.S.; Fabricius, K.E.; de Beer, D.; Uthicke, S. The O2, pH and Ca2+ Microenvironment of Benthic Foraminifera in a High CO2 World. PLoS ONE 2012, 7, e50010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harloff, J.; Mackensen, A. Recent benthic foraminiferal associations and ecology of the Scotia Sea and Argentine Basin. Mar. Micropaleontol. 1997, 31, 1–29. [Google Scholar] [CrossRef]
- Murray, J.W.; Alve, E. The distribution of agglutinated foraminifera in NW European seas: Baseline data for the interpretation of fossil assemblages. Palaeontol. Electron. 2011, 14, 1–41. [Google Scholar]
- Ramajo, L.; Lagos, N.A.; Duarte, C.M. Seagrass Posidonia oceanica diel pH fluctuations reduce the mortality of epiphytic forams under experimental ocean acidification. Mar. Pollut. Bull. 2019, 146, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Boudagher-Fadel, K.M. Biology and Evolutionary History of Larger Benthic Foraminifera. In Evolution and Geological Significance of Larger Benthic Foraminifera; UCL Press: London, UK, 2018; pp. 1–44. ISBN 9781911576938. [Google Scholar]
| Foraminiferal Assemblages of P. crispa Mats | |||||
|---|---|---|---|---|---|
| Source | Df | SS | R2 | F | p |
| site | 3 | 0.3898 | 0.2161 | 1.1028 | 0.313 |
| residual | 12 | 1.4138 | 0.7839 | ||
| total | 15 | 1.8036 | 1.0000 | ||
| Foraminiferal Assemblages of P. oceanica Meadows | |||||
| Source | Df | SS | R2 | F | p |
| sub-habitat | 1 | 4.5640 | 0.3886 | 25.7381 | 0.001 |
| site | 1 | 0.6696 | 0.0570 | 3.7759 | 0.006 |
| sub-habitat:site | 1 | 0.4833 | 0.0411 | 2.7252 | 0.024 |
| residual | 34 | 6.0291 | 0.5133 | ||
| total | 37 | 11.7459 | 1.0000 | ||
| Family | Species | Ecotype 1 | P. crispa Mats | P. oceanica Leaves | P. oceanica Shoots | ||||
|---|---|---|---|---|---|---|---|---|---|
| AVG | STDEV | AVG | STDEV | AVG | STDEV | ||||
| Most abundant | Cibicididae | Lobatula lobatula | B | 3147 | 1590 | 2127 | 1783 | 129 | 218 |
| Homotrematidae | Miniacina miniacea | A* | 1877 | 1366 | 0 | 0 | 4117 | 3602 | |
| Hauerinidae | Unknown | A* | 1245 | 855 | 716 | 1330 | 0 | 0 | |
| Discorbinellidae | Discorbinella bertheloti | B | 472 | 338 | 931 | 1076 | 249 | 362 | |
| Spirillinidae | Sejunctella sp. | A* | 465 | 505 | 15 | 62 | 912 | 899 | |
| Planorbulinida | Planorbulina mediterranensis | A* | 365 | 266 | 353 | 484 | 132 | 252 | |
| Hauerinidae | Miliolinella subrotunda | D* | 169 | 285 | 25 | 67 | 360 | 506 | |
| Hauerinidae | Quinqueloculina seminula | D* | 163 | 201 | 0 | 0 | 281 | 388 | |
| Ammoniidae | Ammonia beccari | B | 86 | 275 | 723 | 605 | 119 | 248 | |
| Trochamminidae | Lepidodeuterammina ochracea | A* | 71 | 161 | 28 | 82 | 357 | 580 | |
| LBF | Peneroplidae | Peneroplis pertusus | SB | 153 | 239 | 0 | 0 | 66 | 143 |
| Peneroplidae | Peneroplis planatus | SB | 17 | 37 | 0 | 0 | 0 | 0 | |
| Soritidae | Sorites orbiculus | SB | 13 | 41 | 37 | 123 | 0 | 0 | |
| Fischerinidae | Vertebralina striata | SB | 0 | 0 | 0 | 0 | 53 | 122 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossbach, F.I.; Merk, B.; Wild, C. High Diversity and Abundance of Foraminifera Associated with Mediterranean Benthic Red Algae Mats. Diversity 2022, 14, 21. https://doi.org/10.3390/d14010021
Rossbach FI, Merk B, Wild C. High Diversity and Abundance of Foraminifera Associated with Mediterranean Benthic Red Algae Mats. Diversity. 2022; 14(1):21. https://doi.org/10.3390/d14010021
Chicago/Turabian StyleRossbach, Felix Ivo, Benedikt Merk, and Christian Wild. 2022. "High Diversity and Abundance of Foraminifera Associated with Mediterranean Benthic Red Algae Mats" Diversity 14, no. 1: 21. https://doi.org/10.3390/d14010021
APA StyleRossbach, F. I., Merk, B., & Wild, C. (2022). High Diversity and Abundance of Foraminifera Associated with Mediterranean Benthic Red Algae Mats. Diversity, 14(1), 21. https://doi.org/10.3390/d14010021








