Impact to Coral Reef Populations at Hā‘ena and Pila‘a, Kaua‘i, Following a Record 2018 Freshwater Flood Event
Abstract
1. Introduction
2. Materials & Methods
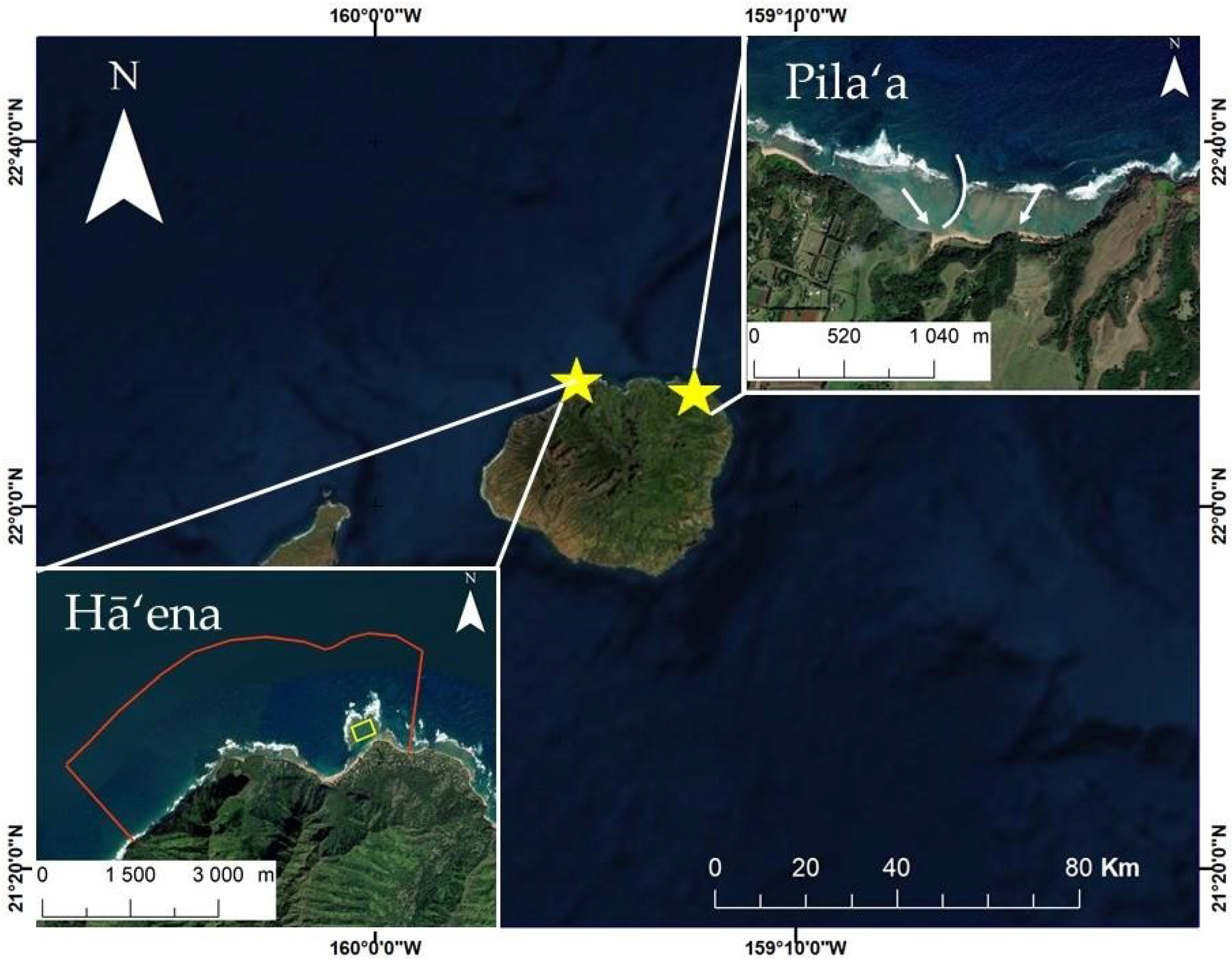
2.1. Site Descriptions
2.1.1. Pila‘a Reef
2.1.2. Hā‘ena Reef
2.2. Station Selection Criteria
2.3. Biological Surveys
