Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish
Abstract
1. Introduction
2. Evidence from Scyphozoan and Cubozoan Populations
2.1. Metapopulations and Spatially Disjunct Ecological Patterns
2.2. Population Genetics
2.3. Morphometrics and Elemental Chemistry
2.4. Biophysical Modelling
2.5. The Contribution of Polyps to Restricting Distributions
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moller, H. Reduction of a larval herring population by a jellyfish predator. Science 1984, 224, 621–622. [Google Scholar] [CrossRef]
- Strand, S.W.; Hamner, W.M. Predatory behavior of Phacellophora camtschatica and size-selective predation upon Aurelia aurita (Scyphozoa: Cnidaria) in Saanich Inlet, British Columbia. Mar. Biol. 1988, 99, 409–414. [Google Scholar] [CrossRef]
- Brodeur, R.D.; Merati, N. Predation on walleye pollock (Theragra chalcogramma) eggs in the western Gulf of Alaska: The roles of vertebrate and invertebrate predators. Mar. Biol. 1993, 117, 483–495. [Google Scholar]
- Arai, M.N. A Functional Biology of Scyphozoa; Chapman & Hall: London, UK, 1997; p. 316. [Google Scholar]
- Kingsford, M.J. Biotic and abiotic structure in the pelagic environment: Importance to small fish. Bull. Mar. Sci. 1993, 53, 393–415. [Google Scholar]
- Doyle, T.K.; Hays, G.C.; Harrod, C.; Houghton, J.D.R. Ecological and Societal Benefits of Jellyfish. In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 105–127. [Google Scholar]
- Lynam, C.P.; Gibbons, M.J.; Axelsen, B.E.; Sparks, C.A.J.; Coetzee, J.; Heywood, B.G.; Brierley, A.S. Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 2006, 16, 492–493. [Google Scholar] [CrossRef] [PubMed]
- Canepa, A.; Fuentes, V.; Sabatés, A.; Piraino, S.; Boero, F.; Gili, J.-M. Pelagia noctiluca in the Mediterranean Sea. In Jellyfish Blooms, 1st ed.; Pitt, K.A., Lucas, C.H., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA, 2014; pp. 237–266. [Google Scholar]
- Uye, S.-I. The Giant Jellyfish Nemopilema nomurai in East Asian marginal Seas. In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany; New York, NY, USA, 2014; pp. 185–206. [Google Scholar]
- Graham, W.M.; Martin, D.L.; Felder, D.L.; Asper, V.L. Ecological and economic implications of a tropical jellyfish invader. Biol. Invasions 2003, 5, 53–69. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Becken, J.S.; Bordehore, C.; Fuentes, V.L.; Pitt, K.A.; Yangihara, A.A. Empowering stakeholders to manage stinging jellyfish: A perspective. Coast. Man. 2018, 46, 1–18. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Pitt, K.A.; Gillanders, B.M. Management of jellyfish fisheries, with special reference to the Order Rhizostomeae. Oceanogr. Mar. Biol. Ann. Rev. 2000, 38, 85–156. [Google Scholar]
- Brotz, L. Jellyfish fisheries—A global assessment. In Global Atlas of Marine Fisheries: A Critical Appraisal of Catches and Ecosystem Impacts; Pauly, D., Zeller, D., Eds.; Island Press: Washington, DC, USA, 2016; pp. 110–124. [Google Scholar]
- Daly, M.; Brugler, M.R.; Cartwright, P.; Collins, A.G.; Dawson, M.N.; Fautin, D.G.; France, S.C.; McFadden, C.S.; Opresko, D.M.; Rodriguez, E.; et al. The phylum Cnidaria: A review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 2007, 1668, 127–182. [Google Scholar] [CrossRef]
