Soil Nematode Communities in Managed and Natural Temperate Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling Procedure
2.3. Analyses of Soil Properties
2.4. Analyses of Nematode Communities
2.5. Statistical Analysis
3. Results
3.1. Effect of Forest Management
3.2. Effect of Forest Age Class
3.3. Mixed Effect of Forest Management × Age
4. Discussion
4.1. Effect of Forest Management
4.2. Effect of Forest Age Class
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scr. Geobot. 1992, 18, 3–258. [Google Scholar]
- Morgan, J.B.; Connolly, E.L. Plant-Soil Interactions: Nutrient Uptake. Nat. Educ. Knowl. 2013, 4, 2. [Google Scholar]
- Prescott, C.; Grayston, S. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. For. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Londo, A.J.; Kushla, J.D.; Carter, R.C. Soil pH and Tree Species Suitability in the South. S. Reg. Ext. For. Reg. Peer Rev. Technol. Bull. 2006, 2, 1–5. [Google Scholar]
- Mayer, M.; Prescott, C.E.; Abaker, W.E.A.; Augusto, L.; Cécillon, L.; Ferreira, G.W.D.; James, J.; Jandl, R.; Katzensteiner, K.; Laclau, J.P.; et al. Tamm Review: Influence of forest management activities on soil organic carbon stocks: A knowledge synthesis. For. Ecol. Manag. 2020, 466, 118127. [Google Scholar] [CrossRef]
- Aponte, C.; García, L.V.; Marañón, T. Tree species effects on nutrient cycling and soil biota: A feedback mechanism favouring species coexistence. For. Ecol. Manag. 2013, 309, 36–46. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.M.; An, S.S.; Sun, H.Y.; Bhople, P.; Chen, Z. Soil physicochemical and microbial characteristics of contrasting land-use types along soil depth gradients. Catena 2018, 162, 345–353. [Google Scholar] [CrossRef]
- Lindtner, P.; Gömöryová, E.; Gömöry, D.; Stašiov, S.; Kubovčík, V. Development of physico-chemical and biological soil properties on the European ground squirrel mounds. Geoderma 2019, 339, 85–93. [Google Scholar] [CrossRef]
- Walter, H.; Straka, H. Arealkunde. Floristisch-historische Geobotanik; Eugen Ulmer: Stuttgart, Germany, 1970. [Google Scholar]
- FAO. Global Forest Resources Assessment; Main Report; FAO: Rome, Italy, 2010. [Google Scholar]
- Pretzsch, H. Diversity and Productivity in Forests. In Forest Diversity and Function: Ecological Studies; Scherer-Lorenzen, M., Körner, C., Schulze, E.-D., Eds.; Springer: Berlin, Germany, 2005; pp. 41–64. [Google Scholar]
- Küster, H. Geschichte des Waldes. In Von der Urzeit bis zur Gegenwart; CH Beck: München, Germany, 1998. [Google Scholar]
- Pretzsch, H. Canopy space filling and tree crown morphology in mixed-species stands compared with monocultures. Carl Olof Tamm Rev. For. Ecol. Manag. 2014, 327, 251–264. [Google Scholar] [CrossRef]
- Franklin, J.F.; Mitchell, R.J.; Palik, B.J. Natural Disturbance and Stand Development Principles for Ecological Forestry. USDA For. Serv. North Res. Station Gen. Tech. Rep. NRS-19 2007, 19, 44. [Google Scholar]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Schall, P.; Gossner, M.M.; Heinrichs, S.; Fischer, M.; Boch, S.; Prati, D.; Jung, K.; Baumgartner, V.; Blaser, S.; Böhm, S.; et al. The impact of even-aged and uneven-aged forest management on regional biodiversity of multiple taxa in European beech forests. J. Appl. Ecol. 2018, 55, 267–378. [Google Scholar] [CrossRef]
- Torras, O.; Saura, S. Effects of silvicultural treatments on forest biodiversity indicators in the Mediterranean. For. Ecol. Manag. 2008, 255, 3322–3330. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Forest management and biodiversity conservation based on natural ecosystem dynamics in northern Europe: The complexity challenge. AMBIO A J. Hum. Environ. 2009, 38, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Odor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.J.; de Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity differences between managed and unmanaged forests: Meta-analysis of species richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef]
- Van den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; de Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 5727768, 194–198. [Google Scholar] [CrossRef]
- Wilschut, R.A.; Geisen, S. Nematodes as Drivers of Plant Performance in Natural Systems. Trends Plant Sci. 2020, 26, 237–347. [Google Scholar] [CrossRef]
- De Goede, R.G.M.; Bongers, T. Nematode community structure in relation to soil and vegetation characteristics. Appl. Soil Ecol. 1994, 1, 29–44. [Google Scholar] [CrossRef]
- Wasilewska, L. Soil invertebrates as bioindicators, with special reference to soil inhabiting nematodes. Russ. J. Nematol. 1997, 5, 113–126. [Google Scholar]
- Ferris, H.; Griffiths, B.S.; Porazinska, D.L.; Powers, T.O.; Wang, K.-H.; Tenuta, M. Reflections on Plant and Soil Nematode Ecology: Past, Present and Future. J. Nematol. 2012, 44, 115–126. [Google Scholar]
- Yeates, G.W.; Bongers, T.; de Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera, outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Šály, R. Pôdy chránenej krajinnej oblasti biosférickej rezervácie Poľana. In Soils of the Protected Lanscape Area—Poľana Biosphere Reserve; VÚPOP: Bratislava, Slovakia, 2000. [Google Scholar]
- Šťastný, P.; Nieplová, E.; Melo, M. Mean Annual Air Temperature. In Landscape Atlas of the Slovak Republic; Miklós, L., Maráky, P., Klinda, J., Eds.; Ministry of Environment of the Slovak Republic, Bratislava & Slovak Environmental Agency: Banská Bystrica, Slovakia, 2002; p. 98. [Google Scholar]
- Ujházy, K.; Hederová, L.; Máliš, F.; Ujházyová, M.; Bosela, M.; Čiliak, M. Overstorey dynamics controls plant diversity in age-class temperate forests. For. Ecol. Manag. 2017, 391, 96–105. [Google Scholar] [CrossRef]
- Seinhorst, J.W. On the killing, fixation and transferring to glycerine of nematodes. Nematologica 1962, 81, 29–32. [Google Scholar] [CrossRef]
- Brzeski, M.W. Nematodes of Tylenchida in Poland and Temperate Europe. In Museum of Institute of Zoology; Polish Academy of Sciences: Warszawa, Poland, 1998. [Google Scholar]
- Loof, P.A.A. Nematoda, Adenophorea Dorylaimida; Spektrum Akademischer Verlag: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Siddiqi, M.R. Tylenchida: Parasites of Plants and Insects, 2nd ed.; CAB International: Wallingford, UK, 2000. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary Nematoda Errantia; Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2005; Volume 1. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary Nematoda Errantia; Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2007; Volume 2. [Google Scholar]
- Andrássy, I. Free-Living Nematodes of Hungary Nematoda Errantia; Hungarian Natural History Museum and Systematic Zoology Research Group of the Hungarian Academy of Sciences: Budapest, Hungary, 2009; Volume 3. [Google Scholar]
- Geraert, E. The Tylenchidae of the World Identification of the Family Tylenchidae Nematoda; Academia Press: Gent, Belgium, 2008. [Google Scholar]
- Geraert, E. The Criconematidae of the World Identification of the Family Criconematidae Nematoda; Academia Press: Gent, Belgium, 2010. [Google Scholar]
- Bongers, T. The maturity index, an ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; de Goede, R.G.M. A framework for soil food web diagnostics, extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Berkelmans, R.; Ferris, H.; Tenuta, M.; van Bruggen, A.H.C. Effects of long term crop management on nematode trophic levels other than plant feeders disappear after 1 year of disruptive soil management. Appl. Soil Ecol. 2003, 23, 223–235. [Google Scholar] [CrossRef]
- Sieriebriennikov, B.; Ferris, H.; de Goede, R.G.M. NINJA: An automated calculation system for nematode-based biological monitoring. Eur. J. Soil Biol. 2014, 61, 90–93. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- StatSoft, Inc STATISTICA data analysis software system, version 12.0, 2013.
- Bethlahny, N. First year effects of timber removal on soil moisture. International Association of Scientific Hydrology. Bulletin 1962, 7, 34–38. [Google Scholar]
- Hashimoto, S.; Suzuki, M. The impact of forest clear-cutting on soil temperature: A comparison between before and after cutting, and between clear-cut and control sites. J. For. Res. 2004, 9, 125–132. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Ma, Y.; Geng, Y.; Huang, Y.; Shi, Y.; Niklaus, P.A.; Schmid, B.; He, J.-H. Effect of clear-cutting silviculture on soil respiration in a subtropical forest of China. J. Plant Ecol. 2013, 6, 335–348. [Google Scholar] [CrossRef]
- Bergholm, J.; Olsson, B.A.; Vegerfors, B.; Persson, T. Nitrogen fluxes after clear-cutting. Ground vegetation uptake and stump/root immobilisation reduce N leaching after experimental liming, acidification and N fertilisation. For. Ecol. Manag. 2015, 342, 64–75. [Google Scholar] [CrossRef]
- Lacroix, E.M.; Petrenko, C.L.; Friedland, A.J. Evidence for Losses from Strongly Bound SOM Pools after Clear Cutting in a Northern Hardwood Forest. Soil Sci. 2016, 181, 202–207. [Google Scholar] [CrossRef]
- Keith, A.M.; Brooker, R.W.; Osler, G.H.R.; Chapman, S.J.; Burslem, D.F.R.P.; van der Wal, R. Strong impacts of belowground tree inputs on soil nematode trophic composition. Soil Biol. Biochem. 2009, 41, 1060–1065. [Google Scholar] [CrossRef]
- Panesar, T.S.; Marshall, V.G.; Barclaz, H.J. Abundance and diversity of soil nematodes in chronosequences of coastal Douglas-fir forests on Vancouver Island, British Columbia. Pedobiologia 2001, 45, 193–212. [Google Scholar] [CrossRef]
- Yeates, G.W. Abundance, diversity, and resilience of nematode assemblages in forest soils. Can. J. For. Res. 2007, 37, 216–225. [Google Scholar] [CrossRef]
- Neher, D.; Weicht, T.R.; Moorhead, D.L.; Sinsabaugh, R.L. Elevated CO2 alters functional attributes of nematode communities in forest soils. Funct. Ecol. 2004, 18, 584–591. [Google Scholar] [CrossRef]
- Scherer-Lorenzen, M.; Palmborg, C.; Prinz, A.; Schulze, E.D. The role of plant diversity and composition for nitrate leaching in grasslands. Ecology 2003, 84, 1539–1552. [Google Scholar] [CrossRef]
- Forge, T.S.; Simard, S.W. Trophic structure of nematode communities, microbial biomass, and nitrogen mineralization in soils of forests and clearcuts in the southern interior of British Columbia. Can. J. Soil Sci. 2001, 40, 401–410. [Google Scholar] [CrossRef]
- Sohlenius, B. Influence of clear-cutting and forest age on the nematode fauna in a Swedish pine forest soil. Appl. Soil Ecol. 2002, 19, 261–277. [Google Scholar] [CrossRef]
- Neher, D.A.; Wu, J.; Barbercheck, M.E.; Anas, O. Ecosystem type affects interpretation of soil nematode community measures. Appl. Soil Ecol. 2005, 30, 47–64. [Google Scholar] [CrossRef]
- De Deyn, G.B.; Raaijmakers, C.E.; van Ruijven, J.; Berendse, F.; van der Putten, W.H. Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 2004, 106, 576–586. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Homolová, Z.; Gömöryová, E. Soil Nematode Fauna and Microbial Characteristics in an Early-Successional Forest Ecosystem. Forests 2019, 10, 888. [Google Scholar] [CrossRef]
- Matlack, G.R. Factors determining the distribution of soil nematodes in a commercial forest landscape. For. Ecol. Manag. 2001, 146, 129–143. [Google Scholar] [CrossRef]
- Magnusson, C. Abundance, distribution and feeding relations of root/fungal feeding nematodes in a Scots pine forest. Ecography 1983, 6, 183–193. [Google Scholar] [CrossRef]
- Renčo, M.; Čerevková, A.; Homolová, Z.; Gömöryová, E. Long-term effects on soil nematode community structure in spruce forests of removing or not removing fallen trees after a windstorm. For. Ecol. Manag. 2015, 356, 243–252. [Google Scholar] [CrossRef]
- Bjørnlund, L.; Vestergård, M.; Johansson, S.; Nyborg, M.; Steffensen, L.; Christensen, S. Nematode communities of natural and managed beech forests–A pilot survey. Pedobiologia 2002, 46, 53–62. [Google Scholar] [CrossRef][Green Version]
- Ettema, C.H.; Bongers, T. Characterization of nematode colonization and succession in disturbed soil using the Maturity Index. Biol. Fertil. Soils 1993, 16, 79–85. [Google Scholar] [CrossRef]
- Háněl, L. Response of soil nematodes inhabiting spruce forests in the Šumava Mountains to disturbance by bark beetles and clear-cutting. For. Ecol. Manag. 2004, 202, 209–225. [Google Scholar] [CrossRef]
- De Goede, R.G.M.; Georgieva, B.C.; Verschoor, B.C.; Kamerman, J. Changes in nematode community structure in a primary succession of blown-out areas in a drift sand landscape. Fundam. Appl. Nematol. 1993, 16, 501–513. [Google Scholar]
- Hodda, M.; Bloemers, G.F.; Lawton, J.H.; Lambshead, P.J.D. The effects of clearing and subsequent land-use on abundance and biomass of soil nematodes in tropical forest. Pedobiologia 1997, 414, 279–294. [Google Scholar]
- Háněl, L. Succession of soil nematodes in pine forests on coal-mining sands near Cottbus, Germany. Appl. Soil Ecol. 2001, 16, 23–34. [Google Scholar] [CrossRef]
- Armendariz, I.; Hernández, M.A.; Jordana, R. Temporal evolution of soil nematode communities in Pinus nigra forests of Navarra, Spain. Fundam. Appl. Nematol. 1996, 19, 561–577. [Google Scholar]
- Yeates, G.W.; Newton, P.C.D.; Ross, D.J. Significant changes in soil microfauna in grazed pasture under elevated carbon dioxide. Biol. Fertil. Soils 2003, 38, 319–326. [Google Scholar] [CrossRef]
- Bengtsson, J.; Lundkvist, H.; Seatre, P.; Sohlenius, B.; Solbreck, B. Effects of organic matter removal on the soil food web: Forestry practices meet ecological theory. Appl. Soil Ecol. 1998, 9, 137–143. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of Biodiversity on Ecosystem Functioning: A Consensus of Current Knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Coleman, D.C.; Whitman, W.B. Linking species richness, biodiversity and ecosystem function in soil systems. Pedobiologia 2005, 49, 479–497. [Google Scholar] [CrossRef]
- Setälä, H.; McLean, M.A. Decomposition rate of organic substrates in relation to the species diversity of soil saprophytic fungi. Oecologica 2004, 139, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Wertz, S.; Degrange, V.; Prosser, J.I.; Poly, F.; Commeaux, C.; Freitag, T.; Guillaumaud, N.; Roux, X.L. Maintenance of soil functioning following erosion of microbial diversity. Environ. Microbiol. 2006, 8, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
| BEE | SPR | UNM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(2,44) | p | Mean | SD | Mean | SD | Mean | SD | |||||
| Soil moisture | 4.619 | 0.015 | * | 54.86 | 15.31 | ab | 42.48 | 7.54 | a | 58.65 | 20.15 | b |
| pH-H2O | 2.011 | 0.147 | 4.86 | 0.35 | 4.69 | 0.29 | 4.93 | 0.34 | ||||
| C% | 0.210 | 0.811 | 9.10 | 2.48 | 9.27 | 2.14 | 9.71 | 3.23 | ||||
| N% | 0.435 | 0.650 | 0.77 | 0.17 | 0.76 | 0.13 | 0.82 | 0.24 | ||||
| C/N | 0.634 | 0.536 | 11.66 | 1.07 | 12.07 | 1.27 | 11.71 | 0.84 | ||||
| Nematode Genera | TG | Forest Stand | Age (BEE + SPR) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEE | SPR | UNM | 0–20 year | 40–60 Year | 100–120 Year | ||||||||
| D% | F% | D% | F% | D% | F% | D% | F% | D% | F% | D% | F% | ||
| Araeolaimida | |||||||||||||
| Aulolaimus | B | 0.2 | 13 | - | - | - | - | 0.1 | 10 | 0.1 | 10 | - | - |
| Chronogaster | B | - | - | 0.1 | 13 | - | - | 0.2 | 20 | - | - | - | - |
| Plectus | B | 6.6 | 100 | 16.3 | 100 | 6.6 | 100 | 15.6 | 100 | 6.9 | 100 | 12.2 | 100 |
| Ceratoplectus | B | 0.7 | 40 | - | - | - | - | 0.6 | 30 | 0.1 | 30 | - | - |
| Wilsonema | B | 1.7 | 80 | 0.7 | 47 | 2.0 | 67 | 1.7 | 70 | 0.9 | 70 | 0.5 | 50 |
| Euteratocephalus | B | 0.1 | 7 | - | - | - | - | 0.1 | 10 | - | - | - | - |
| Rhabditida | |||||||||||||
| Teratocephalus | B | 2.2 | 87 | 1.4 | 40 | 0.3 | 27 | 3.4 | 100 | 0.7 | 50 | 0.6 | 40 |
| Cephalobus | B | 2.3 | 73 | 2.8 | 80 | 3.0 | 67 | 1.5 | 90 | 1.4 | 50 | 5.6 | 90 |
| Eucephalobus | B | 0.4 | 20 | 0.6 | 47 | 2.0 | 80 | 1.0 | 60 | - | - | 0.4 | 40 |
| Acrobeloides | B | 8.3 | 100 | 21.3 | 100 | 16.7 | 73 | 14.2 | 100 | 9.9 | 100 | 23.8 | 100 |
| Chiloplacus | B | 0.4 | 53 | 0.2 | 20 | - | - | 0.3 | 40 | 0.3 | 40 | 0.2 | 30 |
| Cervidellus | B | 6.2 | 93 | 6.7 | 87 | 5.0 | 87 | 5.0 | 100 | 6.4 | 90 | 8.8 | 80 |
| Acrobeles | B | 0.5 | 20 | 0.1 | 7 | 0.1 | 7 | 0.5 | 30 | 0.1 | 10 | - | - |
| Rhabditis | B | 17.3 | 100 | 12.8 | 100 | 15.2 | 100 | 14.7 | 100 | 20.8 | 100 | 7.9 | 100 |
| Diploscapter | B | - | - | 0.6 | 40 | 1.8 | 33 | - | - | 0.3 | 30 | 0.8 | 30 |
| Steinernema | B | 0.7 | 27 | - | - | - | - | 0.7 | 30 | - | - | 0.1 | 10 |
| Aphelenchida | |||||||||||||
| Aphelenchus | F | 1.3 | 33 | 0.3 | 13 | 0.1 | 7 | 0.9 | 40 | 0.6 | 20 | 0.5 | 10 |
| Aphelenchoides | F | 3.3 | 100 | 10.1 | 100 | 4.1 | 67 | 6.4 | 100 | 7.7 | 100 | 7.3 | 100 |
| Tylenchida | |||||||||||||
| Aglenchus | H | 1.1 | 40 | 0.1 | 7 | 0.9 | 27 | - | - | 0.3 | 30 | 1.5 | 40 |
| Coslenchus | H | 0.2 | 20 | 0.1 | 7 | - | - | - | - | 0.2 | 10 | 0.3 | 30 |
| Filenchus | F | 11.2 | 100 | 5.8 | 100 | 10.1 | 100 | 6.0 | 100 | 10.1 | 100 | 9.4 | 100 |
| Tylenchus | H | 0.1 | 7 | - | - | - | - | 0.2 | 10 | - | - | - | - |
| Malenchus | H | 6.2 | 73 | 1.9 | 87 | 6.0 | 93 | 2.8 | 80 | 6.8 | 80 | 2.0 | 80 |
| Tylenchorhynchus | H | 0.4 | 20 | 0.4 | 13 | 0.2 | 13 | 0.9 | 40 | 0.1 | 10 | - | - |
| Pratylenchus | H | 0.3 | 27 | 0.2 | 27 | 0.2 | 7 | 0.3 | 20 | 0.1 | 10 | 0.5 | 50 |
| Helicotylenchus | H | 0.5 | 33 | 0.3 | 60 | 0.7 | 33 | 0.6 | 50 | 0.2 | 60 | 0.4 | 40 |
| Rotylenchus | H | 0.2 | 27 | 0.2 | 20 | 0.3 | 7 | 0.2 | 20 | 0.1 | 20 | 0.2 | 30 |
| Heterodera | H | 0.1 | 7 | - | - | 0.3 | 27 | - | - | - | - | 0.1 | 10 |
| Paratylenchus | H | 5.7 | 100 | 1.6 | 73 | 4.1 | 67 | 1.6 | 80 | 6.2 | 80 | 2.7 | 100 |
| Mesocriconema | H | 0.1 | 20 | - | - | - | - | - | - | 0.1 | 30 | - | - |
| Enoplida | |||||||||||||
| Prismatolaimus | B | 4.5 | 87 | 2.4 | 73 | 1.3 | 67 | 4.5 | 80 | 2.6 | 90 | 2.3 | 70 |
| Tripyla | P | 1.2 | 87 | 0.9 | 73 | 1.1 | 60 | 1.1 | 90 | 1.1 | 80 | 0.8 | 70 |
| Alaimida | |||||||||||||
| Alaimus | B | 1.4 | 93 | 1.3 | 93 | 2.0 | 87 | 1.2 | 90 | 1.8 | 100 | 0.9 | 90 |
| Amphidelus | B | 0 | 7 | - | - | 0.1 | 7 | - | - | 0.1 | 10 | - | - |
| Diphtherophorida | |||||||||||||
| Diphtherophora | F | 0.6 | 60 | 0.2 | 20 | 0.3 | 27 | 0.4 | 30 | 0.4 | 60 | 0.1 | 30 |
| Trichodorus | H | 0.1 | 13 | 0.6 | 47 | 0.9 | 33 | 0.5 | 50 | 0.6 | 40 | - | - |
| Mononchida | |||||||||||||
| Clarkus | P | - | - | - | - | 1.6 | 60 | - | - | - | - | - | - |
| Prionchulus | P | 0.1 | 7 | - | - | 0.2 | 13 | 0.1 | 10 | - | - | - | - |
| Mylonchulus | P | 1.5 | 73 | 1.2 | 73 | 0.7 | 27 | 1.9 | 90 | 1.0 | 70 | 0.9 | 60 |
| Anatonchus | P | 0.5 | 80 | 0.5 | 53 | 1.2 | 67 | 0.2 | 60 | 0.7 | 80 | 0.7 | 60 |
| Dorylaimida | |||||||||||||
| Dorylaimus | O | 0.1 | 7 | - | - | - | - | 0.1 | 10 | - | - | - | - |
| Mesodorylaimus | O | 1.5 | 73 | 0.7 | 53 | 1.2 | 53 | 1.5 | 70 | 0.9 | 90 | 0.5 | 30 |
| Discolaimus | P | - | - | - | - | 0.1 | 7 | - | - | - | - | - | - |
| Crassolabium | O | 0.1 | 7 | - | - | 0.3 | 13 | 0.1 | 10 | - | - | - | - |
| Eudorylaimus | O | 8.0 | 93 | 6.8 | 100 | 7.4 | 100 | 7.0 | 90 | 8.8 | 100 | 6.1 | 100 |
| Aporcelaimellus | O | 0.5 | 47 | 0.4 | 33 | 0.3 | 27 | 0.3 | 40 | 0.5 | 40 | 0.6 | 40 |
| Enchodelus | P | - | - | 0.2 | 13 | 0.4 | 13 | 0.3 | 20 | - | - | - | - |
| Xiphinema | H | 0.1 | 13 | 0.1 | 7 | - | - | 0.1 | 10 | 0 | 10 | 0.1 | 10 |
| Axonchium | H | 0.2 | 33 | 0.1 | 13 | - | - | 0.2 | 40 | 0 | 10 | 0.1 | 20 |
| Tylencholaimus | F | 1.6 | 87 | 0.5 | 40 | 1.3 | 53 | 1.1 | 60 | 0.6 | 70 | 1.1 | 60 |
| Doryllium | F | 0.4 | 13 | - | - | - | - | 0.1 | 10 | 0.4 | 10 | - | - |
| Numbers of genera | 46 | 37 | 38 | 43 | 39 | 34 | |||||||
| BEE | SPR | UNM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(2,44) | p | Mean | SD | Mean | SD | Mean | SD | |||||
| Abundance | 21.502 | 0 | *** | 631.07 | 176.79 | a | 778.33 | 239.53 | a | 311 | 207.14 | b |
| H’gen | 2.529 | 0.092 | 2.40 | 0.46 | 2.24 | 0.25 | 2.40 | 0.22 | ||||
| Total Biomass, mg | 8.984 | 0.001 | *** | 1.51 | 1.19 | a | 1.25 | 0.85 | a | 0.61 | 0.58 | b |
| Bacterivores% | 4.675 | 0.015 | * | 52.48 | 15.21 | a | 65.49 | 12.04 | b | 49.53 | 17.76 | a |
| Fungivores% | 0.179 | 0.837 | 18.67 | 5.97 | 17.17 | 6.31 | 17.72 | 9.17 | ||||
| Herbivores% | 6.802 | 0.003 | ** | 15.48 | 13.99 | a | 6.70 | 6.4 | b | 17.93 | 10.79 | a |
| Omnivores% | 0.771 | 0.469 | 10.20 | 4.24 | 7.93 | 5.02 | 9.81 | 6.57 | ||||
| Predators% | 1.06 | 0.356 | 3.17 | 1.32 | 2.69 | 1.97 | 5.01 | 4.76 | ||||
| Maturity Index | 1.257 | 0.295 | 2.27 | 0.27 | 2.18 | 0.22 | 2.33 | 0.34 | ||||
| Plant Parasitic Index | 0.759 | 0.474 | 2.30 | 0.33 | 2.43 | 0.37 | 2.33 | 0.47 | ||||
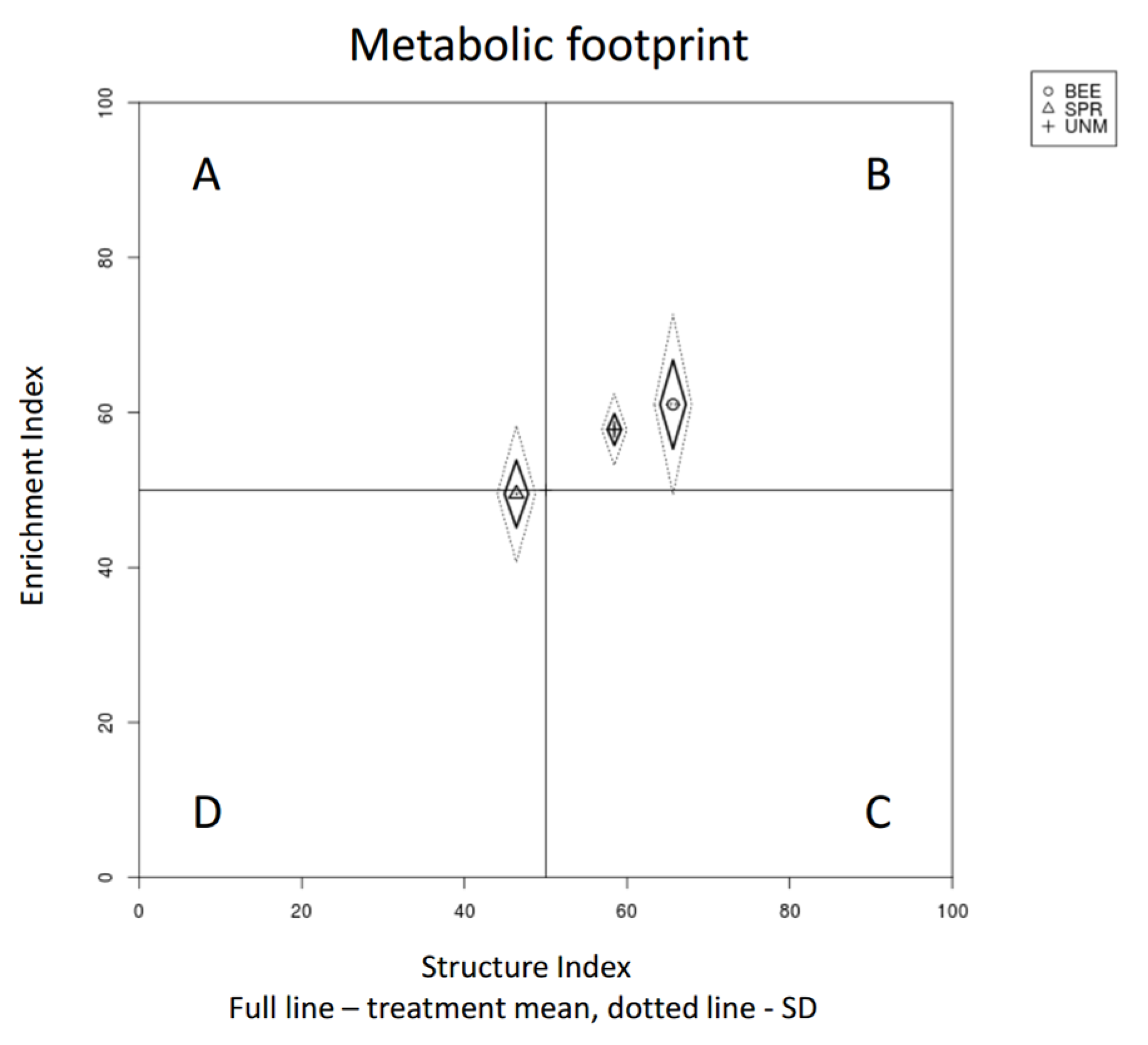
| Enrichment Index | 1.903 | 0.162 | 61.05 | 17.74 | 49.5 | 19.06 | 57.81 | 15.87 | ||||
| Structure Index | 4.304 | 0.02 | * | 65.65 | 10.95 | b | 46.39 | 19.44 | a | 58.44 | 21.6 | ab |
| Basal Index | 3.381 | 0.044 | * | 22.11 | 9.44 | a | 35.93 | 17.27 | b | 26.38 | 14.46 | ab |
| Channel Index | 0.16 | 0.852 | 28.09 | 19.41 | 29.73 | 15.81 | 27.86 | 19.8 | ||||
| Stand | Age | BEE | SPR | 0–20 Year | 40–60 Year | 100–120 Year | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(1,26) | F(2,26) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||||
| Soil moisture | 8.052 | ** | 1.272 | 54.86 | 15.31 | a | 42.48 | 7.54 | b | 46.28 | 8.20 | 46.14 | 13.16 | 53.59 | 17.32 | ||||
| pH-H2O | 2.779 | 6.721 | ** | 4.86 | 0.35 | 4.69 | 0.29 | 4.96 | 0.28 | a | 4.84 | 0.32 | a | 4.53 | 0.23 | b | |||
| Total C | 0.04 | 0.882 | 9.10 | 2.48 | 9.27 | 2.14 | 8.64 | 1.60 | 9.96 | 2.78 | 8.96 | 2.32 | |||||||
| Total N | 0.045 | 1.696 | 0.77 | 0.17 | 0.76 | 0.13 | 0.70 | 0.10 | 0.82 | 0.19 | 0.77 | 0.13 | |||||||
| C/N | 0.903 | 1.167 | 11.66 | 1.07 | 12.07 | 1.27 | 12.22 | 1.12 | 11.93 | 0.97 | 11.43 | 1.38 | |||||||
| Stand | Age | BEE | SPR | 0–20 Year | 40–60 Year | 100–120 Year | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(1,26) | F(2,26) | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||||||
| Abundance | 4.781 | * | 6.469 | ** | 631.07 | 176.79 | a | 778.33 | 239.53 | b | 863.2 | 230.13 | a | 677.0 | 187.42 | b | 573.9 | 141.69 | b |
| H’gen | 6.307 | * | 2.786 | 2.40 | 0.46 | 2.24 | 0.25 | 2.48 | 0.30 | 2.22 | 0.49 | 2.27 | 0.30 | ||||||
| Total Biomass, mg | 0.346 | 7.347 | ** | 1.51 | 1.19 | 1.25 | 0.85 | 1.94 | 1.27 | a | 1.49 | 0.94 | a | 0.72 | 0.19 | b | |||
| Bacterivores% | 7.505 | * | 2.674 | 52.48 | 15.21 | a | 65.49 | 12.04 | b | 63.81 | 9.60 | 51.23 | 17.34 | 61.91 | 15.2 | ||||
| Fungivores% | 0.515 | 1.242 | 18.67 | 5.97 | 17.17 | 6.31 | 15.47 | 4.34 | 19.05 | 6.53 | 19.25 | 6.92 | |||||||
| Herbivores% | 5.365 | * | 0.688 | 15.48 | 13.99 | a | 6.70 | 6.40 | b | 8.12 | 6.04 | 16.6 | 16.76 | 8.55 | 8.01 | ||||
| Omnivores% | 2.949 | 1.292 | 10.20 | 4.24 | 7.93 | 5.02 | 9.18 | 3.98 | 10.3 | 5.31 | 7.72 | 4.86 | |||||||
| Predators% | 1.581 | 1.141 | 3.17 | 1.32 | 2.69 | 1.97 | 3.43 | 1.33 | 2.81 | 1.84 | 2.54 | 1.81 | |||||||
| Maturity Index | 1.472 | 0.275 | 2.27 | 0.27 | 2.18 | 0.22 | 2.27 | 0.25 | 2.20 | 0.32 | 2.21 | 0.18 | |||||||
| Plant Parasitic Index | 1.213 | 0.922 | 2.30 | 0.33 | 2.43 | 0.37 | 2.49 | 0.41 | 2.35 | 0.37 | 2.27 | 0.26 | |||||||
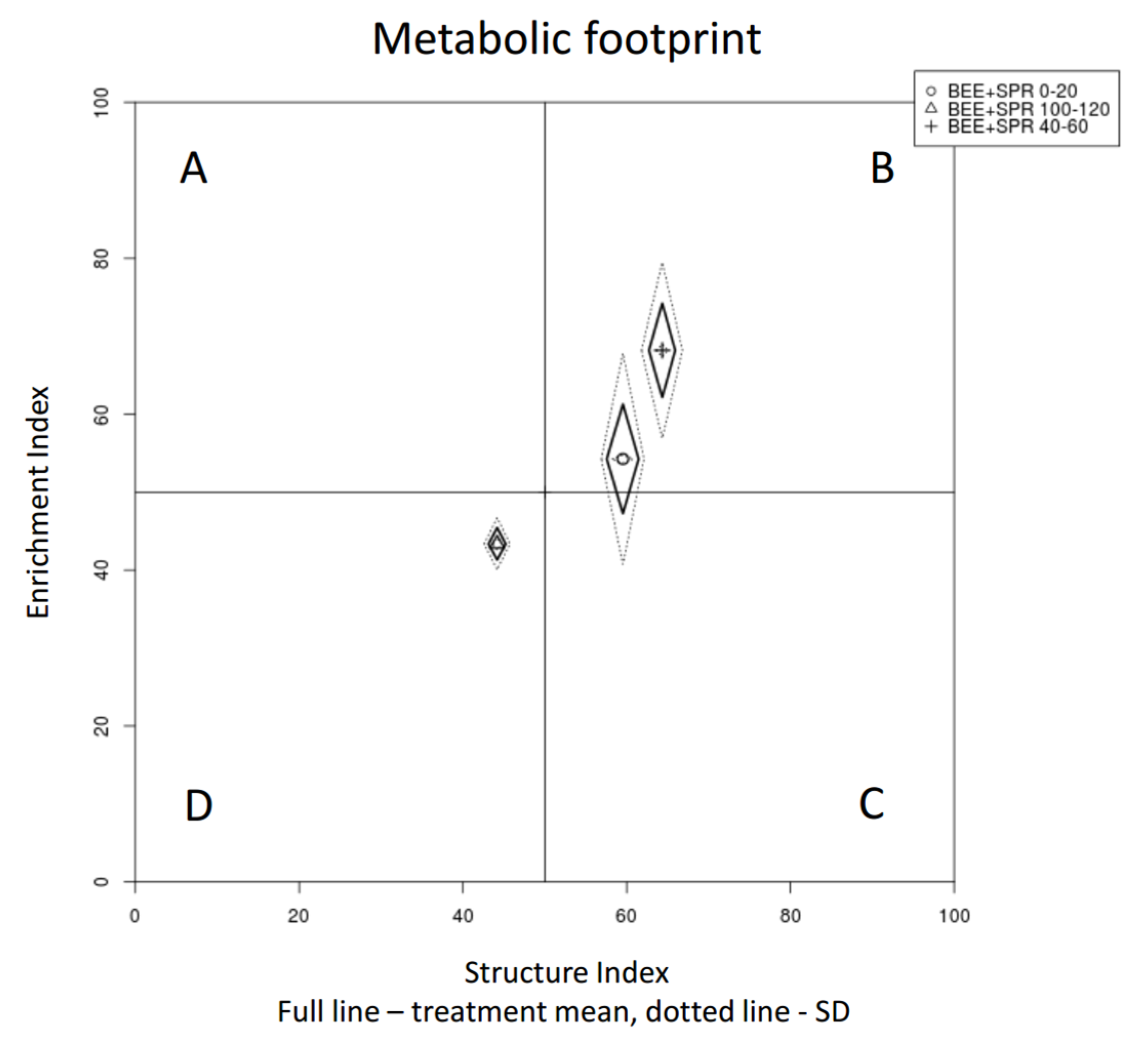
| Enrichment Index | 4.39 | * | 6.628 | ** | 61.05 | 17.74 | a | 49.5 | 19.06 | b | 54.27 | 18.16 | ab | 68.19 | 14.34 | a | 43.37 | 16.92 | b |
| Structure Index | 14.76 | *** | 5.985 | ** | 65.65 | 10.95 | a | 46.39 | 19.44 | b | 59.53 | 13.14 | a | 64.34 | 17.76 | a | 44.18 | 18.68 | b |
| Basal Index | 9.556 | ** | 6.968 | ** | 22.11 | 9.44 | a | 35.93 | 17.27 | b | 26.65 | 11.74 | a | 20.65 | 11.8 | a | 39.76 | 16.49 | b |
| Channel Index | 0.261 | 2.689 | 28.09 | 19.41 | 29.73 | 15.81 | 25.95 | 17.21 | 22.25 | 11.05 | 38.53 | 19.92 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čerevková, A.; Renčo, M.; Miklisová, D.; Gömöryová, E. Soil Nematode Communities in Managed and Natural Temperate Forest. Diversity 2021, 13, 327. https://doi.org/10.3390/d13070327
Čerevková A, Renčo M, Miklisová D, Gömöryová E. Soil Nematode Communities in Managed and Natural Temperate Forest. Diversity. 2021; 13(7):327. https://doi.org/10.3390/d13070327
Chicago/Turabian StyleČerevková, Andrea, Marek Renčo, Dana Miklisová, and Erika Gömöryová. 2021. "Soil Nematode Communities in Managed and Natural Temperate Forest" Diversity 13, no. 7: 327. https://doi.org/10.3390/d13070327
APA StyleČerevková, A., Renčo, M., Miklisová, D., & Gömöryová, E. (2021). Soil Nematode Communities in Managed and Natural Temperate Forest. Diversity, 13(7), 327. https://doi.org/10.3390/d13070327