2.3.1. Fishes and Urchins
2.3.2. Corals
2.3.3. Sarcothelia edmondsoni Mapping Swim Surveys
2.4. Environmental Characterization
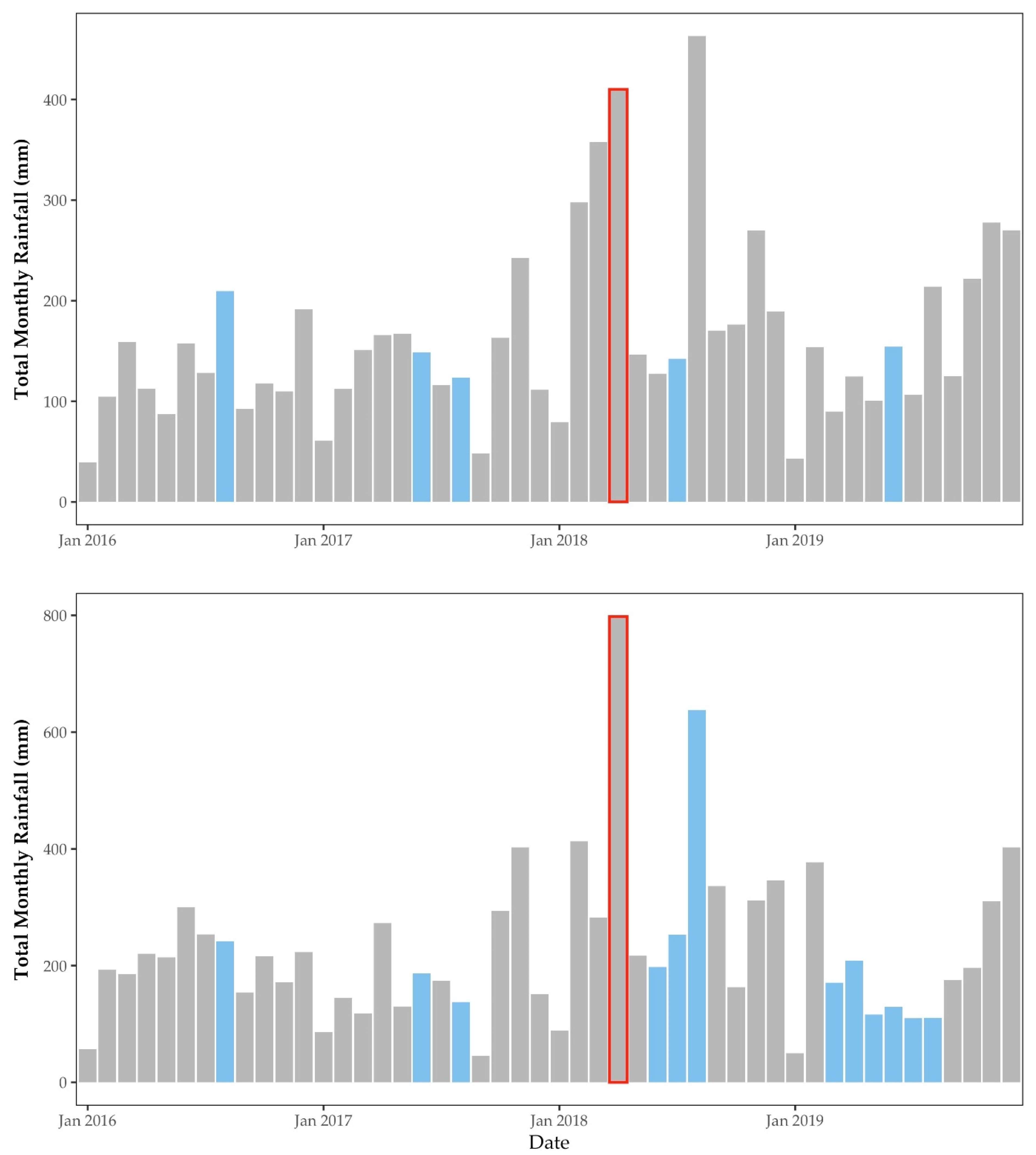
2.4.1. Rainfall
2.4.2. Temperature
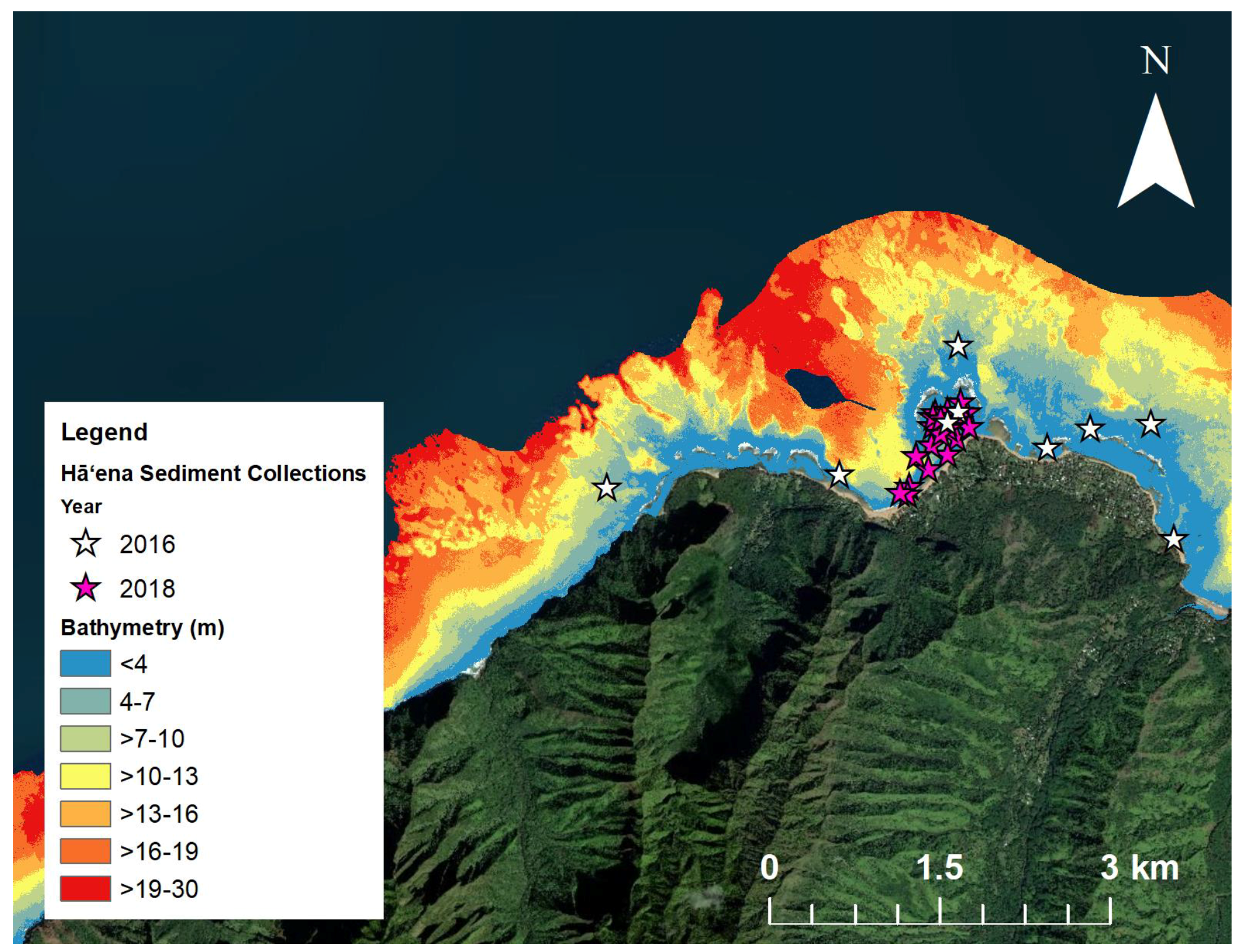
2.4.3. Sediment Characteristics
Sediment Collection
Sediment Grain Size
