High Diversity in Urban Areas: How Comprehensive Sampling Reveals High Ant Species Richness within One of the Most Urbanized Regions of the World
Abstract
1. Introduction
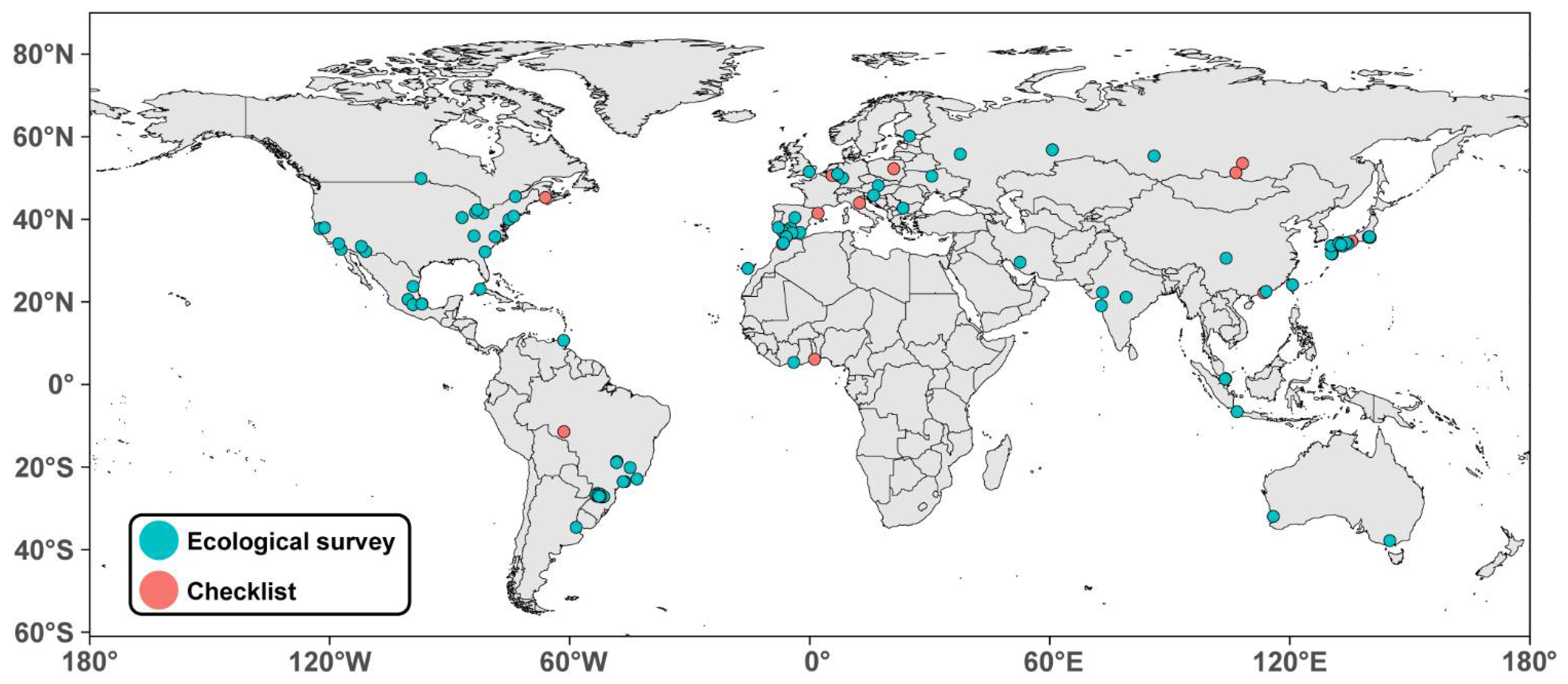
| References | Study Type | Locality | Latitude | Area (km2) | Natives | Exotics | Total |
|---|---|---|---|---|---|---|---|
| Africa (7) | |||||||
| Kouakou et al. 2018 [62] | ecological survey | Abidjan, Ivory Coast | 5.3 | 422 | 170 | 6 | 176 |
| Yeo et al. 2016 [63] | ecological survey | Abidjan, Ivory Coast | 5.3 | 422 | 83 | 8 | 91 |
| Kasseney et al. 2019 [64] | checklist | Lomé (Togo) | 6.1 | 99.1 | 41 | 2 | 43 |
| Taheri et al. 2017 [65] | ecological survey | Tangier, Morocco | 35.76 | 116 | 30 | 8 | 38 |
| Bernard 1958 [66] | checklist | Rabat, Kenitra and Tangier, Morocco | 34.3 to 35.8 | 905 (multiple cities included) | 5 *** | 11 *** | 16 *** |
| Bernard 1974 [67] | ecological survey | Kenitra, Morocco | 34.3 | 112 | 13 | 0 | 13 |
| Reyes-Lopez and Carpintero 2014 [68] | ecological survey | Las Palmas, Canary Islands | 28.2 | 100.6 | 6 | 6 | 12 |
| Asia (42) | |||||||
| Ito et al. 2001 [69] | checklist | Bogor, Java, Indonesia | −6.6 | 118.5 | 202 | 11 | 213 |
| This study | checklist | Macao, SAR, China | 22.2 | 32.9 | 137 | 18 | 155 |
| Leong et al. 2017 [61] | checklist | Macao, SAR, China | 22.2 | 32.9 | 88 | 10 | 98 |
| Rizali et al. 2008 [70] | ecological survey | Bogor, West Java, Indonesia | −6.6 | 118.5 | 82 | 12 | 94 |
| Terayama 2005a,b and 2014 [71,72,73] | ecological survey ** | Tokyo, Japan | 35.7 | 2194.1 | 54 | 0 | 54 |
| Matsumura and Yamane 2012 [74] | ecological survey | Kagoshima City, Japan | 31.6 | 547.6 | 48 | 3 | 51 |
| Liu et al. 2019 [75] | ecological survey | Taichung City, Taiwan | 24.2 | 2215 | ? | ? | 50 * |
| Natuhara 1998 [76] | checklist | Osaka City, Japan | 34.7 | 225.2 | 44 | 5 | 49 |
| Tan and Corlett 2012 [77] | ecological survey | Singapore | 1.3 | 728.3 | 38 | 4 | 42 |
| Harada et al. 2012 [78] | checklist | Isa City, Japan | 32.1 | 392.4 | 40 | 0 | 40 |
| Iwata, Eguchi and Yamane 2005 [79] | ecological survey | Kagoshima City, Japan | 31.6 | 547.6 | 37 | 2 | 39 |
| Yamaguchi 2004 [80] | ecological survey | Chiba, Japan | 35.6 | 271.8 | 37 | 1 | 38 |
| Park et al. 2014a [81] | ecological survey | Fukuoka City, Japan | 33.6 | 343.4 | 35 | 3 | 38 |
| Matsumura and Yamane 2012 [74] | ecological survey | Kagoshima City, Japan | 31.6 | 547.6 | 36 | 2 | 38 |
| Wang et al. 2012 [82] | ecological survey | Shenzhen, Guangdong, China | 22.5 | 2050 | 29 | 6 | 35 |
| Khot et al. 2014 [83] | ecological survey | Mumbai, Maharashtra, India | 19.1 | 603 | 25 | 3 | 28 |
| Yamaguchi 2004 [80] | ecological survey | Tokyo, Japan | 35.7 | 2194.1 | 27 | 1 | 28 |
| Hiroyuki 2012 [84] | checklist | Matsuyama, Japan | 33.8 | 429.4 | 28 | 0 | 28 |
| Harada et al. 2021 [85] | ecological survey | Kagoshima City, Japan | 31.6 | 547.6 | 21 | 6 | 27 |
| Miyake et al. 2002 [86] | ecological survey | Hatsukaichi City, Japan | 34.4 | 489.4 | 22 | 4 | 26 |
| Tan et al. 2009 [87] | ecological survey | Chengdu, Sichuan, China | 30.7 | 885.6 | 24 | 2 | 26 |
| Park et al. 2014b [88] | ecological survey | Hiroshima city, Japan | 34.4 | 906.7 | 21 | 4 | 25 |
| Touyama, Ogata and Sugiyama 2003 [89] | ecological survey | Hatsukaichi and Hiroshima city, Japan | 34.4 | 1395 (multiple cities included) | 23 *** | 1 *** | 24 *** |
| Harada and Yamashita, 2019 [90] | ecological survey | Tokushima, Japan | 34.1 | 191 | 21 | 1 | 22 |
| Yasuda and Koike 2009 | ecological survey | Matsudo, Japan | 35.8 | 61.4 | ? | ? | 22 * |
| Malozemova and Malozemov 1999 [91] | ecological survey | Yekaterinburg, Russia | 56.8 | 495 | 20 | 1 | 21 |
| Harada 2020 [92] | ecological survey | Hioki City, Japan | 31.6 | 253.1 | 16 | 3 | 19 |
| Roshanak et al. 2017 [93] | ecological survey | Shiraz, Iran | 29.6 | 240 | 19 | 0 | 19 |
| Hosoishi et al. 2019 [94] | ecological survey | Fukuoka City, Japan | 33.6 | 343.4 | 17 | 1 | 18 |
| Terayama et al. 2006 [95] | ecological survey | Iwakuni City, Japan | 34.2 | 873.7 | 14 | 2 | 16 |
| Putyatina et al. 2017 [96] | ecological survey | Moscow, Russia | 55.8 | 2511 | 16 | 0 | 16 |
| Kumar and Archana 2008 [97] | ecological survey | Vadodara, Gujarat, India | 22.3 | 220 | 15 | 0 | 15 |
| Harada and Yamashita, 2019 [90] | ecological survey | Kochi, Japan | 33.6 | 309.2 | 15 | 0 | 15 |
| Terayama et al. 2006 [95] | ecological survey | Iwakuni City, Japan | 34.2 | 873.7 | 12 | 2 | 14 |
| Antonov 2008 [98] | checklist | Irkutsk, Baikal, Russia | 52.3 | 277 | 14 | 0 | 14 |
| Harada and Yamashita, 2019 [90] | ecological survey | Takamatsu, Japan | 34.4 | 375.4 | 14 | 0 | 14 |
| Harada and Yamashita, 2019 [90] | ecological survey | Matsuyama City, Japan | 33.8 | 429.4 | 12 | 1 | 13 |
| Hosaka et al. 2019 [99] | ecological survey | Tokyo, Japan | 35.7 | 2194.1 | 11 | 0 | 11 |
| Antonov 2008 [98] | checklist | Gusinoozersk, Baikal, Russia | 51.3 | ? | 7 | 0 | 7 |
| Blinova 2008 [100] | ecological survey | Kemerovo, Russia | 55.4 | 282.3 | 7 | 0 | 7 |
| Meshram et al. 2015 [101] | ecological survey | Nagpur, Maharashtra, India | 21.2 | 393.5 | 25 (genera) | 3 | NA * |
| Yong et al. 2017 [102] | checklist | Pulau Aubin, Singapore | 1.3 | 10.2 | 35 (genera) | 2 (no total mentioned) | NA * |
| Australia (6) | |||||||
| Ossola et al. 2015 [103] | ecological survey | Melbourne, Australia | −37.8 | 9993 | 59 * | 1 * | 60 |
| Heterick et al. 2013 [104] | ecological survey | Perth, Australia | −32.0 | 6418 | 54 | 6 | 60 |
| Majer and Brown 1986 [105] | ecological survey | Perth, Australia | −32.0 | 6418 | 45 | 2 | 47 |
| Callan and Majer 2009 [106] | ecological survey | Perth, Australia | −32.0 | 6418 | 32 | 4 | 36 |
| Heterick et al. 2000 [107] | ecological survey | Perth, Australia | −32.0 | 6418 | 19 | 8 | 27 |
| May and Heterick 2000 [108] | ecological survey | Perth, Australia | −32.0 | 6418 | 18 | 8 | 26 |
| Europe (28) | |||||||
| Radchenko et al. 2019 [109] | checklist | Kyiv, Ukraine | 50.5 | 839 | 55 | 4 | 59 |
| Ordóñez-Urbano, Reyes-López and Carpintero-Ortega 2008 [110] | ecological survey | Córdoba, Sevilla, Málaga and Cádiz, Spain | 36.5–37.9 | >1800 (multiple cities included) | ? | ? | 59 *,*** |
| Antonova and Penev 2006 [111,112,113] | ecological survey | Sofia, Bulgaria | 42.7 | 492 | 54 | 0 | 46 |
| Dauber 1997 [114] | ecological survey | Mainz, Germany | 50.0 | 97.7 | 49 | 0 | 49 |
| Dauber and Eisenbeis [114] | ecological survey | Mainz, Germany | 50.0 | 97.7 | 46 | 0 | 46 |
| Reyes-Lopez and Carpintero 2014 [68] | ecological survey | Cordoba and Seville, Spain | 37.4 and 37.9 | 1393 (multiple cities included) | 39 *** | 5 *** | 44 *** |
| Pisarski and Czechowski 1978 [115] | checklist | Warsaw, Poland | 52.2 | 517.2 | 36 | 1 | 37 |
| Pisarski 1982 [116] | checklist | Warsaw, Poland | 52.2 | 517.2 | 35 | 2 | 37 |
| Ruiz Heras et al. 2011 [117] | ecological survey | Madrid, Spain | 40.4 | 604.3 | 36 | 1 | 37 |
| Klesniakova et al. 2016 [118] | ecological survey | Bratislava, Slovakia | 48.1 | 367.6 | ? | 1 * | 36 * |
| Trigos-Peral et al. 2020 [119] | ecological survey | Warsaw, Poland | 52.2 | 517.2 | 32 | 2 | 34 |
| Reyes-Lopez and Carpintero 2014 [68] | ecological survey | Almeria, Cadiz, Huelva and Malaga, Spain | 36.5 to 37.3 | 859.1 (multiple cities included) | 24 *** | 8 *** | 32 *** |
| Ješovnik and Bujan 2021 [120] | ecological survey | Zagreb, Croatia | 45.8 | 641 | 30 | 0 | 30 |
| Behr, Lippke and Cölln 1996 [121] | ecological survey | Köln (Cologne), Germany | 51.0 | 405.1 | 25 | 3 | 28 |
| Ślipiński et al. 2012 [122] | ecological survey | Warsaw, Poland | 52.2 | 517.2 | 27 | 0 | 27 |
| Behr and Cölln 1993 [123] | checklist | Gönnersdorf, Germany | 50.5 | 5.2 | 27 | 0 | 27 |
| Rigato and Wetterer 2018 [124] | checklist | San Marino | 43.9 | 61.2 | 23 | 0 | 23 |
| Espadaler and López-Soria 1991 [125] | checklist | Sant Cugat, Barcelona, Spain | 41.5 | 48.2 | 22 | 1 | 23 |
| Vepsäläinen, Ikonen and Koivula 2008 [126] | ecological survey | Helsinki, Finland | 60.2 | 213.8 | 17 | 2 | 19 |
| Trigos Peral and Reyes Lopez 2018 [127] | ecological survey | Beja, Portugal | 38.0 | 1146 | 17 | 0 | 17 |
| Vepsäläinen, Ikonen and Koivula 2008 [126] | ecological survey | Helsinki, Finland | 60.2 | 185 | 16 | 0 | 16 |
| Stukalyuk 2017 [128] | ecological survey | Kyiv, Ukraine | 50.5 | 839 | 16 | 0 | 16 |
| Reyes López and Taheri 2018 [129] | checklist | Cádiz, Andalusia, Spain | 36.5 | 12.1 | 6 | 9 | 15 |
| Smith et al. 2006 [130] | ecological survey | London, UK | 51.5 | 1572 | 6 | 0 | 6 |
| Gaspar and Thirion 1978 [131] | checklist | Liege, Belgium | 50.6 | 69.4 | 6 | 0 | 6 |
| N. America (22) | |||||||
| Guénard et al. 2015 [16] | ecological survey | Raleigh, NC, USA | 35.8 | 380 | 77 | 12 | 89 |
| Nuhn and Wright 1979 [132] | checklist | Raleigh, NC, USA | 35.8 | 380 | 50 | 6 | 56 |
| Baena et al. 2019 [133] | ecological survey | Coatepec, Mexico | 19.5 | 255.8 | 51 | 4 | 55 |
| Menke et al. 2011 [134] | ecological survey | Raleigh, NC, USA | 35.8 | 380 | 49 | 5 | 54 |
| Miguelena and Baker 2019 [135] | ecological survey | Tucson, AZ, USA | 32.2 | 623.6 | 45 | 3 | 48 |
| Rocha-Ortega and Castano-Meneses 2015 [136] | ecological survey | Santiago de Querétaro, Mexico | 20.6 | 363 | 45 | 3 | 48 |
| Toennisson et al. 2011 [137] | ecological survey | Knoxville, TN, USA | 36.0 | 270 | 44 | 2 | 46 |
| Suarez et al. 1998 [138] | ecological survey | San Diego, CA, USA | 32.7 | 964.6 | 42 | 4 | 46 |
| Gochnour et al. 2019 [139] | ecological survey | Garden City, Georgia, USA | 32.1 | 37.6 | 32 | 13 | 45 |
| Savage et al. 2015 [140] | ecological survey | New York, NY, USA | 40.7 | 778 | 36 | 6 | 42 |
| Baena et al. 2019 [133] | ecological survey | Xalapa, Mexico | 19.5 | 124.4 | 36 | 4 | 40 |
| Uno S. pers. Comm. In Friedrich and Philpott 2009 [141] | ecological survey | Toledo, USA | 39.9 | 217.1 | ? | ? | 35 * |
| García-Martínez et al. 2019 [142] | ecological survey | Ciudad Victoria, Mexico | 23.7 | 188 | 28 | 4 | 32 |
| Fairweather et al. 2020 [143] | checklist | St-John, NB, Canada | 45.3 | 316 | 30 | 0 | 30 |
| Uno, Cotton and Philpott 2010 [144] | ecological survey | Toledo, USA | 41.7 | 217.1 | 28 | 2 | 30 |
| Ivanov and Keiper 2010 [145] | ecological survey | Cleveland, Oh, USA | 41.5 | 201 | 28 | 1 | 29 |
| Uno, Cotton and Philpott 2010 [144] | ecological survey | Detroit, USA | 42.3 | 370.1 | 26 | 1 | 27 |
| Lessard and Buddle 2005 [146] | ecological survey | Montreal, CAN | 45.5 | 431.5 | 23 | 1 | 24 |
| Clarke, Fisher and LeBuhn 2008 [147] | ecological survey | San Francisco, CAL, USA | 37.8 | 600.6 | 18 | 3 | 21 |
| Buczkowski and Richmond 2012 [10] | ecological survey | West Lafayette, Indiana, USA | 40.4 | 35.8 | 19 | 1 | 20 |
| King and Green 1995 [148] | ecological survey | Philadelphia, PA, USA | 40.0 | 369.6 | 18 | 1 | 19 |
| Staubus et al. 2015 [149] | ecological survey | Claremont, CAL, USA | 34.1 | 34.9 | 12 | 6 | 18 |
| Pećarević et al. 2010 [150] | ecological survey | New York, NY, USA | 40.7 | 778 | 10 | 3 | 13 |
| Thompson and McLachlan 2007 [151] | ecological survey | Winnipeg, Manitoba, Canada | 49.8 | 464.1 | 10 | 0 | 10 |
| Villar and Ríos-Casanova [152] | ecological survey | La Cantera Oriente, Mexico city, Mexico | 19.3 | 0.1 | 8 | 2 | 10 |
| Stahlschmidt and Johnson 2018 [153] | ecological survey | Stockton, CAL, USA | 38.0 | 169 | 4 | 5 | 9 |
| Marussich and Faeth 2009 [154] | ecological survey | Phoenix, AZ, USA | 33.4 | 1341 | 7 | 1 | 8 |
| S. and C. America (31) | |||||||
| Pacheco and Vasconcelos 2007 [155] | ecological survey | Uberlândia, Brazil | −18.9 | 4116 | 137 | 6 | 143 |
| Santos et al. 2019 [156] | ecological survey | Rio de Janeiro, Brazil | −22.9 | 1221 | 116 | 4 | 120 |
| Santos-Silva et al. 2016 [157] | checklist | Cacoal, Rondônia, Brazil | −11.4 | 3793 | 98 | 4 | 102 |
| De Souza et al. 2012 [158] | ecological survey | Mogi das Cruzes, Brazil | −23.5 | 713 | 91 | 1 | 92 |
| Lutinski et al. 2013 [159] | ecological survey | Chapecó, Santa Catarina, Brazil | −27.1 | 624.3 | 89 | 2 | 91 |
| Munhae et al. 2014 [160] | ecological survey | Alto Tietê region, São Paulo, Brazil | −23.5 | 1455 (multiple cities included) | 82 *** | 5 *** | 87 *** |
| Lutinski et al. 2013 [159] | ecological survey | Palmitos, Santa Catarina, Brazil | −27.1 | 350.7 | 81 | 4 | 85 |
| Lutinski et al. 2013 [159] | ecological survey | Campo Erê, Santa Catarina, Brazil | −26.4 | 478.7 | 82 | 3 | 85 |
| Lutinski et al. 2013 [159] | ecological survey | Xanxerê, Santa Catarina, Brazil | −26.9 | 377.8 | 80 | 3 | 83 |
| Lutinski et al. 2013 [159] | ecological survey | São Miguel do Oeste, Santa Catarina, Brazil | −26.7 | 234.4 | 81 | 2 | 83 |
| Lutinski et al. 2013 [159] | ecological survey | Abelardo Luz, Santa Catarina, Brazil | −26.6 | 953.6 | 81 | 2 | 83 |
| Lutinski et al. 2013 [159] | ecological survey | Concórdia, Santa Catarina, Brazil | −27.2 | 800 | 80 | 2 | 82 |
| Lutinski et al. 2013 [159] | ecological survey | Pinhalzinho, Santa Catarina, Brazil | −26.8 | 128.3 | 76 | 4 | 80 |
| Lutinski et al. 2013 [159] | ecological survey | Joaçaba, Santa Catarina, Brazil | −27.1 | 232.4 | 76 | 3 | 79 |
| Morini et al. 2007 [161] | ecological survey | São Paulo, Brazil | −23.6 | 1521.1 | 76 | 3 | 79 |
| Lutinski et al. 2013 [159] | ecological survey | Seara, Santa Catarina, Brazil | −27.1 | 312.5 | 75 | 3 | 78 |
| Iop et al. 2009 [162] | checklist | Xanxerê, Santa Catarina, Brazil | −26.9 | 377.8 | 45 | 2 | 67 |
| Caldart et al. 2012 [163] | ecological survey | Chapecó, Santa Catarina, Brazil | −27.1 | 624.3 | 63 | 3 | 66 |
| Ilha et al. 2017 [164] | ecological survey | Chapecó, Santa Catarina, Brazil | −27.1 | 624.3 | 60 | 3 | 63 |
| Josens et al. 2017 [165] | ecological survey | Buenos Aires, Argentina | −34.6 | 203 | 57 | 3 | 60 |
| Kamura et al. 2007 [166] | ecological survey | Mogi das Cruzes, São Paulo, Brazil | −23.5 | 713 | 49 | 9 | 58 |
| Santiago et al. 2018 [167] | ecological survey | Divinópolis, Minas Gerais, Brazil | −20.1 | 192 | 55 | 0 | 55 |
| De Souza-Campana et al. 2016 [168] | checklist | São Paulo, Brazil | −23.6 | 1521 | 46 | 1 | 47 |
| Piva and de Carvalho Campos 2012 [169] | ecological survey | São Paulo, Brazil | −23.6 | 1521 | 38 | 6 | 44 |
| Simonetti, Brito and Luis 2010 [170] | ecological survey | Havana City, Cuba | 23.1 | 728.3 | ? | ? | 37* |
| Ribeiro et al. 2012 [171] | ecological survey | São Paulo, Brazil | −23.6 | 1521 | 33 | 3 | 36 |
| Lutinski and Mello Garcia 2005 [172] | ecological survey | Chapecó, Santa Catarina, Brazil | −27.1 | 624.3 | 32 | 0 | 32 |
| Lange et al. 2015 [173] | ecological survey | Araguari, Minas Gerais, Brazil | −18.6 | 2730.6 | 21 | 2 | 23 |
| Starr and Ballah 2017 [174] | ecological survey | Port of Spain, Trinidad | 10.7 | 12 | 23 | 0 | 23 |
| Soares et al. 2006 [175] | ecological survey | Uberlândia, Minas Gerais, Brazil | −18.9 | 4116 | 10 | 4 | 14 |
2. Materials and Methods
2.1. Geographic and Climatic Characteristics of Macao
2.2. Sampling Effort and Collection Methods
2.3. Sample Processing
2.4. Imaging
2.5. Mapping Species Distributions and Urban Studies
2.6. Analyses
2.7. Literature Search
2.8. Notes on Invasion Status
2.9. Notes on Records
2.10. Notes on Taxonomy
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. AMBLYOPONINAE
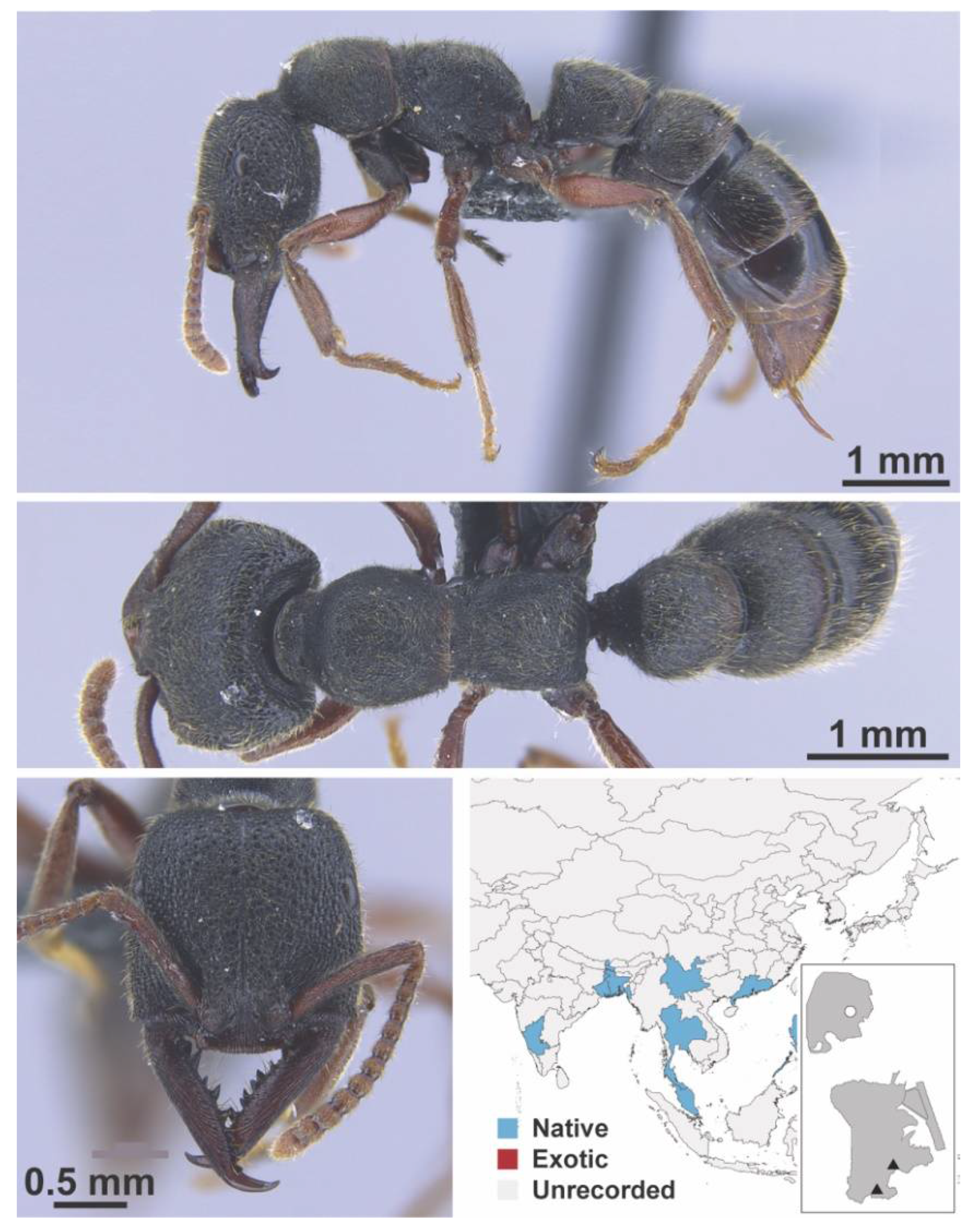
Appendix A.2. DOLICHODERINAE
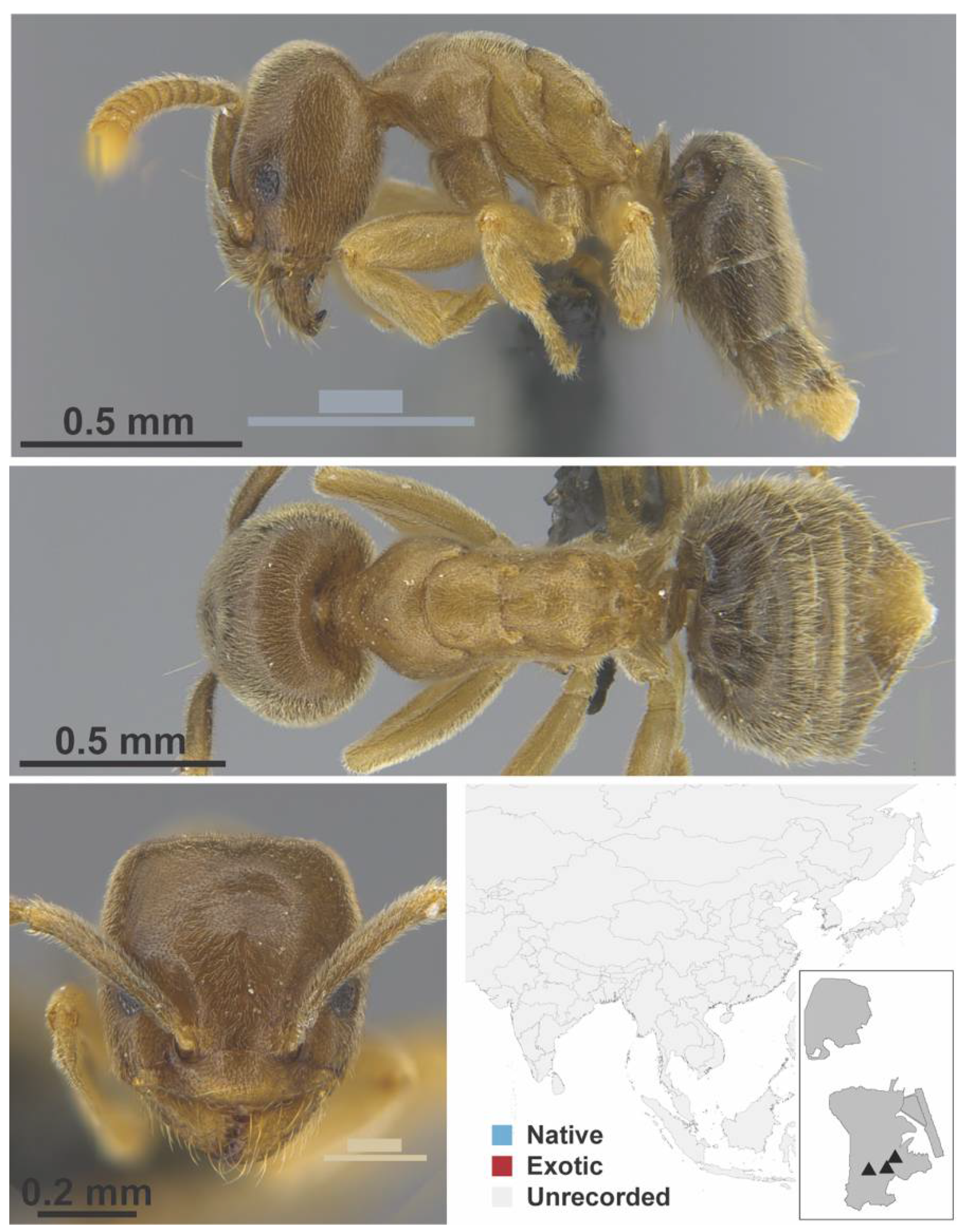
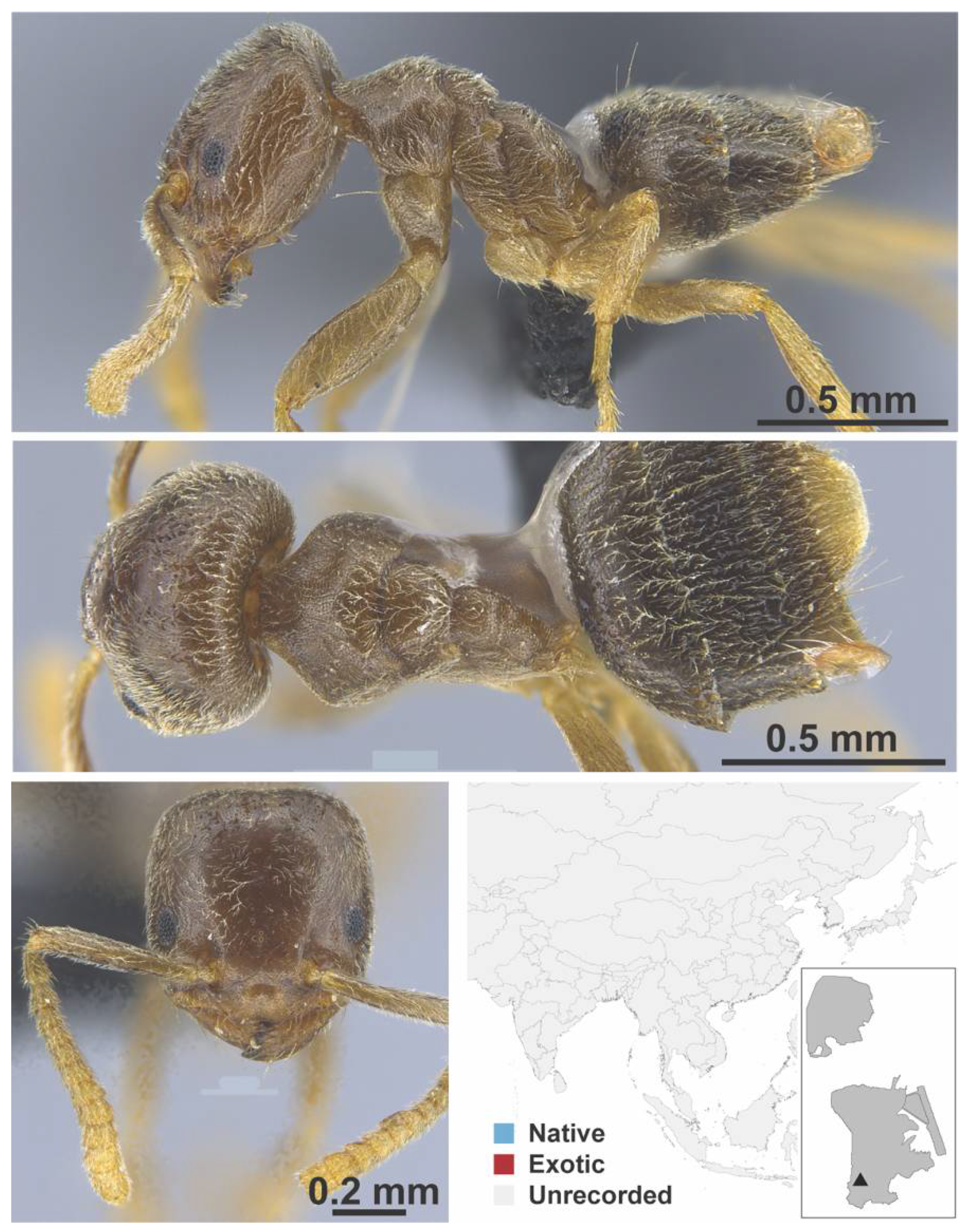
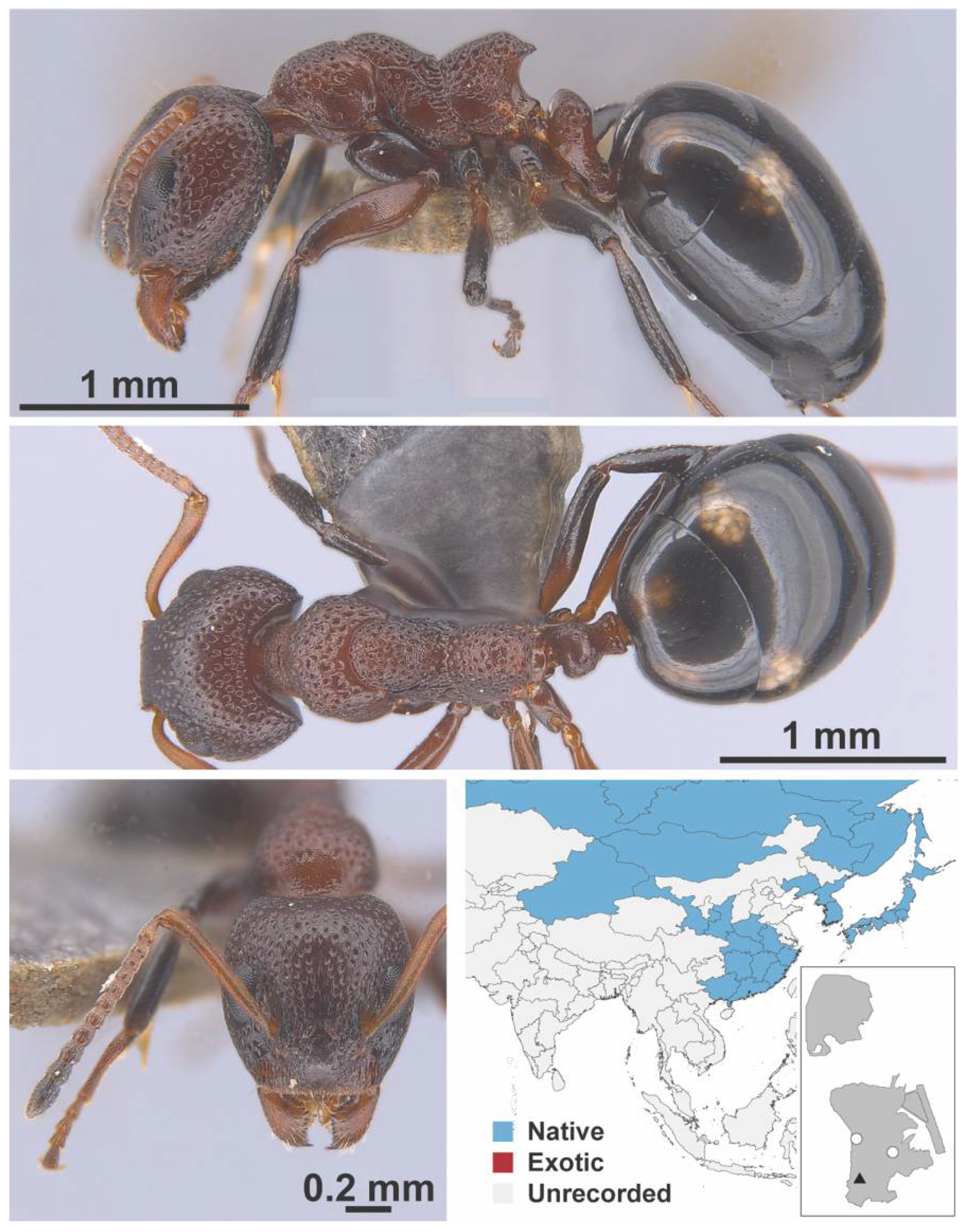

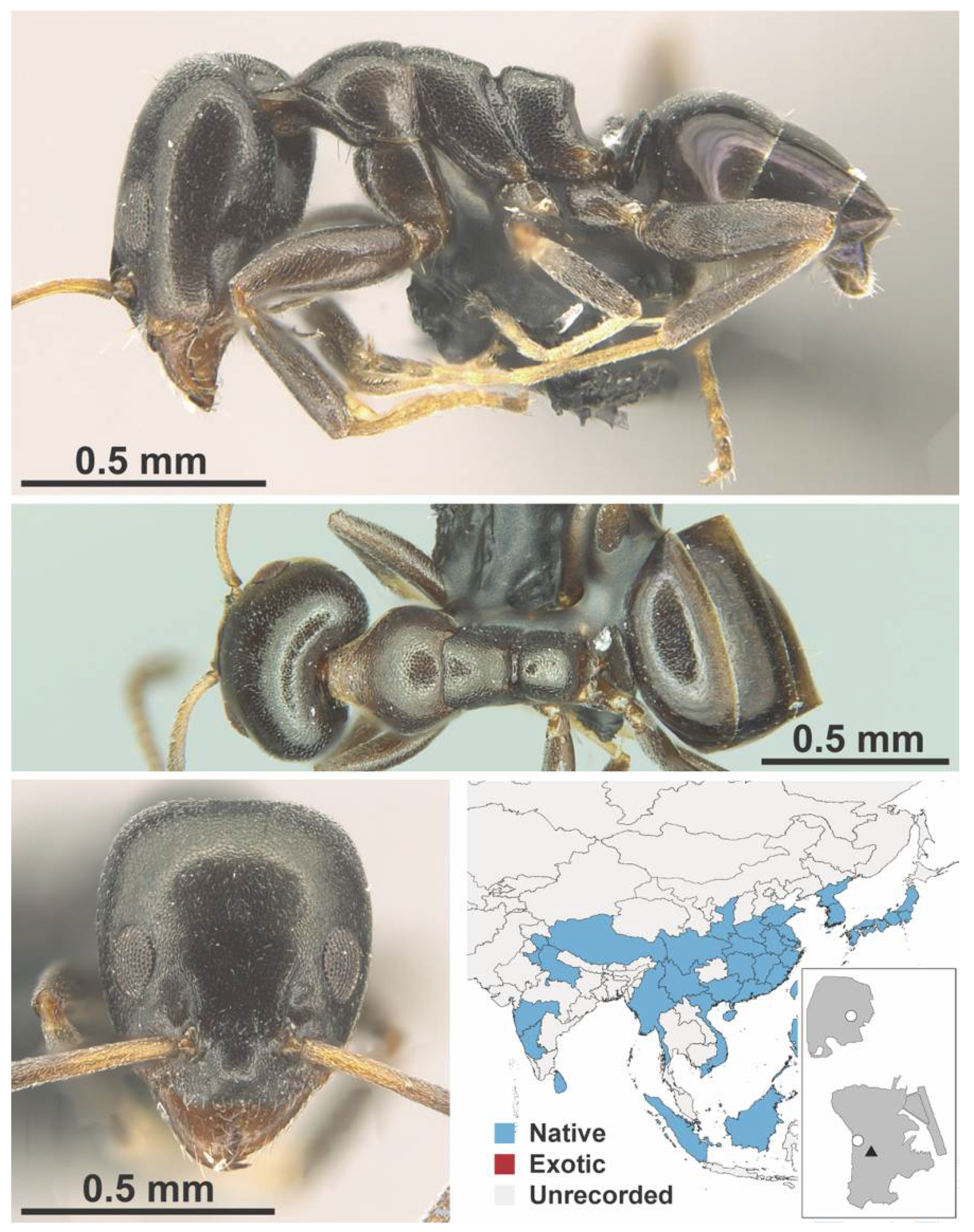
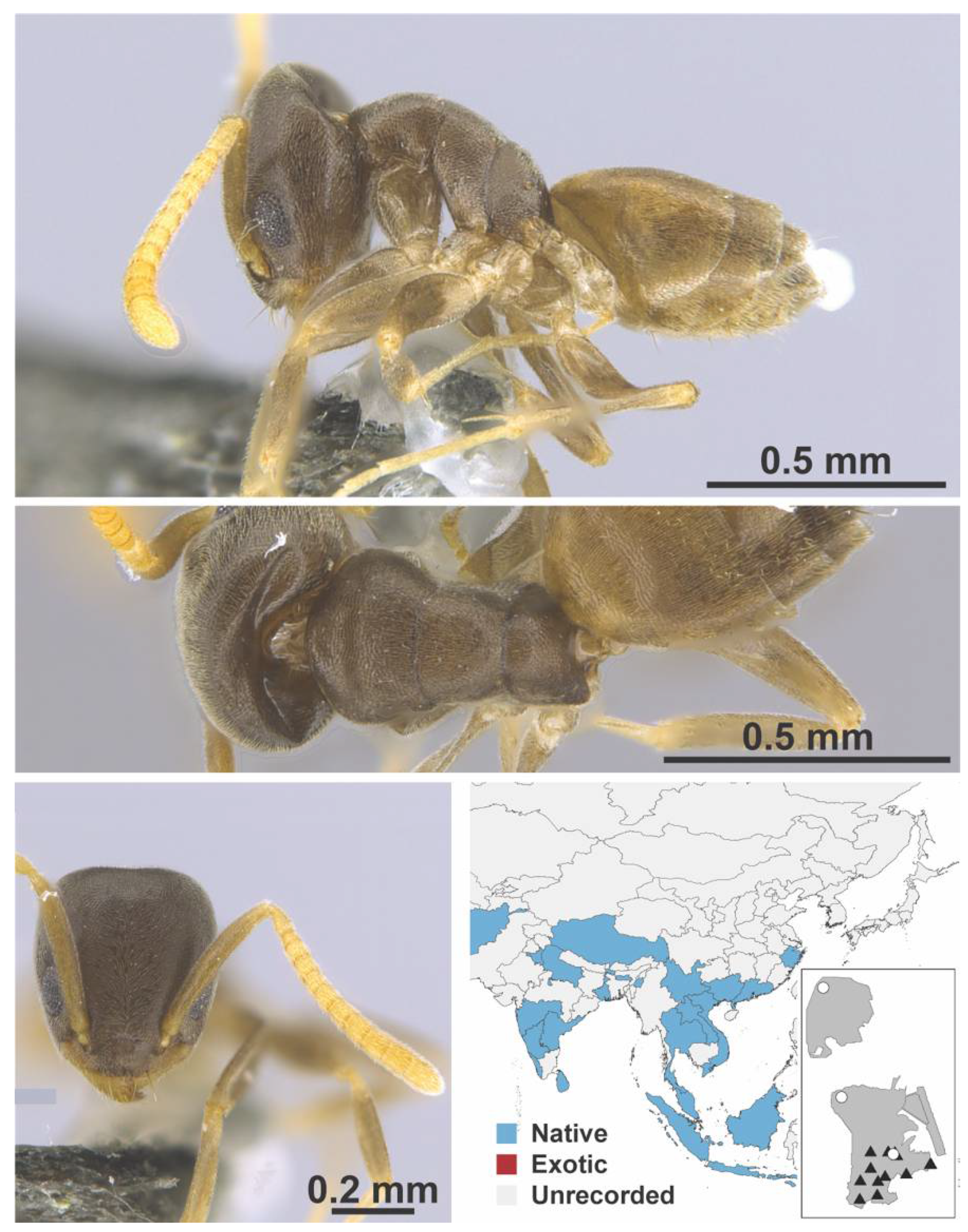
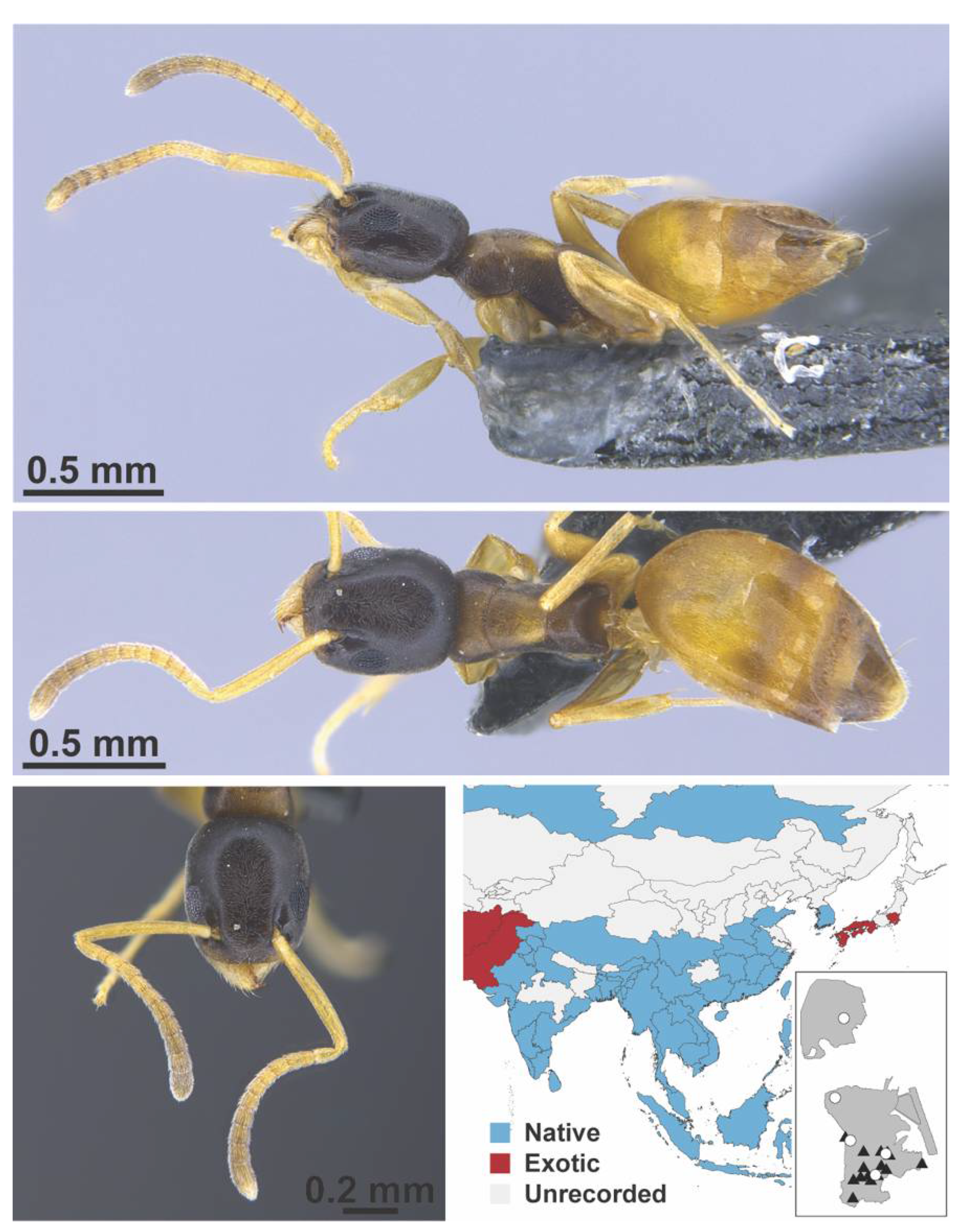

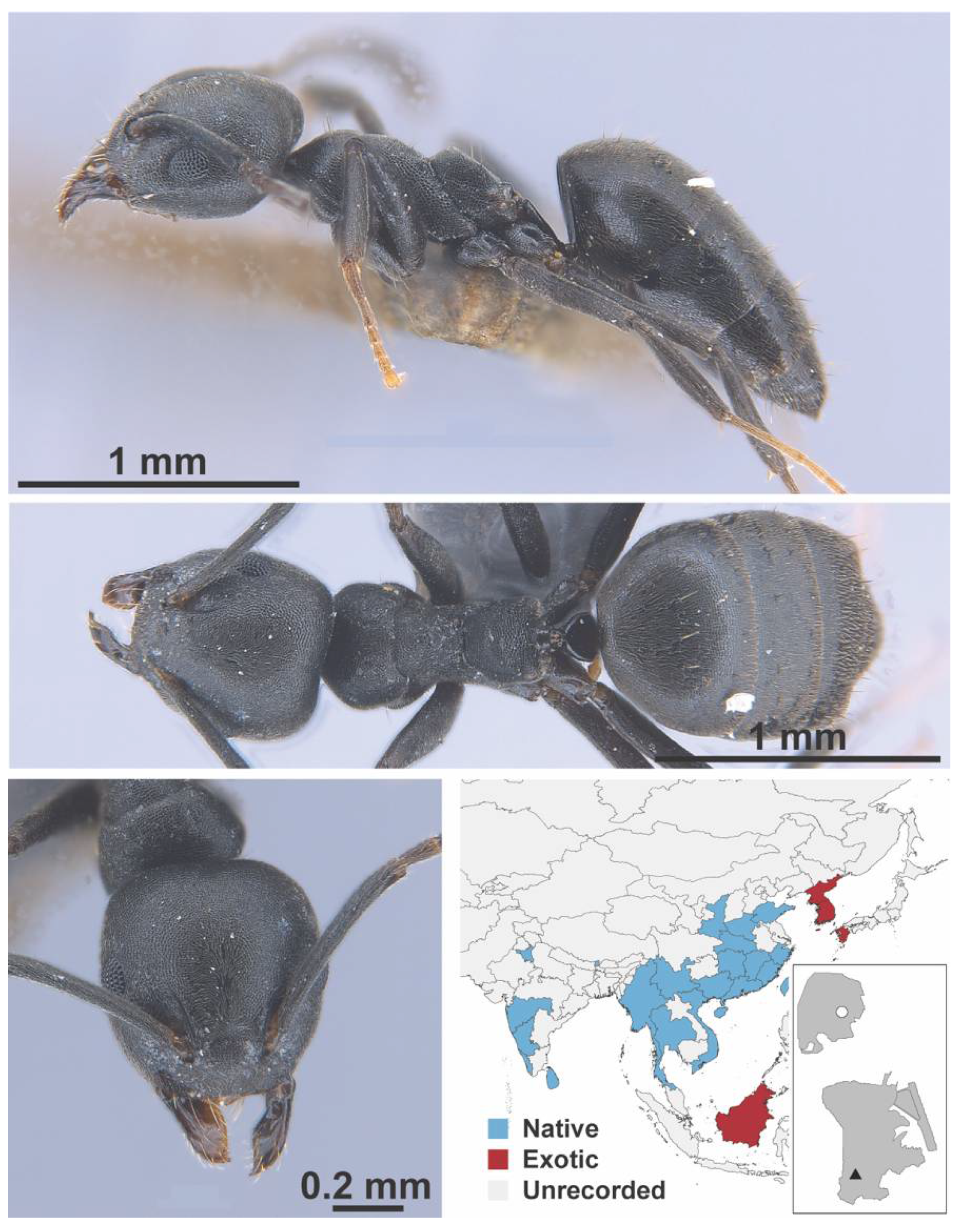
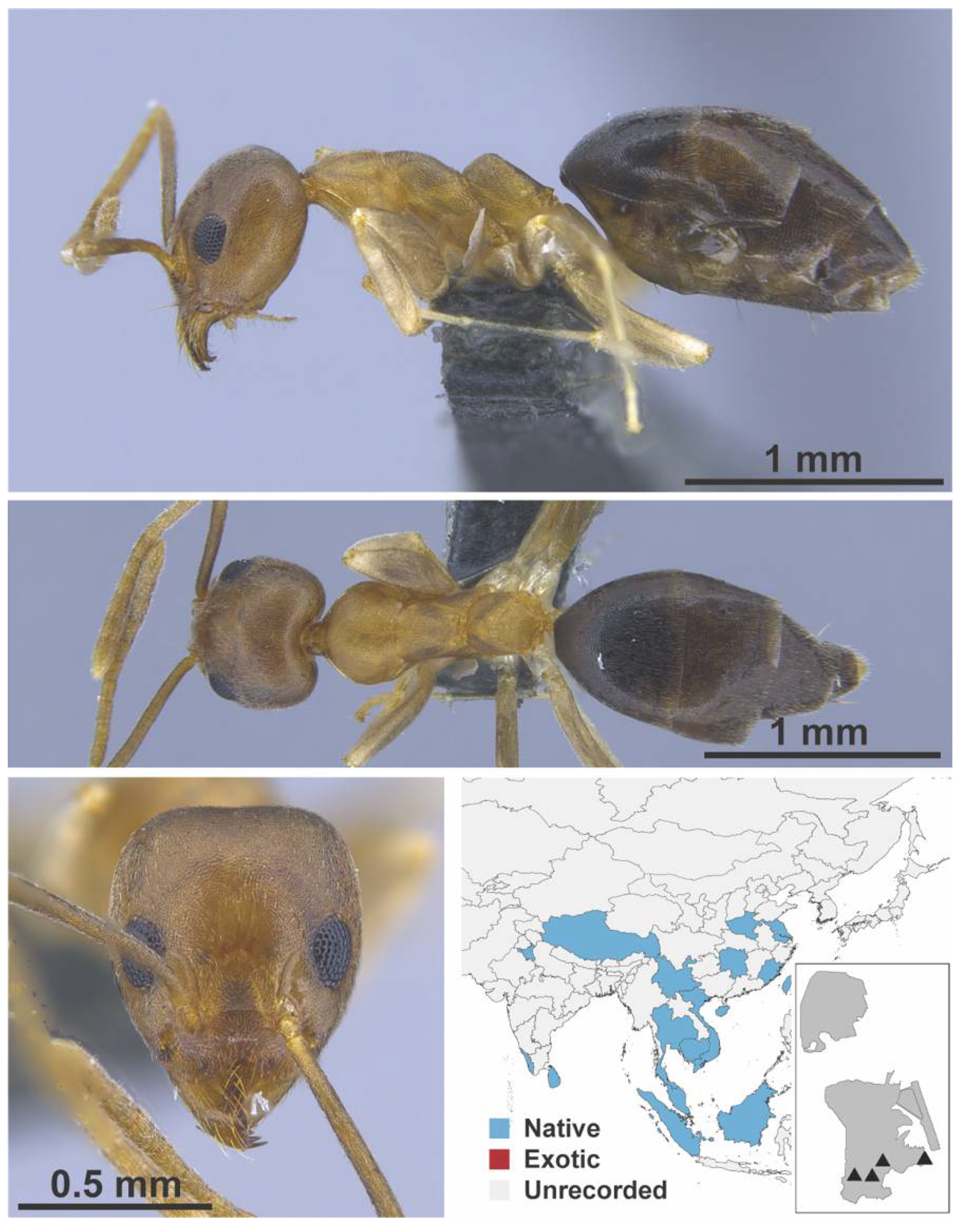
Appendix A.3. DORYLINAE
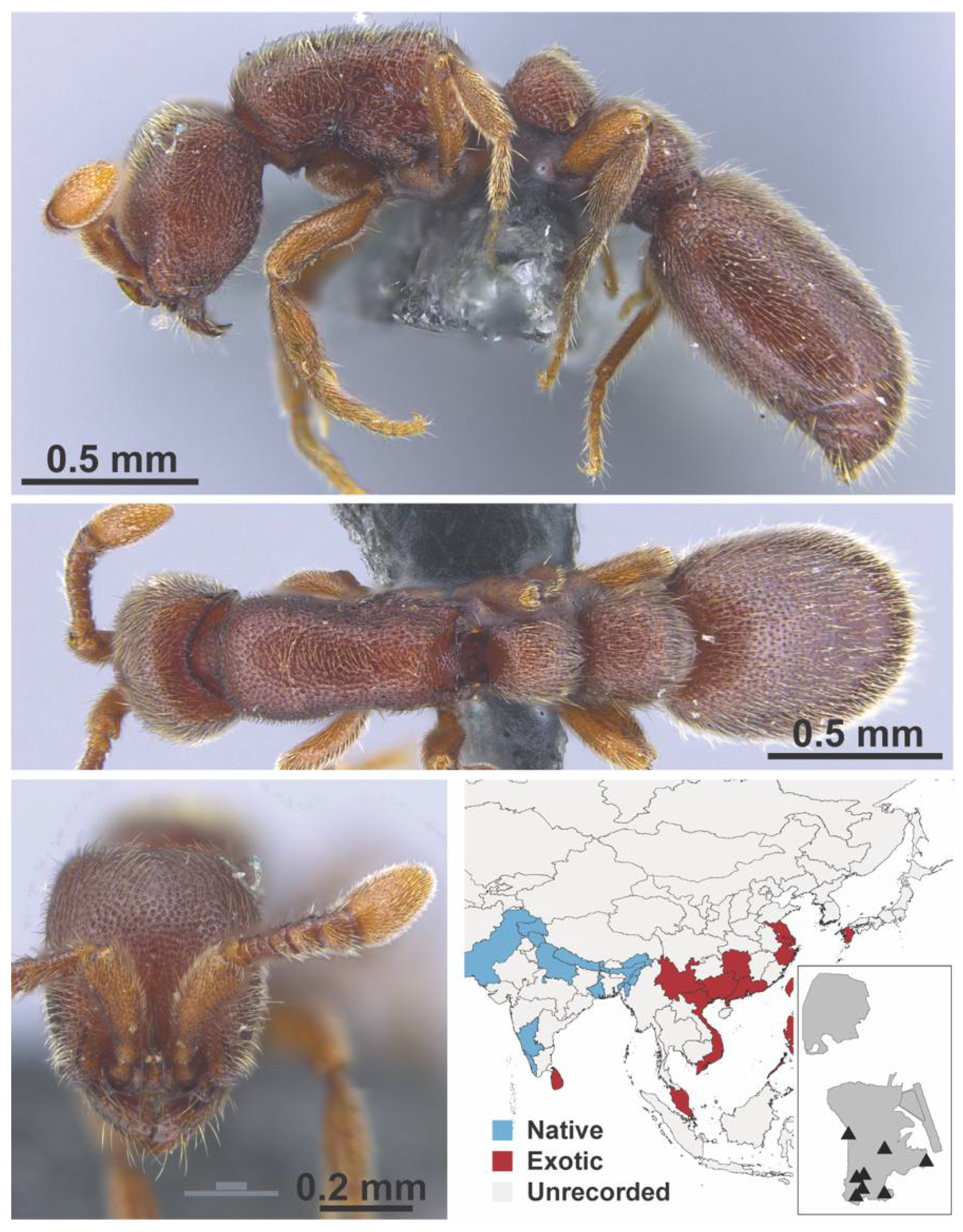
Appendix A.4. FORMICINAE
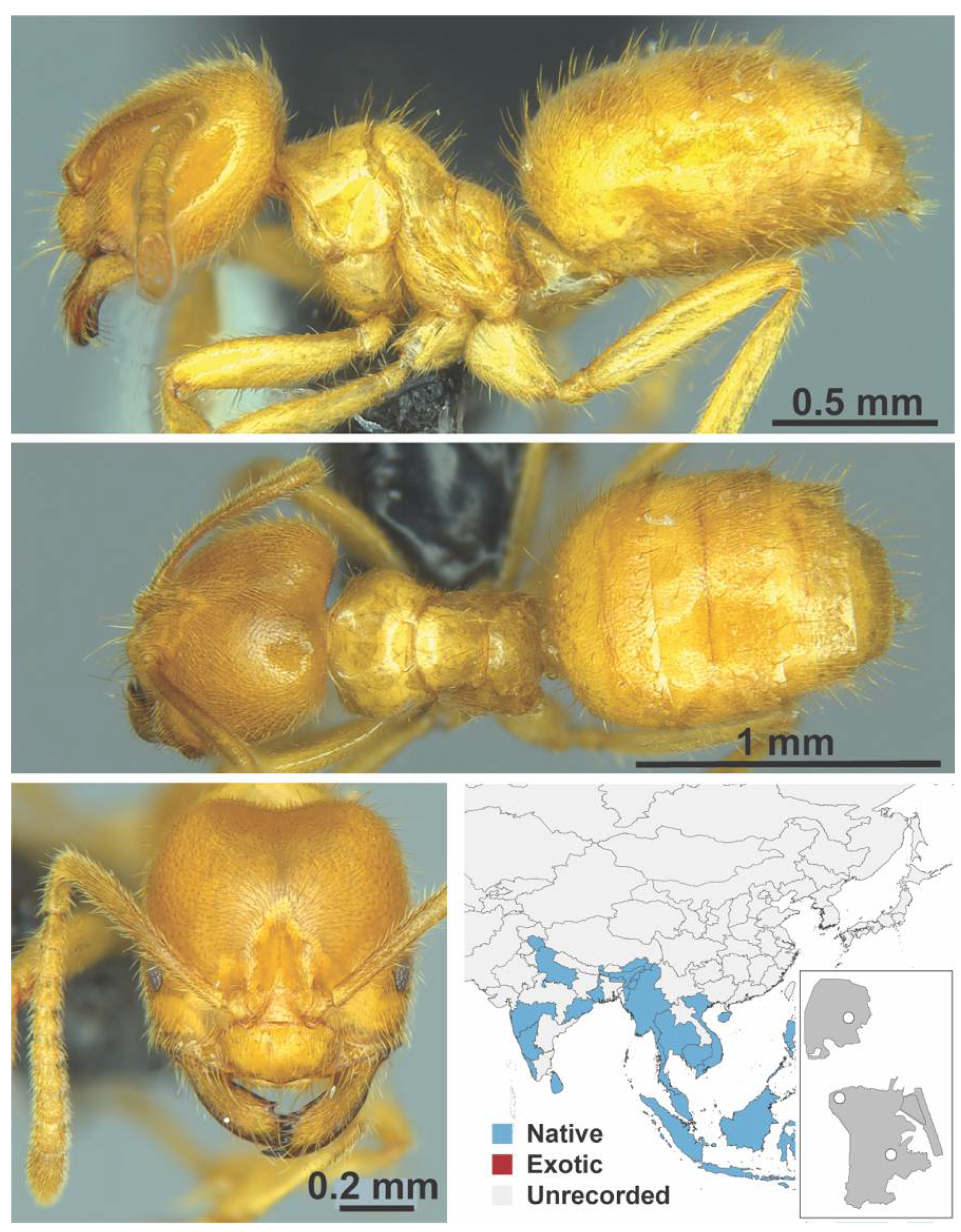
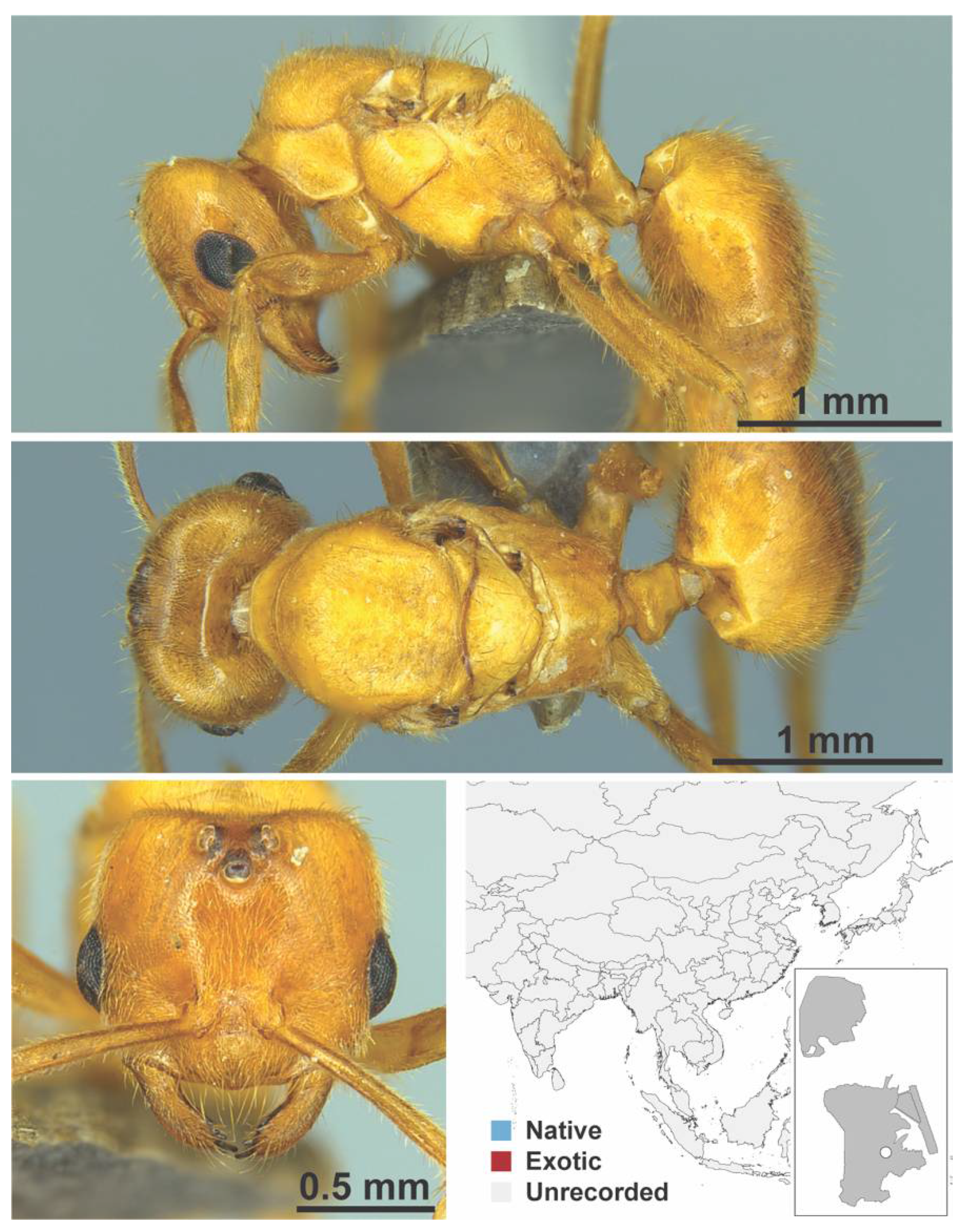
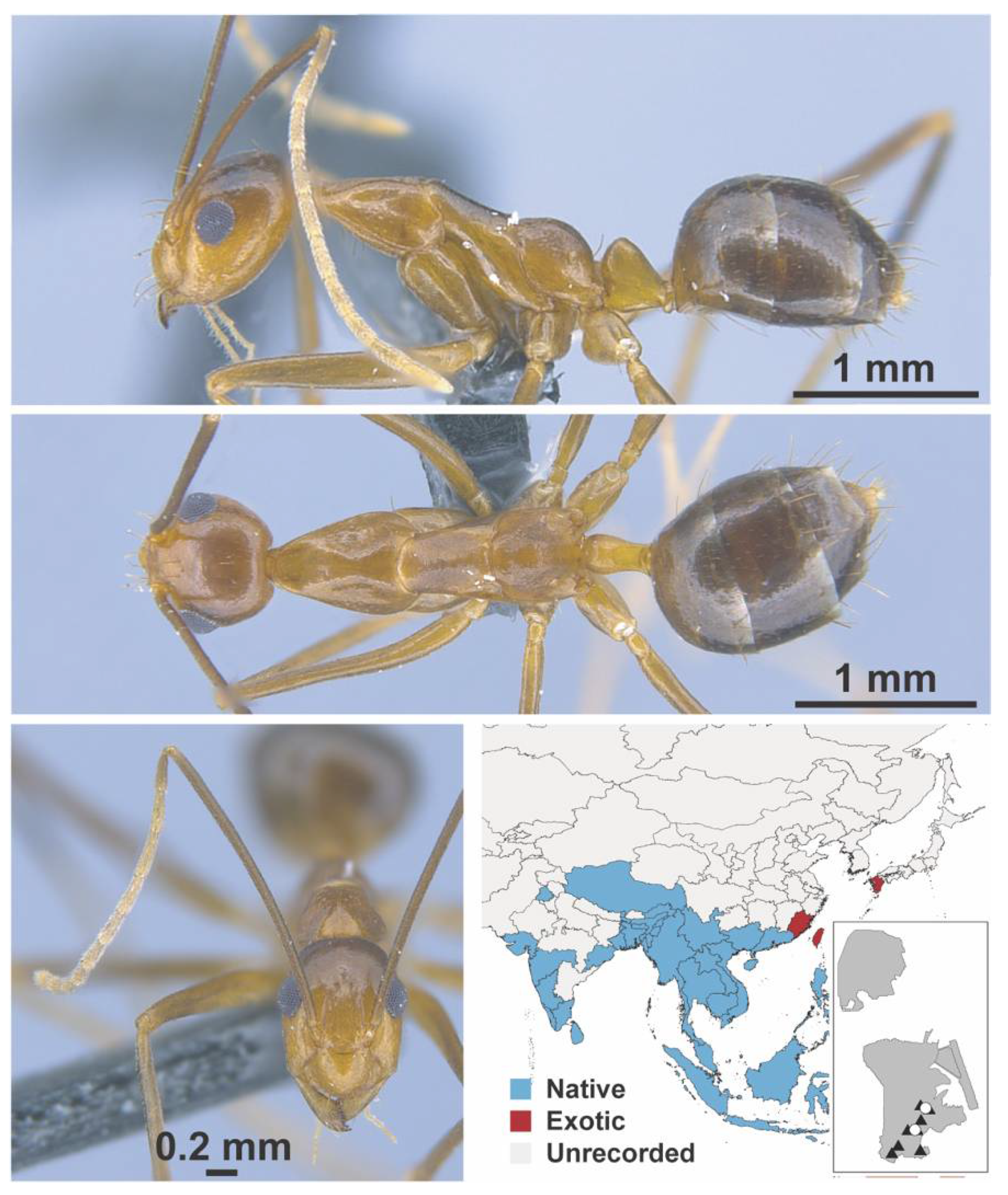
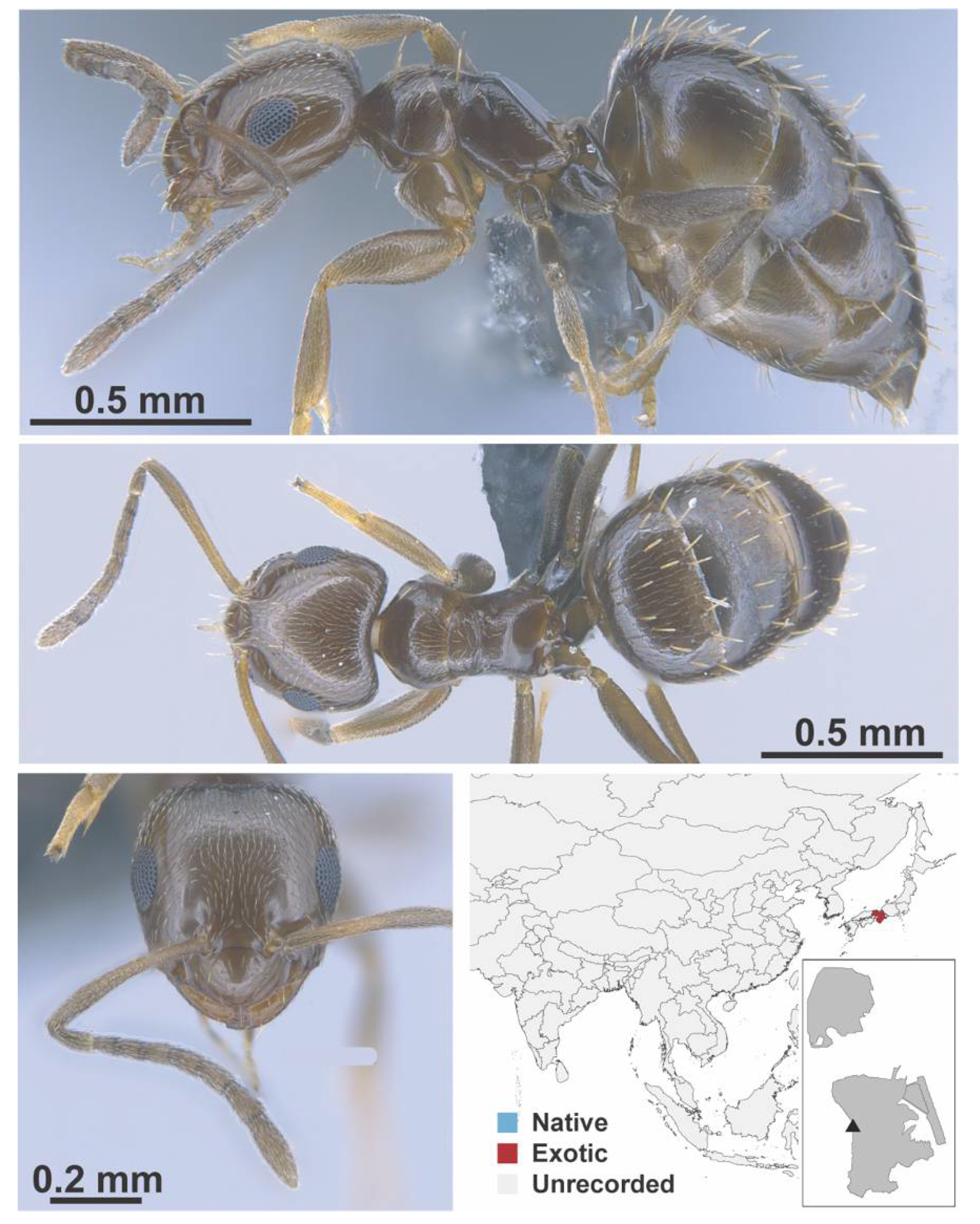
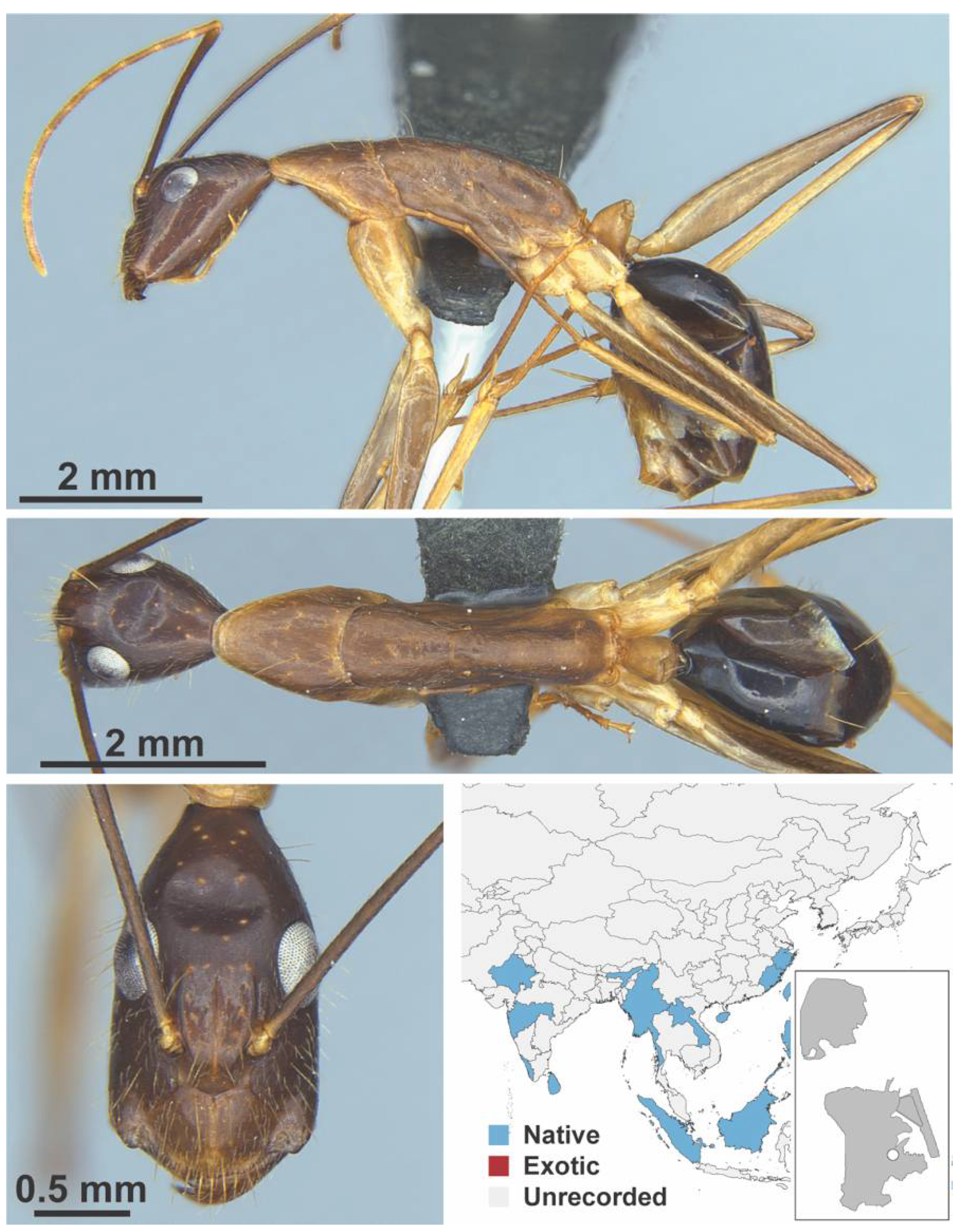