- Dawson, M.N. Some implications of molecular phylogenetics for understanding biodiversity in jellyfishes, with emphasis on Scyphozoa. In Coelenterate Biology 2003; Springer: Dordrecht, The Netherlands, 2004; pp. 249–260. [Google Scholar]
- Collins, A.G.; Jarms, G. WoRMS Cubozoa: World list of Cubozoa (version 2018-04-01). In Species 2000 Naturalis & ITIS Catalogue of Life, 2018 Annual Checklist; Roskov, Y., Abucay, L., Orrell, T., Nicolson, D., Bailly, N., Kirk, P.M., Bourgoin, T., DeWalt, R.E., Decock, W., De Wever, A., et al., Eds.; Species 2000: Leiden, The Netherlands, 2018. [Google Scholar]
- Kingsford, M.J.; Mooney, C.M. The Ecology of Box Jellyfishes (Cubozoa). In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer: Dordrecht, The Netherlands; Berlin/Heidelberg, Germany, 2014; pp. 267–302. [Google Scholar]
- Waples, R.S.; Gaggiotti, O.E. What is a population? An empirical evaluation of some genetic methods for identifying the number of gene pools and their degree of connectivity. Mol. Ecol. 2006, 15, 1419–1439. [Google Scholar] [CrossRef]
- Hutchings, P.; Kingsford, M.J. Biodiversity. In The Great Barrier Reef: Biology, Environment and Management; Hutchings, P., Kingsford, M.J., Hoegh-Guldberg, O., Eds.; CSIRO: Melbourne, Australia, 2019; pp. 183–189. [Google Scholar]
- Abboud, S.S.; Gómez Daglio, L.; Dawson, M.N. A global estimate of genetic and geographic differentiation in macromedusae—implications for identifying the causes of jellyfish blooms. Mar. Ecol. Prog. Ser. 2018, 591, 199–216. [Google Scholar] [CrossRef]
- Lewis, C.; Bentlage, B. Clarifying the identity of the Japanese Habu-kurage, Chironex yamaguchii, sp nov (Cnidaria: Cubozoa: Chirodropida). Zootaxa 2009, 2030, 59–65. [Google Scholar] [CrossRef]
- Sucharitakul, P.; Chomdej, S.; Achalawitkun, T.; Aongsara, S.; Arsiranant, S.; Paiphongpheaw, P.; Chanachon, K. Chirodropid box jellyfish in the Gulf of Thailand. Mar. Biodiv. 2018, 49, 1247–1252. [Google Scholar] [CrossRef]
- Keesing, J.K.; Strzelecki, J.; Stowar, M.; Wakeford, M.; Miller, K.J.; Gershwin, L.A.; Liu, D.Y. Abundant box jellyfish, Chironex sp (Cnidaria: Cubozoa: Chirodropidae), discovered at depths of over 50 m on western Australian coastal reefs. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Nuankanya, S.; Kasetsin, K.; Thunyaporn, P.; Mitila, P.; Supaporn, O.; Usawadee, D.; Bungbai, S.; Sam-ang, L.; Worawut, P.; Satariya, T. Rapid and Accurate Species-Specific PCR for the Identification of Lethal Chironex Box Jellyfish in Thailand. Int. J. Environ. Res. Public Health 2021, 18, 219. [Google Scholar] [CrossRef]
- WoRMS. World Register of Marine Species. Available online: http://www.marinespecies.org (accessed on 12 March 2021).
- Pineda, J.; Hare, J.A.; Sponaugle, S. Larval Transport and Dispersal in the Coastal Ocean and Consequences for Population Connectivity. Oceanography 2007, 20, 22–39. [Google Scholar] [CrossRef]
- Begg, G.A.; Friedland, K.D.; Pearce, J.B. Stock identification and its role in stock assessment and fisheries management: An overview. Fish. Res. 1999, 43, 1–8. [Google Scholar] [CrossRef]
- McManus, M.A.; Woodson, C.B. Plankton distribution and dispersal. J. Exp. Biol. 2012, 215, 1008–1016. [Google Scholar] [CrossRef]