Sediment Composition
2.5. Statistical Analysis
2.5.1. Environmental Data
2.5.2. Community-Level Multivariate Approach
2.5.3. Discrete Population Univariate Approach
3. Results
3.1. Environmental Factors
3.1.1. Rainfall & Temperature
3.1.2. Sediment
3.2. Biological Factors
3.2.1. Community-Level Changes
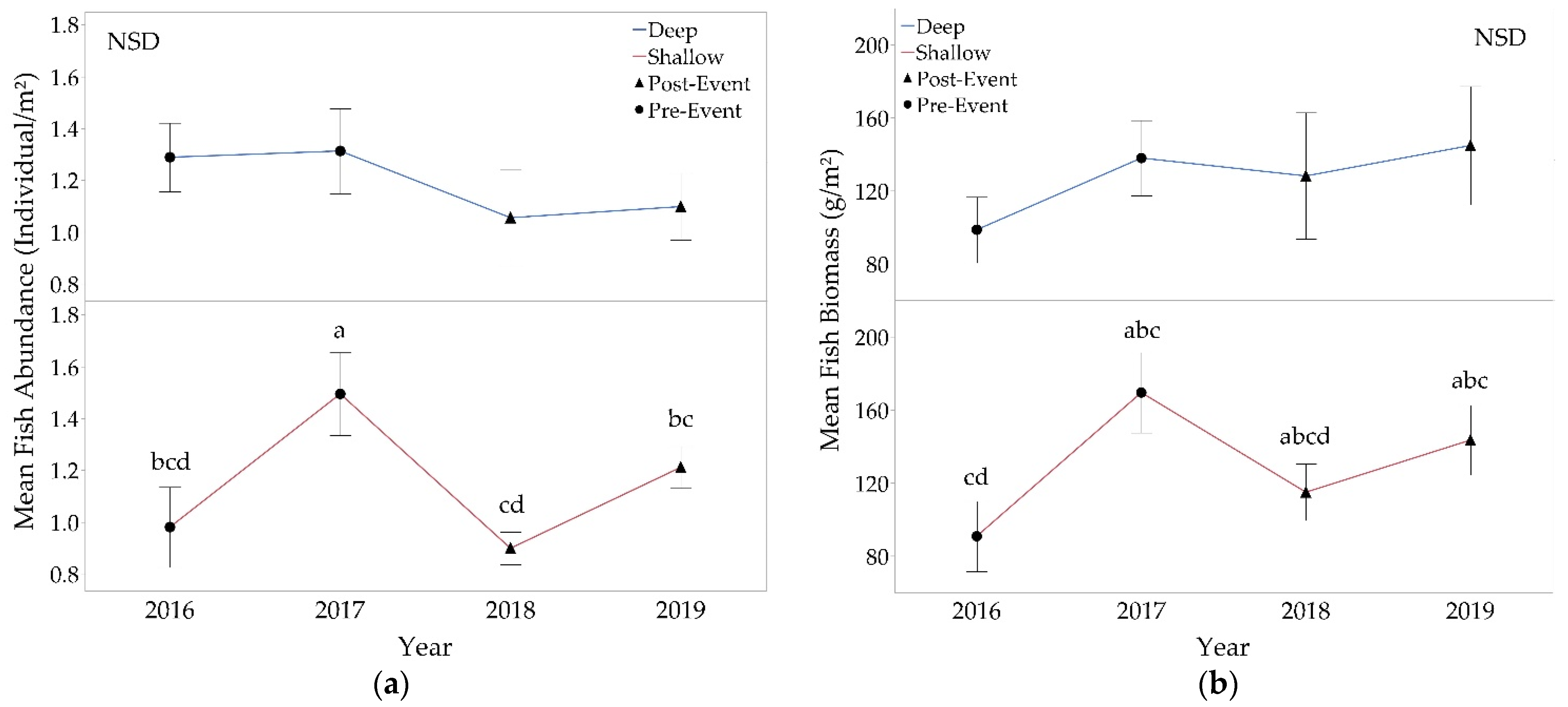
3.2.2. Fishes
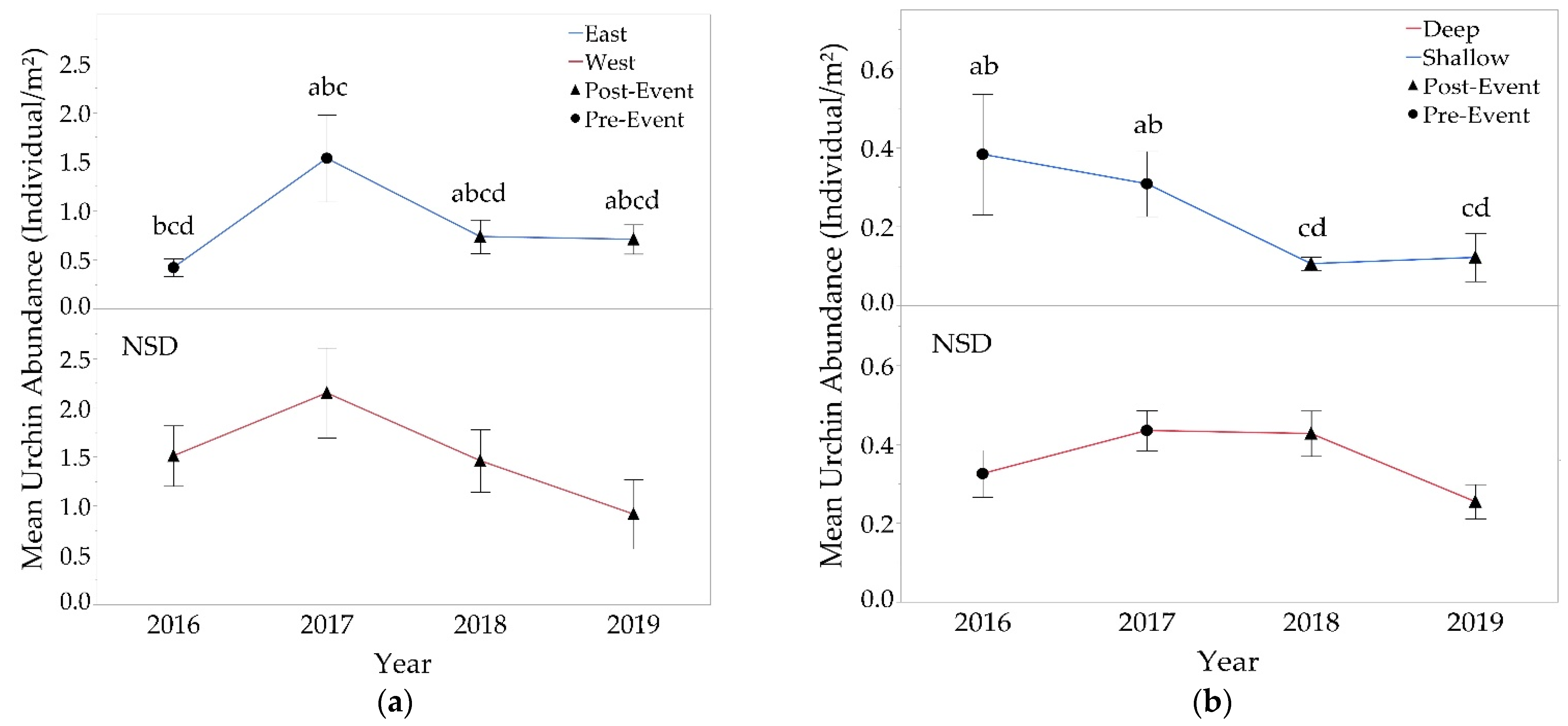
3.2.3. Sea Urchins
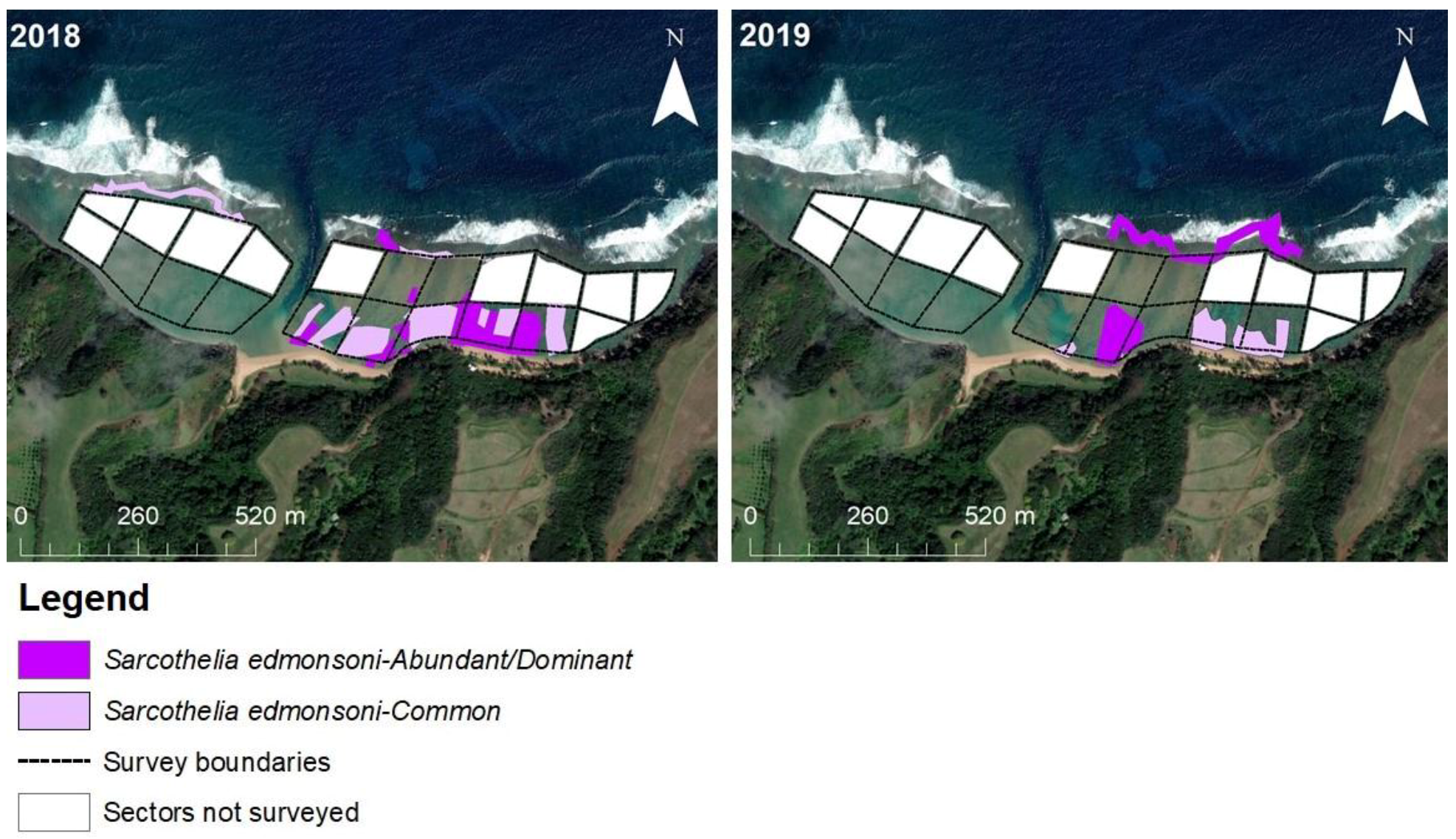
3.2.4. Sarcothelia Edmondsoni
3.2.5. Coral Cover & Bleaching
4. Discussion
4.1. Fish Populations
4.2. Sea Urchin Populations
4.3. Coral
4.3.1. Octocoral Sarcothelia edmondsoni
4.3.2. Coral Bleaching
4.4. Management Implications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
- DAR Acting Director: Brian Nielson
- DAR Kaua‘i Monitoring Team: Ka‘ilikea Shayler, McKenna Allen, and Mia Melamed
- DAR O‘ahu Monitoring Team: Paul Murakawa, Kazuki Kageyama, Haruko Koike, Tiffany Cunanan, Kimberly Fuller, Justin Goggins, and Ryan Okano
- DAR Maui Monitoring Team: Russell Sparks, Kristy Stone, Linda Castro, Tatiana Martinez
- Additional support: Kosta Stamoulis, Jade Delevaux, Angela Richards Donà, Rebecca Weible, Ashley McGowan, Keisha Bahr, Bernard Rodrigues, and Keoki Stender.
