Abstract
Prunus lusitanica L. is a paleotropical relic species with an Ibero-Maghrebian distribution, which is presently considered as an endangered species, recognized by the Natura 2000 Network (92/43/EEC) as a priority habitat for conservation in Europe. The mountains in the Portuguese mainland central region offer the best location for this species to occur. The main objective of this study is to measure the current conservation status of the communities of P. lusitanica, through the collection of field data, such as the number of existing individuals of each population and their location, which will then be comparatively analyzed based on the previous literature, published at least 15 years ago. Soil characterization analysis was carried out and the main threats to conservation were identified. As a result, a decline of approximately 40% was observed in the number of individuals and in the quality of their habitat. The main threats to their conservation were found to be the seasonal occurrence of rural fires and the expansion of invasive species, such as Acacia dealbata Link and Ailanthus altissima (Mill.) Swingle. Finally, we present the main management methodologies that should be considered for the valorization of this important vegetational relic in the central region of mainland Portugal.
1. Introduction
The communities of Prunus lusitanica L. are true paleotropical relics that had their optimal ecological apogee during the Paleogene period [1,2]. Since then, with the evolution of the Mediterranean climate conditions to actual standards in the Iberian Peninsula, characterized mainly by the accentuation of a summer drought period, the Laurissilva forest has undergone a sharp reduction in its occupation area throughout southern Europe and, particularly, in the Mediterranean basin [3,4,5]. For this reason, the communities of P. lusitanica are currently refugees in certain specific locations with very particular conditions, where the orography allows for a greater atmospheric humidity and mild temperatures to occur [6,7]. This forest can be characterized by exhibiting persistent leafy vegetation, similar to what happens today in some well-preserved slopes in the north of the Island of Madeira (Portugal) and in the Canary Islands (Spain) [8]. Although much of the typical Laurissilva flora has disappeared from the European continent, several other species, such as Rhododendron ponticum L., Laurus nobilis L., Ilex aquifolium L. and Myrica faya Aiton, still persist as relic vegetation [1,9].
Evaluated as an Endangered species using the IUCN criteria established in 1998 [10], it is in Portugal that the most well-preserved and extensive communities of P. lusitanica still persist throughout its entire distribution area, with more than 19,000 individuals registered at a national level in 2005, corresponding to approximately 62% of the total global population [11]. Their communities are also recognized as a priority habitat for conservation in Europe through the Sectoral Plan of the Natura 2000 Network (92/43/EEC), called Laurus nobilis arborescent shrubs, of the Azereirais subtype (5230*pt2). However, due to the permanent threats that menace these habitats, a significant reduction in the number of individuals occurred [6]. Among all the threats identified in the Habitat File, the invasion of exotic plants that progressively colonize new areas and the recurrence and severity of rural fires can be highlighted [12,13,14]. In fact, at this moment, the impact of fires is, probably, the major concern for the reduction in the number of individuals and loss of the area occupied by this habitat, particularly in the central region of mainland Portugal [15]. An example of this is the rural fire that occurred in October 2017 and destroyed a significant part of the largest population of P. lusitanica, located in Mata da Margaraça (Arganil, Portugal). As can be seen in Figure 1, the entire area that surrounds the habitat was burnt and is slowly recovering 1 year after the rural fire.

Figure 1.
Mata da Margaraça (Arganil, Portugal) after the rural fire that occurred in October 2017 (picture taken in 2018).
Thus, it is necessary to study and monitor, in detail, the evolution of P. lusitanica nuclei to reverse this situation of decline. The analysis of the edaphoclimatic conditions, as well as the anthropic action, allows us to understand and facilitate the adoption of adequate management methodologies for the valorization of the remaining populations of the continental Laurissilva. Based on these concerns, the main objectives of the present article are to update the population census of P. lusitanica in Serra da Estrela; to identify the main threats to its conservation; to identify management strategies to value this relict habitat.
2. Materials and Methods
2.1. Characterization of the Area under Study
The area under study is located on the reliefs of the central region of mainland Portugal, covering the area occupied by Serra da Estrela, Serra da Lousã, Serra de Alvéolos, Serra Vermelha and Serra do Açor. The lower altitude found in the region is around 220 m and the highest altitude is around 1300 m (Figure 2). In biogeographic terms, the areas under study belong to the Montemuro and Estrela Sierran Sector and the Oretania and Tejo Sector [16]. The geology of the region is mostly formed by Precambrian and Cambrian metasediments that form the schist–greywacke complex, which is intercepted by a set of granitic rocks of hercynian origin [17]. Although a varied mineralogical composition can be found from granodiorites to leucogranites [18], the areas of occurrence of P. lusitanica are dominated by the schist soils, as well as fluvial sedimentary deposits with wavy and pronounced reliefs. These schists are part of the Malpica do Tejo formation and are dated from 500 to 650 million years ago [19]. Most of the soils in the area under study are lithosols, while some of which have evolved into cambisols over the years [20].