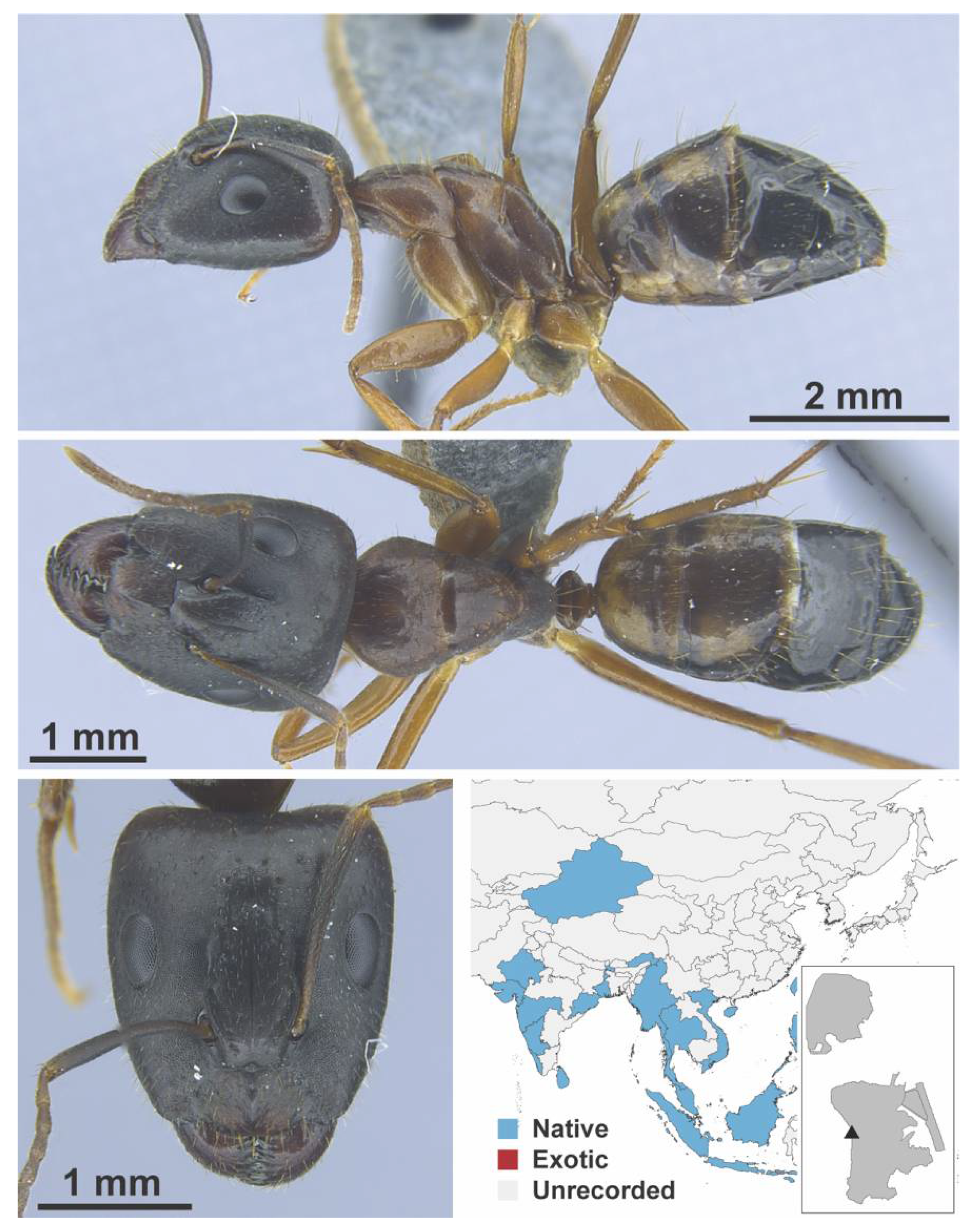
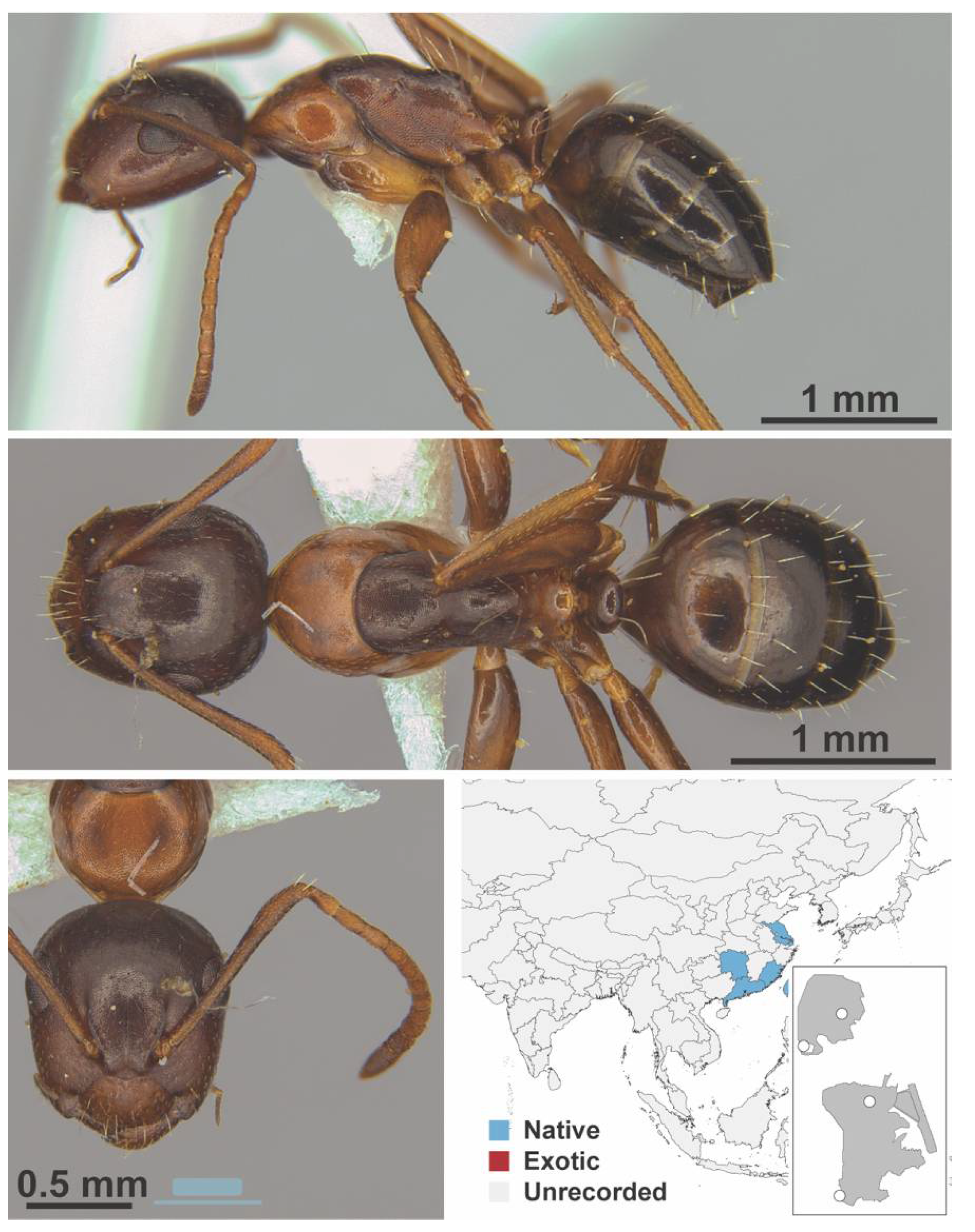


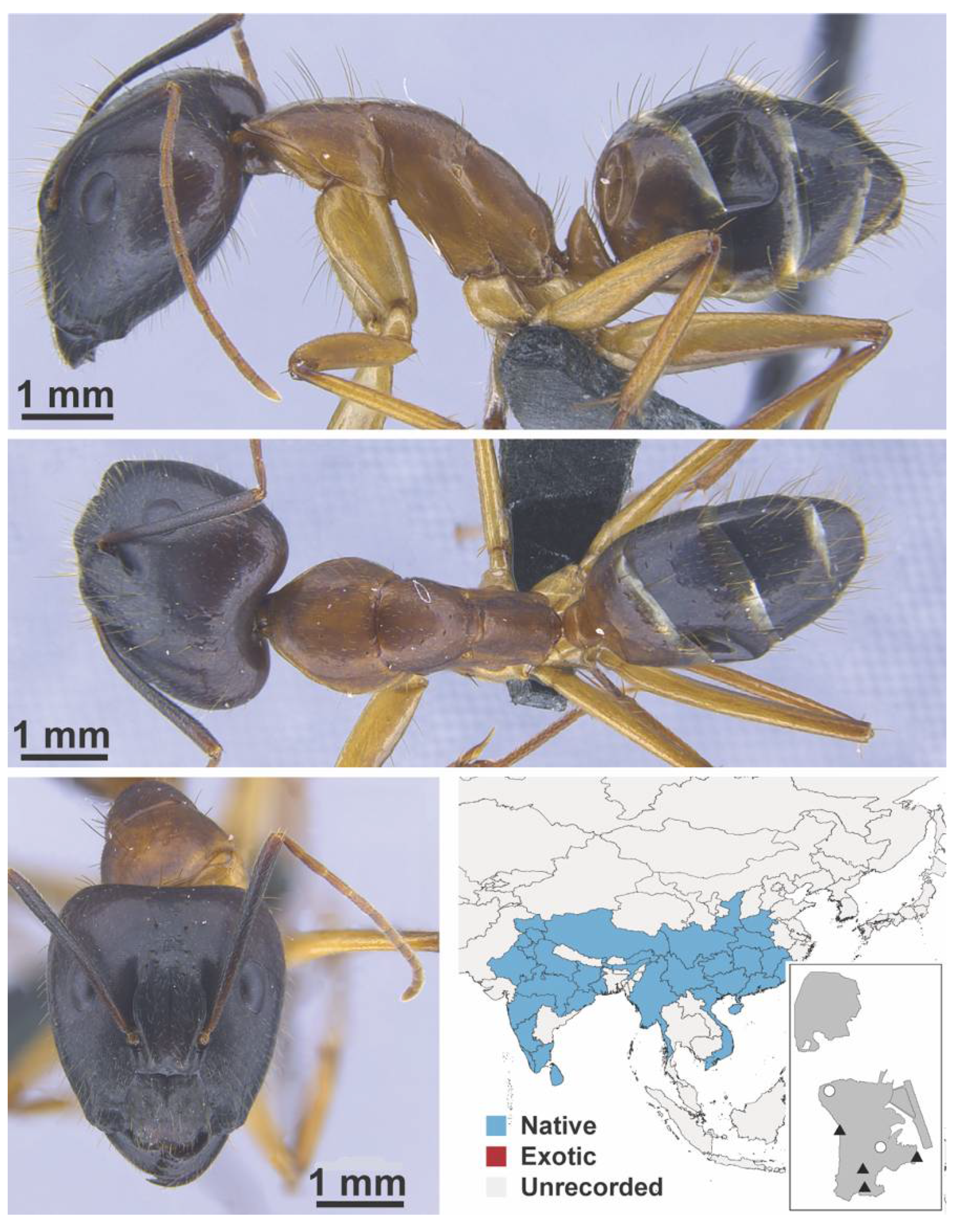
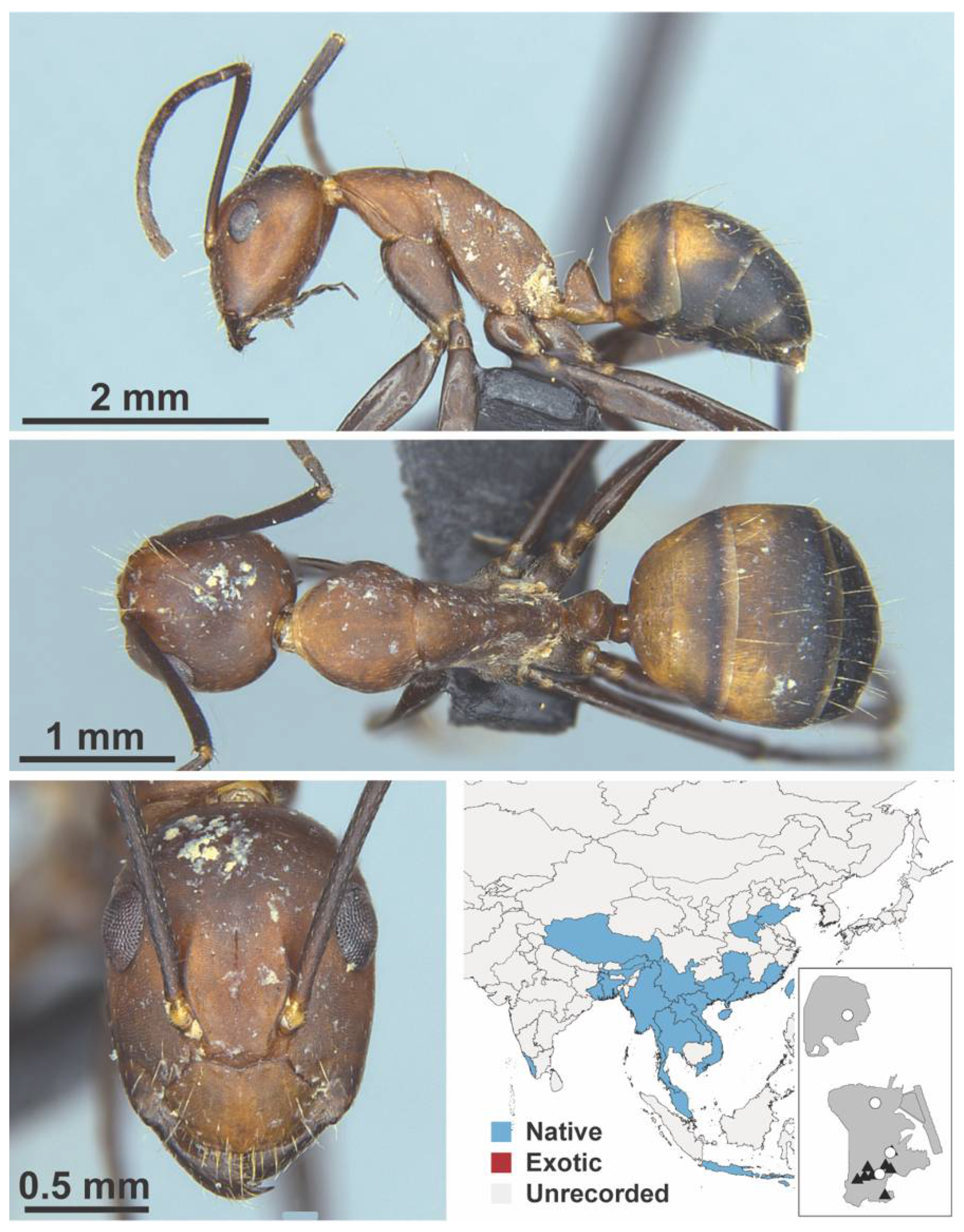
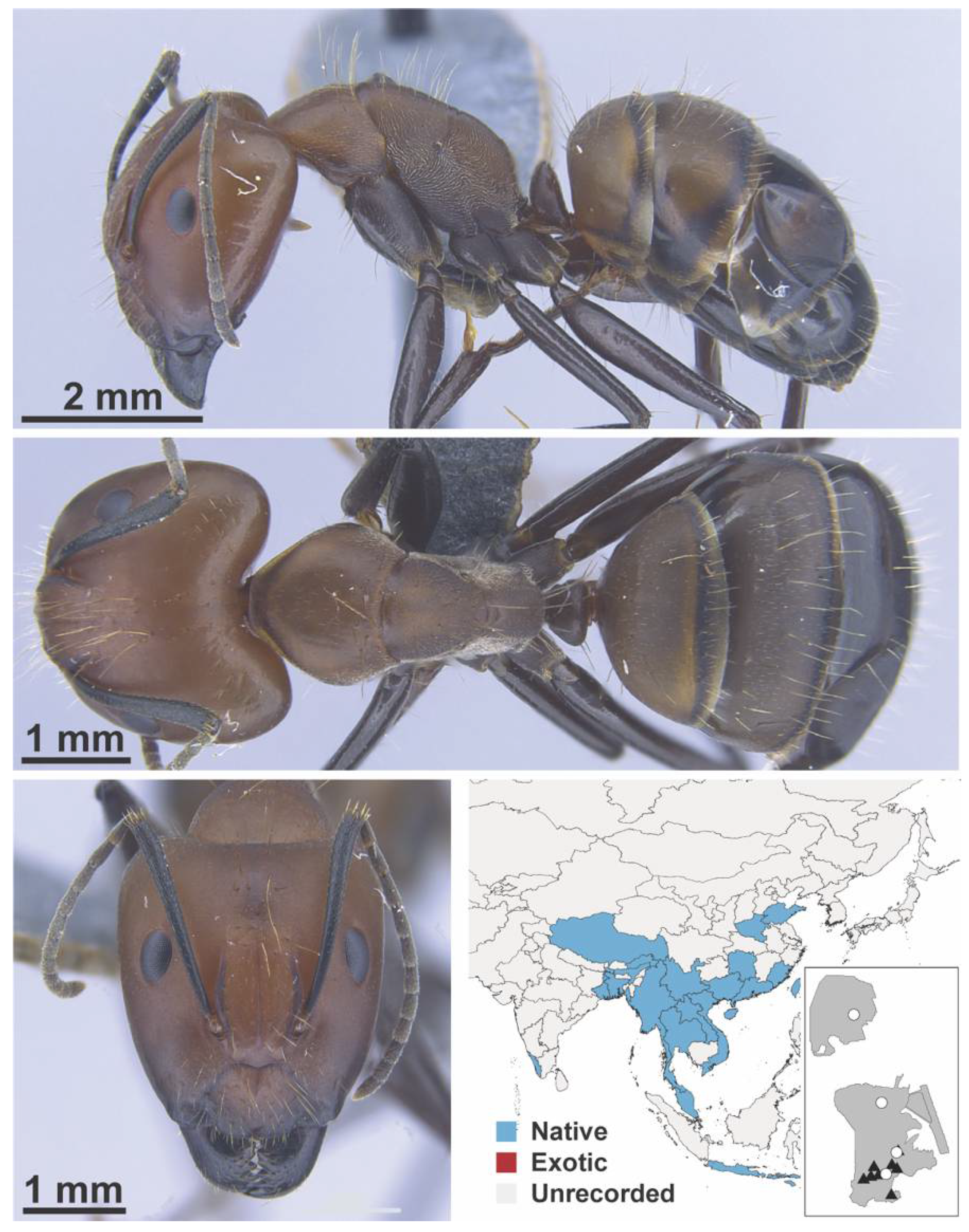
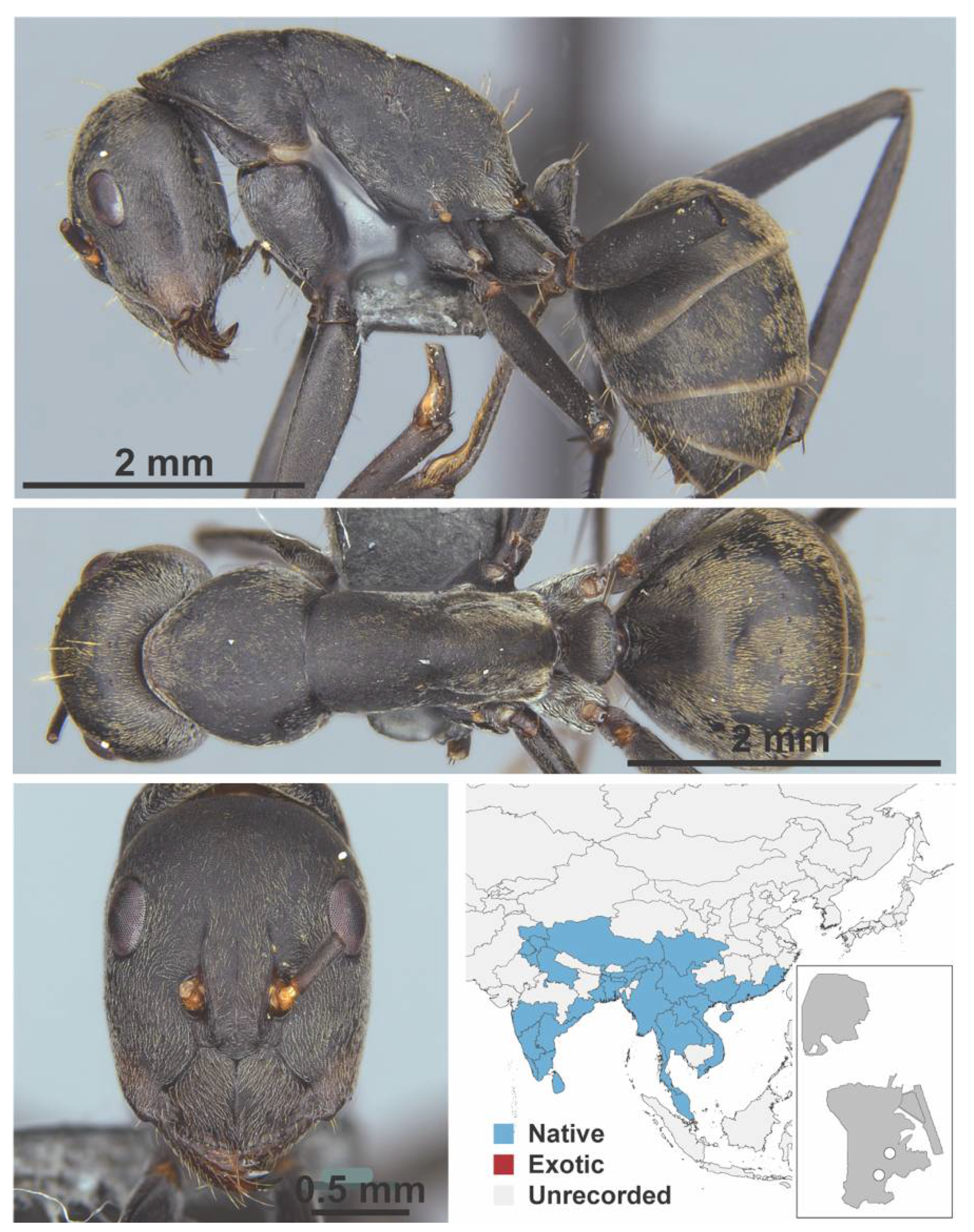

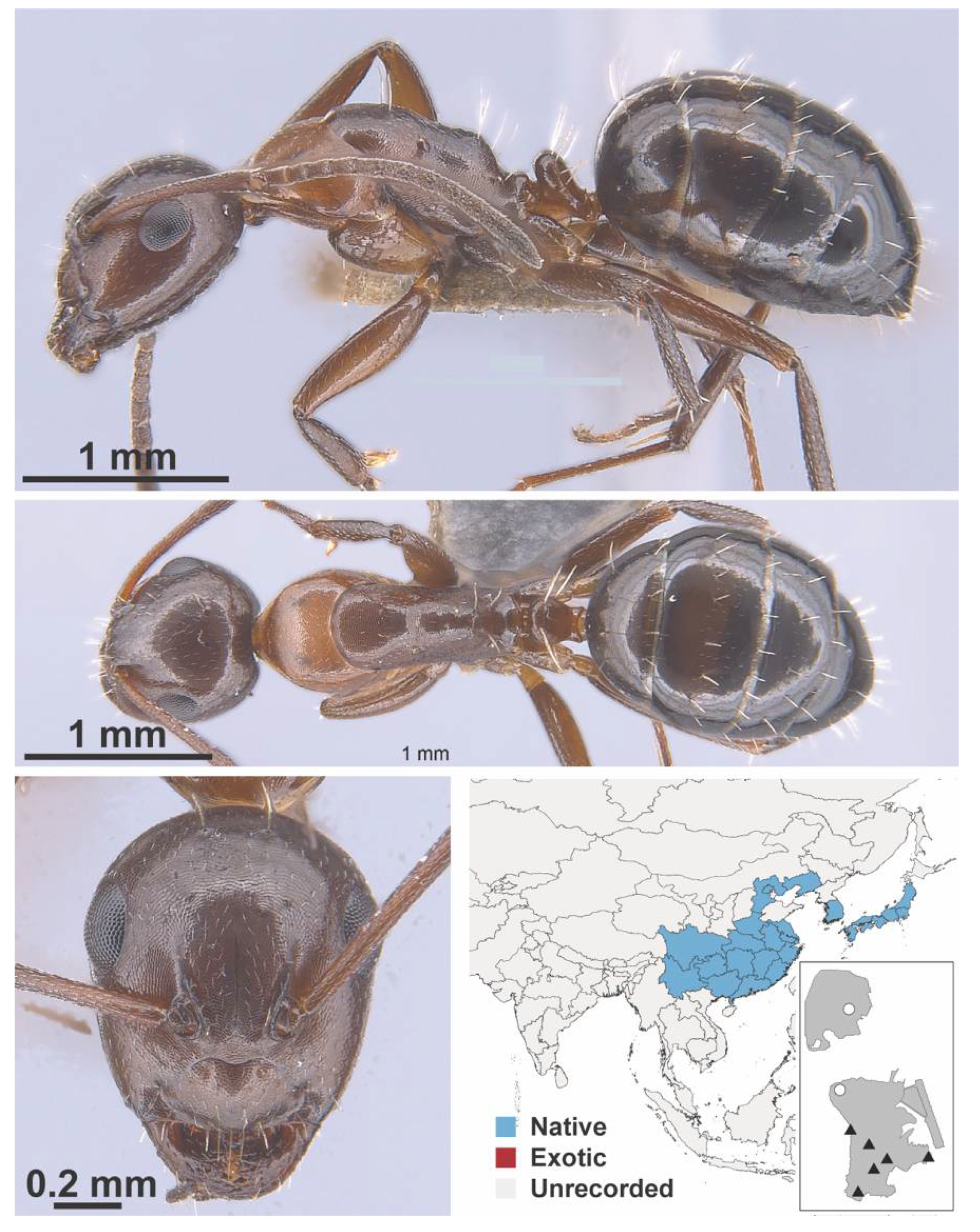

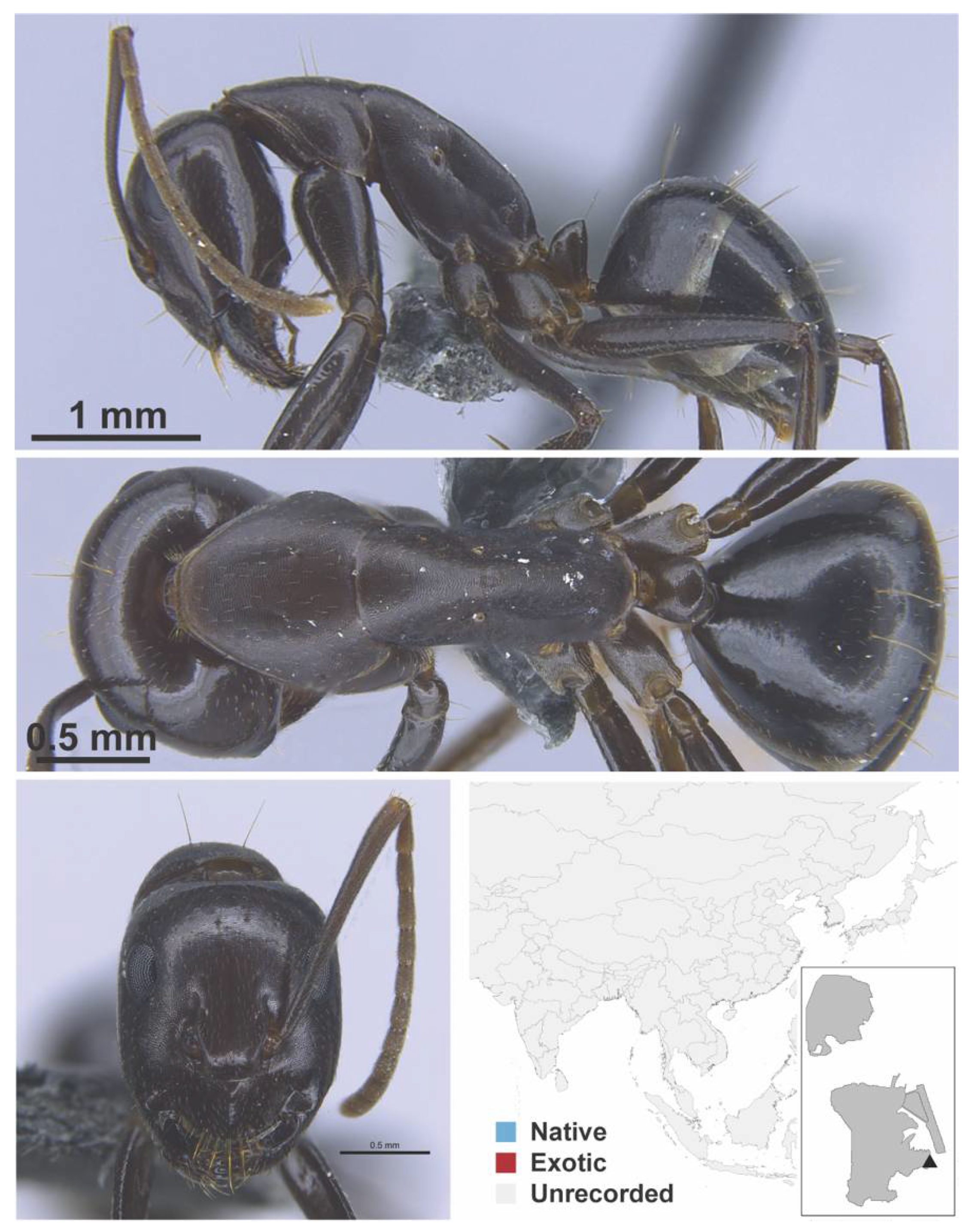
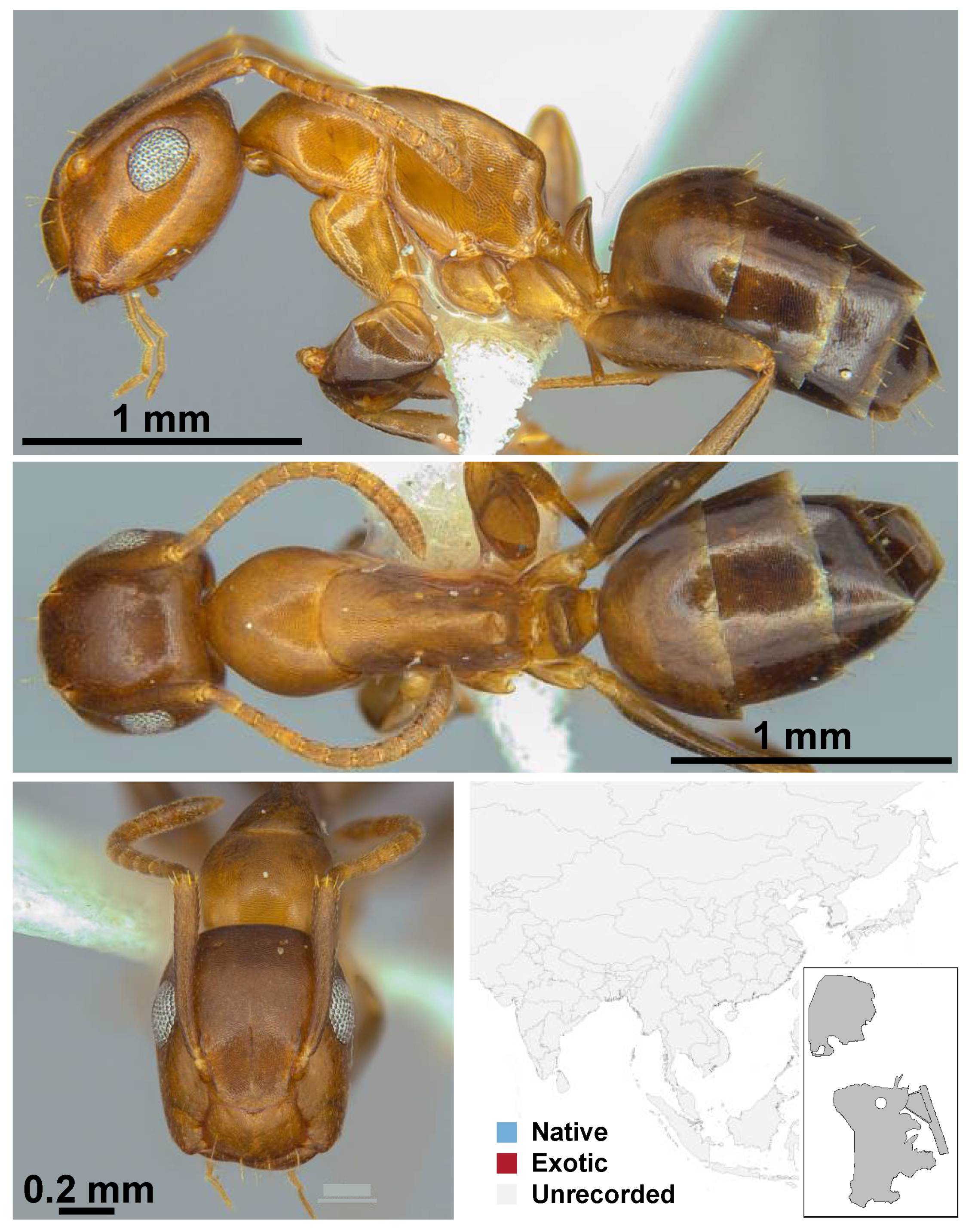
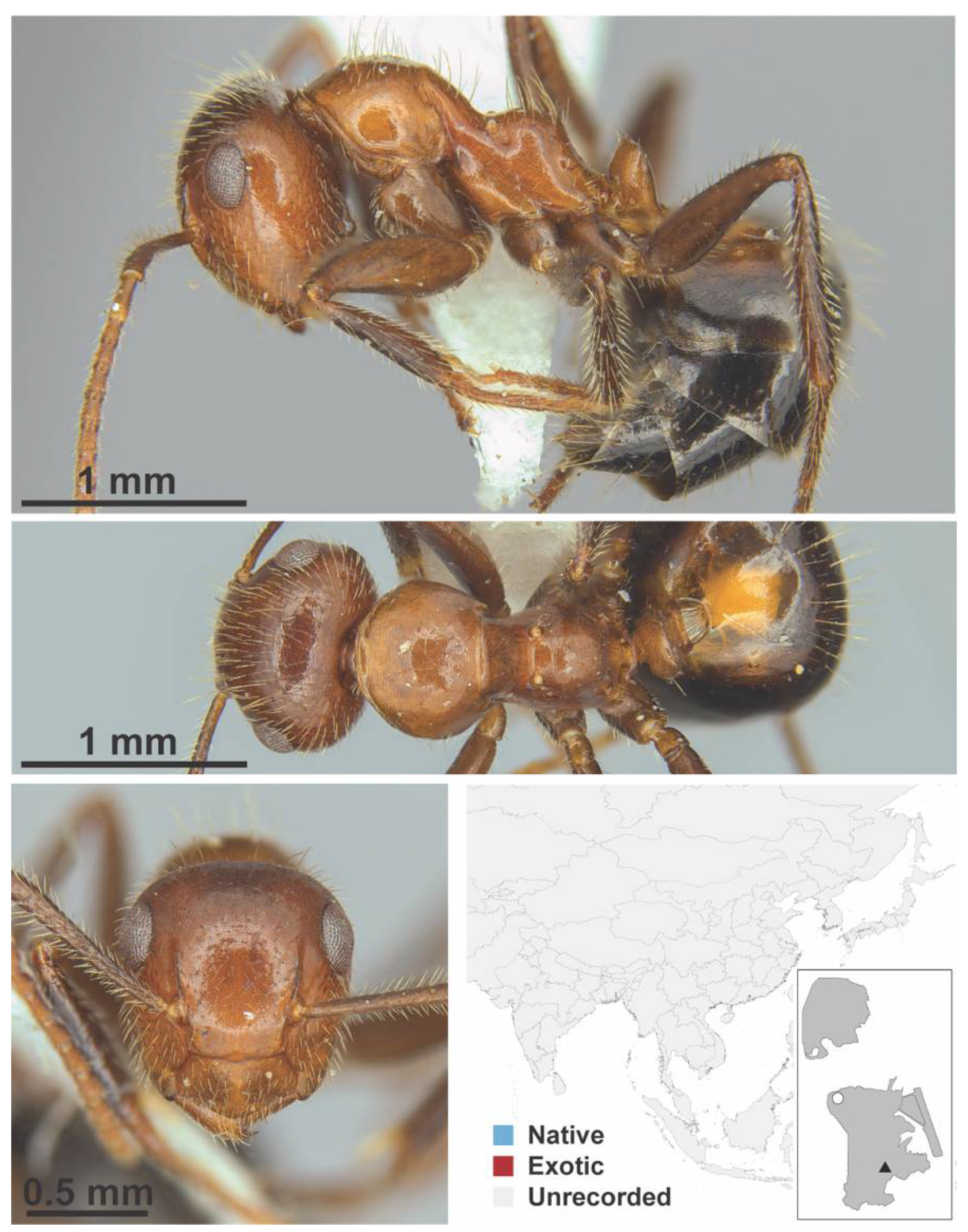
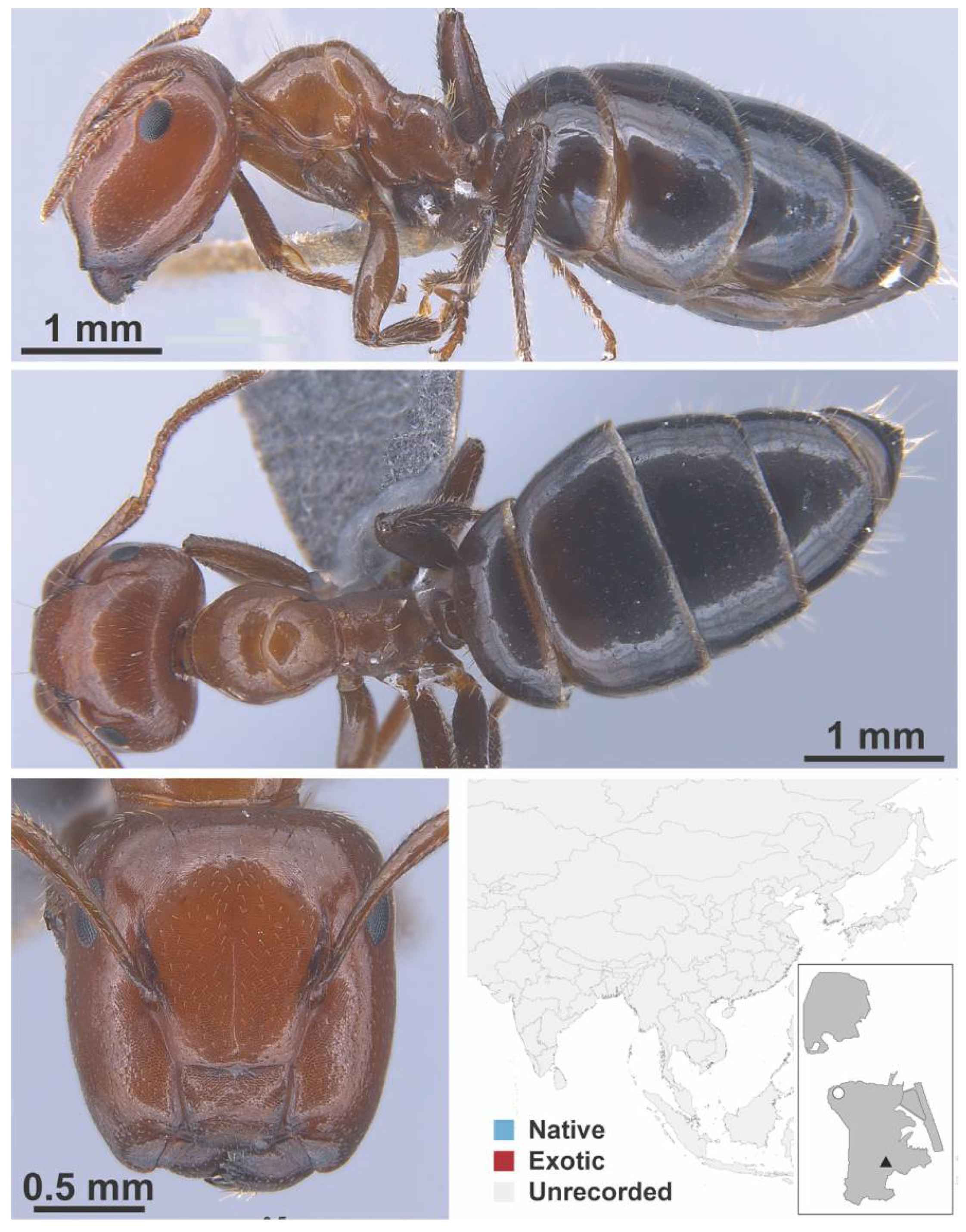
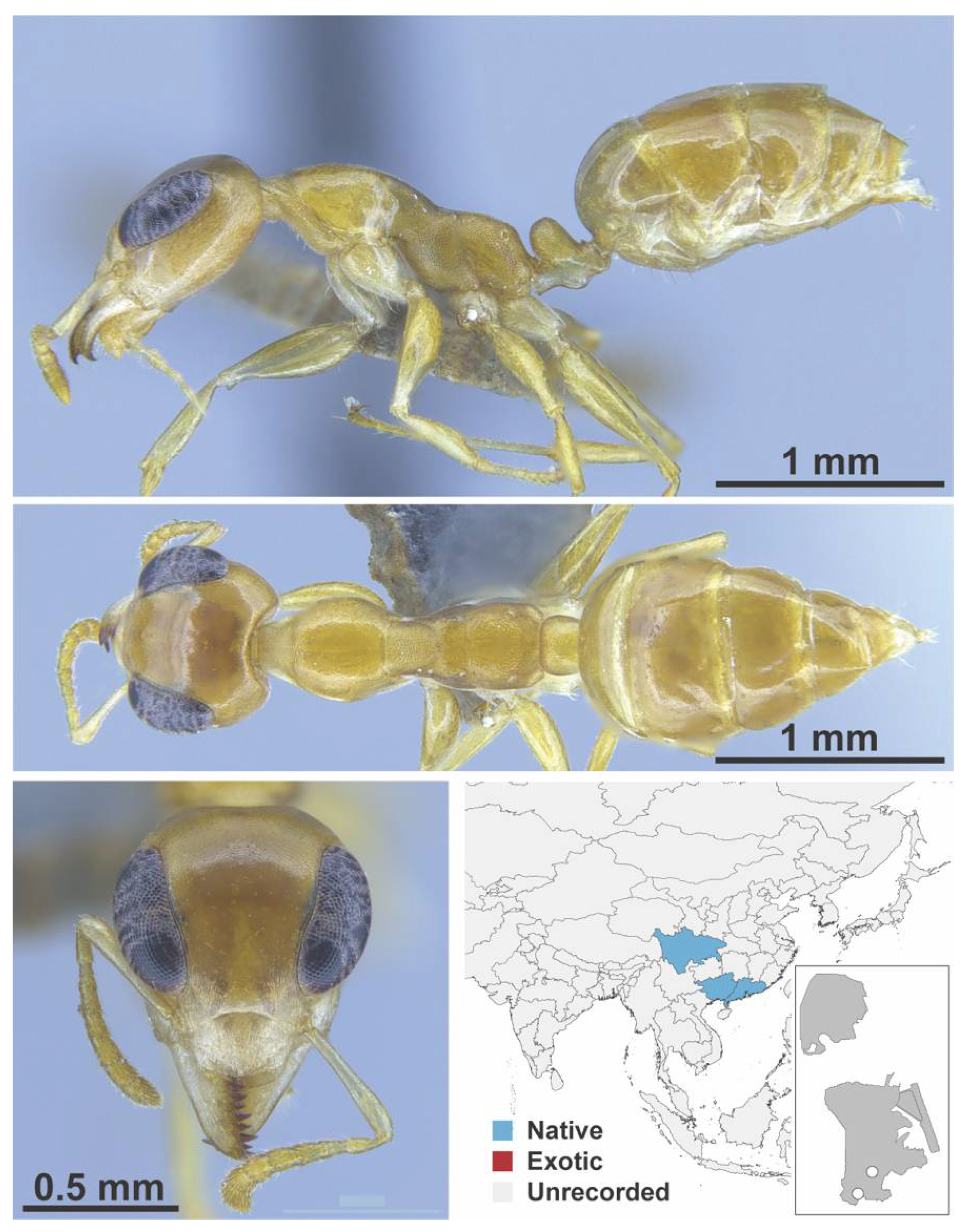
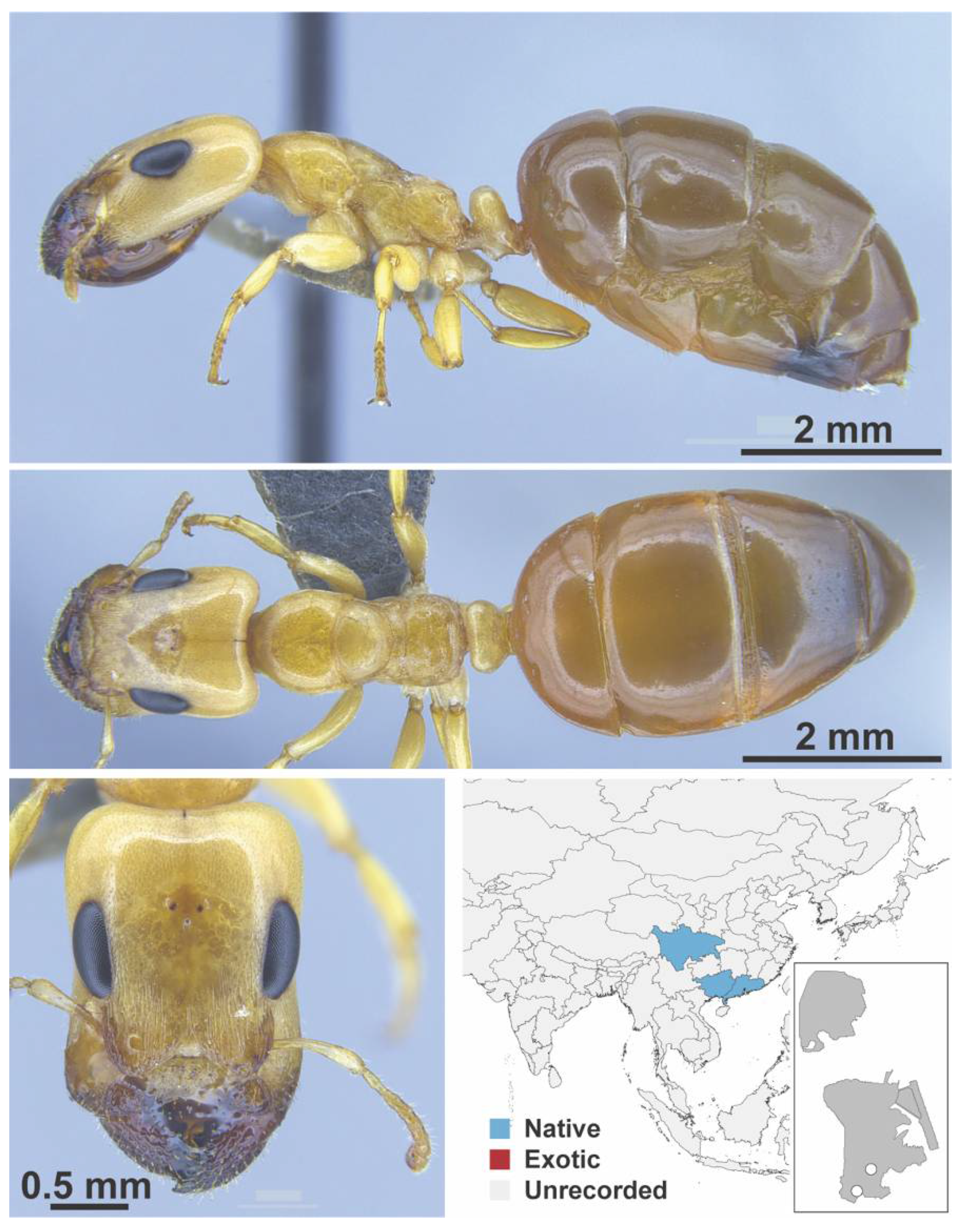
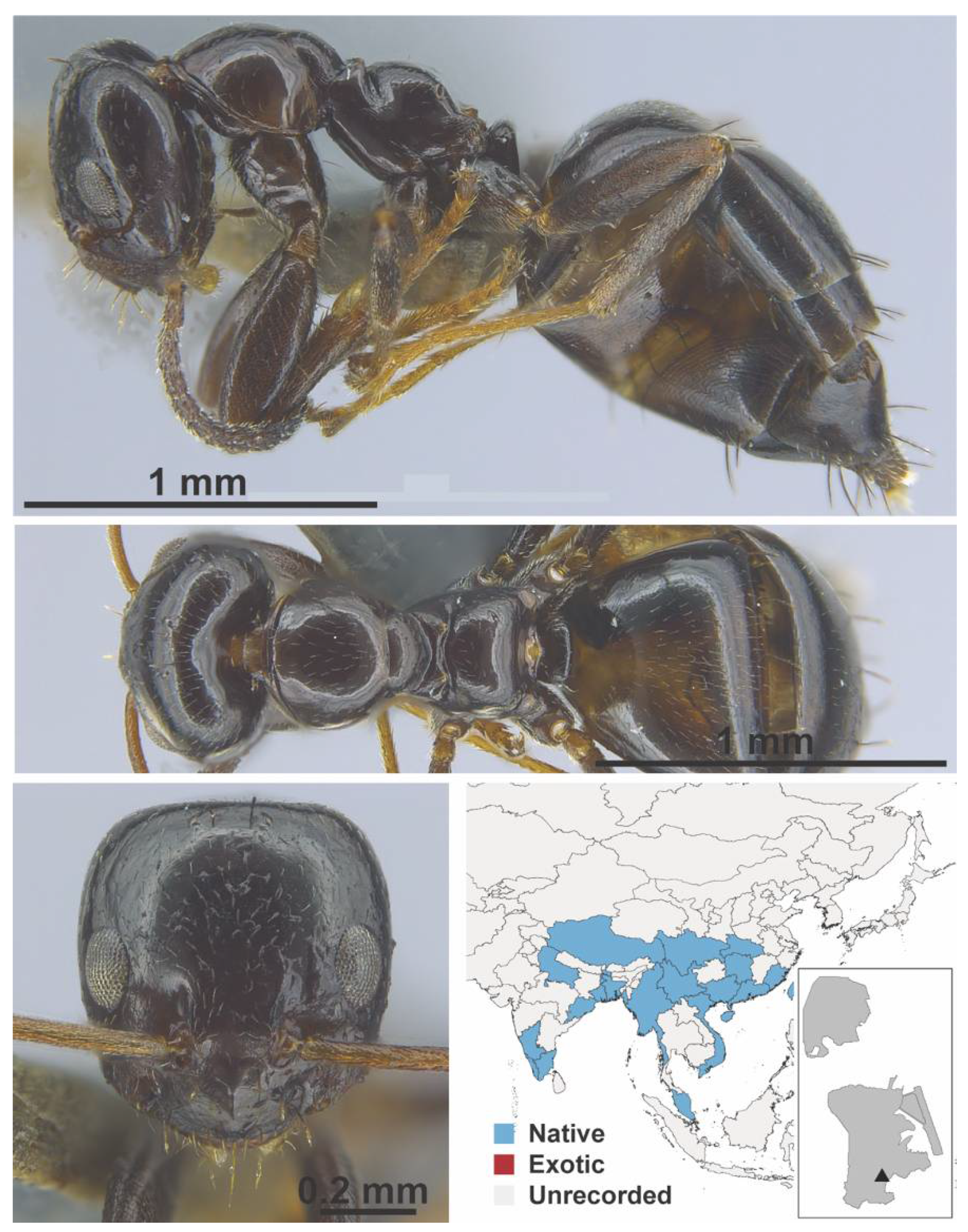
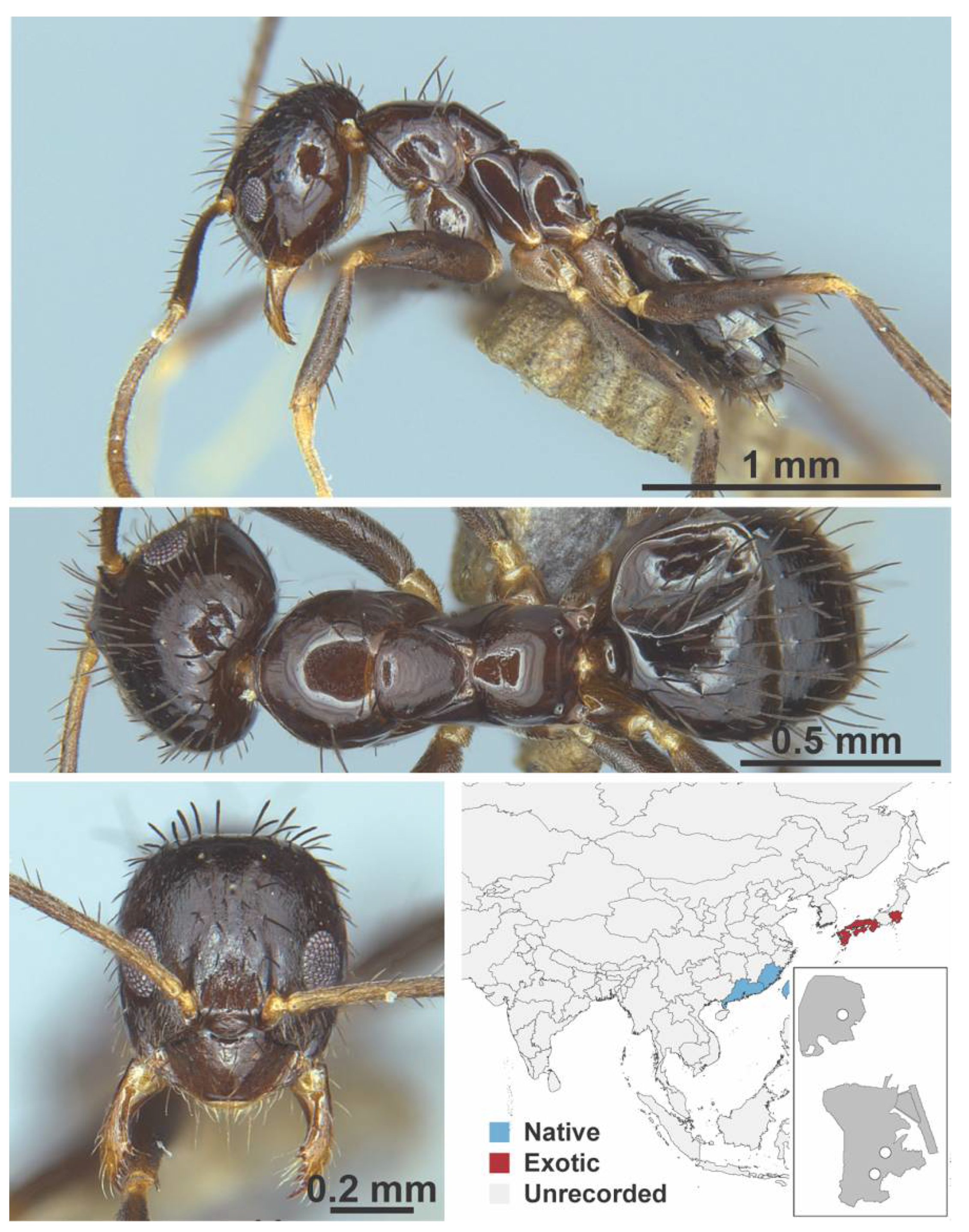
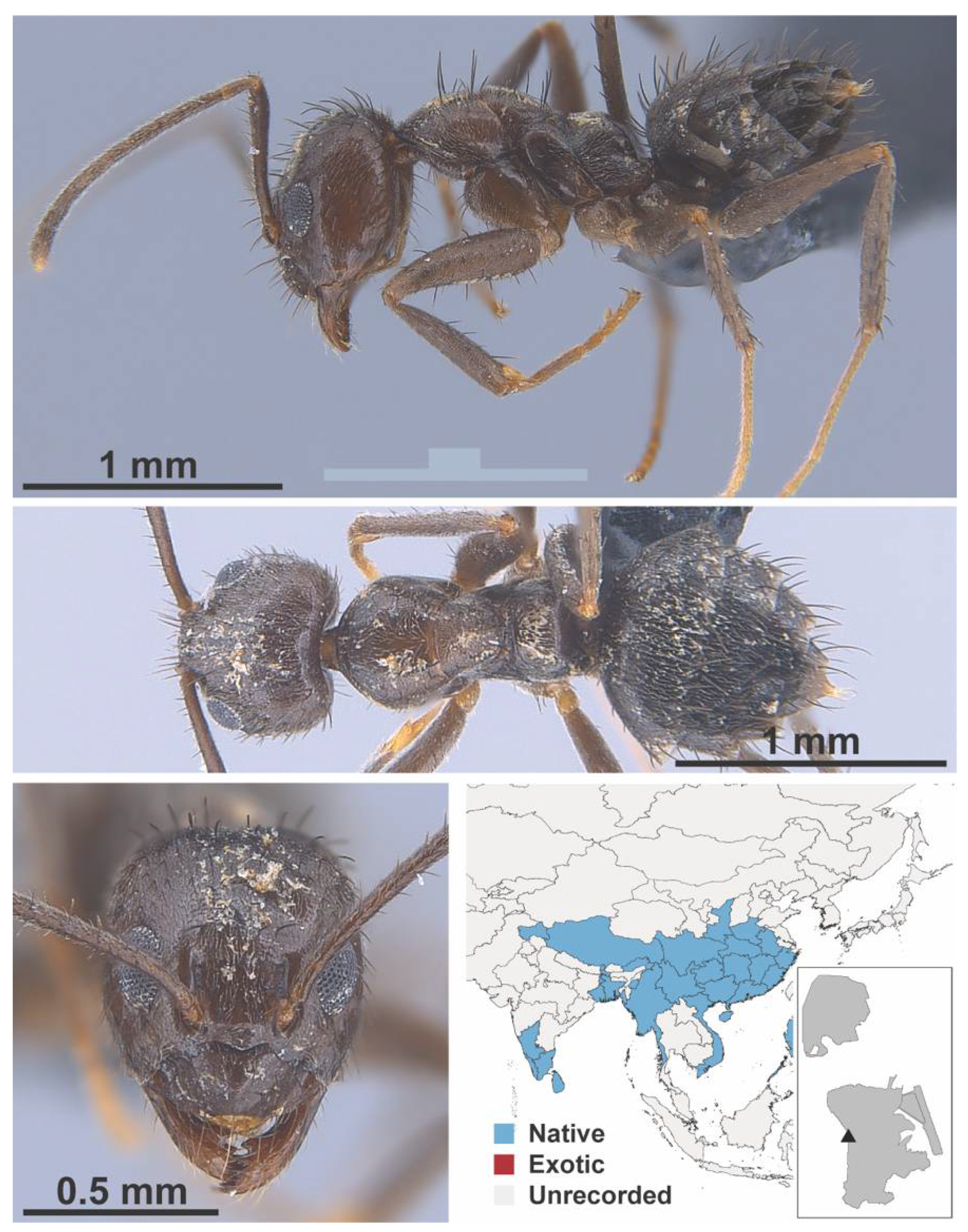
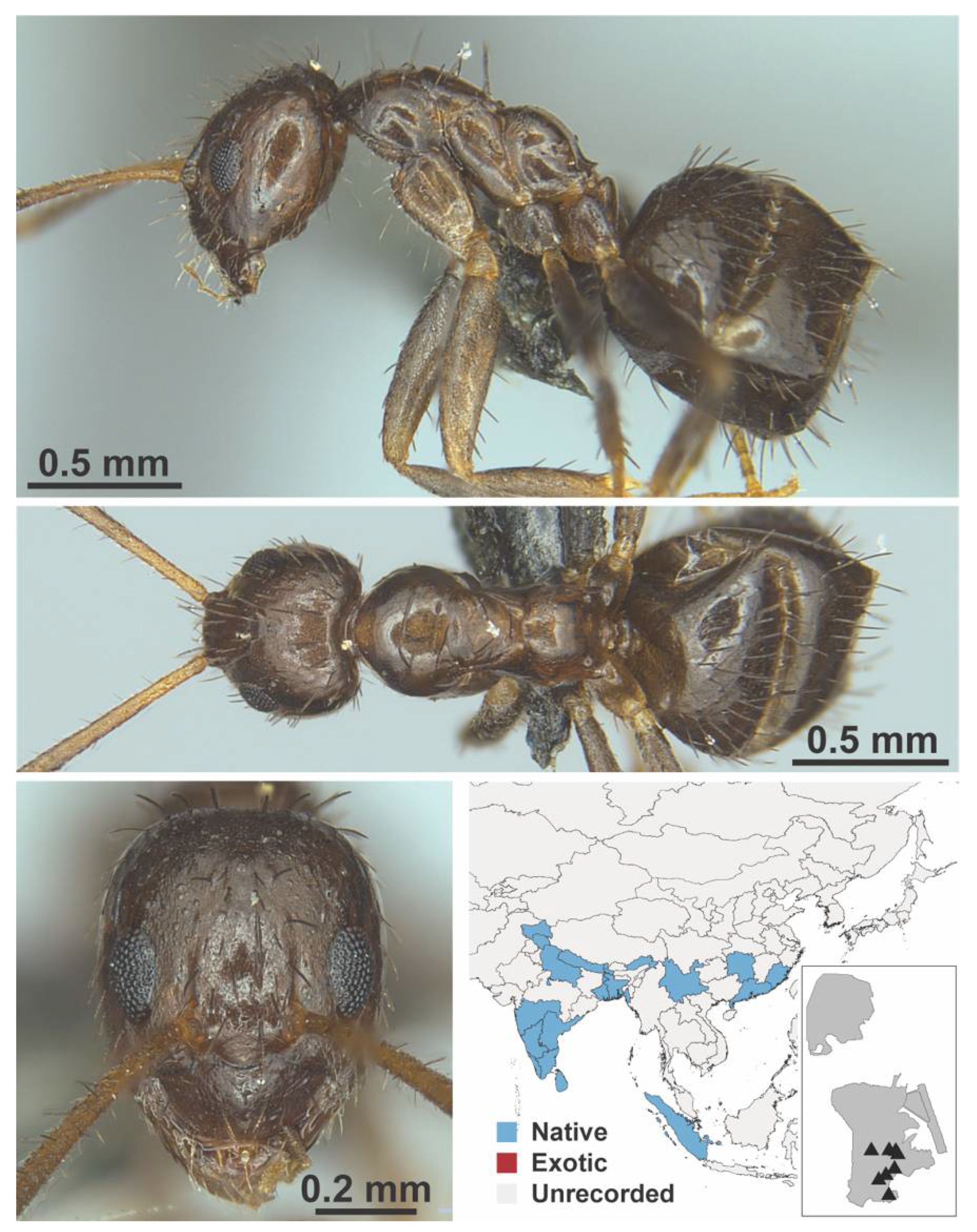
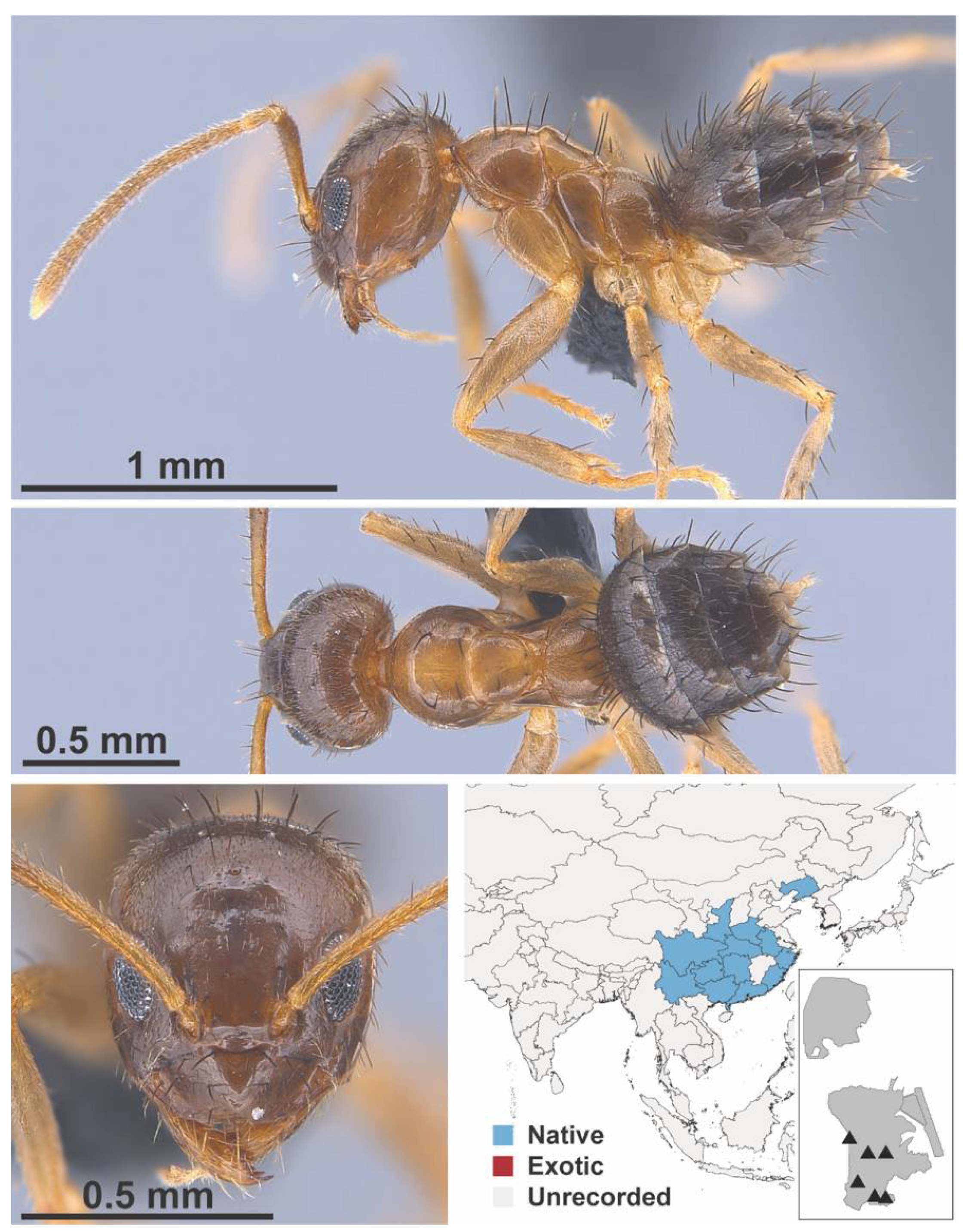
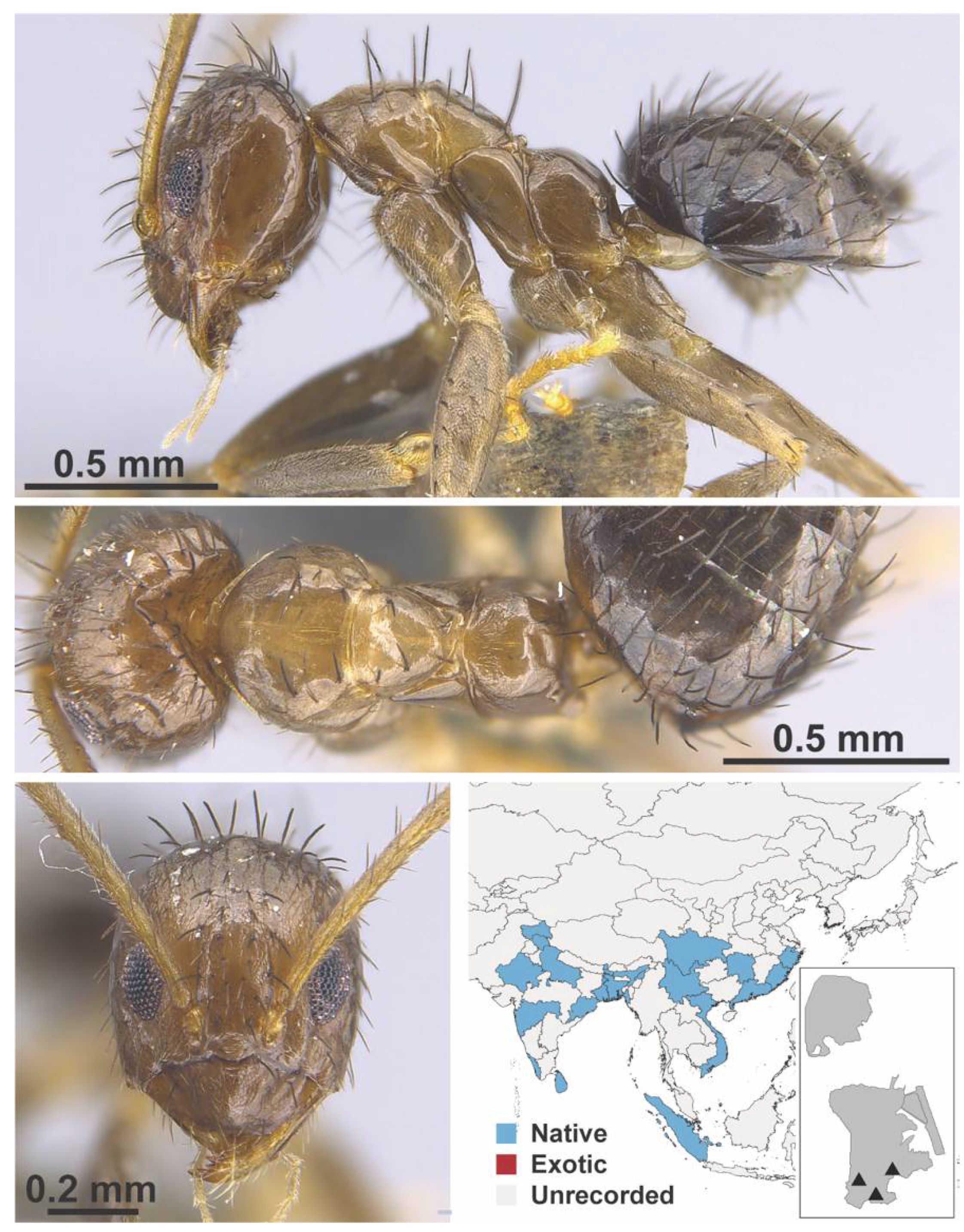
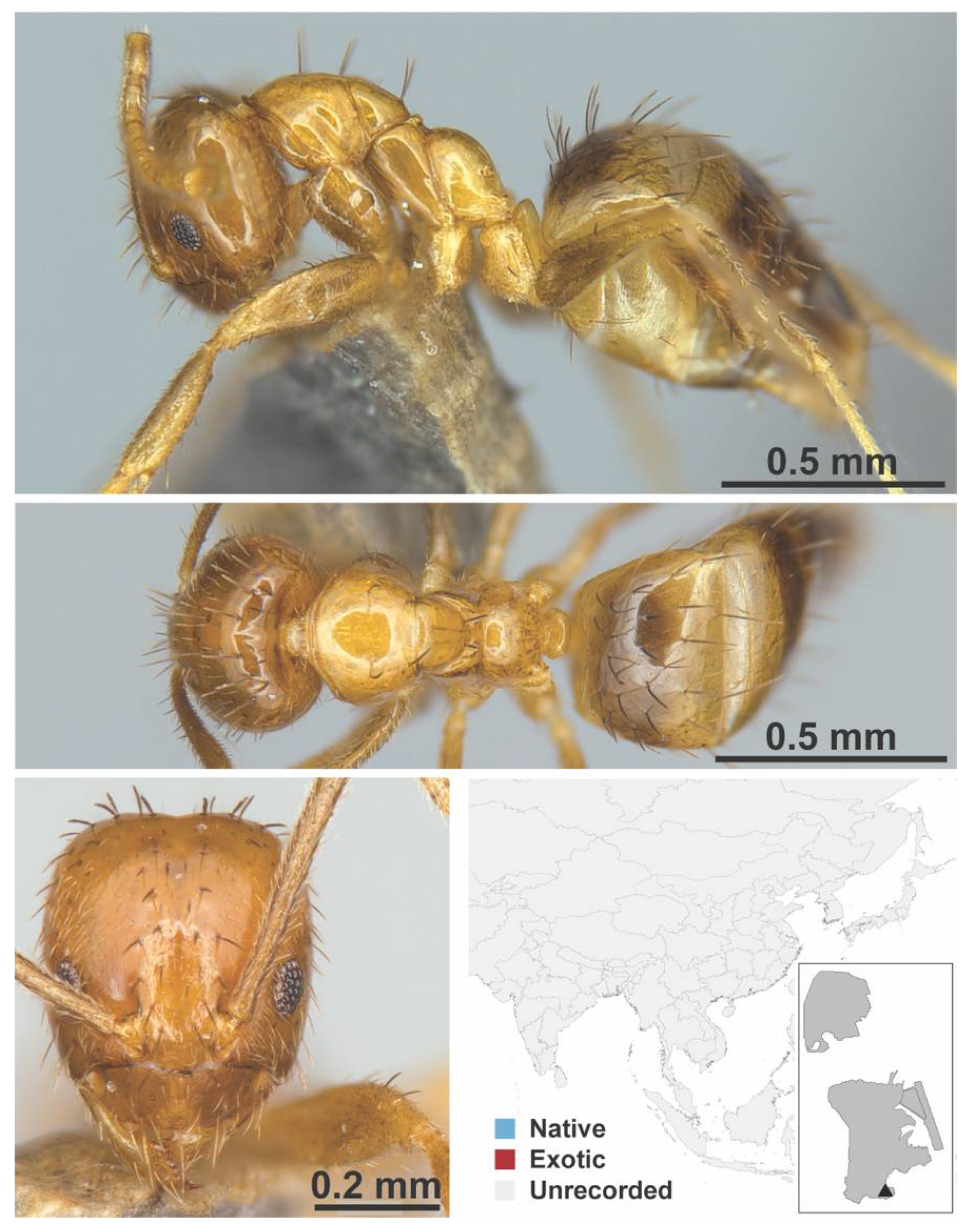
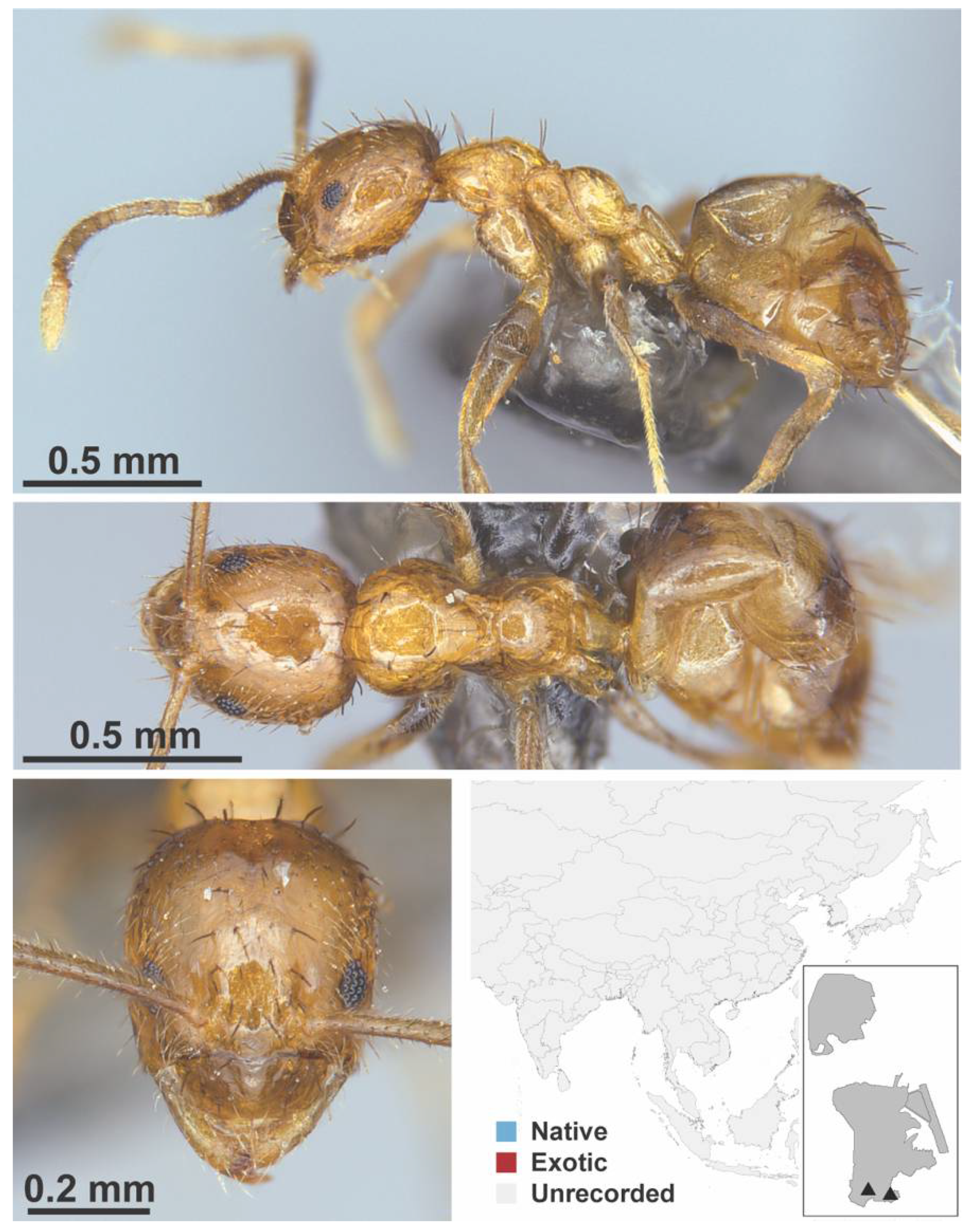

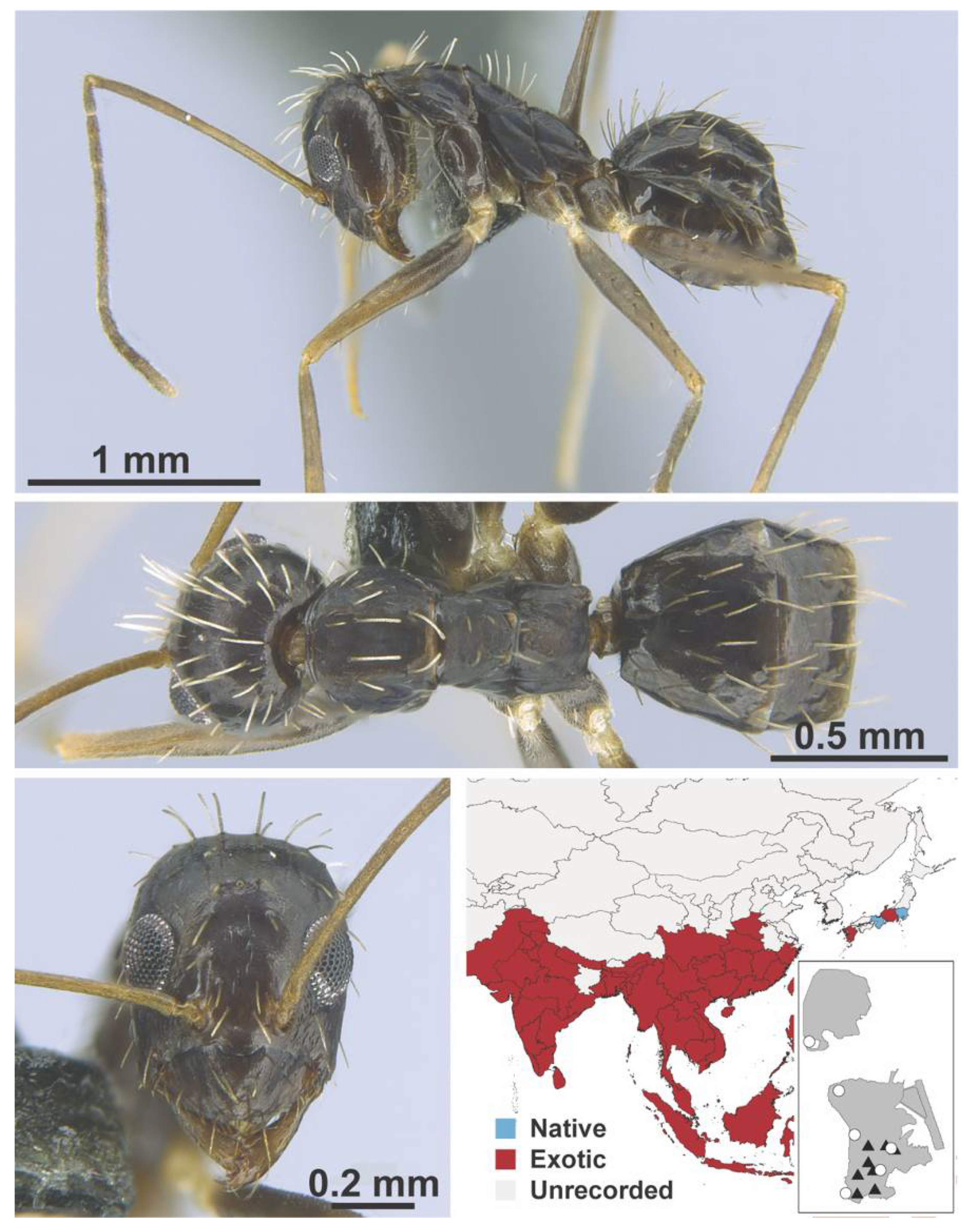
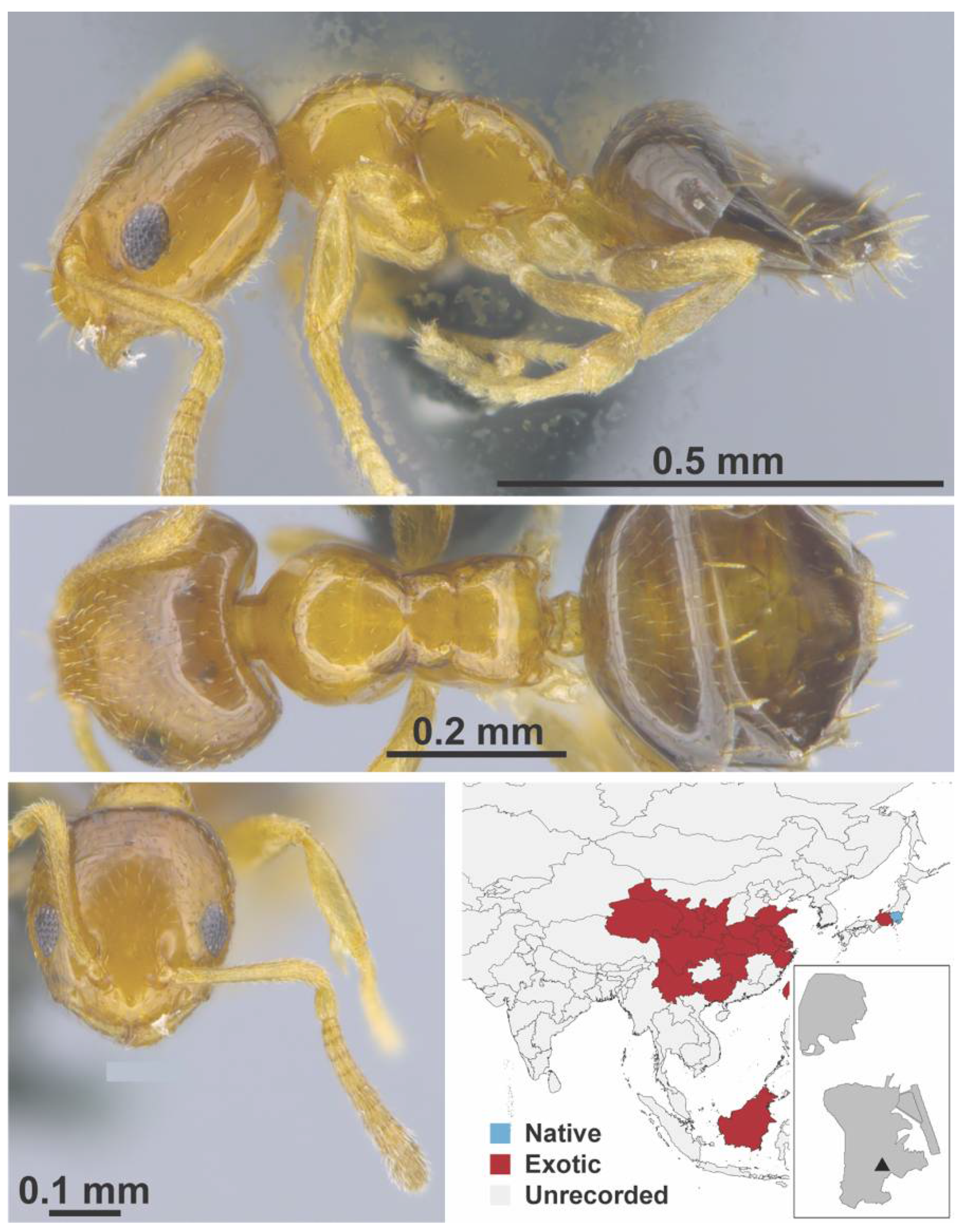
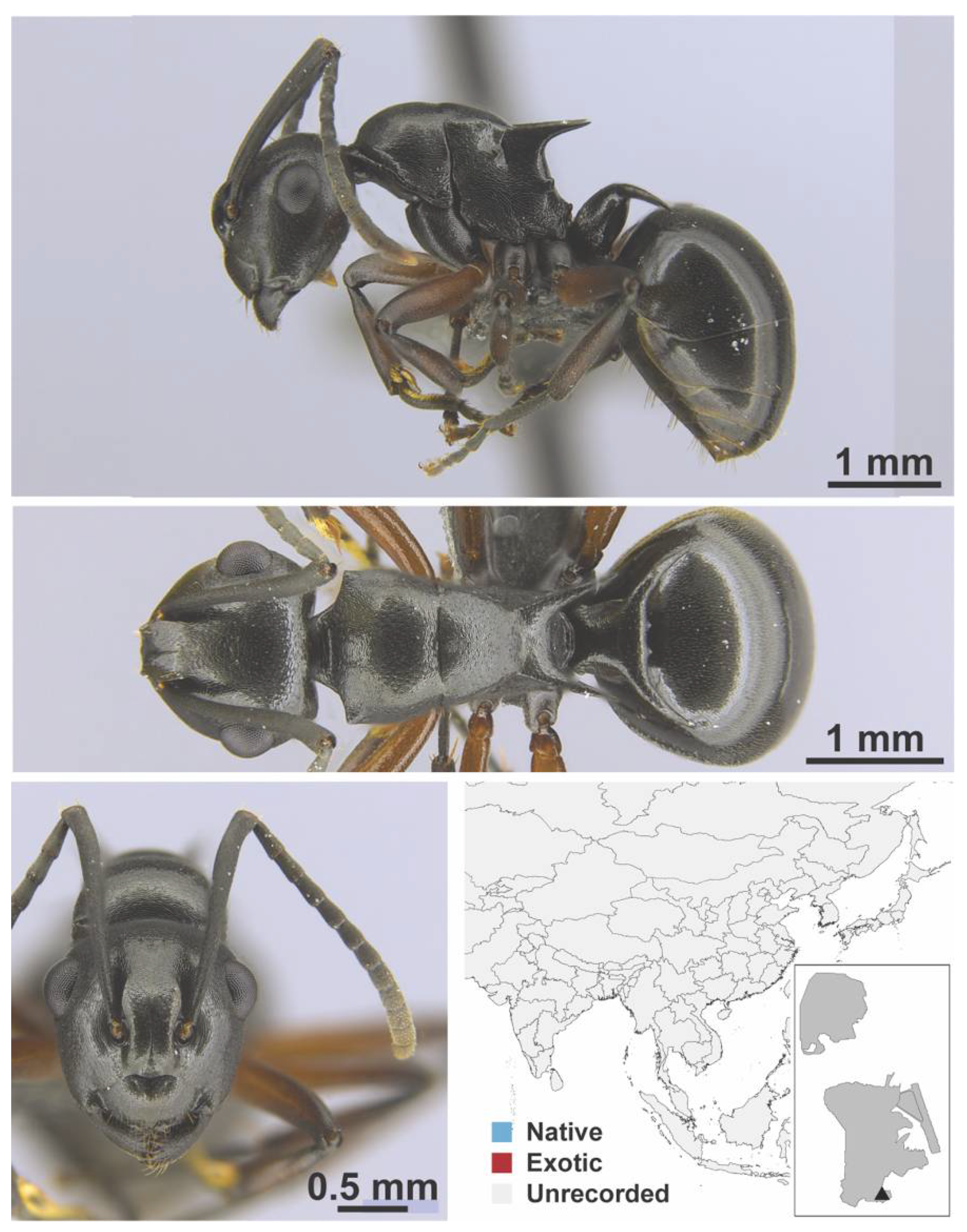
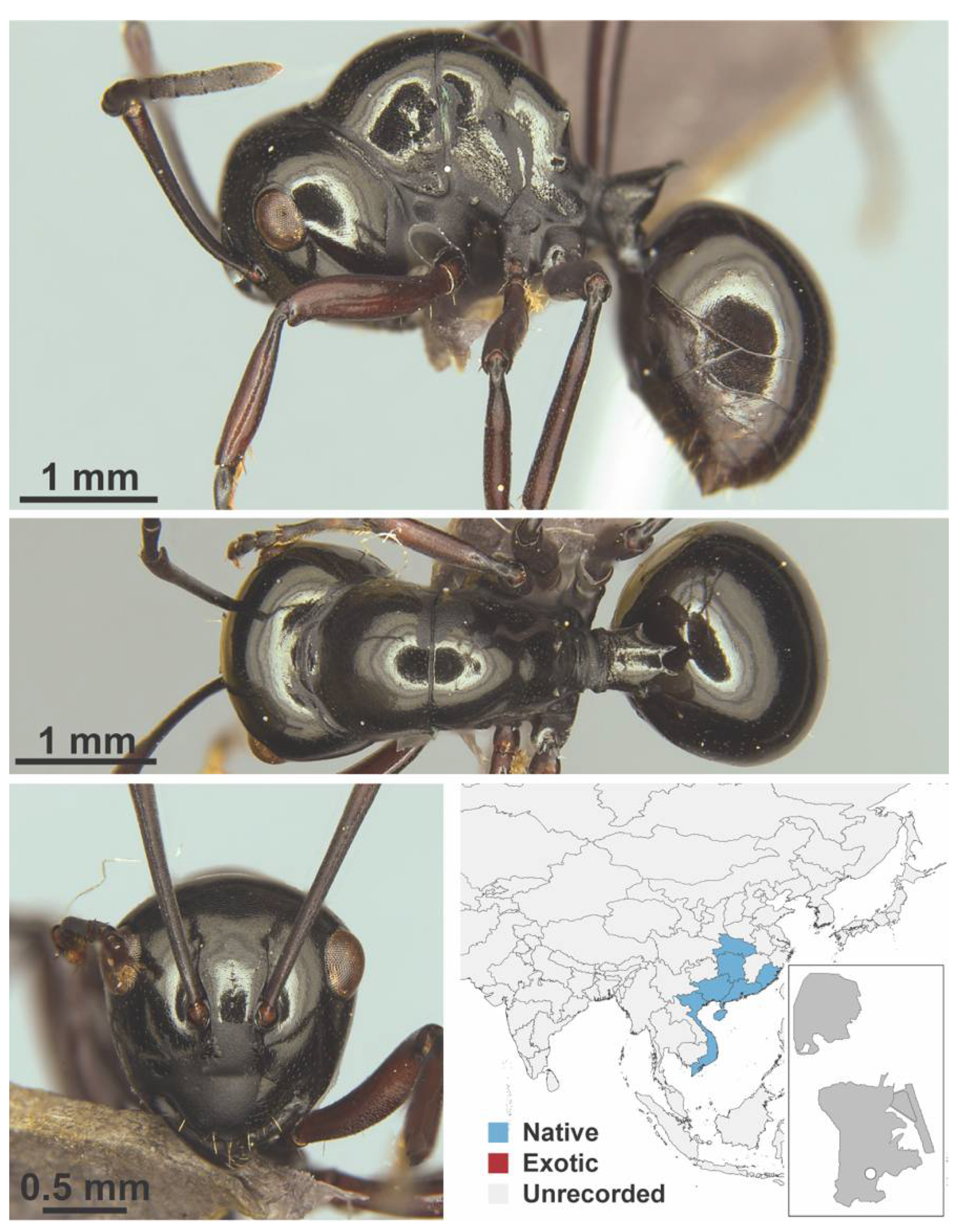
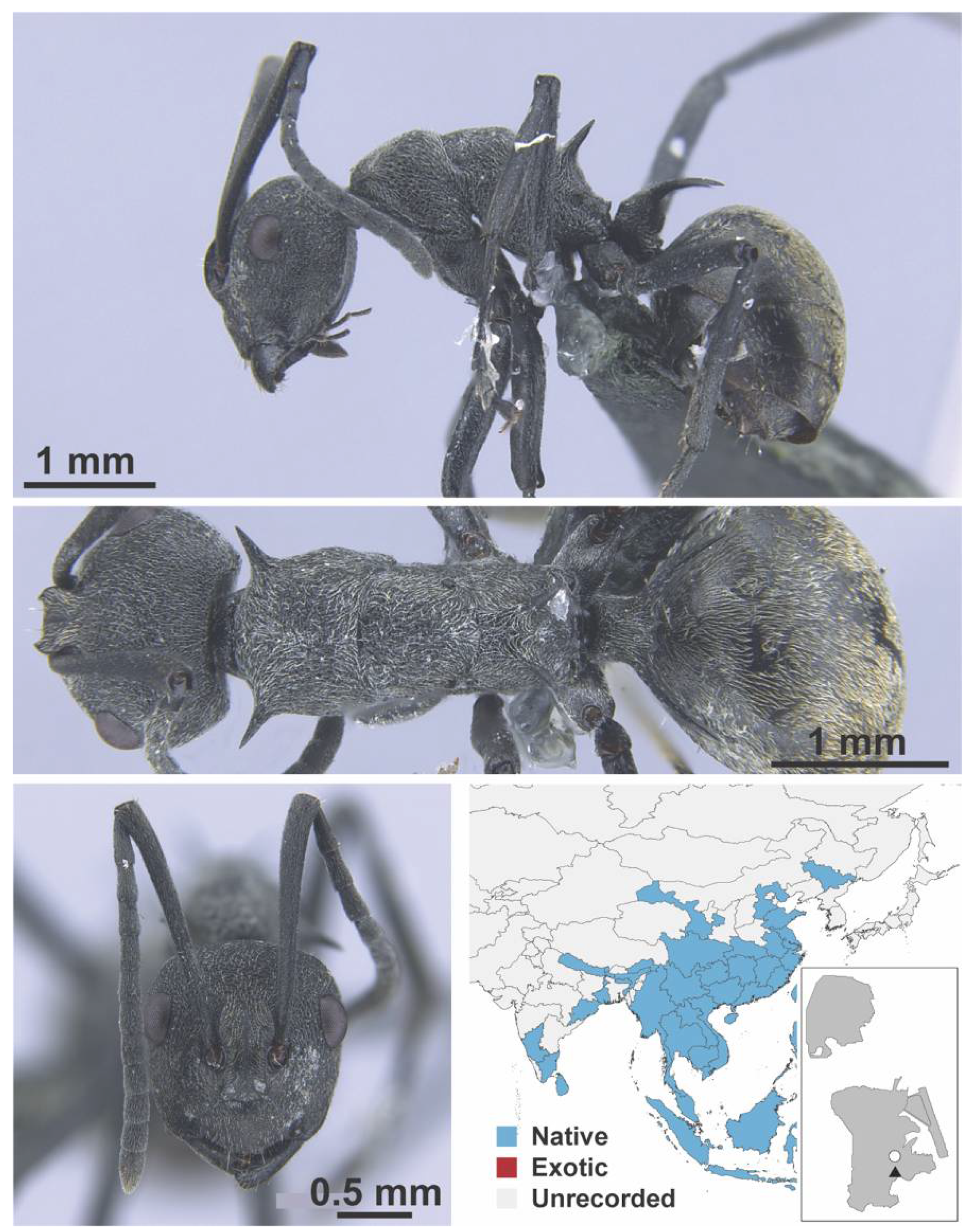
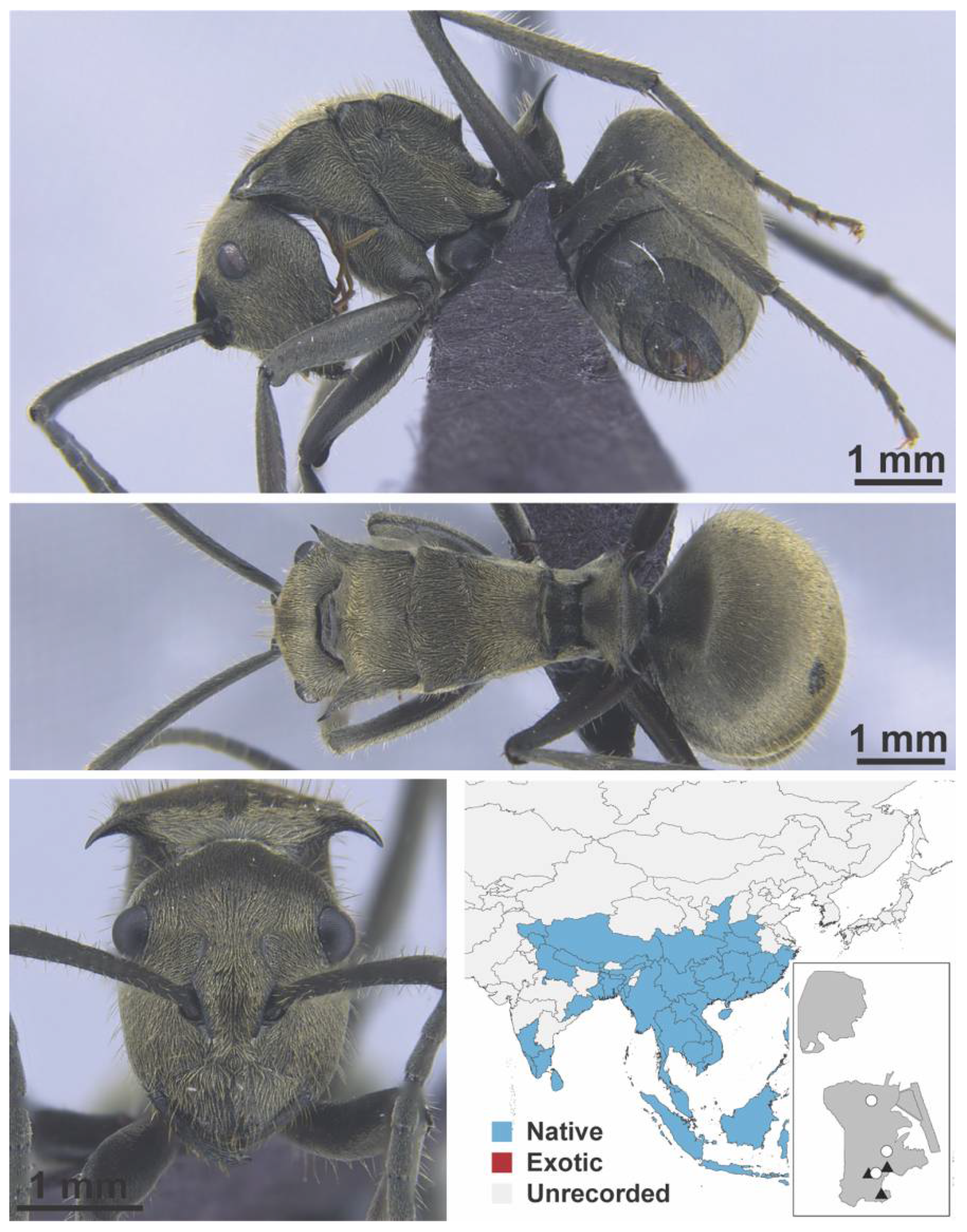
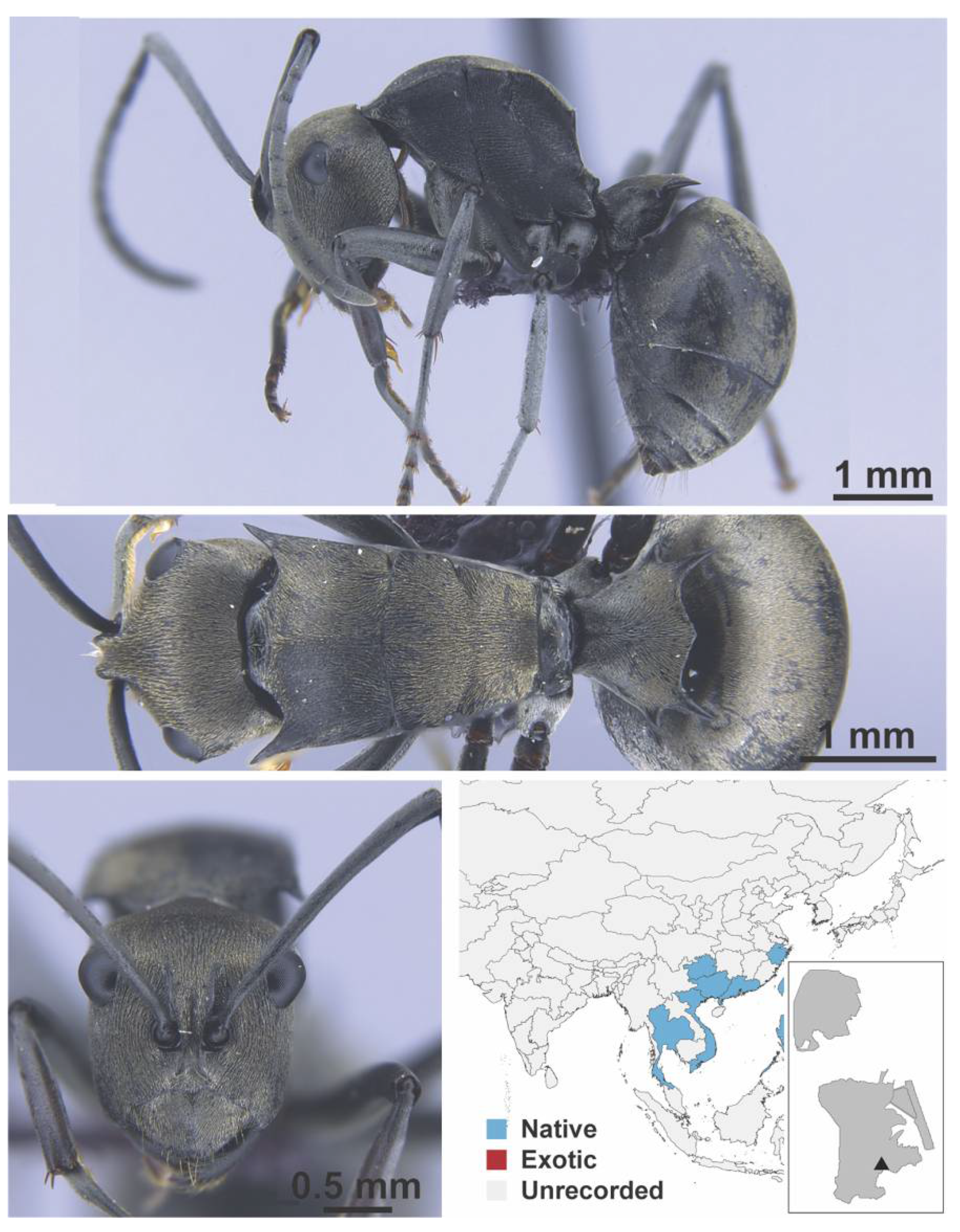
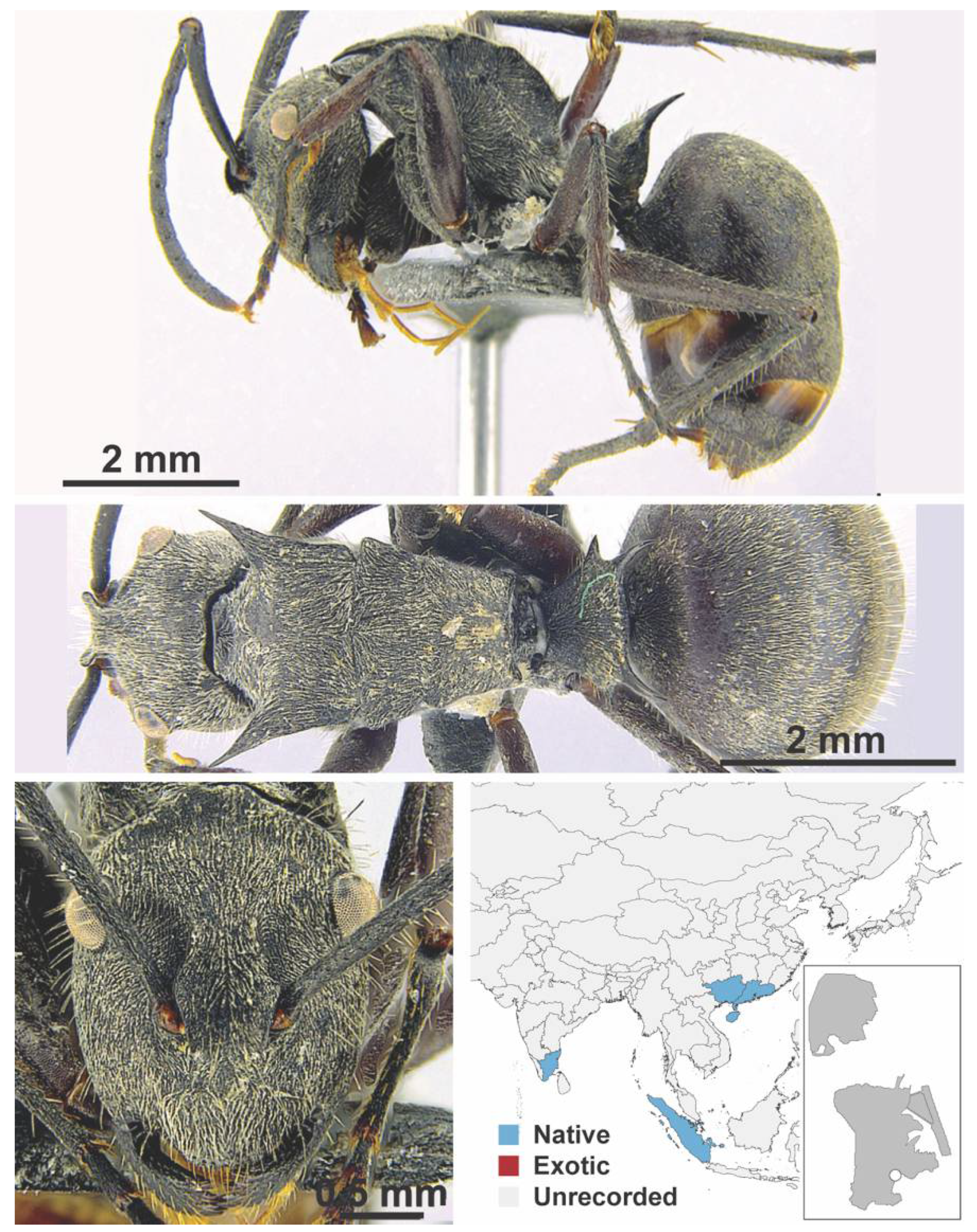

Appendix A.5. LEPTANILLINAE

Appendix A.6. MYRMICINAE
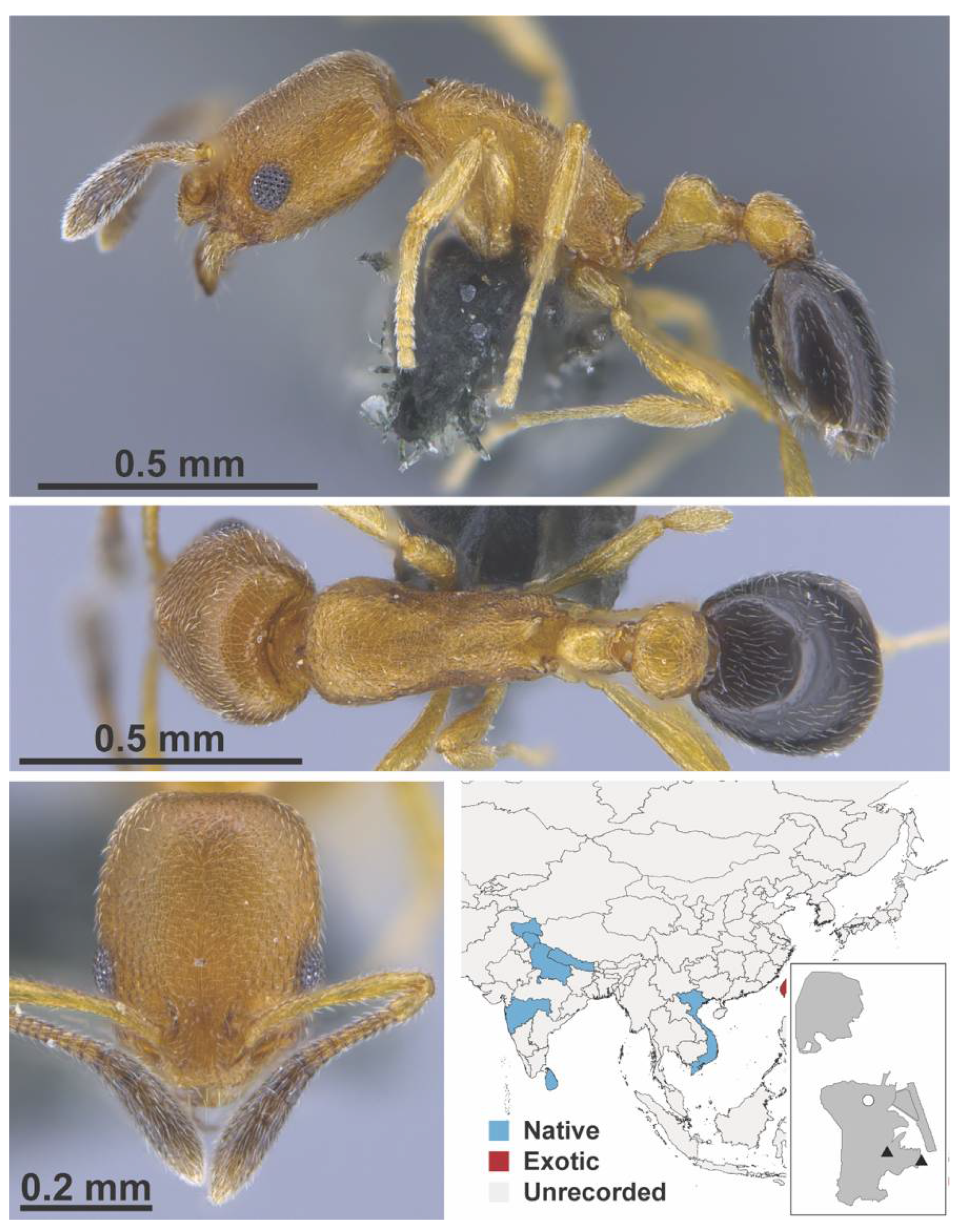

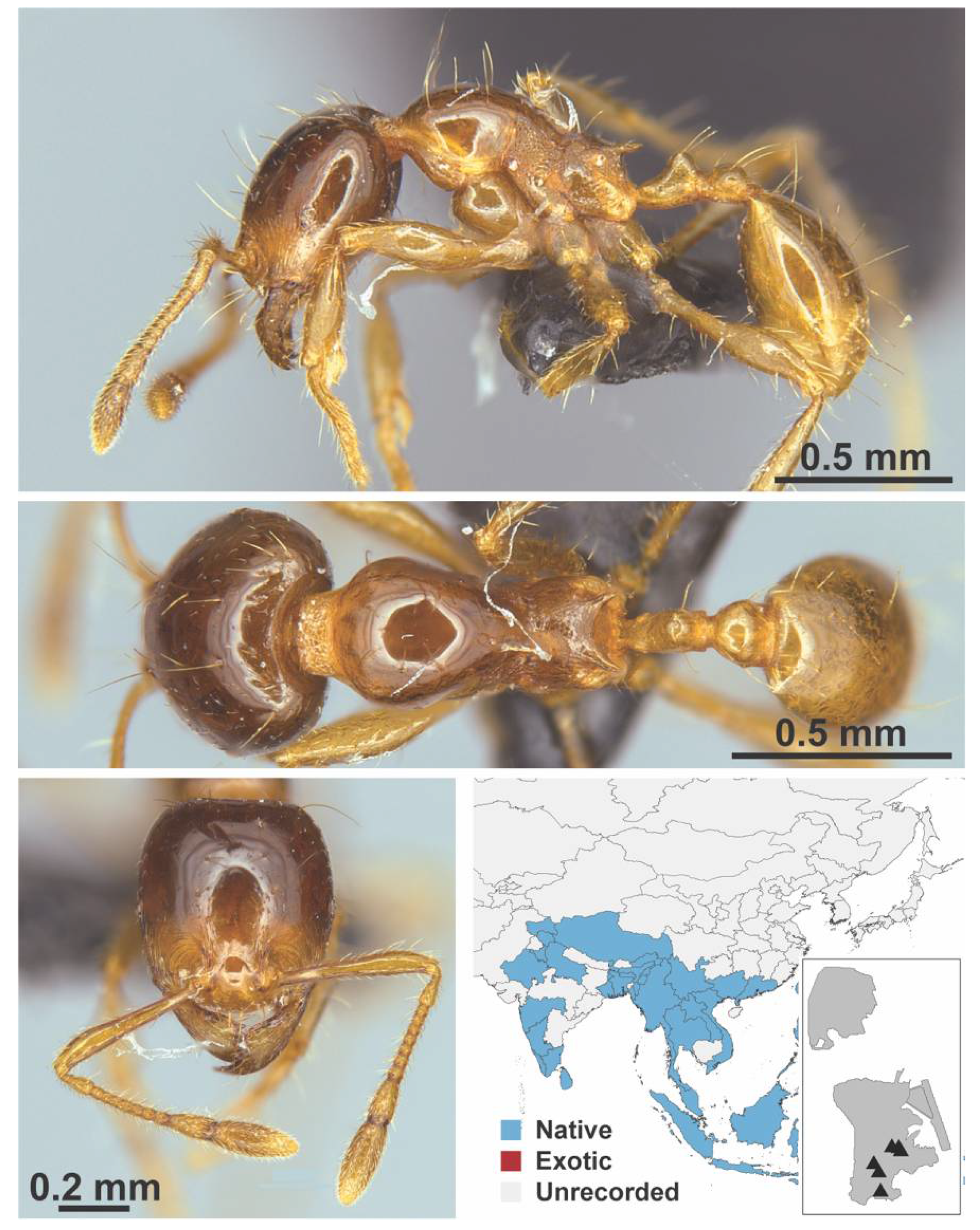
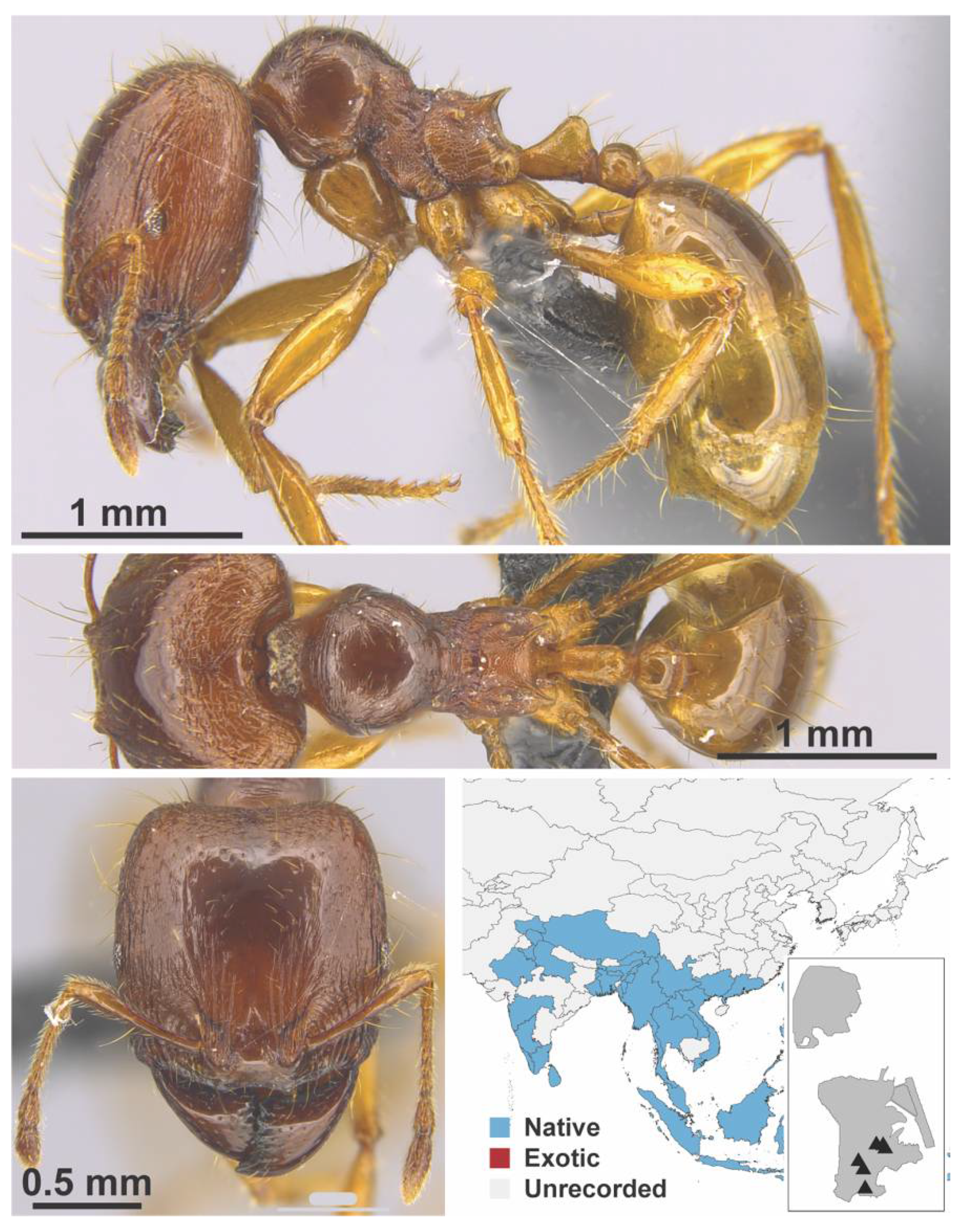

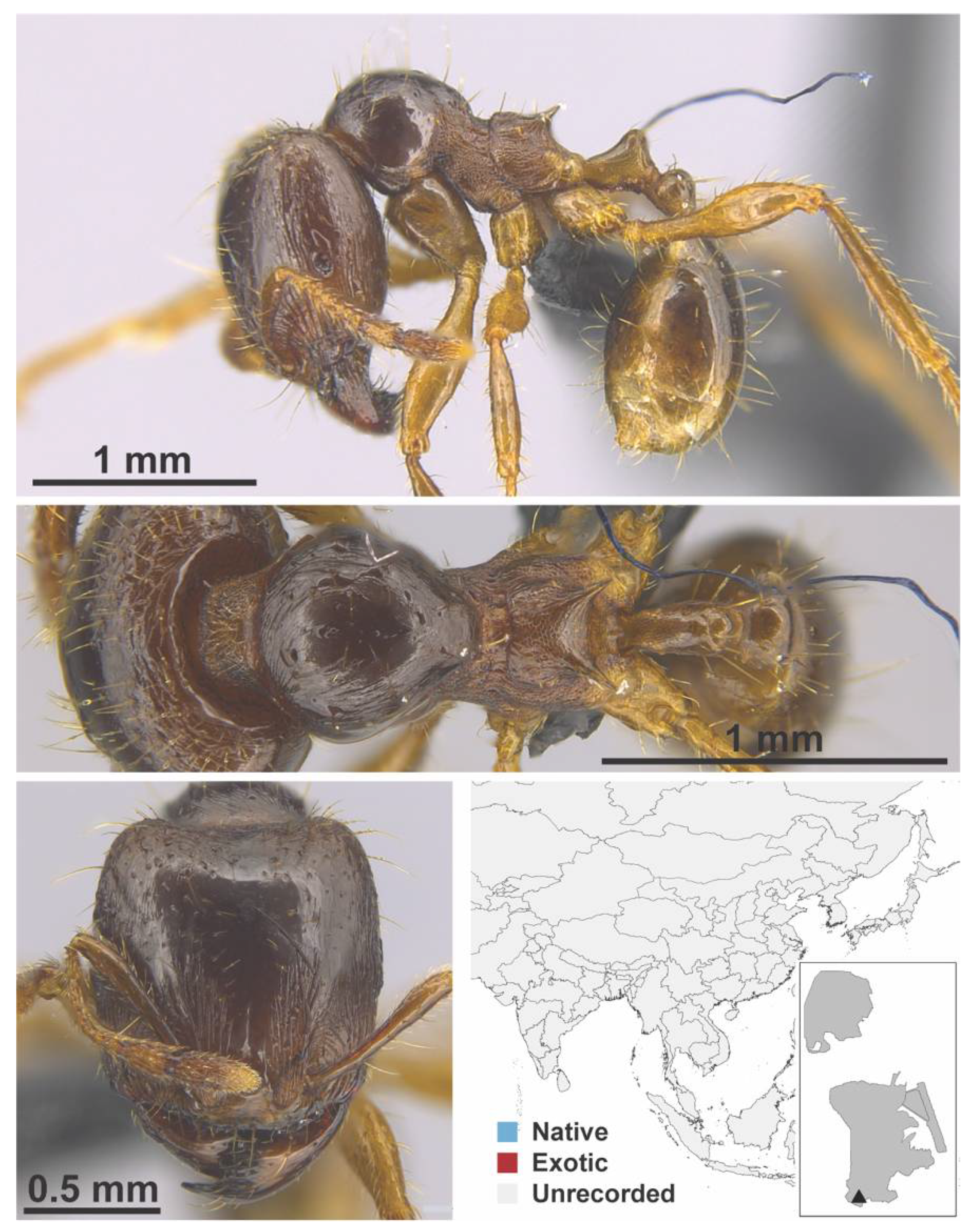
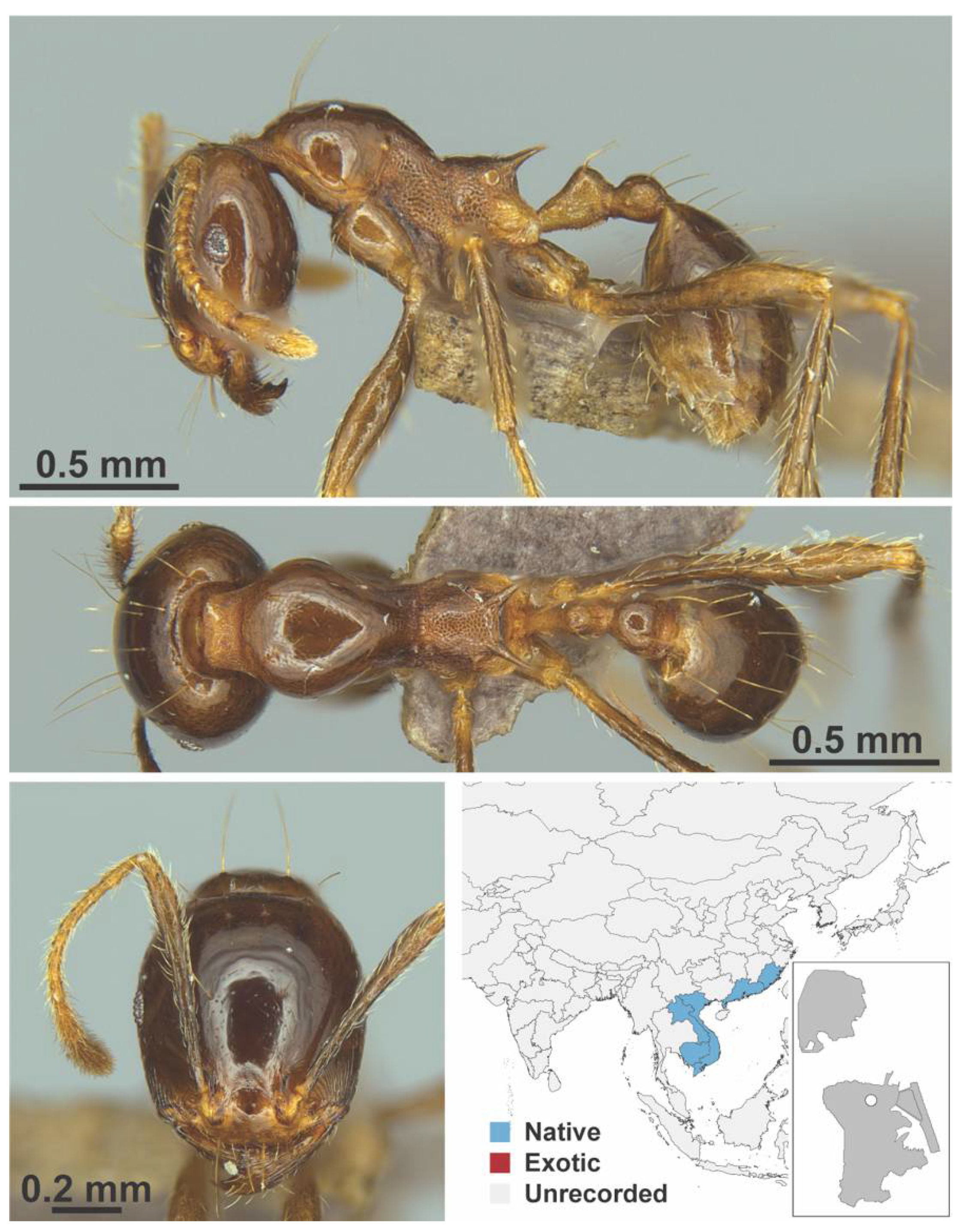


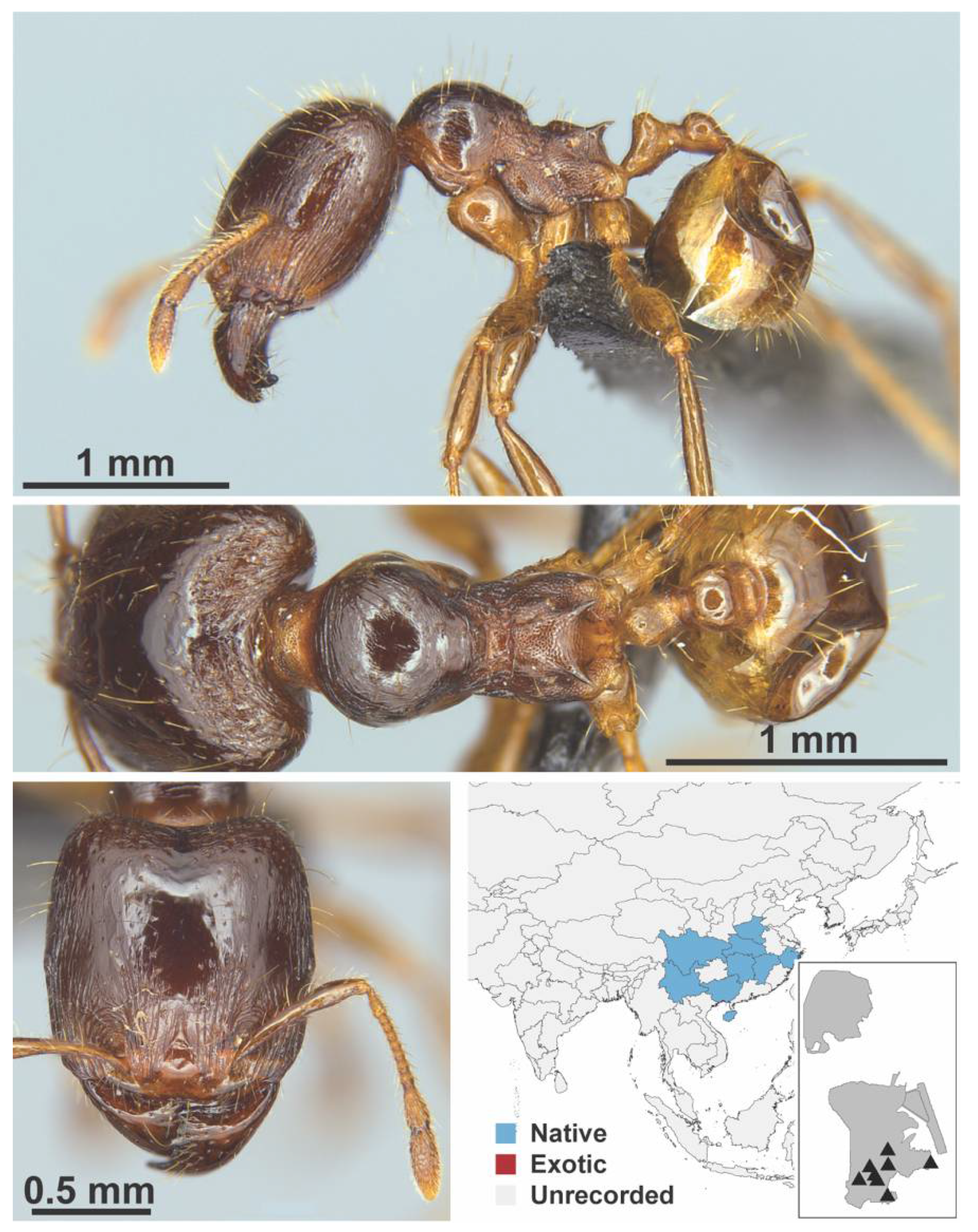
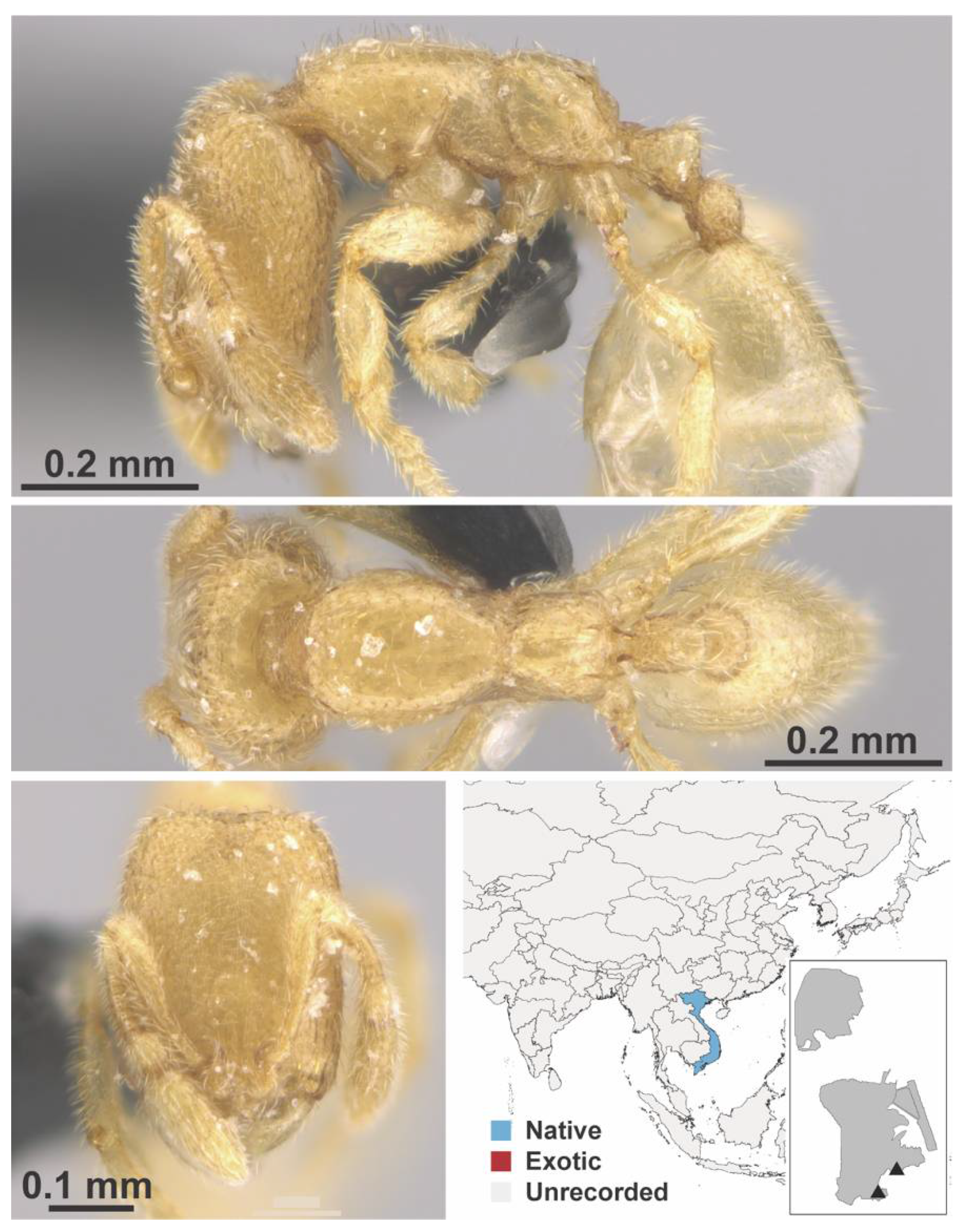
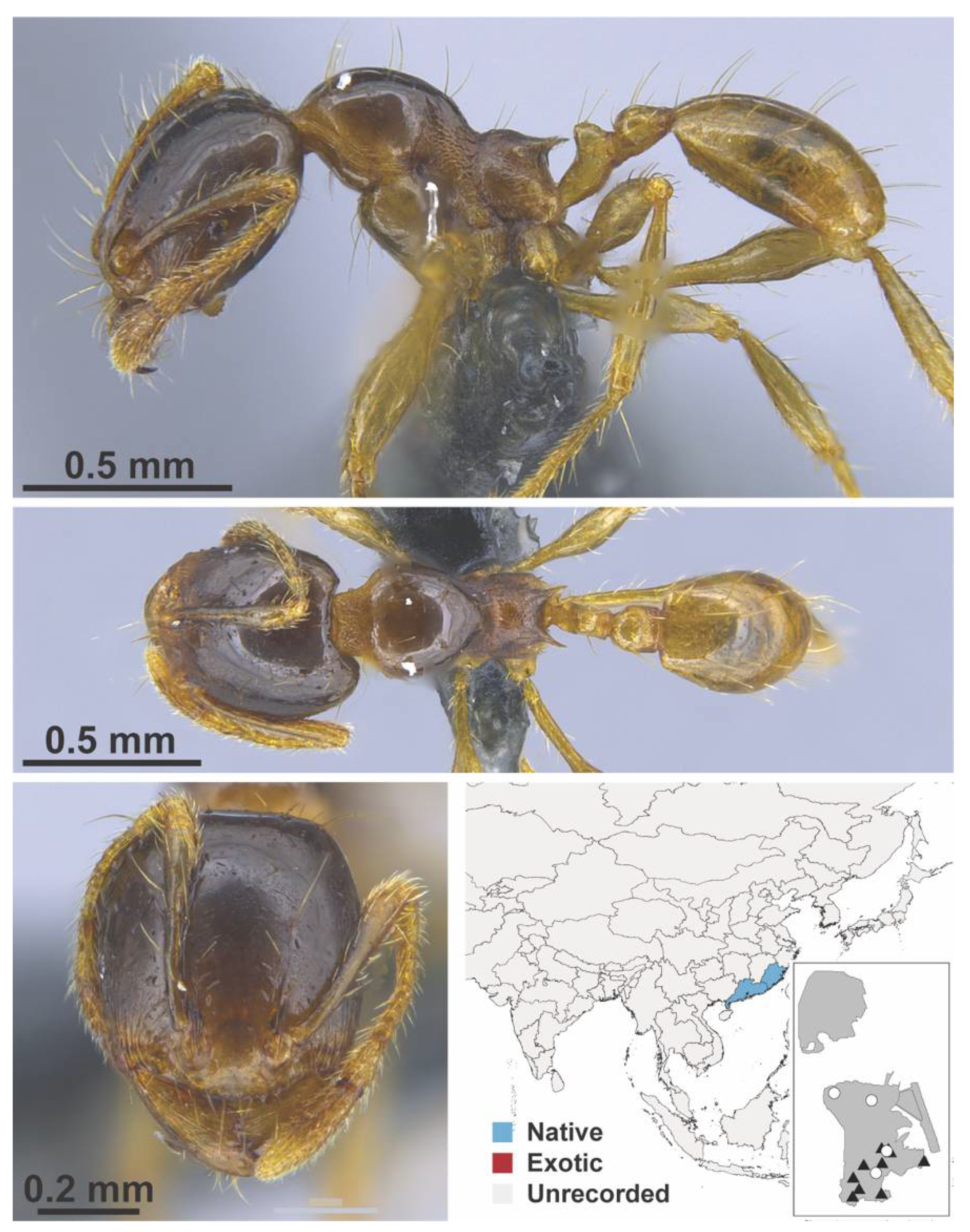
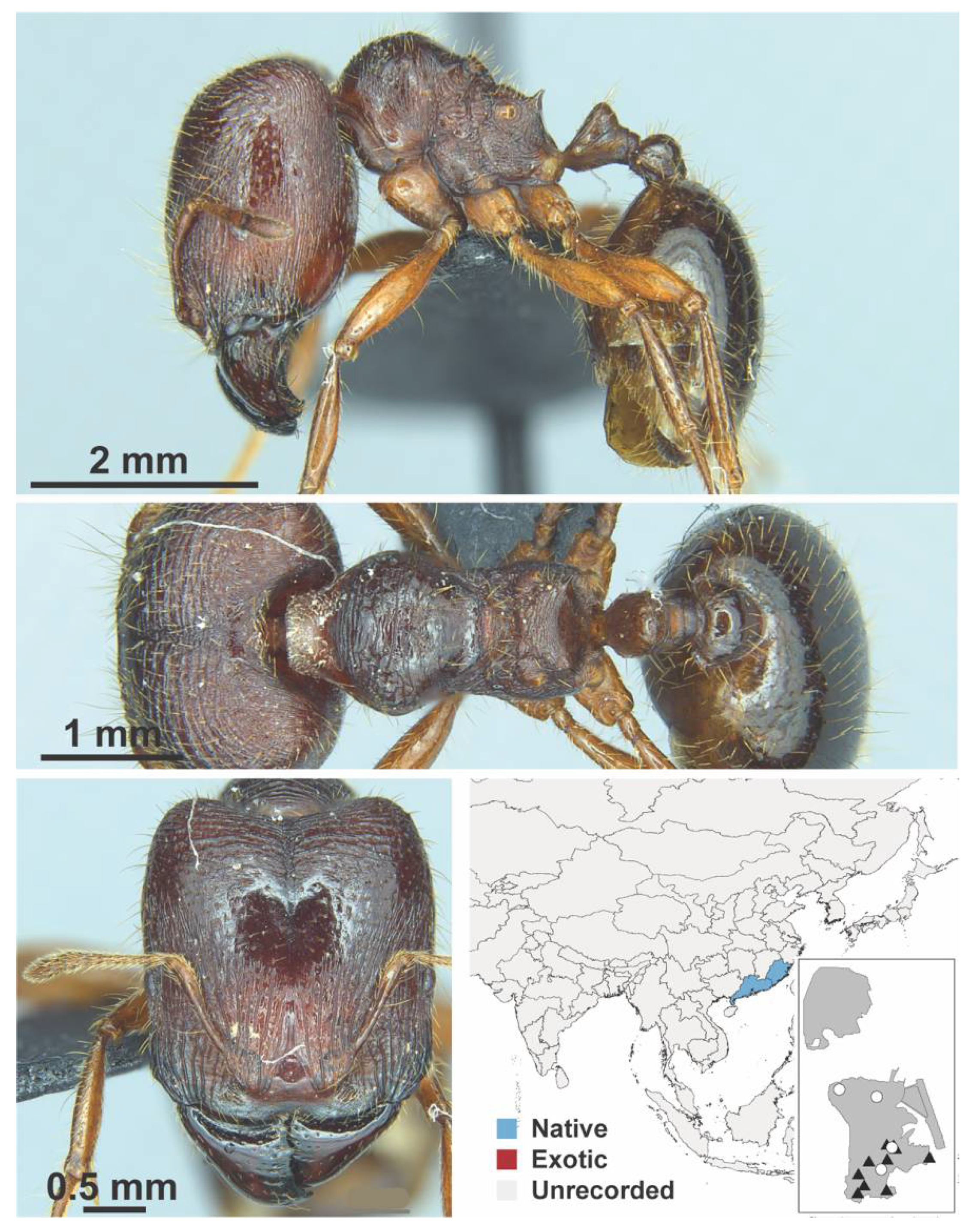
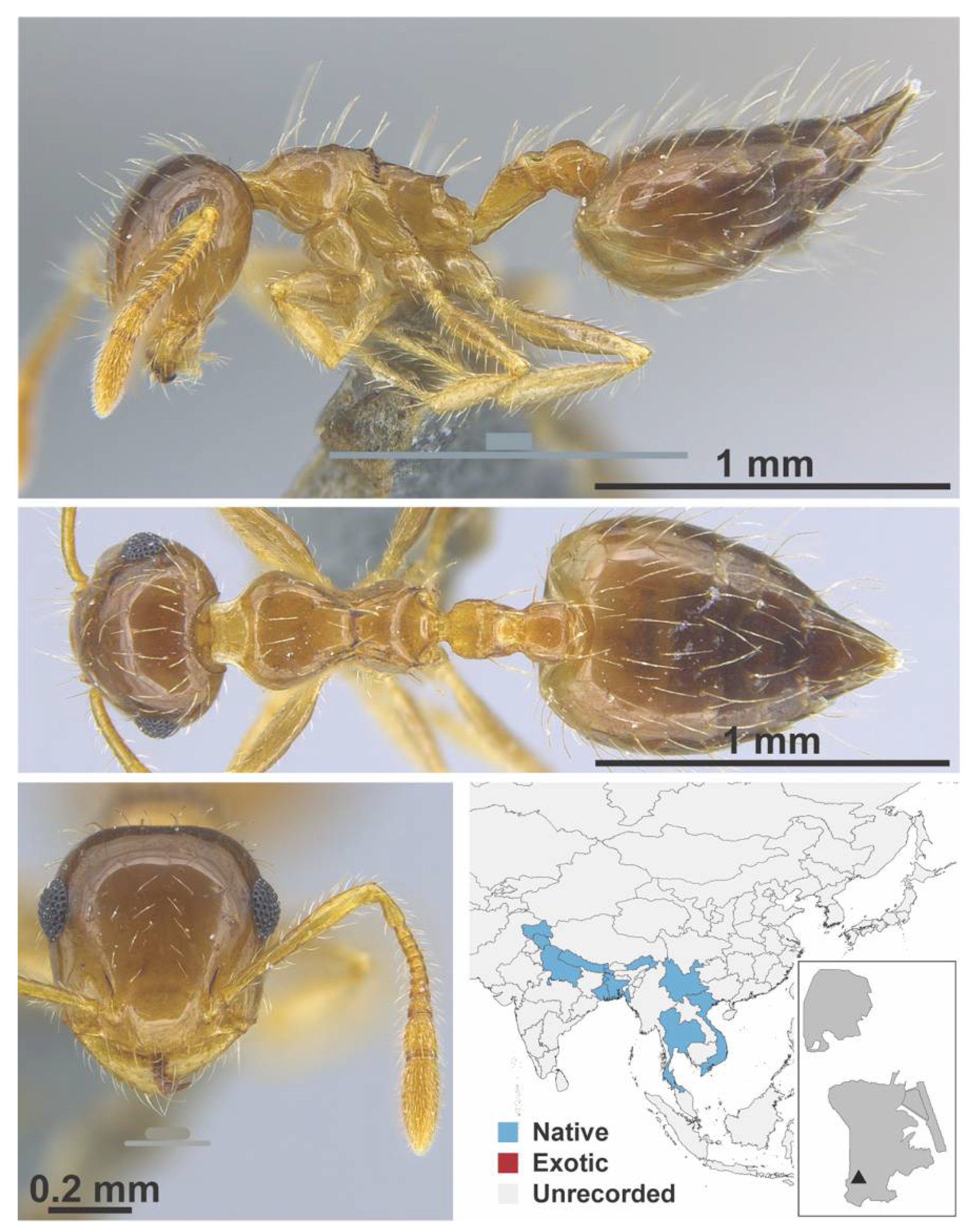

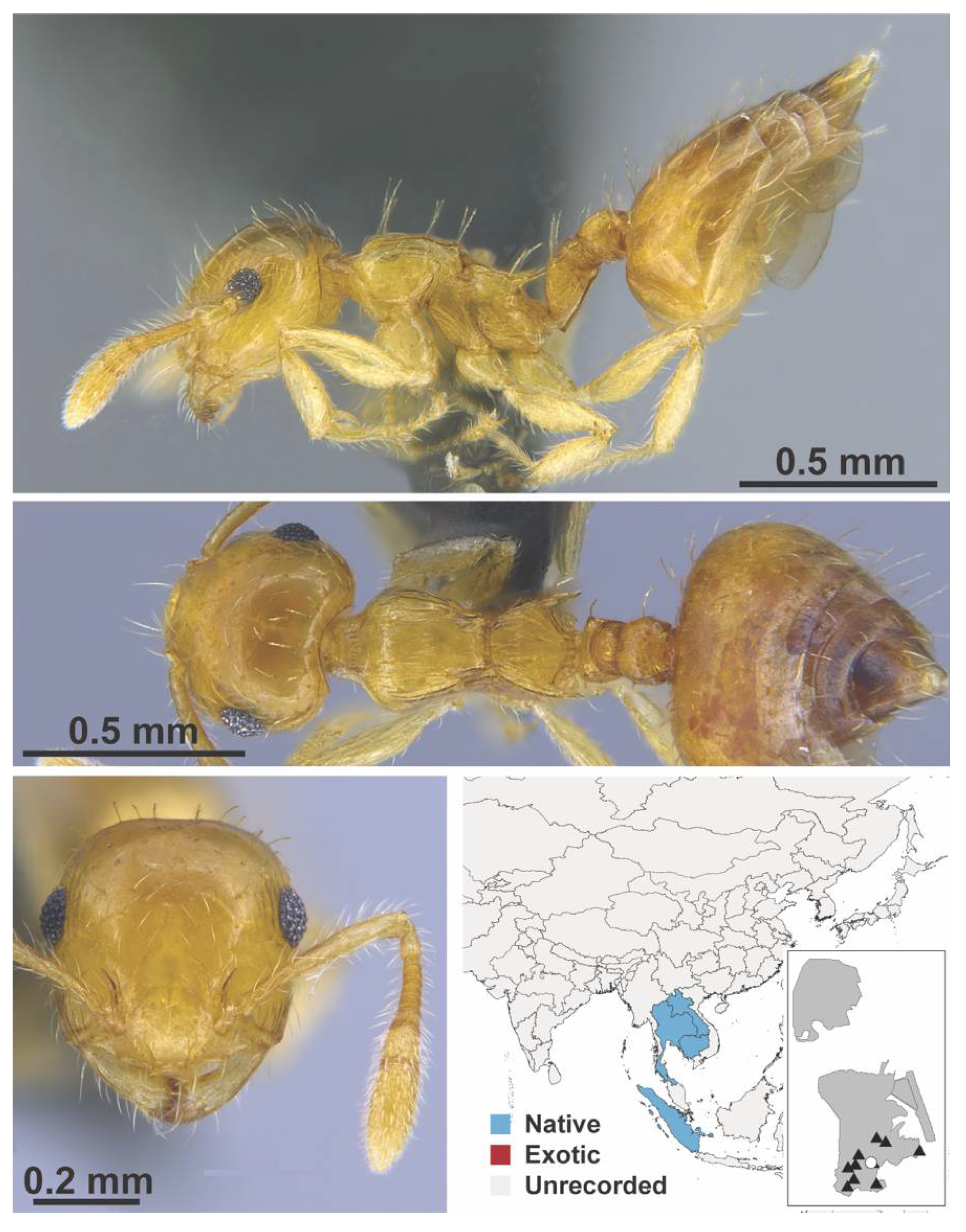
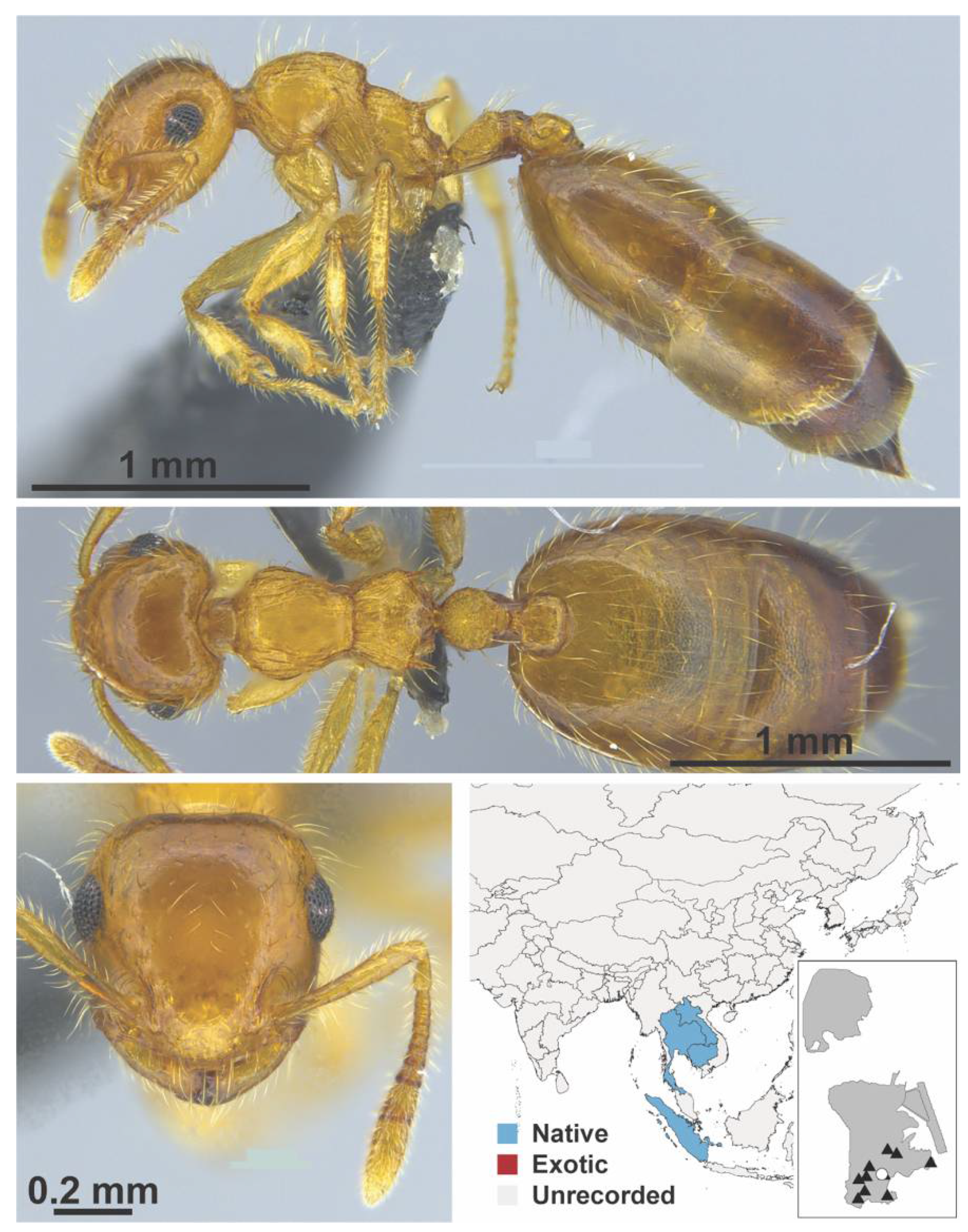
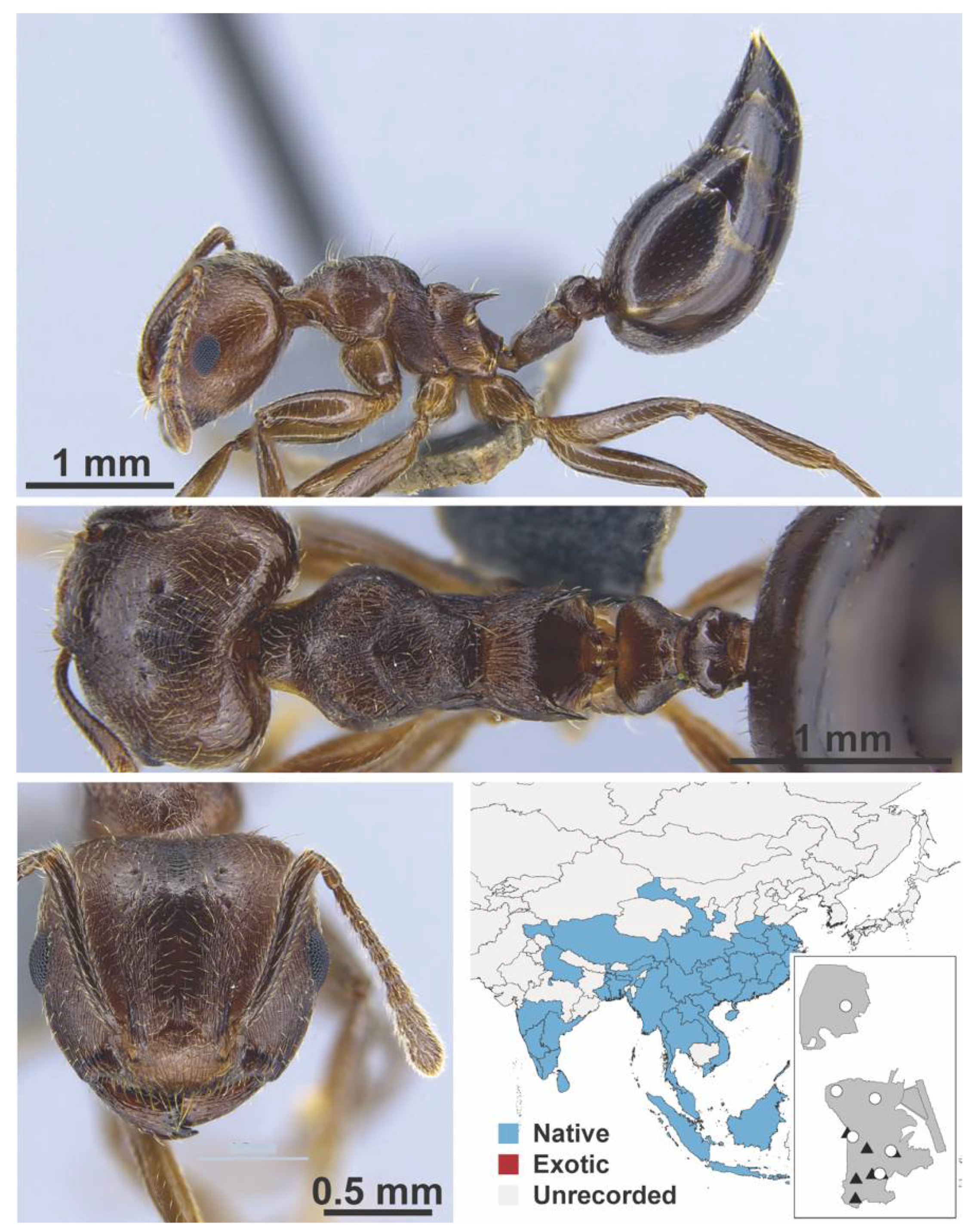

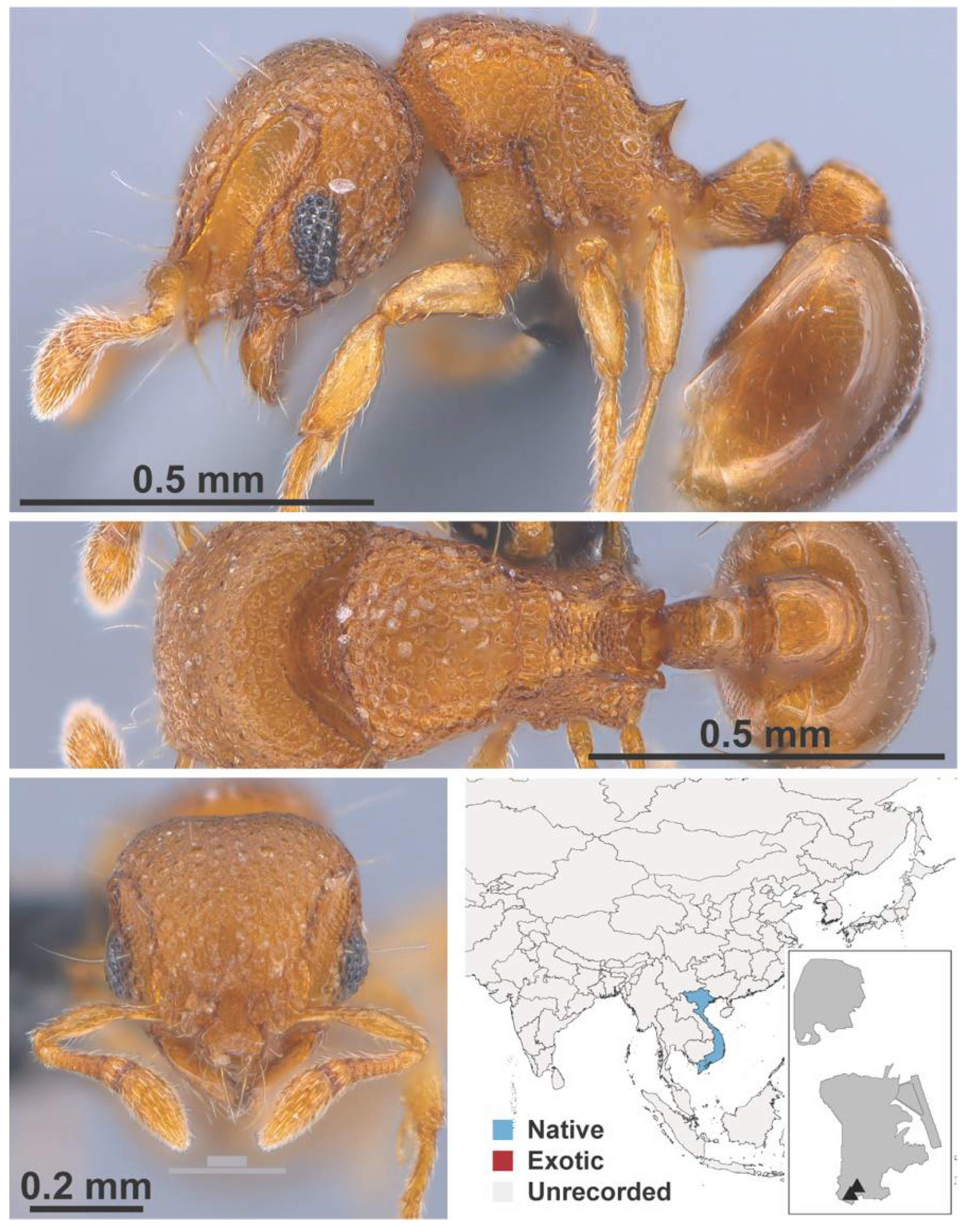
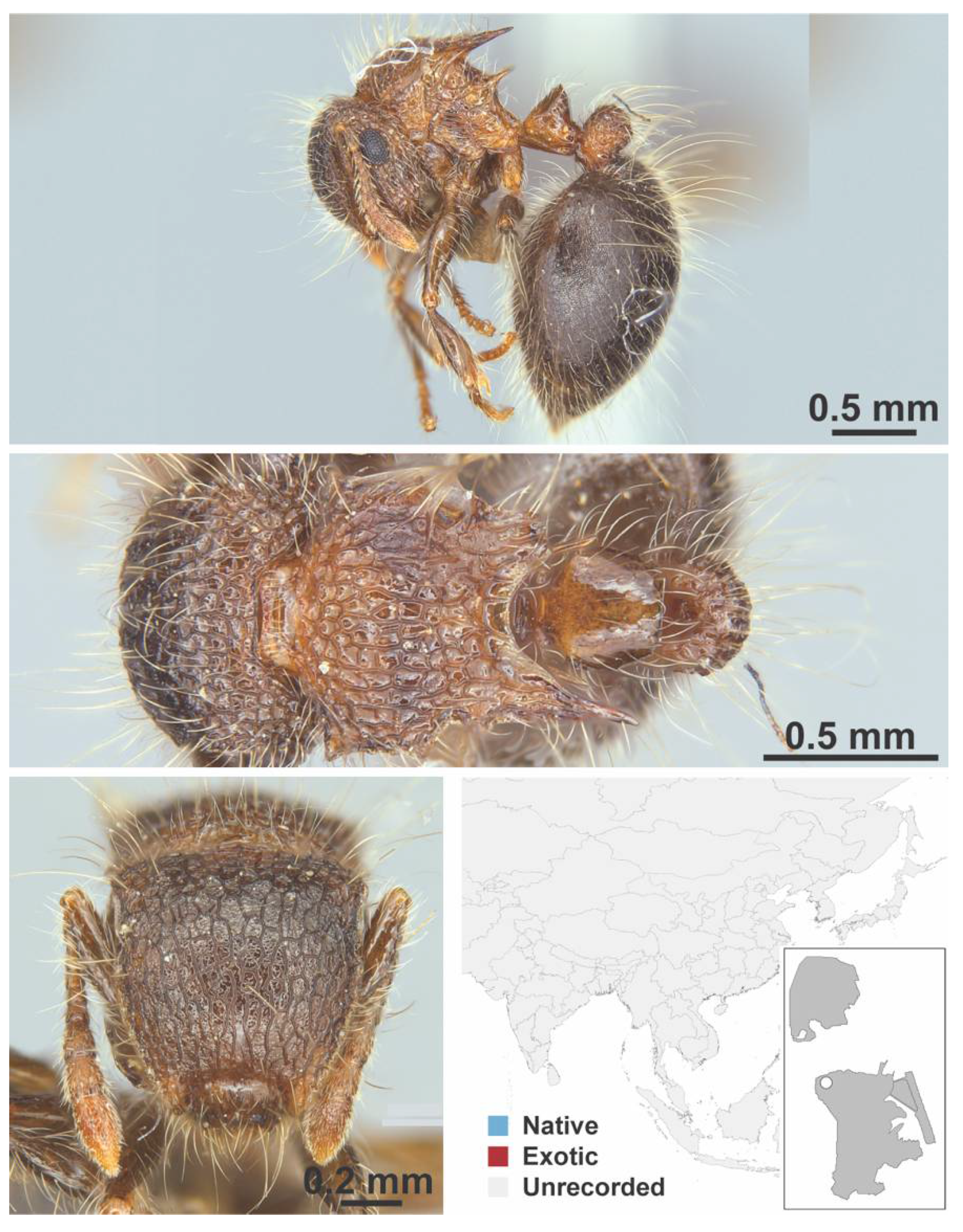
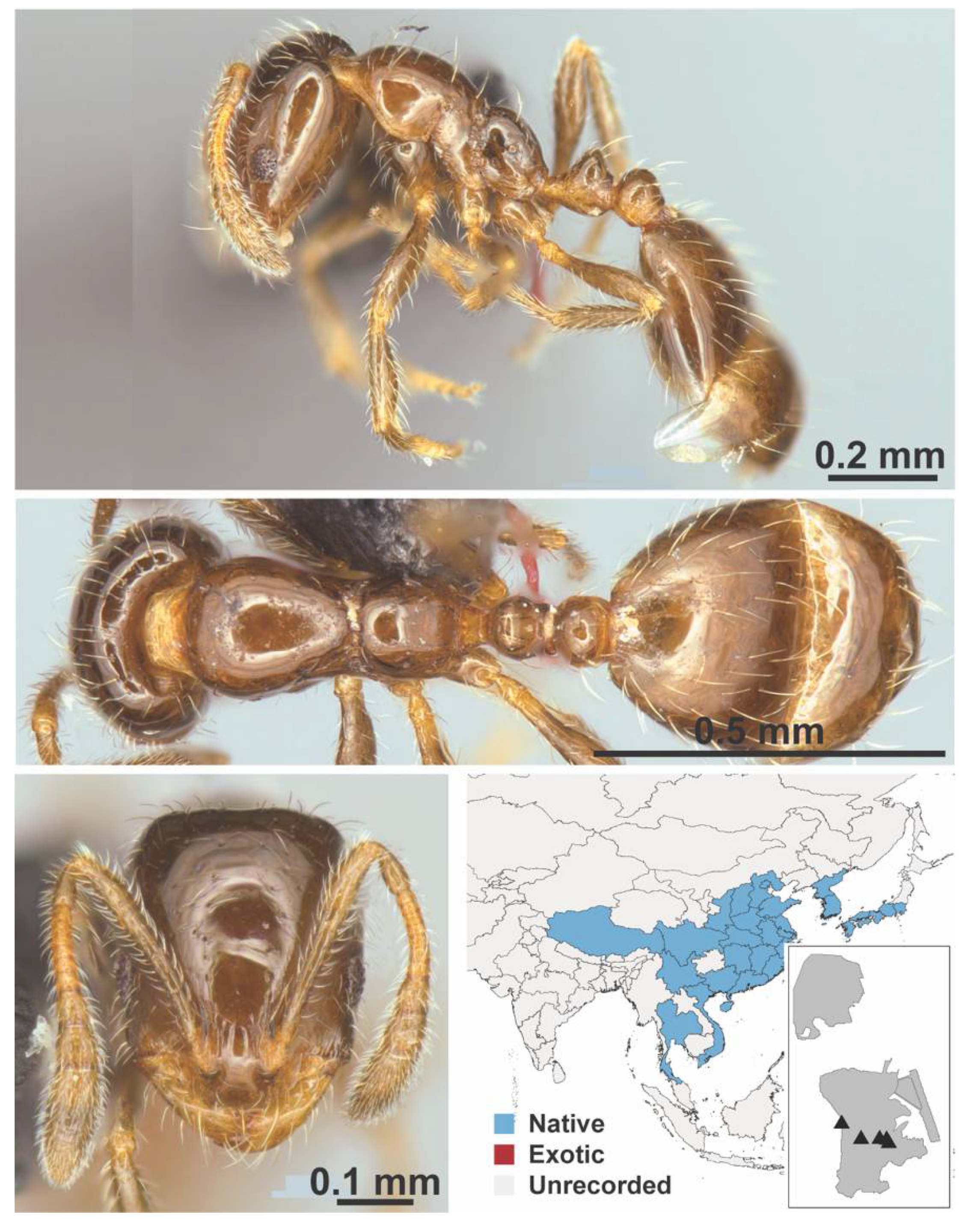
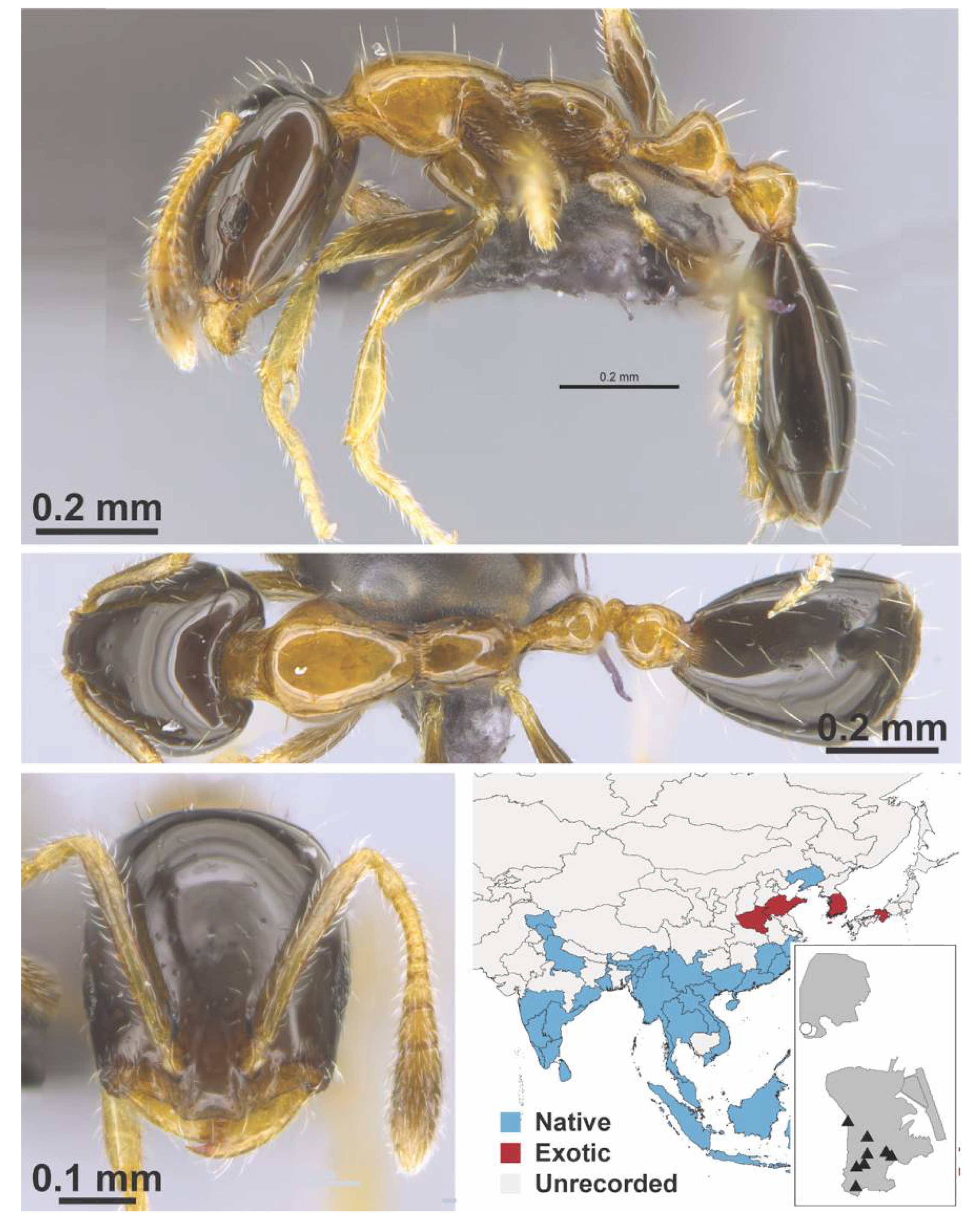
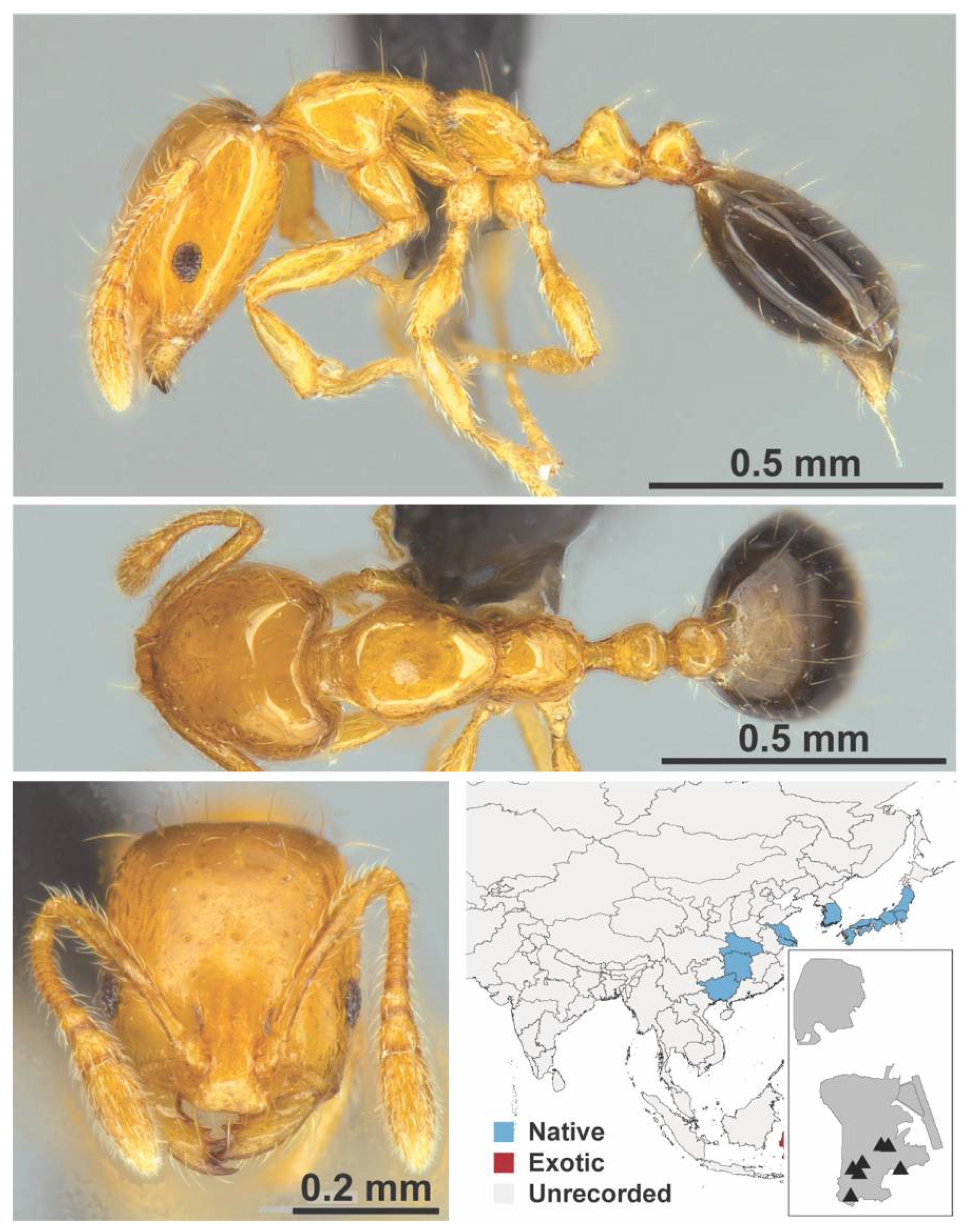


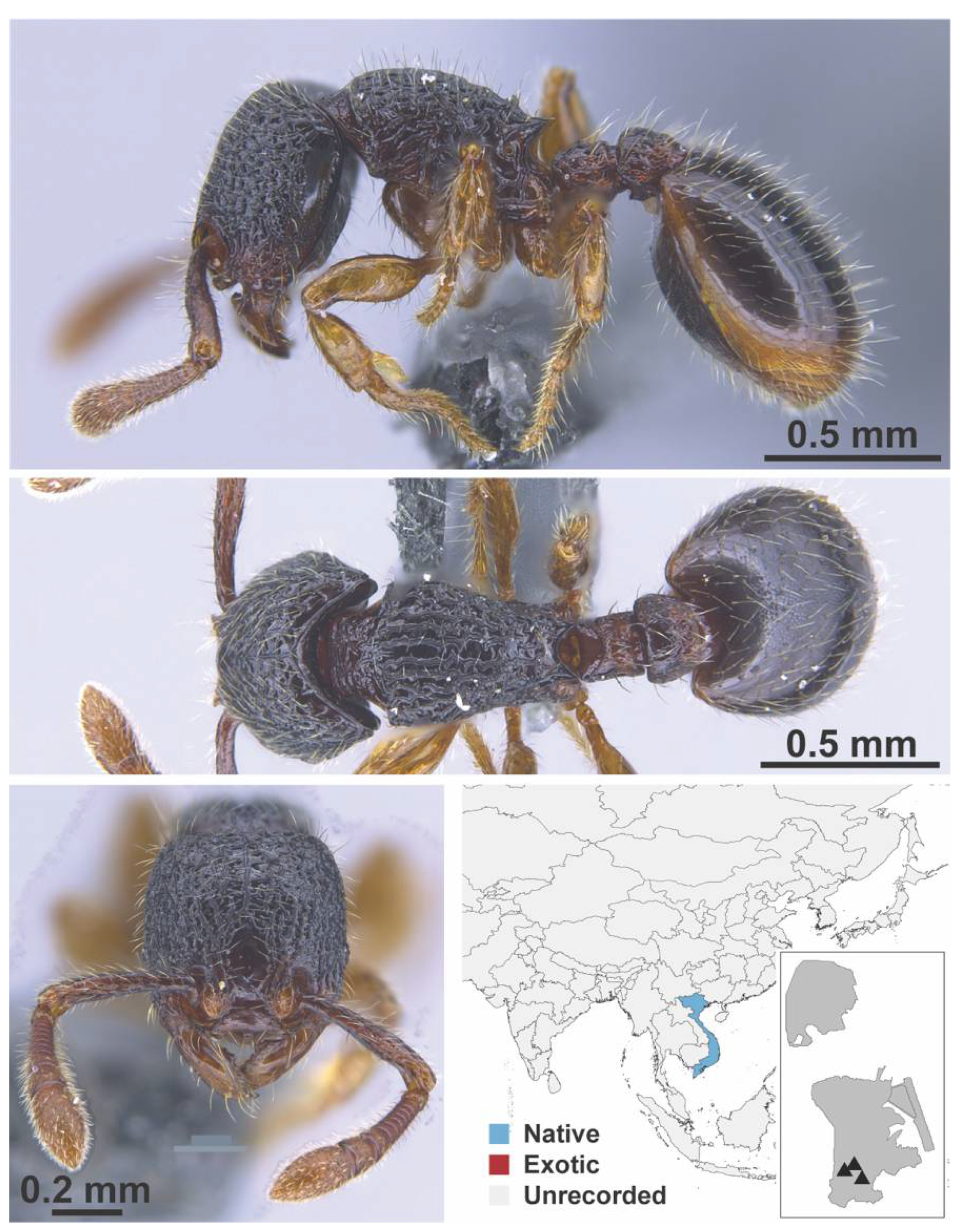
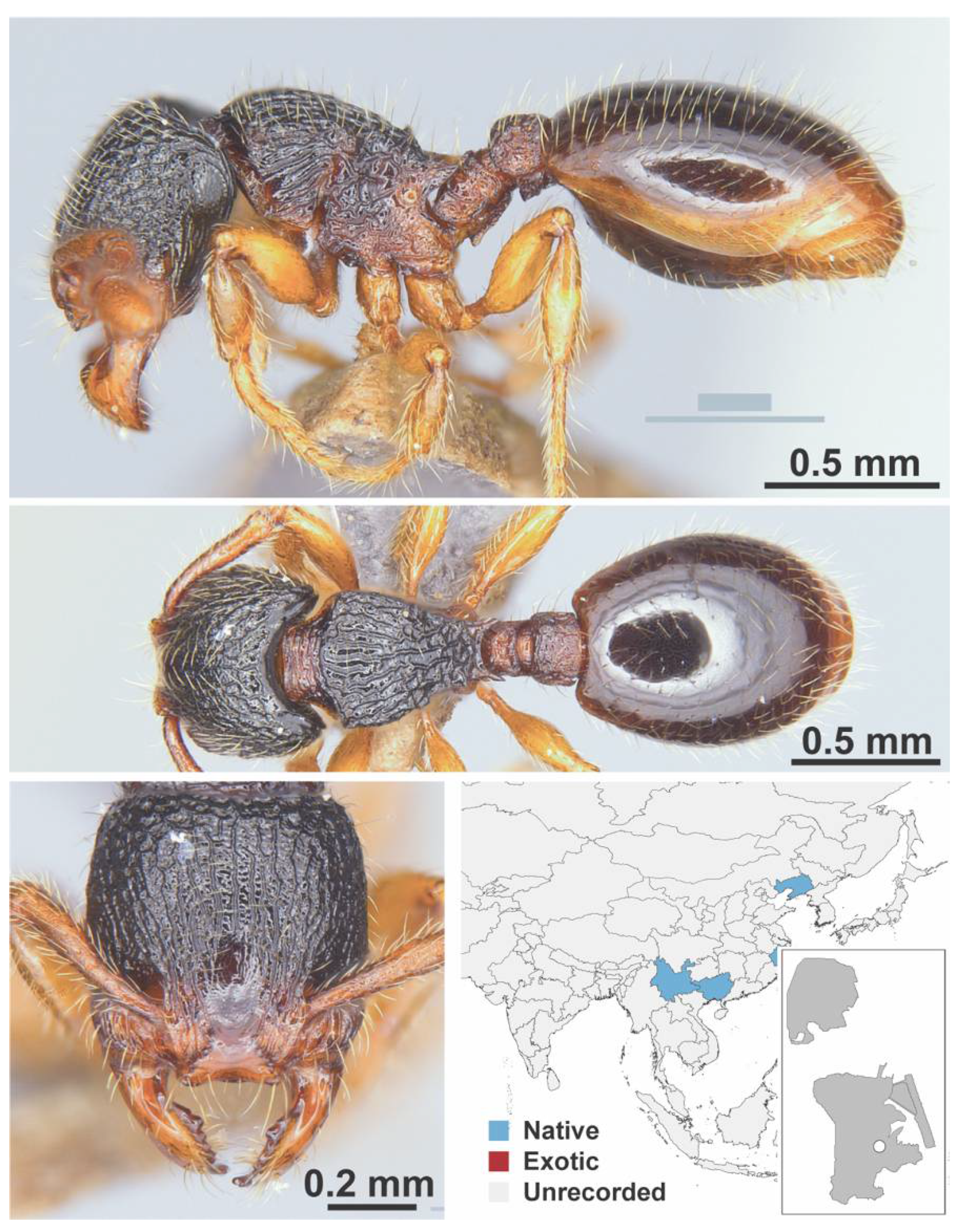

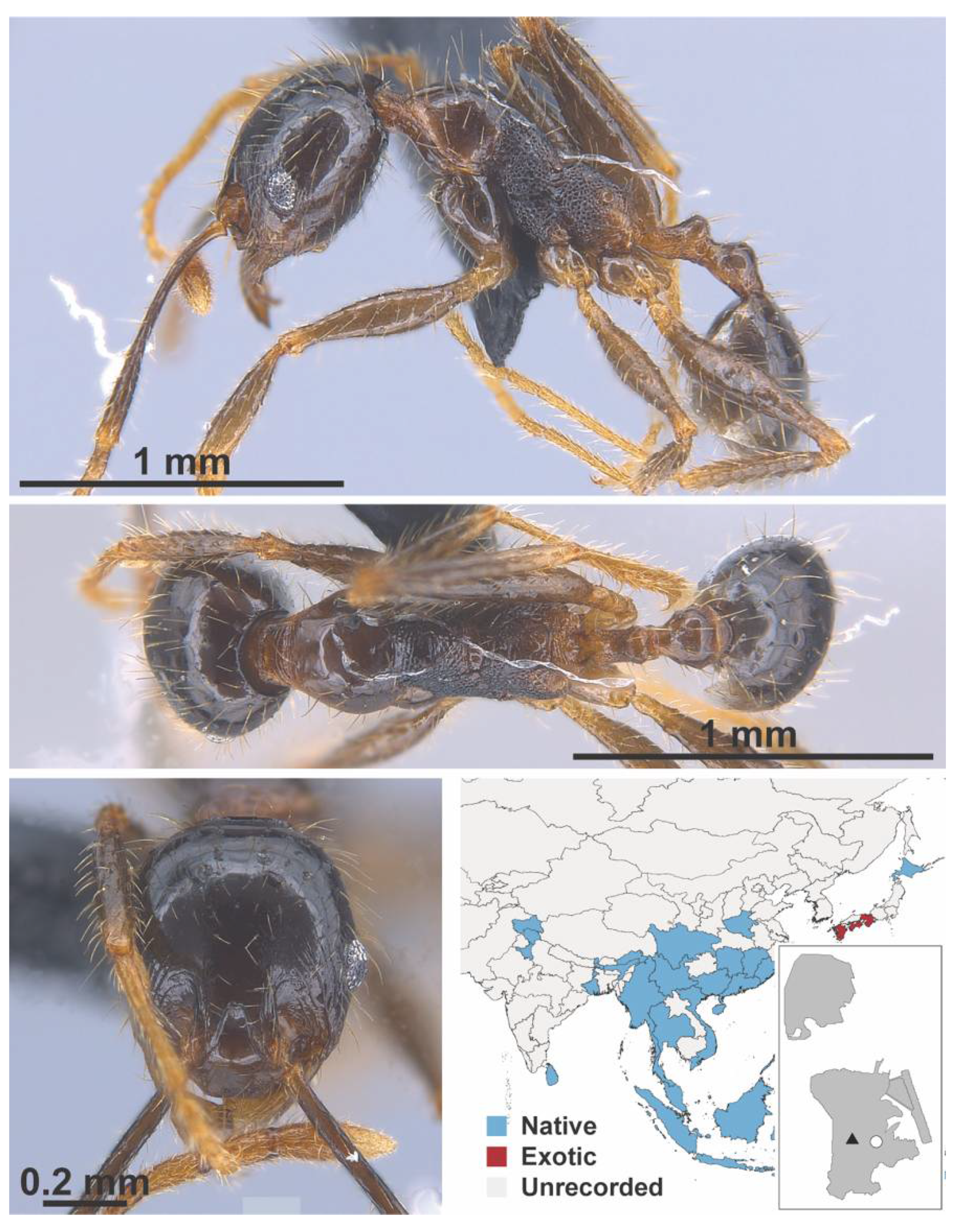
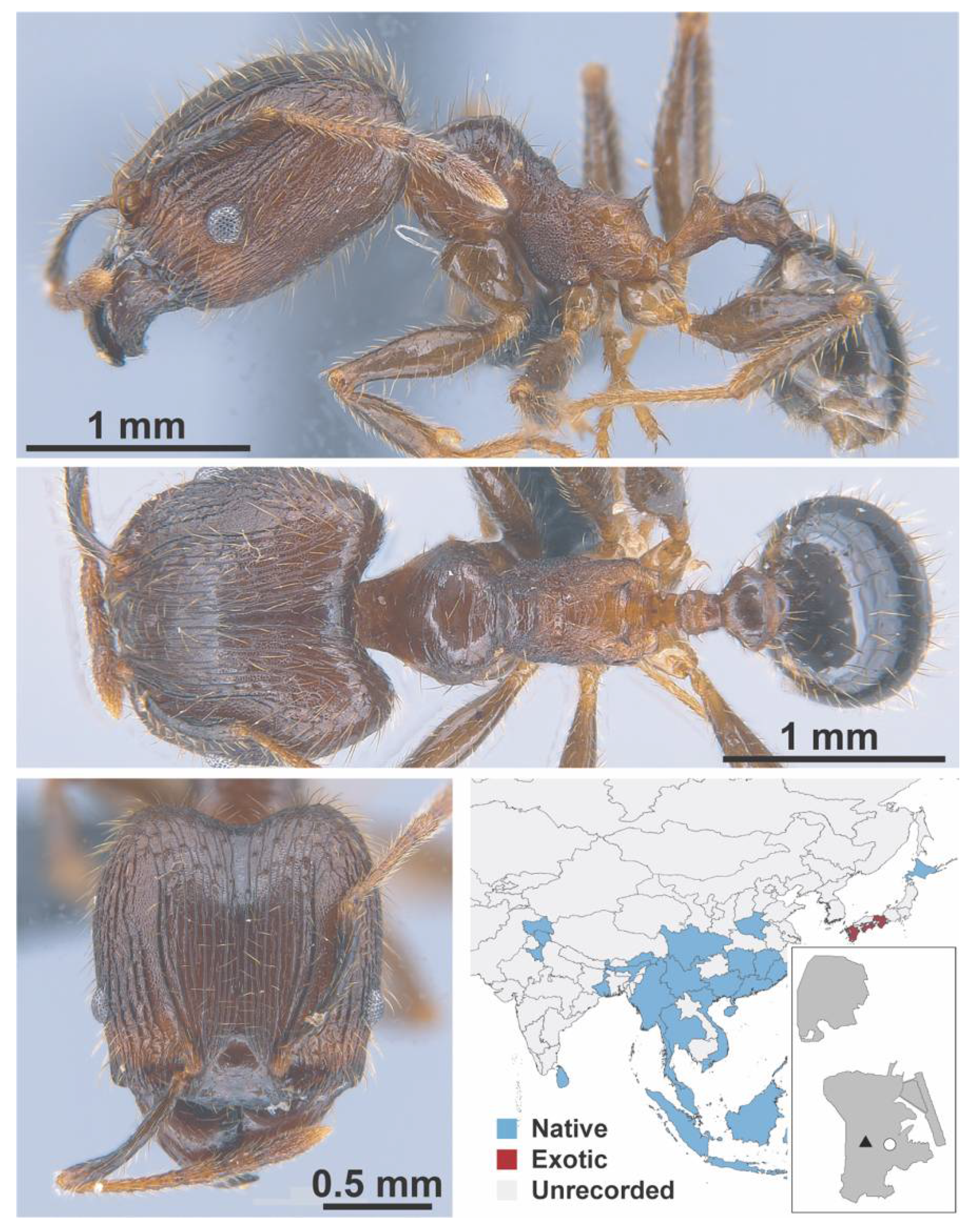
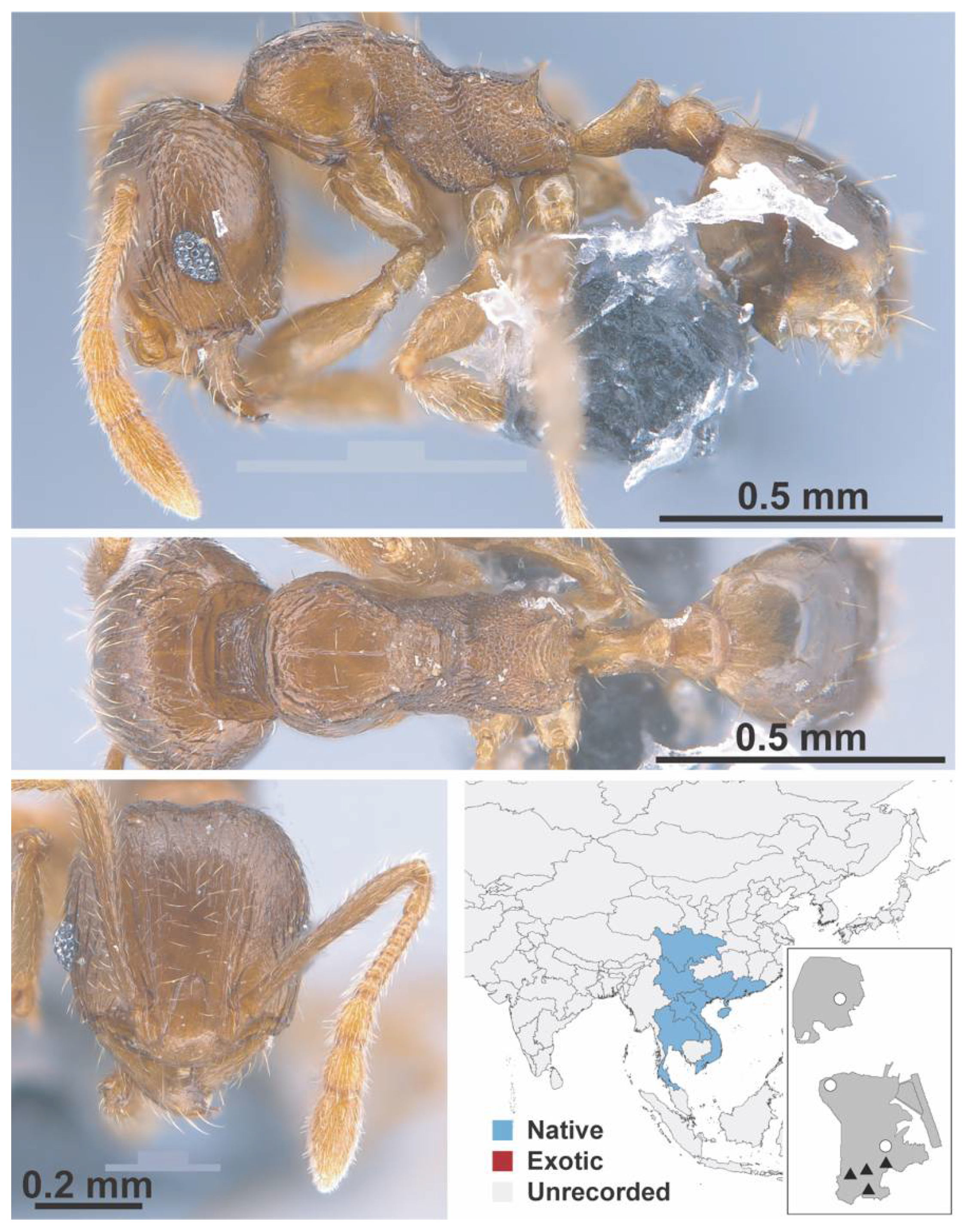
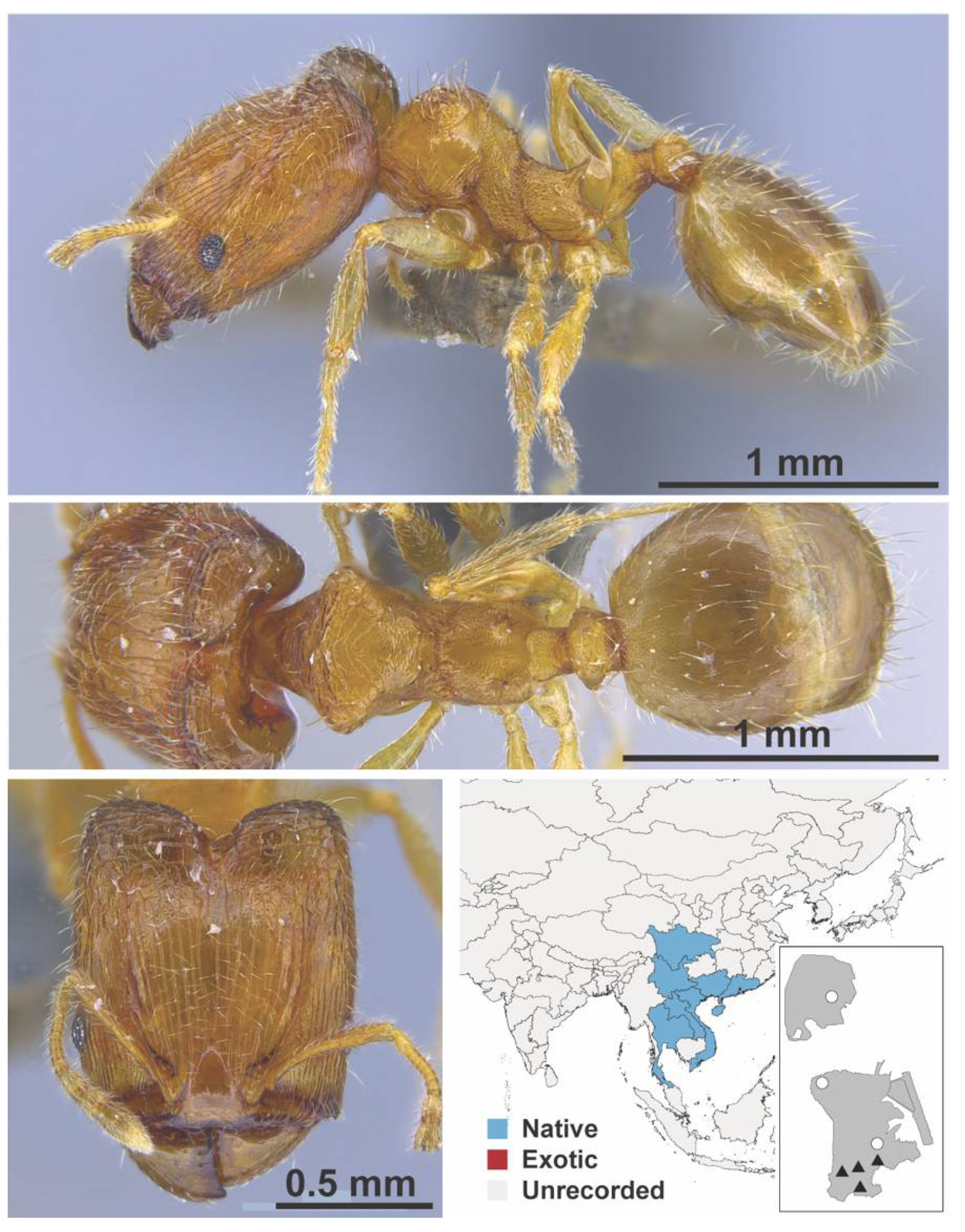

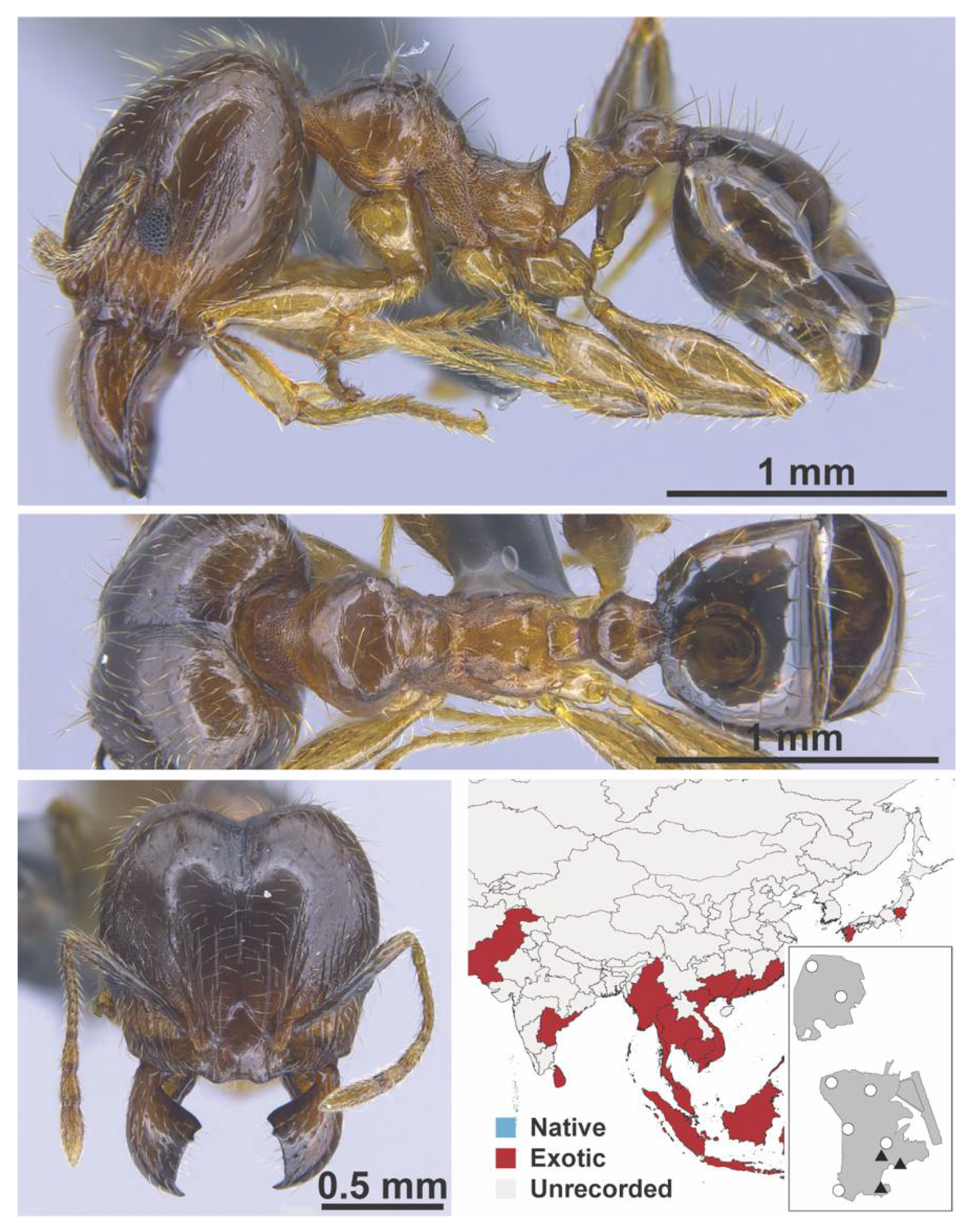

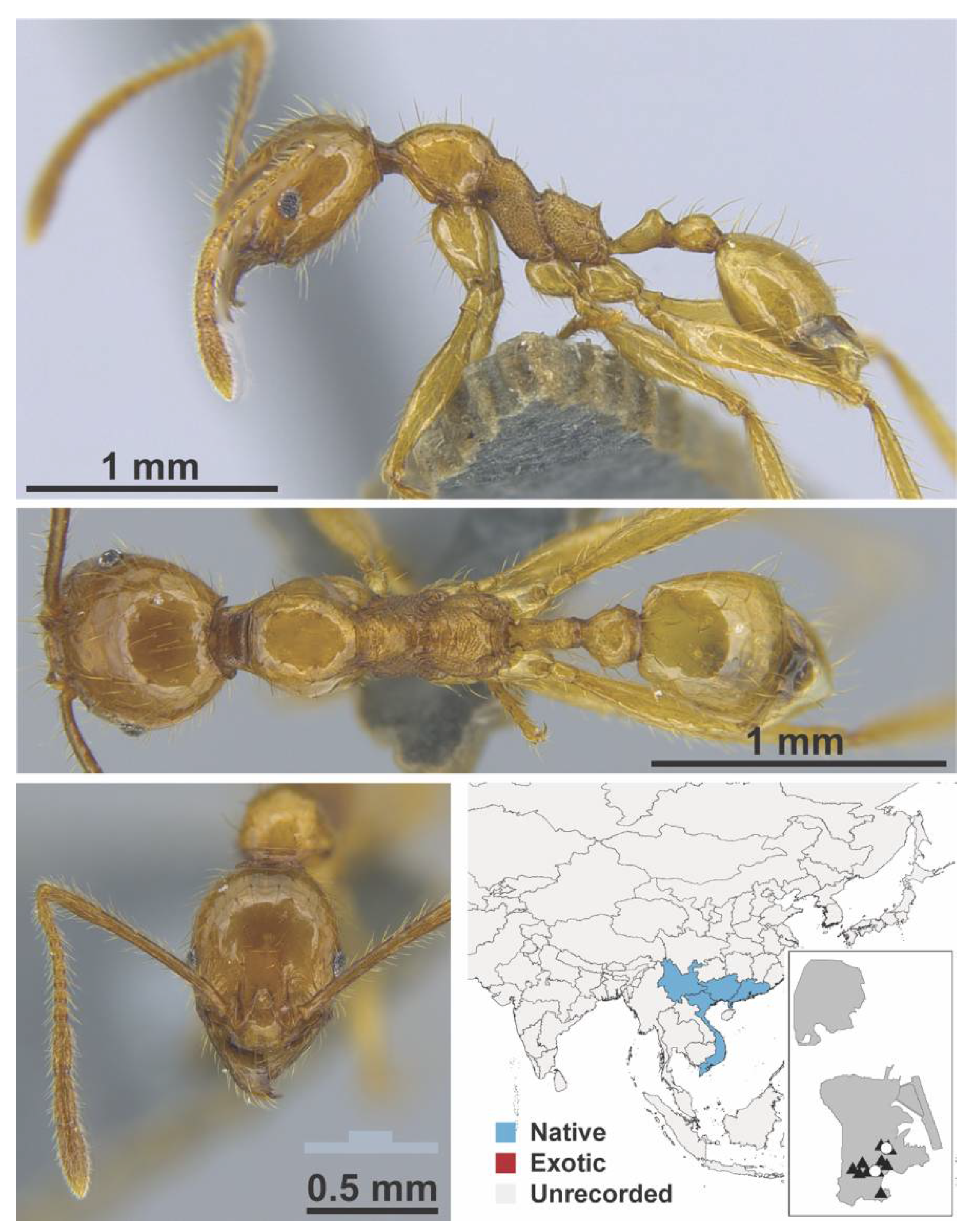

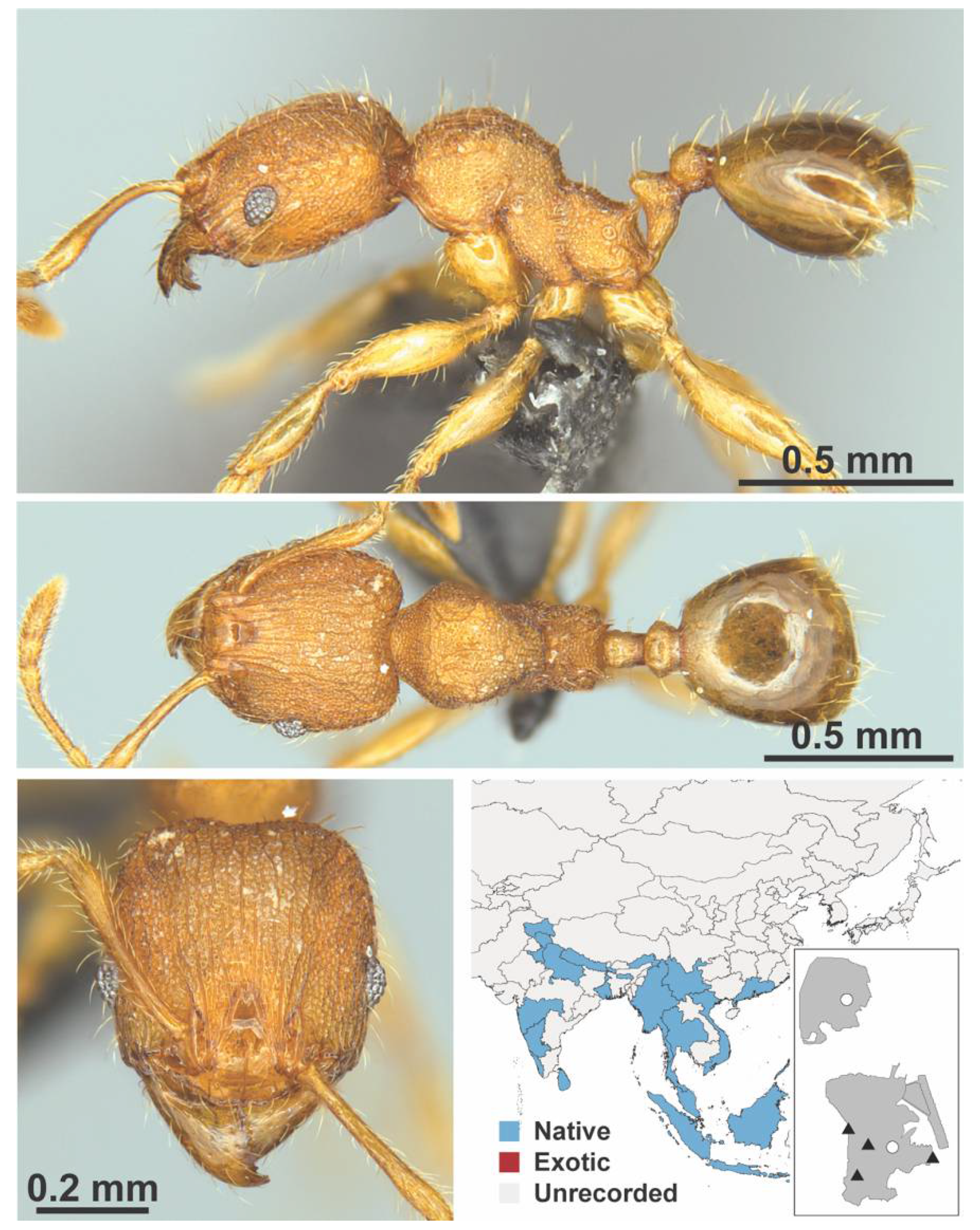
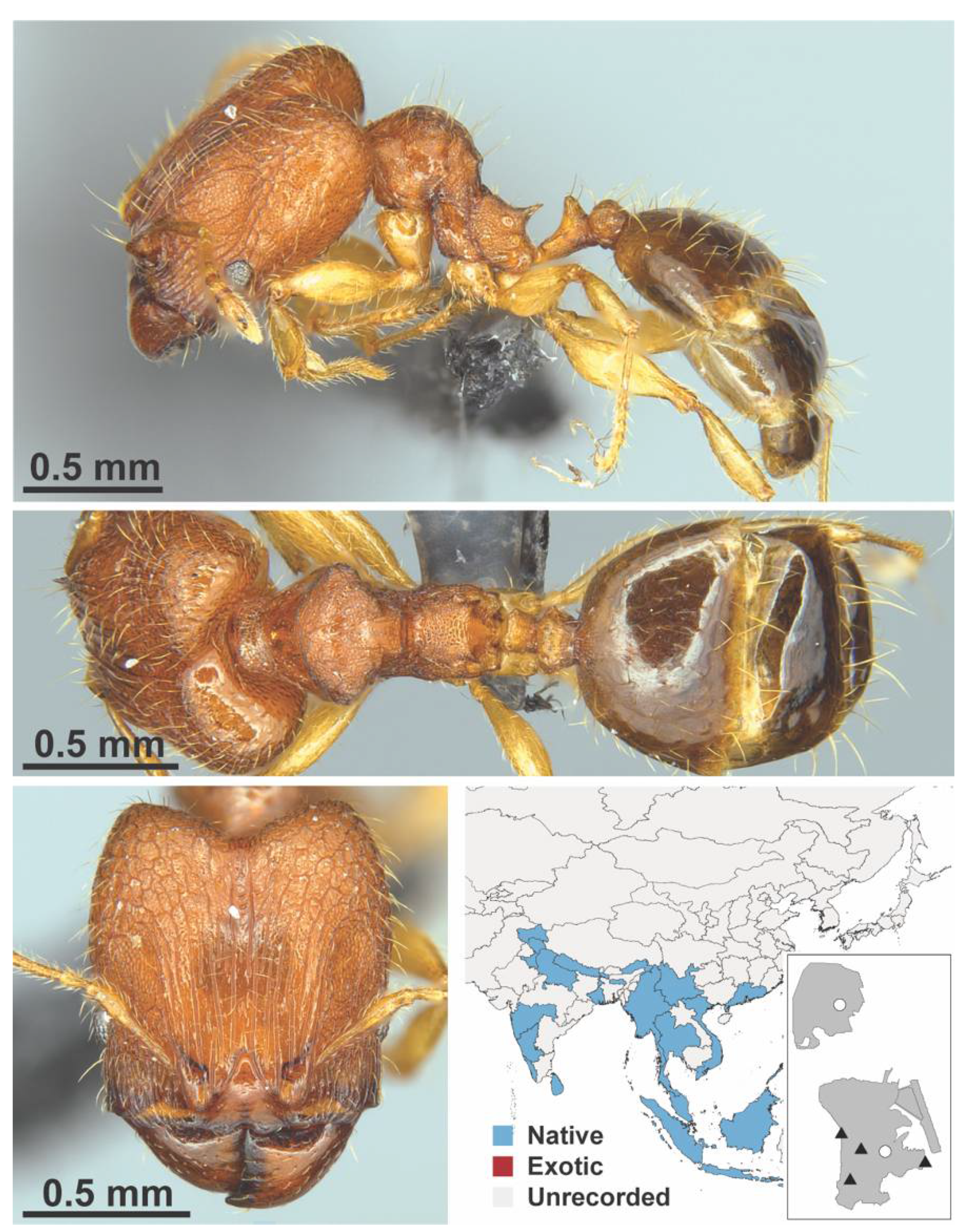

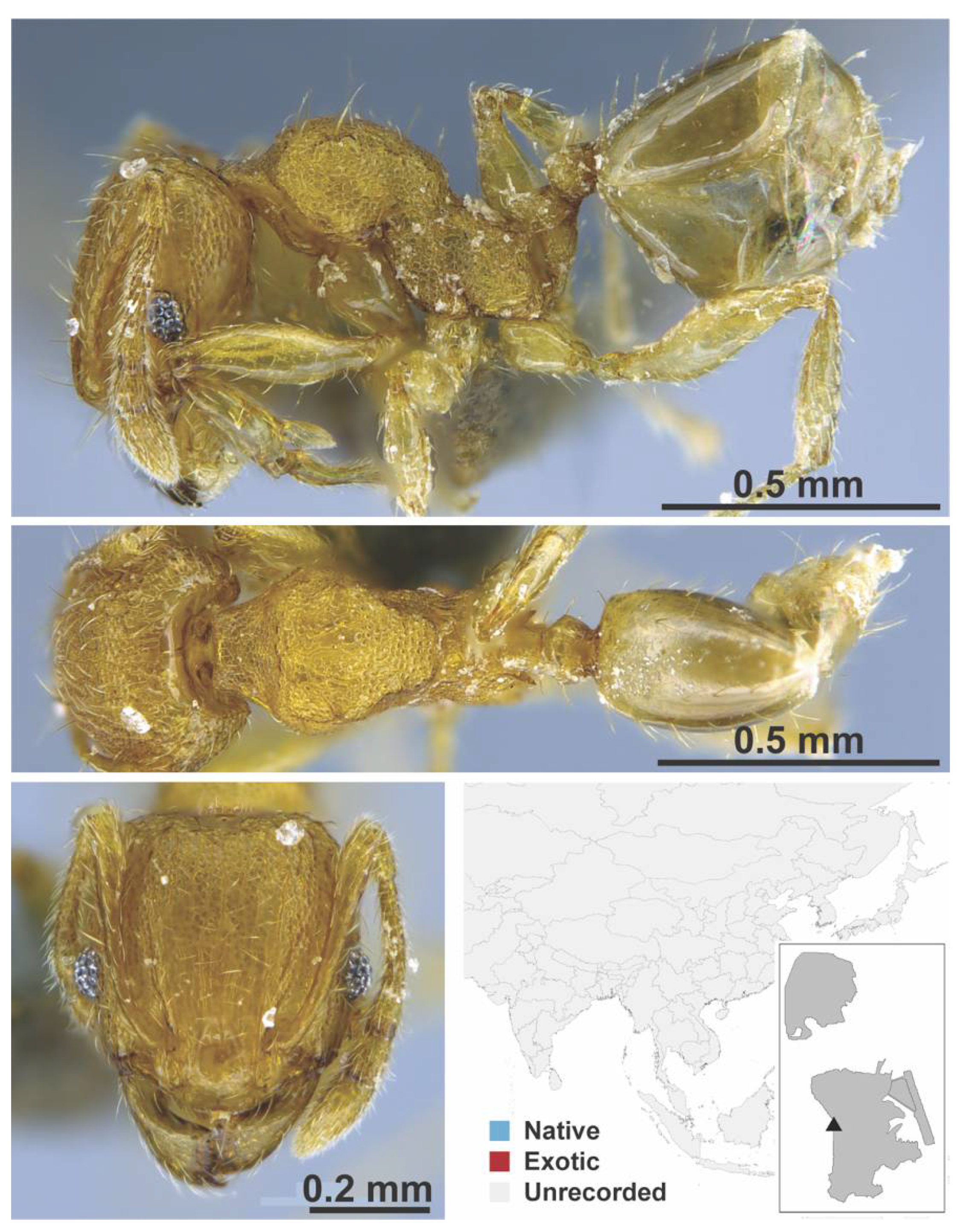
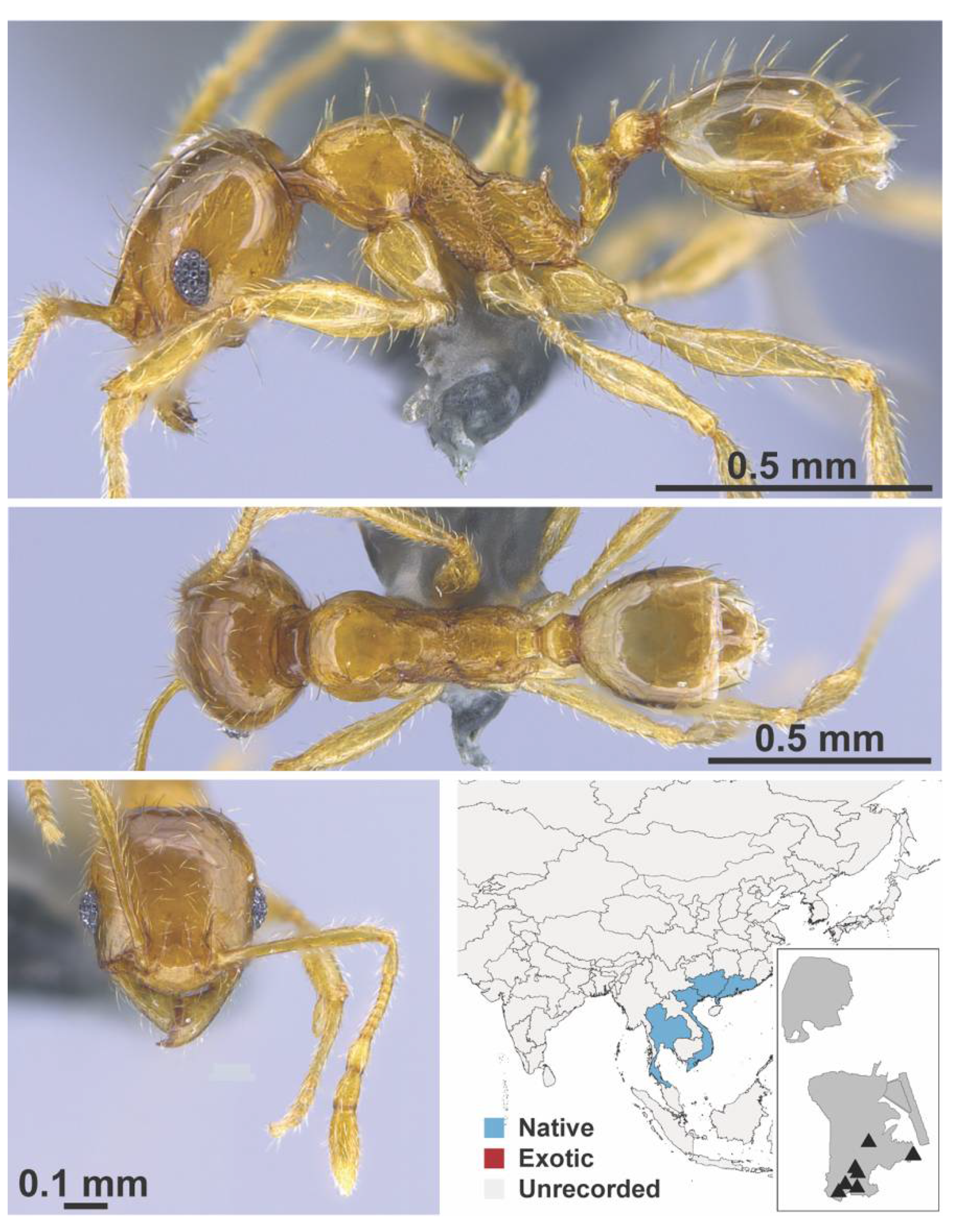

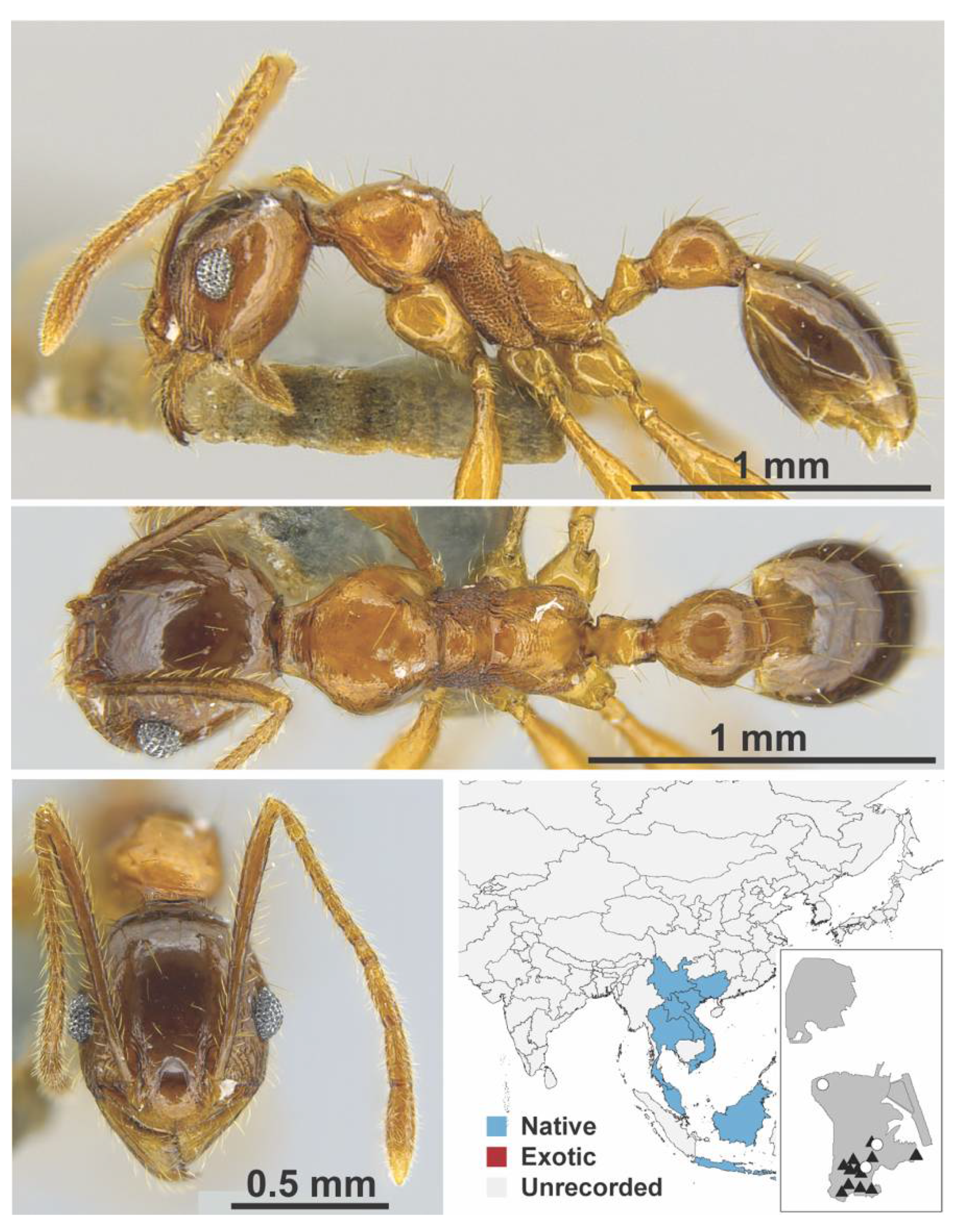

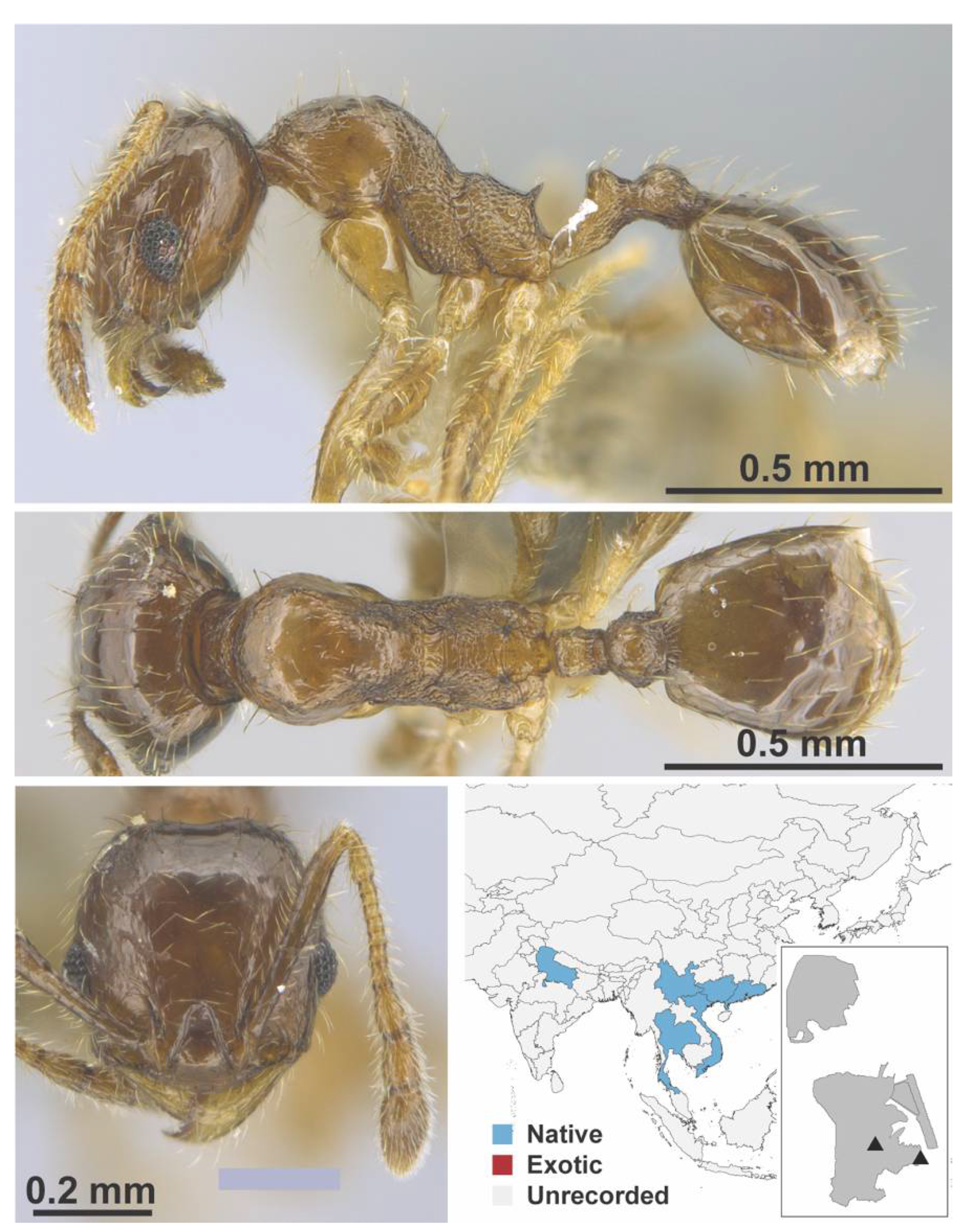
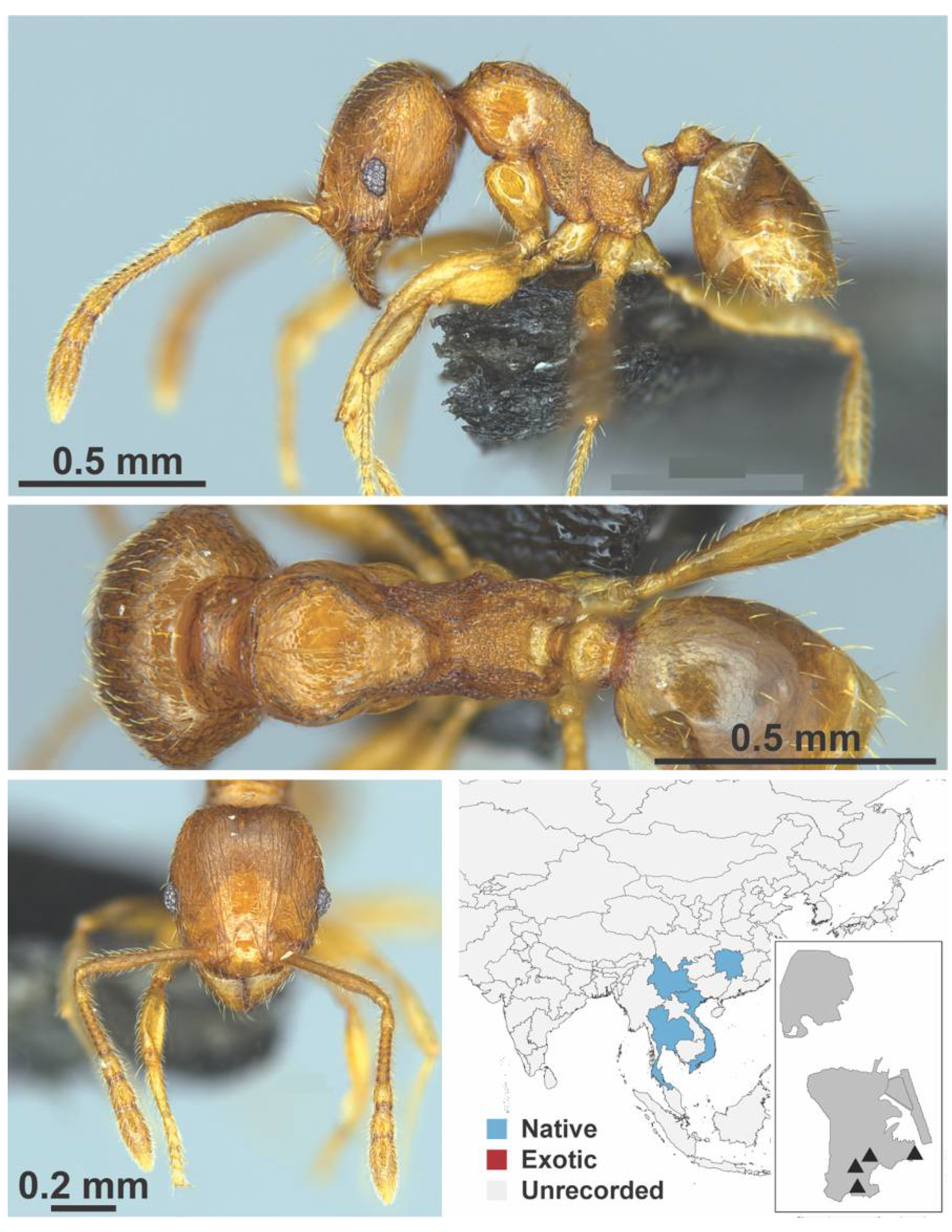
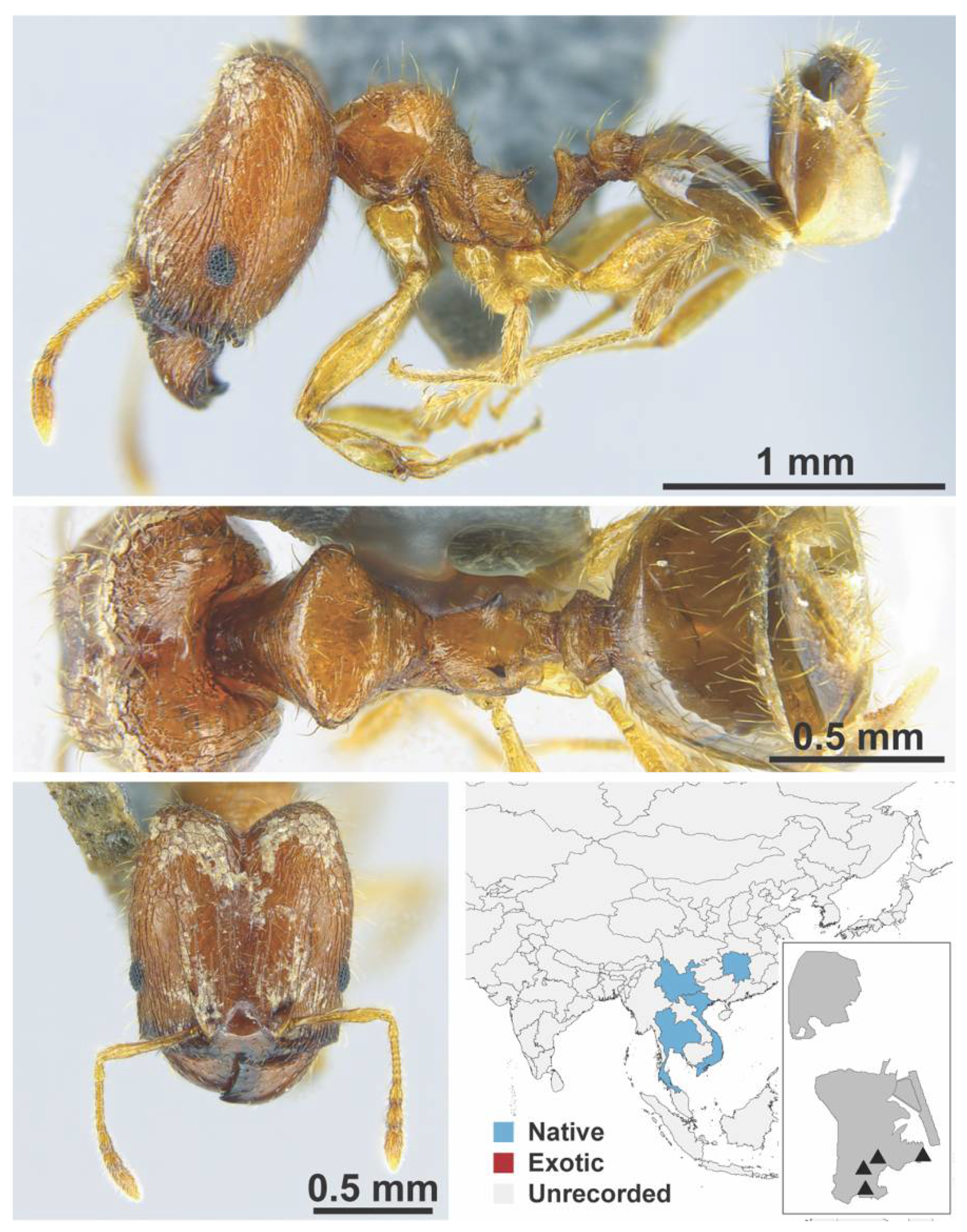
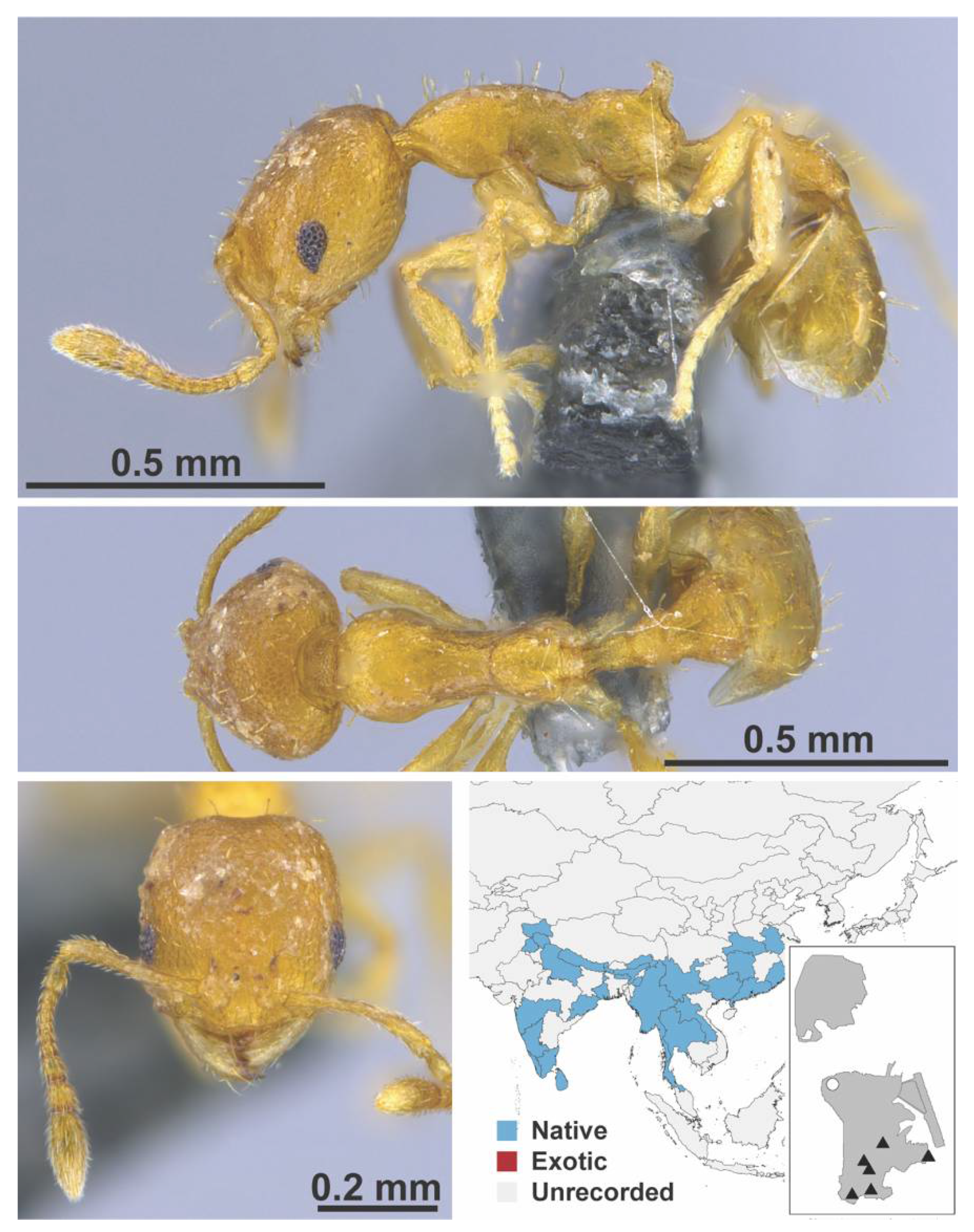
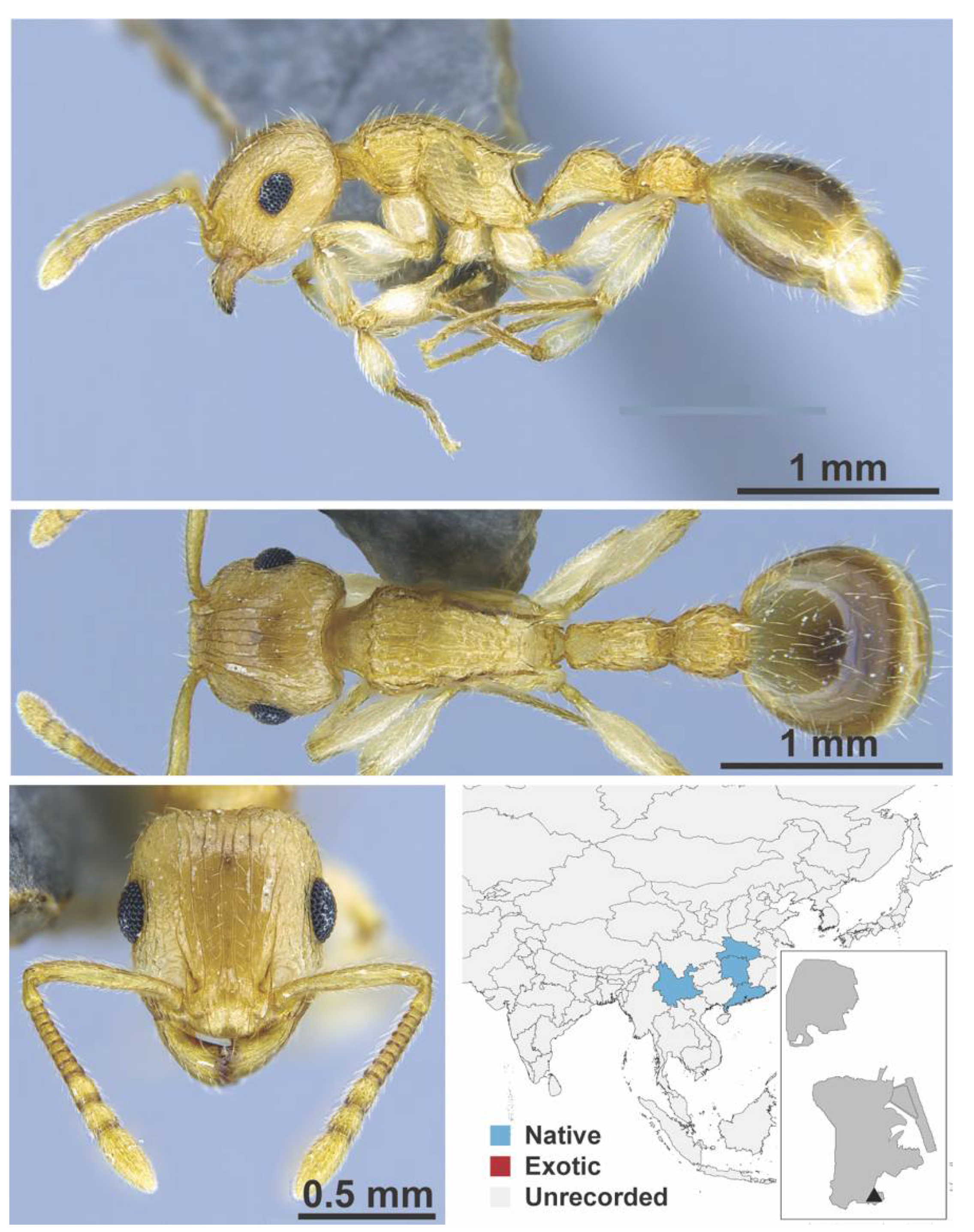
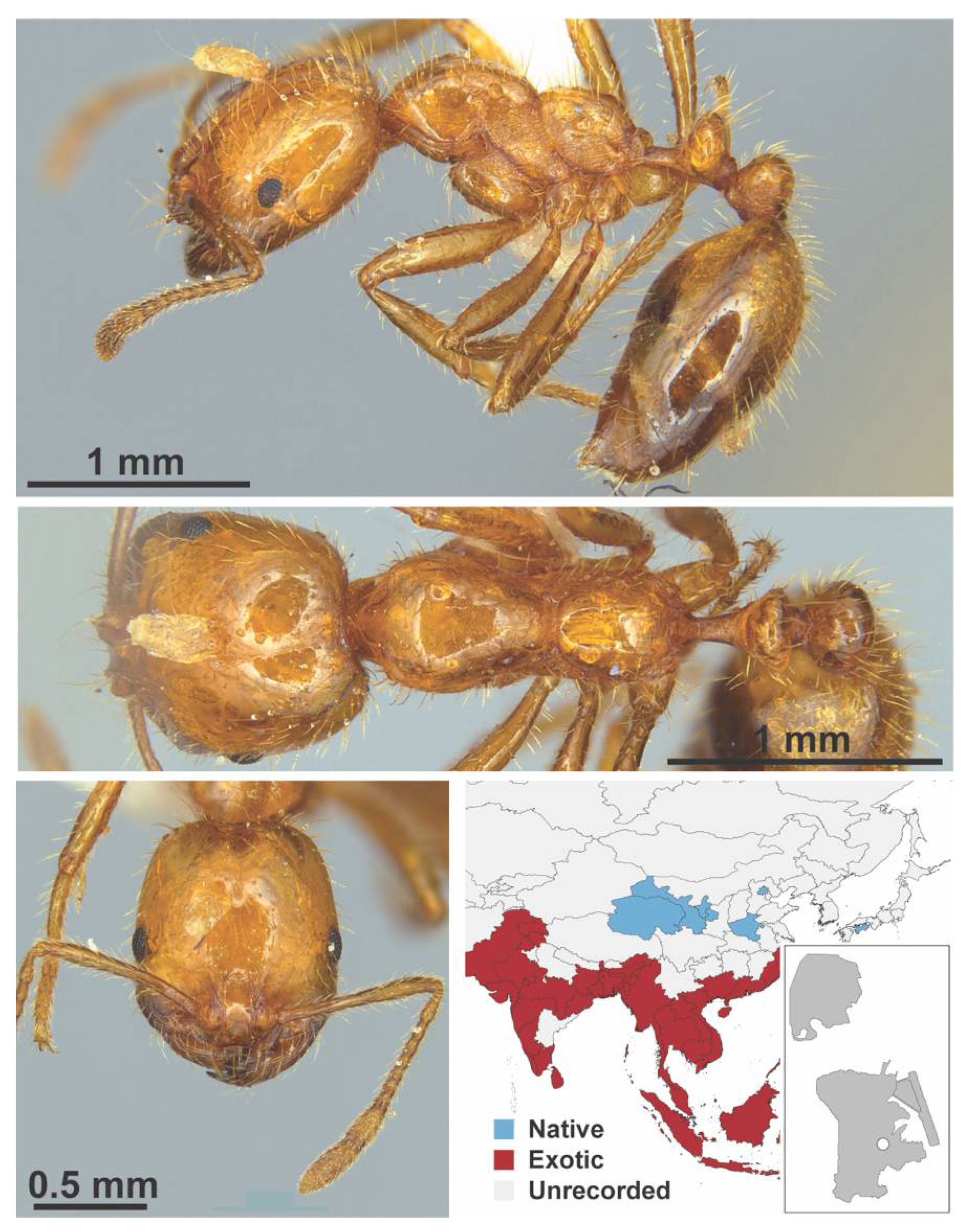
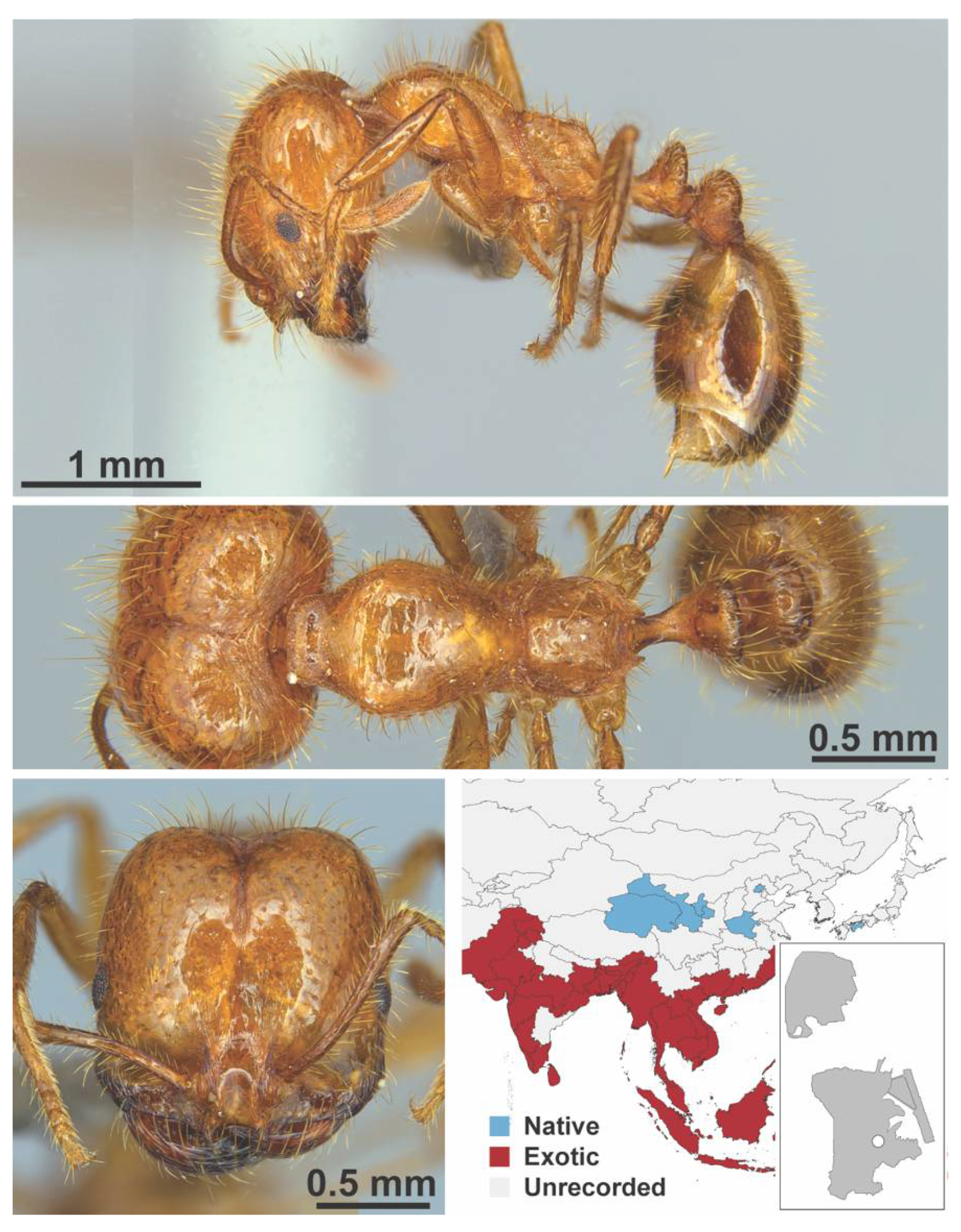
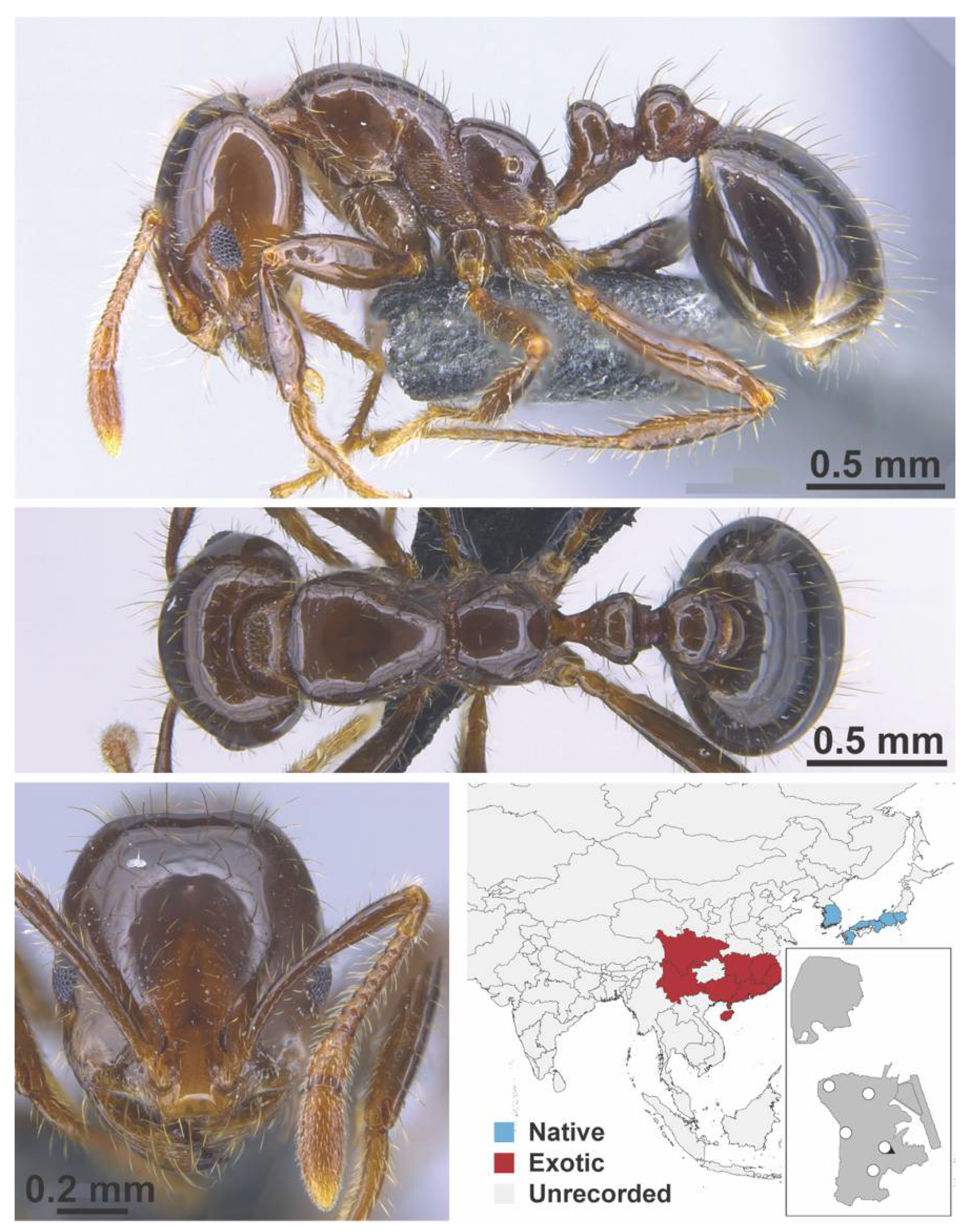
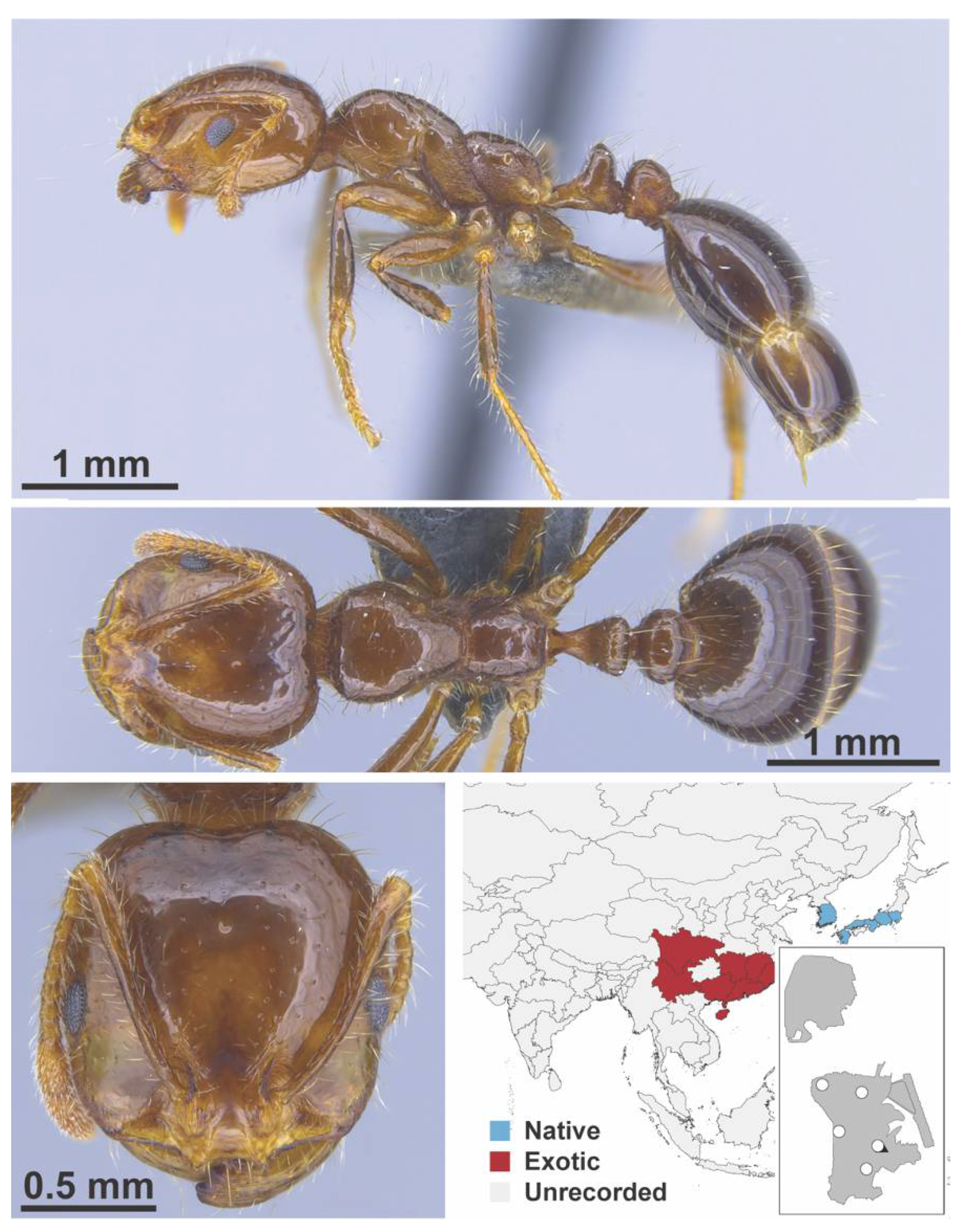
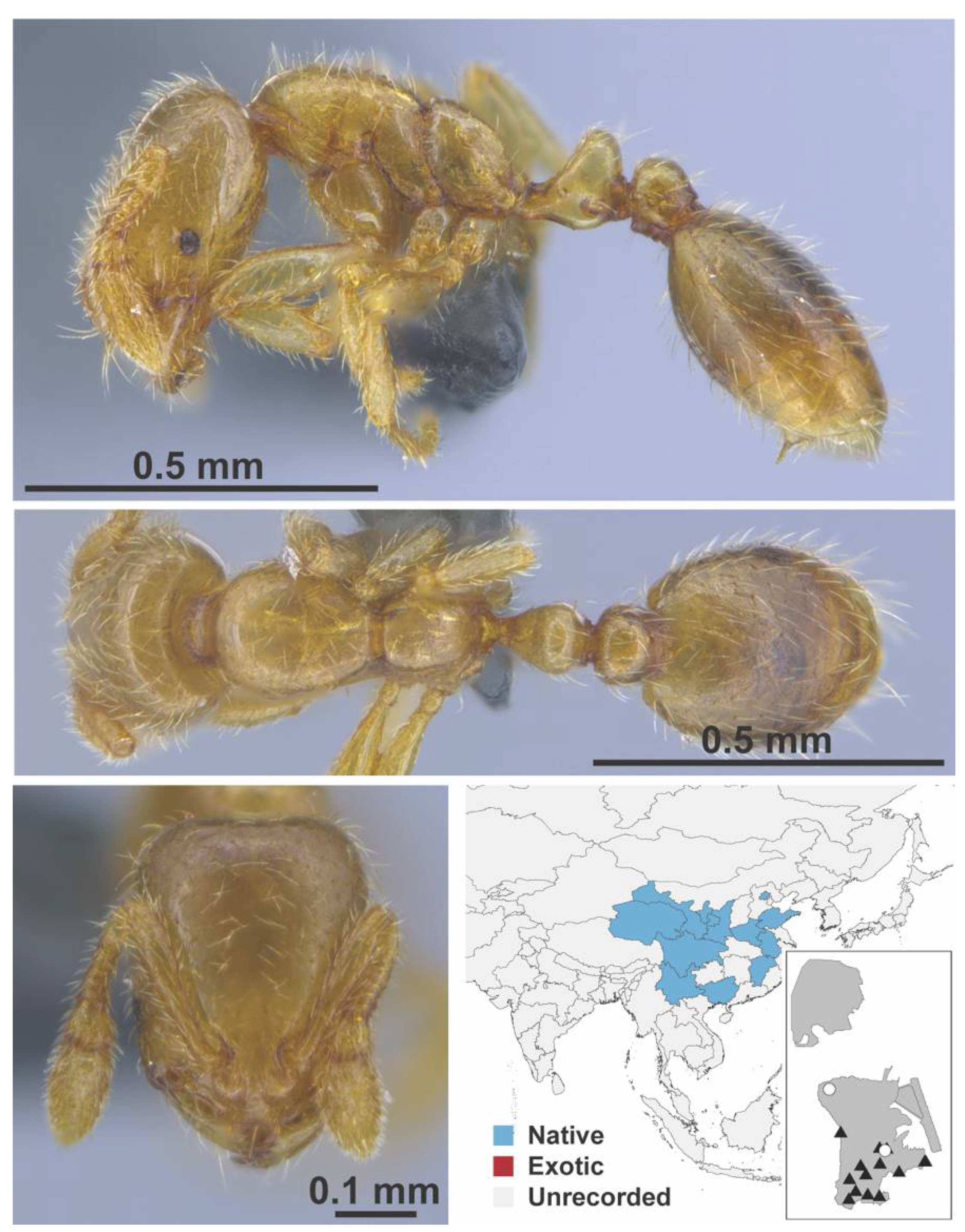