Conflicts of Interest
References
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Burke, L.; Reytar, K.; Spalding, M.; Perry, A. Reefs at Risk Revisited; World Resources Institute: Washington, DC, USA, 2011. [Google Scholar]
- Easterling, D.R. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- Karl, T.R.; Melillo, J.M.; Peterson, T.C.; Hassol, S.J. Global Climate Change Impacts in the United States; Cambridge University Press: New York, NY, USA, 2009. [Google Scholar]
- Mora, C.; Frazier, A.G.; Longman, R.J.; Dacks, R.S.; Walton, M.M.; Tong, E.J.; Sanchez, J.J.; Kaiser, L.R.; Stender, Y.O.; Anderson, J.M.; et al. The projected timing of climate departure from recent variability. Nature 2013, 502, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Giambelluca, T.W.; Chen, Q.; Frazier, A.G.; Price, J.; Chen, Y.-L.; Chu, P.-S.; Eischeid, J.; Delparte, D. Online rainfall atlas of Hawai’i. Bull. Am. Meteorol. Soc. 2013, 94, 313–316. [Google Scholar] [CrossRef]
- Longman, R.J.; Newman, A.J.; Giambelluca, T.W.; Lucas, M. Characterizing the uncertainty and assessing the value of gap-filled daily rainfall data in Hawai‘i. J. Appl. Meteorol. Climatol. 2020, 59, 1261–1276. [Google Scholar] [CrossRef]
- Jokiel, P. Impact of storm waves and storm floods on Hawaiian reefs. In Proceedings of the Tenth International Coral Reef Symposium, Okinawa, Japan, 28 June–2 July 2004; pp. 390–398. [Google Scholar]
- Andersson, A.J.; Kuffner, I.B.; Mackenzie, F.T.; Jokiel, P.L.; Rodgers, K.S.; Tan, A. Net loss of CaCO3 from a subtropical calcifying community due to seawater acidification: Mesocosm-scale experimental evidence. Biogeosciences 2009, 6, 1811–1823. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Bahr, K.D.; Rodgers, K. Synergistic Impacts of Global Warming and Ocean Acidification on Coral Reefs; Pacific Islands Climate Change Cooperative Final Report: Honolulu, Hawai‘i, 2013; p. 63. [Google Scholar]
- Bahr, K.D.; Rodgers, K.S.; Jokiel, P.L. Ocean warming drives decline in coral metabolism while acidification highlights species-specific responses. Mar. Biol. Res. 2018, 14, 924–935. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Coles, S.L. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 1990, 8, 155–162. [Google Scholar] [CrossRef]
- Jokiel, P.L. Temperature stress and coral bleaching. In Coral Health and Disease; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 401–425. [Google Scholar]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Ward, S.; Harrison, P.; Hoegh-Guldberg, O. Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. In Proceedings of the Ninth International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; pp. 1123–1128. [Google Scholar]
- Bahr, K.D.; Jokiel, P.L.; Rodgers, K.S. The 2014 coral bleaching and freshwater flood events in Kāne‘ohe Bay, Hawai‘i. PeerJ 2015, 3, e1136. [Google Scholar] [CrossRef] [PubMed]
- Banner, A.H. A Fresh-Water “Kill” on the Coral Reefs of Hawaii; Hawai‘i Institute of Marine Biology, University of Hawai‘i: Kāne‘ohe, HI, USA, 1968. [Google Scholar]
- Jones, A.M.; Berkelmans, R. Flood impacts in Keppel Bay, Southern Great Barrier Reef in the aftermath of cyclonic rainfall. PLoS ONE 2014, 9, e84739. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Hunter, C.L.; Taguchi, S.; Watarai, L. Ecological impact of a fresh-water “reef kill” in Kaneohe Bay, Oahu, Hawai‘i. Coral Reefs 1993, 12, 177–184. [Google Scholar] [CrossRef]
- Mayfield, A.B.; Gates, R.D. Osmoregulation in anthozoan–dinoflagellate symbiosis. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2007, 147, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, P.; Grey, S.C. The effects of storms, heavy rain, and sedimentation on the shallow coral reefs of St. John, US Virgin Islands. Hydrobiologia 2014, 734. [Google Scholar] [CrossRef]
- Sheridan, C.; Grosjean, P.; Leblud, J.; Palmer, C.; Kushmaro, A.; Eeckhaut, I. Sedimentation rapidly induces an immune response and depletes energy stores in a hard coral. Coral Reefs 2014, 33, 1067–1076. [Google Scholar] [CrossRef]
- Bessell-Browne, P.; Negri, A.P.; Fisher, R.; Clode, P.L.; Duckworth, A.; Jones, R. Impacts of turbidity on corals: The relative importance of light limitation and suspended sediments. Mar. Pollut. Bull. 2017, 117, 161–170. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Coles, S.L. Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar. Biol. 1977, 43, 201–208. [Google Scholar] [CrossRef]
- Coles, S.; Jokiel, P.L. Synergistic effects of temperature, salinity and light on the hermatypic coral Montipora verrucosa. Mar. Biol. 1978, 49, 187–195. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Smith, G.J. The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. J. