Biodiversity Monitoring in Mediterranean Marine Protected Areas: Scientific and Methodological Challenges
Abstract
1. Introduction
2. Monitoring as an Inventory
3. Monitoring as Surveillance
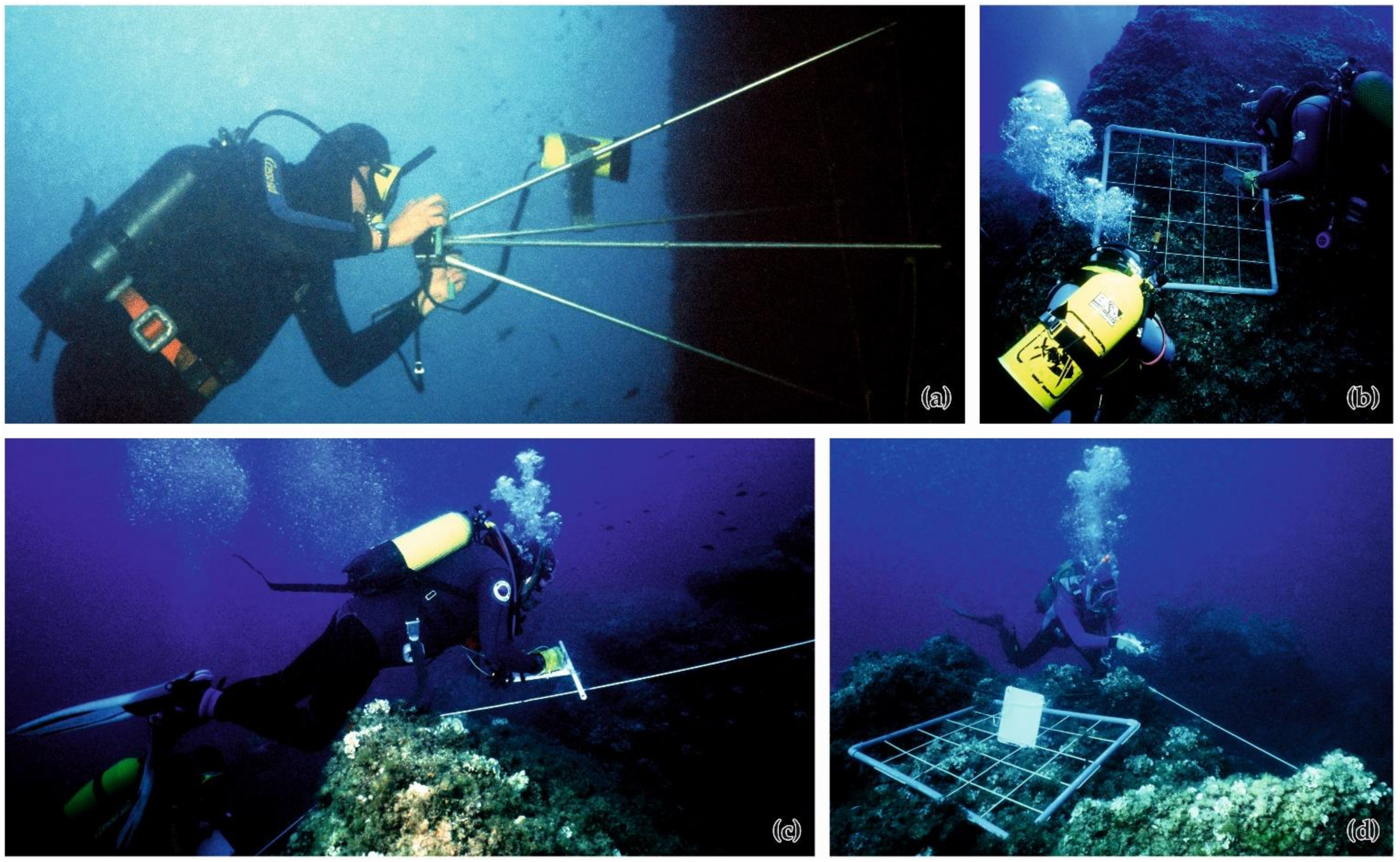
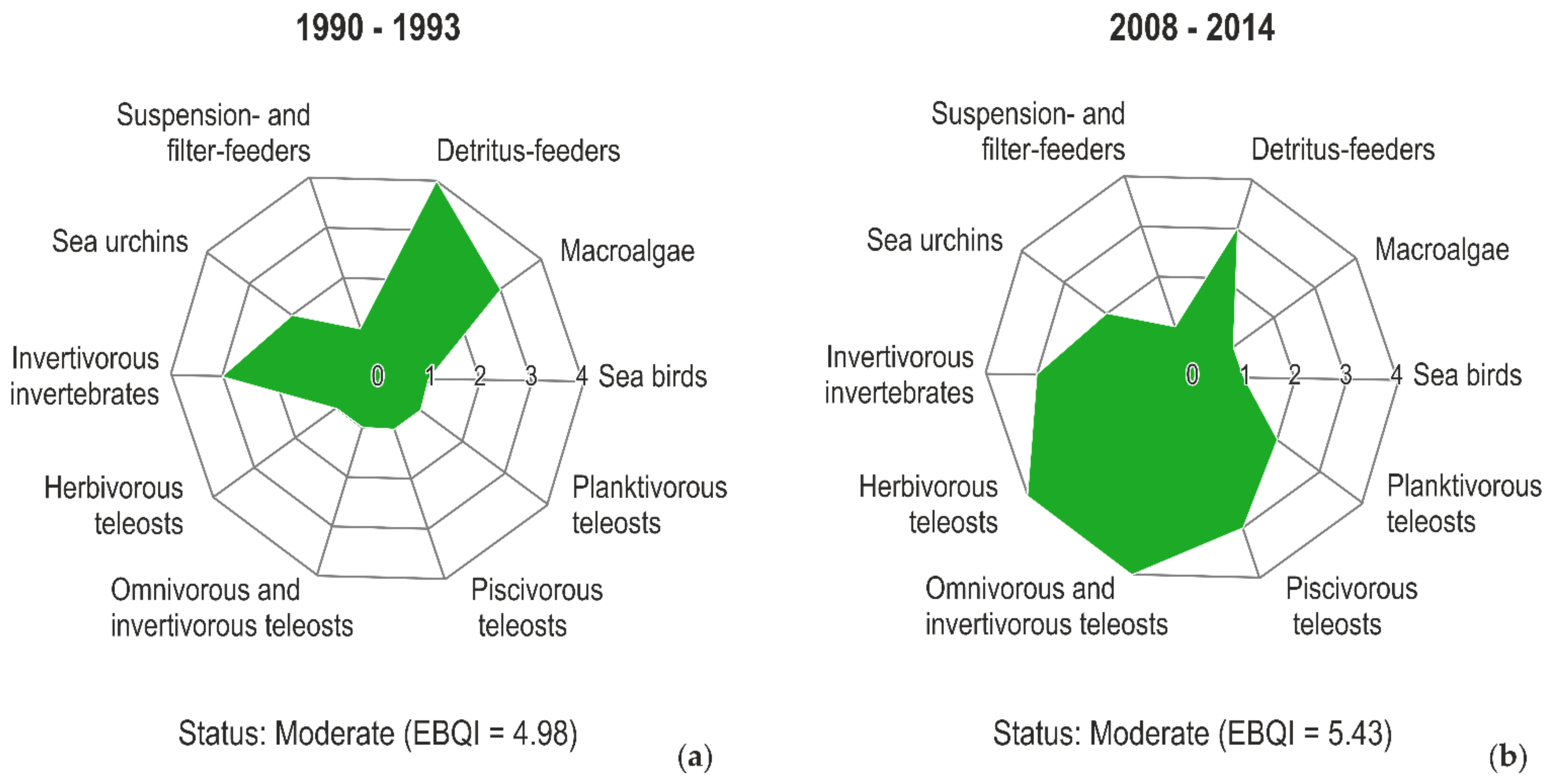
4. What to Monitor?
- contain numerous species;
- large dimensions;
- wide distribution in the different habitats of the MPA;
- sufficiently simple identification;
- easy and productive sampling, possibly with non-destructive methods.
5. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeLong, D.C., Jr. Defining biodiversity. Wildl. Soc. Bull. 1996, 24, 738–749. [Google Scholar]
- Boudouresque, C.F. Insights into the diversity of the biodiversity concept. Sci. Rep. Port-Cros Natl. Park 2014, 28, 65–86. [Google Scholar]
- Swingland, I.R. Biodiversity, definition of. In Encyclopedia of Biodiversity; Levin, S.A., Ed.; Academic Press: New York, NY, USA, 2001; Volume 1, pp. 377–391. [Google Scholar]
- Boudouresque, C.F.; Verlaque, M. Nature conservation, Marine Protected Areas, sustainable development and the flow of invasive species to the Mediterranean Sea. Sci. Rep. Port-Cros Natl. Park 2005, 21, 29–54. [Google Scholar]
- Eble, J.A.; Daly-Engel, T.S.; DiBattista, J.D.; Koziol, A.; Gaither, M.R. Marine environmental DNA: Approaches, applications, and opportunities. Adv. Mar. Biol. 2020, 86, 141–169. [Google Scholar] [PubMed]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- Ortega-Rubio, A.; Olmos-Martínez, E.; Blázquez, M.C. Socioecology and biodiversity conservation. Diversity 2021, 13, 442. [Google Scholar] [CrossRef]
- Ormond, R.F.G. Marine biodiversity: Causes and consequences. J. Mar. Biol. Assoc. U. K. 1996, 76, 151–152. [Google Scholar] [CrossRef]
- Sperandii, M.G.; Barták, V.; Carboni, M.; Acosta, A.T.R. Getting the measure of the biodiversity crisis in Mediterranean coastal habitats. J. Ecol. 2021, 109, 1224–1235. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea biodiversity between the legacy from the past and a future of change. In Life in the Mediterranean Sea: A Look at Habitat Changes; Stambler, N., Ed.; Nova Science: New York, NY, USA, 2012; pp. 1–55. [Google Scholar]
- Boudouresque, C.F.; Ruitton, S.; Bianchi, C.N.; Chevaldonné, P.; Fernandez, C.; Harmelin-Vivien, M.; Ourgaud, M.; Pasqualini, V.; Perez, T.; Pergent, G.; et al. Terrestrial versus marine diversity of ecosystems—and the winner is: The marine realm. In Proceedings of the 5th Mediterranean Symposium on Marine Vegetation, Portorož, Slovenia, 27–28 October 2014; Langar, H., Bouafif, C., Ouerghi, A., Eds.; UNEP/MAP–RAC/SPA: Tunis, Tunisia, 2014; pp. 11–25. [Google Scholar]
- Kidd, A.H.; Kidd, R.M. Aquarium visitors’ perceptions and attitudes toward the importance of marine biodiversity. Psychol. Rep. 1997, 81 (Suppl. 3), 1083–1088. [Google Scholar] [CrossRef]
- Pittman, S.; Kneib, R.; Simenstad, C.; Nagelkerken, I. Seascape ecology: Application of landscape ecology to the marine environment. Mar. Ecol. Prog. Ser. 2011, 427, 187–302. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Training scientific divers—Italian style. Ocean Chall. 2000, 10, 25–29. [Google Scholar]
- Cattaneo-Vietti, R.; Mojetta, A. The essential role of diving in marine biology. Bull. Environ. Life Sci. 2021, 3, 1–44. [Google Scholar]
- Gatti, G.; Bianchi, C.N.; Parravicini, V.; Rovere, A.; Peirano, A.; Montefalcone, M.; Massa, F.; Morri, C. Ecological change, sliding baselines and the importance of historical data: Lessons from combining observational and quantitative data on a temperate reef over 70 years. PLoS ONE 2015, 10, e0118581. [Google Scholar] [CrossRef]
- Gravina, M.F.; Bonifazi, A.; Del Pasqua, M.; Giampaoletti, J.; Lezzi, M.; Ventura, D.; Giangrande, A. Perception of changes in marine benthic habitats: The relevance of taxonomic and ecological memory. Diversity 2020, 12, 480. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Bianchi, C.N. Une idée neuve: La protection des espèces marines. In GIS Posidonie: Plus de 30 Ans au Service de la Protection et de la Gestion du Milieu Marin; Le Diréach, L., Boudouresque, C.F., Eds.; GIS Posidonie: Marseille, France, 2013; pp. 85–91. [Google Scholar]
- Jackson, J.B.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- O’Hara, C.C.; Frazier, M.; Halpern, B.S. At-risk marine biodiversity faces extensive, expanding, and intensifying human impacts. Science 2021, 372, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Nyström, M.; Norström, A.V.; Blenckner, T.; de la Torre-Castro, M.; Eklöf, J.S.; Folke, C.; Österblom, H.; Steneck, R.S.; Thyresson, M.; Troell, M. Confronting feedbacks of degraded marine ecosystems. Ecosystems 2012, 15, 695–710. [Google Scholar] [CrossRef]
- Roberson, L.A.; Beyer, H.L.; O’Hara, C.; Watson, J.E.M.; Dunn, D.C.; Halpern, B.S.; Klein, C.J.; Frazier, M.R.; Kuempel, C.D.; Williams, B.; et al. Multinational coordination required for conservation of at least 90% of marine species. Glob. Chang. Biol. 2021, 27, 6206–6216. [Google Scholar] [CrossRef] [PubMed]
- Meinesz, A. Protéger la Biodiversité Marine; Odile Jacob: Paris, France, 2021; pp. 1–331. [Google Scholar]
- Lotze, H.K. Marine biodiversity conservation. Curr. Biol. 2021, 31, R1190–R1195. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Estim, A.; Shaleh, S.R.M. Human relationship with the ocean needs a new paradigm. Acad. Lett. 2021, 2941. [Google Scholar] [CrossRef]
- Weigel, J.Y.; Mannle, K.O.; Bennett, N.J.; Carter, E.; Westlund, L.; Burgener, V.; Hoffman, Z.; Da Silva, A.S.; Kane, E.A.; Sanders, J.; et al. Marine protected areas and fisheries: Bridging the divide. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 199–215. [Google Scholar] [CrossRef]
- Guidetti, P.; Sala, E. Community-wide effects of marine reserves in the Mediterranean Sea. Mar. Ecol. Prog. Ser. 2007, 335, 43–56. [Google Scholar] [CrossRef]
- Fraschetti, S.; Guarnieri, G.; Bevilacqua, S.; Terlizzi, A.; Boero, F. Protection enhances community and habitat stability: Evidence from a Mediterranean Marine Protected Area. PLoS ONE 2013, 8, e81838. [Google Scholar] [CrossRef] [PubMed]
- García-Charton, J.A.; Pérez-Ruzafa, A.; Marcos, C.; Claudet, J.; Badalamenti, F.; Benedetti-Cecchi, L.; Falcón, J.M.; Milazzo, M.; Schembri, P.J.; Stobart, B.; et al. Effectiveness of European Atlanto-Mediterranean MPAs: Do they accomplish the expected effects on populations, communities and ecosystems? J. Nat. Conserv. 2008, 16, 193–221. [Google Scholar] [CrossRef]
- Scianna, C.; Niccolini, F.; Bianchi, C.N.; Guidetti, P. Applying organization science to assess the management performance of Marine Protected Areas: An exploratory study. J. Environ. Manag. 2018, 223, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Scianna, C.; Niccolini, F.; Giakoumi, S.; Di Franco, A.; Gaines, S.D.; Bianchi, C.N.; Scaccia, L.; Bava, S.; Cappanera, V.; Charbonnel, E.; et al. Organization Science improves management effectiveness of Marine Protected Areas. J. Environ. Manag. 2019, 240, 285–292. [Google Scholar] [CrossRef]
- Earle, S.A. Sea Change: A Message of the Oceans; Random House: New York, NY, USA, 1996; pp. 1–362. [Google Scholar]
- Campbell, L.M.; Gray, N.J. Area expansion versus effective and equitable management in international marine protected areas goals and targets. Mar. Policy 2019, 100, 192–199. [Google Scholar] [CrossRef]
- Jameson, S.C.; Tupper, M.H.; Ridley, J.M. The three screen doors: Can marine “protected” areas be effective? Mar. Pollut. Bull. 2002, 44, 1117–1183. [Google Scholar] [CrossRef]
- Costanza, R.; Daly, H.E. Natural capital and sustainable development. Conserv. Biol. 1992, 6, 37–46. [Google Scholar] [CrossRef]
- Jones, M. (Ed.) Accounting for Biodiversity; Routledge: London, UK, 2014; pp. 1–322. [Google Scholar]
- Hutto, R.L.; Belote, R.T. Distinguishing four types of monitoring based on the questions they address. For. Ecol. Manag. 2013, 289, 183–189. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Bianchi, C.N. Il monitoraggio della biodiversità nelle Aree Marine Protette: Considerazioni scientifiche e metodologiche. Not. Soc. Ital. Biol. Mar. 2002, 41, 89–95. [Google Scholar]
- Foley, M.M.; Halpern, B.S.; Micheli, F.; Armsby, M.H.; Caldwell, M.R.; Crain, C.M.; Prahler, E.; Rohr, N.; Sivas, D.; Beck, M.W.; et al. Guiding ecological principles for marine spatial planning. Mar. Policy 2010, 34, 955–966. [Google Scholar] [CrossRef]
- Lee, S.T.; Kelly, M.; Langlois, T.J.; Costello, M.J. Baseline seabed habitat and biotope mapping for a proposed marine reserve. PeerJ 2015, 3, e1446. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N. Climate change and biological response in the marine benthos. Proc. Ital. Assoc. Oceanol. Limnol. 1997, 12, 3–20. [Google Scholar]
- Pickett, S.T.A.; Kolasa, J.; Armesto, J.J.; Collins, S.L. The ecological concept of disturbance and its expression at various hierarchical levels. Oikos 1989, 54, 129–136. [Google Scholar] [CrossRef]
- Pickett, S.T.A.; White, P.S. (Eds.) The Ecology of Natural Disturbance and Patch Dynamics; Academic Press: San Diego, CA, USA, 1986; pp. 1–472. [Google Scholar]
- Montefalcone, M.; Parravicini, V.; Bianchi, C.N. Quantification of coastal ecosystem resilience. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D.S., Eds.; Elsevier Academic Press: Waltham, MA, USA, 2011; Volume 10 (Ecohydrology and Restoration), pp. 49–70. [Google Scholar]
- Bianchi, C.N.; Azzola, A.; Parravicini, V.; Peirano, A.; Morri, C.; Montefalcone, M. Abrupt change in a subtidal rocky reef community coincided with a rapid acceleration of sea water warming. Diversity 2019, 11, 215. [Google Scholar] [CrossRef]
- Oprandi, A.; Mucerino, L.; De Leo, F.; Bianchi, C.N.; Morri, C.; Azzola, A.; Benelli, F.; Besio, G.; Ferrari, M.; Montefalcone, M. Effects of a severe storm on seagrass meadows. Sci. Total Environ. 2020, 748, 141373. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Boero, F.; Fonda Umani, S.; Morri, C.; Vacchi, M. Succession and change in marine ecosystems. Biol. Mar. Mediterr. 1998, 5, 117–135. [Google Scholar]