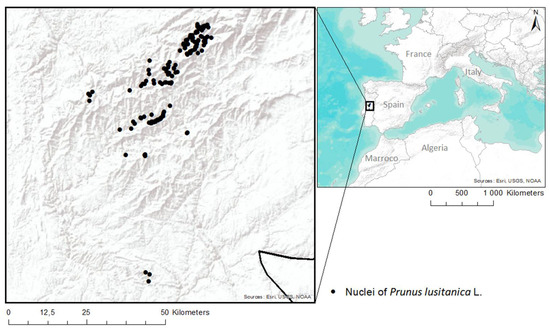
Figure 2.
Location of the areas under study.
At the bioclimatic level, the area is located in the transition from the mediterranean to the temperate macrobioclimate (Table 1). The municipality of Mação represents the southern limit of the natural distribution area of P. lusitanica in mainland Portugal and is under the influence of a humid thermomediterranean bioclimate [21]. The northern limit of the area is part of the municipality of Seia and is influenced by a humid mesotemperate bioclimate (Figure 3).

Table 1.
Bioclimatic data of the area under study: T—annual average temperature in Celsius degrees; P—average annual rainfall in millimeters; Tp—positive annual temperature in Celsius degrees; Pp—positive rainfall in millimeters; Io—annual ombrothermic index; It—thermicity index; Ic—simple continentality index (index based on Rivas-Martínez et al. [21]).
These conditions are favorable for the presence of Quercus robur subsp. broteroana O. Schwartz that constituted the potential climatophilic vegetation of much of the area under study, of the association Viburno tini-Quercetum broteroanae (Br.Bl., P. Silva, and Rozeira, 1955) J.C. Costa, Capelo, Honrado, Aguiar and Lousã 2002 corr. J.C. Costa and Monteiro-Henriques 2012. The communities of Alnus lusitanica Vít, Douda and Mandák, from the association Scrophulario scorodoniae-Alnetum glutinosae Br.-Bl., P. Silva and Rozeira 1955, appear in an edafo-hygrophilic position. In a secondary position, or as an edaphoxerophilous vegetation, are communities of Quercus pyrenaica Willd., Q. suber L. and Q. rotundifolia Lam., from the associations Arbuto unedonis-Quercetum pyrenaicae (Rivas-Goday in Rivas-Goday, Esteve, Galiano, Rigual and Rivas-Martínez, 1960) Rivas-Martínez 1987, Sanguisorbo hybridae-Quercetum suberis Rivas-Goday in Rivas-Goday, Borja, Esteve, Galiano, Rigual and Rivas-Martínez 1960 and Teucrium salviastri-Quercetum rotundifoliae Pinto-Gomes, Ladero, Cano, Meireles, Aguiar and P. Ferreira 2010, respectively [22].
However, the landscape is quite altered, dominating the stands of maritime pine (Pinus pinaster Aiton) and eucalyptus (Eucalyptus globulus Labill.). The communities of P. lusitanica in the Portuguese central region are ecologically positioned between the amyals of Scrophulario scorodoniae-Alnetum glutinosae and the oak trees of Viburno tini-Quercetum broteroanae, integrating with the slopes or as the first stage of replacement of these forests. Thus, the association Frangulo alni-Prunetum lusitanicae C. Lopes, J.C. Costa, Lousã and Capelo in J.C. Costa, C. Lopes, Capelo and Lousã 2000 often occupies a tempori-hygrophilic position or a climatophilic position, with water being compensated at a ground level or at an atmospheric level by the fog.
2.2. Data Collection
The interpretation of plant communities followed the phytosociological information based on the landscape and sigmatist approach presented by the Zurich-Montpellier Phytosociology School, as proposed by Braun-Blanquet [23], Géhu and Rivas-Martínez [24] and updated by Rivas-Martínez [25]. Taxonomic and sintaxonomic nomenclature followed the work of Rivas-Martínez et al. [26] and was complemented by more recent works, namely those by Costa et al. [22], Mucina et al. [27] and Taleb and Fennane [28]. For the identification of plant material, the works presented by Castroviejo et al. [29], Coutinho [30], Franco [31] and Franco and Rocha Afonso [32] were used. The biogeographical and bioclimatic framework followed the work presented by Rivas-Martínez et al. [16] and Rivas-Martínez et al. [21].
To count the number of individuals of P. lusitanica, field trips were conducted from 2018 to 2020, during which their geographical coordinates were recorded using GPS equipment with a precision of 1 m, as well as the number of individuals observed in each nucleus. These individuals were separated into two groups: more than 1.5 m and less than 1.5 m height. Regeneration over the year itself was not taken into account due to its small size. Although pollen crossing between individuals can occur at a distance of several kilometers, in this study, each population nuclei was differentiated when any specimen was more than 100 m away.
