Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria)
Abstract
1. Introduction
2. Materials and Methods
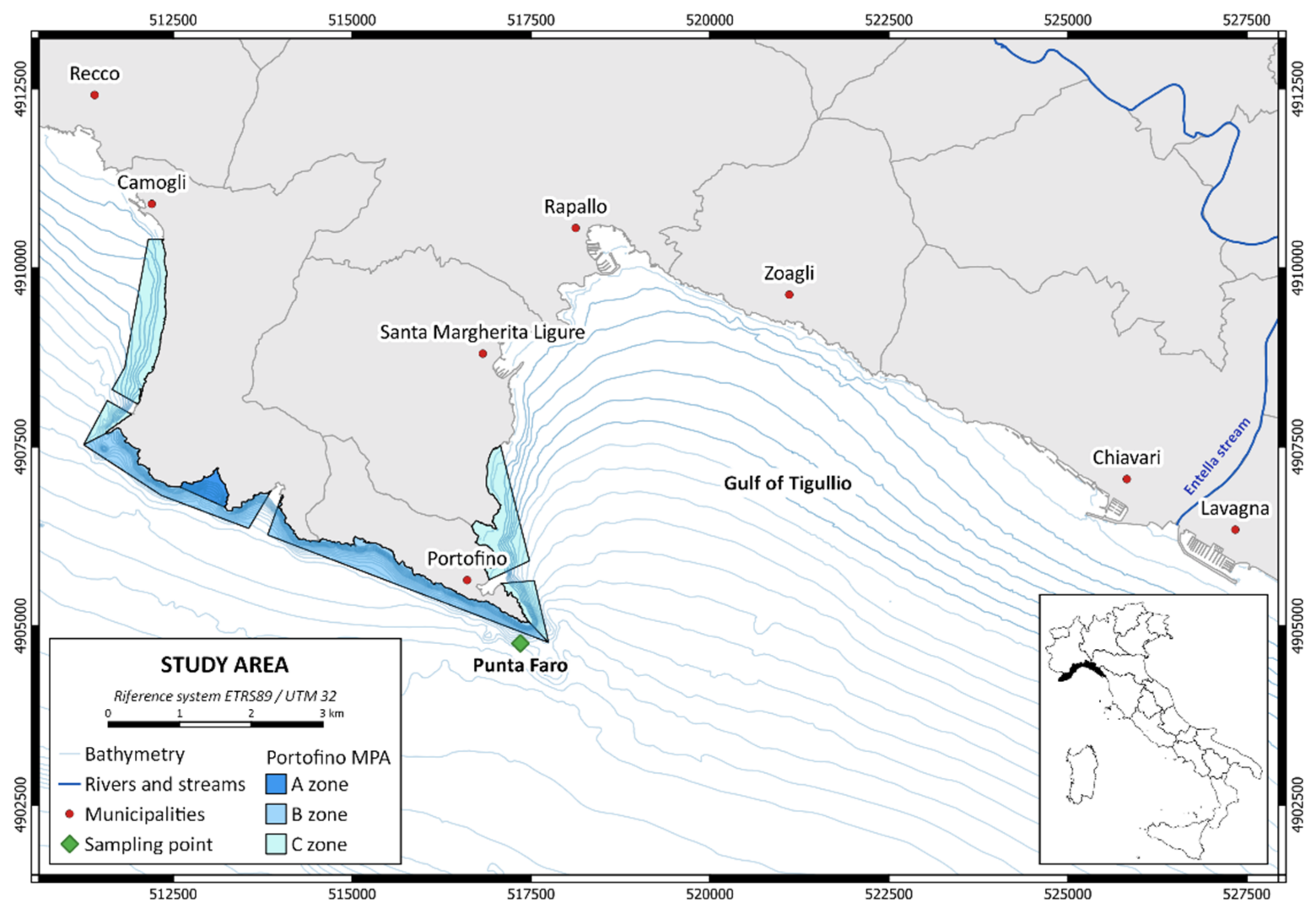
2.1. Study Site
2.2. Field Activity
2.2.1. Environmental Features
2.2.2. Zooplankton Sampling and Laboratory Procedures
2.3. Abundance to Biomass Conversion
2.4. Data Processing
2.5. Modelling Approach
2.5.1. Model Outputs
Mixed Trophic Impact
Ecological Network Analysis
Emergy Analysis
3. Results
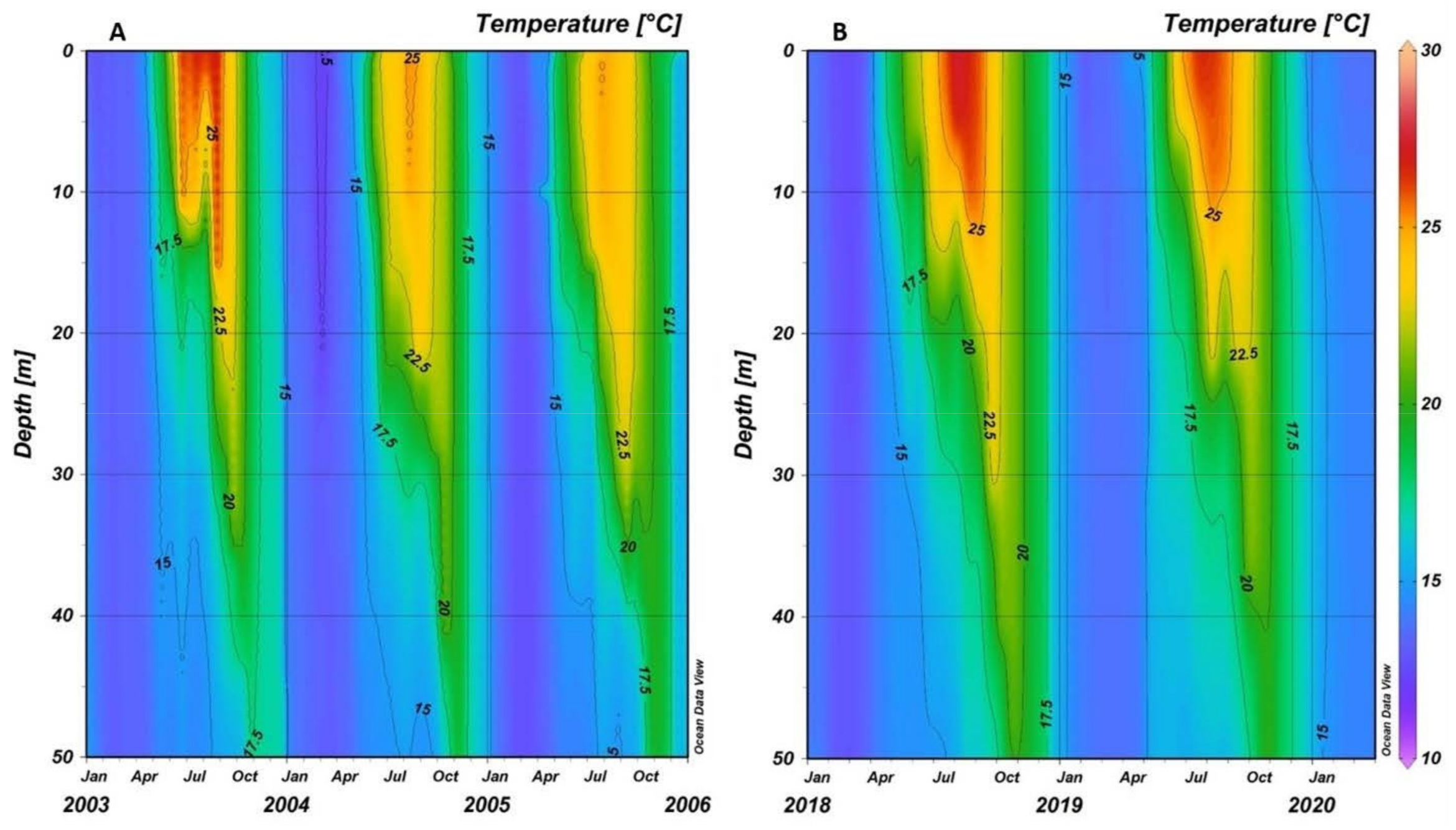
3.1. Environmental Features
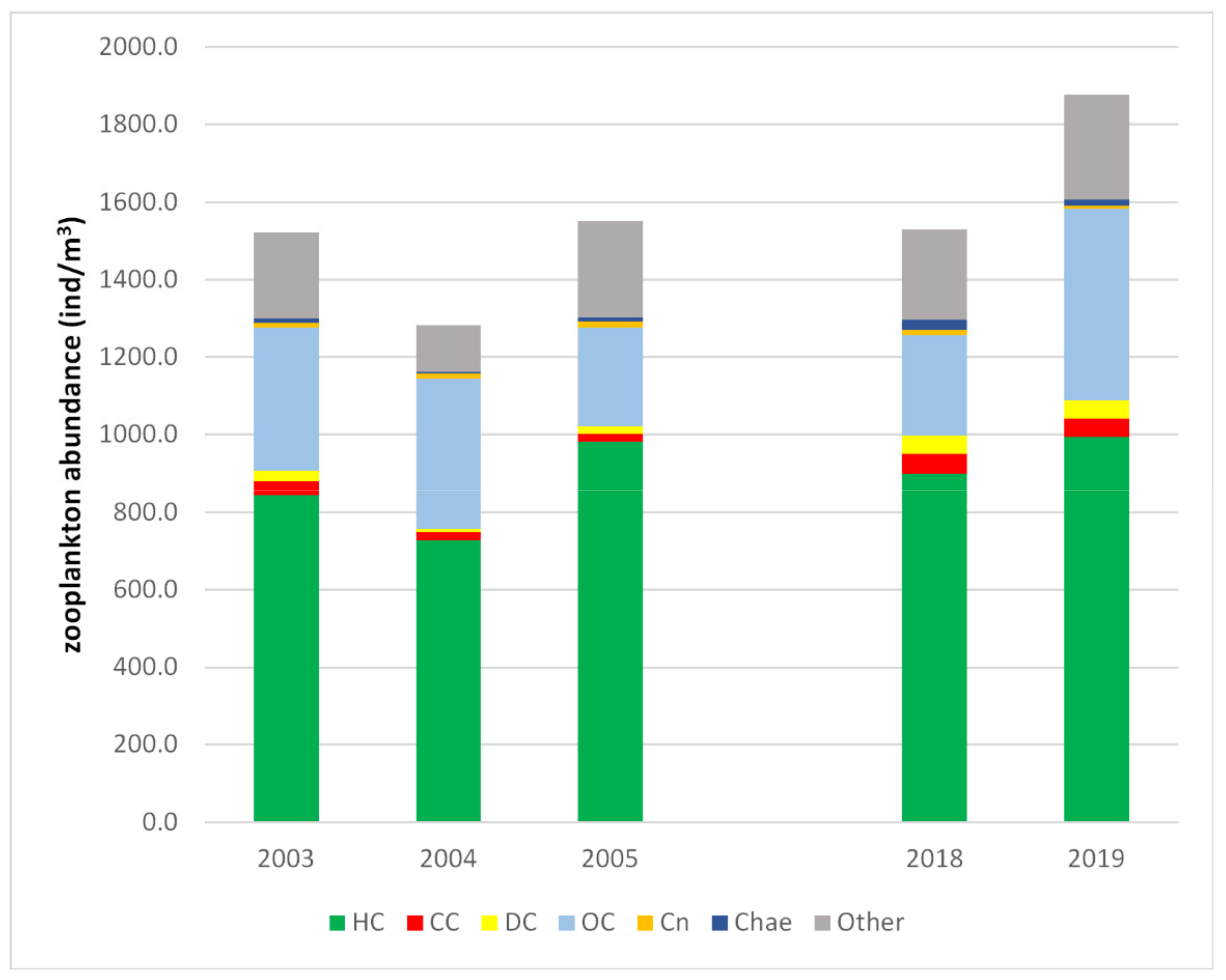
3.2. Zooplankton Community
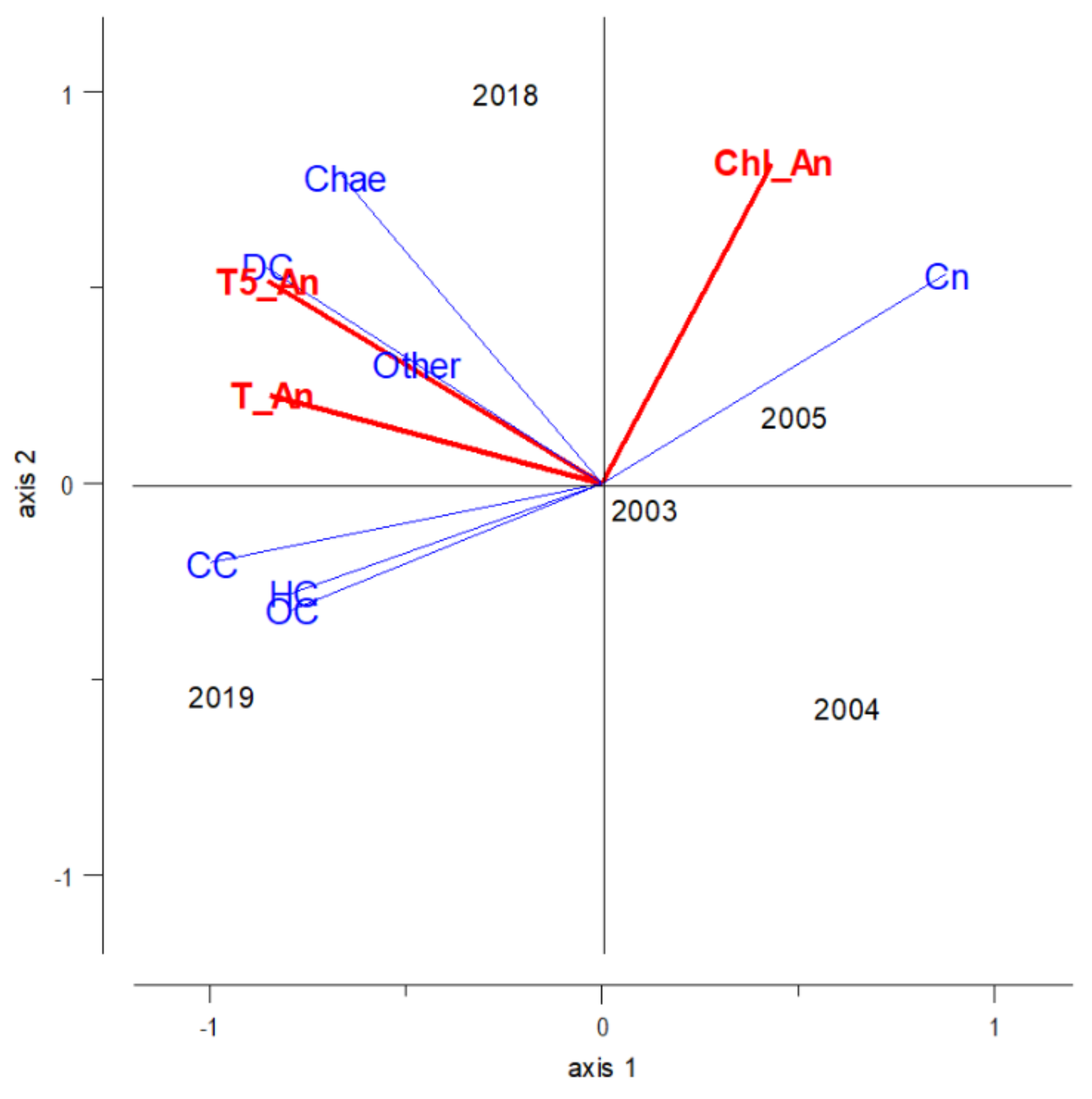
3.3. Influence of Environmental Features on Zooplankton
3.4. Modelling Approach
3.4.1. Mixed Trophic Impact
3.4.2. Ecological Network Analysis
3.4.3. Emergy Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Functional Group | Species/Taxa | IC (mgC) |
|---|---|---|
| HC | Acartia clausi | 0.0029 |
| Acartia italica | 0.0029 | |
| Acartia margalefi | 0.0029 | |
| Acartia teclae | 0.0029 | |
| Calocalanus contractus | 0.0029 | |
| Calocalanus styliremis | 0.0029 | |
| Calocalanus tenuis | 0.0029 | |
| Clausocalanus arcuicornis | 0.0029 | |
| Clausocalanus furcatus | 0.0029 | |
| Clausocalanus lividus | 0.0029 | |
| Clausocalanus parapergens | 0.0029 | |
| Clausocalanus paululus | 0.0029 | |
| Clausocalanus pergens | 0.0029 | |
| Clausocalanus spp. | 0.0029 | |
| Ctenocalanus vanus | 0.0029 | |
| Euterpina acutifrons | 0.0029 | |
| Nannocalanus minor | 0.0049 | |
| Paracalanus denudatus | 0.0019 | |
| Paracalanus nanus | 0.0019 | |
| Paracalanus parvus | 0.0019 | |
| Paracalanus spp. | 0.0019 | |
| Temora stylifera | 0.0102 | |
| Juvenile copepods | 0.0038 | |
| CC | Candacia armata | 0.0020 |
| Corycaeus spp. | 0.0022 | |
| DC | Microsetella sp. | 0.0012 |
| Oncaea spp. | 0.0022 | |
| Scolecithricidae | 0.0063 | |
| OC | Centropages kroyeri | 0.0065 |
| Centropages typicus | 0.0065 | |
| Isias clavipes | 0.0068 | |
| Oithona nana | 0.0004 | |
| Oithona plumifera | 0.0004 | |
| Oithona similis | 0.0004 | |
| Pleuromamma abdominalis | 0.1000 | |
| Pleuromamma gracilis | 0.0020 | |
| Cn | Cnidaria | 0.1885 |
| Juvenile Cnidarians | 0.1885 | |
| Che | Chaetognatha | 0.1885 |
| Other | Evadne spinifera | 0.0017 |
| Evadne spp. | 0.0017 | |
| Penilia avirostris | 0.0017 | |
| Podon spp. | 0.0017 | |
| Thecosomata | 2.41 × 10−5 | |
| Appendicularia | 0.0030 | |
| Thaliacea | 0.0028 | |
| Fisch larvae | 0.0016 | |
| Other Juvenile | Malacostraca | 0.0016 |
| Polychaeta | 0.0016 | |
| Bivalvia larvae | 0.0016 | |
| Bryozoa larvae | 0.0016 | |
| Echinodermata | 0.0016 | |
| Cirripedia | 0.0016 | |
| Crustacea | 0.0016 |
| Model Parameters | ||||||
|---|---|---|---|---|---|---|
| Functional Group | Species | P/B | Q/B | U/Q | References | |
| Chla | 1.278 | |||||
| HC | Herbivorous copepods | 0.04 | 0.631 | 0.53 | [31,80,81] | |
| Paracalanus spp. | 0.116 | 0.667 | 0.53 | |||
| Temora spp. | Temora stylifera | 0.04 | 0.223 | 0.53 | ||
| CC | Candacia spp. | Candacia armata | 0.04 | 0.631 | 0.53 | |
| Corycaeus spp. | 0.108 | 0.289 | 0.323 | |||
| Euchaeta spp. | 0.04 | 0.631 | 0.53 | |||
| DC | Microsetella sp. | 0.04 | 0.631 | 0.53 | ||
| Oncaea spp. | 0.04 | 0.631 | 0.53 | |||
| Scolecithricella spp. | 0.04 | 0.631 | 0.53 | |||
| OC | Centropages spp. | 0.108 | 0.289 | 0.323 | ||
| Omivores Cyclopoida | 0.055 | 0.297 | 0.53 | |||
| Isias spp. | Isias clavipes | 0.04 | 0.631 | 0.53 | ||
| Pleuromamma spp. | Pleuromamma abdominalis | 0.04 | 0.631 | 0.53 | ||
| Pleuromamma gracilis | 0.04 | 0.631 | 0.53 | |||
| Cn | Cnidaria | 0.25 | 0.192 | 0.195 | ||
| Che | Chaetognata | 0.25 | 0.192 | 0.195 | ||
| Other | Cladocera | 0.793 | 1.452 | 0.496 | ||
| Thecosomata | 0.25 | 0.192 | 0.195 | |||
| Appendicularia | 0.494 | 14.012 | 0.604 | |||
| Thaliacea | 1.35 | 1.392 | 0.22 | |||
| 2003 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional Group | Trophic Refs. | Species/Taxa | Winter | Spring | Summer | Autumn | ||||
| Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | |||
| HC | [82,83,84,85,86] | Acartia clausi | 14.59 | 20 | 184.46 | 50 | 45.19 | 50 | 3.93 | 33 |
| Acartia italica | - | - | - | - | - | - | - | - | ||
| Acartia margalefi | - | - | - | - | - | - | - | - | ||
| Acartia teclae | - | - | - | - | - | - | - | - | ||
| Calocalanus contractus | - | - | - | - | - | - | - | - | ||
| Calocalanus styliremis | 35.08 | 40 | 24.88 | 50 | 24.59 | 50 | 29.01 | 50 | ||
| Calocalanus tenuis | - | - | - | - | - | - | - | - | ||
| Clausocalanus arcuicornis | 19.94 | 20 | 3.13 | 33 | 7.36 | 33 | 6.17 | 33 | ||
| Clausocalanus furcatus | 19.97 | 40 | 4.55 | 33 | 39.26 | 50 | 31.95 | 50 | ||
| Clausocalanus lividus | 3.71 | 40 | 2.11 | 17 | - | - | 1.68 | 17 | ||
| Clausocalanus parapergens | - | - | - | - | - | - | - | - | ||
| Clausocalanus paululus | 56.35 | 60 | 7.91 | 33 | 145 | 50 | 20.90 | 50 | ||
| Clausocalanus pergens | 38.40 | 40 | 41.22 | 50 | 5.67 | 50 | 15.96 | 50 | ||
| Clausocalanus spp. | 47.97 | 20 | 12.22 | 33 | 28.54 | 26 | 19.94 | 50 | ||
| Ctenocalanus vanus | 8.60 | - | 10.32 | 17 | 0.92 | 17 | 0.67 | 17 | ||
| Euterpina acutifrons | 22.57 | 60 | 17.20 | 33 | 5.67 | 33 | 17.09 | 50 | ||
| Nannocalanus minor | - | - | - | - | - | - | 2.18 | 17 | ||
| Paracalanus denudatus | 2.32 | 40 | 22.05 | 33 | - | - | - | - | ||
| Paracalanus nanus | 12.41 | 40 | 2.29 | 17 | 15.14 | 50 | 12.06 | 50 | ||
| Paracalanus parvus | 54.57 | 40 | 128.65 | 50 | 23.46 | 50 | 43.87 | 50 | ||
| Paracalanus spp. | 7.80 | 20 | 11.95 | 50 | 3.40 | 17 | 2.09 | 33 | ||
| Temora stylifera | 3.11 | - | - | - | 5.47 | 50 | 10.29 | 50 | ||
| Juvenile copepods | 784.09 | 40 | 1251.86 | 50 | 414.17 | 50 | 643.67 | 50 | ||
| CC | [87,88,89,90] | Candacia armata | - | - | - | - | 2.22 | 17 | - | - |
| Corycaeus spp. | 15.15 | 40 | 9.95 | 50 | 24.17 | 50 | 46.03 | 50 | ||
| DC | [87,91,92,93] | Microsetella sp. | - | - | - | - | 1.65 | 17 | - | - |
| Oncaea spp. | 11.71 | 20 | 11.95 | 50 | 3.93 | 33 | 7.34 | 50 | ||
| Scolecithricidae | - | - | - | - | 2.45 | 17 | 1.09 | 17 | ||
| OC | [82,83,94,95,96] | Centropages kroyeri | - | - | 6.88 | 45 | 8.36 | 55 | - | - |
| Centropages typicus | 13.61 | 20 | 27.69 | 54 | 5.39 | 10 | 4.89 | 09 | ||
| Isias clavipes | - | 20 | 14.87 | 59 | 7.41 | 30 | 2.73 | 11 | ||
| Oithona nana | 3.27 | - | - | - | - | - | - | - | ||
| Oithona plumifera | - | - | 6.88 | 19 | 19.92 | 55 | 9.39 | 26 | ||
| Oithona similis | 80.18 | 40 | 102.19 | 46 | 17.16 | 08 | 23.02 | 10 | ||
| Pleuromamma abdominalis | - | - | - | - | - | - | - | - | ||
| Pleuromamma gracilis | - | - | - | - | - | - | 0.67 | 17 | ||
| Cn | [19,97,98] | Cnidaria | 5.31 | 60 | 15.06 | 50 | 19.55 | 50 | 10.40 | 50 |
| Juvenile Cnidarians | - | - | - | - | - | - | - | - | ||
| Che | [99,100,101,102] | Chaetognatha | 3.61 | 60 | 8.59 | 50 | 12.81 | 50 | 23.47 | 50 |
| Other | [103,104,105] | Evadne spinifera | 1.10 | - | 117.42 | 50 | 11.66 | 50 | 0.21 | 33 |
| Evadne spp. | 0.21 | - | 3.35 | 50 | 7.79 | 50 | - | - | ||
| Penilia avirostris | - | - | 2.82 | 50 | 73.14 | 50 | 0.26 | 33 | ||
| Podon spp. | 11.66 | 40 | 11.03 | 50 | 2.88 | 50 | - | - | ||
| Thecosomata | 14.90 | 60 | 75.60 | 50 | 19.08 | 50 | 21.64 | 50 | ||
| Appendicularia | 69.22 | 60 | 156.26 | 50 | 184.08 | 50 | 61.64 | 50 | ||
| Thaliacea | 0.94 | 40 | 24.78 | 50 | 2.40 | 50 | 4.08 | 33 | ||