- Gemmell, B.J.; Colin, S.P.; Costello, J.H. Widespread utilization of passive energy recapture in swimming medusae. J. Exp. Biol. 2018, 22, 1–5. [Google Scholar] [CrossRef]
- Larson, R.J. Riding Langmuir circulations and swimming in circles: A novel form of clustering behavior by the scyphomedusa Linuche unguiculata. Mar. Biol. 1992, 112, 229–235. [Google Scholar] [CrossRef]
- Shanks, A.L.; Graham, W.M. Orientated swimming in the jellyfish Stomolophus meleagris L. Agassiz (Scyphozoan: Rhizostomida). J. Exp. Mar. Biol. Ecol. 1987, 108, 159–169. [Google Scholar] [CrossRef]
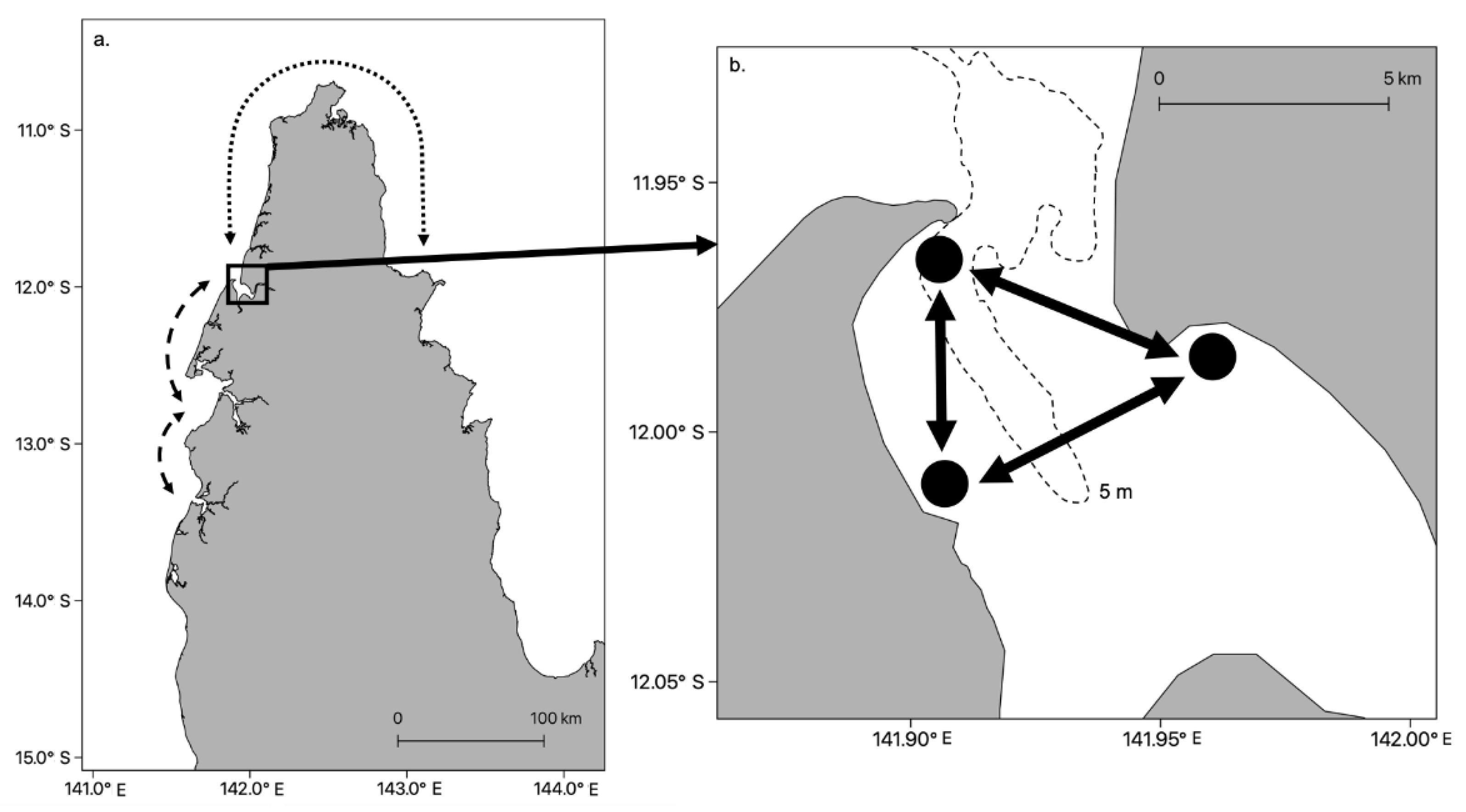
- Schlaefer, J.A.; Wolanski, E.; Yadav, S.; Kingsford, M.J. Behavioural maintenance of highly localised jellyfish (Copula sivickisi, class Cubozoa) populations. Mar. Biol. 2020, 167. [Google Scholar] [CrossRef]
- Schlaefer, J.A.; Wolanski, E.; Kingsford, M.J. Swimming behaviour can maintain localised jellyfish (Chironex fleckeri: Cubozoa) populations. Mar. Ecol. Prog. Ser. 2018, 591, 287–302. [Google Scholar] [CrossRef]
- Nilsson, D.E.; Gislen, L.; Coates, M.M.; Skogh, C.; Garm, A. Advanced optics in a jellyfish eye. Nature 2005, 435, 201–205. [Google Scholar] [CrossRef]
- Hamner, W.M.; Hauri, I.R. Long-distance horizontal migrations of zooplankton (Scyphomedusae: Mastigias). Limnol. Oceanogr. 1981, 26, 414–423. [Google Scholar]
- Hamner, W.M.; Hamner, P.P.; Strand, S.W. Sun-compass migration by Aurelia aurita (Scyphozoa): Population rentention and reproduction in Saanich Inlet, British Columbia. Mar. Biol. 1994, 119, 347–356. [Google Scholar] [CrossRef]
- Matsumoto, G.I. Observations on the anatomy and behaviour of the cubozoan Carybdea rastonii Haacke. Mar. Freshw. Behav. Physiol. 1995, 26, 139–148. [Google Scholar] [CrossRef]
- Hartwick, R.F. Observations on the anatomy, behaviour, reproduction and life cycle of the cubozoan Carybdea sivickisi. Hydrobiologia 1991, 216–217, 171–179. [Google Scholar] [CrossRef]
- Hamner, W.M.; Jones, M.S.; Hamner, P.P. Swimming, feeding, circulation and vision in the Australian box jellyfish, Chironex fleckeri (Cnidaria: Cubozoa). Mar. Freshw. Res. 1995, 46, 985–990. [Google Scholar] [CrossRef]
- Garm, A.; O’Connor, M.; Parkefelt, L.; Nilsson, D.E. Visually guided obstacle avoidance in the box jellyfish Tripedalia cystophora and Chiropsella bronzie. J. Exp. Biol. 2007, 210, 3616–3623. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Seymour, J.E.; O’Callaghan, M.D. Abundance patterns of cubozoans on and near the Great Barrier Reef. Hydrobiologia 2012, 690, 257–268. [Google Scholar] [CrossRef]
- Gordon, M.R.; Seymour, J.E. Quantifying movement of the tropical Australian cubozoan Chironex fleckeri using acoustic telemetry. Hydrobiologia 2009, 616, 87–97. [Google Scholar] [CrossRef]
- Buskey, E. Behavioral adaptations of the cubozoan medusa Tripedalia cystophora for feeding on copepod (Dioithona oculata) swarms. Mar. Biol. 2003, 142, 225–232. [Google Scholar] [CrossRef]
- Garm, A.; Bielecki, J.; Petie, R.; Nilsson, D.E. Opposite Patterns of Diurnal Activity in the Box Jellyfish Tripedalia cystophora and Copula sivickisi. Biol. Bull. 2012, 222, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.E. Field behavior of Tripedalia cystophora (class Cubozoa). Mar. Freshw. Behav. Physiol. 1996, 27, 175–188. [Google Scholar] [CrossRef]