For the characterization of the pollen, scanning electron microscopy was carried out on a Phenom Pro X instrument. The P. lusitanica L. pollen was deposited on carbon adhesive tabs with a diameter of 12 mm. For the hydration of pollen grains, the carbon adhesive was placed in a humid chamber overnight. The pollen samples were photographed in high resolution (10 kV).
In order to understand the different existing soil conditions, five sampling points were randomly selected in four different zones: P1—habitat in good condition; P2—degraded habitat; P3—areas surrounding the habitat; P4—areas dominated by acacias. This approach resulted in the selection and collection of composite samples, separated into three depth horizons: 0–5 cm; 5–15 cm; 15–30 cm. In the sampling points with shallow soil, it was possible to collect only the first two most superficial horizons. The determination of the organic matter (OM) content was achieved using the muffle method following the procedure established by Goldin [33], with some modifications: beforehand, the samples were dried in an oven at 105 °C for a period of 24 h to eliminate all the water present in the samples [34]. After this period, the ceramic crucibles with the samples were placed in a muffle furnace and incinerated at a temperature of 550 °C for 3 h. Subsequently, the set (crucible + waste) was placed in a desiccator and then weighed. The organic matter content was determined due to the mass loss of the incinerated residue, considering the material lost by burning in the temperature range of from 105 °C to 550 °C, according to the formula (P = weight of the sample (g) after heating to 105 °C; C = tare of the crucible (g); T = weight of ash + crucible (g)), as presented in Equation (1):
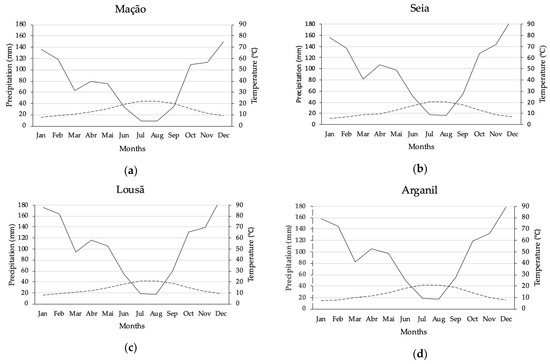

Figure 3.
Thermopluviometric graphics of the areas under study: (a) Mação; (b) Seia; (c) Lousã; (d) Arganil (data acquired at Portal do Clima web platform, available at www.portaldoclima.pt, accessed on 14 June 2021, in accordance with the methodology presented by Walter and Lieth (1967) and Walter (2012) [34,35]).
2.3. Ecological Modeling
Based on prior knowledge of the ecological conditions of P. lusitanica, modeling was carried out using the Maxent 3.4.4 program for the area under study [11,35]. This information was complemented with field data collected during the period from 2018 to 2020. Eight climate variables from WorldClim (version 2.1) were used for ecological modeling: Tavg—average temperature; Tmax—maximum temperature; Tmin—minimum temperature; elev—elevation; prec—precipitation; srad—solar radiation; vapr—water vapor pressure; wind—wind speed [36]. The bioclimatic variables (1970–2000) used to calculate the current distribution have a spatial resolution of 30 s (~1 km2).
3. Results and Discussion
The fieldwork resulted in the observation of almost 10,000 individuals of P. lusitanica in the central region of mainland Portugal (Table 2). Despite the rural fire that occurred in October 2017, Serra do Açor continues to have the greatest representativeness of this species, corresponding to approximately 50% of the individuals in the area under study. With almost 3000 individuals, the União de Freguesias de Vide e Cabeça faces serious problems with the invasion of exotic species, such as Acacia dealbata Link., Acacia melanoxylon R. Br. and Ailanthus altissima (Mill.) Swingle. In the municipality of Mação, which includes the lowest number of cherry trees, with around 70 individuals, constitutes the southernmost territory in Portugal, where the presence of thermophilic elements can still be detected, such as Pistacia lentiscus, Myrtus communis and Smilax aspera in the Ribeira do Aziral and Ribeira do Fraguedo.

Table 2.
Size of the population nuclei of P. lusitanica.
These data show a reduction of approximately 6500 individuals in the area under study, corresponding to a loss of approximately 40% in comparison with the results published by Calleja [11]. In general, in the well-preserved areas, there were no problems with the pollination of flowers, or with the seed production, or with the natural germination (Figure 4). However, we verified a low survival of young plants. Occasionally, there were problems with the presence of herbivorous animals, such as deer, namely in Ribeira do Gondufo (Seia), where it is possible to observe some of the oldest cherry trees in Portugal, although the natural regeneration is practically nil.

Figure 4.
Details of P. lusitanica. From left to right: flowering; fruiting; natural regeneration.
At the infraspecific and even specific levels, the pollen of the genus Prunus is one of the most difficult to differentiate due to its morphological similarities [11,37]. According to the present research, pollen from P. lusitanica is rarely studied, and there is only one database with its typification [38]. Through our analyzes we confirm that Prunus lusitanica L. pollen is monad, tricolporate (three apertures furrows, pores indistinct or obscure) and isopolar. In polar view is circular or triangular and subprolate in equatorial view (Figure 5). In equatorial view the apertures are visible. The exine is strongly striate with some puncta between the striae. Furrows are wide, with irregular margins, granulate ornamentation with saliente intine. The size of this pollen type is average, around 36-40 µm. The pollen of several taxa of the genus Prunus is heavy, which is why pollination by the honeybee (Apis mellifera Linneus) is important for the development of a high number of fruits, as is the case with the cultivation of the cherry trees [39,40].
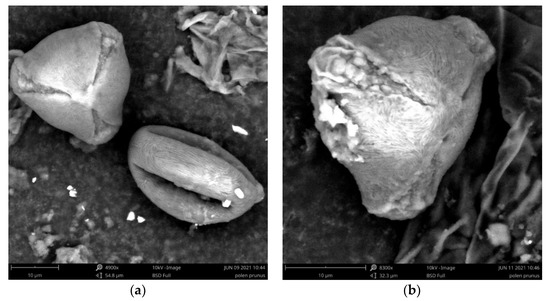
Figure 5.
P. lusitanica pollen grains under the Scanning Electron Microscope (SEM), collected in Mata da Margaraça. (a) Tricolpate pollen in polar view on the left side and equatorial view on the right side. (b) Tricolpate pollen in polar view—detail of the exine sculpturing with some puncta between the striae.
The strong mortality of young plants may be associated with climatic or edaphic factors. In the different sampled areas, the horizon A (0–5 cm) of the soil showed a high organic matter content, while the second sampled horizon (5–15 cm) showed a significant reduction in organic matter content (Figure 6). Although some samples from the third horizon (15–30 cm) were not taken due to the weak thickness of the soil, this was the horizon that showed the lowest accumulated values. Although the areas P2 and P3 were sometimes presented as clearings between the shrub cover, the areas dominated by A. dealbata showed an even lower organic matter content in horizon A. This fact is mainly due to the lack of other flora, kept away by the physical and allelopathic competition effect of the acacias, as well as the reduced amount of organic matter produced by A. dealbata stands [41].
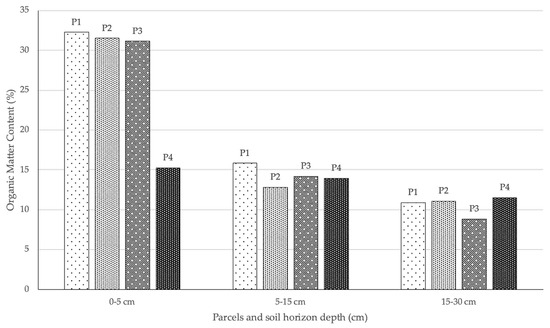
Figure 6.
Percentage of organic matter per plot and depth of sampling (P1—habitat with good conservation status; P2—habitat with poor conservation status; P3—periphery of habitat 5230*pt2; P4—areas dominated by A. dealbata).
Several nuclei of P. lusitanica from the central region of mainland Portugal are currently in regression due to the strong expansion of exotic plants with an invasive character. Table 3 presents the main species identified in the area under study, as well as the most appropriate control method for each species [42]. Of all species, A. dealbata can be highlighted, which occurs inside or in the vicinity of all the areas under study, occupying the slopes and margins of the water lines (Figure 7a). Another identified aspect was the formation of seeds in Hakea sericea Schrader from plants that germinated 1 year after the occurrence of a rural fire (Figure 7b). On the other hand, although E. globulus does not have an invasive behavior in much of the national territory, in territories with high humidity and after the passage of fire, it presents a high dispersion and germination of seeds, resulting in plant densities which sometimes completely cover the soil (Figure 7c).

Table 3.
Main exotic plants with invasive behavior identified in the areas under study.

Figure 7.
Exotic plants with invasive behavior in the areas under study. (a) Invasion of the banks of the River Alvôco with A. dealbata; (b) H. sericea fruiting near Ribeira do Aziral (Mação); (c) aggressive seminal regeneration of E. globulus in Serra da Estrela. (These photographs are by Mauro Raposo).
In addition to the urgent need to control invasive plants in the region under study, it is necessary to avoid the introduction of other species that could invade new areas, as has happened in other areas of Europe [43,44]. Rural fires promote the appearance of heliophilous shrubs, which increases the risk of rural fires recurring in short time periods [45]. In this sense, selective control with vegetation cover is proposed, in order to promote the growth of the characteristic species of habitat 5230, such as Laurus nobilis, Rhododendron ponticum, Myrica faya, Arbutus unedo, Viburnum tinus, Ilex aquifolium, Rosa sempervirens and Smilax aspera, among others, avoiding the negative impacts of chemical control [46].
On the other hand, to create buffer zones and fire breaks in a landscape marked by forest production stands, discontinuities must be created through the promotion of native forest species, such as Q. robur subsp. broteroana, Q. pyrenaica, Q. suber or even Castanea sativa, Prunus avium and Arbutus unedo. The landscape mosaic contributes not only to lowering the risk of fires but also to the greater sustainability and resilience of the landscape. Thus, planting native forests in the areas surrounding habitat 5230 will contribute significantly to reducing the risk of fire, when compared to the high rates of flammability of resinous species and species with high essential oil content, such as P. pinaster and E. globulus, respectively [47,48]. Although the strong contribution of the analyzed threats to the decrease in the number of individuals of P. lusitanica, recolonization through plantations is an effective method and has already been tested and used with other species [49,50]. To meet the potential area of occurrence of the species, a model was created using current ecological data, presented in Figure 8.
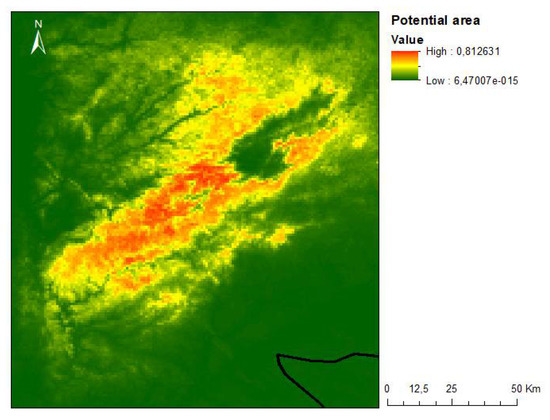
Figure 8.
Map of the potential occurrence area of P. lusitanica in the central region of mainland Portugal.
According to the results obtained, the most determining factor in the geographic distribution of P. lusitanica in the central region of mainland Portugal is precipitation. Other noteworthy factors are solar radiation and the average annual temperature (Figure 9). These results are in accordance with the ecological characterizations carried out in previous works, namely on the north-facing slopes, with mild temperatures and higher humidity [51,52,53,54]. Following the achievements of the present research, however, the aim is to identify integrative policies with local communities instead of on a restrictive basis, as is the case in other places in the Mediterranean basin with lush vegetation [55].
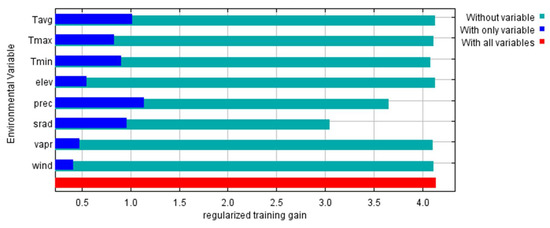
Figure 9.
Importance of ecological factors in modeling the potential occurrence area of P. lusitanica in Serra da Estrela (Tavg—average temperature; Tmax—maximum temperature; Tmin—minimum temperature; elev—elevation; prec—precipitation; srad—solar radiation; vapr—water vapor pressure; wind—wind speed).
4. Conclusions
The communities of P. lusitanica in the central region of mainland Portugal are in sharp decline, with the loss of approximately 40% of individuals in 15 years. Several conservation projects have contributed to recovering this species and avoiding aggravating the evaluation criteria to Critically Endangered. Thus, it is expected that, in the near future, the assessment of Endangered will be maintained due to the adequacy of the A2c criterion. However, stricter conservation policies and replication actions are needed based on the experiences that were already carried out, such as the Life-Relict Project (NAT/PT/000754). Thus, it is not enough to conserve the species, but instead, its habitat and the surrounding areas must be conserved, allowing better flow between plants and animals. This study shows the need for the rapid integration of P. lusitanica in the Red List of Vascular Plants of Mainland Portugal, thus allowing a better transmission of the value of this species to the instruments of territorial management.
Author Contributions
Conceptualization, M.A.M.R. and C.J.P.-G.; methodology, M.A.M.R., A.G. and L.J.R.N.; software, S.d.R. and R.Q.-C.; validation, S.d.R., F.M.V.P. and C.J.P.-G.; formal analysis, M.A.M.R. and A.G.; investigation, M.A.M.R., L.J.R.N. and R.Q.-C.; data curation, M.A.M.R., S.d.R., R.Q.-C., L.J.R.N. and C.J.P.-G.; writing—original draft preparation, M.A.M.R.; writing—review and editing, S.d.R. and F.M.V.P.; visualization, A.G. and M.A.M.R.; supervision, S.d.R., F.M.V.P. and C.J.P.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by National Funds through the Foundation for Science and Technology (FCT) under the Project UIDB/05183/2020. L.J.R.N. was supported by Prometheus—Research Unit on Energy, Materials and Environment for Sustainability, UIDP/05975/2020, funded by national funds through FCT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request to the corresponding author.
Acknowledgments
This research received a contribution from the European Commission’s LIFE program, through the Life-Relict Project (NAT/PT/000754), to the municipalities of Mação, Pampilhosa da Serra and Freguesia de Vide e Cabeça for their collaboration in the fieldwork. We would like to thank researcher Helena Ribeiro from the Department of Geosciences, Environment and Spatial Plannings of the Faculty of Sciences University of Porto for her contributions to pollen characterization.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aguiar, C.; Pinto, B. Paleo-História e História Antiga das Florestas de PORTUGAL Continental: Até à IDADE Média; Público: Lisbon, Portugal, 2007; pp. 15–53. [Google Scholar]
- Barrón, E. Evolución de las floras terciarias en la península ibérica. Mongr. Jard. Bot. Córdoba 2003, 11, 63–74. [Google Scholar]
- Barrón, E.; Rivas-Carballo, R.; Postigo-Mijarra, J.M.; Alcalde-Olivares, C.; Vieira, M.; Castro, L.; Pais, J.; Valle-Hernández, M. The cenozoic vegetation of the Iberian Peninsula: A synthesis. Rev. Palaeobot. Palynol. 2010, 162, 382–402. [Google Scholar] [CrossRef]
- Juaristi, C.M. El paisaje vegetal ibérico durante el quaternario. Monogr. Jard. Bot. Córdoba 2003, 11, 75–93. [Google Scholar]
- Pignatti, S. Evolutionary trends in mediterranean flora and vegetation. Vegetatio 1978, 37, 175–185. [Google Scholar] [CrossRef]
- Calleja, J.; Ollero, H. Análisis e interpretación geobotánica de la estructura y composición florística de las loreras ibéricas. Ecologia 2009, 22, 45–71. [Google Scholar]
- Pulido, F.; Valladares, F.; Calleja, J.A.; Moreno, G.; González-Bornay, G. Tertiary relict trees in a mediterranean climate: Abiotic constraints on the persistence of Prunus lusitanica at the eroding edge of its range. J. Biogeogr. 2008, 35, 1425–1435. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, F.; Guzmán, B.; Valido, A.; Vargas, P.; Arroyo, J. Late neogene history of the laurel tree (Laurus L., Lauraceae) based on phylogeographical analyses of mediterranean and macaronesian populations. J. Biogeogr. 2009, 36, 1270–1281. [Google Scholar] [CrossRef] [Green Version]
- Kondraskov, P.; Schütz, N.; Schüßler, C.; de Sequeira, M.M.; Guerra, A.S.; Caujapé-Castells, J.; Jaén-Molina, R.; Marrero-Rodríguez, Á.; Koch, M.A.; Linder, P.; et al. Biogeography of mediterranean hotspot biodiversity: Re-evaluating the “Tertiary Relict” hypothesis of macaronesian laurel forests. PLoS ONE 2015, 10, e0132091. [Google Scholar] [CrossRef] [PubMed]
- Vivero, J.L. IUCN Red List of Threatened Species: Prunus lusitanica Subsp. Lusitanica. Available online: https://www.iucnredlist.org/en (accessed on 6 June 2020).
- Calleja, J.A. Geobotánica, Estructura Demográfica, Consevación Y Biología Predispersiva de Prunus lusitanica L. (Loro) en La Península Ibérica. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2006. [Google Scholar]
- Monteiro, A.T.; Gonçalves, J.; Fernandes, R.F.; Alves, S.; Marcos, B.; Lucas, R.; Teodoro, A.C.; Honrado, J.P. Estimating invasion success by non-native trees in a national park combining worldview-2 very high resolution satellite data and species distribution models. Diversity 2017, 9, 6. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.J.R.; Raposo, M.A.M.; Meireles, C.I.R.; Gomes, C.J.P.; Ribeiro, N.M.C.A. The impact of rural fires on the development of invasive species: Analysis of a case study with acacia dealbata link, in Casal Do Rei (Seia, Portugal). Environments 2021, 8, 44. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Raposo, M.A.M.; Meireles, C.I.R.; Pinto Gomes, C.J.; Ribeiro, N.M.C.A. Fire as a selection agent for the dissemination of invasive species: Case study on the evolution of forest coverage. Environments 2020, 7, 57. [Google Scholar] [CrossRef]
- Turco, M.; Jerez, S.; Augusto, S.; Tarín-Carrasco, P.; Ratola, N.; Jiménez-Guerrero, P.; Trigo, R.M. Climate drivers of the 2017 devastating fires in portugal. Sci. Rep. 2019, 9, 13886. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Martínez, S.; Penas, Á.; Díaz González, T.E.; Cantó, P.; del Río, S.; Costa, J.C.; Herrero, L.; Molero, J. Biogeographic units of the Iberian Peninsula and Baelaric Islands to district level. A concise synopsis. In The Vegetation of the Iberian Peninsula: Volume 1; Loidi, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 131–188. ISBN 978-3-319-54784-8. [Google Scholar]
- Marques, J.E.; Marques, J.M.; Chaminé, H.I.; Carreira, P.M.; Fonseca, P.E.; Monteiro Santos, F.A.; Moura, R.; Samper, J.; Pisani, B.; Teixeira, J.; et al. Conceptualizing a mountain hydrogeologic system by using an integrated groundwater assessment (Serra Da Estrela, Central Portugal): A Review. Geosci. J. 2013, 17, 371–386. [Google Scholar] [CrossRef]
- Sant’Ovaia, H.; Olivier, P.; Ferreira, N.; Noronha, F.; Leblanc, D. Magmatic structures and kinematics emplacement of the variscan granites from Central Portugal (Serra Da Estrela and Castro Daire Areas). J. Struct. Geol. 2010, 32, 1450–1465. [Google Scholar] [CrossRef]
- Sá, A.A.; Gutiérrez-Marco, J.C.; Meireles, C.; García-Bellido, D.C.; Rábano, I. A revised correlation of lower ordovician sedimentary rocks in the Central Iberian Zone, Portugal and Spain. In STRATI 2013: First International Congress on Stratigraphy: On the Cutting Edge of Stratigraphy; Springer: Berlin, Germany, 2013. [Google Scholar]
- Connor, S.E.; Araújo, J.; van der Knaap, W.O.; van Leeuwen, J.F.N. A long-term perspective on biomass burning in the Serra Da Estrela, Portugal. Quat. Sci. Rev. 2012, 55, 114–124. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, Á.; del Río, S.; Díaz González, T.E.; Rivas-Sáenz, S. Bioclimatology of the Iberian Peninsula and the Balearic Islands. In The Vegetation of the Iberian Peninsula: Volume 1; Loidi, J., Ed.; Springer: Cham, Switzerland, 2017; pp. 29–80. ISBN 978-3-319-54784-8. [Google Scholar]
- Costa, J.C.; Neto, C.; Aguiar, C.; Capelo, J.; Espírito Santo, M.D.; Honrado, J.J.; Gomes, C.P.; Monteiro-Henriques, T.; Sequeira, M.; Lousã, M. Vascular plant communities in Portugal (continental, the Azores and Madeira). Glob. Geobot. 2012, 2, 1–180. [Google Scholar]
- Braun-Blanquet, J. Fitosociología. Bases Para El Estudio de Las Comunidades Vegetales; Blume: Madrid, Spain, 1979. [Google Scholar]
- Géhu, J.M.; Rivas-Martínez, S. Notions fondamentales de phytosociologia. Ber. Int. Symp. Int. 1981, 4–5, 5–33. [Google Scholar]
- Rivas-Martínez, S. Avances En Geobotánica. Discurso de Apertura Del Curso 2005; Real Academia Farmacia: Madrid, Spain, 2005. [Google Scholar]
- Rivas-Martínez, S.; Díaz, T.E.; Fernández-González, F.; Izco, J.; Loidi, J.; Lousã, M.; Penas, A. Vascular plant communities of Spain and Portugal. Addenda to the syntaxonomical checklist of 2001, part II. Itinera Geobot. 2002, 15, 433–922. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.-P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Taleb, M.S.; Fennane, M. Vascular Plant Communities of Morocco: Phytosociology, Ecology and Geography; Springer: Berlin, Germany, 2019; ISBN 978-3-319-93703-8. [Google Scholar]
- Castroviejo, S. Flora Ibérica. Plantas Vasculares Da Península Ibérica e Ilhas Baleares; CSIC: Madrid, Spain, 1986. [Google Scholar]
- Coutinho, A.X.P. Flora de Portugal (Plantas vasculares); Bertrand: Lisbon, Portugal, 1939. [Google Scholar]
- Franco, J.A. Nova Flora de Portugal (Continente e Açores) Volume 1-Fascículos 2; Escolar Editora: Vila Franca de Xira, Portugal, 1971. [Google Scholar]
- Franco, J.A.; Rocha-Afonso, M.L. Nova Flora de Portugal (Continente e Açores) Volume III Fascísculo I; Escolar Editora: Vila Franca de Xira, Portugal, 1994. [Google Scholar]
- Goldin, A. Reassessing the use of loss-on-ignition for estimating organic matter content in noncalcareous soils. Commun. Soil Sci. Plant Anal. 1987, 18, 1111–1116. [Google Scholar] [CrossRef]
- Rodella, A.A.; Alcarde, J.C. Avaliação de materiais orgânicos empregados como fertilizantes. Sci. Agric. 1994, 51, 556–562. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-Km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Liu, L.; Niu, Y.; Zhao, S.; Zhang, S.; Wang, Y.; Liao, K. Pollen morphology of selected apricot (prunus) taxa. Palynology 2021, 45, 95–102. [Google Scholar] [CrossRef]
- Auer, W.; Prunus lusitanica. In PalDat—A Palynological Database. Available online: https://www.paldat.org/pub/Prunus_lusitanica/304059;jsessionid=EA915573AE592ED5F577CE42A8223CC6 (accessed on 26 May 2021).
- Chauvin, J.-E. Etude de Quatre Familles de Demi-Frères Issues de Croisements Intraspécifiques Chez le Prunier Domestique; Ecole Nationale Supérieure Agronomique: Alger, Algeria, 1985. [Google Scholar]
- Guitián, J.; Guitián, P.; Sánchez, J.M. Reproductive biology of twoprunus species (rosaceae) in the Northwest Iberian Peninsula. Plant Syst. Evol. 1993, 185, 153–165. [Google Scholar] [CrossRef]
- Lorenzo, P.; Pereira, C.S.; Rodríguez-Echeverría, S. Differential impact on soil microbes of allelopathic compounds released by the invasive acacia dealbata link. Soil Biol. Biochem. 2013, 57, 156–163. [Google Scholar] [CrossRef]
- Marchante, H.; Morais, M.; Freitas, H.; Marchante, E. Guia Prático Para a Identificação de Plantas Invasoras Em Portugal; Imprensa da Universidade de Coimbra: Coimbra, Portugal, 2014; ISBN 978-989-26-0786-3. [Google Scholar]
- Laface, V.L.A.; Musarella, C.M.; Cano Ortiz, A.; Quinto Canas, R.; Cannavò, S.; Spampinato, G. Three new alien taxa for Europe and a chorological update on the alien vascular flora of Calabria (Southern Italy). Plants 2020, 9, 1181. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Gioria, M. Plant invasions. J. Plant Ecol. 2018, 11, 1–3. [Google Scholar] [CrossRef]
- Raposo, M.A.M.; Gomes, C.J.P.; Nunes, L.J.R. Selective shrub management to preserve mediterranean forests and reduce the risk of fire: The case of Mainland Portugal. Fire 2020, 3, 65. [Google Scholar] [CrossRef]
- Piñar Fuentes, J.C.; Leiva, F.; Cano-Ortiz, A.; Musarella, C.M.; Quinto-Canas, R.; Pinto-Gomes, C.J.; Cano, E. Impact of grass cover management with herbicides on biodiversity, soil cover and humidity in olive groves in the Southern Iberian. Agronomy 2021, 11, 412. [Google Scholar] [CrossRef]
- Fernandes, P.M.; Guiomar, N.; Rossa, C.G. Analysing eucalypt expansion in Portugal as a fire-regime modifier. Sci. Total Environ. 2019, 666, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, F.; Esposito, A.; Altieri, S.; Battipaglia, G. Fire severity influences ecophysiological responses of pinus pinaster ait. Front. Plant Sci. 2019, 10, 539. [Google Scholar] [CrossRef]
- Rumpf, S.B.; Hülber, K.; Wessely, J.; Willner, W.; Moser, D.; Gattringer, A.; Klonner, G.; Zimmermann, N.E.; Dullinger, S. Extinction debts and colonization credits of non-forest plants in the European Alps. Nat. Commun. 2019, 10, 4293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittaker, R.J.; Bush, M.B.; Richards, K. Plant recolonization and vegetation succession on the Krakatau Islands, Indonesia. Ecol. Monogr. 1989, 59, 59–123. [Google Scholar] [CrossRef]
- Lara, F.; Garilleti, R.; Calleja, J.A. La Vegetación de Ribera de la Mitad Norte Española, 2nd ed.; Centro de Estudios y Experimentación de Obras Públicas: Madrid, Spain, 2007; ISBN 978-84-7790-440-3. [Google Scholar]
- Franco, J.A. O Azereiro e as Ginjeiras Bravas, Coimbra, 1964—Biblioteca nacional digital. Bol. Soc. Port. Ciênc. Nat. 1964, 10, 66–90. [Google Scholar]
- Ladero, M. Prunus lusitanica L. (rosaceae) en la Península Ibérica. Anal. Inst. Bot. A. J. Cavanilles 1976, 33, 207–218. [Google Scholar]
- Calleja, J.A.; Garzón, M.B.; Ollero, H.S. A quaternary perspective on the conservation prospects of the tertiary relict tree Prunus lusitanica L. J. Biogeogr. 2009, 36, 487–498. [Google Scholar] [CrossRef]
- Sattout, E.; Caligari, P.D.S. Forest biodiversity assessment in relic ecosystem: Monitoring and management practice implications. Diversity 2011, 3, 531–546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).