- Pool, R. Ecologists flirt with chaos. Science 1989, 243, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.H. A Critique for Ecology; Cambridge University Press: Cambridge, UK, 1991; pp. 1–366. [Google Scholar]
- Margalef, R. Our Biosphere; Ecology Institute: Oldendorfe/Luhe, Germany, 1997; pp. 1–194. [Google Scholar]
- Van Meerbeek, K.; Jucker, T.; Svenning, J.C. Unifying the concepts of stability and resilience in ecology. J. Ecol. 2021, 109, 3114–3132. [Google Scholar] [CrossRef]
- Coulson, T. We live in a changing world, but that shouldn’t mean we abandon the concept of equilibrium. Ecol. Lett. 2021, 24, 3–5. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Climate change and biological response in Mediterranean Sea ecosystems—A need for broad-scale and long-term research. Ocean. Chall. 2004, 13, 32–36. [Google Scholar]
- Ros, J.; Gili, J.M. Four decades of research on the Medes Islands. Contrib. Sci. 2015, 11, 75–83. [Google Scholar]
- Montefalcone, M.; Giovannetti, E.; Lasagna, R.; Parravicini, V.; Morri, C.; Albertelli, G. Diachronic analysis of a Posidonia oceanica meadows in the Marine Protected Area of Bergeggi. Biol. Mar. Mediterr. 2007, 14, 82–83. [Google Scholar]
- Rees, H.C.; Maddison, B.C.; Middleditch, D.J.; Patmore, J.R.; Gough, K.C. The detection of aquatic animal species using environmental DNA—a review of eDNA as a survey tool in ecology. J. Appl. Ecol. 2014, 51, 1450–1459. [Google Scholar] [CrossRef]
- Pawlowski, J.; Apothéloz-Perret-Gentil, L.; Altermatt, F. Environmental DNA: What’s behind the term? Clarifying the terminology and recommendations for its future use in biomonitoring. Mol. Ecol. 2020, 29, 4258–4264. [Google Scholar] [CrossRef]
- Pawlowski, J.; Bruce, K.; Panksep, K.; Aguirre, F.I.; Amafitano, S.; Apothéloz-Perret-Gentil, L.; Baussant, T.; Bouchez, A.; Carugati, L.; Cermakova, K.; et al. Environmental DNA metabarcoding for benthic monitoring: A review of sediment sampling and DNA extraction methods. Sci. Total Environ. 2021, 151783, in press. [Google Scholar] [CrossRef]
- Stat, M.; Huggett, M.J.; Bernasconi, R.; DiBattista, J.D.; Berry, T.E.; Newman, S.J.; Harvey, E.S.; Bunce, M. Ecosystem biomonitoring with eDNA: Metabarcoding across the tree of life in a tropical marine environment. Sci. Rep. 2017, 7, 12240. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, E.; Loiseau, N.; Valentini, A.; Arnal, V.; Boissery, P.; Dejean, T.; Deter, J.; Guellati, N.; Holon, F.; Juhel, J.B.; et al. Environmental DNA metabarcoding reveals and unpacks a biodiversity conservation paradox in Mediterranean marine reserves. Proc. R. Soc. B 2021, 288, 20210112. [Google Scholar] [CrossRef] [PubMed]
- Madduppa, H.; Cahyani, N.K.D.; Anggoro, A.W.; Subhan, B.; Jefri, E.; Sani, L.M.I.; Arafat, D.; Akbar, N.; Bengen, D.G. eDNA metabarcoding illuminates species diversity and composition of three phyla (chordata, mollusca and echinodermata) across Indonesian coral reefs. Biodivers. Conserv. 2021, 30, 3087–3114. [Google Scholar] [CrossRef]
- Mugnai, F.; Meglécz, E.; CoMBoMed Group; Abbiati, M.; Bavestrello, G.; Bertasi, F.; Bo, M.; Capa, M.; Chenuil, A.; Colangelo, M.A.; et al. Are well-studied marine biodiversity hotspots still blackspots for animal barcoding? Glob. Ecol. Conserv. 2021, 32, e01909. [Google Scholar] [CrossRef]
- Stowell, D.; Sueur, J. Ecoacoustics: Acoustic sensing for biodiversity monitoring at scale. Remote Sens. Ecol. Conserv. 2020, 6, 217–219. [Google Scholar] [CrossRef]
- Duarte, C.M.; Chapuis, L.; Collin, S.P.; Costa, D.P.; Devassy, R.P.; Eguiluz, V.M.; Erbe, C.; Gordon, T.A.C.; Halpern, B.S.; Harding, H.R.; et al. The soundscape of the Anthropocene ocean. Science 2021, 371, eaba4658. [Google Scholar] [CrossRef]
- Sueur, J.; Krause, B.; Farina, A. Acoustic biodiversity. Curr. Biol. 2021, 31, R1172–R1173. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, N.; Martire, M.L.; Farina, A.; Danovaro, R. Marine soundscape as an additional biodiversity monitoring tool: A case study from the Adriatic Sea (Mediterranean Sea). Ecol. Indic. 2017, 83, 13–20. [Google Scholar] [CrossRef]
- Brunoldi, M.; Bozzini, G.; Casale, A.; Corvisiero, P.; Grosso, D.; Magnoli, N.; Alessi, J.; Bianchi, C.N.; Mandich, A.; Morri, C.; et al. A permanent automated real-time passive acoustic monitoring system for bottlenose dolphin conservation in the Mediterranean Sea. PLoS ONE 2016, 11, e0145362. [Google Scholar] [CrossRef]
- Alessi, J.; Mandich, A.; Wurtz, M.; Paoli, C.; Bianchi, C.N.; Morri, C.; Povero, P.; Brunoldi, M.; Bozzini, G.; Casale, A.; et al. Eavesdropping on dolphins: Investigating the habits of bottlenose dolphins (Tursiops truncatus) through fixed acoustic stations. PLoS ONE 2019, 14, e0226023. [Google Scholar] [CrossRef]
- Di Iorio, L.; Audax, M.; Deter, J.; Holon, F.; Lossent, J.; Gervaise, C.; Boissery, P. Biogeography of acoustic biodiversity of NW Mediterranean coralligenous reefs. Sci. Rep. 2021, 11, 16991. [Google Scholar] [CrossRef]
- La Manna, G.; Picciulin, M.; Crobu, A.; Perretti, F.; Ronchetti, F.; Manghi, M.; Ruiu, A.; Ceccherelli, G. Marine soundscape and fish biophony of a Mediterranean marine protected area. PeerJ 2021, 9, e12551. [Google Scholar] [CrossRef]
- Desiderà, E.; Guidetti, P.; Panzalis, P.; Navone, A.; Valentini-Poirrier, C.A.; Boissery, P.; Gervaise, C.; Di Iorio, L. Acoustic fish communities: Sound diversity of rocky habitats reflects fish species diversity. Mar. Ecol. Prog. Ser. 2019, 608, 183–197. [Google Scholar] [CrossRef]
- Pieretti, N.; Danovaro, R. Acoustic indexes for marine biodiversity trends and ecosystem health. Philos. Trans. R. Soc. B 2020, 375, 20190447. [Google Scholar] [CrossRef]
- Mooney, T.A.; Di Iorio, L.; Lammers, M.; Lin, T.H.; Nedelec, S.L.; Parsons, M.; Radford, C.; Urban, E.; Stanley, J. Listening forward: Approaching marine biodiversity assessments using acoustic methods. R. Soc. Open Sci. 2020, 7, 201287. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, P.J. Technological advances in biodiversity monitoring: Applicability, opportunities and challenges. Curr. Opin. Environ. Sustain. 2020, 45, 36–41. [Google Scholar] [CrossRef]
- Cappo, M.; Harvey, E.; Shortis, M. Counting and measuring fish with baited video techniques—an overview. In Cutting-Edge Technologies in Fish and Fisheries Science; Lyle, J.M., Furlani, D.M., Buxton, C.D., Eds.; Australian Society for Fish Biology: Hobart, Australia, 2006; pp. 101–114. [Google Scholar]
- La Manna, G.; Guala, I.; Grech, D.; Perretti, F.; Ronchetti, F.; Manghi, M.; Ruiu, A.; Ceccherelli, G. Performance of a baited underwater video system vs. the underwater visual census technique in assessing the structure of fish assemblages in a Mediterranean marine protected area. Mediterr. Mar. Sci. 2021, 22, 480–495. [Google Scholar] [CrossRef]
- Neigel, J.E. Species–area relationships and marine conservation. Ecol. Appl. 2003, 13 (Suppl. 1), 138–145. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C.; Pichon, M.; Benzoni, F.; Colantoni, P.; Baldelli, G.; Sandrini, M. Dynamics and pattern of coral recolonization following the 1998 bleaching event in the reefs of the Maldives. In Proceedings of the 10th International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004; Suzuki, Y., Nakamori, T., Hidaka, M., Kayanne, H., Casareto, B.E., Nadaoka, K., Yamano, H., Tsuchiya, M., Eds.; Japanese Coral Reef Society: Tokyo, Japan, 2006; pp. 30–37. [Google Scholar]
- Bianchi, C.N.; Dando, P.R.; Morri, C. Increased biodiversity of sessile epibenthos at subtidal hydrothermal vents: Seven hypotheses based on observations at Milos Island, Aegean Sea. Adv. Oceanogr. Limnol. 2011, 2, 1–31. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Cocito, S.; Diviacco, G.; Dondi, N.; Fratangeli, F.; Montefalcone, M.; Parravicini, V.; Rovere, A.; Sgorbini, S.; Vacchi, M.; et al. The park never born: Outcome of a quarter of a century of inaction on the sea-floor integrity of a proposed but not established Marine Protected Area. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1209–1228. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Ardizzone, G.D.; Belluscio, A.; Colantoni, P.; Diviacco, G.; Morri, C.; Tunesi, L. Benthic cartography. Biol. Mar. Mediterr. 2004, 11, 347–370. [Google Scholar]
- Ozenda, P. Ecological Mapping and Its Applications; Masson: Paris, France, 1986; pp. 1–160. [Google Scholar]
- Appolloni, L.; Sandulli, R.; Bianchi, C.N.; Russo, G.F. Spatial analyses of an integrated landscape-seascape territorial system: The case of the overcrowded Gulf of Naples, Southern Italy. J. Environ. Account. Manag. 2018, 6, 365–380. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Parravicini, V.; Montefalcone, M.; Rovere, A.; Morri, C. The challenge of managing marine biodiversity: A practical toolkit for a cartographic, territorial approach. Diversity 2012, 4, 419–452. [Google Scholar] [CrossRef]
- Coley, K. Mapping the seafloor with geodesy. Mar. Technol. Report. 2016, 59, 28–31. [Google Scholar]
- Marion, A.F. Esquisse d’une topographie zoologique du Golfe de Marseille. Ann. Mus. Hist. Nat. Marseille 1883, 1, 6–108. [Google Scholar]
- Vatova, A. Le zoocenosi bentoniche dell’Adriatico. Boll. Pesca Piscic. Idrobiol. 1946, 1, 131–135. [Google Scholar]
- Colantoni, P. Mapping of the Posidonia oceanica meadows. Riv. Marittima 1995, 12, 116–122. [Google Scholar]
- Wedding, L.M.; Lepczyk, C.A.; Pittman, S.J.; Friedlander, A.M.; Jorgensen, S. Quantifying seascape structure: Extending terrestrial spatial pattern metrics to the marine realm. Mar. Ecol. Prog. Ser. 2011, 427, 219–232. [Google Scholar] [CrossRef]
- Augier, H. Inventory and Classification of the Marine Benthic Biocoenoses of the Mediterranean; (Nature and Environment Series 25); Council of Europe: Strasbourg, France, 1982; pp. 1–57.
- Canessa, M.; Montefalcone, M.; Bavestrello, G.; Povero, P.; Coppo, S.; Morri, C.; Bianchi, C.N. Fishery maps contain approximate but useful information for inferring the distribution of marine habitats of conservation interest. Estuar. Coast. Shelf Sci. 2017, 187, 74–83. [Google Scholar] [CrossRef]
- Rowan, G.S.; Kalacska, M. A review of remote sensing of submerged aquatic vegetation for non-specialists. Remote Sens. 2021, 13, 623. [Google Scholar] [CrossRef]
- Pandian, P.K.; Ruscoe, J.P.; Shields, M.; Side, J.C.; Harris, R.E.; Kerr, S.A.; Bullen, C.R. Seabed habitat mapping techniques: An overview of the performance of various systems. Mediterr. Mar. Sci. 2009, 10, 29–44. [Google Scholar] [CrossRef]
- Lindenbaum, C.; Bennell, J.D.; Rees, E.I.S.; McClean, D.; Cook, W.; Wheeler, A.J.; Sanderson, W.G. Small-scale variation within a Modiolus modiolus (Mollusca: Bivalvia) reef in the Irish Sea: I. Seabed mapping and reef morphology. J. Mar. Biol. Assoc. U. K. 2008, 88, 133–141. [Google Scholar] [CrossRef]
- Poursanidis, D.; Traganos, D.; Reinartz, P.; Chrysoulakis, N. On the use of Sentinel-2 for coastal habitat mapping and satellite-derived bathymetry estimation using downscaled coastal aerosol band. Int. J. Appl. Earth Obs. Geoinf. 2019, 80, 58–70. [Google Scholar] [CrossRef]
- Pasqualini, V.; Pergent-Martini, C.; Fernandez, C.; Pergent, G. The use of airborne remote sensing for benthic cartography: Advantages and reliability. Int. J. Remote Sens. 1997, 18, 1167–1177. [Google Scholar] [CrossRef]
- Minghelli, A.; Vadakke-Chanat, S.; Chami, M.; Guillaume, M.; Migne, E.; Grillas, P.; Boutron, O. Estimation of bathymetry and benthic habitat composition from hyperspectral remote sensing data (BIODIVERSITY) using a semi-analytical approach. Remote Sens. 2021, 13, 1999. [Google Scholar] [CrossRef]
- Chust, G.; Galparsoro, I.; Borja, A.; Franco, J.; Uriarte, A. Coastal and estuarine habitat mapping, using LIDAR height and intensity and multi-spectral imagery. Estuar. Coast. Shelf Sci. 2008, 78, 633–643. [Google Scholar] [CrossRef]
- Jaubert, J.M.; Chisholm, J.R.; Minghelli-Roman, A.; Marchioretti, M.; Morrow, J.H.; Ripley, H.T. Re-evaluation of the extent of Caulerpa taxifolia development in the northern Mediterranean using airborne spectrographic sensing. Mar. Ecol. Prog. Ser. 2003, 263, 75–82. [Google Scholar] [CrossRef][Green Version]
- Ventura, D.; Bonifazi, A.; Gravina, M.F.; Belluscio, A.; Ardizzone, G. Mapping and classification of ecologically sensitive marine habitats using unmanned aerial vehicle (UAV) imagery and object-based image analysis (OBIA). Remote Sens. 2018, 10, 1331. [Google Scholar] [CrossRef]
- Mumby, P.J.; Edwards, A.J. Mapping marine environments with IKONOS imagery: Enhanced spatial resolution can deliver greater thematic accuracy. Remote Sens. Environ. 2002, 82, 248–257. [Google Scholar] [CrossRef]
- Rende, S.F.; Bosman, A.; Di Mento, R.; Bruno, F.; Lagudi, A.; Irving, A.D.; Dattola, L.; Di Giambattista, L.; Lanera, P.; Proietti, R.; et al. Ultra-high-resolution mapping of Posidonia oceanica (L.) Delile meadows through acoustic, optical data and object-based image classification. J. Mar. Sci. Eng. 2020, 8, 647. [Google Scholar] [CrossRef]
- Vassallo, P.; Bianchi, C.N.; Paoli, C.; Holon, F.; Navone, A.; Bavestrello, G.; Cattaneo-Vietti, R.; Morri, C. A predictive approach to benthic marine habitat mapping: Efficacy and management implications. Mar. Pollut. Bull. 2018, 131, 218–232. [Google Scholar] [CrossRef]
- Mata, D.; Úbeda, J.; Fernández-Sánchez, A. Modelling of the reef benthic habitat distribution within the Cabrera National Park (Western Mediterranean Sea). Ann. GIS 2021, 27, 285–298. [Google Scholar] [CrossRef]
- Fanelli, E.; Delbono, I.; Ivaldi, R.; Pratellesi, M.; Cocito, S.; Peirano, A. Cold-water coral Madrepora oculata in the eastern Ligurian Sea (NW Mediterranean): Historical and recent findings. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 965–975. [Google Scholar] [CrossRef]
- Cocito, S.; Bianchi, C.N.; Degl’Innocenti, F.; Forti, S.; Morri, C.; Sgorbini, S.; Zattera, A. The use of environmental descriptors for the ecological analysis of the coastal marine landscape—An example. SItE Atti 1991, 13, 65–68. [Google Scholar]
- Musard, O.; Le Dû-Blayo, L.; Francour, P.; Beurier, J.P.; Feunteun, E.; Talassinos, L. (Eds.) Underwater Seascapes: From Geographical to Ecological Perspectives; Springer: Cham, Switzerland, 2014; pp. 1–312. [Google Scholar]
- Rovere, A.; Parravicini, V.; Firpo, M.; Morri, C.; Bianchi, C.N. Combining geomorphologic, biological and accessibility values for marine natural heritage evaluation and conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 541–552. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Zurlini, G. Criteri e prospettive di una classificazione ecotipologica dei sistemi marini costieri italiani. Acqua Aria 1984, 8, 785–796. [Google Scholar]
- Rovere, M.; Parravicini, V.; Donato, M.; Riva, C.; Diviacco, G.; Coppo, S.; Firpo, M.; Bianchi, C.N. Indagini sulle secche di Punta Manara: Un esempio di approccio ecotipologico. Biol. Mar. Mediterr. 2006, 13, 210–211. [Google Scholar]
- Rovere, A.; Vacchi, M.; Parravicini, V.; Morri, C.; Bianchi, C.N.; Firpo, M. Bringing geoheritage underwater: Methodological approaches to evaluation and mapping. Trav. Rech. Inst. Géogr. Lausanne 2010, 35, 65–80. [Google Scholar]
- Rovere, A.; Parravicini, V.; Vacchi, M.; Montefalcone, M.; Morri, C.; Bianchi, C.N.; Firpo, M. Geo-environmental cartography of the Marine Protected Area “Isola di Bergeggi” (Liguria, NW Mediterranean Sea). J. Maps 2010, 6, 505–519. [Google Scholar] [CrossRef]
- Rovere, A.; Vacchi, M.; Parravicini, V.; Bianchi, C.N.; Zouros, N.; Firpo, M. Bringing geoheritage underwater: Definitions, methods, and application in two Mediterranean marine areas. Environ. Earth Sci. 2011, 64, 133–142. [Google Scholar] [CrossRef]
- Gili, J.M.; Zabala, M.; Ros, J.D.; Olivella, I.; Ballesteros, E. Cartografia Bionòmica de les Illes Medes; Institut d’Estudis Catalans, Arxius de la Secció de Ciènces LXXIII: Barcelona, Spain, 1984. [Google Scholar]
- Parravicini, V.; Montefalcone, M.; Lasagna, R.; Rovere, A.; Morri, C.; Bianchi, C.N. Carta delle Biocenosi Marine dell’Area Marina Protetta ‘Isola di Bergeggi’; Aree Protette di Bergeggi: Bergeggi, Italy, 2007. [Google Scholar]
- Morri, C.; Bianchi, C.N.; Damiani, V.; Peirano, A.; Romeo, G.; Tunesi, L. Carta delle Biocenosi Marine Bentiche tra Punta della Chiappa e Sestri Levante (Mar Ligure); Enea, Centro Ricerche Energia Ambiente di Santa Teresa: La Spezia, Italy, 1986. [Google Scholar]
- Navone, A.; Bianchi, C.N. Riserva Marina di Tavolara Capo Coda Cavallo (Sardegna NE): Carta Bionomica dei Fondi Marini; Studio Navone: Olbia, Italy, 1992. [Google Scholar]
- Bianchi, C.N.; Morri, C.; Ugolini, U.; Sara, G.; Ferdeghini, F. Carta delle Biocenosi Marine; Parco Naturale Regionale di Portovenere: Portovenere, Italy, 2002. [Google Scholar]
- Diviacco, G.; Tunesi, L.; Bianchi, C.N.; Morri, C.; Cattaneo-Vietti, R. Carta dei Principali Popolamenti Bentonici; Area Marina Protetta di Portofino: Santa Margherita Ligure, Italy, 2004. [Google Scholar]
- Bianchi, C.N.; Morri, C. Carta delle Maggiori Biocenosi Bentiche delle Coste Pugliesi; Enea, Centro Ricerche Energia Ambiente di Santa Teresa: La Spezia, Italy, 1989. [Google Scholar]
- Bianchi, C.N.; Cinelli, F.; Morri, C. Carta Bionomica dei Mari Toscani; Regione Toscana: Firenze, Italy, 1993. [Google Scholar]
- Parenzan, P. Carte Biocenotiche dei Mari Pugliesi; Congedo: Galatina, Italy, 1983. [Google Scholar]
- Bianchi, C.N.; Peirano, A. Atlante delle Fanerogame Marine della Liguria: Posidonia Oceanica e Cymodocea Nodosa; ENEA, Centro Ricerche Ambiente Marino: La Spezia, Italy, 1995; pp. 1–146. [Google Scholar]
- Ardizzone, G.; Belluscio, A.; Criscoli, A. Atlante degli Habitat dei Fondali Marini del Lazio; Sapienza Università Editrice: Roma, Italy, 2018; pp. 1–390. [Google Scholar]
- Coppo, S.; Diviacco, G.; Montepagano, E. Nuovo Atlante degli Habitat Marini della Liguria; Regione Liguria: Genova, Italy, 2020; pp. 1–89. [Google Scholar]
- Burel, F.; Baudry, J.; Clergeau, P.; Constant, P.; Eybert, M.C. Approche spatiale des phénomènes écologiques: Échelle et hiérarchie. Bull. Ecol. 1992, 23, 93–101. [Google Scholar]
- Levin, S.A. The problem of pattern and scale in ecology. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Peterson, D.L.; Parker, V.T. (Eds.) Ecological Scale: Theory and Applications; Columbia University Press: New York, NY, USA, 1998; pp. 1–615. [Google Scholar]
- Sutherland, J.P. Multiple stable points in natural communities. Am. Nat. 1974, 108, 859–873. [Google Scholar] [CrossRef]
- Rahel, F.J. The hierarchical nature of community persistence: A problem of scale. Am. Nat. 1990, 136, 328–344. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Cinelli, F.; Morri, C. La carta bionomica dei mari toscani: Introduzione, criteri informativi e note esplicative. Atti Soc. Toscana Sci. Nat. Mem. Ser. A 1996, 102, 255–270. [Google Scholar]
- De Biasi, A.M. Biology of the Meloria shoals: Bionomic characterisation along three pilot transects. Biol. Mar. Mediterr. 1999, 6, 372–375. [Google Scholar]
- Rovere, A.; Ferraris, F.; Parravicini, V.; Navone, A.; Morri, C.; Bianchi, C.N. Characterization and evaluation of a marine protected area: Tavolara Punta Coda Cavallo (Sardinia, NW Mediterranean). J. Maps 2013, 9, 279–288. [Google Scholar] [CrossRef]
- Parravicini, V.; Rovere, A.; Vassallo, P.; Micheli, F.; Montefalcone, M.; Morri, C.; Paoli, C.; Albertelli, G.; Fabiano, M.; Bianchi, C.N. Understanding relationships between conflicting human uses and coastal ecosystems status: A geospatial modeling approach. Ecol. Indic. 2012, 19, 253–263. [Google Scholar] [CrossRef]
- Azzola, A.; Bavestrello, G.; Bertolino, M.; Bianchi, C.N.; Bo, M.; Enrichetti, F.; Morri, C.; Oprandi, A.; Toma, M.; Montefalcone, M. You cannot conserve a species that has not been found: The case of the marine sponge Axinella polypoides in Liguria, Italy. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 737–747. [Google Scholar] [CrossRef]
- Montefalcone, M.; Azzola, A. Ampliamento delle Conoscenze sulla Distribuzione e sullo Stato di Conservazione della Specie Prioritaria Scyllarides latus (Magnosa) nell’Area Marina Protetta delle Cinque Terre; Parco Nazionale delle Cinque Terre: Riomaggiore, Italy, 2020; pp. 1–54. [Google Scholar]
- Montefalcone, M.; Baudana, M.; Venturini, S.; Lasagna, R.; Bianchi, C.N.; Albertelli, G. Spatial distribution of Posidonia oceanica meadows in the Portofino marine protected area. Biol. Mar. Medit. 2006, 13, 90–91. [Google Scholar]
- Navone, A.; Deiana, A.; Panzalis, P.; Holon, F.; Sposino, P.; Pasta, S.; Parravicini, V.; Rovere, A.; Vacchi, M. Carta degli Habitat dell’Area Marina Protetta di Tavolara-Punta Coda Cavallo (Sardegna Nord-Orientale); Area Marina Protetta di Tavolara-Punta Coda Cavallo: Olbia, Italy, 2014. [Google Scholar]
- Montefalcone, M.; Oprandi, A. Caratterizzazione Cartografica e Definizione della Qualità Ecologica dell’Habitat Marino Prioritario Praterie di Posidonia oceanica nell’Area Marina Protetta delle Cinque Terre; Parco Nazionale delle Cinque Terre: Riomaggiore, Italy, 2020; pp. 1–96. [Google Scholar]
- Ardizzone, G.; Belluscio, A.; Maiorano, L. Long-term change in the structure of a Posidonia oceanica landscape and its reference for a monitoring plan. Mar. Ecol. 2006, 27, 299–309. [Google Scholar] [CrossRef]
- Barsanti, M.; Delbono, I.; Ferretti, O.; Peirano, A.; Bianchi, C.N.; Morri, C. Measuring change of Mediterranean coastal biodiversity: Diachronic mapping of the meadow of the seagrass Cymodocea nodosa (Ucria) Ascherson in the Gulf of Tigullio (Ligurian Sea, NW Mediterranean). Hydrobiologia 2007, 580, 35–41. [Google Scholar] [CrossRef]
- Descamp, P.; Holon, F.; Ballesta, L.; Guilbert, A.; Guillot, M.; Boissery, P.; Raimondino, V.; Deter, J. Fast and easy method for seagrass monitoring: Application of acoustic telemetry to precision mapping of Posidonia oceanica beds. Mar. Pollut. Bull. 2011, 62, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Montefalcone, M.; Rovere, A.; Parravicini, V.; Albertelli, G.; Morri, C.; Bianchi, C.N. Evaluating change in seagrass meadows: A time-framed comparison of side-scan sonar maps. Aquat. Bot. 2013, 104, 204–212. [Google Scholar] [CrossRef]
- Del Rosso, D. Cartografia Diacronica della Prateria di Posidonia oceanica Prospiciente il Comune di Bergeggi (SV): Analisi Critica di Affidabilità. Master’s Thesis, University of Genoa, Genoa, Italy, 2006. [Google Scholar]
- Leriche, A.; Boudouresque, C.F.; Bernard, G.; Bonhomme, P.; Denis, J. A one-century suite of seagrass bed maps: Can we trust ancient maps? Estuar. Coast. Shelf Sci. 2004, 59, 353–362. [Google Scholar] [CrossRef]
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G.; et al. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef] [PubMed]
- Burgos, E.; Montefalcone, M.; Ferrari, M.; Paoli, C.; Vassallo, P.; Morri, C.; Bianchi, C.N. Ecosystem functions and economic wealth: Trajectories of change in seagrass meadows. J. Clean. Prod. 2017, 168, 1108–1119. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Azzola, A.; Bertolino, M.; Betti, F.; Bo, M.; Cattaneo-Vietti, R.; Cocito, S.; Montefalcone, M.; Morri, C.; Oprandi, A.; et al. Consequences of the marine climate and ecosystem shift of the 1980-90s on the Ligurian Sea biodiversity (NW Mediterranean). Eur. Zool. J. 2019, 86, 458–487. [Google Scholar] [CrossRef]
- Cocito, S.; Sgorbini, S.; Bianchi, C.N. Aspects of the biology of the bryozoan Pentapora fascialis in the north-western Mediterranean. Mar. Biol. 1998, 131, 73–82. [Google Scholar] [CrossRef]
- Cocito, S.; Chiantore, M. Monitoring of natural animal populations. Biol. Mar. Mediterr. 2004, 11 (Suppl. 1), 309–345. [Google Scholar]
- Pressey, R.L. Conservation planning and biodiversity: Assembling the best data for the job. Conserv. Biol. 2004, 18, 1677–1681. [Google Scholar] [CrossRef]
- Azzola, A.; Bianchi, C.N.; Morri, C.; Oprandi, A.; Peirano, A.; Montefalcone, M. Population structure change in a temperate reef coral after a quarter of century. Estuar. Coast. Shelf Sci. 2021. under review. [Google Scholar]
- Grorud-Colvert, K.; Lester, S.E.; Airamé, S.; Neeley, E.; Gaines, S.D. Communicating marine reserve science to diverse audiences. Proc. Natl. Acad. Sci. USA 2010, 107, 18306–18311. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, C.N.; Peirano, A.; Salvati, E.; Morri, C. Assessing interannual and decadal changes in marine epibenthic assemblages through UW photography: An example from Punta Mesco, Ligurian Sea. Arch. Oceanogr. Limnol. 2001, 22, 83–86. [Google Scholar]
- Bianchi, C.N.; Pronzato, R.; Cattaneo-Vietti, R.; Benedetti-Cecchi, L.; Morri, C.; Pansini, M.; Chemello, R.; Milazzo, M.; Fraschetti, S.; Terlizzi, A.; et al. Mediterranean marine benthos: A manual of methods for its sampling and study. 6: Hard bottoms. Biol. Mar. Mediterr. 2004, 11 (Suppl. 1), 185–215. [Google Scholar]
- Mallet, D.; Pelletier, D. Underwater video techniques for observing coastal marine biodiversity: A review of sixty years of publications (1952–2012). Fish. Res. 2014, 154, 44–62. [Google Scholar] [CrossRef]
- Cánovas-Molina, A.; Montefalcone, M.; Bavestrello, G.; Cau, A.; Bianchi, C.N.; Morri, C.; Canese, S.; Bo, M. A new ecological index for the status of mesophotic megabenthic assemblages in the Mediterranean based on ROV photography and video footage. Cont. Shelf Res. 2016, 121, 13–20. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bo, M.; Morri, C.; Montefalcone, M.; Toma, M.; Bavestrello, G.; Tunesi, L.; Canese, S.; Giusti, M.; Salvati, E.; et al. Assessing the environmental status of temperate mesophotic reefs: A new, integrated methodological approach. Ecol. Indic. 2019, 102, 218–229. [Google Scholar] [CrossRef]
- Parravicini, V.; Morri, C.; Ciribilli, G.; Montefalcone, M.; Albertelli, G.; Bianchi, C.N. Size matters more than method: Visual quadrats vs. photography in measuring human impact on Mediterranean rocky reef communities. Estuar. Coast. Shelf Sci. 2009, 81, 358–367. [Google Scholar] [CrossRef]
- La Mesa, G.; Vacchi, M. Benthic fishes. Biol. Mar. Mediterr. 2004, 11, 371–405. [Google Scholar]
- Morrisey, D.J.; Turner, S.J.; MacDiarmid, A.B. Subtidal assemblages of soft substrata. In Studying Temperate Marine Environments; Kingsford, M., Battershill, C., Eds.; Canterbury University Press: Christchurch, New Zealand, 1998; pp. 194–226. [Google Scholar]
- Hamner, W.M. Underwater observations of blue-water plankton: Logistics, techniques, and safety procedures for divers at sea. Limnol. Oceanogr. 1975, 20, 1045–1051. [Google Scholar] [CrossRef]
- Azzola, A.; Atzori, F.; Bianchi, C.N.; Cadoni, N.; Frau, F.; Mora, F.; Morri, C.; Oprandi, A.; Orrù, P.E.; Montefalcone, M. Variability between observers does not hamper detecting change over time in a temperate reef. Mar. Environ. Res. 2021. under review. [Google Scholar]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, Q.D.; Knapp, S.; Stevenson, D.W.; Stevenson, J.; Blum, S.D.; Boom, B.M.; Borisy, G.G.; Buizer, J.L.; De Carvalho, M.R.; Cibrian, A.; et al. Mapping the biosphere: Exploring species to understand the origin, organization and sustainability of biodiversity. Syst. Biodivers. 2012, 10, 1–20. [Google Scholar] [CrossRef]
- International HapMap Consortium. A haplotype map of the human genome. Nature 2005, 437, 1299–1320. [Google Scholar] [CrossRef]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, H.R.; Leader-Williams, N.; Milner-Gulland, E.J.; Stuart, S.N. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Ballesteros, E.; Ben Maiz, N.; Boisset, F.; Bouladier, E.; Cinelli, F.; Cirik, S.; Cormaci, M.; Jeudy de Grissac, A.; Laborel, J.; et al. Livre Rouge “Gérard Vuigner” des Végétaux, Peuplements et Paysages Menacés de Méditerranée; UNEP/MAP: Athens, Greece, 1990; pp. 1–205. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Clucas, B.; McHugh, K.; Caro, T. Flagship species on covers of US conservation and nature magazines. Biodivers. Conserv. 2008, 17, 1517–1528. [Google Scholar] [CrossRef]
- Albert, C.; Luque, G.M.; Courchamp, F. The twenty most charismatic species. PLoS ONE 2018, 13, e0199149. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Avon, M.; Gravez, V. Les Espèces Marines à Protéger en Méditerranée; GIS Posidonie: Marseille, France, 1991; pp. 1–448. [Google Scholar]
- La Posta, S.; Mazzola, A.; Relini, G. Protezione della fauna marina e introduzione di specie alloctone. Boll. Mus. Ist. Biol. Univ. Genova 1992, 56–57, 1–281. [Google Scholar]
- Small, E. The new Noah’s Ark: Beautiful and useful species only. Part 1. Biodiversity conservation issues and priorities. Biodiversity 2011, 12, 232–247. [Google Scholar] [CrossRef]
- Small, E. The new Noah’s Ark: Beautiful and useful species only. Part 2. The chosen species. Biodiversity 2012, 13, 37–53. [Google Scholar] [CrossRef]
- Wilson, E.O. Biophilia; Harvard University Press: Cambridge, MA, USA, 1986; pp. 1–176. [Google Scholar]
- Kellert, S.R. The Value of Life: Biological Diversity and Human Society; Island Press: Washington, DC, USA, 1996; pp. 1–263. [Google Scholar]
- Montefalcone, M.; Vassallo, P.; Gatti, G.; Parravicini, V.; Paoli, C.; Morri, C.; Bianchi, C.N. The exergy of a phase shift: Ecosystem functioning loss in seagrass meadows of the Mediterranean Sea. Estuar. Coast. Shelf Sci. 2015, 156, 186–194. [Google Scholar] [CrossRef]
- Jørgensen, S.E.; Ludovisi, A.; Nielsen, S.N. The free energy and information embodied in the amino acid chains of organisms. Ecol. Model. 2010, 221, 2388–2392. [Google Scholar] [CrossRef]
- Barua, M. Mobilizing metaphors: The popular use of keystone, flagship and umbrella species concepts. Biodivers. Conserv. 2011, 20, 1427–1440. [Google Scholar] [CrossRef]
- Ducarme, F.; Luque, G.M.; Courchamp, F. What are “charismatic species” for conservation biologists. BioSci. Master Rev. 2013, 10, 1–8. [Google Scholar]
- Mannino, A.M.; Balistreri, P. Citizen science: A successful tool for monitoring marine biodiversity. Eur. J. Phicol. 2019, 54 (Suppl. 1), 99. [Google Scholar]
- Monk, J.; Ierodiaconou, D.; Bellgrove, A.; Laurenson, L. Using community-based monitoring with GIS to create habitat maps for a marine protected area in Australia. J. Mar. Biol. Assoc. U. K. 2008, 88, 865–871. [Google Scholar] [CrossRef]
- Kelly, R.; Fleming, A.; Pecl, G.T.; von Gönner, J.; Bonn, A. Citizen science and marine conservation: A global review. Philos. Trans. R. Soc. B 2020, 375, 20190461. [Google Scholar] [CrossRef]
- Kasten, P.; Jenkins, S.R.; Christofoletti, R.A. Participatory monitoring—A citizen science approach for coastal environments. Front. Mar. Sci. 2021, 8, 681969. [Google Scholar] [CrossRef]
- Piraino, S.; Fanelli, G.; Boero, F. Variability of species’ roles in marine communities: Change of paradigms for conservation priorities. Mar. Biol. 2002, 140, 1067–1074. [Google Scholar]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean bioconstructions along the Italian coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar]
- Braeckman, U.; Rabaut, M.; Vanaverbeke, J.; Degraer, S.; Vincx, M. Protecting the commons: The use of subtidal ecosystem engineers in marine management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2014, 24, 275–286. [Google Scholar] [CrossRef]
- Gambi, M.C.; Fresi, E.; Giangrande, A. Descrittori efficaci di comunità bentoniche. Nat. Sicil. S. IV 1982, 6 (Suppl. 3), 489–497. [Google Scholar]
- Morri, C.; Bellan-Santini, D.; Giaccone, G.; Bianchi, C.N. Principles of bionomy: Definition of assemblages and use of taxonomic descriptors (macrobenthos). Biol. Mar. Mediterr. 2004, 11 (Suppl. 1), 573–600. [Google Scholar]
- Batavia, C.; Nelson, M.P.; Bruskotter, J.T.; Jones, M.S.; Yanco, E.; Ramp, D.; Bekoff, M.; Wallach, A.D. Emotion as a source of moral understanding in conservation. Conserv. Biol. 2021, 35, 1380–1387. [Google Scholar] [CrossRef]
- Rovere, A.; Bellati, S.; Parravicini, V.; Firpo, M.; Morri, C.; Bianchi, C.N. Abiotic and biotic links work two ways: Effects on the deposit at the cliff foot induced by mechanical action of date mussel harvesting (Lithophaga lithophaga). Estuar. Coast. 2009, 32, 333–339. [Google Scholar] [CrossRef]
- Boudouresque, C.F.; Médail, F.; Ponel, P.; Astruch, P.; Barcelo, A.; Blanfuné, A.; Changeux, T.; Chevaldonné, P.; Cheylan, G.; Le Diréach, L.; et al. Species-based or ecosystem-based approaches to conservation practices: Lessons from the Port-Cros National Park (South-East France, Mediterranean Sea). Life Environ. 2020, 70, 89–112. [Google Scholar]
- Bellan-Santini, D.; Lacaze, J.C.; Poizat, C. Les Biocénoses Marines et Littorales de Méditerranée: Synthèse, Menaces et Perspectives; Muséum National d’Histoire Naturelle: Paris, France, 1994; pp. 1–146. [Google Scholar]
- Cochrane, S.K.J.; Andersen, J.H.; Berg, T.; Blanchet, H.; Borja, A.; Carstensen, J.; Elliott, M.; Hummel, H.; Niquil, N.; Renaud, P.E. What is marine biodiversity? Towards common concepts and their implications for assessing biodiversity status. Front. Mar. Sci. 2016, 3, 248. [Google Scholar] [CrossRef]
- Borja, A.; Ranasinghe, A.; Weiseberg, S.B. Assessing ecological integrity in marine waters, using multiple indices and ecosystem components: Challenges for the future. Mar. Pollut. Bull. 2009, 59, 1–4. [Google Scholar] [CrossRef]
- Bavestrello, G.; Bertolino, M.; Betti, F.; Bianchi, C.N.; Cattaneo-Vietti, R.; Montefalcone, M.; Morri, C. New perspectives in the study of Mediterranean coralligenous assemblages. Biol. Mar. Mediterr. 2016, 23, 170–173. [Google Scholar]
- Oprandi, A.; Bianchi, C.N.; Karayali, O.; Morri, C.; Rigo, I.; Montefalcone, M. RESQUE: A novel comprehensive approach to compare the performance of different indices in evaluating seagrass health. Ecol. Indic. 2021, 131, 108118. [Google Scholar] [CrossRef]
- Ballesteros, E.; Torras, X.; Pinedo, S.; Garcia, M.; Mangialajo, L.; De Torres, M. A new methodology based on littoral community cartography dominated by macroalgae for the implementation of the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Gobert, S.; Sartoretto, S.; Rico-Raimondino, V.; Andral, B.; Chery, A.; Lejeune, P.; Boissery, P. Assessment of the ecological status of Mediterranean French coastal waters as required by the Water Framework Directive using the Posidonia oceanica Rapid Easy Index: PREI. Mar. Pollut. Bull. 2009, 58, 1727–1733. [Google Scholar] [CrossRef]
- Piazzi, L.; Gennaro, P.; Cecchi, E.; Bianchi, C.N.; Cinti, M.F.; Gatti, G.; Guala, I.; Morri, C.; Sartoretto, F.; Serena, F.; et al. Ecological status of coralligenous assemblages: Ten years of application of the ESCA index from local to wide scale validation. Ecol. Indic. 2021, 121, 107077. [Google Scholar] [CrossRef]
- Paoli, C.; Morten, A.; Bianchi, C.N.; Morri, C.; Fabiano, M.; Vassallo, P. Capturing ecological complexity: OCI, a novel combination of ecological indices as applied to benthic marine habitats. Ecol. Indic. 2016, 66, 86–102. [Google Scholar] [CrossRef]
- Gatti, G.; Bianchi, C.N.; Morri, C.; Montefalcone, M.; Sartoretto, S. Coralligenous reefs state along anthropized coasts: Application and validation of the COARSE index, based on a rapid visual assessment (RVA) approach. Ecol. Indic. 2015, 52, 567–576. [Google Scholar] [CrossRef]
- Thibaut, T.; Blanfuné, A.; Boudouresque, C.F.; Personnic, S.; Ruitton, S.; Ballesteros, E.; Bellan-Santini, D.; Bianchi, C.N.; Bussotti, S.; Cebrian, E.; et al. An ecosystem-based approach to assess the status of Mediterranean algae-dominated shallow rocky reefs. Mar. Pollut. Bull. 2017, 117, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Mairi, H.; Jessica, C.W.; Erin, L.; Carly, N.C. Lessons from other disciplines for setting management thresholds for biodiversity conservation. Conserv. Biol. 2021, in press. [Google Scholar] [CrossRef]
- Mancini, I.; Rigo, I.; Oprandi, A.; Montefalcone, M.; Morri, C.; Peirano, A.; Vassallo, P.; Paoli, C.; Bianchi, C.N. What biotic indices tell us about ecosystem change: Lessons from the seagrass Posidonia oceanica. Indices application on historical data. Life Environ. 2020, 70, 55–61. [Google Scholar]
- Vassallo, P.; Paoli, C.; Aliani, S.; Cocito, S.; Morri, C.; Bianchi, C.N. Benthic diversity patterns and predictors: A study case with inferences for conservation. Mar. Pollut. Bull. 2020, 150, 110748. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A.; Forni, G.; Silvestri, C. The Mediterranean intercalibration exercise on soft-bottom benthic invertebrates with special emphasis on the Italian situation. Mar. Ecol. 2009, 30, 495–504. [Google Scholar] [CrossRef]
- Bianchi, C.N. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 2007, 580, 7–21. [Google Scholar] [CrossRef]
- Loreau, M.; Barbier, M.; Filotas, E.; Gravel, D.; Isbell, F.; Miller, S.J.; Montoya, J.M.; Wang, S.; Aussenac, R.; Germain, R.; et al. Biodiversity as insurance: From concept to measurement and application. Biol. Rev. 2021, 96, 2333–2354. [Google Scholar] [CrossRef]
- Lewin, R. In ecology change brings stability. Science 1986, 234, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Morri, C.; Bianchi, C.N. Recent changes in biodiversity in the Ligurian Sea (NW Mediterranean): Is there a climatic forcing? In Mediterranean Ecosystems: Structure and Processes; Faranda, F.M., Guglielmo, L., Spezie, G., Eds.; Springer: Milan, Italy, 2001; pp. 375–384. [Google Scholar]
- Montefalcone, M.; Morri, C.; Bianchi, C.N.; Bavestrello, G.; Piazzi, L. The two facets of species sensitivity: Stress and disturbance on coralligenous assemblages in space and time. Mar. Pollut. Bull. 2017, 117, 229–238. [Google Scholar] [CrossRef]
- Gissi, E.; Manea, E.; Mazaris, A.D.; Fraschetti, S.; Almpanidou, V.; Bevilacqua, S.; Coll, M.; Guarnieri, G.; Lloret-Lloret, E.; Pascual, M.; et al. A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 2021, 755, 142564. [Google Scholar] [CrossRef]
- Heip, C.; Keegan, B.F.; Lewis, J.R. Long-Term Changes in Coastal Benthic Communities; (Developments in Hydrobiology series); Springer: Berlin/Heidelberg, Germany, 1987; Volume 38, pp. 1–340. [Google Scholar]
- García-Barón, I.; Giakoumi, S.; Santos, M.B.; Granado, I.; Louzao, M. The value of time-series data for conservation planning. J. Appl. Ecol. 2021, 58, 608–619. [Google Scholar] [CrossRef]
- Proença, V.; Martin, L.J.; Pereira, H.M.; Fernandez, M.; McRae, L.; Belnap, J.; Böhm, M.; Brummitt, N.; García-Moreno, J.; Gregory, R.D.; et al. Global biodiversity monitoring: From data sources to essential biodiversity variables. Biol. Conserv. 2017, 213, 256–263. [Google Scholar] [CrossRef]
- Pearson, T.H.; Josefson, A.B.; Rosenberg, R. Petersen’s benthic stations revisited. I: Is the Kattegatt becoming eutrophic? J. Exp. Mar. Biol. Ecol. 1985, 92, 157–206. [Google Scholar] [CrossRef]
- Barry, J.P.; Baxter, C.H.; Sagarin, R.D.; Gilman, S.E. Climate-related, long-term faunal changes in a California rocky intertidal community. Science 1995, 267, 672–675. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, K. A re-assessment of rocky sublittoral biota at Hilsea Point Rock after fifty years. J. Mar. Biol. Assoc. U. K. 2005, 85, 1009–1010. [Google Scholar] [CrossRef]
- Parravicini, V.; Micheli, F.; Montefalcone, M.; Morri, C.; Villa, E.; Castellano, M.; Povero, P.; Bianchi, C.N. Conserving biodiversity in a human-dominated world: Degradation of marine sessile communities within a protected area with conflicting human uses. PLoS ONE 2013, 8, e75767. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Corsini-Foka, M.; Morri, C.; Zenetos, A. Thirty years after: Dramatic change in the coastal marine ecosystems of Kos Island (Greece), 1981–2013. Mediterr. Mar. Sci. 2014, 15, 482–497. [Google Scholar] [CrossRef]
- Gatti, G.; Bianchi, C.N.; Montefalcone, M.; Venturini, S.; Diviacco, G.; Morri, C. Observational information on a temperate reef community helps understanding the marine climate and ecosystem shift of the 1980–90s. Mar. Pollut. Bull. 2017, 114, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Montefalcone, M.; De Falco, G.; Nepote, E.; Canessa, M.; Bertolino, M.; Bavestrello, G.; Morri, C.; Bianchi, C.N. Thirty year ecosystem trajectories in a submerged marine cave under changing pressure regime. Mar. Environ. Res. 2018, 137, 98–110. [Google Scholar] [CrossRef]
- Giakoumi, S.; McGowan, J.; Mills, M.; Beger, M.; Bustamante, R.H.; Charles, A.; Christie, P.; Fox, M.; Garcia-Borboroglu, P.; Gelcich, S.; et al. Revisiting “success” and “failure” of marine protected areas: A conservation scientist perspective. Front. Mar. Sci. 2018, 5, 223. [Google Scholar] [CrossRef]
- Grorud-Colvert, K.; Sullivan-Stack, J.; Roberts, C.; Constant, V.; Horta e Costa, B.; Pike, E.P.; Kingston, N.; Laffoley, D.; Sala, E.; Claudet, J.; et al. The MPA Guide: A framework to achieve global goals for the ocean. Science 2021, 373, 1215. [Google Scholar] [CrossRef]
- Boero, F. The study of species in the era of biodiversity: A tale of stupidity. Diversity 2010, 2, 115–126. [Google Scholar] [CrossRef]
- Southward, A.J. The importance of long time-series in understanding the variability of natural systems. Helgol. Wiss. Meeresunters. 1995, 49, 329–333. [Google Scholar] [CrossRef]
- McDonald, T. Changes over time and changes yet to come. Ecol. Manag. Restor. 2019, 20, 181. [Google Scholar] [CrossRef]
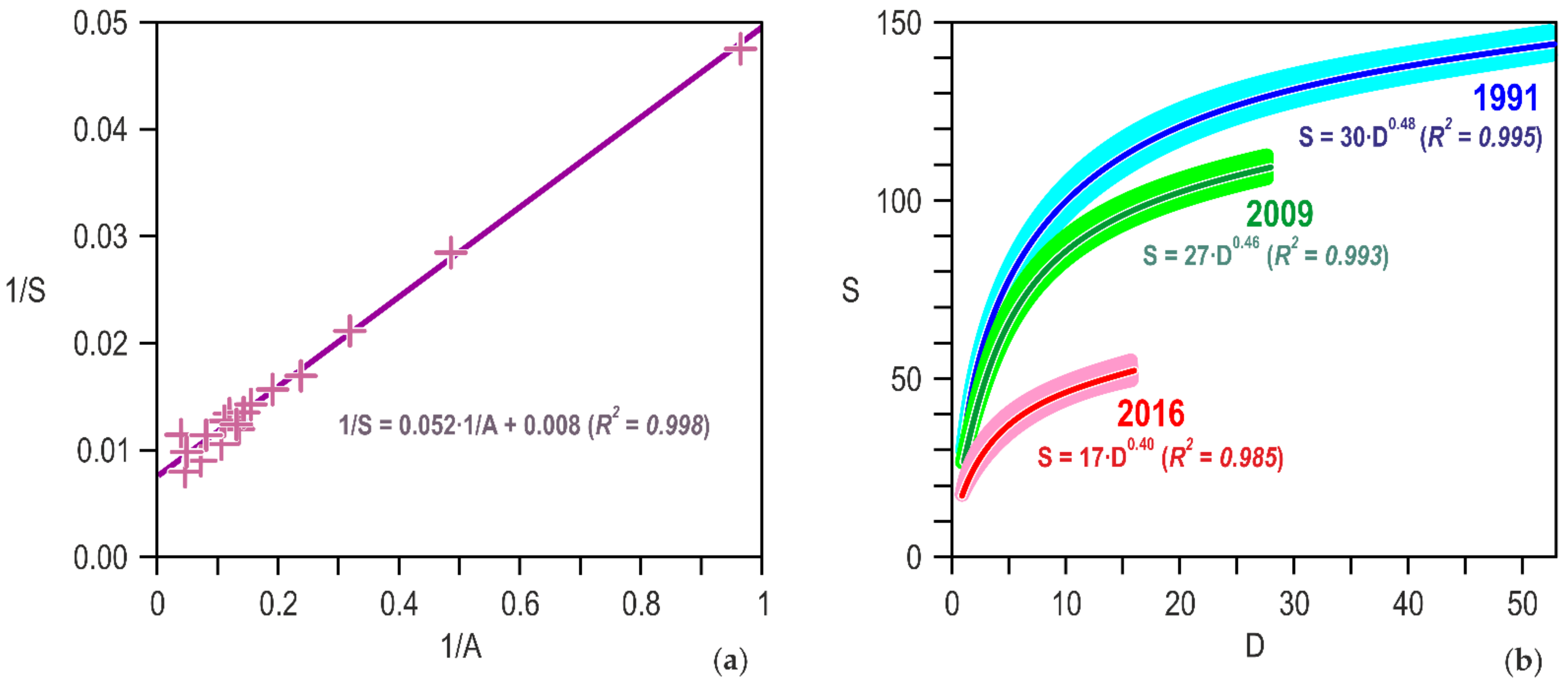
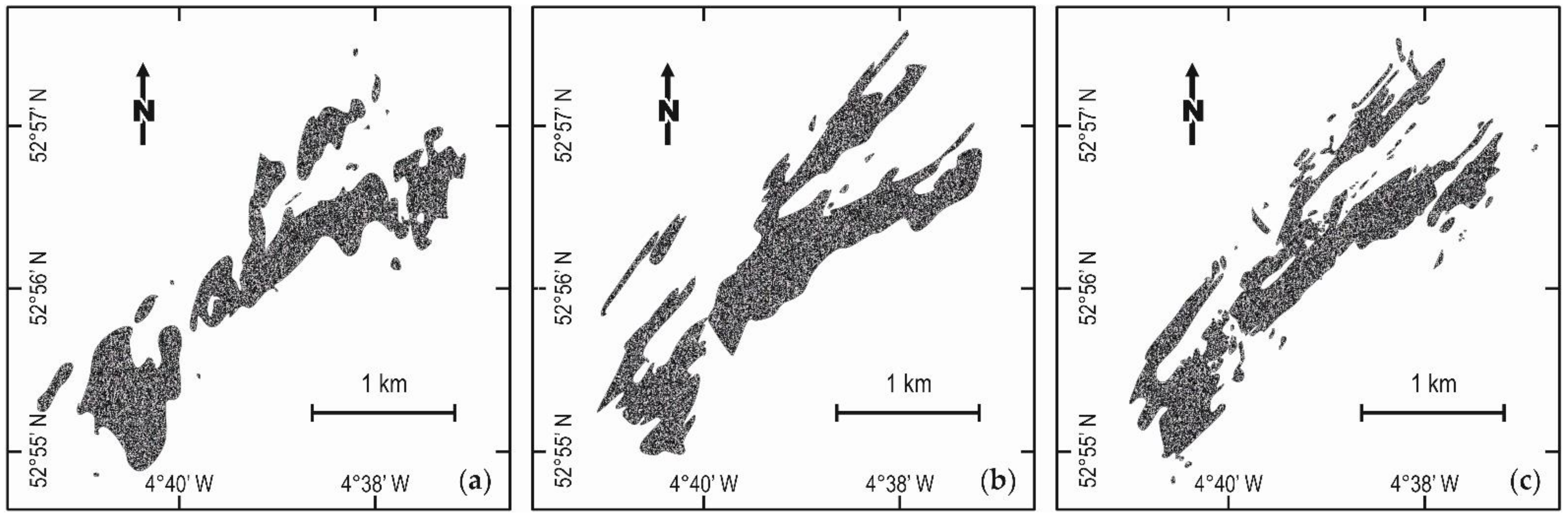
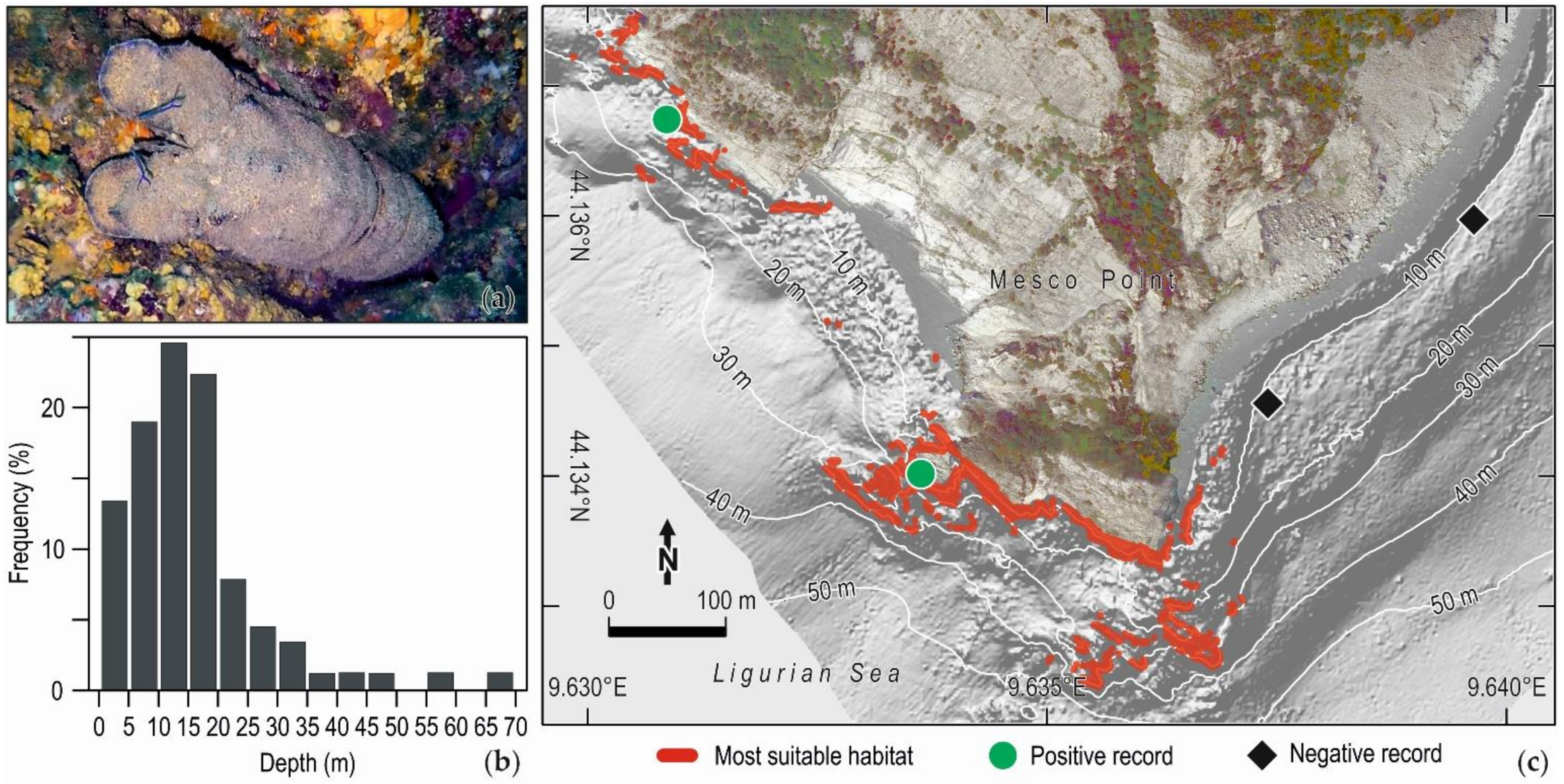
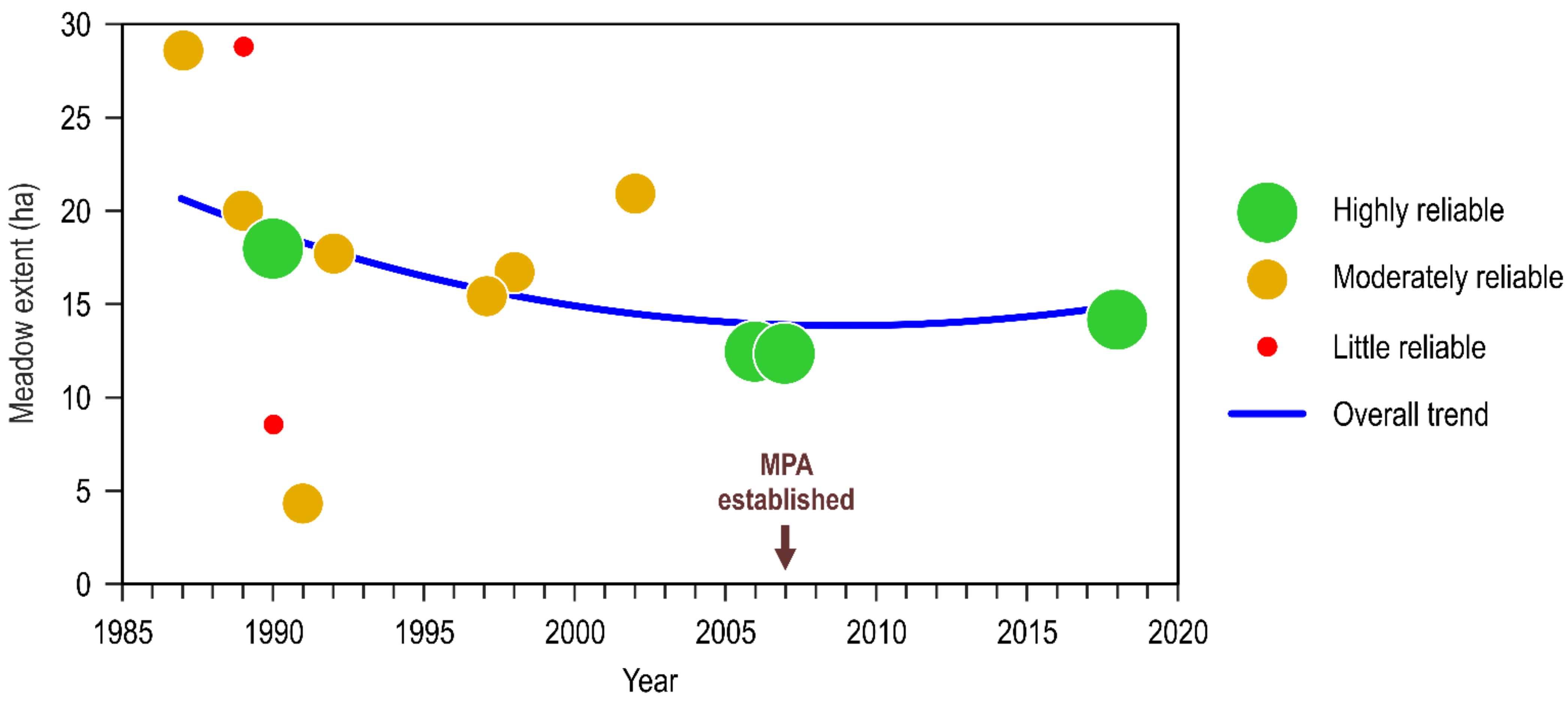

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, C.N.; Azzola, A.; Cocito, S.; Morri, C.; Oprandi, A.; Peirano, A.; Sgorbini, S.; Montefalcone, M. Biodiversity Monitoring in Mediterranean Marine Protected Areas: Scientific and Methodological Challenges. Diversity 2022, 14, 43. https://doi.org/10.3390/d14010043
Bianchi CN, Azzola A, Cocito S, Morri C, Oprandi A, Peirano A, Sgorbini S, Montefalcone M. Biodiversity Monitoring in Mediterranean Marine Protected Areas: Scientific and Methodological Challenges. Diversity. 2022; 14(1):43. https://doi.org/10.3390/d14010043
Chicago/Turabian StyleBianchi, Carlo Nike, Annalisa Azzola, Silvia Cocito, Carla Morri, Alice Oprandi, Andrea Peirano, Sergio Sgorbini, and Monica Montefalcone. 2022. "Biodiversity Monitoring in Mediterranean Marine Protected Areas: Scientific and Methodological Challenges" Diversity 14, no. 1: 43. https://doi.org/10.3390/d14010043
APA StyleBianchi, C. N., Azzola, A., Cocito, S., Morri, C., Oprandi, A., Peirano, A., Sgorbini, S., & Montefalcone, M. (2022). Biodiversity Monitoring in Mediterranean Marine Protected Areas: Scientific and Methodological Challenges. Diversity, 14(1), 43. https://doi.org/10.3390/d14010043