- Garm, A.; Bielecki, J. Swim pacemakers in box jellyfish are modulated by the visual input. J. Comp. Physiol. A 2008, 194, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Garm, A.; Oskarsson, M.; Nilsson, D.E. Box Jellyfish Use Terrestrial Visual Cues for Navigation. Curr. Biol. 2011, 21, 798–803. [Google Scholar] [CrossRef]
- Olson, R.R.; McPherson, R. Potential vs. realized larval dispersal: Fish predation on larvae of the ascidian Lissoclinum patella (Gottschaldt). J. Exp. Mar. Biol. Ecol. 1987, 110, 245–246. [Google Scholar] [CrossRef]
- Gerlach, G.; Atema, J.; Raupach, M.J.; Deister, F.; Muller, A.; Kingsford, M.J. Cryptic species of cardinalfish with evidence for old and new divergence. Coral Reefs 2016, 35, 437–450. [Google Scholar] [CrossRef]
- Brill, R.W.; Block, B.A.; Boggs, C.H.; Bigelow, K.A.; Freund, E.V.; Marcinek, D.J. Horizontal movements and depth distribution of large adult yellowfin tuna (Thunnus albacares) near the Hawaiian Islands, recorded using ultrasonic telemetry: Implications for the physiology and ecology of the species. Mar. Biol. 1999, 133, 385–408. [Google Scholar] [CrossRef]
- Almany, G.R.; Berumen, M.L.; Thorrold, S.R.; Planes, S.; Jones, G.P. Local replenishment of coral reef fish populations in a marine reserve. Science 2007, 316, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Haddon, M.; Willis, T.J. Morphometric and meristic comparison of orange roughy (Hoplstethus atlanticus: Trachichthyidae) from the Puysegur Bank and Lord Howe Rise, New Zealand, and its implication for stock structure. Mar. Biol. 1995, 123, 19–27. [Google Scholar] [CrossRef]
- Small, C.G. The Statistical Theory of Shape; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Fowler, A.J.; Gillanders, B.M.; Hall, K.C. Relationship between elemental concentration and age from otoliths of adult snapper (Pagrus auratus, Sparidae): Implications for movement and stock structure. Mar. Freshw. Res. 2005, 56, 661–676. [Google Scholar] [CrossRef]
- Wolanski, E.; Kingsford, M.J. Oceanographic and behavioural assumptions in models of the fate of coral and coral reef fish larvae. J. Roy. Soc. Int. 2014, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
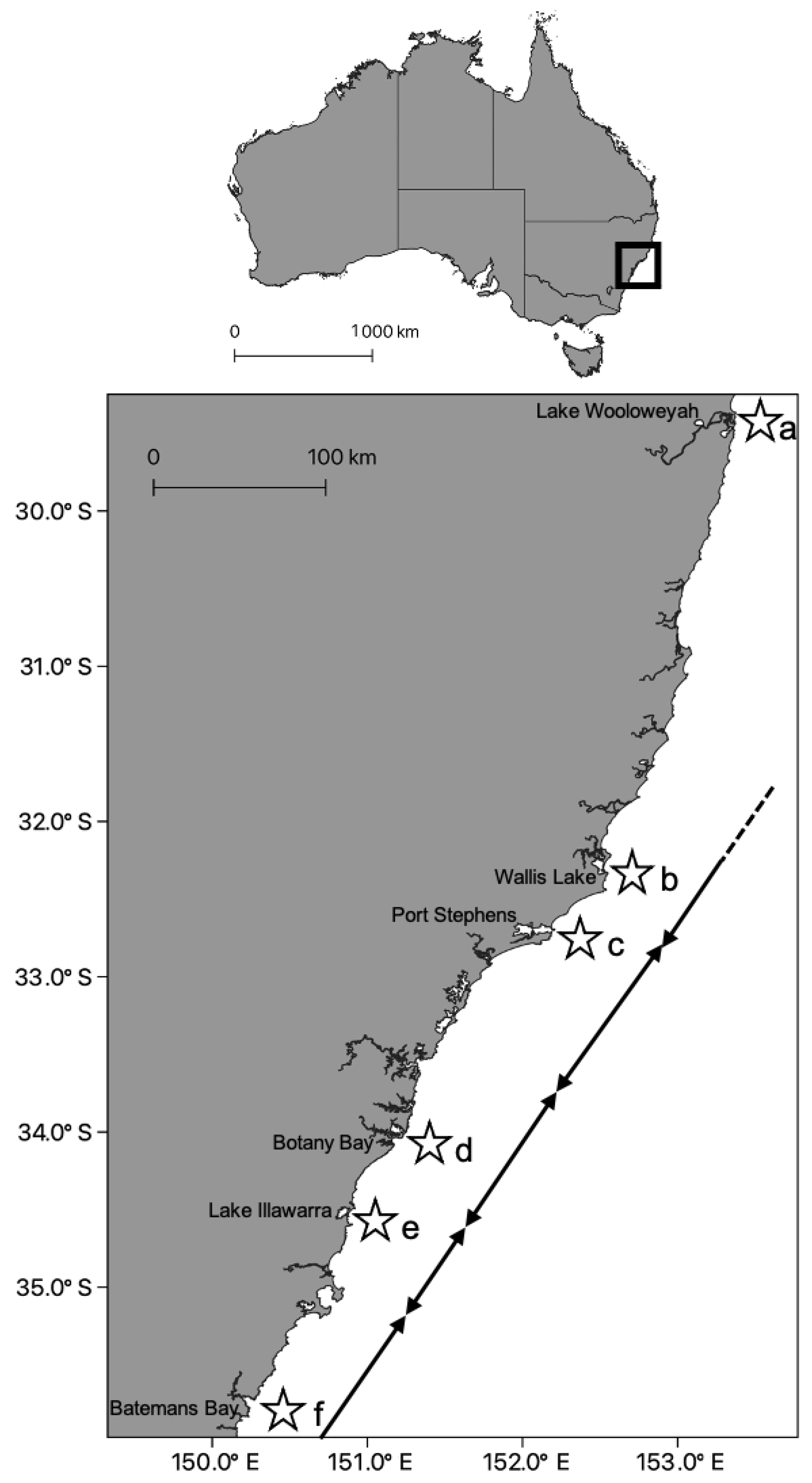
- Pitt, K.A.; Kingsford, M.J. Geographic separation of stocks of the edible jellyfish, Catostylus mosaicus (Rhizostomeae) in New South Wales, Australia. Mar. Ecol. Prog. Ser. 2000, 196, 143–155. [Google Scholar] [CrossRef]
- Dawson, M.N. Incipient speciation of Catostylus mosaicus (Scyphozoa, Rhizostomeae, Catostylidae), comparative phylogeography, and biogeography in south-east Australia. J. Biogeogr. 2005, 31, 1–19. [Google Scholar] [CrossRef]
- Dawson, M.N. Morphological and molecular redescription of Catostylus mosaicus (Scyphozoa: Rhizostomeae: Catostylidae) from south-east Australia. J. Mar. Biol. Assoc. 2005, 85, 723–731. [Google Scholar] [CrossRef]
- Ben Faleh, A.R.; Ben Othmen, A.; Deli, T.; Annabi, A.; Said, K. High genetic homogeneity of the moon jelly Aurelia aurita (Scyphozoa, Semaeostomeae) along the Mediterranean coast of Tunisia. Afr. J. Mar. Sci. 2009, 31, 73–80. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, Z.; Liu, D. Genetic characterization of the scyphozoan jellyfish Aurelia spp. in Chinese coastal waters using mitochondrial markers. Biochem. Syst. Ecol. 2015, 60, 15–23. [Google Scholar] [CrossRef]
- De Angelis, S.A.; Stampar, S.N.; Maronna, M.M.; Morandini, A.C. Absence of cryptic species and population structure in Lychnorhiza lucerna (Cnidaria) from southwestern Atlantic Ocean. Genome 2017, 60, 925–926. [Google Scholar]
- Lee, P.L.; Dawson, M.N.; Neill, S.P.; Robins, P.E.; Houghton, J.D.; Doyle, T.K.; Hays, G.C. Identification of genetically and oceanographically distinct blooms of jellyfish. J. R. Soc. Interface 2013, 10, 20120920. [Google Scholar] [CrossRef]
- Glynn, F.; Houghton, J.D.; Provan, J. Population genetic analyses reveal distinct geographical blooms of the jellyfish Rhizostoma octopus (Scyphozoa). Biol. J. Linn. Soc. 2015, 116, 582–592. [Google Scholar] [CrossRef]
- Miller, B.J.; Von der Heyden, S.; Gibbons, M.J. Significant population genetic structuring of the holoplanktic scyphozoan Pelagia noctiluca in the Atlantic Ocean. Afr. J. Mar. Sci. 2012, 34, 425–430. [Google Scholar] [CrossRef]
- Stopar, K.; Ramšak, A.; Trontelj, P.; Malej, A. Lack of genetic structure in the jellyfish Pelagia noctiluca (Cnidaria: Scyphozoa: Semaeostomeae) across European seas. Mol. Phylogenet. Evol. 2010, 57, 417–428. [Google Scholar] [CrossRef]
- Dawson, M.N.; Hamner, W.M. Geographic variation and behavioural evolution in marine plankton: The case of Mastigias (Scyphozoa, Rhizostomeae). Mar. Biol. 2003, 143, 1161–1174. [Google Scholar] [CrossRef]
- Mamet, L.N.G.; Daglio, L.G.; García-De León, F.J. High genetic differentiation in the edible cannonball jellyfish (cnidaria: Scyphozoa: Stomolophus spp.) from the Gulf of California, Mexico. Fish. Res. 2019, 219, 105328. [Google Scholar] [CrossRef]
- Dawson, M.N.; Cieciel, K.; Decker, M.B.; Hays, G.C.; Lucas, C.L. Population-level perspectives on global change: Genetic and demographic analyses indicate various scales, timing, and causes of scyphozoan jellyfish blooms. Biol. Invasions 2015, 17, 851–867. [Google Scholar] [CrossRef]
- Lawley, J.W.; Ames, C.L.; Bentlage, B.; Yanagihara, A.; Goodwill, R.; Kayal, E.; Hurwitz, K.; Collins, A.G. Box jellyfish Alatina alata has a circumtropical distribution. Biol. Bull. 2016, 231, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Mooney, C.J.; Kingsford, M.J. Statolith morphometrics as a tool to distinguish among populations of three cubozoan species. Hydrobiologia 2017, 787, 111–121. [Google Scholar] [CrossRef]
- Mooney, C.J.; Kingsford, M.J. Discriminating populations of medusae (Chironex fleckeri, Cubozoa) using statolith microchemistry. Mar. Freshw. Res. 2016, 68, 1144–1152. [Google Scholar] [CrossRef]
- Dawson, M.N.; Hamner, W.M. Rapid evolutionary radiation of marine zooplankton in peripheral environments. Proc. Natl. Acad. Sci. USA 2005, 102, 9235–9240. [Google Scholar] [CrossRef] [PubMed]
- Galil, B.S.; Spanier, E.; Ferguson, W.W. The scyphomedusae of the mediterrannean coast of Israel, including two lessepsian migrants new to the Mediterranean. Zoo. Mededel. 1990, 64, 95–105. [Google Scholar]
- Brown, W.M.; George, M.; Wilson, A.C. Rapid evolution of animal mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef]
- Peplow, L.M.; Kingsford, M.J.; Seymour, J.E.; van Oppen, M.J.H. Eight microsatellite loci for the Irukandji syndrome-causing carybdeid jellyfish, Carukia barnesi (Cubozoa, Cnidaria). Mol. Ecol. Resour. 2009, 9, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, J.P.; Seymour, J.; Cross, T.F. Isolation and characterization of seven polymorphic microsatellite loci in the box jellyfish (Chironex fleckeri, Cubozoa, Cnidaria). Mol. Ecol. Notes 2006, 6, 41–43. [Google Scholar] [CrossRef]
- Aungtonya, C.; Xiao, J.; Zhang, X.; Wutthituntisil, N. The Genus Chiropsoides (Chirodropida: Chiropsalmidae) from the Andaman Sea, Thai waters. Acta Oceanol. Sin. 2018, 30, 119–125. [Google Scholar] [CrossRef]
- Bolte, B.; Goldsbury, J.; Huerlimann, R.; Jerry, D.; Kingsford, M.J. Validation of eDNA as a viable method of detection for dangerous cubozoan jellyfish. Environ. DNA 2021. [Google Scholar] [CrossRef]
- Berta, A.; Sumich, J.L.; Kovacs, K.M. Population structure and dynamics. In Marine Mammals—Evolutionary Biology; Berta, A., Sumich, J.L., Kovacs, K.M., Eds.; Academic Press: Burlington, MA, USA, 2015; pp. 416–445. [Google Scholar]
- Holland, B.S.; Dawson, M.N.; Crow, G.L.; Hofmann, D.K. Global phylogeography of Cassiopea (Scyphozoa: Rhizostomeae): Molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar. Biol. 2004, 146, 1119–1128. [Google Scholar] [CrossRef]
- Bolton, T.F.; Graham, W.M. Morphological variation among populations of an invasive jellyfish. Mar. Ecol. Prog. Ser. 2004, 278, 125–139. [Google Scholar] [CrossRef]
- Campana, S.E.; Chouinard, G.A.; Hanson, J.M.; Frechet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Mooney, C.J.; Kingsford, M.J. Statolith Morphometrics Can Discriminate among Taxa of Cubozoan Jellyfishes. PLoS ONE 2016, 11, 16. [Google Scholar] [CrossRef]
- Tiemann, H.; Sotje, I.; Becker, A.; Jarms, G.; Epple, M. Calcium sulfate hemihydrate (bassanite) statoliths in the cubozoan Carybdea sp. Zool. Anz. 2006, 245, 13–17. [Google Scholar] [CrossRef]
- Barz, K.; Hinrichsen, H.H.; Hirche, H.J. Scyphozoa in the Bornholm Basin (central Baltic Sea)-the role of advection. J. Mar. Syst. 2006, 60, 167–176. [Google Scholar] [CrossRef]
- Chen, K.; Ciannelli, L.; Decker, B.B.; Ladd, C.; Cheng, W.; Zhou, Z.; Chan, K.-S. Reconstructing Source-Sink Dynamics in a Population with a Pelagic Dispersal Phase. PLoS ONE 2014, 9, e95316. [Google Scholar] [CrossRef]
- Fossett, S.; Gleiss, A.C.; Chalumeau, J.; Bastian, T. Current-oriented swimming by jellyfish and its role in bloom maintenance. Curr. Biol. 2015, 25, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hopley, D.; Smithers, S.G.; Parnell, K.E. The Geomorpholgy of the Great Barrier Reef; Cambridge University Press: New York, NY, USA, 2007; p. 532. [Google Scholar]
- Shahrestani, S.; Bi, H. Settlement and survival of Chrysaora chesapeakei polyps: Implications for adult abundance. Mar. Ecol. Progr. Ser. 2018, 601, 139–151. [Google Scholar] [CrossRef]
- Marques, R.; Cantou, M.; Soriano, S.; Molinero, J.C.; Bonnet, D. Mapping distribution and habitats of Aurelia sp. polyps in Thau lagoon, north-western Mediterranean Sea (France). Mar. Biol. 2015, 162, 1441–1449. [Google Scholar] [CrossRef]
- Colin, S.P.; Kremer, P. Population maintenance of the scyphozoan Cyanea sp. settled planulae and the distribution of medusae in the Niantic River, Connecticut, USA. Estuaries 2002, 25, 70–75. [Google Scholar] [CrossRef]
- Toyokawa, M.; Aoki, K.; Yamada, S.; Yasuda, A.; Murata, Y.; Kikuchi, T. Distribution of ephyrae and polyps of jellyfish Aurelia aurita (Linnaeus 1758) sensu lato in Mikawa Bay, Japan. J. Oceanogr. 2011, 67, 209–218. [Google Scholar] [CrossRef]
- Van Walraven, L.; Driessen, F.; van Bleijswijk, J.; Bol, A.; Luttikhuizen, P.C.; Coolen, J.W.; van der Veer, H.W. Where are the polyps? Molecular identification, distribution and population differentiation of Aurelia aurita jellyfish polyps in the southern North Sea area. Mar. Biol. 2016, 163, 1–13. [Google Scholar] [CrossRef]
- Hartwick, R.F. Distributional ecology and behavior of the early life stages of the box-jellyfish Chironex fleckeri. Hydrobiologia 1991, 216–217, 181–188. [Google Scholar] [CrossRef]

| Behaviour | Species | Method | Study |
|---|---|---|---|
| (Order) | |||
| Scyphozoa | |||
| Sun-compass migration | Aurelia aurita (Semaeostomeae) | Field observation | Hamner et al. [36] |
| Mastigias (Rhizostomae) | Field observation | Hamner & Hauri [35] | |
| Wind and currents and bottom avoidance | Stomolophus meleagris (Rhizostomae) | Field observation | Shanks & Graham [31] |
| Cubozoa | |||
| Obstacle avoidance | Carybdea rastonii (Carybdeida) | Field observations (SCUBA) and laboratory experiments | Matsumoto [37] |
| Chironex fleckeri (Chirodropida) | Laboratory experiments | Hamner et al. [39] | |
| Field observations | Schlaefer et al. [33] | ||
| Chiropsella bronzie (Chirodropida) | Laboratory experiments | Garm et al. [40] | |
| Tripedalia cystophora (Carybdeida) | Laboratory experiments | Garm et al. [40] | |
| Maintain positions near the shore | C. rastonii | Field observations (SCUBA) | Matsumoto [37] |
| Opportunistic sampling (e.g., Surf Life Saver plankton tows and verbal records) | Kingsford et al. [41] | ||
| Electronic tagging | Gordon & Seymour [42] | ||
| Field observations and biophysical modelling | Schlaefer et al. [33] | ||
| Rheotaxis | C. fleckeri | Field observations | Schlaefer et al. [33] |
| C. bronzie | Laboratory experiments | Garm et al. [40] | |
| T. cystophora | Laboratory experiments | Garm et al. [40] | |
| Laboratory experiments | Buskey [43] | ||
| Copula sivickisi (Carybdeida) | Laboratory experiments | Schlaefer et al. [32] | |
| Diel activity—nocturnal | C. sivickisi | Field observations (SCUBA) | Hartwick [38] |
| Field sampling (plankton tows and SCUBA) and laboratory experiments | Garm et al. [40] | ||
| Laboratory experiments | Schlaefer et al. [32] | ||
| Attach to substrate | C. sivickisi | Laboratory observations | Hartwick [38] |
| Laboratory Experiments | Garm et al. [44] | ||
| Laboratory experiments and in-field video recording | Schlaefer et al. [32] | ||
| Rest on the bottom | C. rastoni | Field observations | Matsumoto [37] |
| Swim toward bioluminescent plankton | C. sivickisi | Laboratory experiments | Garm et al. [44] |
| Exhibit habitat preference | C. sivickisi | Laboratory experiments | Garm et al. [44] |
| Laboratory experiments | Schlaefer et al. [32] | ||
| Diel activity—diurnal | T. cystophora | Field sampling (snorkel and SCUBA) and laboratory experiments | Garm et al. [44] |
| Maintain positions in light shafts | T. cystophora | Field observations (snorkel) | Stewart [45] |
| Laboratory experiments | Buskey [43] | ||
| Laboratory experiments and in-field video recordings | Garm & Bielecki [46] | ||
| Orient via the mangrove canopy | T. cystophora | Eye orientation measurements, optical modelling and in-field experiments | Garm et al. [47] |
| Species | Class | Methods | Spatial Scale | Range (Km) | Source |
|---|---|---|---|---|---|
| Aurelia aurita | Scyphozoa | Genetics * | 100s of kms | >1000 | Ben Faleh et al. [59] |
| Aurelia spp. | Scyphozoa | Genetics * | 10s to hundreds of kms | >1000 | Dong et al. [60] |
| Lychnorhiza lucerna | Scyphozoa | Genetics * | 1000s of kms | >1000 | De Angelis et al. [61] |
| Catostylus mosaicus | Scyphozoa | Ecological # | 10s of kms | >1000 | Pitt & Kingsford [56] |
| Catostylus mosaicus | Scyphozoa | Genetics * | 10s of kms | >1000 | Dawson [57] |
| Rhizostoma octopus | Scyphozoa | Genetics * | 100s of kms | >1000 | Lee et al. [62] |
| Rhizostoma octopus | Scyphozoa | Genetics * | 100s of kms | >1000 | Glynn et al. [63] |
| Pelagia sp. | Scyphozoa | Genetics * | 1000s of kms | >1000 | Miller et al. [64] |
| Pelagia sp. | Scyphozoa | Genetics * | 1000s of kms | >1000 | Stopar et al. [65] |
| Mastigias papua | Scyphozoa | Genetics * | 10s of kms | >1000 | Dawson & Hamner [66] |
| Stomolophus spp. | Scyphozoa | Genetics * | 100s of kms | >1000 | Mamet et al. [67] |
| Chrysaora melanaster | Scyphozoa | Genetics * | 1000s of km | >1000 | Dawson et al. [68] |
| Cyanea sp. | Scyphozoa | Genetics * | 100s of kms | >1000 | Abboud et al. [20] |
| Alatina alata | Cubozoa | Genetics * | 1000s of kms | >1000 | Lawley [69] |
| Chironex fleckeri | Cubozoa | Biophysical # | Hundreds of meters to kms | >1000 | Schlaefer et al. [33] |
| Chironex fleckeri | Cubozoa | Statolith morphology # | 10s to hundreds of kms | >1000 | Mooney & Kingsford [70] |
| Chironex fleckeri | Cubozoa | Elemental chemistry # | Kms to 10s of kms | >1000 | Mooney & Kingsford [71] |
| Copula sivicksi | Cubozoa | Statolith morphology # | 10s to hundreds of kms | >1000 | Mooney & Kingsford [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kingsford, M.J.; Schlaefer, J.A.; Morrissey, S.J. Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish. Diversity 2021, 13, 174. https://doi.org/10.3390/d13040174
Kingsford MJ, Schlaefer JA, Morrissey SJ. Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish. Diversity. 2021; 13(4):174. https://doi.org/10.3390/d13040174
Chicago/Turabian StyleKingsford, Michael J., Jodie A. Schlaefer, and Scott J. Morrissey. 2021. "Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish" Diversity 13, no. 4: 174. https://doi.org/10.3390/d13040174
APA StyleKingsford, M. J., Schlaefer, J. A., & Morrissey, S. J. (2021). Population Structures and Levels of Connectivity for Scyphozoan and Cubozoan Jellyfish. Diversity, 13(4), 174. https://doi.org/10.3390/d13040174