| Fisch larvae | 0.52 | 20 | 4.71 | 50 | 2.14 | 50 | 0.31 | 33 | ||
| Other Juvenile | Malacostraca | 20.86 | 40 | 8.42 | 50 | 6.85 | 50 | 9.51 | 50 | |
| Polychaeta | 5.96 | 40 | 0.68 | 50 | 1.15 | 50 | 5.76 | 50 | ||
| Bivalvia larvae | 23.84 | 40 | 58.61 | 50 | 2.67 | 50 | 2.35 | 50 | ||
| Bryozoa larvae | 5.44 | 40 | 11.66 | 50 | - | 0.26 | 33 | |||
| Echinodermata | 6.12 | 40 | 27.86 | 50 | 1.15 | 50 | 1.52 | 50 | ||
| Cirripedia | 0.05 | - | 0.05 | 17 | 0.05 | 17 | - | - | ||
| Crustacea | 10.87 | 40 | 2.67 | 50 | 4.55 | 50 | 1.46 | 50 | ||
| 2004 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional Group | Trophic Refs. | Species/Taxa | Winter | Spring | Summer | Autumn | ||||
| Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | |||
| HC | [82,83,84,85,86] | Acartia clausi | 3.35 | 25 | 55.99 | 50 | 8.36 | 50 | 18.90 | 33 |
| Acartia italica | - | - | - | - | - | - | - | - | ||
| Acartia margalefi | - | - | - | - | - | - | - | - | ||
| Acartia teclae | - | - | - | - | - | - | - | - | ||
| Calocalanus contractus | - | - | - | - | - | - | - | - | ||
| Calocalanus styliremis | 60.48 | 50 | 9.95 | 50 | 24.96 | 50 | 8.53 | 67 | ||
| Calocalanus tenuis | - | - | - | - | - | - | - | - | ||
| Clausocalanus arcuicornis | 4.56 | 25 | 3.14 | 33 | 4.42 | 50 | - | - | ||
| Clausocalanus furcatus | 4.87 | 50 | - | - | 2.03 | 33 | 12.43 | 33 | ||
| Clausocalanus lividus | 7.59 | 50 | 1.33 | 17 | - | - | - | - | ||
| Clausocalanus parapergens | - | - | - | - | - | - | - | - | ||
| Clausocalanus paululus | 27.27 | 75 | 17.17 | 50 | 20.51 | 33 | 33.77 | 67 | ||
| Clausocalanus pergens | 38.40 | 50 | 46.15 | 50 | 12.23 | 33 | 3.36 | 67 | ||
| Clausocalanus spp. | 12.87 | 25 | 6.02 | 17 | 7.87 | 33 | 55.77 | 67 | ||
| Ctenocalanus vanus | - | - | - | - | - | - | - | - | ||
| Euterpina acutifrons | 18.46 | 75 | 5.86 | 33 | 2.73 | 33 | 1.00 | 33 | ||
| Nannocalanus minor | - | - | 0.51 | 17 | - | - | - | - | ||
| Paracalanus denudatus | 8.68 | 50 | - | - | - | - | - | - | ||
| Paracalanus nanus | 11.93 | 50 | 3.27 | 33 | 3.04 | 17 | 2.00 | 33 | ||
| Paracalanus parvus | 56.13 | 50 | 40.74 | 50 | 60.45 | 50 | 17.79 | 67 | ||
| Paracalanus spp. | 6.70 | 25 | 18.02 | 50 | 9.09 | 33 | - | - | ||
| Temora stylifera | - | - | - | - | 2.74 | 33 | 2.09 | 33 | ||
| Juvenile copepods | 1057.50 | 50 | 1211.49 | 50 | 413.90 | 50 | 555.83 | 1.00 | ||
| CC | [87,88,89,90] | Candacia armata | - | - | 0.51 | 17 | 1.12 | 17 | - | - |
| Corycaeus spp. | 6.33 | 50 | 7.23 | 50 | 7.28 | 50 | 29.88 | 67 | ||
| DC | [87,91,92,93] | Microsetella sp. | - | - | - | - | - | - | - | - |
| Oncaea spp. | 10.05 | 25 | 3.27 | 33 | 2.73 | 33 | - | - | ||
| Scolecithricidae | - | - | - | - | 1.21 | 17 | - | - | ||
| OC | [82,83,94,95,96] | Centropages kroyeri | - | - | - | - | 1.36 | 17 | - | - |
| Centropages typicus | 8.93 | 25 | 32.10 | 50 | 12.85 | 50 | 2.36 | 33 | ||
| Isias clavipes | 1.77 | 25 | 1.59 | 17 | 11.91 | 33 | 2.36 | 33 | ||
| Oithona nana | - | - | 52.13 | 50 | 4.80 | 33 | 4.17 | 33 | ||
| Oithona plumifera | - | - | - | - | 5.00 | 50 | 7.72 | 67 | ||
| Oithona similis | 80.89 | 50 | 73.20 | 50 | 12.06 | 50 | 8.53 | 67 | ||
| Pleuromamma abdominalis | - | - | - | - | - | - | - | - | ||
| Pleuromamma gracilis | - | - | - | - | - | - | - | - | ||
| Cn | [19,97,98] | Cnidaria | 16.42 | 75 | 14.06 | 50 | 15.84 | 50 | 12.63 | 67 |
| Juvenile Cnidarians | - | - | - | - | - | - | - | - | ||
| Che | [99,100,101,102] | Chaetognatha | 4.13 | 75 | 3.61 | 50 | 1.88 | 50 | 8.00 | 67 |
| Other | [103,104,105] | Evadne spinifera | - | - | 5.38 | 33 | 8.36 | 50 | 2.67 | 33 |
| Evadne spp. | - | - | 0.21 | 17 | 3.55 | 50 | 0.47 | 33 | ||
| Penilia avirostris | - | - | 0.31 | 17 | 35.97 | 50 | 1.57 | 67 | ||
| Podon spp. | 19.81 | 50 | 12.49 | 50 | 3.82 | 50 | 1.49 | 67 | ||
| Thecosomata | 12.97 | 75 | 35.71 | 50 | 9.67 | 50 | 8.16 | 67 | ||
| Appendicularia | 27.76 | 75 | 86.94 | 50 | 136.82 | 50 | 86.42 | 67 | ||
| Thaliacea | 2.25 | 50 | 9.31 | 33 | 0.73 | 50 | 3.29 | 67 | ||
| Fisch larvae | 0.05 | 25 | 3.08 | 33 | 1.15 | 50 | 0.16 | 67 | ||
| Other Juvenile | Malacostraca | 18.14 | 50 | 11.61 | 50 | 5.23 | 50 | 2.12 | 1.00 | |
| Polychaeta | 3.14 | 50 | 1.46 | 50 | 1.88 | 50 | 1.57 | 1.00 | ||
| Bivalvia larvae | 5.33 | 50 | 12.01 | 50 | 1.57 | 50 | 0.71 | 67 | ||
| Bryozoa larvae | 4.65 | 50 | 1.93 | 50 | 0.05 | 33 | - | - | ||
| Echinodermata | 5.86 | 50 | 1.93 | 50 | 0.94 | 33 | 0.78 | 1.00 | ||
| Cirripedia | - | - | - | - | 0.10 | 17 | - | - | ||
| Crustacea | 10.19 | 50 | 1.10 | 50 | 1.46 | 50 | 1.49 | 1.00 | ||
| 2005 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional Group | Trophic Refs. | Species/Taxa | Winter | Spring | Summer | Autumn | ||||
| Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | |||
| HC | [82,83,84,85,86] | Acartia clausi | 131.20 | 75 | 183.61 | 50 | 42.75 | 40 | 4.30 | 25 |
| Acartia italica | - | - | - | - | 3.27 | 20 | - | - | ||
| Acartia margalefi | - | - | - | - | - | - | - | - | ||
| Acartia teclae | - | - | - | - | - | - | - | - | ||
| Calocalanus contractus | - | - | - | - | - | - | - | - | ||
| Calocalanus styliremis | 46.90 | 75 | 24.54 | 50 | 8.48 | 40 | 12.33 | 25 | ||
| Calocalanus tenuis | - | - | - | - | - | - | - | - | ||
| Clausocalanus arcuicornis | 6.84 | 50 | 7.69 | 17 | 4.14 | 40 | 3.28 | 50 | ||
| Clausocalanus furcatus | 11.35 | 50 | - | - | 1.42 | 20 | 33.23 | 50 | ||
| Clausocalanus lividus | 10.40 | 50 | 4.06 | 33 | 3.63 | 20 | 8.61 | 25 | ||
| Clausocalanus parapergens | - | - | 4.03 | 17 | - | - | - | - | ||
| Clausocalanus paululus | 29.44 | 75 | 6.31 | 33 | 9.08 | 40 | 7.67 | 25 | ||
| Clausocalanus pergens | 28.66 | 75 | 23.15 | 50 | 17.06 | 40 | 3.72 | 25 | ||
| Clausocalanus spp. | 32.73 | 75 | 45.86 | 50 | 61.35 | 60 | 31.71 | 50 | ||
| Ctenocalanus vanus | 3.75 | 25 | - | - | 3.27 | 20 | - | - | ||
| Euterpina acutifrons | 14.59 | 50 | 2.40 | 33 | - | - | 7.03 | 50 | ||
| Nannocalanus minor | - | - | - | - | - | - | - | - | ||
| Paracalanus denudatus | - | - | - | - | 10.89 | 20 | - | - | ||
| Paracalanus nanus | 4.54 | 25 | 15.38 | 17 | 1.42 | 20 | 6.33 | 23 | ||
| Paracalanus parvus | 47.09 | 50 | 174.69 | 50 | 129.95 | 60 | 46.68 | 12 | ||
| Paracalanus spp. | - | - | 5.16 | 17 | 18.05 | 60 | - | - | ||
| Temora stylifera | - | - | 0.87 | 17 | 1.42 | 20 | 1.24 | 35 | ||
| Juvenile copepods | 906.54 | 75 | 1266.04 | 50 | 686.01 | 60 | 515.98 | 50 | ||
| CC | [87,88,89,90] | Candacia armata | 13.56 | 27 | 7.75 | 16 | 10.56 | 21 | 17.68 | 36 |
| Corycaeus spp. | 4.18 | 50 | 31.73 | 33 | 15.30 | 60 | 18.51 | 50 | ||
| DC | [87,91,92,93] | Microsetella sp. | - | - | - | - | - | - | - | - |
| Oncaea spp. | 22.31 | 75 | 16.30 | 33 | 1.36 | 20 | 1.24 | 25 | ||
| Scolecithricidae | - | - | - | - | - | - | - | - | ||
| OC | [82,83,94,95,96] | Centropages kroyeri | - | - | 3.19 | 17 | 3.27 | 20 | - | - |
| Centropages typicus | 5.56 | 25 | 43.32 | 50 | 18.83 | 60 | 1.13 | 25 | ||
| Isias clavipes | 0.91 | 25 | - | - | 7.26 | 20 | - | - | ||
| Oithona nana | 2.11 | 25 | 9.41 | 33 | 3.27 | 20 | 1.13 | 25 | ||
| Oithona plumifera | 2.11 | 25 | 10.35 | 33 | 13.89 | 40 | 29.64 | 50 | ||
| Oithona similis | 78.39 | 75 | 74.55 | 50 | 33.57 | 40 | 39.12 | 50 | ||
| Pleuromamma abdominalis | - | - | - | - | - | - | - | - | ||
| Pleuromamma gracilis | - | - | - | - | - | - | 4.96 | 25 | ||
| Cn | [19,97,98] | Cnidaria | 4.18 | 75 | 31.73 | 50 | 15.30 | 60 | 18.51 | 50 |
| Juvenile Cnidarians | - | - | - | - | - | - | - | - | ||
| Che | [99,100,101,102] | Chaetognatha | 5.02 | 75 | 1.57 | 33 | 6.27 | 40 | 34.11 | 50 |
| Other | [103,104,105] | Evadne spinifera | - | - | 26.82 | 33 | 16.68 | 34 | 5.80 | 12 |
| Evadne spp. | - | - | 2.77 | 17 | 8.21 | 60 | 1.25 | 50 | ||
| Penilia avirostris | 0.10 | 25 | 1.88 | 17 | 217.53 | 60 | 46.97 | 50 | ||
| Podon spp. | 9.10 | 75 | 10.19 | 50 | 10.40 | 60 | 0.71 | 50 | ||
| Thecosomata | 13.17 | 75 | 38.01 | 50 | 19.81 | 60 | 26.35 | 50 | ||
| Appendicularia | 73.98 | 75 | 198.03 | 50 | 157.10 | 60 | 99.91 | 50 | ||
| Thaliacea | - | - | 16.76 | 50 | 22.06 | 60 | 50.19 | 50 | ||
| Fisch larvae | 0.21 | 25 | 2.67 | 50 | 0.31 | 40 | 0.24 | 50 | ||
| Other Juvenile | Malacostraca | 9.41 | 75 | 8.84 | 50 | 8.52 | 60 | 2.27 | 50 | |
| Polychaeta | 4.91 | 75 | 1.20 | 50 | 1.20 | 60 | 4.78 | 50 | ||
| Bivalvia larvae | 9.41 | 75 | 9.51 | 50 | 3.29 | 60 | 3.92 | 50 | ||
| Bryozoa larvae | 3.35 | 50 | 4.08 | 33 | 0.05 | 20 | 0.63 | 50 | ||
| Echinodermata | 5.23 | 75 | 3.71 | 50 | 0.73 | 40 | 11.61 | 50 | ||
| Cirripedia | - | - | 0.31 | 17 | 0.10 | 20 | - | - | ||
| Crustacea | 26.56 | 75 | 0.84 | 50 | 0.63 | 40 | 1.88 | 50 | ||
| 2018 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional Group | Trophic Refs. | Species/Taxa | Winter | Spring | Summer | Autumn | ||||
| Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | |||
| HC | [82,83,84,85,86] | Acartia clausi | 25.46 | 50 | 6.17 | 33 | 10.14 | 60 | 9.79 | 67 |
| Acartia italica | - | - | - | - | - | - | - | - | ||
| Acartia margalefi | - | - | - | - | - | - | 0.25 | 67 | ||
| Acartia teclae | 5.66 | 25 | - | - | 1.77 | 20 | - | - | ||
| Calocalanus contractus | - | - | 1.83 | 17 | 3.54 | 20 | - | - | ||
| Calocalanus styliremis | 12.73 | 75 | 20.49 | 50 | 15.13 | 60 | 22.03 | 67 | ||
| Calocalanus tenuis | - | - | 0.91 | 17 | 1.77 | 0.20 | 0.10 | 33 | ||
| Clausocalanus arcuicornis | 21.29 | 75 | 9.97 | 33 | 5.29 | 40 | 4.43 | 33 | ||
| Clausocalanus furcatus | 16.16 | 50 | 7.21 | 33 | 18.24 | 40 | 24.14 | 67 | ||
| Clausocalanus lividus | - | - | 1.42 | 17 | 3.54 | 20 | 4.30 | 67 | ||
| Clausocalanus parapergens | - | - | - | - | - | - | - | - | ||
| Clausocalanus paululus | 70.14 | 75 | 20.38 | 50 | 12.38 | 20 | 8.03 | 33 | ||
| Clausocalanus pergens | 36.82 | 50 | 11.53 | 33 | 9.26 | 60 | 7.24 | 67 | ||
| Clausocalanus spp. | 14.01 | 50 | 18.00 | 33 | 39.53 | 60 | 7.24 | 67 | ||
| Ctenocalanus vanus | 11.97 | 75 | 0.98 | 17 | 3.54 | 20 | 3.06 | 67 | ||
| Euterpina acutifrons | 26.32 | 75 | 12.31 | 17 | 1.09 | 20 | 5.28 | 67 | ||
| Nannocalanus minor | - | - | - | - | 1.09 | 20 | - | - | ||
| Paracalanus denudatus | - | - | - | - | - | - | - | - | ||
| Paracalanus nanus | 11.08 | 50 | - | - | 1.09 | 20 | 0.85 | 33 | ||
| Paracalanus parvus | 90.57 | 75 | 104.94 | 50 | 88.21 | 60 | 10.44 | 33 | ||
| Paracalanus spp. | - | - | 2.93 | 17 | 2.65 | 20 | - | - | ||
| Temora stylifera | 2.93 | 25 | 5.73 | 33 | 11.01 | 40 | 3.40 | 33 | ||
| Juvenile copepods | 1332.10 | 75 | 947.31 | 50 | 633.76 | 60 | 366.42 | 67 | ||
| CC | [87,88,89,90] | Candacia armata | - | - | - | - | - | - | - | - |
| Corycaeus spp. | 27.84 | 75 | 46.84 | 50 | 52.12 | 60 | 43.17 | 67 | ||
| DC | [87,91,92,93] | Microsetella sp. | - | - | - | - | - | - | - | - |
| Oncaea spp. | 35.94 | 75 | 23.57 | 50 | 21.15 | 60 | 18.78 | 67 | ||
| Scolecithricidae | - | - | - | - | - | - | 6.27 | 33 | ||
| OC | [82,83,94,95,96] | Centropages kroyeri | - | - | - | - | - | - | - | - |
| Centropages typicus | 36.88 | 75 | 15.77 | 50 | 3.98 | 20 | 2.09 | 33 | ||
| Isias clavipes | 10.15 | 25 | - | - | 1.39 | 20 | - | - | ||
| Oithona nana | 38.50 | 50 | 5.62 | 17 | - | - | - | - | ||
| Oithona plumifera | 8.06 | 50 | 11.52 | 50 | 10.23 | 40 | 19.44 | 67 | ||
| Oithona similis | 23.32 | 50 | 52.28 | 50 | 5.72 | 40 | 15.32 | 67 | ||
| Pleuromamma abdominalis | - | - | - | - | - | - | - | - | ||
| Pleuromamma gracilis | - | - | - | - | - | - | 0.85 | 33 | ||
| Cn | [19,97,98] | Cnidaria | 6.79 | 75 | 35.97 | 50 | 9.51 | 60 | 6.32 | 67 |
| Juvenile Cnidarians | - | - | - | - | - | - | - | - | ||
| Che | [99,100,101,102] | Chaetognatha | 26.73 | 75 | 54.15 | 50 | 8.70 | 60 | 23.44 | 67 |
| Other | [103,104,105] | Evadne spinifera | - | - | 79.76 | 33 | 34.90 | 60 | 11.72 | 33 |
| Evadne spp. | - | - | 12.54 | 50 | 18.48 | 60 | 2.75 | 33 | ||
| Penilia avirostris | - | - | 14.47 | 33 | 169.58 | 60 | 53.61 | 67 | ||
| Podon spp. | 1.70 | 50 | 21.84 | 50 | 6.52 | 60 | 0.92 | 67 | ||
| Thecosomata | 32.61 | 75 | 77.86 | 50 | 17.26 | 60 | 12.13 | 67 | ||
| Appendicularia | 32.61 | 75 | 109.32 | 50 | 136.49 | 60 | 67.87 | 67 | ||
| Thaliacea | 10.97 | 75 | 10.70 | 50 | 9.92 | 60 | 46.68 | 67 | ||
| Fisch larvae | 0.20 | 25 | 1.90 | 50 | 2.85 | 40 | 0.31 | 67 | ||
| Other Juvenile | Malacostraca | 9.24 | 75 | 8.90 | 50 | 5.77 | 60 | 1.83 | 67 | |
| Polychaeta | 5.37 | 75 | 1.83 | 50 | 0.41 | 40 | 2.96 | 67 | ||
| Bivalvia larvae | 11.62 | 75 | 9.61 | 50 | 1.36 | 40 | 1.12 | 33 | ||
| Bryozoa larvae | 10.87 | 75 | 24.63 | 50 | - | - | - | - | ||
| Echinodermata | 9.99 | 75 | 14.85 | 50 | 3.94 | 60 | 11.21 | 67 | ||
| Cirripedia | - | - | - | - | 0.14 | 20 | - | - | ||
| Crustacea | 14.88 | 75 | 2.65 | 50 | 1.49 | 60 | 0.51 | 67 | ||
| 2019 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional Group | Trophic Refs. | Species/Taxa | Winter | Spring | Summer | Autumn | ||||
| Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | Ab. | Freq. (%) | |||
| HC | [82,83,84,85,86] | Acartia clausi | 8.52 | 33 | 40.54 | 75 | 13.48 | 75 | 0.64 | 20 |
| Acartia italica | - | - | - | - | - | - | - | - | ||
| Acartia margalefi | - | - | - | - | 2.18 | 25 | 1.22 | 20 | ||
| Acartia teclae | - | - | - | - | - | - | - | - | ||
| Calocalanus contractus | 3.41 | 17 | - | - | - | - | - | - | ||
| Calocalanus styliremis | 31.31 | 50 | 7.83 | 50 | 16.14 | 75 | 39.04 | 60 | ||
| Calocalanus tenuis | 1.42 | 17 | - | - | - | - | 1.29 | 20 | ||
| Clausocalanus arcuicornis | 1.21 | 17 | 19.07 | 50 | 14.48 | 50 | - | - | ||
| Clausocalanus furcatus | 9.32 | 50 | 9.64 | 50 | 36.69 | 50 | 55.71 | 60 | ||
| Clausocalanus lividus | 2.57 | 17 | 5.23 | 25 | - | - | - | - | ||
| Clausocalanus parapergens | - | - | - | - | - | - | - | - | ||
| Clausocalanus paululus | 47.13 | 50 | 42.03 | 75 | 10.29 | 50 | 17.21 | 20 | ||
| Clausocalanus pergens | 49.41 | 50 | 131.02 | 75 | 3.25 | 50 | - | - | ||
| Clausocalanus spp. | 8.29 | 17 | 38.15 | 75 | 43.20 | 75 | 27.32 | 60 | ||
| Ctenocalanus vanus | 3.71 | 33 | 9.44 | 25 | 4.30 | 50 | 2.43 | 20 | ||
| Euterpina acutifrons | 60.11 | 50 | 1.81 | 25 | 1.62 | 25 | 5.67 | 40 | ||
| Nannocalanus minor | 2.92 | 33 | - | - | - | - | 3.69 | 20 | ||
| Paracalanus denudatus | 1.25 | 17 | - | - | - | - | - | - | ||
| Paracalanus nanus | 1.42 | 17 | - | - | 3.76 | 50 | 1.21 | 20 | ||
| Paracalanus parvus | 50.41 | 50 | 26.61 | 50 | 80.71 | 75 | 42.24 | 60 | ||
| Paracalanus spp. | 2.63 | 17 | 9.44 | 25 | - | - | - | - | ||
| Temora stylifera | 4.28 | 17 | - | - | 17.24 | 75 | 18.61 | 40 | ||
| Juvenile copepods | 1170.71 | 50 | 2064.95 | 75 | 534.42 | 75 | 614.77 | 60 | ||
| CC | [87,88,89,90] | Candacia armata | - | - | - | - | - | - | 1.85 | 20 |
| Corycaeus spp. | 14.62 | 33 | 5.73 | 25 | 64.11 | 75 | 65.79 | 60 | ||
| DC | [87,91,92,93] | Microsetella sp. | - | - | - | - | - | - | - | - |
| Oncaea spp. | 13.06 | 50 | 8.33 | 50 | 35.70 | 75 | 21.07 | 60 | ||
| Scolecithricidae | - | - | 6.79 | 25 | - | - | 0.64 | 20 | ||
| OC | [82,83,94,95,96] | Centropages kroyeri | - | - | - | - | - | - | - | - |
| Centropages typicus | 8.09 | 33 | 58.89 | 75 | 37.28 | 75 | 1.33 | 20 | ||
| Isias clavipes | 2.50 | 33 | 4.92 | 25 | - | - | - | - | ||
| Oithona nana | 8.00 | 50 | 20.68 | 50 | 5.86 | 50 | 9.45 | 60 | ||
| Oithona plumifera | 1.70 | 17 | - | - | 3.74 | 50 | 46.67 | 60 | ||
| Oithona similis | 27.46 | 50 | 83.79 | 50 | 15.54 | 75 | 55.76 | 60 | ||
| Pleuromamma abdominalis | - | - | - | - | - | - | 1.21 | 20 | ||
| Pleuromamma gracilis | - | - | 1.31 | 25 | - | - | 1.33 | 20 | ||
| Cn | [19,97,98] | Cnidaria | 5.16 | 50 | 5.98 | 75 | 13.79 | 75 | 4.42 | 60 |
| Juvenile Cnidarians | - | - | 2.65 | 50 | 0.14 | 25 | 0.14 | 20 | ||
| Che | [99,100,101,102] | Chaetognatha | 10.94 | 50 | 7.34 | 75 | 11.96 | 75 | 29.62 | 60 |
| Other | [103,104,105] | Evadne spinifera | 1.29 | 33 | 10.60 | 50 | 14.62 | 75 | 0.34 | 20 |
| Evadne spp. | - | - | 2.17 | 50 | 18.28 | 75 | 0.61 | 20 | ||
| Penilia avirostris | - | - | 8.42 | 50 | 425.24 | 75 | 52.38 | 60 | ||
| Podon spp. | 6.59 | 50 | 4.01 | 75 | 8.97 | 75 | 0.20 | 20 | ||
| Thecosomata | 12.30 | 50 | 9.72 | 75 | 25.27 | 75 | 19.57 | 60 | ||
| Appendicularia | 62.64 | 50 | 119.44 | 75 | 172.37 | 75 | 53.13 | 60 | ||
| Thaliacea | 4.62 | 50 | 6.45 | 75 | 31.12 | 75 | 10.87 | 60 | ||
| Fisch larvae | 0.204 | 33 | 2.310 | 50 | 1.223 | 50 | 0.272 | 40 | ||
| Other Juvenile | Malacostraca | 5.16 | 50 | 12.70 | 75 | 5.16 | 75 | 14.61 | 60 | |
| Polychaeta | 4.01 | 50 | 0.48 | 50 | 0.82 | 75 | 12.03 | 60 | ||
| Bivalvia larvae | 4.21 | 50 | 4.01 | 75 | 1.97 | 75 | 1.56 | 60 | ||
| Bryozoa larvae | 0.54 | 50 | 4.14 | 75 | 0.14 | 25 | 1.29 | 20 | ||
| Echinodermata | 11.28 | 50 | 2.38 | 75 | 1.49 | 75 | 7.95 | 60 | ||
| Cirripedia | 0.14 | 17 | - | - | 0.20 | 50 | - | - | ||
| Crustacea | 15.35 | 50 | 4.01 | 50 | 0.54 | 50 | 3.87 | 40 | ||
References
- Buitenhuis, E.; Le Quéré, C.; Aumont, O.; Beaugrand, G.; Bunker, A.; Hirst, A.; Ikeda, T.; O’Brien, T.; Piontkovski, S.; Straile, D. Biogeochemical Fluxes through Mesozooplankton. Glob. Biogeochem. Cycles 2006, 20. [Google Scholar] [CrossRef]
- De Senerpont Domis, L.N.; Elser, J.J.; Gsell, A.S.; Huszar, V.L.M.; Ibelings, B.A.S.W.; Jeppesen, E.; Kosten, S.; Mooij, W.M.; Roland, F.; Sommer, U.; et al. Plankton Dynamics under Different Climatic Conditions in Space and Time. Freshw. Biol. 2013, 58, 463–482. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.S. Resurrecting the Ecological Underpinnings of Ocean Plankton Blooms. Ann. Rev. Mar. Sci. 2014, 6, 167–194. [Google Scholar] [CrossRef]
- Webber, M.; Edwards-Myers, E.; Campbell, C.; Webber, D. Phytoplankton and Zooplankton as Indicators of Water Quality in Discovery Bay, Jamaica. Hydrobiologia 2005, 545, 177–193. [Google Scholar] [CrossRef]
- White, S.; Rakhesh, M.; Sarma, V.S.; Rajanna, B.; Raman, A.V. Discriminating Zooplankton Assemblages through Multivariate Methods: A Case for a Tropical Polluted Harbour and Bar-Built Estuary. Chem. Ecol. 2006, 22, 225–237. [Google Scholar] [CrossRef]
- Valdés, L.; Moral, M. Time-Series Analysis of Copepod Diversity and Species Richness in the Southern Bay of Biscay off Santander, Spain, in Relation to Environmental Conditions. ICES J. Mar. Sci. 1998, 55, 783–792. [Google Scholar] [CrossRef]
- Valdés, L.; Harris, R.; Ikeda, T.; McKinnell, S.; Peterson, W.T. The Role of Zooplankton in Global Ecosystem Dynamics: Comparative Studies from the World Oceans. ICES J. Mar. Sci. 2004, 61, 441–444. [Google Scholar] [CrossRef][Green Version]
- Hooff, R.C.; Peterson, W.T. Copepod Biodiversity as an Indicator of Changes in Ocean and Climate Conditions of the Northern California Current Ecosystem. Limnol. Oceanogr. 2006, 51, 2607–2620. [Google Scholar] [CrossRef]
- Blandin, P. Bioindicateurs et Diagnostic Des Systèmes Écologiques. Bull. Ecol. 1986, 17, 215–307. [Google Scholar]
- Boucher, J.; Ibanez, F.; Prieur, L. Daily and Seasonal Variations in the Spatial Distribution of Zooplankton Populations in Relation to the Physical Structure in the Ligurian Sea Front. J. Mar. Res. 1987, 45, 133–173. [Google Scholar] [CrossRef]
- Bonnet, D.; Frid, C. Seven Copepod Species Considered as Indicators of Water-Mass Influence and Changes: Results from a Northumberland Coastal Station. ICES J. Mar. Sci. 2004, 61, 485–491. [Google Scholar] [CrossRef]
- Beaugrand, G.; IbaHez, F. Monitoring Marine Plankton Ecosystems. II: Long-Term Changes in North Sea Calanoid Copepods in Relation to Hydro-Climatic Variability. Mar. Ecol. Prog. Ser. 2004, 284, 35–47. [Google Scholar] [CrossRef]
- Perry, R.I.; Batchelder, H.P.; Mackas, D.L.; Chiba, S.; Durbin, E.; Greve, W.; Verheye, H.M. Identifying Global Synchronies in Marine Zooplankton Populations: Issues and Opportunities. ICES J. Mar. Sci. 2004, 61, 445–456. [Google Scholar] [CrossRef]
- Jamet, J.-L.; Jean, N.; Bogé, G.; Richard, S.; Jamet, D. Plankton Succession and Assemblage Structure in Two Neighbouring Littoral Ecosystems in the North-West Mediterranean Sea. Mar. Freshw. Res. 2005, 56, 69–83. [Google Scholar] [CrossRef]
- Philippart, C.; Anadón, R.; Danovaro, R.; Dippner, J.; Drinkwater, K.; Hawkins, S.; Oguz, T.; O’Sullivan, G.; Reid, P.C. Impacts of Climate Change on European Marine Ecosystems: Observations, Expectations and Indicators. J. Exp. Mar. Biol. Ecol. 2011, 400, 52–69. [Google Scholar] [CrossRef]
- Beaugrand, G.; Brander, K.M.; Alistair Lindley, J.; Souissi, S.; Reid, P.C. Plankton Effect on Cod Recruitment in the North Sea. Nature 2003, 426, 661–664. [Google Scholar] [CrossRef]
- Alcaraz, M.; Saiz, E.; Estrada, M. Excretion of Ammonia by Zooplankton and Its Potential Contribution to Nitrogen Requirements for Primary Production in the Catalan Sea (NW Mediterranean). Mar. Biol. 1994, 119, 69–76. [Google Scholar] [CrossRef]
- Ribera d’Alcala, M.; Conversano, F.; Corato, F.; Licandro, P.; Mangoni, O.; Marino, D.; Mazzocchi, M.G.; Modigh, M.; Montresor, M.; Nardella, M.; et al. Seasonal Patterns in Plankton Communities in Pluriannual Time Series at a Coastal Mediterranean Site (Gulf of Naples): An Attempt to Discern Recurrences and Trends. Sci. Mar. 2004, 68, 65–83. [Google Scholar] [CrossRef]
- Molinero, J.C.; Ibanez, F.; Souissi, S.; Buecher, E.; Dallot, S.; Nival, P. Climate Control on the Long-Term Anomalous Changes of Zooplankton Communities in the Northwestern Mediterranean. Glob. Chang. Biol. 2008, 14, 11–26. [Google Scholar] [CrossRef]
- Morabito, G.; Mazzocchi, M.G.; Salmaso, N.; Zingone, A.; Bergami, C.; Flaim, G.; Accoroni, S.; Basset, A.; Bastianini, M.; Belmonte, G.; et al. Plankton Dynamics across the Freshwater, Transitional and Marine Research Sites of the LTER-Italy Network. Patterns, Fluctuations, Drivers. Sci. Total Environ. 2018, 627, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Doglioli, A.M.; Griffa, A.; Magaldi, M.G. Numerical Study of a Coastal Current on a Steep Slope in Presence of a Cape: The Case of the Promontorio Di Portofino. J. Geophys. Res. Ocean. 2004, 109. [Google Scholar] [CrossRef]
- Doglioli, A.M.; Magaldi, M.G.; Vezzulli, L.; Tucci, S. Development of a Numerical Model to Study the Dispersion of Wastes Coming from a Marine Fish Farm in the Ligurian Sea (Western Mediterranean). Aquaculture 2004, 231, 215–235. [Google Scholar] [CrossRef]
- Cattaneo-Vietti, R.; Cappanera, V.; Castellano, M.; Povero, P. Yield and Catch Changes in a Mediterranean Small Tuna Trap: A Warming Change Effect? Mar. Ecol. 2015, 36, 155–166. [Google Scholar] [CrossRef]
- Vietti, R.C.; Albertelli, G.; Aliani, S.; Bava, S.; Bavestrello, G.; Cecchi, L.B.; Bianchi, C.N.; Bozzo, E.; Capello, M.; Castellano, M.; et al. The Ligurian Sea: Present Status, Problems and Perspectives. Chem. Ecol. 2010, 26, 319–340. [Google Scholar] [CrossRef]
- Parravicini, V.; Micheli, F.; Montefalcone, M.; Morri, C.; Villa, E.; Castellano, M.; Povero, P.; Bianchi, C.N. Conserving Biodiversity in a Human-Dominated World: Degradation of Marine Sessile Communities within a Protected Area with Conflicting Human Uses. PLoS ONE 2013, 8, e75767. [Google Scholar] [CrossRef]
- Povero, P.; Misic, C.; Castellano, M.; Ruggieri, N.; Fabiano, M. Response of a Coastal Marine Ecosystem to Atmospheric Forcing (Portofino, Ligurian Sea). In Proceedings of the First Italian IGBP Conference, Paestum (Salerno), Italy, 14–16 November 2002. [Google Scholar]
- Ruggieri, N.; Castellano, M.; Misic, C.; Gasparini, G.; Cattaneo-Vietti, R. Seasonal and Interannual Dynamics of a Coastal Ecosystem (Portofino, Ligurian Sea) in Relation to Meteorological Constraints. Geophys. Res. Abstr. 2006, 8, 07774. [Google Scholar]
- Holm-Hansen, O.; Lorenzen, C.J.; Holmes, R.W.; Strickland, J.D.H. Fluorometric Determination of Chlorophyll. ICES J. Mar. Sci. 1965, 30, 3–15. [Google Scholar] [CrossRef]
- Frontier, S. Calcul de l’erreur Sur Un Comptage de Zooplancton. J. Exp. Mar. Biol. Ecol. 1972, 8, 121–132. [Google Scholar] [CrossRef]
- Fabiano, M.; Danovaro, R.; Fraschetti, S. A Three-Year Time Series of Elemental and Biochemical Composition of Organic Matter in Subtidal Sandy Sediments of the Ligurian Sea (Northwestern Mediterranean). Cont. Shelf Res. 1995, 15, 1453–1469. [Google Scholar] [CrossRef]
- D’Alelio, D.; Libralato, S.; Wyatt, T.; Ribera d’Alcalà, M. Ecological-Network Models Link Diversity, Structure and Function in the Plankton Food-Web. Sci. Rep. 2016, 6, 21806. [Google Scholar] [CrossRef] [PubMed]
- Little, W.; Copley, N. WHOI Silhouette DIGITIZER Version 1.0 User’s Guide; Woods Hole Oceanographic Institution: Woods Hole, MA, USA, 2003. [Google Scholar] [CrossRef]
- Bednaršek, N.; Možina, J.; Vogt, M.; O’Brien, C.; Tarling, G. The Global Distribution of Pteropods and Their Contribution to Carbonate and Carbon Biomass in the Modern Ocean. Earth Syst. Sci. Data 2012, 4, 167–186. [Google Scholar] [CrossRef]
- Larson, R.J. Water Content, Organic Content, and Carbon and Nitrogen Composition of Medusae from the Northeast Pacifi. J. Exp. Mar. Biol. Ecol. 1986, 99, 107–120. [Google Scholar] [CrossRef]
- Zuur, A.; Ieno, E.; Smith, G. Analysing Ecological Data; Springer Science and Business Media LLC: New York, NY, USA, 2007; Volume 75, ISBN 978-0-387-45967-7. [Google Scholar]
- Paoli, C.; Morten, A.; Bianchi, C.N.; Morri, C.; Fabiano, M.; Vassallo, P. Capturing Ecological Complexity: OCI, a Novel Combination of Ecological Indices as Applied to Benthic Marine Habitats. Ecol. Indic. 2016, 66, 86–102. [Google Scholar] [CrossRef]
- Christensen, V. A Model of Trophic Interactions in the North Sea in 1981, the Year of the Stomach. Dana 2003, 11, 1–28. [Google Scholar]
- Christensen, V.; Walters, C. Ecopath With Ecosim: Methods, Capabilities and Limitations. Ecol. Model. 2004, 172, 109–139. [Google Scholar] [CrossRef]
- Leontief, W.W. The Structure of {American} Economy, {1919–1939}; An Empirical Application of Equilibrium Analysis; Oxford University Press: New York, NY, USA, 1951. [Google Scholar]
- Ulanowicz, R.E.; Puccia, C.J. Mixed trophic impacts in ecosystems. Coenoses 1990, 5, 7–16. [Google Scholar]
- Libralato, S.; Christensen, V.; Pauly, D. A Method for Identifying Keystone Species in Food Web Models. Ecol. Model. 2006, 195, 153–171. [Google Scholar] [CrossRef]
- Ulanowicz, R.E. Growth and Development: Ecosystem Phenomenology; Springer: New York, NY, USA, 1986. [Google Scholar]
- Finn, T.J. Measures of Ecosystem Structure and Function Derived from Analysis of Flows. J. Theor. Biol. 1976, 56, 363–380. [Google Scholar] [CrossRef]
- Ulanowicz, R.E.; Abarca-Arenas, L.G. An Informational Synthesis of Ecosystem Structure and Function. Ecol. Model. 1997, 95, 1–10. [Google Scholar] [CrossRef]
- Odum, H.T. Environmental Accounting. Emergy and Environmental Decision Making; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
- Vassallo, P.; Paoli, C.; Buonocore, E.; Franzese, P.P.; Russo, G.F.; Povero, P. Assessing the Value of Natural Capital in Marine Protected Areas: A Biophysical and Trophodynamic Environmental Accounting Model. Ecol. Model. 2017, 355, 12–17. [Google Scholar] [CrossRef]
- Paoli, C.; Povero, P.; Burgos, E.; Dapueto, G.; Fanciulli, G.; Massa, F.; Scarpellini, P.; Vassallo, P. Natural Capital and Environmental Flows Assessment in Marine Protected Areas: The Case Study of Liguria Region (NW Mediterranean Sea). Ecol. Model. 2018, 368, 121–135. [Google Scholar] [CrossRef]
- De La Fuente, G.; Asnaghi, V.; Chiantore, M.; Thrush, S.; Povero, P.; Vassallo, P.; Petrillo, M.; Paoli, C. The Effect of Cystoseira Canopy on the Value of Midlittoral Habitats in NW Mediterranean, an Emergy Assessment. Ecol. Model. 2019, 404, 1–11. [Google Scholar] [CrossRef]
- Mattei, F.; Buonocore, E.; Franzese, P.P.; Scardi, M. Global Assessment of Marine Phytoplankton Primary Production: Integrating Machine Learning and Environmental Accounting Models. Ecol. Model. 2021, 451, 109578. [Google Scholar] [CrossRef]
- Jorgensen, S.E.; Nielsen, S.N.; Meyer, H.F. Emergy, Environ, Exergy and Ecological Modelling. Ecol. Model. 1995, 77, 99–109. [Google Scholar] [CrossRef]
- McGowan, J.A. Climate and Change in Oceanic Ecosystems: The Value of Time-Series Data. Trends Ecol. Evol. 1990, 5, 293–299. [Google Scholar] [CrossRef]
- Ménard, F.J.; Fromentin, M.; Goy, J.; Dallot, S. Temporal Fluctuations of Doliolid Abundance in the Bay of Ville-Franche-Sur-Mer (Northwestern Mediterranean Sea) from 1967 to 1990. Oceanol. Acta 1997, 20, 733–742. [Google Scholar]
- Fevre-Lehoerff, G.; Ibanez, F.; Poniz, P.; Fromentin, J.-M. Hydroclimatic Relationships with Planktonic Time Series from 1975 to 1992 in the North Sea off Gravelines, France. Mar. Ecol. Ser. 1995, 129, 269–281. [Google Scholar] [CrossRef]
- Fromentin, J.; Planque, B. Calanus and Environment in the Eastern North Atlantic. II. Influence of the North Atlantic Oscillation on C. Finmarchicus and C. Helgolandicus. Mar. Ecol. Prog. Ser. 1996, 134, 111–118. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, S.; Licandro, P.; van Damme, C.J.G.; Hufnagl, M. Effect of Zooplankton on Fish Larval Abundance and Distribution: A Long-Term Study on North Sea Herring (Clupea Harengus). ICES J. Mar. Sci. 2015, 72, 2569–2577. [Google Scholar] [CrossRef]
- Monteiro, M.; Azeiteiro, U.M.; Martinho, F.; Pardal, M.A.; Primo, A.L. Long-Term Changes of Ichthyoplankton Communities in an Iberian Estuary Are Driven by Varying Hydrodynamic Conditions. J. Plankton Res. 2021, 43, 33–45. [Google Scholar] [CrossRef]
- Pucher-Petković, T. Recherches Sur La Production Primaire et La Densite Des Populations Du Phyfcoplancton En Adriatique Moyenne (1962–1967) (Research on Primary Production and Population Density of Phycoplankton in the Middle Adriatic). Rapp. Comm. Int. Mer. Medit. 1971, 20, 339–343. [Google Scholar]
- Goy, J.; Pie, O. Long-Term Fluctuations of Pelagia Noctiluca (Cnidaria, Scyphomedusa) in the Western Mediterranean Sea: Prediction by Climatic Variables. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 36, 269–279. [Google Scholar] [CrossRef]
- Ménard, F.S.; Dallot, G.T.; Braconnot, J.C. Temporal Fluctuations of Two Mediterranean Salp Populations From1967 to 1990. Analysis of the Influence of Environmental Variables Using a Markov Chain Model. Mar. Ecol. Prog. Ser. 1994, 104, 139–152. [Google Scholar] [CrossRef]
- Cataletto, B.; Feoli, E.; Umani, S.F.; Cheng-Yong, S. Eleven Years of Time-Series Analysis on the Net-Zooplankton Community in the Gulf of Trieste. ICES J. Mar. Sci. 1995, 52, 669–678. [Google Scholar] [CrossRef]
- Solic, M.; Krstulovic, N.; Marasović, I.; Baranovic, A.; Puciier-Petkovic, T.; Vucetic, T. Analysis of Time Sertes of Planktonic Communities in the Adriatic Sea: Distinguishing between Natural and Man-Induced Changes. Oceanol. Acta 1997, 20, 131–143. [Google Scholar]
- Licandro, P.; Ibanez, F. Changes of Zooplankton Communities in the Gulf of Tigullio (Ligurian Sea, Western Mediterranean) from 1985 to 1995. Influence of Hydroclimatic Factors. J. Plankton Res. 2000, 22, 2225–2253. [Google Scholar] [CrossRef]
- García-Comas, C.; Stemmann, L.; Ibanez, F.; Berline, L.; Mazzocchi, M.G.; Gasparini, S.; Picheral, M.; Gorsky, G. Zooplankton Long-Term Changes in the NW Mediterranean Sea: Decadal Periodicity Forced by Winter Hydrographic Conditions Related to Large-Scale Atmospheric Changes? J. Mar. Syst. 2011, 87, 216–226. [Google Scholar] [CrossRef]
- Mazzocchi, M.G.; Licandro, P.; Dubroca, L.; Di Capua, I.; Saggiomo, V. Zooplankton Associations in a Mediterranean Long-Term Time-Series. J. Plankton Res. 2011, 33, 1163–1181. [Google Scholar] [CrossRef]
- Vandromme, P.; Stemmann, L.; Berline, L.; Gasparini, S.; Mousseau, L.; Prejger, F.; Passafiume, O.; Guarini, J.-M.; Gorsky, G. Inter-Annual Fluctuations of Zooplankton Communities in the Bay of Villefranche-Sur-Mer from 1995 to 2005 (Northern Ligurian Sea, France). Biogeosciences 2011, 8, 3143–3158. [Google Scholar] [CrossRef]
- Berline, L.; Siokou-Frangou, I.; Marasović, I.; Vidjak, O.; de Puelles, M.L.F.; Mazzocchi, M.G.; Assimakopoulou, G.; Zervoudaki, S.; Fonda-Umani, S.; Conversi, A.; et al. Intercomparison of Six Mediterranean Zooplankton Time Series. Prog. Oceanogr. 2012, 97, 76–91. [Google Scholar] [CrossRef]
- Fullgrabe, L.; Grosjean, P.; Gobert, S.; Lejeune, P.; Leduc, M.; Engels, G.; Dauby, P.; Boissery, P.; Richir, J. Zooplankton Dynamics in a Changing Environment: A 13-Year Survey in the Northwestern Mediterranean Sea. Mar. Environ. Res. 2020, 159, 104962. [Google Scholar] [CrossRef]
- D’Alelio, D.; Mazzocchi, M.G.; Montresor, M.; Sarno, D.; Zingone, A.; Di Capua, I.; Franzè, G.; Margiotta, F.; Saggiomo, V.; Ribera d’Alcalà, M. The Green–Blue Swing: Plasticity of Plankton Food-Webs in Response to Coastal Oceanographic Dynamics. Mar. Ecol. 2015, 36, 1155–1170. [Google Scholar] [CrossRef]
- Marouan, M.; Niquil, N.; Grami, B.; Mejri, K.; Haraldsson, M.; Chaalali, A.; Pringault, O.; Hlaili, A. A New Type of Plankton Food Web Functioning in Coastal Waters Revealed by Coupling Monte Carlo Markov Chain Linear Inverse Method and Ecological Network Analysis. Ecol. Indic. 2019, 104, 67–85. [Google Scholar] [CrossRef]
- Betti, F.; Venturini, S.; Merotto, L.; Cappanera, V.; Ferrando, S.; Aicardi, S.; Mandich, A.; Castellano, M.; Povero, P. Population Trends of the Fan Mussel Pinna Nobilis from Portofino MPA (Ligurian Sea, Western Mediterranean Sea) before and after a Mass Mortality Event and a Catastrophic Storm. Eur. Zool. J. 2021, 88, 18–25. [Google Scholar] [CrossRef]
- Misic, C.; Fabiano, M. Ectoenzymatic Activity and Its Relationship to Chlorophyll- a and Bacteria in the Gulf of Genoa (Ligurian Sea, NW Mediterranean). J. Mar. Syst. 2006, 60, 193–206. [Google Scholar] [CrossRef]
- Misic, C.; Castellano, M.; Covazzi Harriague, A. Organic Matter Features, Degradation and Remineralisation at Two Coastal Sites in the Ligurian Sea (NW Mediterranean) Differently Influenced by Anthropogenic Forcing. Mar. Environ. Res. 2011, 72, 67–74. [Google Scholar] [CrossRef]
- Coppari, M.; Ferrier-Pagès, C.; Castellano, M.; Massa, F.; Olivari, E.; Bavestrello, G.; Povero, P.; Bo, M. Seasonal Variation of the Stable C and N Isotopic Composition of the Mesophotic Black Coral Antipathella Subpinnata (Ellis \& Solander, 1786). Estuar. Coast. Shelf Sci. 2020, 233, 106520. [Google Scholar]
- D’Ortenzio, F.; Ribera d’Alcalà, M. On the Trophic Regimes of the Mediterranean Sea: A Satellite Analysis. Biogeosciences 2009, 6, 139–148. [Google Scholar] [CrossRef]
- Frangoulis, C.; Grigoratou, M.; Zoulias, T.; Hannides, C.C.S.; Pantazi, M.; Psarra, S.; Siokou, I. Expanding Zooplankton Standing Stock Estimation from Meso- to Metazooplankton: A Case Study in the N. Aegean Sea (Mediterranean Sea). Cont. Shelf Res. 2017, 149, 151–161. [Google Scholar] [CrossRef]
- Anjusha, A.; Jyothibabu, R.; Jagadeesan, L.; Mohan, A.P.; Sudheesh, K.; Krishna, K.; Ullas, N.; Deepak, M. Trophic Efficiency of Plankton Food Webs: Observations from the Gulf of Mannar and the Palk Bay, Southeast Coast of India. J. Mar. Syst. 2013, 115, 40–61. [Google Scholar] [CrossRef]
- Hannides, C.C.S.; Popp, B.N.; Close, H.G.; Benitez-Nelson, C.R.; Ka’apu-Lyons, C.A.; Gloeckler, K.; Wallsgrove, N.; Umhau, B.; Palmer, E.; Drazen, J.C. Seasonal Dynamics of Midwater Zooplankton and Relation to Particle Cycling in the North Pacific Subtropical Gyre. Prog. Oceanogr. 2020, 182, 102266. [Google Scholar] [CrossRef]
- Vassallo, P.; Fabiano, M. Trophodynamic Variations on Microtidal North Mediterranean Sandy Beaches. Oceanologia 2005, 47, 351–364. [Google Scholar]
- Vassallo, P.; Paoli, C.; Schiavon, G.; Albertelli, G.; Fabiano, M. How Ecosystems Adapt to Face Disruptive Impact? The Case of a Commercial Harbor Benthic Community. Ecol. Indic. 2013, 24, 431–438. [Google Scholar] [CrossRef]
- D’Alelio, D.; Montresor, M.; Mazzocchi, M.G.; Margiotta, F.; Sarno, D.; D’Alcalà, M.R. Plankton Food-Webs: To What Extent Can They Be Simplified? Adv. Oceanogr. Limnol. 2016, 7, 67–92. [Google Scholar] [CrossRef]
- Cianelli, D.; D’Alelio, D.; Uttieri, M.; Sarno, D.; Zingone, A.; Zambianchi, E.; d’Alcalà, M.R. Disentangling Physical and Biological Drivers of Phytoplankton Dynamics in a Coastal System. Sci. Rep. 2017, 7, 15868. [Google Scholar] [CrossRef] [PubMed]
- Martynova, D.; Kazus, N.; Bathmann, U.; Graeve, M.; Sukhotin, A. Seasonal Abundance and Feeding Patterns of Copepods Temora Longicornis, Centropages Hamatus and Acartia Spp. in the White Sea (66° N). Polar Biol. 2011, 34, 1175–1195. [Google Scholar] [CrossRef]
- Wiadnyana, N.N.; Wiadnyana, N.W.; Rassoulzadegan, F. Selective Feeding of Acartia Clausi and Centropages Typicus on Microzooplankton. Mar. Ecol. Prog. Ser. 1989, 53, 37–45. [Google Scholar] [CrossRef]
- Razouls, C.; Kouwenberg, J.; Desreumaux, N. Diversity and Geographic Distribution of Marine Planktonic Copepods (Sensu lato) A Pan-European Species-Directories Infrastructure (PESI) View Project. 2005. Available online: https://doi.org/10.13140/RG.2.1.2077.4241 (accessed on 15 December 2021).
- Swadling, K.M.; Slotwinski, A.; Davies, C.; Beard, J.; McKinnon, A.D.; Coman, F.; Murphy, N.; Tonks, M.; Rochester, W.; Conway, D.V.P.; et al. Australian Marine Zooplankton: A Taxonomic Guide and Atlas. Available online: http://www.imas.utas.edu.au/zooplankton (accessed on 15 December 2021).
- Dam, H.G.; Lopes, R.M. Omnivory in the Calanoid Copepod Temora Longicornis: Feeding, Egg Production and Egg Hatching Rates. J. Exp. Mar. Biol. Ecol. 2003, 292, 119–137. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Watanabe, Y.; Ishida, H.; Harimoto, T.; Furusawa, K.; Suzuki, S.; Ishizaka, J.; Ikeda, T.; Takahashi, M. Mac Community and Trophic Structures of Pelagic Copepods down to Greater Depths in the Western Subarctic Pacific (WEST-COSMIC). Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 1007–1025. [Google Scholar] [CrossRef]
- Kouwenberg, J. Copepod Distribution in Relation to Seasonal Hydrographics and Spatial Structure in the North-Western Mediterranean (Golfe Du Lion). Estuar. Coast. Shelf Sci. 1994, 38, 69–90. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Onbé, T. Evidence of Selective Feeding on Larvaceans by the Pelagic Copepod Candacia Bipinnata (Calanoida: Candaciidae). J. Plankton Res. 1989, 11, 869–872. [Google Scholar] [CrossRef]
- López-Urrutia, Á.; Harris, R.P.; Smith, T. Predation by Calanoid Copepods on the Appendicularian Oikopleura Dioica. Limnol. Oceanogr. 2004, 49, 303–307. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Kubo, N.; Okada, M.; Gushima, K. Attachment and Feeding of Pelagic Copepods on Larvacean Houses. J. Oceanogr. 1993, 49, 115–120. [Google Scholar] [CrossRef]
- Nishida, S.; Ohtsuka, S. Ultrastructure of the Mouthpart Sensory Setae in Mesopelagic Copepods of the Family Scolecitrichidae. Plankt. Biol. Ecol. 1997, 44, 81–90. [Google Scholar]
- Maar, M.; Visser, A.; Nielsen, T.G.; Stips, A.; Saito, H. Turbulence and Feeding Behaviour Affect the Vertical Distributions of Oithona Similis and Microsetella Norwegica. Mar. Ecol. Ser. 2006, 313, 157–172. [Google Scholar] [CrossRef][Green Version]
- Calbet, A.; Carlotti, F.; Gaudy, R. The Feeding Ecology of the Copepod Centropages Typicus (Kröyer). Prog. Oceanogr. 2007, 72, 137–150. [Google Scholar] [CrossRef]
- Turner, J.T. The Importance of Small Planktonic Copepods and Their Roles in Pelagic Marine Food Webs. Zool. Stud. 2004, 43, 255–266. [Google Scholar]
- Lampitt, R.S.; Gamble, J.C. Diet and Respiration of the Small Planktonic Marine Copepod Oithona Nana. Mar. Biol. 1982, 66, 185–190. [Google Scholar] [CrossRef]
- Boero, F.; Bucci, C.; Colucci, A.M.R.; Gravili, C.; Stabili, L. Obelia (Cnidaria, Hydrozoa, Campanulariidae): A Microphagous, Filter-Feeding Medusa. Mar. Ecol. 2007, 28, 178–183. [Google Scholar] [CrossRef]
- Purcell, J.E. A Review of Cnidarians and Ctenophores Feeding on Competitors in the Plankton. Hydrobiologia 1991, 216, 335–342. [Google Scholar] [CrossRef]
- Pearre, S., Jr. Vertical Migration and Feeding in Sagitta Elegans Verrill. Ecology 1973, 54, 300–314. [Google Scholar] [CrossRef]
- Giesecke, R.; González, H.E. Feeding of Sagitta Enflata and Vertical Distribution of Chaetognaths in Relation to Low Oxygen Concentrations. J. Plankton Res. 2004, 26, 475–486. [Google Scholar] [CrossRef]
- Kehayias, G.; Kourouvakalis, D. Diel Vertical Migration and Feeding of Chaetognaths in Coastal Waters of the Eastern Mediterranean. Biologia 2010, 65, 301–308. [Google Scholar] [CrossRef]
- Bonnet, D.; Lindeque, P.K.; Harris, R.P. Sagitta Setosa Predation on Calanus Helgolandicus in the English Channel. J. Plankton Res. 2010, 32, 725–737. [Google Scholar] [CrossRef]
- Katechakis, A.; Stibor, H. Feeding Selectivities of the Marine Cladocerans Penilia Avirostris, Podon Intermedius and Evadne Nordmanni. Mar. Biol. 2004, 145, 529–539. [Google Scholar] [CrossRef]
- Lipej, L.; Mozetič, P.; Turk, V.; Malej, A. The Trophic Role of the Marine Cladoceran Penilia Avirostris in the Gulf of Trieste. Hydrobiologia 1997, 360, 197–203. [Google Scholar] [CrossRef]
- Atienza, D.; Saiz, E.; Calbet, A. Feeding Ecology of the Marine Cladoceran Penilia Avirostris: Natural Diet, Prey Selectivity and Daily Ration. Mar. Ecol. Ser. 2006, 315, 211–220. [Google Scholar] [CrossRef]


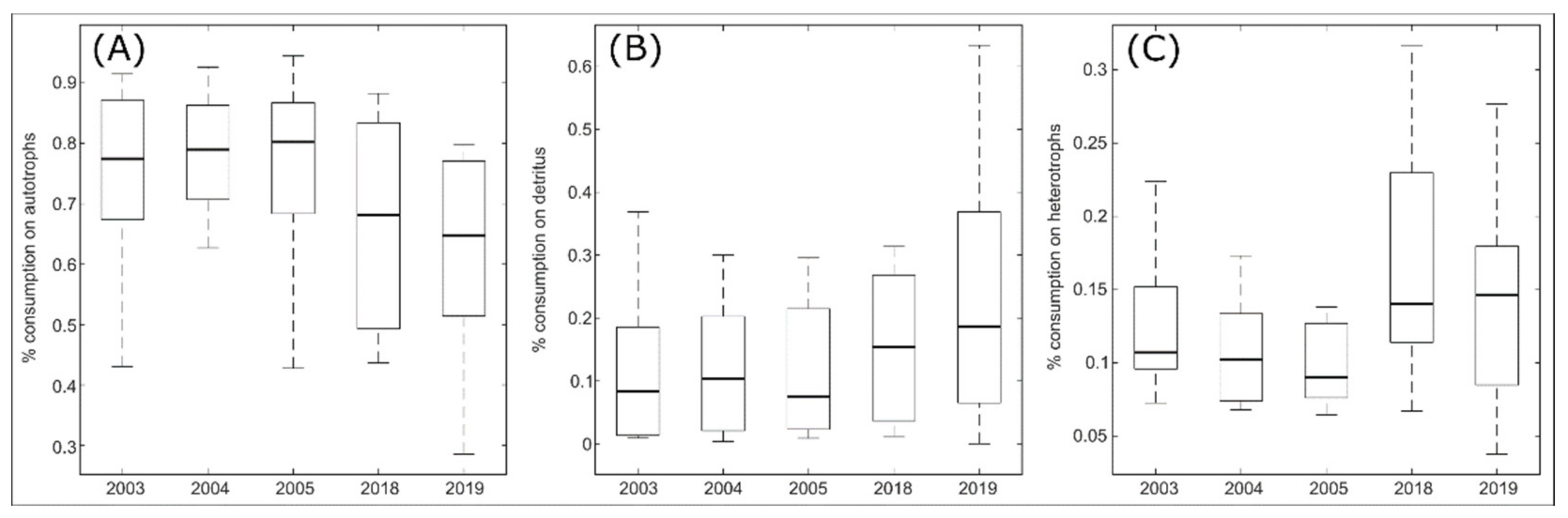
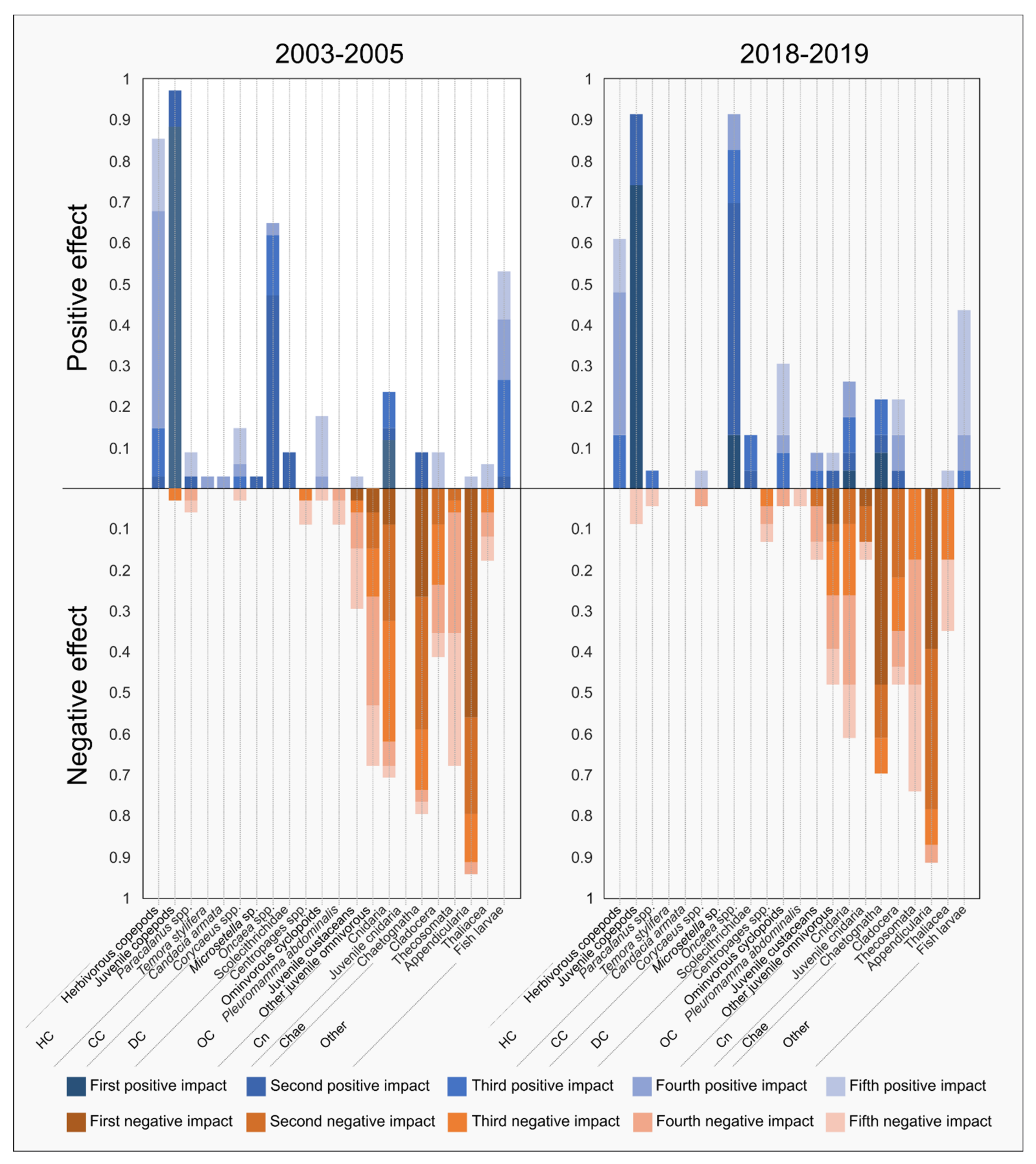
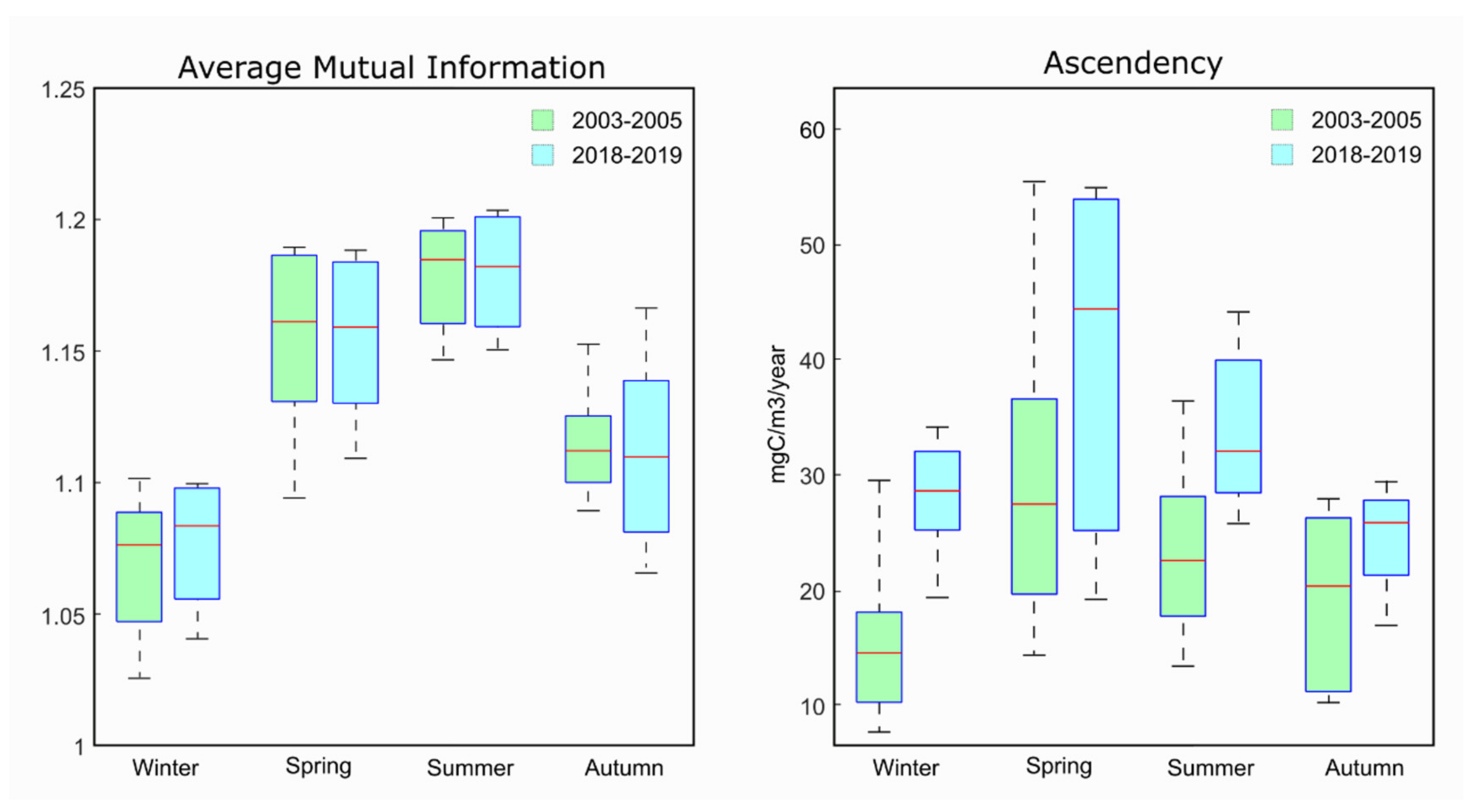
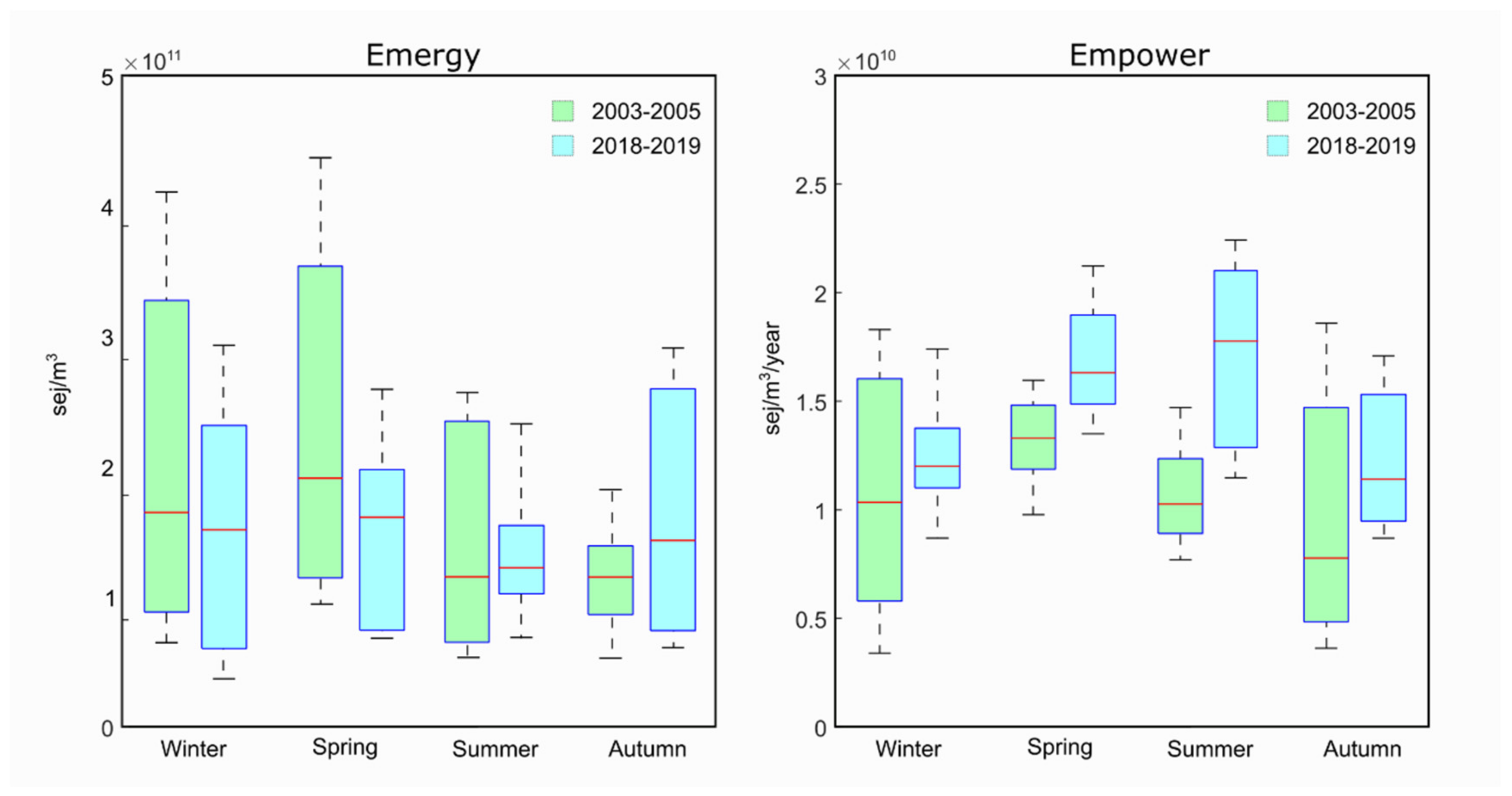
| Groups Number | TL Mean | TL Max | Production (Sum) | Respiration (Tot) | Flows to Detritus | TST | |
|---|---|---|---|---|---|---|---|
| mgC/m3/year | |||||||
| 2003 | |||||||
| Minimum | 16.00 | 1.26 | 1.61 | 7.40 | 2.00 | 5.23 | 12.91 |
| Maximum | 22.00 | 1.98 | 2.41 | 31.06 | 8.44 | 29.15 | 45.12 |
| Mean | 19.83 | 1.67 | 2.10 | 17.57 | 4.50 | 12.76 | 28.90 |
| St.dev. | 1.59 | 0.26 | 0.22 | 9.94 | 2.24 | 8.46 | 12.43 |
| 2004 | |||||||
| Minimum | 13.00 | 1.31 | 1.49 | 7.61 | 1.86 | 4.94 | 12.17 |
| Maximum | 23.00 | 1.79 | 2.35 | 28.26 | 6.52 | 26.53 | 42.17 |
| Mean | 18.45 | 1.53 | 1.95 | 16.13 | 3.66 | 13.94 | 26.87 |
| St.dev. | 2.46 | 0.17 | 0.28 | 10.08 | 1.36 | 9.40 | 10.22 |
| 2005 | |||||||
| Minimum | 15.00 | 1.23 | 1.41 | 12.71 | 1.42 | 6.83 | 20.22 |
| Maximum | 20.00 | 1.94 | 2.48 | 25.96 | 10.48 | 20.14 | 53.01 |
| Mean | 17.82 | 1.62 | 2.00 | 17.21 | 4.90 | 12.13 | 30.10 |
| St.dev. | 1.60 | 0.20 | 0.27 | 4.15 | 2.30 | 4.13 | 9.00 |
| 2018 | |||||||
| Minimum | 16.00 | 1.31 | 1.77 | 8.13 | 2.59 | 4.17 | 15.44 |
| Maximum | 22.00 | 2.22 | 2.45 | 47.97 | 7.40 | 41.42 | 56.06 |
| Mean | 19.09 | 1.75 | 2.18 | 22.13 | 4.54 | 16.37 | 33.36 |
| St.dev. | 1.76 | 0.27 | 0.19 | 14.05 | 1.61 | 12.69 | 15.38 |
| 2019 | |||||||
| Minimum | 17.00 | 1.38 | 2.00 | 8.01 | 1.31 | 4.33 | 11.23 |
| Maximum | 22.00 | 2.26 | 2.66 | 36.93 | 13.67 | 17.64 | 50.45 |
| Mean | 19.58 | 1.77 | 2.26 | 21.12 | 5.28 | 10.75 | 28.93 |
| St.dev. | 1.44 | 0.24 | 0.19 | 5.85 | 3.29 | 3.96 | 10.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vassallo, P.; Bellardini, D.; Castellano, M.; Dapueto, G.; Povero, P. Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria). Diversity 2022, 14, 19. https://doi.org/10.3390/d14010019
Vassallo P, Bellardini D, Castellano M, Dapueto G, Povero P. Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria). Diversity. 2022; 14(1):19. https://doi.org/10.3390/d14010019
Chicago/Turabian StyleVassallo, Paolo, Daniele Bellardini, Michela Castellano, Giulia Dapueto, and Paolo Povero. 2022. "Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria)" Diversity 14, no. 1: 19. https://doi.org/10.3390/d14010019
APA StyleVassallo, P., Bellardini, D., Castellano, M., Dapueto, G., & Povero, P. (2022). Structure and Functionality of the Mesozooplankton Community in a Coastal Marine Environment: Portofino Marine Protected Area (Liguria). Diversity, 14(1), 19. https://doi.org/10.3390/d14010019