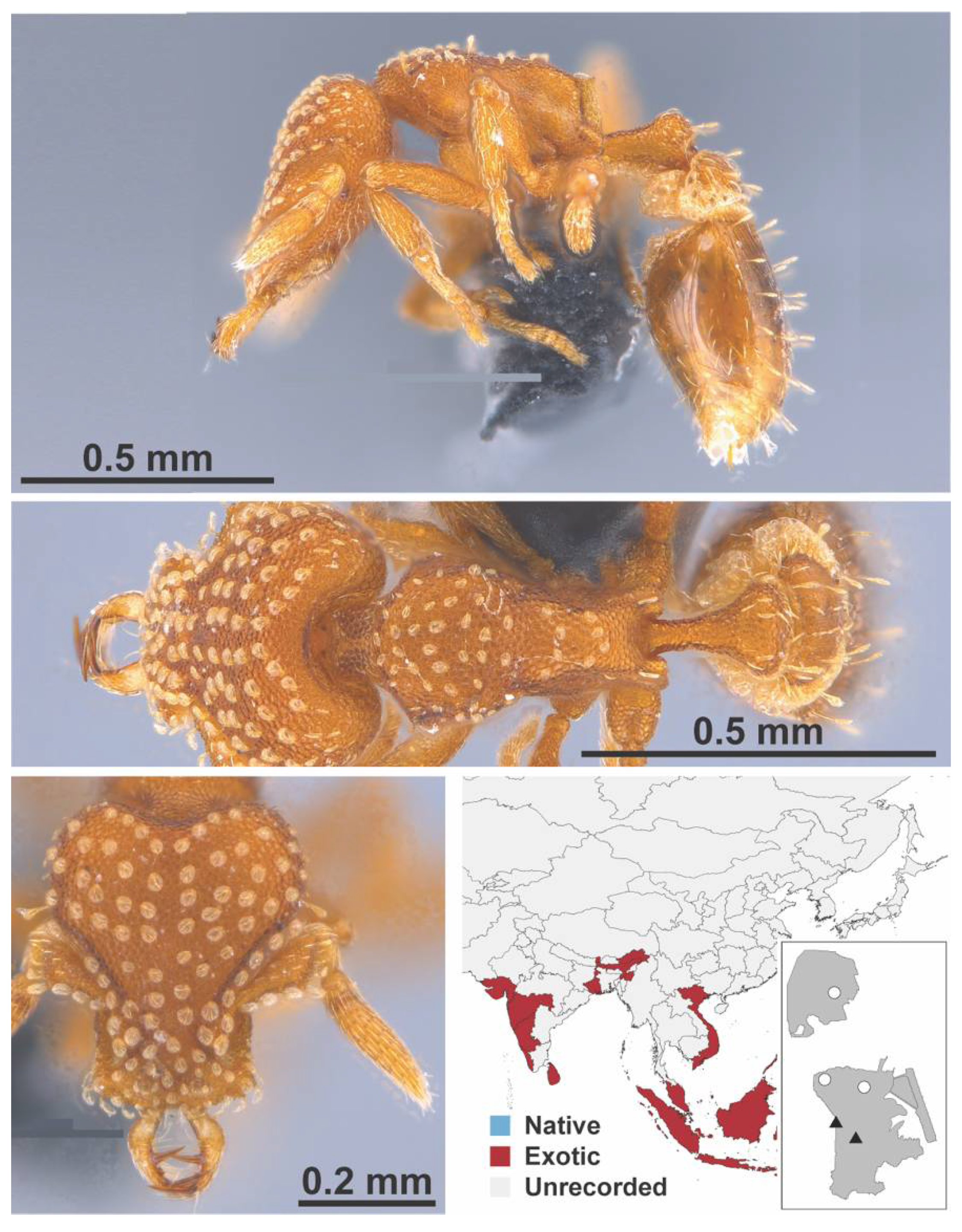
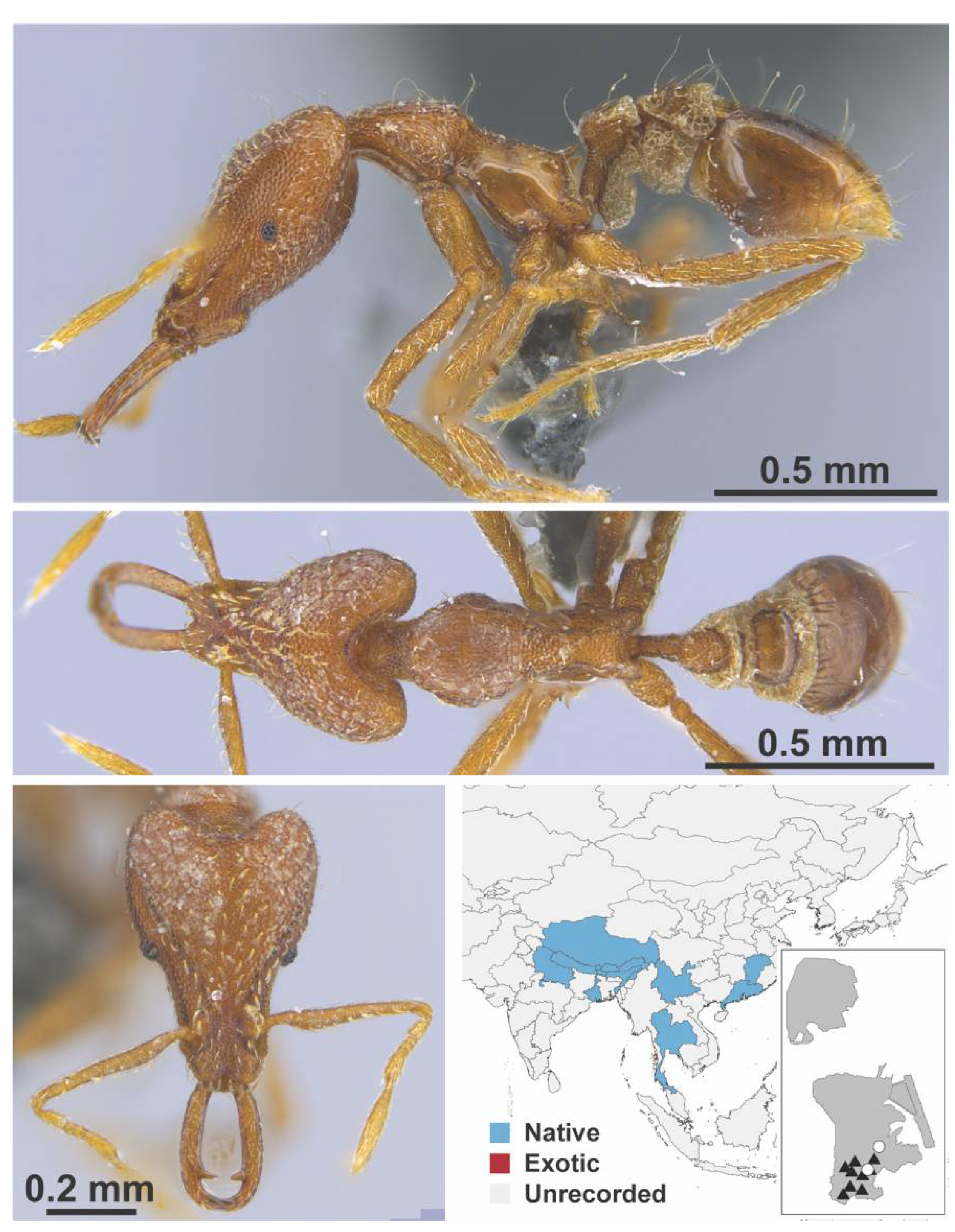
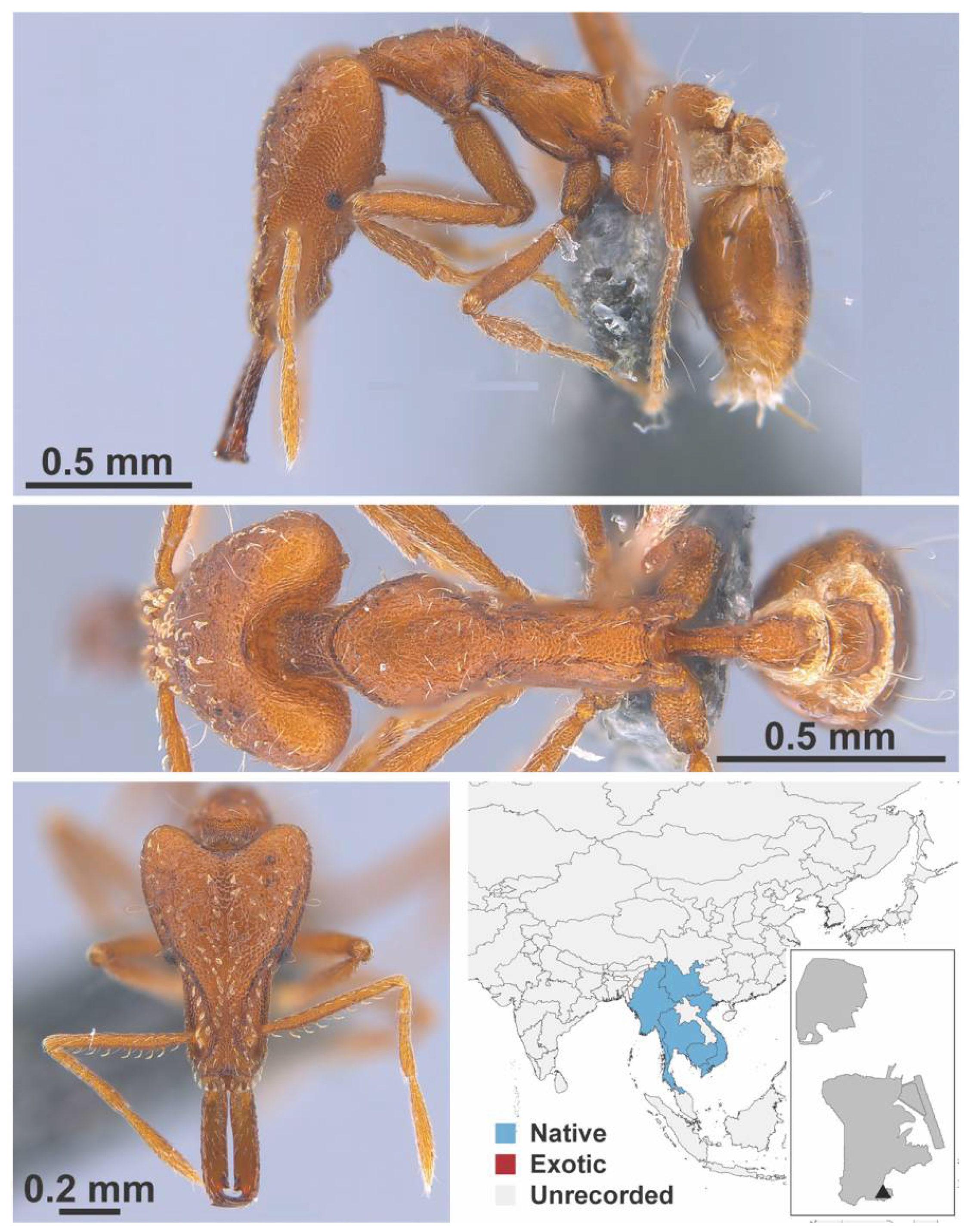
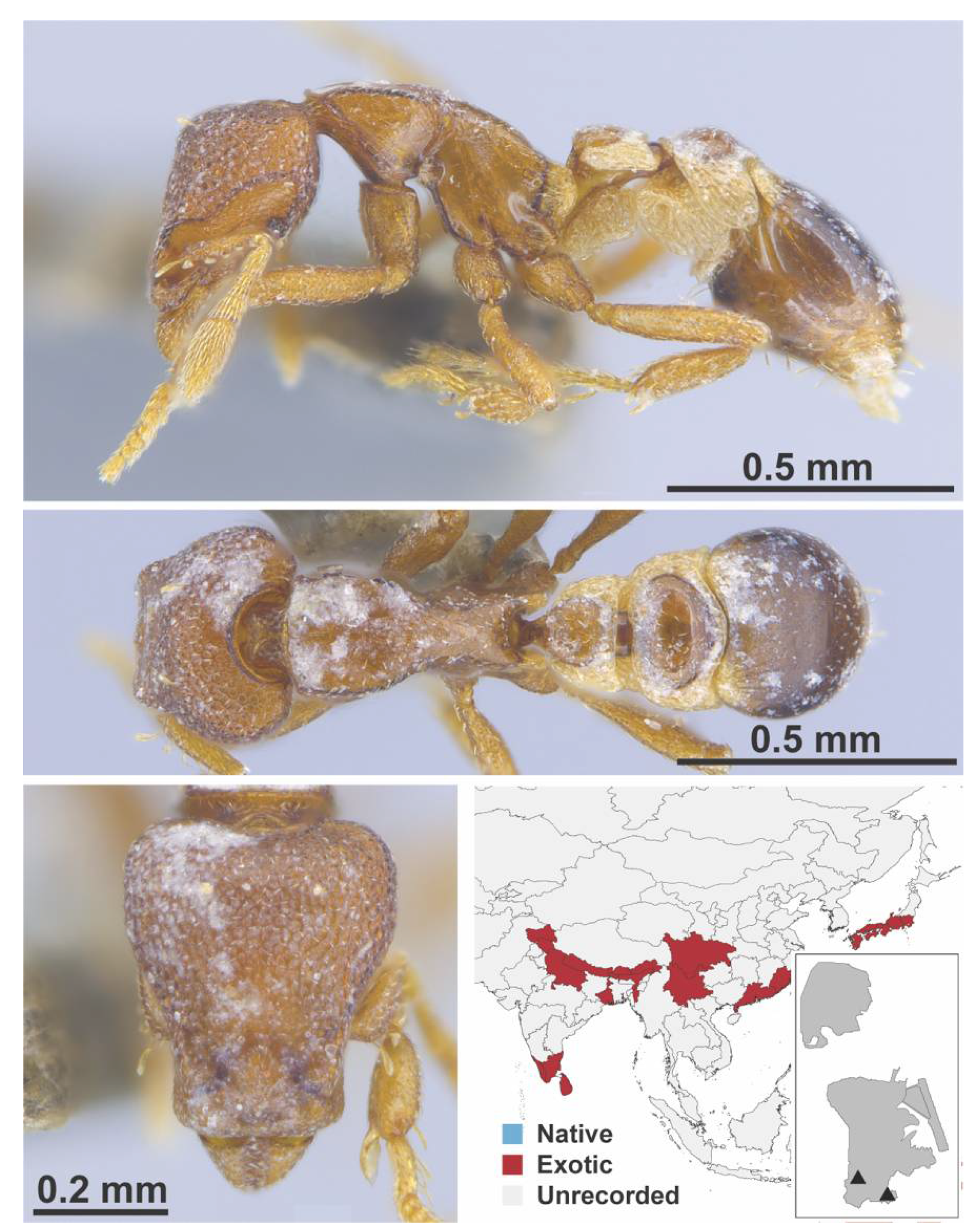
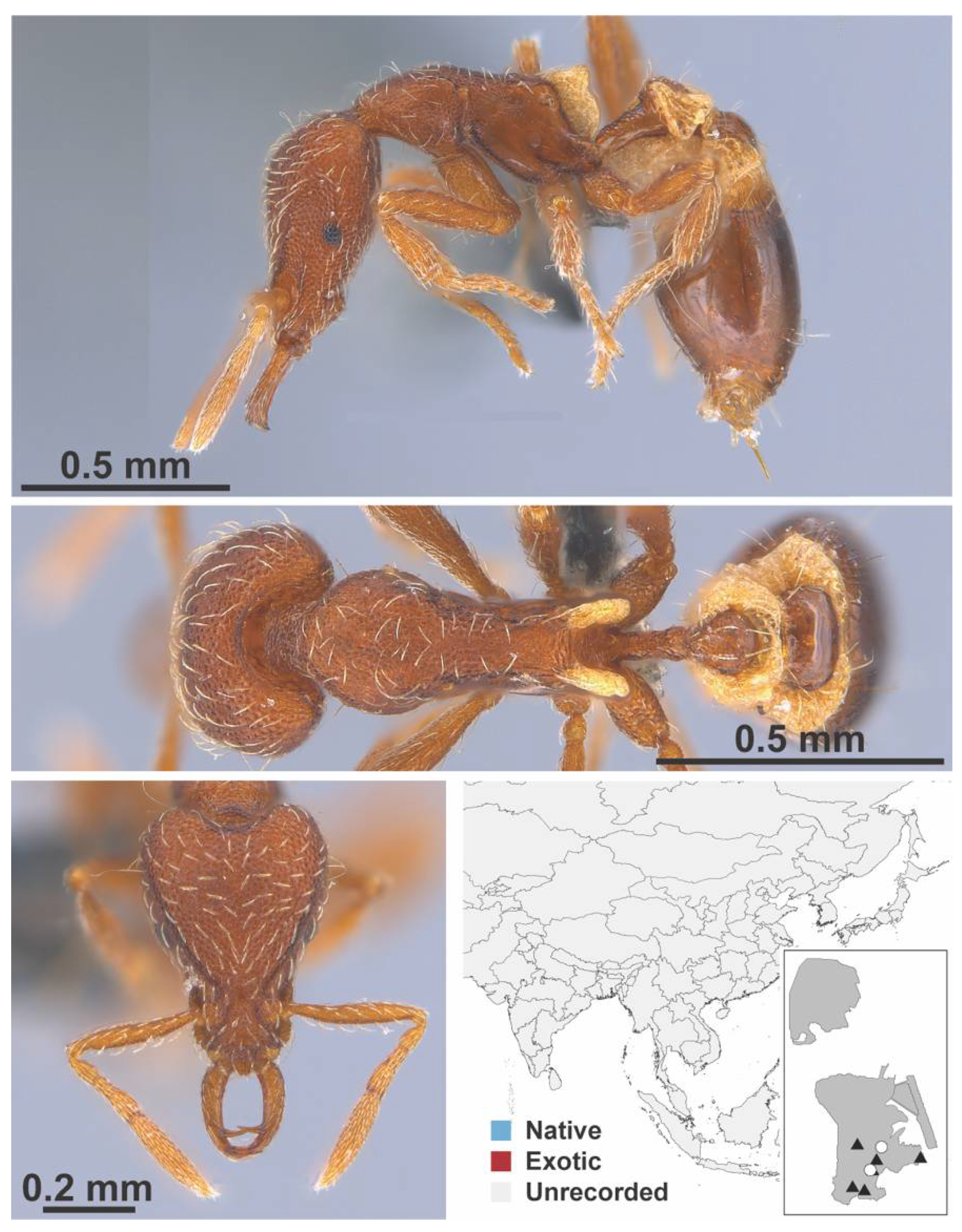

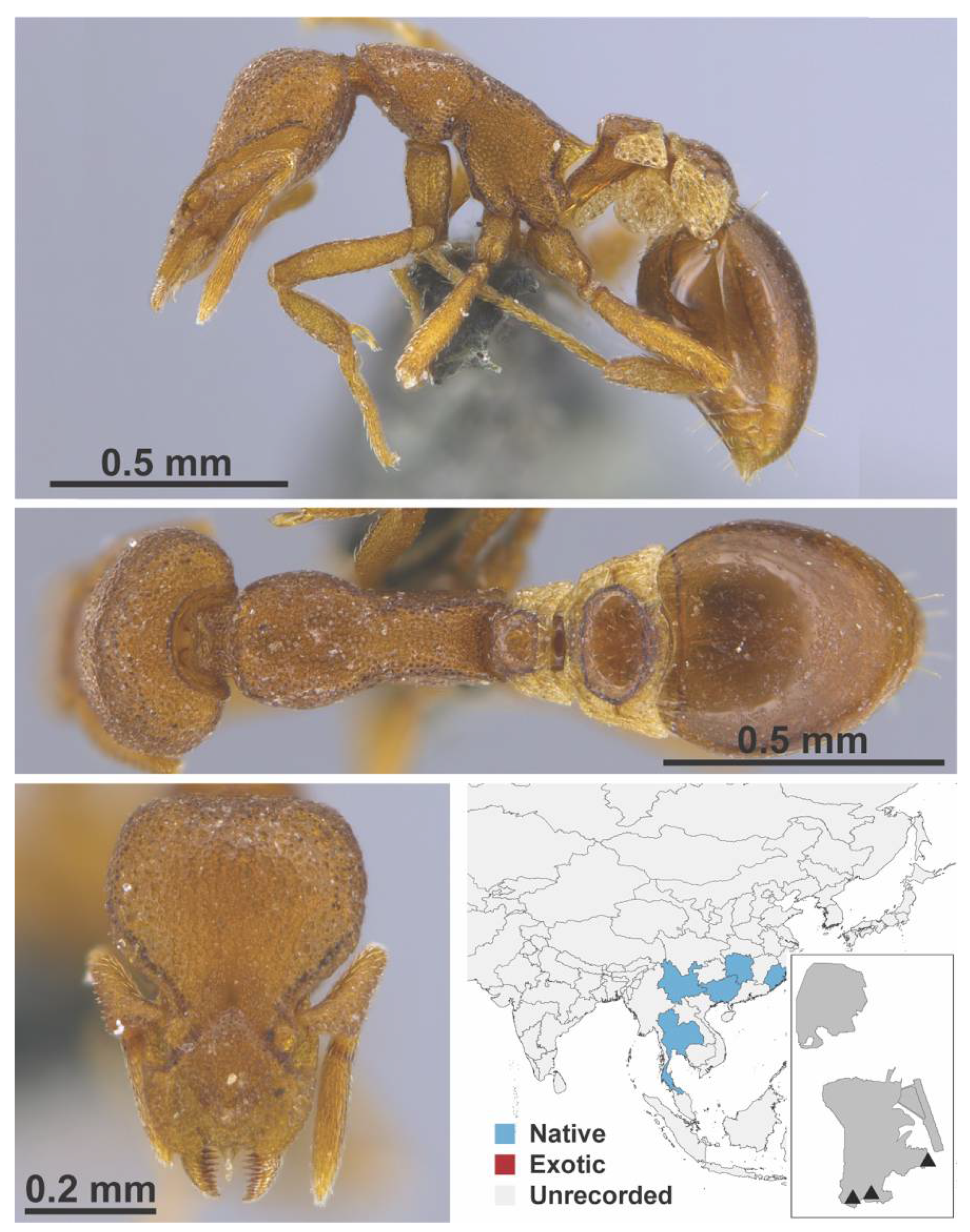

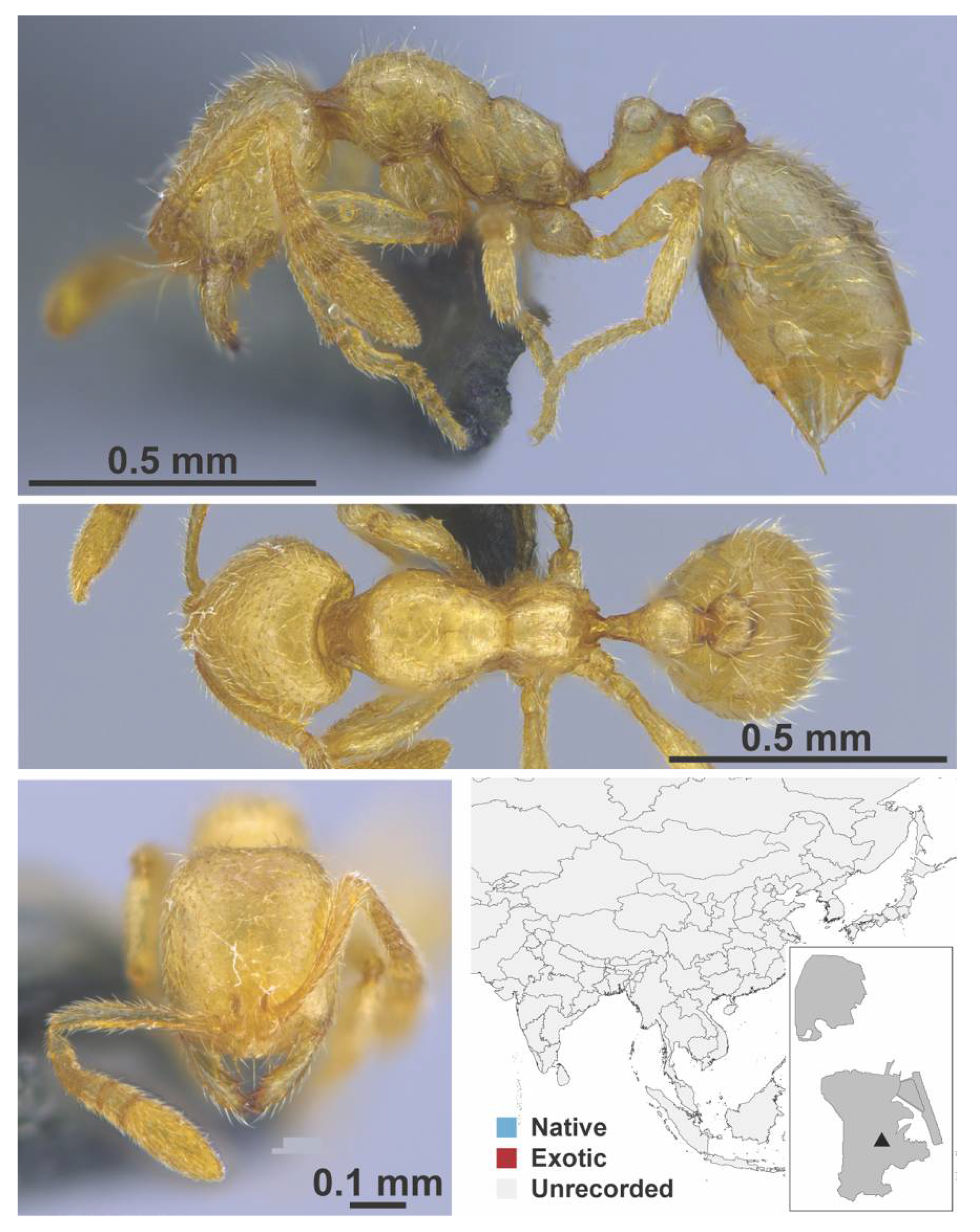

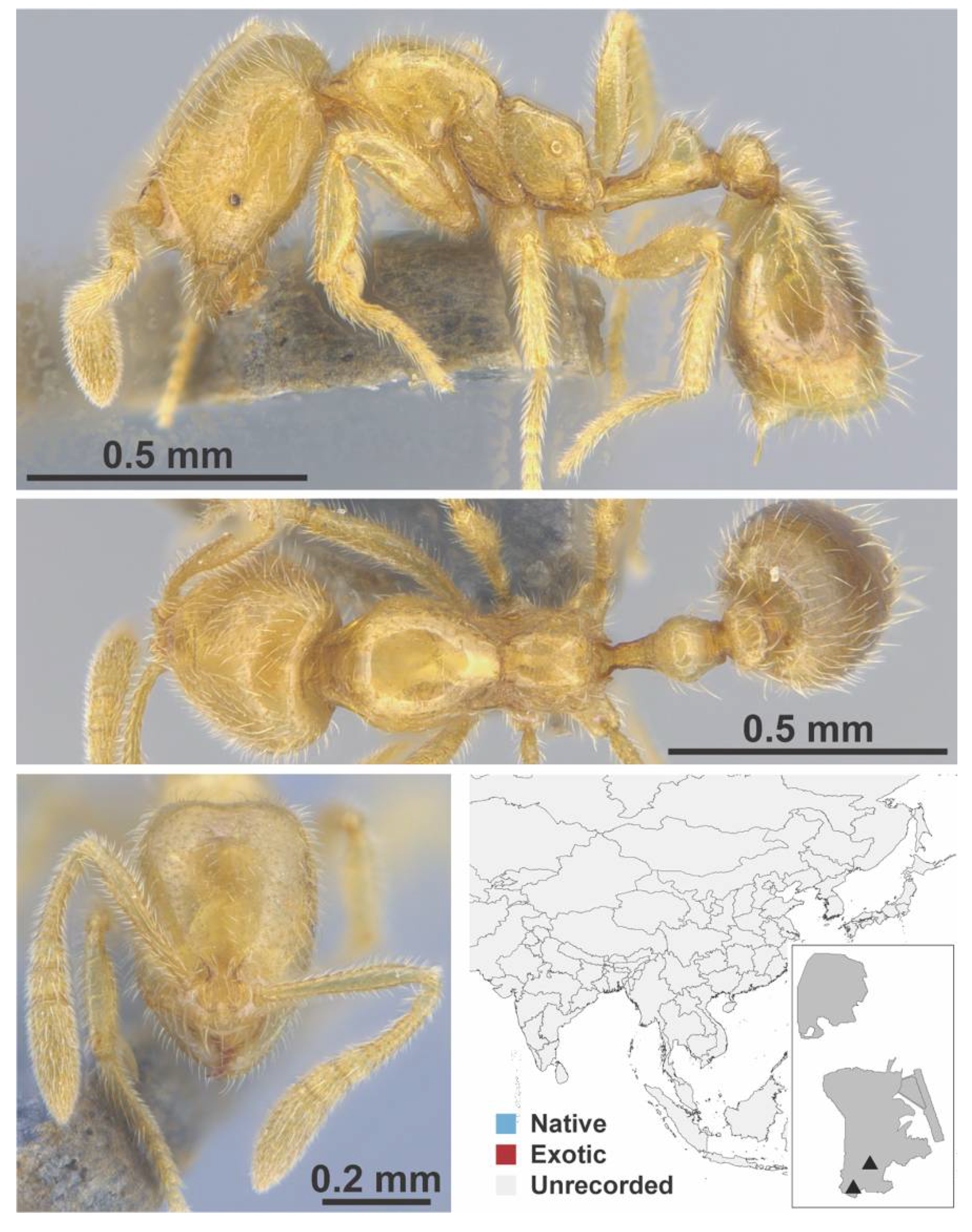
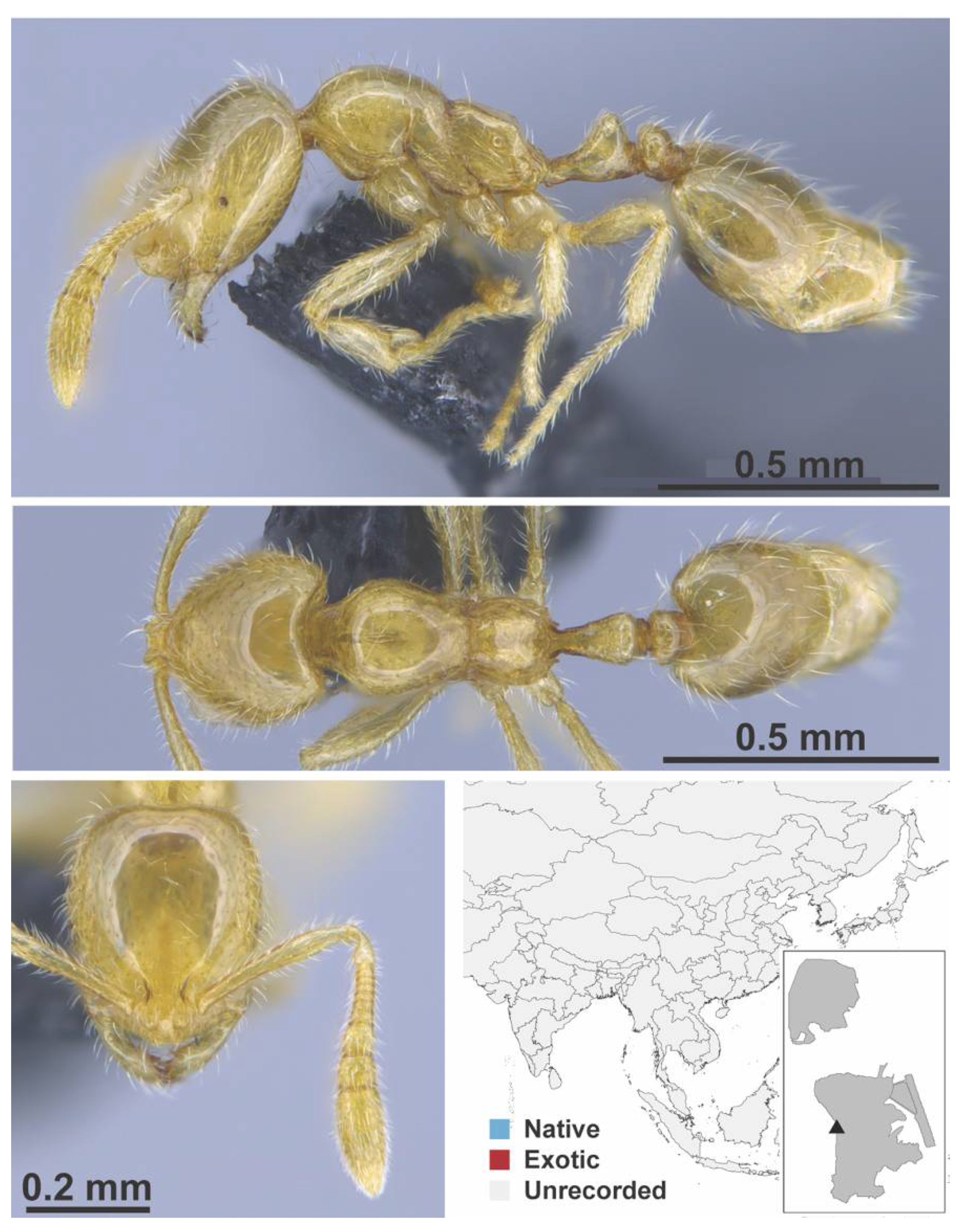
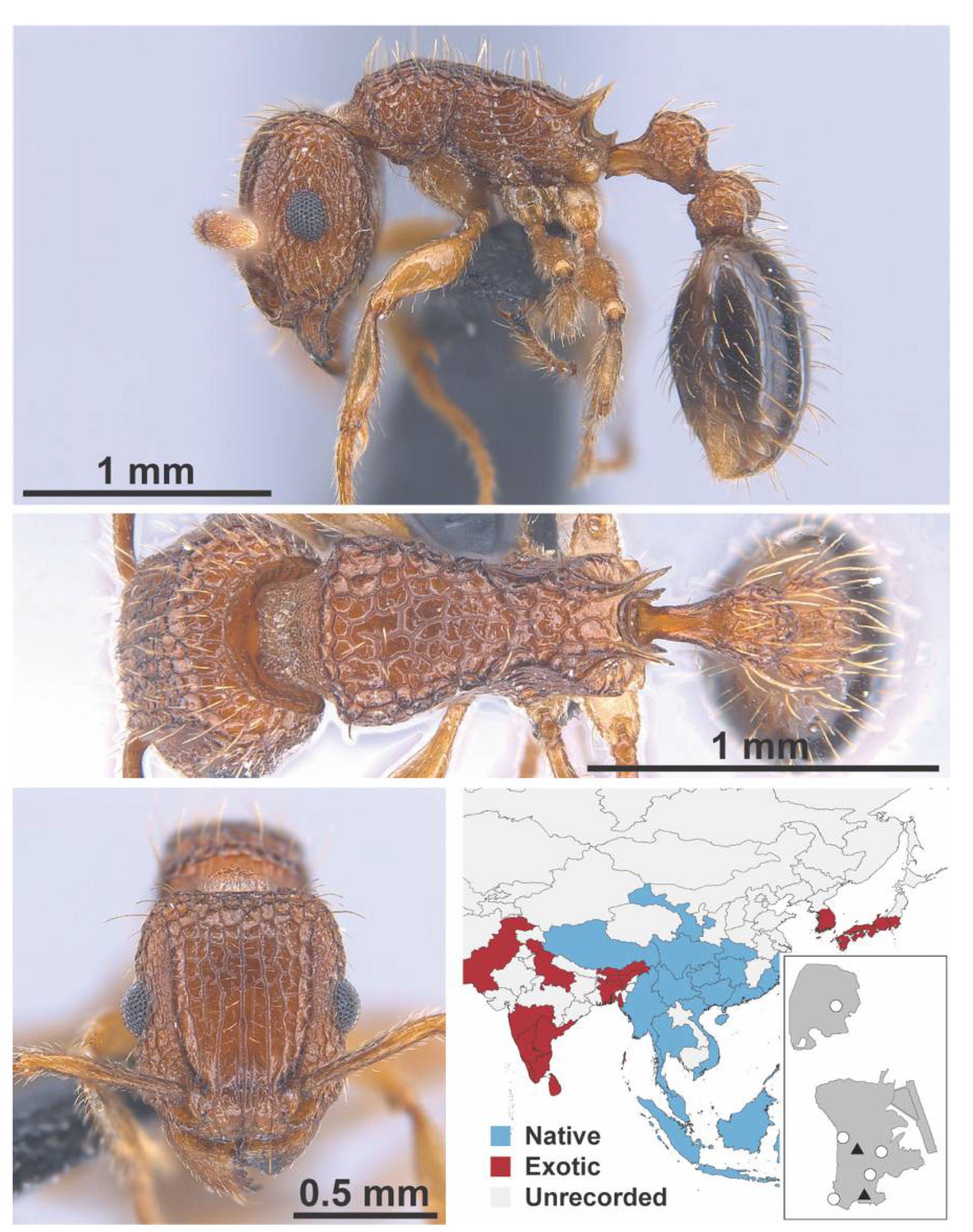
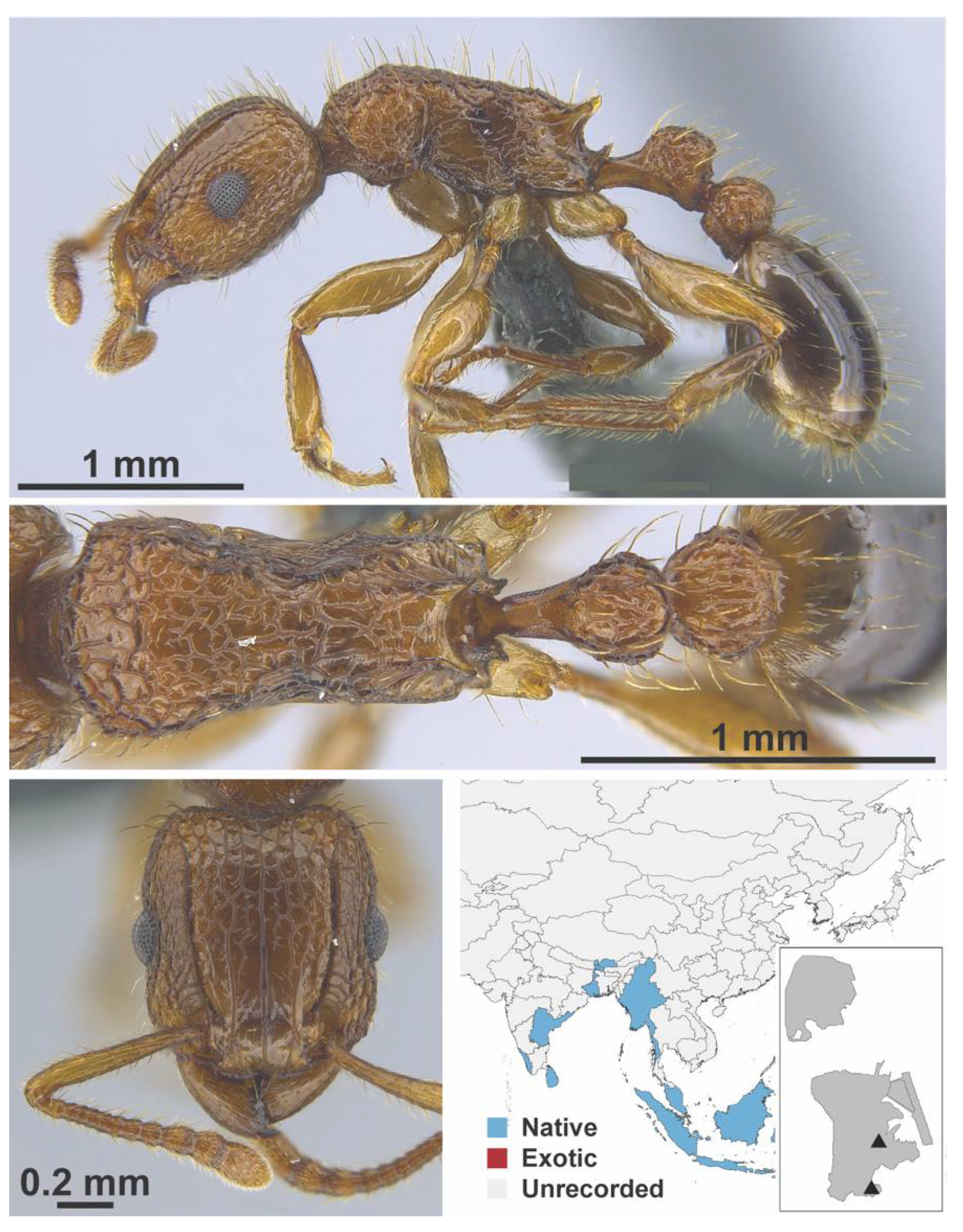

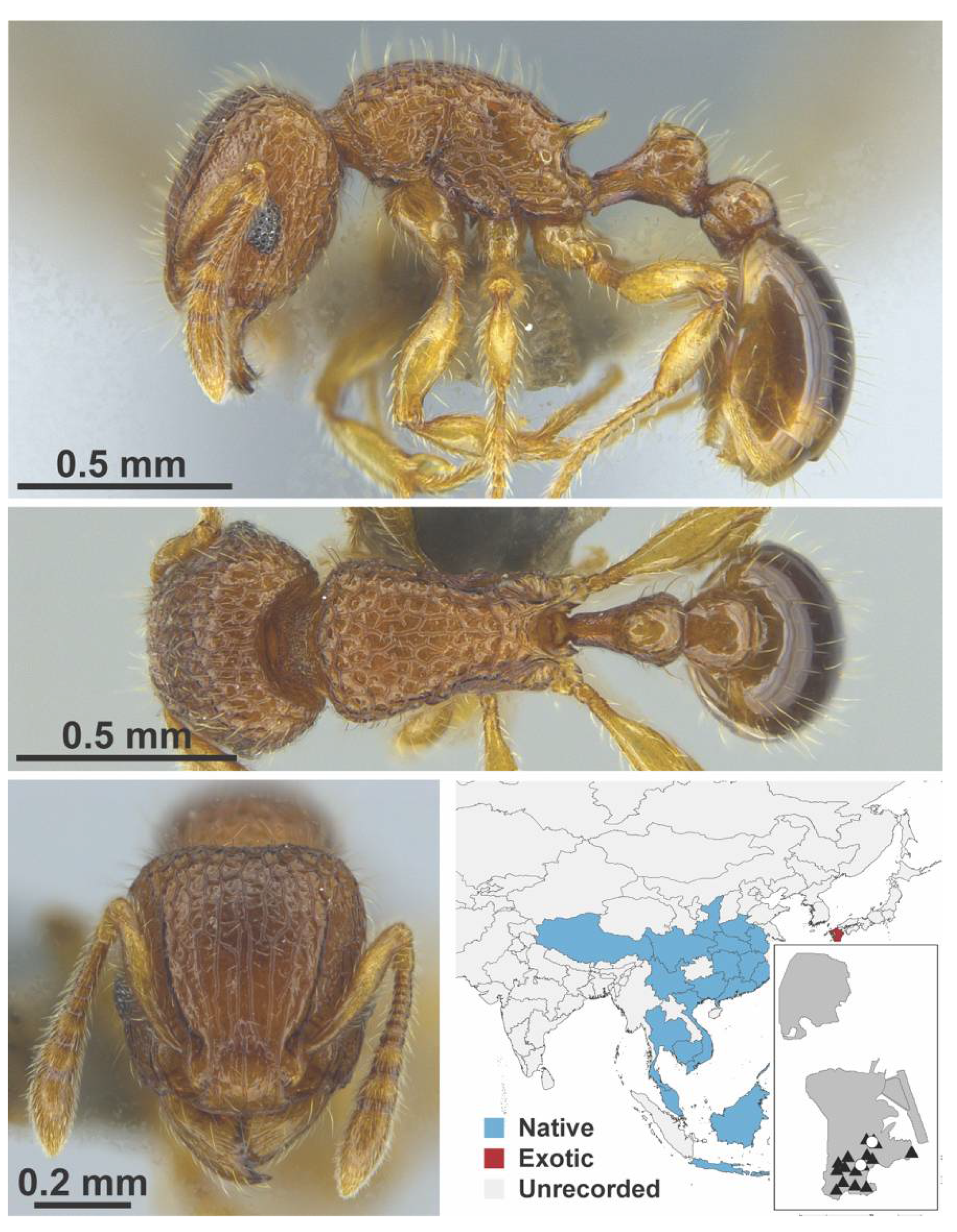



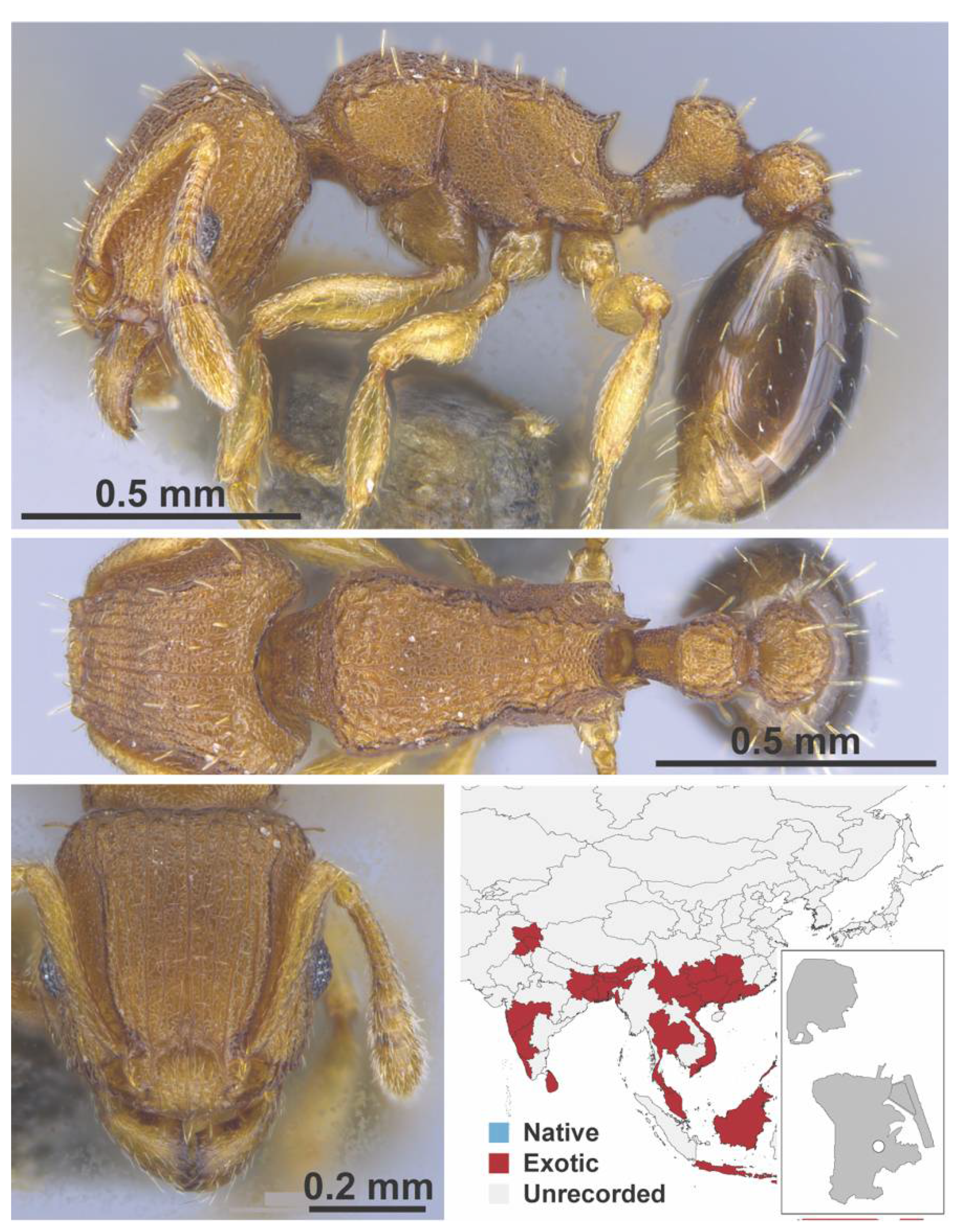
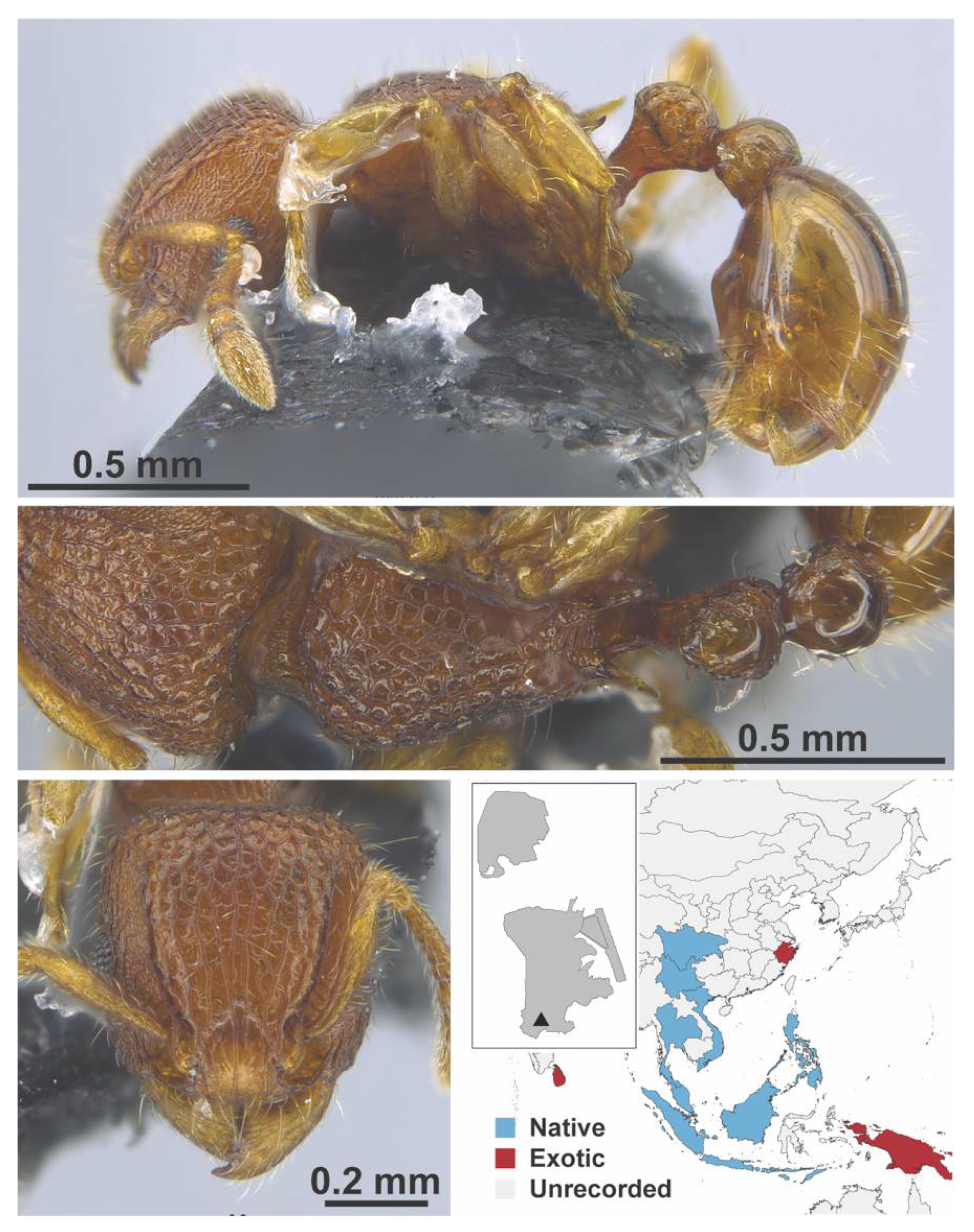

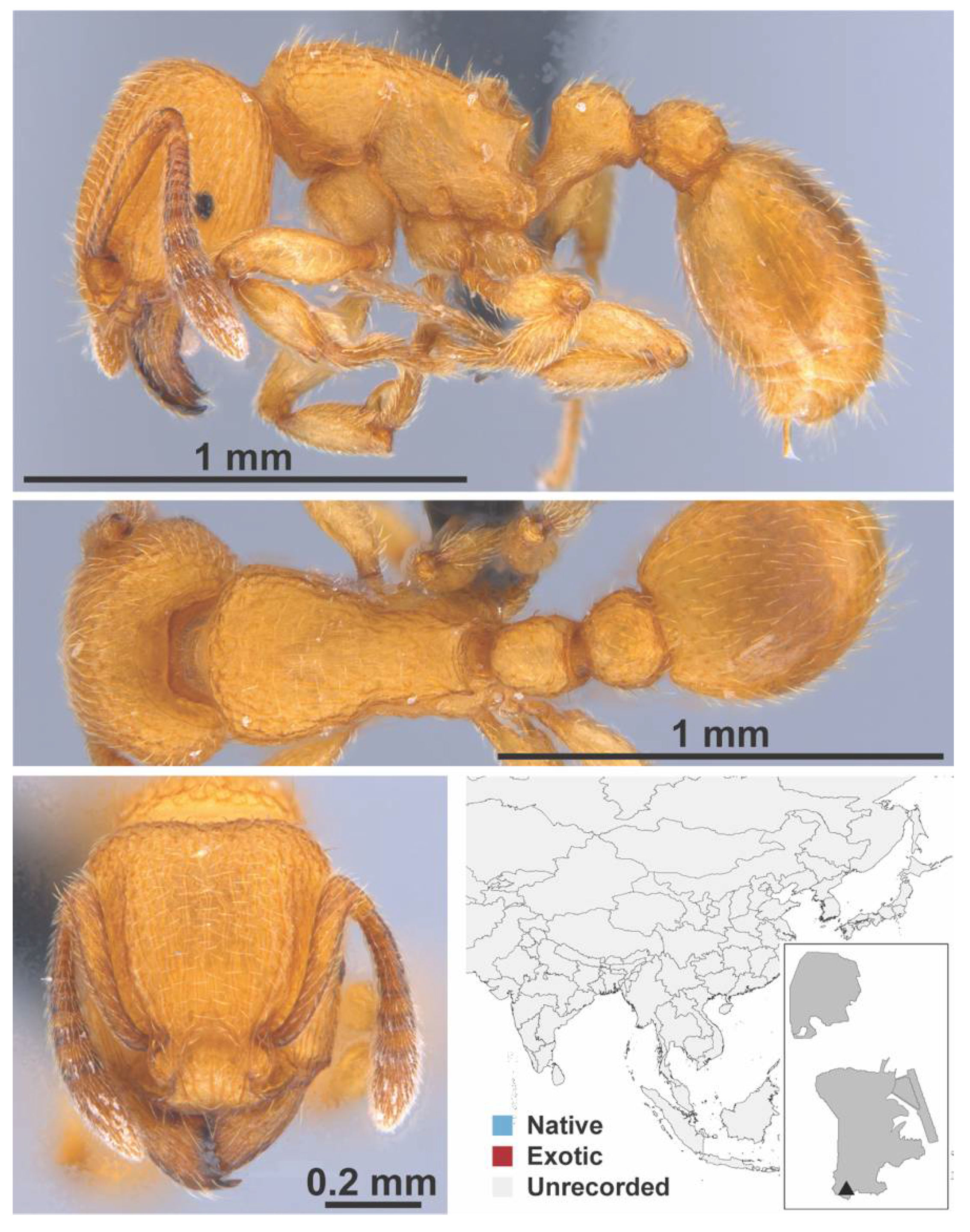
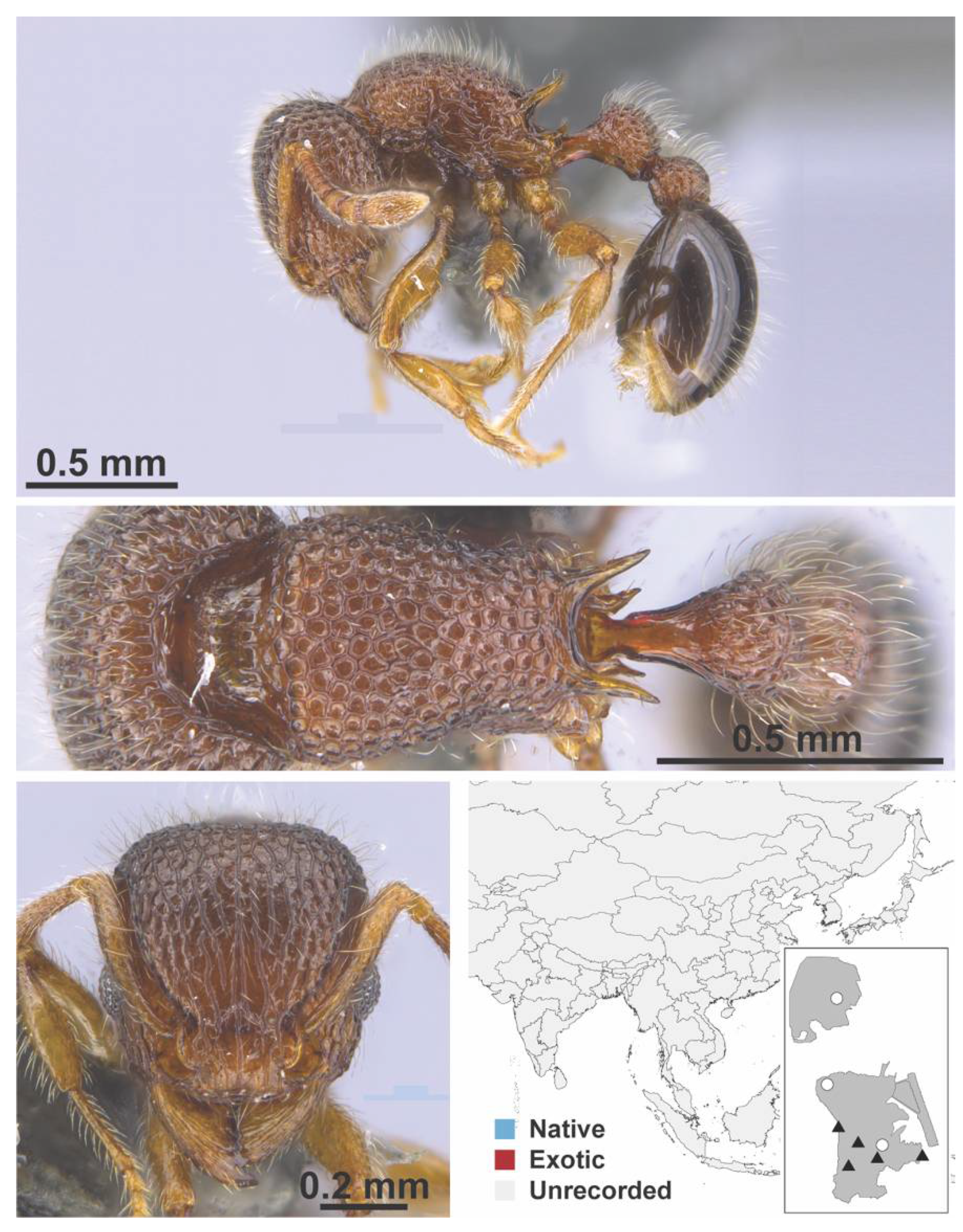
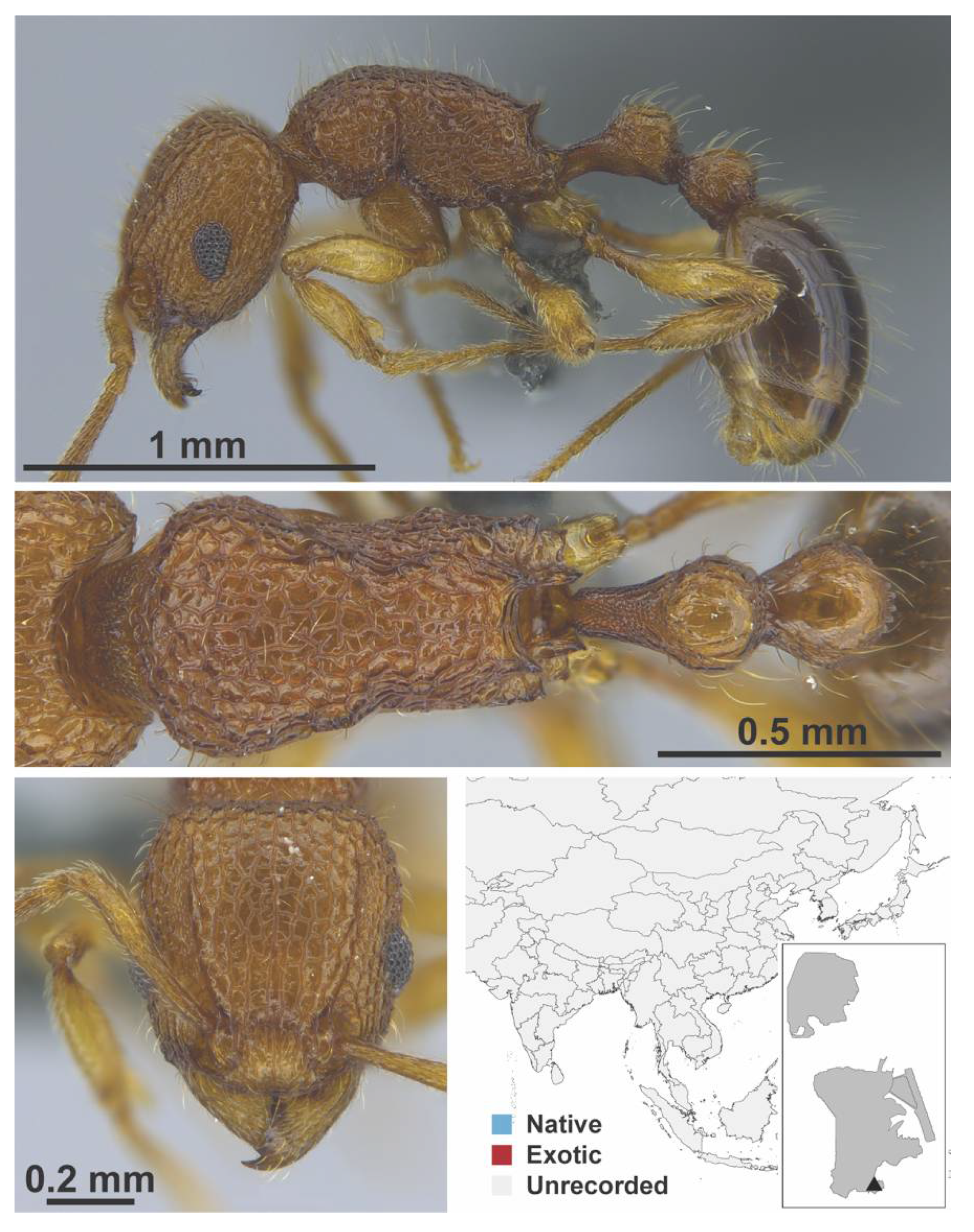
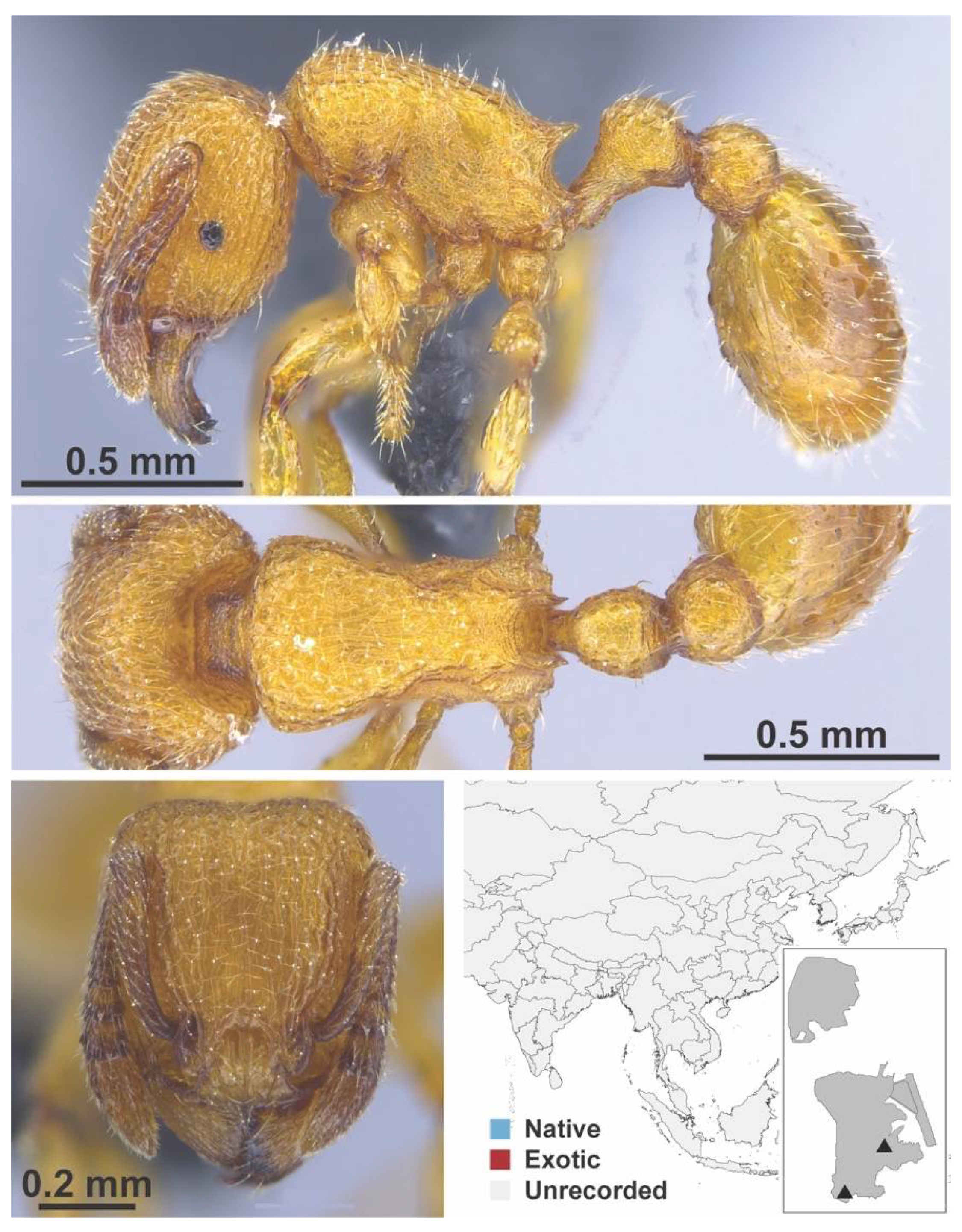

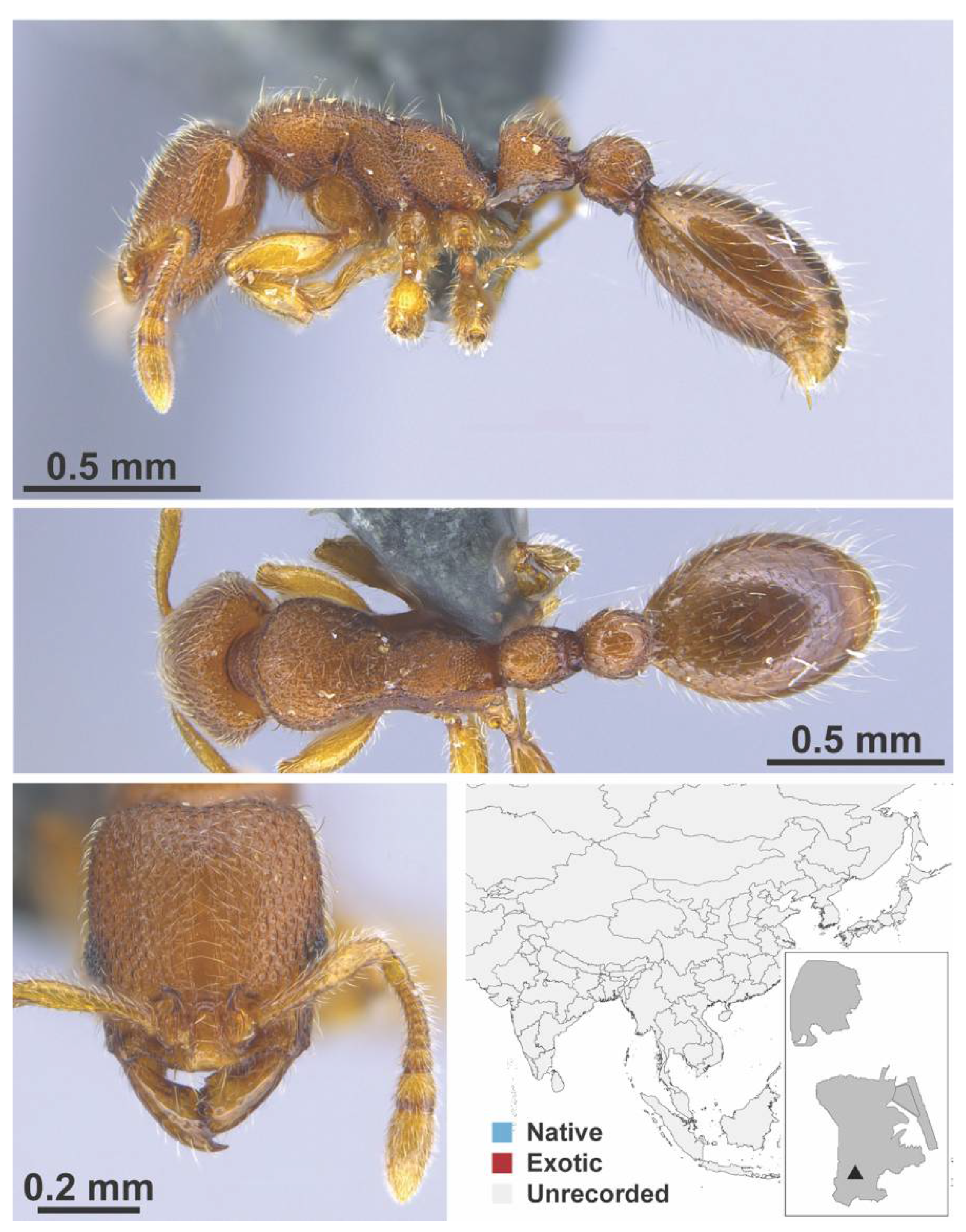
Appendix A.7. PONERINAE
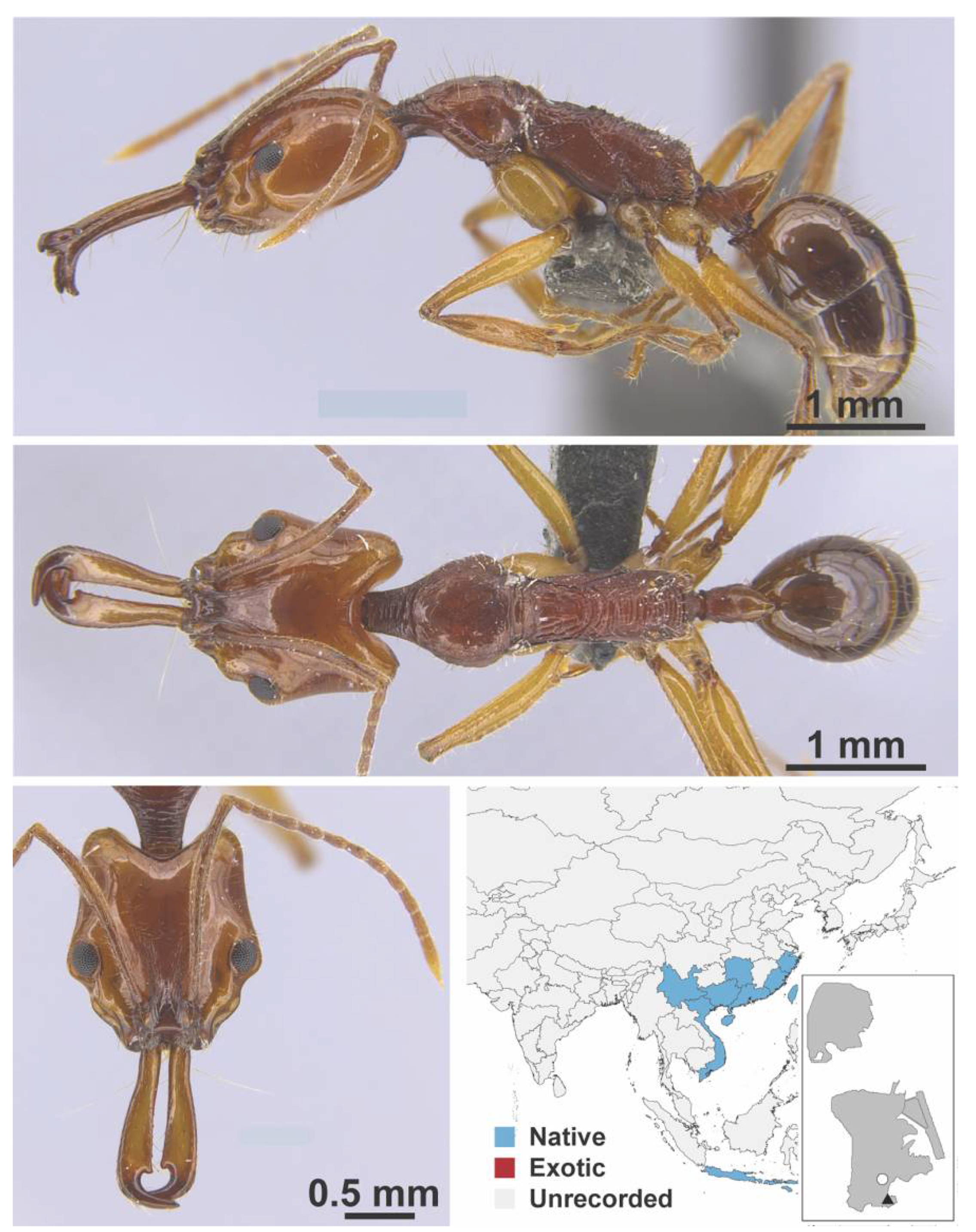
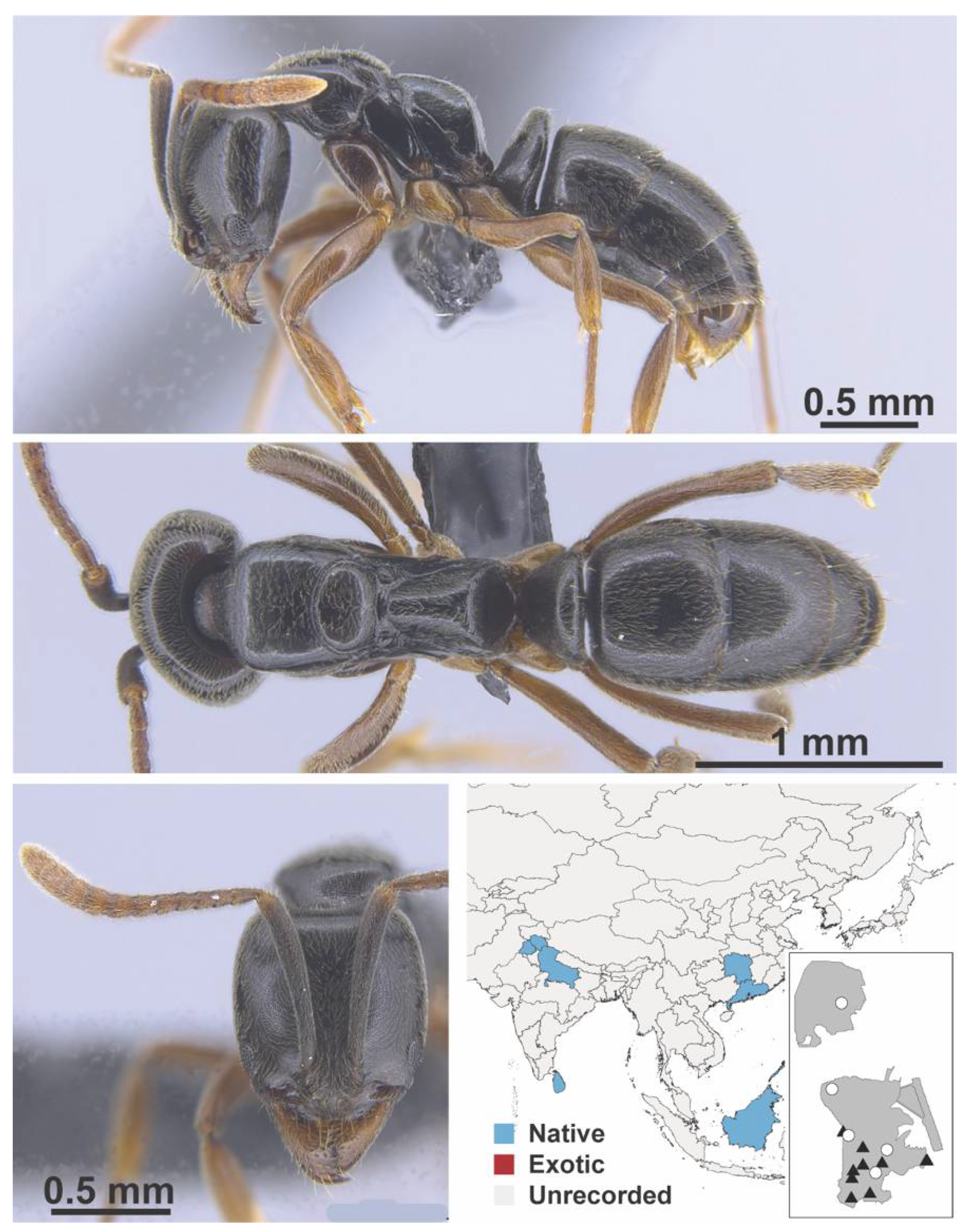

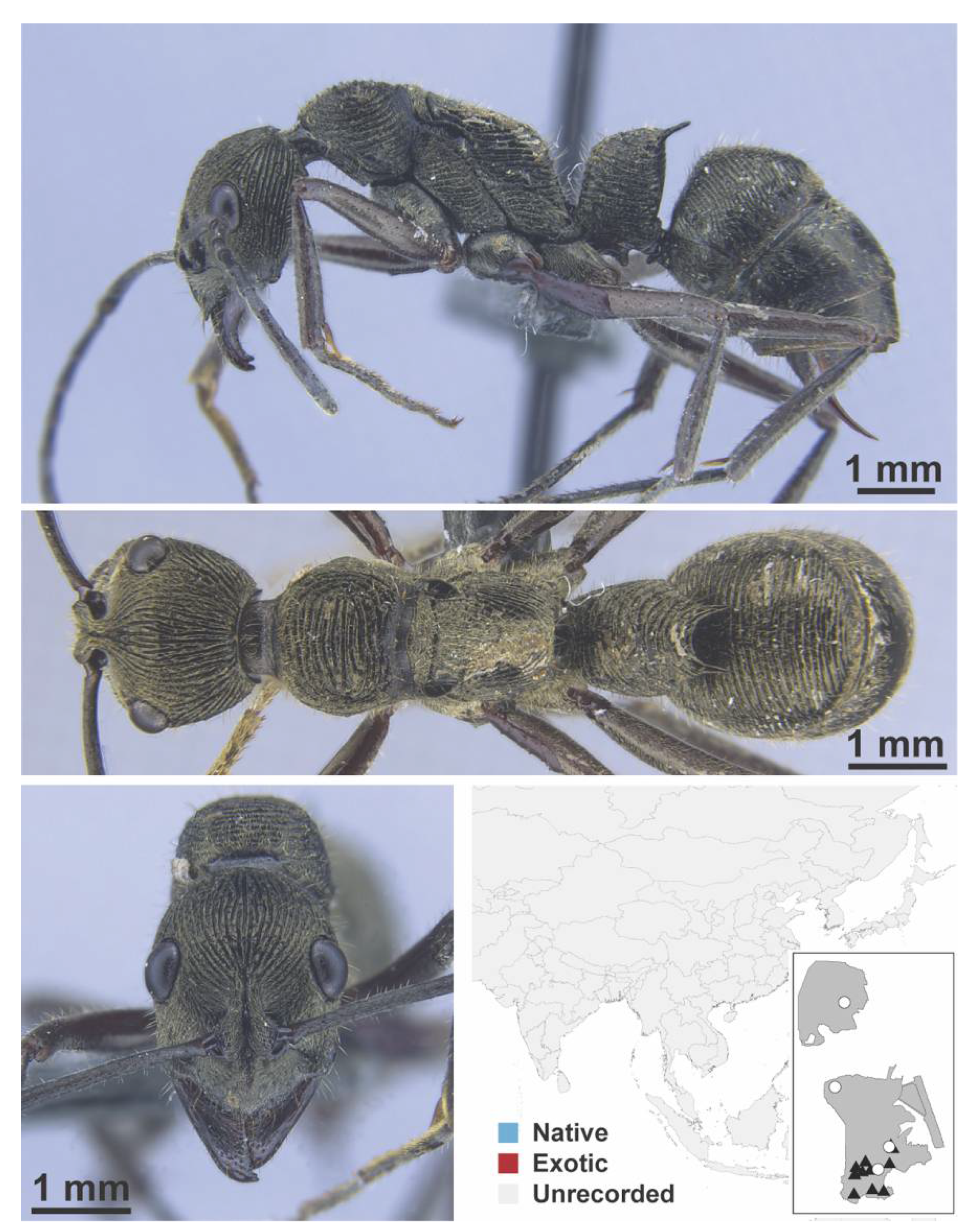
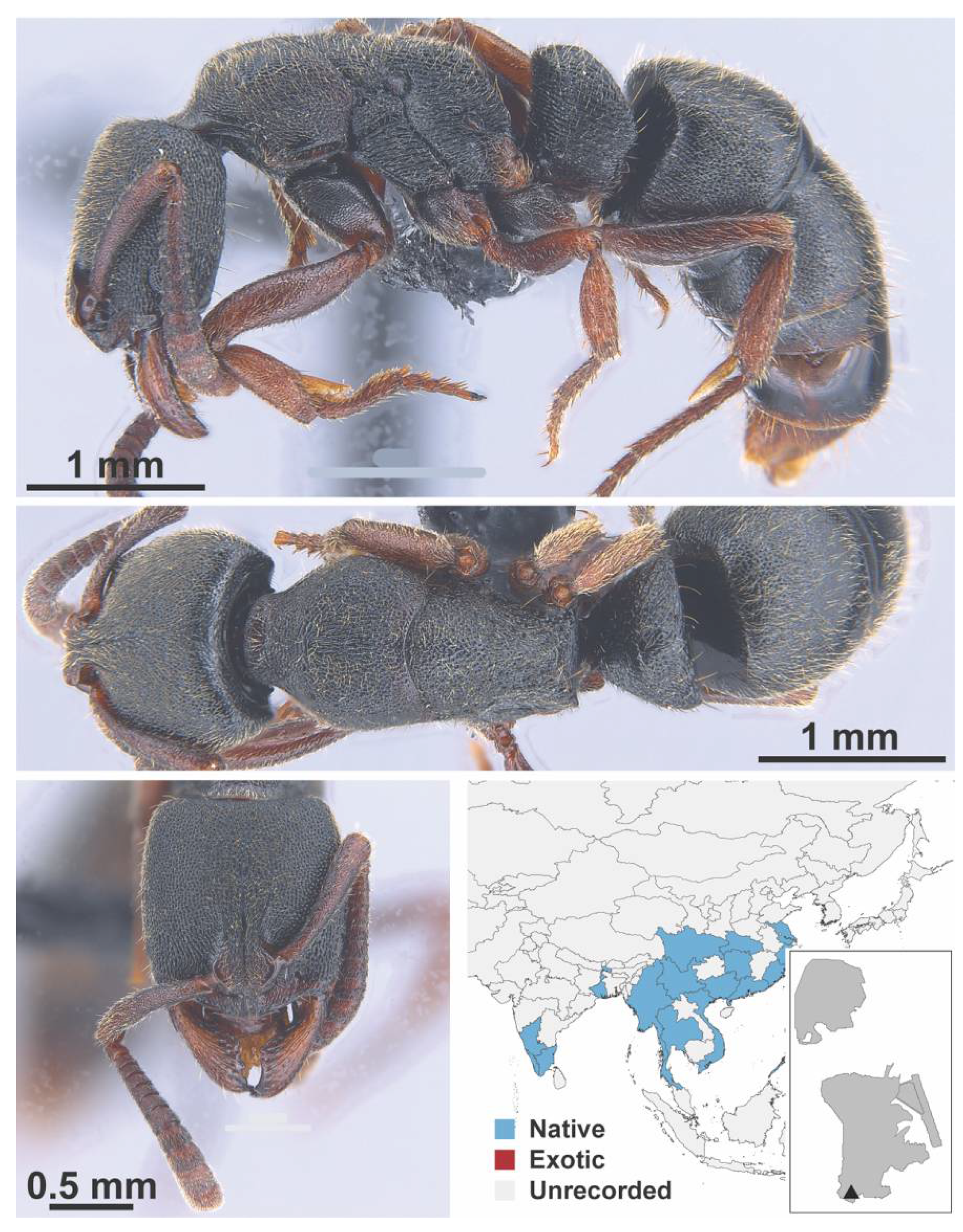
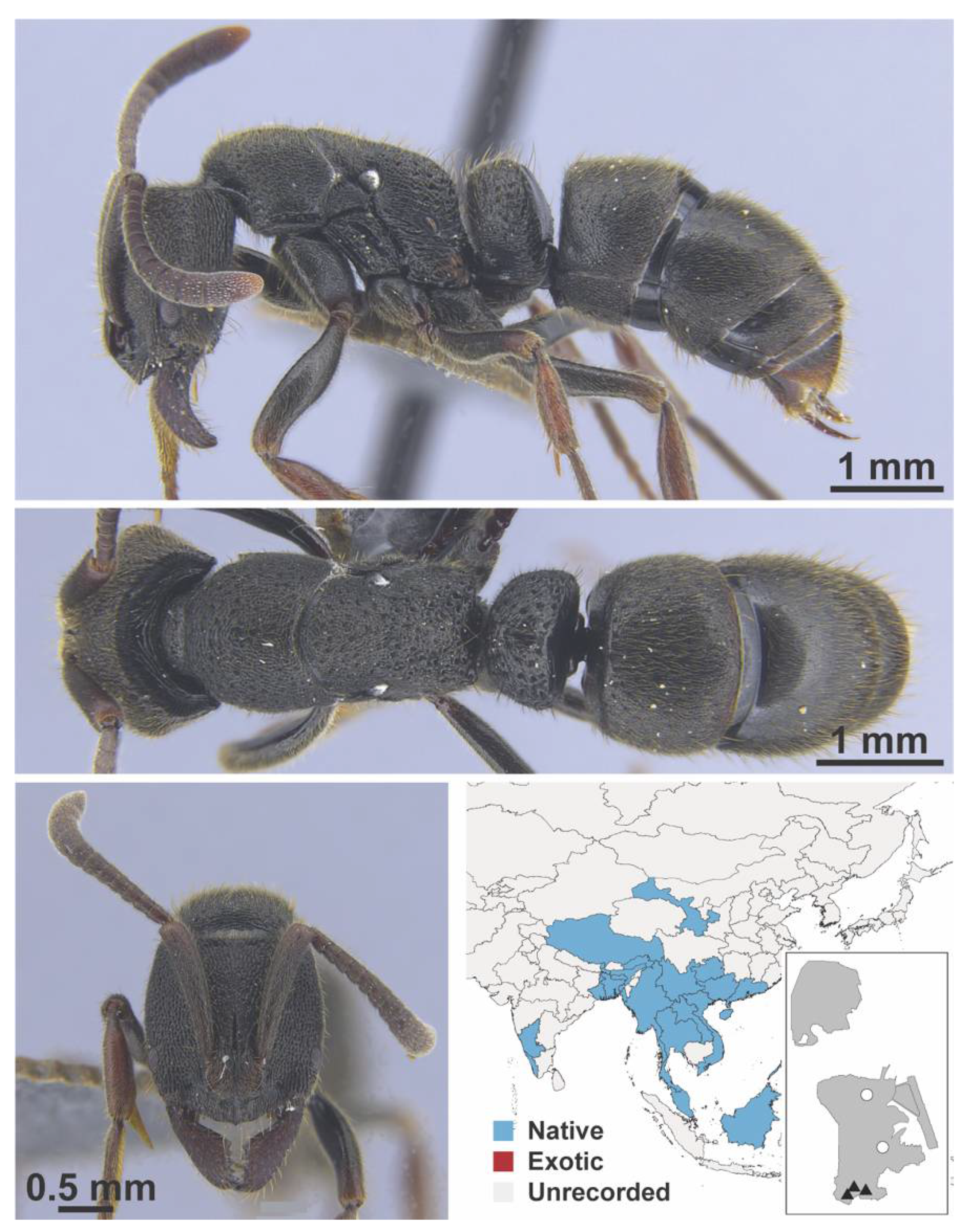
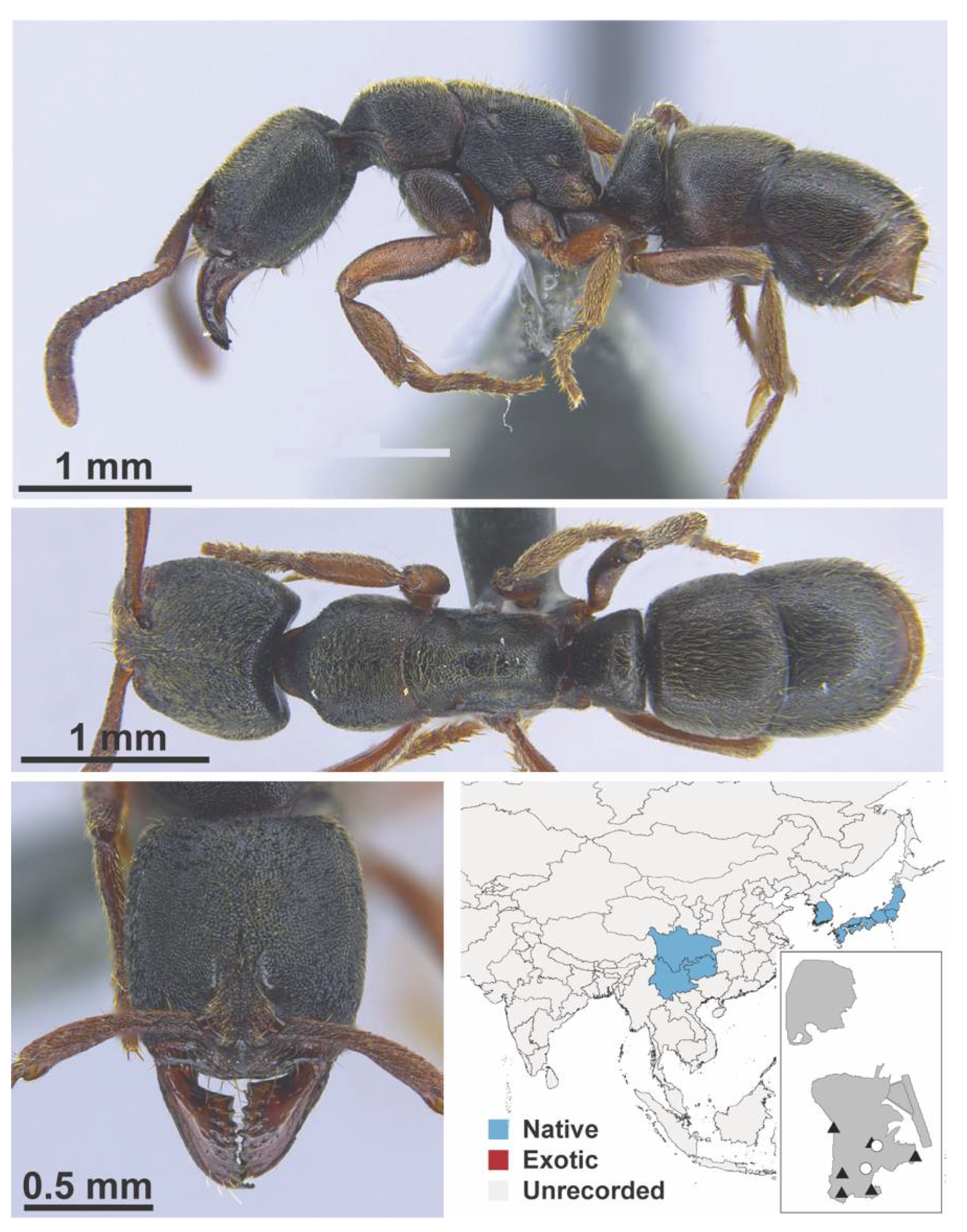
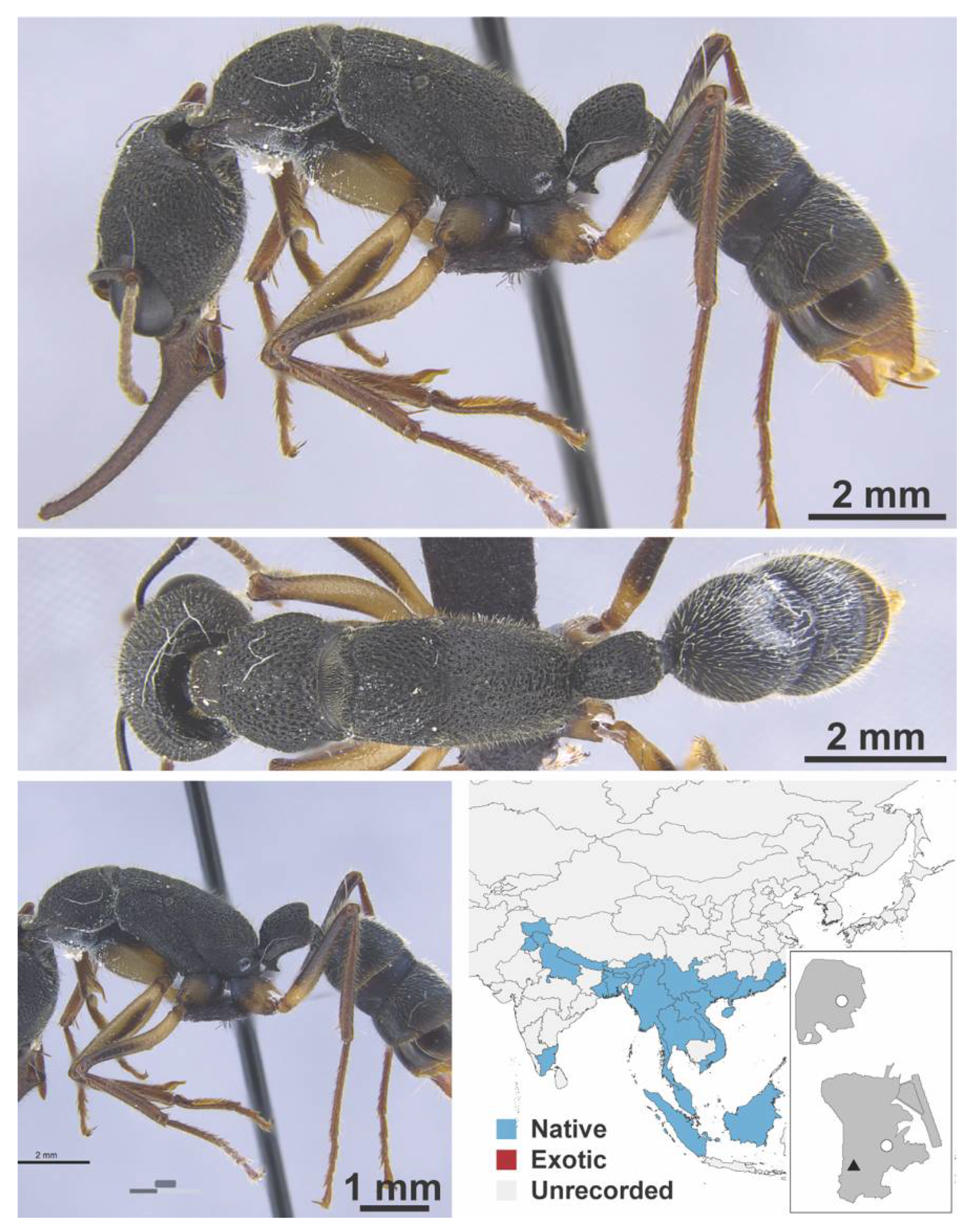
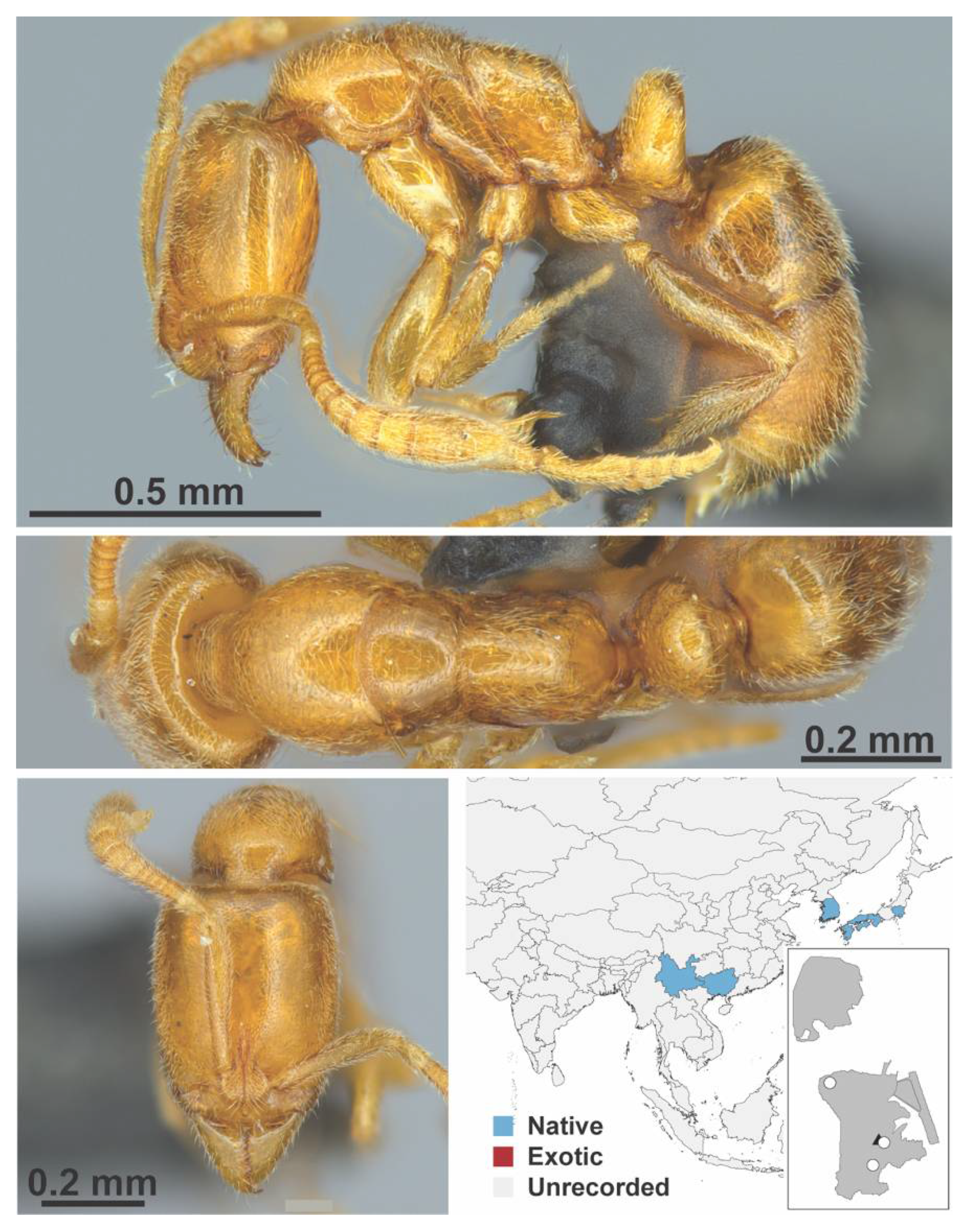

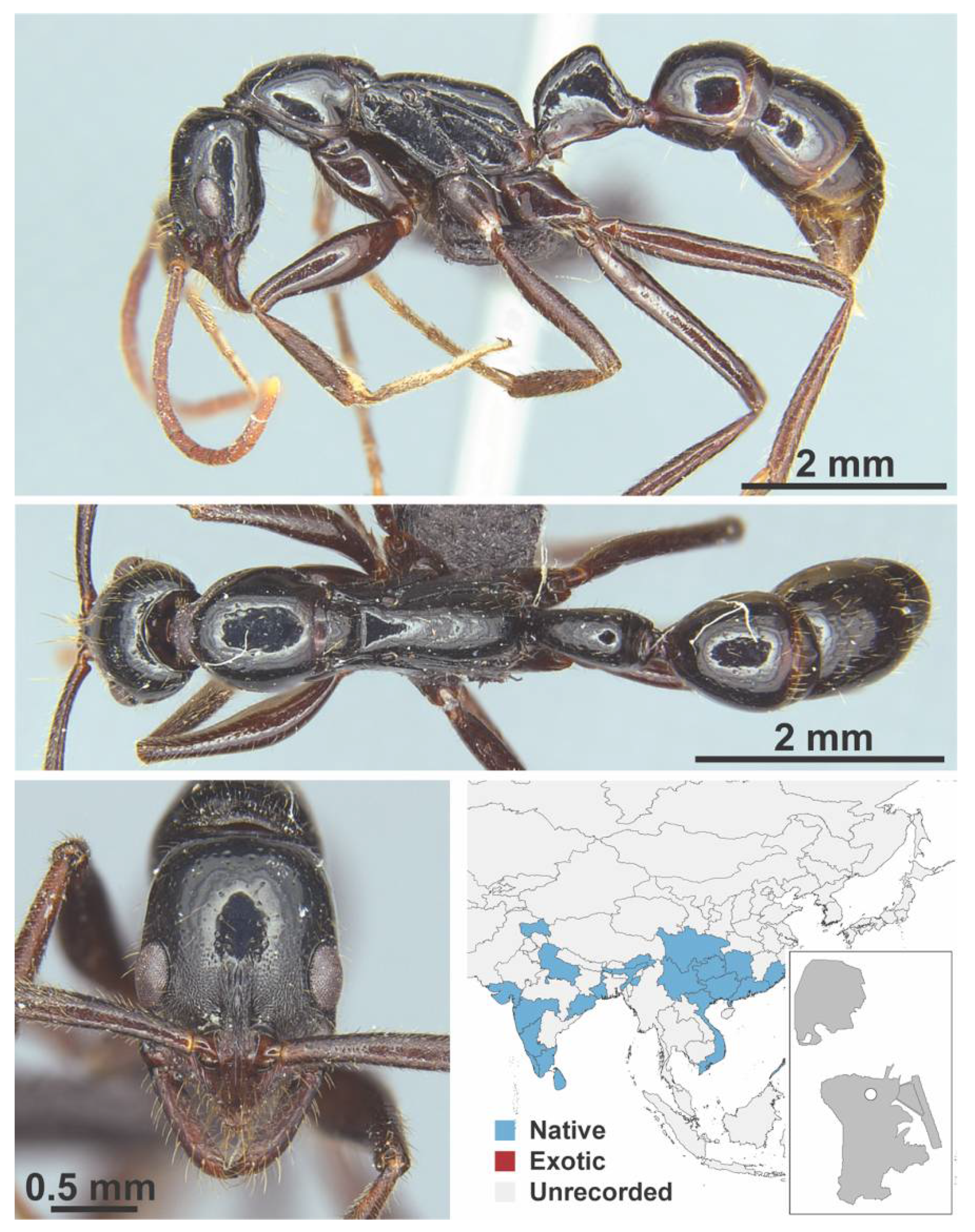
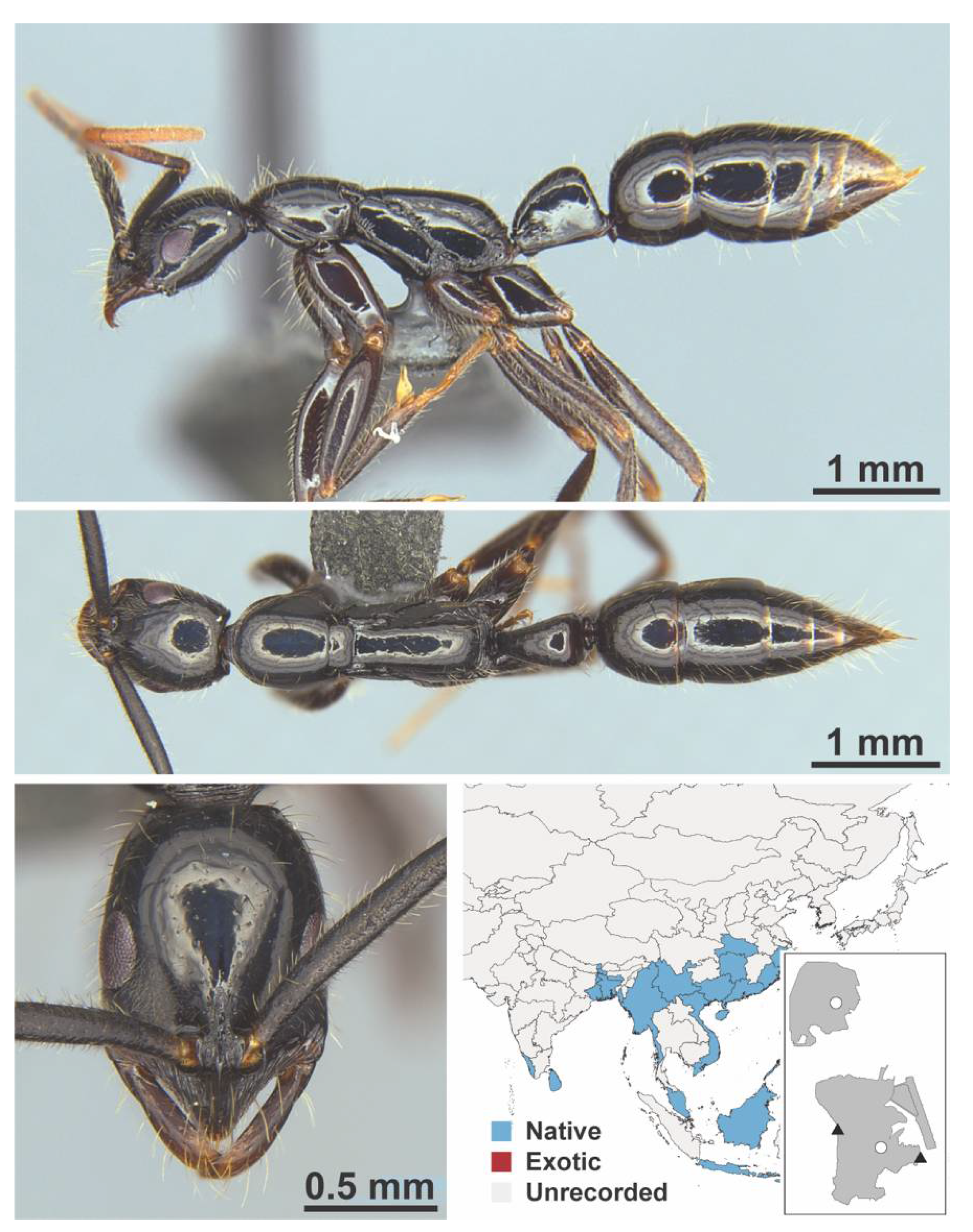
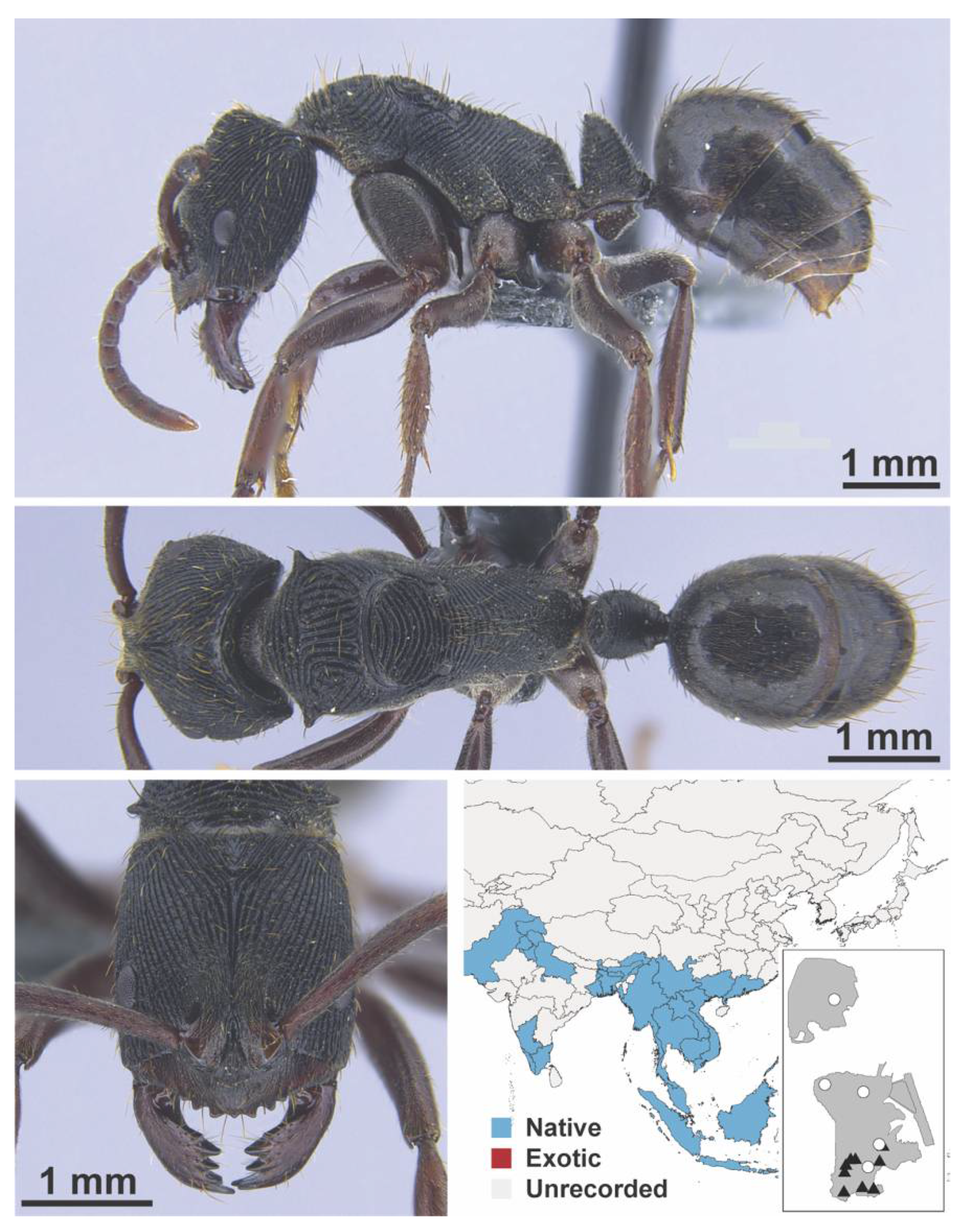
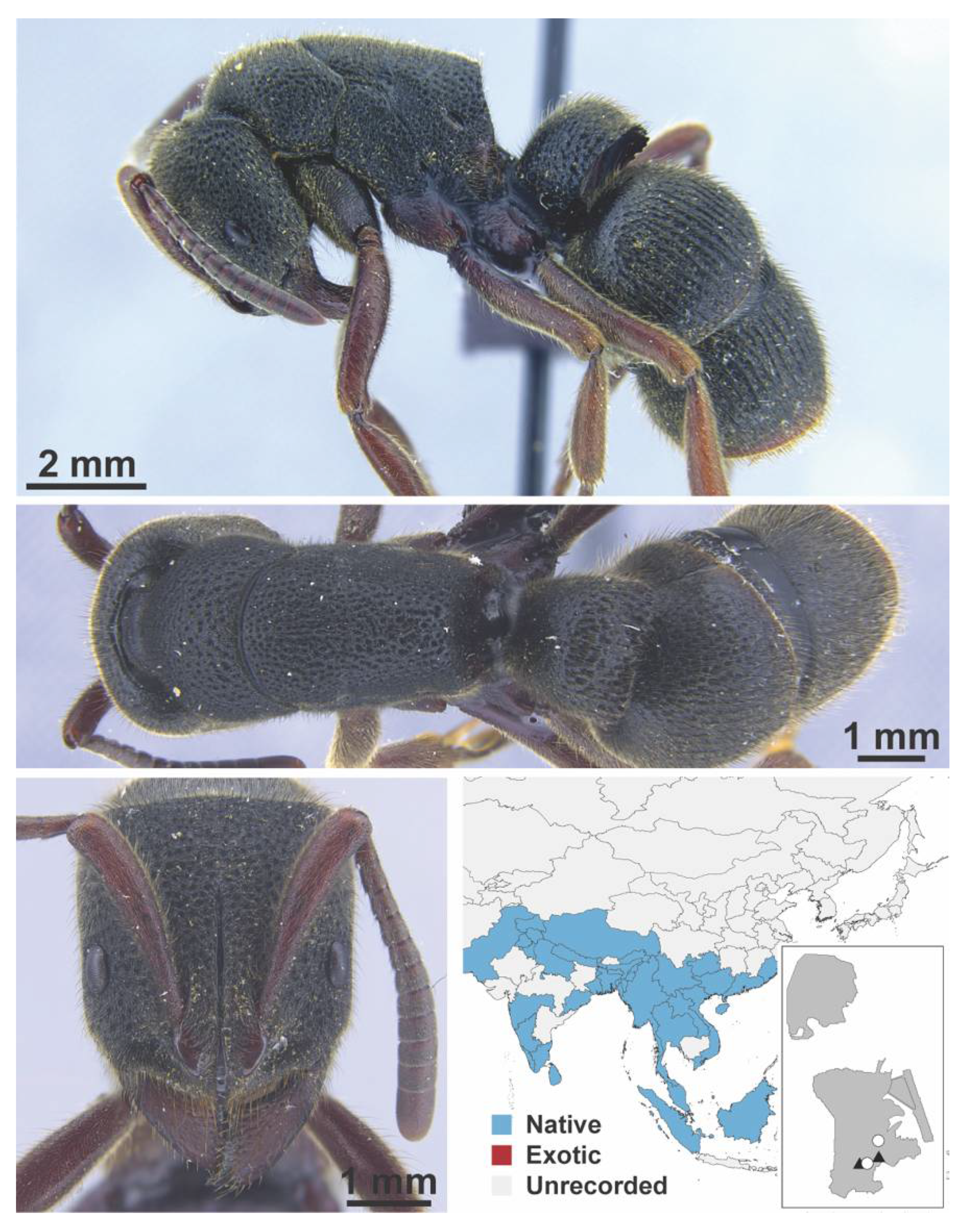
Appendix A.8. PROCERATIINAE

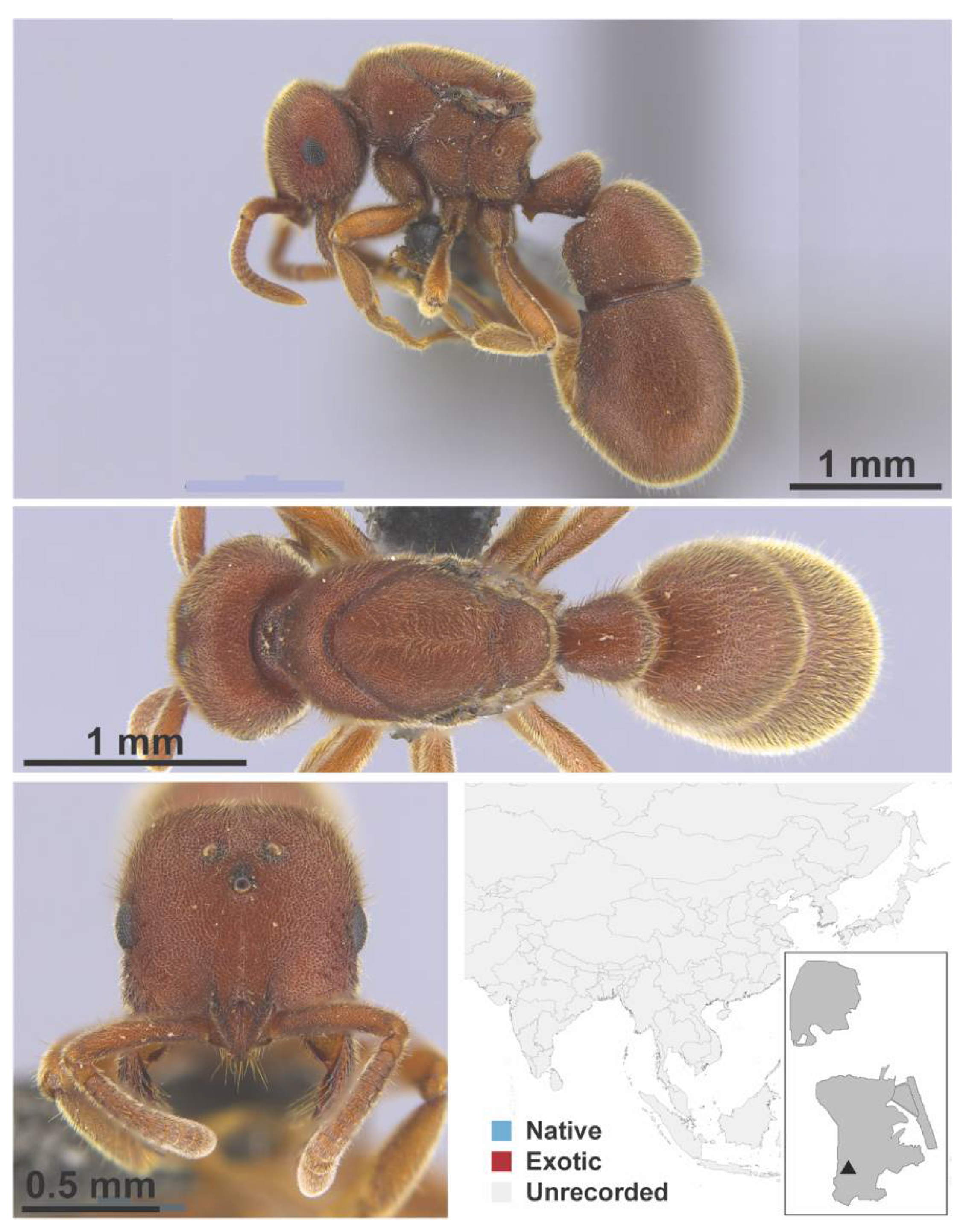
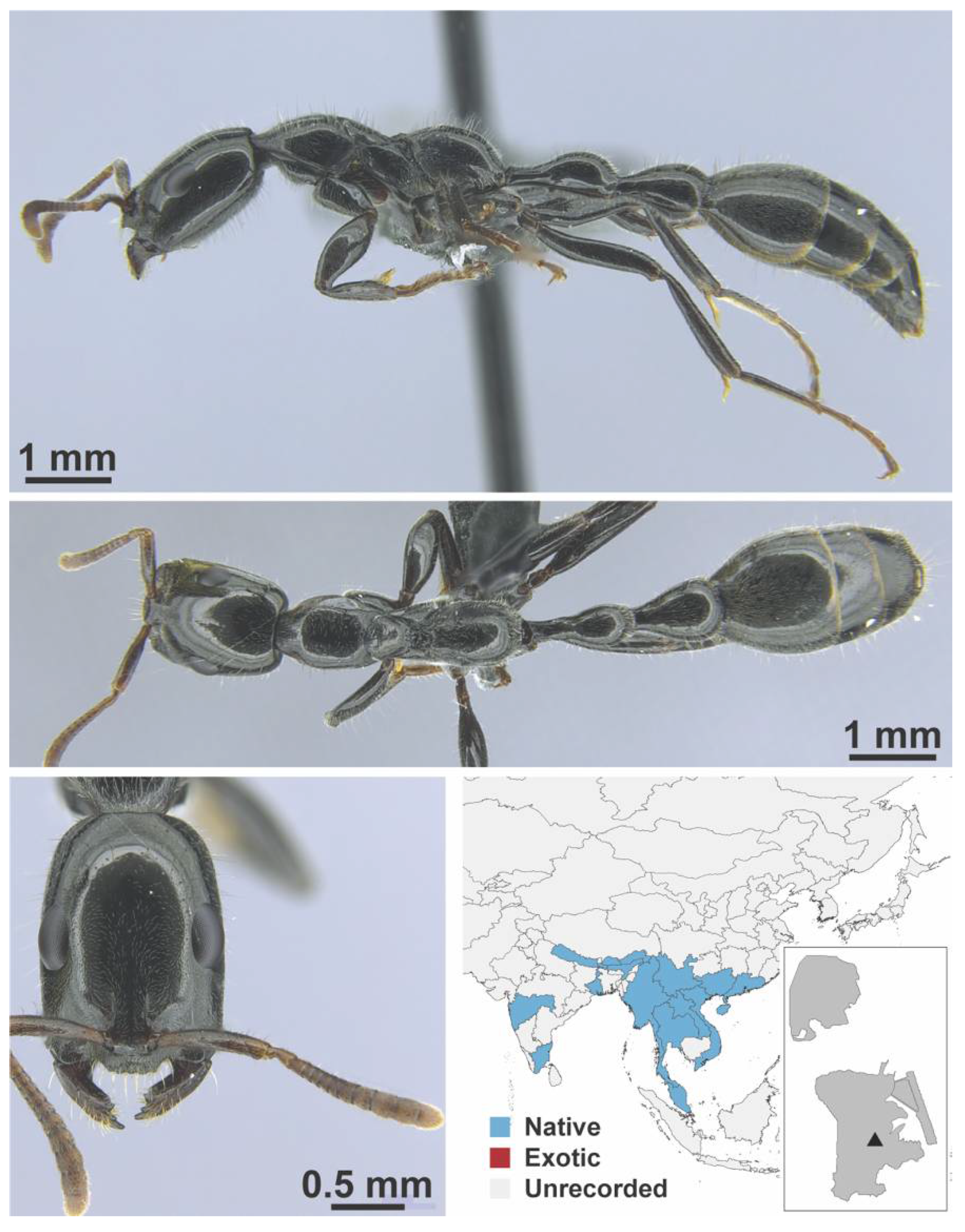
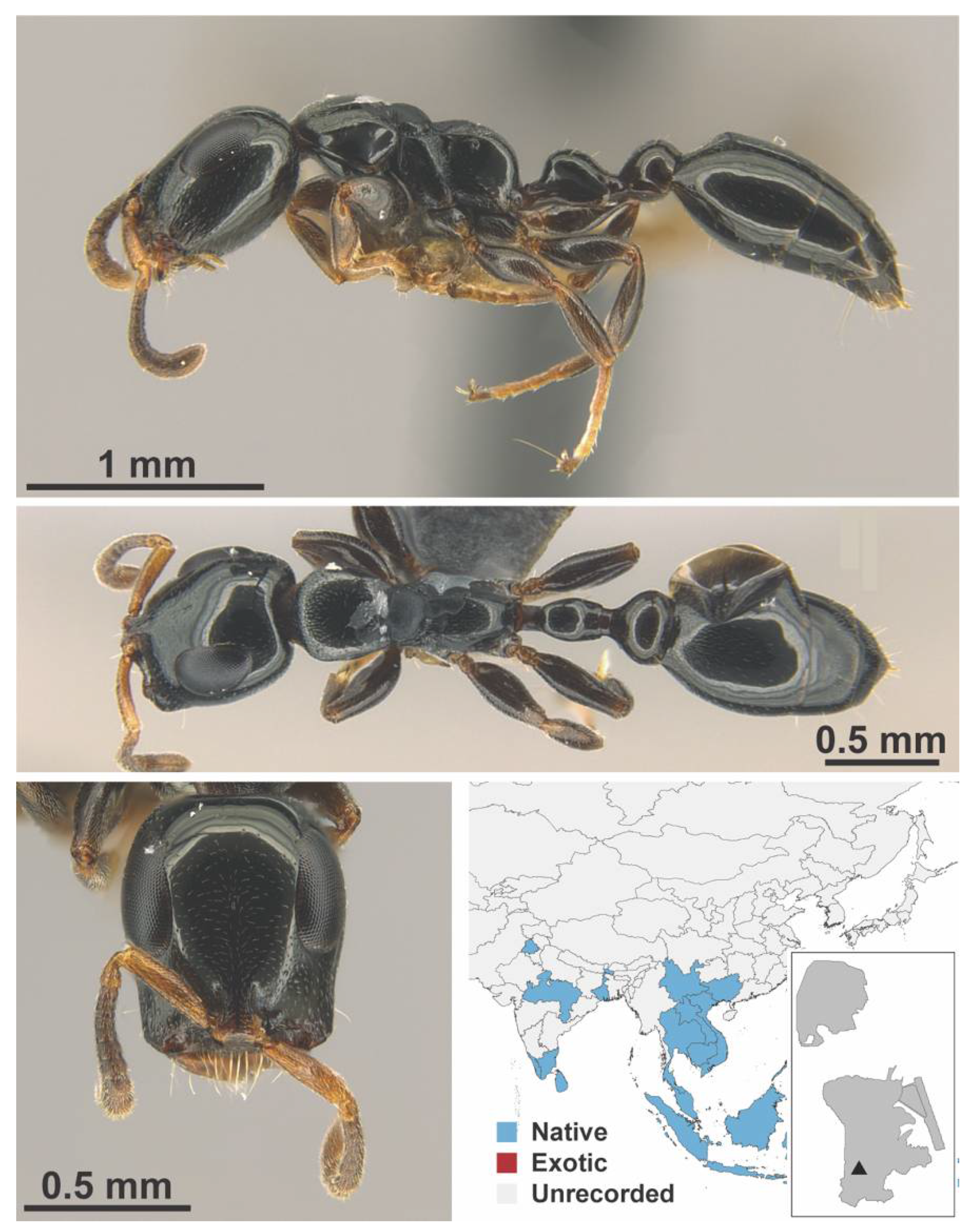
Appendix A.9. PSEUDOMYRMICINAE

References
- United Nations. World Urbanization Prospects 2018, United Nations: New York, NY, USA, 2019.
- Güneralp, B.; Seto, K.C. Futures of global urban expansion: Uncertainties and implications for biodiversity conservation. Environ. Res. Lett. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Batty, M. The size, scale, and shape of cities. Science (80-. ) 2008, 319, 769–771. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Knop, E. Biotic homogenization of three insect groups due to urbanization. Glob. Chang. Biol. 2016, 22, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Fiera, C. Biodiversity of Collembola in urban soils and their use as bioindicators for pollution. Pesqui. Agropecuária Bras. 2009, 44, 868–873. [Google Scholar] [CrossRef]
- Meyer, L.A.; Sullivan, S.M.P. Bright lights, big city: Influences of ecological light pollution on reciprocal stream-riparian invertebrate fluxes. Ecol. Appl. 2013, 23, 1322–1330. [Google Scholar] [CrossRef]
- Pompeu, P.S.; Alves, C.B.M.; Callisto, M. The effects of urbanization on biodiversity and water quality in the Rio das Velhas Basin, Brazil. Am. Fish. Soc. Symp. 2005, 47, 11–22. [Google Scholar]
- Buczkowski, G.; Richmond, D.S. The effect of urbanization on ant abundance and diversity: A temporal examination of factors affecting biodiversity. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Wang, H.F.; López-Pujol, J.; Meyerson, L.A.; Qiu, J.X.; Wang, X.K.; Ouyang, Z.Y. Biological invasions in rapidly urbanizing areas: A case study of Beijing, China. Biodivers. Conserv. 2011, 20, 2483–2509. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; Macivor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. Bioscience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Ives, C.D.; Lentini, P.E.; Threlfall, C.G.; Ikin, K.; Shanahan, D.F.; Garrard, G.E.; Bekessy, S.A.; Fuller, R.A.; Mumaw, L.; Rayner, L.; et al. Cities are hotspots for threatened species. Glob. Ecol. Biogeogr. 2016, 25, 117–126. [Google Scholar] [CrossRef]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.G.; Cilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 1–8. [Google Scholar] [CrossRef]
- Guénard, B.; Cardinal-De Casas, A.; Dunn, R.R. High diversity in an urban habitat: Are some animal assemblages resilient to long-term anthropogenic change? Urban Ecosyst. 2015, 18, 449–463. [Google Scholar] [CrossRef]
- Donnelly, R.; Marzluff, J.M. Importance of reserve size and landscape context to urban bird conservation. Conserv. Biol. 2004, 18, 733–745. [Google Scholar] [CrossRef]
- Evans, K.L.; Newson, S.E.; Gaston, K.J. Habitat influences on urban avian assemblages. Ibis (Lond.) 2009, 151, 19–39. [Google Scholar] [CrossRef]
- Shwartz, A.; Muratet, A.; Simon, L.; Julliard, R. Local and management variables outweigh landscape effects in enhancing the diversity of different taxa in a big metropolis. Biol. Conserv. 2013, 157, 285–292. [Google Scholar] [CrossRef]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Kowarik, I. On the role of alien species in urban flora and vegetation. In Urban Ecology: An International Perspective on the Interaction Between Humans and Nature; Marzluff, J., Shulenberger, E., Endlicher, W., Alberti, M., Bradley, G., Ryan, C., ZumBrunnen, C., Simon, U., Eds.; Springer: New York, NY, USA, 2008; p. 802. ISBN 9780387734118. [Google Scholar]
- Ricotta, C.; La Sorte, F.A.; Pyšek, P.; Rapson, G.L.; Celesti-Grapow, L.; Thompson, K. Phyloecology of urban alien floras. J. Ecol. 2009, 97, 1243–1251. [Google Scholar] [CrossRef]
- Costello, C.; Springborn, M.; McAusland, C.; Solow, A. Unintended biological invasions: Does risk vary by trading partner? J. Environ. Econ. Manag. 2007, 54, 262–276. [Google Scholar] [CrossRef]
- Wittig, R. The origin and development of the urban flora of Central Europe. Urban Ecosyst. 2004, 7, 323–329. [Google Scholar] [CrossRef]
- Von Der Lippe, M.; Kowarik, I. Do cities export biodiversity? Traffic as dispersal vector across urban-rural gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- Dawson, W.; Moser, D.; Van Kleunen, M.; Kreft, H.; Pergl, J.; Pyšek, P.; Weigelt, P.; Winter, M.; Lenzner, B.; Blackburn, T.M.; et al. Global hotspots and correlates of alien species richness across taxonomic groups. Nat. Ecol. Evol. 2017, 1, 1–7. [Google Scholar] [CrossRef]
- Lu, J.; Li, S.P.; Wu, Y.; Jiang, L. Are Hong Kong and Taiwan stepping-stones for invasive species to the mainland of China? Ecol. Evol. 2018, 8, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.C.; Fisher, B.L. The role of ants in conservation monitoring: If, when, and how. Biol. Conserv. 2006, 132, 166–182. [Google Scholar] [CrossRef]
- Agosti, D.; Majer, J.D.; Alonso, L.E.; Schultz, T.R. Standard Methods for Measuring and Monitoring Biodiversity; Smithsonian Institution Press: Washington, DC, USA, 2000; ISBN 1560988584. [Google Scholar]
- Hölldobler, B.; Wilson, E.O. The Ants; Belknap Press of Harvard University Press: Cambridge, MA, USA, 1990; Volume N1, ISBN 0674040759. [Google Scholar]
- Lach, L.; Parr, C.L.L.; Abbott, K. Ant Ecology, 1st ed.; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Andersen, A.N.; Majer, J.D. Ants show the way Down Under: Invertebrates as bioindicators in land management. Front. Ecol. Environ. 2004, 2, 291–298. [Google Scholar] [CrossRef]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Holway, D.A.; Lach, L.; Suarez, A.V.; Tsutsui, N.D.; Case, T.J. The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 2002, 33, 181–233. [Google Scholar] [CrossRef]
- Holway, D.A. Edge effects of an invasive species across a natural ecological boundary. Biol. Conserv. 2005, 121, 561–567. [Google Scholar] [CrossRef]
- Human, K.G.; Gordon, D.M. Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 1996, 105, 405–412. [Google Scholar] [CrossRef]
- Gerlach, J. Impact of the invasive crazy ant Anoplolepis gracilipes on Bird Island, Seychelles. J. Insect Conserv. 2004, 8, 15–25. [Google Scholar] [CrossRef]
- Allen, C.R.; Birge, H.E.; Slater, J.; Wiggers, E. The invasive ant, Solenopsis invicta, reduces herpetofauna richness and abundance. Biol. Invasions 2017, 19, 713–722. [Google Scholar] [CrossRef]
- Ness, J.H.; Bronstein, J.L. The effects of invasive ants on prospective ant mutualists. Biol. Invasions 2004, 6, 445–461. [Google Scholar] [CrossRef]
- O’Dowd, D.J.; Green, P.T.; Lake, P.S. Invasional “meltdown” on an oceanic island. Ecol. Lett. 2003, 6, 812–817. [Google Scholar] [CrossRef]
- Chan, K.H.; Guénard, B. Ecological and socio-economic impacts of the red import fire ant, Solenopsis invicta (Hymenoptera: Formicidae), on urban agricultural ecosystems. Urban Ecosyst. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Lee, C.Y. Tropical Household Ants: Pest Status, Species Diversity, Foraging Behavior, and Baiting Studies. In Proceedings of the 4th International Conference on Urban Pests, Charleston, SC, USA, 7–10 July 2002. [Google Scholar]
- Klotz, J.H.; Mangold, J.R.; Vail, K.M.; Davis, L.R.; Patterson, R.S. A survey of the urban pest ants (Hymenoptera: Formicidae) of Peninsular Florida. Florida Entomol. 1995, 78, 109–118. [Google Scholar] [CrossRef]
- Fowler, H.G.; Bueno, O.C.; Sadatsune, T.; Montelli, A.C. Ants as potential vectors of pathogens in hospitals in the state of sao paulo, brazil. Int. J. Trop. Insect Sci. 1993, 14, 367–370. [Google Scholar] [CrossRef]
- Beatson, S.H. Pharaoh’s ants as pathogen vectors in hospitals. Lancet 1972, 1, 425–427. [Google Scholar] [CrossRef]
- Brühl, C.A.; Gunsalam, G.; Linsenmair, K.E. Stratification of ants (Hymenoptera, Formicidae) in a primary rain forest in Sabah, Borneo. J. Trop. Ecol. 1998, 14, 285–297. [Google Scholar] [CrossRef]
- Martins, M.F.d.O.; Thomazini, M.J.; Baretta, D.; Brown, G.G.; da Rosa, M.G.; Zagatto, M.R.G.; Santos, A.; Nadolny, H.S.; Cardoso, G.B.X.; Niva, C.C.; et al. Accessing the subterranean ant fauna (Hymenoptera: Formicidae) in native and modified subtropical landscapes in the neotropics. Biota Neotrop. 2020, 20, 1–16. [Google Scholar] [CrossRef]
- Wilkie, K.T.R.; Mertl, A.L.; Traniello, J.F.A. Species diversity and distribution patterns of the ants of Amazonian ecuador. PLoS ONE 2010, 5, 1–12. [Google Scholar] [CrossRef]
- Wong, M.K.L.; Guénard, B. Subterranean ants: Summary and perspectives on field sampling methods, with notes on diversity and ecology (Hymenoptera: Formicidae). Myrmecol. News 2017, 25, 1–16. [Google Scholar] [CrossRef]
- Houadria, M.; Menzel, F. Digging deeper into the ecology of subterranean ants: Diversity and niche partitioning across two continents. Diversity 2021, 13, 53. [Google Scholar] [CrossRef]
- Santos, M.N. Research on urban ants: Approaches and gaps. Insectes Soc. 2016, 63, 359–371. [Google Scholar] [CrossRef]
- Leong, C.M.; Shiao, S.F.; Guénard, B. Ants in the city, a preliminary checklist of Formicidae (Hymenoptera) in Macau, one of the most heavily urbanized regions of the world. Asian Myrmecol. 2017, 9, 1–20. [Google Scholar] [CrossRef]
- Brassard, F.; Leong, C.M.; Chan, H.H.; Guénard, B. A new subterranean species and an updated checklist of Strumigenys (Hymenoptera, formicidae) from Macao SAR, China, with a key to species of the greater bay area. Zookeys 2020, 970, 63–116. [Google Scholar] [CrossRef]
- Wong, T.L.; Guénard, B. Review of ants from the genus Polyrhachis Smith (Hymenoptera:Formicidae:Formicinae) in Hong Kong and Macau, with notes on their natural history. Asian Myrmecol. 2021; 13, 1–70. [Google Scholar] [CrossRef]
- Hui, E.C.M.; Li, X.; Chen, T.; Lang, W. Deciphering the spatial structure of China’s megacity region: A new bay area—The Guangdong-Hong Kong-Macao Greater Bay Area in the making. Cities 2018, 105, 1–13. [Google Scholar] [CrossRef]
- Statistics and Census Service Yearbook of Statistics 2019. Available online: www.dsec.gov.mo (accessed on 23 July 2021).
- Grydehoj, A. Making Ground, Losing Space: Land Reclamation and Urban Public Space in Island Cities. Urban Isl. Stud. 2015, 1, 96–117. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Q.; Zhou, S.; Li, J.S.; Chen, G.Q. Urban economy’s carbon flow through external trade: Spatial-temporal evolution for Macao. Energy Policy 2017, 110, 69–78. [Google Scholar] [CrossRef]
- Ptak, R.; Souza, G.B. The Survival of Empire: Portuguese Trade and Society in China and the South China Sea, 1630–1754. J. Am. Orient. Soc. 1988, 108, 1630–1754. [Google Scholar] [CrossRef]
- Leong, C.M.; Yamane, S.; Guénard, B. Lost in the city: Discovery of the rare ant genus Leptanilla (Hymenoptera: Formicidae) in Macau with description of Leptanilla macauensis sp. nov. Asian Myrmecol. 2018, 10, 1–16. [Google Scholar] [CrossRef]
- Kouakou, L.M.M.; Yeo, K.; Kone, M.; Ouattara, K.; Kouakou, A.K.; Delsinne, T.; Dekoninck, W. Espaces verts comme une alternative de conservation de la biodiversité en villes: Le cas des fourmis (Hyménoptère: Formicidae) dans le district d’Abidjan (Côte d’Ivoire). J. Appl. Biosci. 2019, 131, 13359–13381. [Google Scholar] [CrossRef]
- Yeo, K.; Dekoninck, W.; Kouakou, L.M.; Ouattara, K.; Konate, S. Detecting intruders: Assessment of the anthropophilic ant fauna (Hymenoptera: Formicidae) in the city of Abidjan and along access roads in Banco National Park (Côte d’Ivoire). J. Entomol. Zool. Stud. JEZS 2016, 4, 351–359. [Google Scholar]
- Kasseney, B.D.; N’tie, T.B.; Nuto, Y.; Wouter, D.; Yeo, K.; Glitho, I.A. Diversity of ants and termites of the botanical garden of the university of lomé, Togo. Insects 2019, 10, 218. [Google Scholar] [CrossRef]
- Taheri, A.; Wetterer, J.K.; Reyes-López, J. Tramp ants of Tangier, Morocco. Trans. Am. Entomol. Soc. 2017, 143, 267–270. [Google Scholar] [CrossRef]
- Bernard, F. Fourmis des villes et fourmis du bled entre Rabat et Tanger. Société Sci. Nat. Phys. Maroc 1958, 38, 131–142. [Google Scholar]
- Bernard, F. Les Fourmis des rues de Kenitra (Maroc) [Hym.]. Bull. Société Entomol. Fr. 1974, 79, 178–183. [Google Scholar]
- Reyes-López, J.; Carpintero, S. Comparison of the exotic and native ant communities (Hymenoptera: Formicidae) in urban green areas at inland, Coastal and insular sites in Spain. Eur. J. Entomol. 2014, 111, 421–428. [Google Scholar] [CrossRef]
- Ito, F.; Yamane, S.; Eguchi, K.; Noerdjito, W.A.; Kahono, S.; Tsuji, K.; Ohkawara, K.; Yamauchi, K.; Nishida, T.; Nakamura, K. Ant Species Diversity in the Bogor Botanic Garden, West Java, Indonesia, with Descriptions of Two New Species of the Genus Leptanilla (Hymenoptera, Formicidae). Tropics 2001, 10, 379–404. [Google Scholar] [CrossRef][Green Version]
- Rizali, A.; Bos, M.M.; Buchori, D.; Yamane, S.; Schulze, C.H. Ants in Tropical Urban Habitats: The Myrmecofauna in a Densely Populated Area of Bogor, West Java, Indonesia. HAYATI J. Biosci. 2008, 15, 77–84. [Google Scholar] [CrossRef]
- Terayama, M. Ants from the Akasaka Imperial Gardens, Tokyo. Mem. Natl. Sci. Museum 2005, 39, 239–243. [Google Scholar]
- Terayama, M. Ants from the Tokiwamatsu Imperial Gardens, Tokyo. Mem. Natl. Sci. Museum Tokyo 2005, 39, 245–247. [Google Scholar]
- Terayama, M. Ants (Formicidae) from the imperial Palace, Tokyo. Mem. Natl. Sci. Museum Tokyo 2014, 50, 527–535. [Google Scholar]
- Matsumura, S.; Yamane, S. Species composition and dominant species of ants in Jigenji Park, Kagoshima City, Japan. Nat. Kagoshima 2012, 38, 99–107. [Google Scholar]
- Liu, K.L.; Peng, M.H.; Hung, Y.C.; Neoh, K.B. Effects of park size, peri-urban forest spillover, and environmental filtering on diversity, structure, and morphology of ant assemblages in urban park. Urban Ecosyst. 2019, 22, 643–656. [Google Scholar] [CrossRef]
- Natuhara, Y. Ant faunae in Osaka City and three sites in Osaka Prefecture. ARI J. Myrmecol. Soc. Jpn. 1998, 22, 1–5. [Google Scholar]
- Tan, C.K.W.; Corlett, R.T. Scavenging of dead invertebrates along an urbanisation gradient in Singapore. Insect Conserv. Divers. 2012, 5, 138–145. [Google Scholar] [CrossRef]
- Harada, Y.; Koto, S.; Kawaguchi, N.; Sato, K.; Setoguchi, T.; Muranaga, R.; Yamashita, H.; Yo, A.; Yamane, S. Ants of Jusso, Isa City, Kagoshima Prefecture, southwestern Japan. Bull. Biogeogr. Soc. Jpn. 2012, 67, 143–152. [Google Scholar]
- Iwata, K.; Eguchi, K.; Yamane, S. A Case Study on Urban Ant Fauna of Southern Kyusyu, Japan, with Notes on a New Monitoring Protocol (Insecta, Hymenoptera, Formicidae). J. Asia. Pac. Entomol. 2005, 8, 263–272. [Google Scholar] [CrossRef]
- Yamaguchi, T. Influence of urbanization on ant distribution in parks of Tokyo and Chiba City, Japan I. Analysis of ant species richness. Ecol. Res. 2004, 19, 209–216. [Google Scholar] [CrossRef]
- Park, S.H.; Hosoishi, S.; Ogata, K.; Kasuya, E. Changes of species diversity of ants over time: A case study in two urban parks. J. Fac. Agric. Kyushu Univ. 2014, 59, 71–76. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Xu, Z. Distribution patterns of ant species in Shenzhen City. J. Southwest For. Univ. 2012, 32, 69–74. [Google Scholar]
- Khot, K.; Quadros, G.; Somani, V. Ant Diversity in an urban garden at Mumbai, Maharashtra. In Proceedings of the National Conference on Biodiversity: Status and Challenges in Conservation-FAVEO, Thane, India, 29–30 November 2013; 2013, pp. 121–125. [Google Scholar]
- Hiroyuki, Y. List of species of Hymenoptera and Diptera in Matsuyama City, Ehime Prefecture, Shikoku, Japan. Comm. Surv. Nat. Environ. Matsuyama City 2012, 167–176. [Google Scholar]
- Harada, Y.; Yamasaki, M.; Saito, N.; Higasayama, U. Spreading of Technomyrmex brunneus in Shiroyama Park, Kagoshima City, southern Japan. Nat. Kagoshima 2021, 47, 275–279. [Google Scholar]
- Miyake, K. Effect of Argentine ant invasions on Japanese ant fauna in Hiroshima Prefecture, western Japan: Preliminary report (Hymenoptera: Formicidae). Sociobiology 2002, 39, 65–474. [Google Scholar]
- Tan, S.; Wei, H.; Liu, D. An investigation of ant species in houses and courtyards in the Chengdu area. Sichuan J. Zool. 2009, 28, 870–873. [Google Scholar]
- Park, S.H.; Hosoishi, S.; Ogata, K. Long-term impacts of argentine ant invasion of urban parks in Hiroshima, Japan. J. Ecol. Environ. 2014, 37, 123–129. [Google Scholar] [CrossRef][Green Version]
- Touyama, Y.; Ogata, K.; Sugiyama, T. The Argentine ant, Linepithema humile, in Japan: Assessment of impact on species diversity of ant communities in urban environments. Entomol. Sci. 2003, 6, 57–62. [Google Scholar] [CrossRef]
- Harada, Y.; Yamashita, M. Ants in the parks of the ruins of castles in Shikoku, western Japan. Nat. Kagoshima 2019, 46, 171–175. [Google Scholar]
- Malozemova, L.A.; Malozemov, Y.A. Ecological Peculiarities of Ants in Urbanized Areas. Russ. J. Ecol. 1999, 30, 283–286. [Google Scholar]
- Harada, Y. Ant fauna in Joyama Park, Hioki City, Kagoshima Prefecture, Japan, after the Technomyrmex brunneus invasion. Nat. Kagoshima 2020, 47, 173–178. [Google Scholar]
- Roshanak, T.; Pashaei Rad, S.; Shokri, M. Faunistic investigation of ant (Hymenoptera: Formicidae) in the vicinity of Shiraz. Environ. Sci. 2017, 15, 75–91. [Google Scholar]
- Hosoishi, S.; Rahman, M.M.; Murakami, T.; Park, S.H.; Kuboki, Y.; Ogata, K. Winter activity of ants in an urban area of western Japan. Sociobiology 2019, 66, 414–419. [Google Scholar] [CrossRef]
- Terayama, M. Effects of the invasive ants Linepithema humile (Hymenoptera: Formicidae) on native ant and homopterous insect ommunities in Japan. ARI J. Myrmecol. Soc. Jpn. 2006, 28, 13–27. [Google Scholar]
- Putyatina, T.S.; Perfilieva, K.S.; Zakalyukina, Y.V. Typification of urban habitats, with ant assemblages of Moscow city taken as an example. Entomol. Rev. 2017, 97, 1053–1062. [Google Scholar] [CrossRef]
- Kumar, D.; Mishra, A. Ant community variation in urban and agricultural ecosystems in Vadodara District (Gujarat State), western India. Asian Myrmecol. 2008, 2, 85–93. [Google Scholar]
- Antonov, I.A. Ant Assemblages of Two Cities with Different Ecological Conditions in Southern Cisbaikalia. Russ. J. Ecol. 2008, 39, 454–456. [Google Scholar] [CrossRef]
- Hosaka, T.; Di, L.; Eguchi, K.; Numata, S. Ant assemblages on littered food waste and food removal rates in urban–suburban parks of Tokyo. Basic Appl. Ecol. 2019, 37, 1–9. [Google Scholar] [CrossRef]
- Blinova, S.V. Changes in ant assemblages under conditions of a large industrial center. Russ. J. Ecol. 2008, 39, 148–150. [Google Scholar] [CrossRef]
- Meshram, H.; Chavahan, P.; Sangode, V.; Khandwekar, V. Distribution of ant fauna in different terrestrial ecosystem in and around Nagpur city, Maharashtra India. J. Entomol. Zool. Stud. 2015, 3, 215–218. [Google Scholar]
- Yong, G.W.J.; Wong, M.K.L.; Soh, E.J.Y. A preliminary checklist of the ant genera of Pulau Ubin, Singapore, from rapid opportunistic sampling (Hymenoptera: Formicidae). Nat. Singap. 2017, 10, 55–66. [Google Scholar]
- Ossola, A.; Nash, M.A.; Christie, F.J.; Hahs, A.K.; Livesley, S.J. Urban habitat complexity affects species richness but not environmental filtering of morphologically-diverse ants. PeerJ 2015, 3, 1–19. [Google Scholar] [CrossRef]
- Heterick, B.E.; Lythe, M.; Smithyman, C. Urbanisation factors impacting on ant (Hymenoptera: Formicidae) biodiversity in the Perth metropolitan area, Western Australia: Two case studies. Urban Ecosyst. 2013, 16, 145–173. [Google Scholar] [CrossRef]
- Majer, J.D.; Brown, K.R. The effects of urbanization on the ant fauna of the Swan Coastal Plain near Perth, Western Australia. J. R. Soc. West. Aust. 1986, 69, 13–17. [Google Scholar]
- Callan, S.K.; Mater, J.D. Impacts of an incursion of African Big-headed Ants, Pheidole megacephala (Fabricius), in urban bushland in Perth, Western Australia. Pacific Conserv. Biol. 2009, 15, 102–115. [Google Scholar] [CrossRef]
- Heterick, B.E.; Majer, J.D. Influence of Argentine and coastal brown ant (Hymenoptera: Formicidae) invasions on ant communities in Perth gardens, Western Australia. Urban Ecosyst. 2000, 4, 277–292. [Google Scholar] [CrossRef]
- May, J.E.; Heterick, B.E. Effects of the coastal brown ant Pheidole megacephala (Fabricius), on the ant fauna of the Perth metropolitan region, Western Australia. Pacific Conserv. Biol. 2000, 6, 81–85. [Google Scholar] [CrossRef]
- Radchenko, A.G.; Stukalyuk, S.V.; Netsvetov, M.V. Ants (Hymenoptera, Formicidae) of Kyiv. Entomol. Rev. 2019, 99, 753–773. [Google Scholar] [CrossRef]
- Ordóñez-Urbano, C.; Reyes López, J.; Carpintero-Ortega, S. ¿Una especie alóctona se puede ser “rara”?: El caso de Pyramica membranifera (Hymenoptera, Formicidae). Bol. Soc. Entomológica Aragon. 2008, 42, 321–323. [Google Scholar]
- Antonova, V.; Penev, L. Change in the zoogeographical structure of ants caused by urban pressure in the Sofia region (Bulgaria). Mymecol. News 2006, 8, 271–276. [Google Scholar]
- Penev, L.; Stoyanov, I.; Dedov, I.; Antonova, V. Patterns of Urbanisation in the City of Sofia as Shown by Carabid Beetles (Coleoptera, Carabidae), Ants (Hymenoptera, Formicidae), and Terrestrial Gastropods (Mollusca, Gastropoda Terrestria). In Back to the Roots and Back to the Future: Towards A New Synthesis Amongst Taxonomic, Ecological and Biogeographical Approaches in Carabidology, Proceedings of the XIII European Carabidologists Meeting, Blagoevgrad, Bulgaria, 20–24 August 2007; Pensoft: Moscow, Russia, 2008; pp. 483–509. [Google Scholar]
- Antonova, V.; Lyubomir, P. Classification of Ant Assemblages (Hymenoptera: Formicidae) in Green Areas of Sofia. Acta Zool. Bulg. 2008, 2, 103–110. [Google Scholar] [CrossRef]
- Dauber, J.; Eisenbeis, G. Untersuchungen zur Ameisenfauna einer urbanen Landschaft am Beispiel der Stadt Mainz. Abh. Ber. Naturkundemus. Görlitz 1997, 69, 237–244. [Google Scholar]
- Pisarski, B.; Czechowski, W. Influence de la pression urbaine sur la myrmécofaune. Memorab. Zool. 1978, 29, 109–128. [Google Scholar]
- Pisarski, B. Ants (Hymenoptera, Formicoidea) of Warzaw and Mazovia. Memorab. Zool. 1982, 36, 73–90. [Google Scholar]
- Heras, P.R.; Ibáñez, M.D.M.; Sañudo, F.J.C.; Martínez, M.d.l.Á.V. Primeros datos de Formícidos (Hymenoptera, Formicidae) en parques urbanos de Madrid. Boletín la Asoc. Española Entomol. 2011, 35, 93–112. [Google Scholar]
- Klesniaková, M.; Holecová, M.; Pavlíková, A. Interesting ant species in the urban greenery of Bratislava. Folia Faun. Slovaca 2016, 21, 235–238. [Google Scholar]
- Trigos-Peral, G.; Rutkowski, T.; Witek, M.; Ślipiński, P.; Babik, H.; Czechowski, W. Three categories of urban green areas and the effect of their different management on the communities of ants, spiders and harvestmen. Urban Ecosyst. 2020, 23, 803–818. [Google Scholar] [CrossRef]
- Ješovnik, A.; Bujan, J. Wooded areas promote species richness in urban parks. Urban Ecosyst. 2021, 1–11. [Google Scholar] [CrossRef]
- Behr, D.; Lippke, S.; Cölln, K. Zur Kenntnis der Ameisen von Köln (Hymenoptera, Formicidae). Dechen. Beih. 1996, 35, 215–232. [Google Scholar]
- Ślipiński, P.; ZMihorski, M.; Czechowski, W. Species diversity and nestedness of ant assemblages in an urban environment. Eur. J. Entomol. 2012, 109, 197–206. [Google Scholar] [CrossRef]
- Behr, D.; Cölln, K. Zur Ameisenfauna (Hymenoptera, Formicidae) von Gönnersdorf. Dendrocopos 1993, 20, 148–160. [Google Scholar]
- Rigato, F.; Wetterer, J.K. Ants (Hymenoptera: Formicidae) of San Marino. Nat. Hist. Sci. 2018, 5. [Google Scholar] [CrossRef]
- Espadaler, X.; López-Soria, L. Rareness of certain Mediterranean ant species: Fact or artifact? Insectes Soc. 1991, 38, 365–377. [Google Scholar] [CrossRef]
- Vepsäläinen, K.; Ikonen, H.; Koivula, M.J. The structure of ant assemblages in an urban area of Helsinki, southern Finland. Ann. Zool. Fennici 2008, 45, 109–127. [Google Scholar] [CrossRef]
- Trigos-Peral, G.; Reyes-López, J.L. Las hormigas como bioindicadores para la gestión de zonas verdes urbanas en el sur de la Península Ibérica. Propuesta de grupos funcionales. Iberomyrmex 2016, 8, 37–38. [Google Scholar]
- Stukalyuk, S.V. Stratification of the ant species (Hymenoptera, Formicidae) in the urban broadleaf woodlands of the city of Kiev. Entomol. Rev. 2017, 97, 320–343. [Google Scholar] [CrossRef]
- Reyes López, J.L.; Taheri, A. First checklist of ants (Hymenoptera, Formicidae) of urban green areas in Cádiz (Andalusia, Spain). Boletín Asoc. Española Entomol. 2018, 42, 217–223. [Google Scholar]
- Smith, J.; Chapman, A.; Eggleton, P. Baseline biodiversity surveys of the soil macrofauna of London’s green spaces. Urban Ecosyst. 2006, 9, 337–349. [Google Scholar] [CrossRef]
- Gaspar, C.; Thirion, C. Modification des populations d’Hyménoptères sociaux dans des milieux anthropogènes. Memorab. Zool. 1978, 29, 61–77. [Google Scholar]
- Nuhn, T.P.; Wright, C.G. An Ecological Survey of Ants (Hymenoptera: Formicidae) in a Landscaped Suburban Habitat. Am. Midl. Nat. 1979, 102, 353–362. [Google Scholar] [CrossRef]
- Baena, M.L.; Escobar, F.; Valenzuela, J.E. Diversity snapshot of green–gray space ants in two Mexican cities. Int. J. Trop. Insect Sci. 2020, 40, 239–250. [Google Scholar] [CrossRef]
- Menke, S.B.; Guénard, B.; Sexton, J.O.; Weiser, M.D.; Dunn, R.R.; Silverman, J. Urban areas may serve as habitat and corridors for dry-adapted, heat tolerant species; an example from ants. Urban Ecosyst. 2011, 14, 135–163. [Google Scholar] [CrossRef]
- Miguelena, J.G.; Baker, P.B. Effects of Urbanization on the Diversity, Abundance, and Composition of Ant Assemblages in an Arid City. Environ. Entomol. 2019, 48, 836–846. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Castaño-Meneses, G. Effects of urbanization on the diversity of ant assemblages in tropical dry forests, Mexico. Urban Ecosyst. 2015, 18, 1373–1388. [Google Scholar] [CrossRef]
- Toennisson, T.A.; Sanders, N.J.; Klingeman, W.E.; Vail, K.M. Influences on the structure of suburban ant (Hymenoptera: Formicidae) communities and the abundance of Tapinoma sessile. Environ. Entomol. 2011, 40, 1397–1404. [Google Scholar] [CrossRef]
- Suarez, A.V.; Bolger, D.T.; Case, T.J. Effects of fragmentation and invasion on native ant communities in coastal southern California. Ecology 1998, 79, 2041. [Google Scholar] [CrossRef]
- Gochnour, B.M.; Suiter, D.R.; Booher, D. Ant (Hymenoptera: Formicidae) Fauna of the Marine Port of Savannah, Garden City, Georgia (USA). J. Entomol. Sci. 2019, 54, 417–429. [Google Scholar] [CrossRef]
- Savage, A.M.; Hackett, B.; Guénard, B.; Youngsteadt, E.K.; Dunn, R.R. Fine-scale heterogeneity across Manhattan’s urban habitat mosaic is associated with variation in ant composition and richness. Insect Conserv. Divers. 2015, 8, 216–228. [Google Scholar] [CrossRef]
- Friedrich, R.; Philpott, S.M. Nest-site limitation and nesting resources of ants (Hymenoptera: Formicidae) in urban green spaces. Environ. Entomol. 2009, 38, 600–607. [Google Scholar] [CrossRef]
- García-Martínez, M.A.; Vanoye-Eligio, V.; Leyva-Ovalle, O.R.; Zetina-Córdoba, P.; Aguilar-Méndez, M.J.; Rosas-Mejía, M. Diversity of ants (hymenoptera: Formicidae) in a sub-montane and sub-tropical cityscape of northeastern Mexico. Sociobiology 2019, 66, 440–447. [Google Scholar] [CrossRef]
- Fairweather, A.D.; Lewis, J.H.; Hunt, L.; McAlpine, D.F.; Smith, M.A. Ants (Hymenoptera: Formicidae) of Rockwood Park, New Brunswick: An Assessment of Species Richness and Habitat. Northeast. Nat. 2020, 27, 576–584. [Google Scholar] [CrossRef]
- Uno, S.; Cotton, J.; Philpott, S.M. Diversity, abundance, and species composition of ants in urban green spaces. Urban Ecosyst. 2010, 13, 425–441. [Google Scholar] [CrossRef]
- Ivanov, K.; Keiper, J. Ant (Hymenoptera: Formicidae) diversity and community composition along sharp urban forest edges. Biodivers. Conserv. 2010, 19, 3917–3933. [Google Scholar] [CrossRef]
- Lessard, J.-P.; Christopher, M. The effects of urbanization on ant assemblages (Hymenoptera: Formicidae) associated with the Molson Nature Reserve, Quebec Jean-Philippe. Entomol. Soc. Can. 2005, 137, 215–225. [Google Scholar] [CrossRef]
- Clarke, K.M.; Fisher, B.L.; Lebuhn, G. The influence of urban park characteristics on ant (Hymenoptera, Formicidae) communities. Urban Ecosyst. 2008, 11, 317–334. [Google Scholar] [CrossRef]
- King, T.G.; Green, S.C. Factors affecting the distribution of pavement ants (Hymenoptera: Formicidae) in Atlantic coast urban fields. Entomol. News 1995, 106, 224–228. [Google Scholar]
- Staubus, W.J.; Boyd, E.S.; Adams, T.A.; Spear, D.M.; Dipman, M.M.; Meyer, W.M. Ant communities in native sage scrub, non-native grassland, and suburban habitats in Los Angeles County, USA: Conservation implications. J. Insect Conserv. 2015, 19, 669–680. [Google Scholar] [CrossRef]
- Pećarević, M.; Danoff-Burg, J.; Dunn, R.R. Biodiversity on broadway—enigmatic diversity of the societies of ants (Formicidae) on the streets of New York City. PLoS ONE 2010, 5, 1–8. [Google Scholar] [CrossRef]
- Thompson, B.; McLachlan, S. The effects of urbanization on ant communities and myrmecochory in Manitoba, Canada. Urban Ecosyst. 2007, 10, 43–52. [Google Scholar] [CrossRef]
- Villar, P.G.; Ríos-Casanova, L. Diversidad de hormigas (Hymenoptera: Formicidae) de La Cantera Oriente, una reserva dentro de la ciudad de México. Dugesiana 2020, 27, 29–36. [Google Scholar]
- Stahlschmidt, Z.R.; Johnson, D. Moving targets: Determinants of nutritional preferences and habitat use in an urban ant community. Urban Ecosyst. 2018, 21, 1151–1158. [Google Scholar] [CrossRef]
- Marussich, W.A.; Faeth, S.H. Effects of urbanization on trophic dynamics of arthropod communities on a common desert host plant. Urban Ecosyst. 2009, 12, 265–286. [Google Scholar] [CrossRef]
- Pacheco, R.; Vasconcelos, H.L. Invertebrate conservation in urban areas: Ants in the Brazilian Cerrado. Landsc. Urban Plan. 2007, 81, 193–199. [Google Scholar] [CrossRef]
- Santos, M.N.; Delabie, J.H.C.; Queiroz, J.M. Biodiversity conservation in urban parks: A study of ground-dwelling ants (Hymenoptera: Formicidae) in Rio de Janeiro City. Urban Ecosyst. 2019, 22, 927–942. [Google Scholar] [CrossRef]
- Santos-Silva, L.; Vicente, R.E.; Feitosa, R.M. Ant species (Hymenoptera, Formicidae) of forest fragments and urban areas in a meridional amazonian landscape. Check List 2016, 12, 1–7. [Google Scholar] [CrossRef]
- De Souza, D.R.; Dos Santos, S.G.; De Munhae, C.B.; De Morini, M.S.C. Diversity of epigeal ants (Hymenoptera: Formicidae) in urban areas of Alto Tietê. Sociobiology 2012, 59, 703–717. [Google Scholar] [CrossRef]
- Lutinski, J.A.; Lopes, B.C.; de Morais, A.B.B. Diversidade de formigas urbanas (Hymenoptera: Formicidae) de dez cidades do sul do Brasil. Biota Neotrop. 2013, 13, 332–342. [Google Scholar] [CrossRef]
- Munhae, C.d.B.; Souza-Campana, D.R.d.; Kamura, C.M.; Castro Morini, M.S.d. Ant communities (Hymenoptera: Formicidae) in urban centers of the Alto Tietê, São Paulo, Brazil. Arq. Inst. Biol. (Sao Paulo) 2015, 82, 1–5. [Google Scholar] [CrossRef]
- Morini, M.S.d.C.; Munhae, C.d.B.; Leung, R.; Candiani, D.F.; Voltolini, J.C. Comunidades de formigas (Hymenoptera, Formicidae) em fragmentos de Mata Atlântica situados em áreas urbanizadas. Iheringia. Série Zool. 2007, 97, 246–252. [Google Scholar] [CrossRef]
- Iop, S.; Lutinski, J.A.; Roberto, F.; Garcia, M. Formigas urbanas da cidade de Xanxerê, Santa Catarina, Brasil. Biotemas 2009, 22, 55–64. [Google Scholar] [CrossRef][Green Version]
- Matheus Caldart, V.; Iop, S.; Antonio Lutinski, J.; Roberto Mello Garcia, F. Diversidade de formigas (Hymenoptera, Formicidae) do perímetro urbano do município de Chapecó, Santa Catarina, Brasil. Rev. Bras. Zoociências 2012, 14, 81–94. [Google Scholar]
- Ilha, C.; Maestri, R.; Antonio Lutinski, J. Assembleias de formigas (Hymenoptera: Formicidae) em áreas verdes urbanas de Chapecó, Santa Catarina. Acta Ambient. Catarin. 2017, 14, 39–50. [Google Scholar]
- Josens, R.; Sola, F.; Lois-Milevicich, J.; Mackay, W. Urban ants of the city of Buenos Aires, Argentina: Species survey and practical control. Int. J. Pest Manag. 2017, 63, 1–11. [Google Scholar] [CrossRef]
- Kamura, C.M.; Morini, M.S.C.; Figueiredo, C.J.; Bueno, O.C.; Campos-Farinha, A.E.C. Ant communities (Hymenoptera: Formicidae) in an urban ecosystem near the Atlantic Rainforest. Braz. J. Biol. 2007, 67, 635–641. [Google Scholar] [CrossRef][Green Version]
- Santiago, G.S.; Campos, R.B.F.; Ribas, C.R. How does landscape anthropization affect the myrmecofauna of urban forest fragments? Sociobiology 2018, 65, 441–448. [Google Scholar] [CrossRef]
- De Souza-Campana, D.R.; Da Silva, O.G.M.; Menino, L.; Morini, M.S.D.C. Epigaeic ant (hymenoptera: Formicidae) communities in urban parks located in atlantic forest biome. Check List 2016, 12, 1967. [Google Scholar] [CrossRef]
- Piva, A.; Campos, A.E.D.C. Ant community structure (Hymenoptera: Formicidae) in two neighborhoods with different urban profiles in the city of São Paulo, Brazil. Psyche (Lond.) 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Alfonso Simonetti, J.; Matienzo Brito, Y.; Vázquez Moreno, L. Fauna de hormigas (Hymenoptera: Formicidae) asociadas a un sistema de producción agrícola urbano. Fitosanidad 2010, 14, 153–158. [Google Scholar]
- Ribeiro, F.M.; Sibinel, N.; Ciocheti, G.; Campos, A.E.C. Analysis of ant communities comparing two methods for sampling ants in an urban park in the city of São Paulo, Brazil. Sociobiology 2012, 59, 971–984. [Google Scholar] [CrossRef]
- Lutinski, J.A.; Roberto Mello Garcia, F. Análise faunística de Formicidae (Hymenoptera: Apocrita) em ecossistema degradado no município de Chapecó, Santa Catarina. Biotemas 2005, 18, 73–86. [Google Scholar]
- Lange, D.; Vilela, A.; Erdogmus, G.; Barbosa, A. Temporal dynamic of foraging of epigeic ants in an urban forest fragment. Bioscience 2015, 31, 1501–1511. [Google Scholar] [CrossRef]
- Starr, C.K.; Ballah, S.T. Urban Ant Fauna of Port of Spain, Trinidad, West Indies. Living World J. Trinidad Tobago F. Nat. Club 2017, 49–50. [Google Scholar]
- Soares, N.S.; Almeida, L.D.O.; Gonçalves, C.A.; Marcolino, M.T.; Bonetti, E.A.M. Survey of ants (Hymenoptera: Formicidae) in the urban area of Uberlândia, MG, Brazil. Neotrop. Entomol. 2006, 35, 324–328. [Google Scholar] [CrossRef][Green Version]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Macao Meteorological and Geophysical Bureau Macao Climate: 30-year Statistics of Some Meteorological Elements. Available online: https://www.smg.gov.mo/en/subpage/348/page/252 (accessed on 1 June 2021).
- Governo da Região Administrativa Especial de Macau Dirreção dos Serviços de Cartografia e Cadastro Land Area Statistics. Available online: http:/www.dscc.gov.mo/ENG/knowledge/geo_statistic.html (accessed on 23 June 2021).
- IACM Macao Forest. Joint Publishing (Hong Kong). 2017; ISBN1 978-962-04-4138-7. ISBN2 962-04-4138-9. [Google Scholar]
- Booher, D.; Macgown, J.A.; Hubbell, S.P.; Duffield, R.M. Density and Dispersion of Cavity Dwelling Ant Species in Nuts of Eastern US Forest Floors. Trans. Am. Entomol. Soc. 2017, 143, 79–93. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Liu, C.; Guénard, B.; Garcia, F.H.; Yamane, S.; Blanchard, B.; Yang, D.R.; Economo, E. New records of ant species from Yunnan, China. Zookeys 2015, 477, 17–78. [Google Scholar] [CrossRef]
- Guénard, B.; Weiser, M.D.; Dunn, R.R. Global models of ant diversity suggest regions where new discoveries are most likely are under disproportionate deforestation threat. Proc. Natl. Acad. Sci. USA 2012, 109, 7368–7373. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016. [Google Scholar] [CrossRef]
- Guénard, B.; Weiser, M.D.; Gómez, K.; Narula, N.; Economo, E.P. The Global Ant Biodiversity Informatics (GABI) database: Synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae). Myrmecol. News 2017, 24, 83–89. [Google Scholar] [CrossRef]
- Janicki, J.; Narula, N.; Ziegler, M.; Guénard, B.; Economo, E.P. Visualizing and interacting with large-volume biodiversity data using client-server web-mapping applications: The design and implementation of antmaps.org. Ecol. Inform. 2016, 32, 185–193. [Google Scholar] [CrossRef]
- Wheeler, W.M. Chinese ants. Bull. Mus. Comp. Zool. 1921, 64, 529–547. [Google Scholar]
- Wheeler, W.M. Ants collected by Professor F. Silvestri in China. Boll. Lab. Zool. Gen. Agrar. R. Ist. Super. Agrar. Portici 1928, 22, 3–38. [Google Scholar]
- Wheeler, W.M. A list of the known Chinese ants. Peking Nat. Hist. Bull. 1930, 5, 53–81. [Google Scholar]
- Wu, C.F. Catalogus Insectorum Sinensium, Volume VI; The Fan Memorial Institute of Biology, Yenching University: Beijing, China, 1941. [Google Scholar]
- Tang, K.L.; Pierce, M.P.; Guénard, B. Review of the genus Strumigenys (Hymenoptera, Formicidae, Myrmicinae) in Hong Kong with the description of three new species and the addition of five native and four introduced species records. Zookeys 2019, 831, 1–48. [Google Scholar] [CrossRef]
- Zhou, S.-Y. Ants of Guangxi; Guangxi Normal University Press: Guangxi, China, 2001. [Google Scholar]
- Xu, Z. A systematic study on Chinese species of the ant genus Oligomyrmex Mayr (Hymenoptera: Formicidae). Acta Zootaxonomica Sin. 2003, 28, 310–322. [Google Scholar]
- Hua, L. List of Chinese insects Vol. IV; Sun Yatsen University Press: Guangzhou, China, 2006; ISBN 9787306017017. [Google Scholar]
- Eguchi, K. A revision of Northern Vietnamese species of the ant genus Pheidole (Insecta: Hymenoptera: Formicidae: Myrmicinae). Zootaxa 2008, 1902, 1–118. [Google Scholar] [CrossRef]
- Bharti, H.; Kumar, R. Five new species of Dilobocondyla (Hymenoptera: Formicidae) with a revised key to the known species. Asian Myrmecol. 2013, 5, 29–44. [Google Scholar]
- Dlussky, G.M.; Radchenko, A.G. The ants (Hymenoptera, Formicidae) of Vietnam. Subfamily Pseudomyrmicinae. Subfamily Myrmicinae (Tribes Calyptomyrmecini, Meranoplini, Cataulacini). Available online: GBIF.org (accessed on 21 July 2021).
- Fisher, B.L. A new species of Probolomyrmex from Madagascar. In Advances in Ant Systematics (Hymenoptera: Formicidae): Homage to E.O. Wilson—50 Years of Contributions; American Entomological Institute: Gainesville, FL, USA, 2007. [Google Scholar]
- Shattuck, S.O.; Gunawardene, N.R.; Heterick, B. A revision of the ant genus Probolomyrmex (Hymenoptera: Formicidae: Proceratiinae) in Australia and Melanesia. Zootaxa 2012, 3444, 40–50. [Google Scholar] [CrossRef]
- Taylor, R.W. A monographic revision of the rare tropicopolitan ant genus Probolomyrmex Mayr (Hymenoptera: Formicidae). Trans. R. Entomol. Soc. Lond. 1965, 117, 345–365. [Google Scholar] [CrossRef]
- Economo, E.P.; Narula, N.; Friedman, N.R.; Weiser, M.D.; Guénard, B. Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Nadkarni, N.M.; Longino, J.T. Invertebrates in Canopy and Ground Organic Matter in a Neotropical Montane Forest, Costa Rica. Biotropica 1990, 286–289. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Solar, R.R.C. Hypogaeic pitfall traps: Methodological advances and remarks to improve the sampling of a hidden ant fauna. Insectes Soc. 2010, 57, 261–266. [Google Scholar] [CrossRef]
- Ryder Wilkie, K.T.; Mertl, A.L.; Traniello, J.F.A. Biodiversity below ground: Probing the subterranean ant fauna of Amazonia. Naturwissenschaften 2007, 94, 725–731. [Google Scholar] [CrossRef]
- Tschinkel, W.R. Back to basics: Sociometry and sociogenesis of ant societies (Hymenoptera: Formicidae). Myrmecol. News 2011, 14, 49–54. [Google Scholar]
- Tschinkel, W.R. Insect sociometry, a field in search of data. Insectes Soc. 1991, 38, 77–82. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Ellison, A.M.; Dunn, R.R.; Sanders, N.J. Counting ants (Hymenoptera: Formicidae): Biodiversity sampling and statistical analysis for myrmecologists. Myrmecolo. News 2011, 15, 13–19. [Google Scholar]
- Longino, J.T.; Coddington, J.; Colwell, R.K. The ant fauna of a tropical rain forest: Estimating species richness three different ways. Ecology 2002, 83, 689–702. [Google Scholar] [CrossRef]
- Lee, R.H.; Guénard, B. Choices of sampling method bias functional components estimation and ability to discriminate assembly mechanisms. Methods Ecol. Evol. 2019, 10, 867–878. [Google Scholar] [CrossRef]
- Yusah, K.M.; Fayle, T.M.; Harris, G.; Foster, W.A. Optimizing diversity assessment protocols for high canopy ants in tropical rain forest. Biotropica 2012, 44, 73–81. [Google Scholar] [CrossRef]
- Kaspari, M.; Pickering, J.; Windsor, D. The reproductive flight phenology of a neotropical ant assemblage. Ecol. Entomol. 2001, 50, 382–390. [Google Scholar] [CrossRef]
- Chong, C.S.; Li, D. Effects of forest disturbance on ant diversity in Singapore. Dep. Biol. Sci. Natl. Univ. Singap. 2003, 1–4. [Google Scholar]
- Bourguignon, T.; Dahlsjö, C.A.L.; Jacquemin, J.; Gang, L.; Wijedasa, L.S.; Evans, T.A. Ant and termite communities in isolated and continuous forest fragments in Singapore. Insectes Soc. 2017, 64, 505–514. [Google Scholar] [CrossRef]
- MacGown, J.A.; Hill, J.G.; Deyrup, M.A. Brachymyrmex patagonicus (Hymenoptera: Formicidae), an emerging pest species in the southeastern United States. Fla. Entomol. 2007, 90, 457–464. [Google Scholar] [CrossRef]
- Guénard, B. First record of the emerging global pest Brachymyrmex patagonicus Mayr 1868 (Hymenoptera: Formicidae) from continental Asia. Asian Myrmecol. 2018, 10, 1–6. [Google Scholar] [CrossRef]
- Tschinkel, W.R. The ecological nature of the fire ant: Some aspects of colony function and some unanswered questions. In Fire Ants and Leafcutter Ants: Biology and Management; Lofgren, C.S., Vander Meer, R.K., Eds.; Avalon Publishing: New York, NY, USA, 1986; pp. 72–87. [Google Scholar]
- Tschinkel, W.R. Distribution of the fire ants Solenopsis invicta and S. geminata (Hymenoptera: Formicidae) in northern Florida in relation to habitat and disturbance. Ann. Entomol. Soc. Am. 1988, 81, 76–81. [Google Scholar] [CrossRef]
- Tschinkel, W.R.; King, J.R. The Role of Habitat in the Persistence of Fire Ant Populations. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Hoffmann, B.D.; Parr, C.L. An invasion revisited: The African big-headed ant (Pheidole megacephala) in northern Australia. Biol. Invasions 2008, 10, 1171–1181. [Google Scholar] [CrossRef]
- Porter, S.D.; Savignano, D.A. Invasion of polygyne fire ants decimates native ants and disrupts arthropod community. Ecology 1990, 71, 2095–2106. [Google Scholar] [CrossRef]
- Wetterer, J.K. Biology and Impacts of Pacific Island Invasive Species. 3. The African Big-Headed Ant, Pheidole megacephala (Hymenoptera: Formicidae). Pac. Sci. 2007, 61, 437–456. [Google Scholar] [CrossRef]
- Lee, R.H.; Wang, C.L.; Guénard, B. The ecological implications of rubber-based agroforestry: Insect conservation and invasion control. J. Appl. Ecol. 2020, 57, 1605–1618. [Google Scholar] [CrossRef]
- Liu, C.; Fischer, G.; Garcia, F.H.; Yamane, S.; Liu, Q.; Peng, Y.Q.; Economo, E.P.; Guénard, B.; Pierce, N.E. Ants of the Hengduan Mountains: A new altitudinal survey and updated checklist for Yunnan Province highlight an understudied insect biodiversity hotspot. Zookeys 2020, 978, 1–171. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Cassey, P.; Blackburn, T. The role of propagule pressure in explaining species invasions. Trends Ecol. Evol. 2005, 20, 223–228. [Google Scholar] [CrossRef]
- Ellison, A.M. Out of Oz: Opportunities and challenges for using ants (Hymenoptera: Formicidae) as biological indicators in north-temperate cold biomes. Myrmecol. News 2012, 17, 105–119. [Google Scholar]
- Brady, S.G.; Ward, P.S. Morphological phylogeny of army ants and other dorylomorphs (Hymenoptera: Formicidae). Syst. Entomol. 2005, 30, 593–618. [Google Scholar] [CrossRef]
- Boswell, G.P.; Britton, N.F.; Franks, N.R. Habitat fragmentation, percolation theory and the conservation of a keystone species. Proc. R. Soc. B Biol. Sci. 1998, 265, 1921–1925. [Google Scholar] [CrossRef]
- Schöning, C.; Kinuthia, W.; Boomsma, J.J. Does the Afrotropical army ant Dorylus (Anomma) molestus go extinct in fragmented forests? J. East Afr. Nat. Hist. 2006, 95. [Google Scholar] [CrossRef]
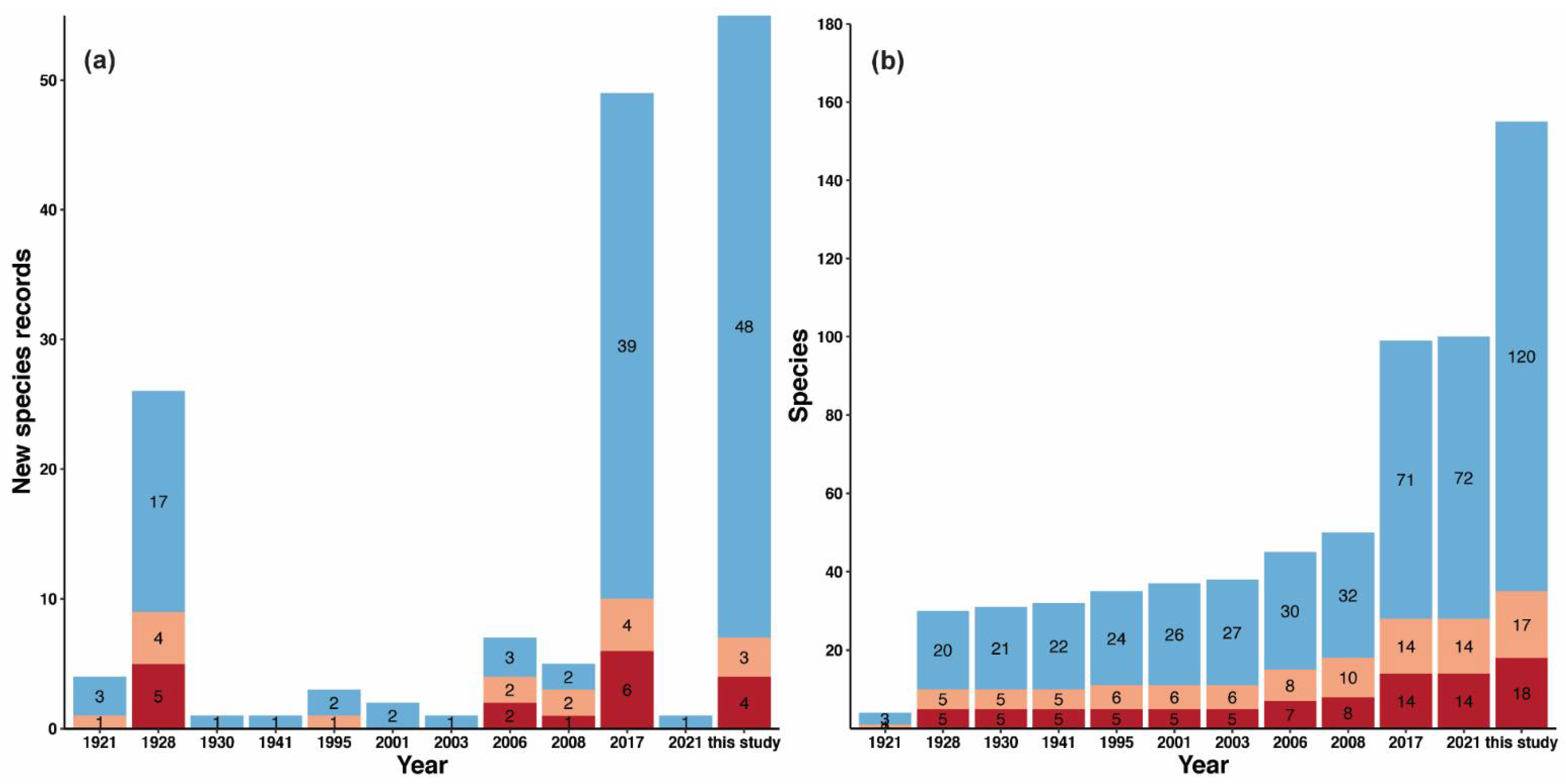
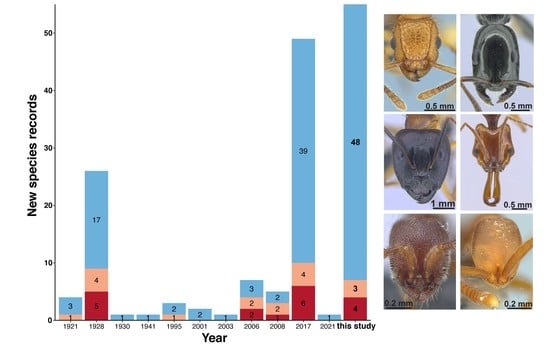
| Studies | Species | Subspecies | Morphospecies | Total per Year | Cumulative Total |
|---|---|---|---|---|---|
| Wheeler 1921 [188] | 4 | 0 | 0 | 4 | 4 |
| Wheeler 1928 [189] | 24 | 2 | 0 | 26 | 30 |
| Wheeler 1930 [190] | 1 | 0 | 0 | 1 | 31 |
| Wu 1941 [191] | 0 | 1 | 0 | 1 | 32 |
| Tang et al. 1995 [192] | 3 | 0 | 0 | 3 | 35 |
| Zhou 2001 [193] | 2 | 0 | 0 | 2 | 37 |
| Xu 2003 [194] | 1 | 0 | 0 | 1 | 38 |
| Hua 2006 [195] | 6 | 1 | 0 | 7 | 45 |
| Eguchi 2008 [196] | 5 | 0 | 0 | 5 | 50 |
| Leong, Shaijo and Guénard 2017 [53] | 41 | 1 | 7 | 49 | 99 |
| Wong and Guénard 2021 [55] | 1 | 0 | 0 | 1 | 100 |
| This study | 40 | 0 | 15 | 55 | 155 |
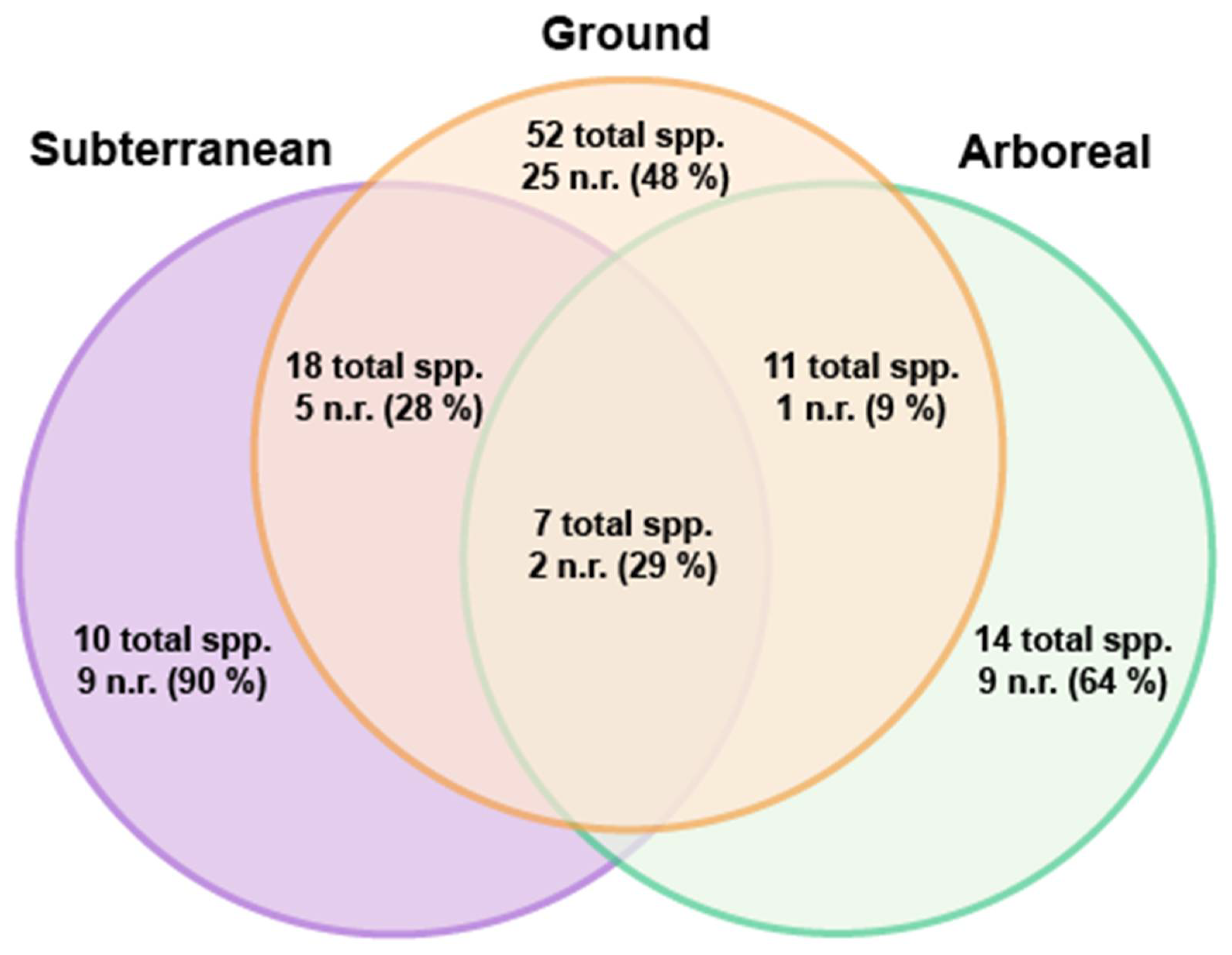
| Subfamily | Genus | Species | Status | Year of First Published Record | Arboreal | Ground | Subterranean |
|---|---|---|---|---|---|---|---|
| AMBLYOPONINAE | Stigmatomma | nr. rothneyi | native | 1928 | - | X | - |
| DOLICHODERINAE | Chronoxenus | dalyi | native | 1928 | |||
| walshi | native | 2006 | |||||
| wroughtonii | native | 1995 | |||||
| wroughtonii formosensis | native | 2006 | |||||
| † morpho1 | native | 2021 | - | X | X | ||
| † morpho2 | native | 2021 | - | X | X | ||
| Dolichoderus | taprobanae | native | 1928 | ||||
| nr. sibiricus | native | 2017 | X | - | - | ||
| Iridomyrmex | sp. anceps cplx * | tramp | 2021 | - | X | - | |
| Ochetellus | glaber | tramp | 1928 | - | X | - | |
| Tapinoma | indicum | native | 2017 | X | X | X | |
| melanocephalum | tramp | 1921 | X | X | X | ||
| sp. 1 FB * | native | 2021 | X | - | - | ||
| Technomyrmex | brunneus | tramp | 1995 | - | X | - | |
| horni * | native | 2021 | - | X | - | ||
| DORYLINAE | Ooceraea | biroi | exotic | 2017 | - | X | X |
| FORMICINAE | Acropyga | acutiventris | native | 2017 | |||
| sauteri | native | 1928 | |||||
| sp. mo02 | native | 2017 | |||||
| Anoplolepis | gracilipes | exotic | 1928 | X | X | - | |
| Brachymyrmex | patagonicus * | exotic | 2021 | X | - | - | |
| Camponotus | albosparus | native | 1928 | ||||
| carin * | native | 2021 | |||||
| irritans * | native | 2021 | - | X | - | ||
| lighti | native | 2017 | |||||
| mitis | native | 1928 | X | X | - | ||
| nicobarensis | native | 2017 | X | X | - | ||
| parius | native | 1921 | |||||
| vitiosus | native | 2017 | X | - | - | ||
| variegatus | tramp | 2006 | |||||
| variegatus dulcis | native | 1928 | |||||
| variegatus proles | native | 1941 | |||||
| sp. 1 FB * | native | 2021 | - | X | - | ||
| Colobopsis | nr. nipponica | native | 2017 | ||||
| nr. vitrea | native | 2017 | X | - | - | ||
| Gesomyrmex | howardi * | native | 2021 | ||||
| Lepisiota | rothneyi | native | 1921 | - | X | - | |
| Nylanderia | amia | tramp | 1928 | ||||
| bourbonica | tramp | 2006 | - | X | - | ||
| indica | native | 1928 | - | X | - | ||
| sharpii | native | 2021 | X | X | X | ||
| taylori * | native | 2021 | - | X | - | ||
| vividula | exotic | 2006 | |||||
| yerburyi | native | 1928 | |||||
| sp. 3 BG * | - | X | - | ||||
| sp. 6 BG * | native | 2021 | - | X | - | ||
| Paraparatrechina | sauteri | native | 2006 | ||||
| sp.1 BG * | native | 2021 | - | X | X | ||
| Paratrechina | longicornis | exotic | 1928 | - | X | X | |
| Plagiolepis | alluaudi * | exotic | 2021 | X | - | - | |
| Polyrhachis | confusa * | native | 2021 | - | X | - | |
| demangei | native | 2017 | |||||
| dives | native | 1995 | X | - | - | ||
| illaudata | native | 2017 | X | X | - | ||
| latona * | native | 2021 | - | X | - | ||
| tyrannica | native | 2021 | |||||
| Pseudolasius | risii * | native | 2021 | ||||
| LEPTANILLINAE | Leptanilla | macaoensis | native | 2017 | |||
| MYRMICINAE | Cardiocondyla | minutior | exotic | 2017 | - | X | - |
| wroughtonii * | tramp | 2021 | X | - | - | ||
| Carebara | affinis * | native | 2021 | - | X | X | |
| capreola | native | 2003 | |||||
| diversa | native | 1921 | - | - | X | ||
| diversa laotina | native | 2017 | |||||
| melasolena * | native | 2021 | - | X | X | ||
| sangi * | native | 2021 | - | - | X | ||
| zengchengensis | native | 2017 | - | X | X | ||
| Crematogaster | binghamii * | native | 2021 | - | X | - | |
| biroi | native | 1928 | |||||
| dohrni | native | 1928 | |||||
| ferrarii | native | 2017 | X | X | - | ||
| macaoensis | native | 1928 | |||||
| quadriruga | native | 2017 | - | X | - | ||
| rogenhoferi | native | 2017 | X | - | - | ||
| Dilobocondyla | propotriangulata * | native | 2021 | X | - | - | |
| Mayriella | granulata * | native | 2021 | - | X | - | |
| Meranoplus | sp. mo01 nr. bicolor | native | 2017 | ||||
| Monomorium | chinense * | native | 2021 | X | X | X | |
| intrudens * | tramp | 2021 | X | X | - | ||
| floricola | tramp | 2017 | X | X | X | ||
| pharaonis | exotic | 2017 | - | X | X | ||
| sp. psw-cn01 | native | 2021 | - | X | - | ||
| Myrmecina | nomurai * | native | 2021 | - | X | - | |
| sinensis | native | 2017 | |||||
| Pheidole | elongicephala * | native | 2021 | - | - | X | |
| fervens | tramp | 2008 | - | X | X | ||
| hongkongensis | native | 2008 | - | X | - | ||
| indica | tramp | 1928 | |||||
| megacephala | exotic | 2008 | X | X | X | ||
| ochracea | native | 2017 | - | X | X | ||
| parva | tramp | 2008 | - | X | X | ||
| pieli* | native | 2021 | - | X | X | ||
| taipoana | native | 2008 | - | X | X | ||
| tumida | native | 2017 | X | X | X | ||
| nodus | tramp | 2017 | - | X | - | ||
| vulgaris * | native | 2021 | - | X | - | ||
| zoceana * | native | 2021 | - | X | - | ||
| nr. ryukyuensis * | native | 2021 | - | - | X | ||
| Recurvidris | recurvispinosa | native | 2017 | - | X | - | |
| Rotastruma | stenoceps * | native | 2021 | - | X | - | |
| Solenopsis | geminata | exotic | 1928 | ||||
| invicta | exotic | 2006 | - | X | - | ||
| jacoti | native | 2017 | - | X | X | ||
| Strumigenys | emmae | exotic | 2017 | - | X | - | |
| elegantula | native | 2020 | - | X | - | ||
| exilirhina | native | 2017 | - | X | - | ||
| feae | native | 2020 | - | X | - | ||
| membranifera | exotic | 1928 | - | X | - | ||
| minutula | native | 2017 | - | X | - | ||
| ‡nepalensis | exotic | 2017 | - | X | - | ||
| sauteri | native | 2020 | - | X | - | ||
| subterranea | native | 2020 | - | - | X | ||
| Syllophopsis | nr. cryptobia * | native | 2021 | - | - | X | |
| sp. mo01 nr sechellensis | native? | 2017 | - | X | X | ||
| sp. 1 BMW * | native | 2021 | - | X | X | ||
| sp. 2 BMW * | native | 2021 | - | X | - | ||
| Tetramorium | bicarinatum | tramp | 2017 | X | X | - | |
| indicum * | native | 2021 | X | - | - | ||
| insolens * | exotic | 2021 | - | X | - | ||
| kraepelini | tramp | 2017 | - | X | X | ||
| lanuginosum | exotic | 1928 | - | X | - | ||
| nipponense | native | 2017 | X | X | - | ||
| parvispinum | native | 2017 | |||||
| simillimum | exotic | 2017 | |||||
| tonganum * | exotic | 2021 | X | - | - | ||
| wroughtonii * | native | 2021 | - | X | - | ||
| nr. elisabethae * | native | 2021 | - | - | X | ||
| sp.1 obseum gr. | native | 2017 | - | X | - | ||
| sp. 2 JF * | native | 2021 | X | - | - | ||
| sp. 9 JF * | native | 2021 | - | - | X | ||
| Vollenhovia | sp.1 BG * | native | 2021 | - | X | - | |
| sp. 2 BG * | native | 2021 | - | X | - | ||
| PONERINAE | Anochetus | risii | native | 2017 | - | X | - |
| Bothroponera | rubiginosa | native | 1928 | ||||
| Brachyponera | luteipes | tramp | 1928 | ||||
| obscurans | native | 1928 | - | X | X | ||
| Buniapone | amblyops * | native | 2021 | - | - | X | |
| Diacamma | sp. 1 | native | 2017 | X | X | - | |
| Ectomomyrmex | annamitus * | native | 2021 | - | - | X | |
| astutus | native | 2001 | |||||
| leeuwenhoecki | native | 2017 | - | X | - | ||
| Euponera | pilosior | native | 2017 | - | - | X | |
| sharpi | native | 1928 | |||||
| Harpegnathos | venator | native | 2001 | - | X | - | |
| venator rugosus | native | 1928 | |||||
| Hypoponera | exoecata | native | 2017 | - | X | - | |
| sp. mo01 | native | 2017 | X | X | - | ||
| Leptogenys | chinensis | native | 2017 | ||||
| peuqueti | native | 1928 | - | X | - | ||
| Odontoponera | denticulata | native | 2017 | - | X | - | |
| Pseudoneoponera | rufipes | native | 1930 | - | X | - | |
| PROCERATIINAE | Probolomyrmex | dabermanii* | native | 2021 | |||
| Proceratium | sp. cf. bruelheidei * | native | 2021 | - | X | - | |
| PSEUDOMYRMICINAE | Tetraponera | allaborans | native | 2017 | |||
| binghami * | native | 2021 | X | X | - | ||
| nitida * | native | 2021 | X | - | - |
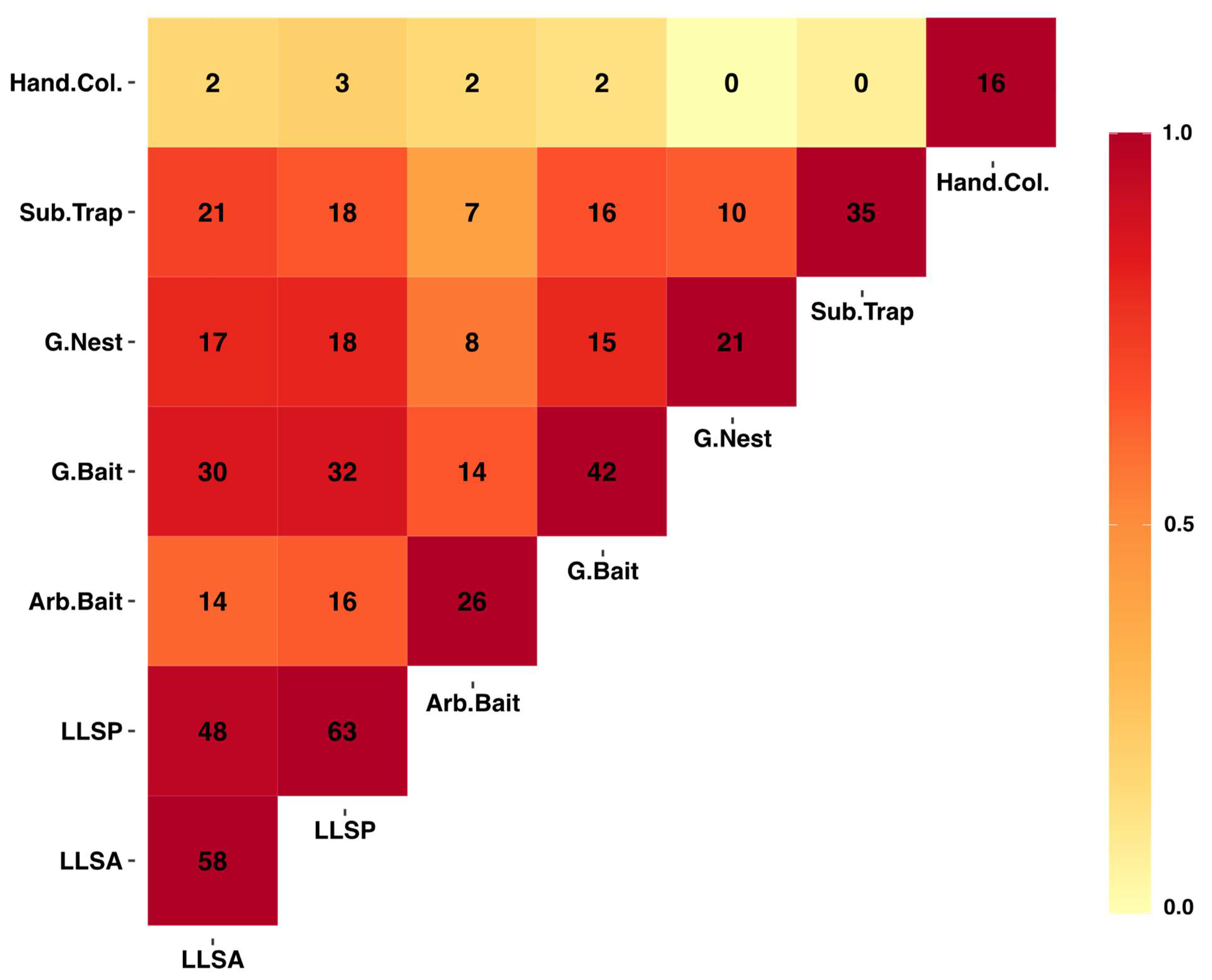
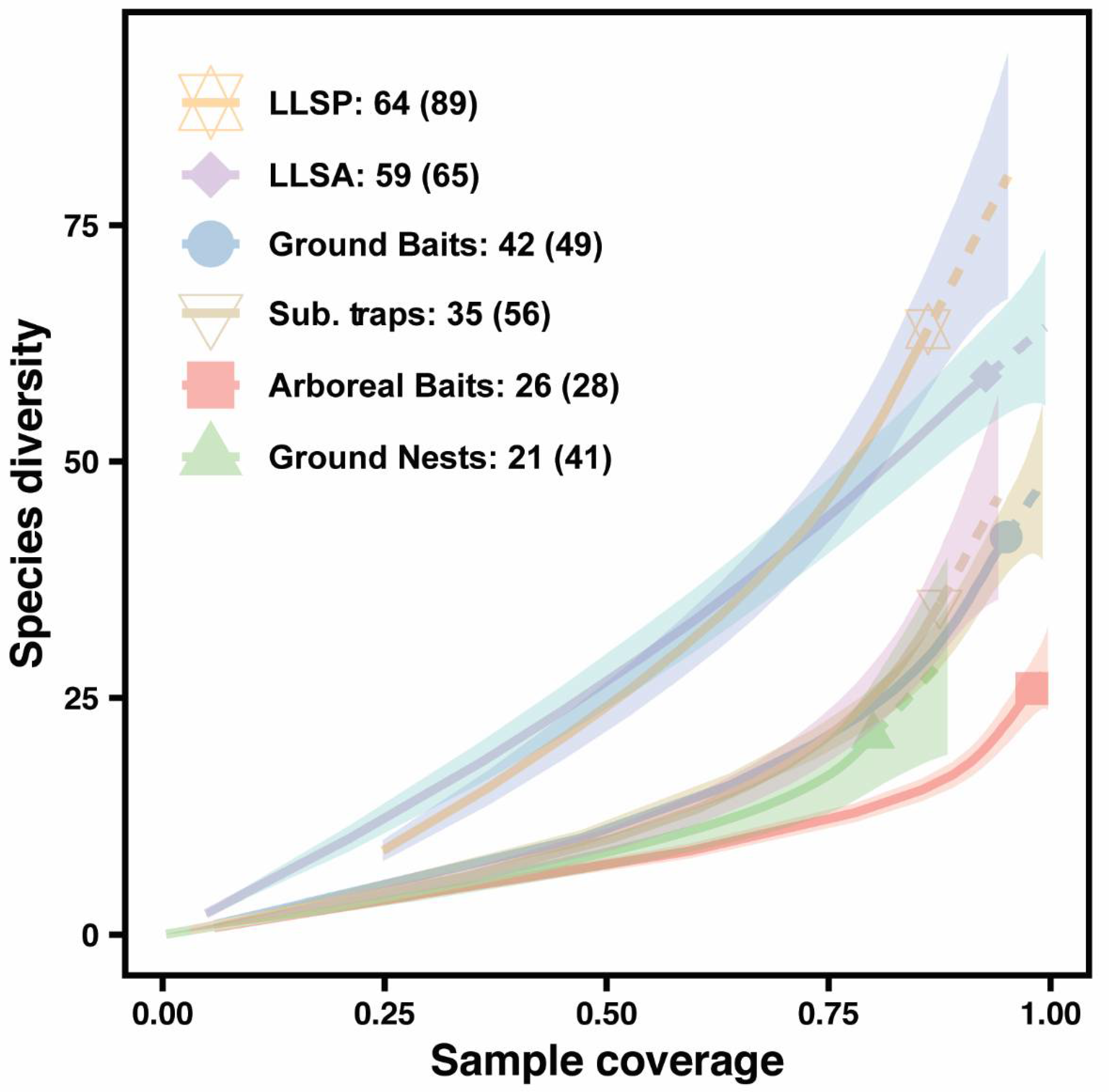
| Sampling Method | Observed Richness | Sampling Completeness | Estimated Richness |
|---|---|---|---|
| Arboreal Bait | 26 | 0.98 | 28.49 |
| Ground Bait | 42 | 0.95 | 49.17 |
| Ground Nest | 21 | 0.80 | 41.14 |
| Leaf Litter Sampling (Standardized area) | 59 | 0.93 | 64.70 |
| Leaf Litter Sampling (Species pool) | 64 | 0.86 | 88.80 |
| Subterranean trap | 35 | 0.88 | 56.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brassard, F.; Leong, C.-M.; Chan, H.-H.; Guénard, B. High Diversity in Urban Areas: How Comprehensive Sampling Reveals High Ant Species Richness within One of the Most Urbanized Regions of the World. Diversity 2021, 13, 358. https://doi.org/10.3390/d13080358
Brassard F, Leong C-M, Chan H-H, Guénard B. High Diversity in Urban Areas: How Comprehensive Sampling Reveals High Ant Species Richness within One of the Most Urbanized Regions of the World. Diversity. 2021; 13(8):358. https://doi.org/10.3390/d13080358
Chicago/Turabian StyleBrassard, François, Chi-Man Leong, Hoi-Hou Chan, and Benoit Guénard. 2021. "High Diversity in Urban Areas: How Comprehensive Sampling Reveals High Ant Species Richness within One of the Most Urbanized Regions of the World" Diversity 13, no. 8: 358. https://doi.org/10.3390/d13080358
APA StyleBrassard, F., Leong, C.-M., Chan, H.-H., & Guénard, B. (2021). High Diversity in Urban Areas: How Comprehensive Sampling Reveals High Ant Species Richness within One of the Most Urbanized Regions of the World. Diversity, 13(8), 358. https://doi.org/10.3390/d13080358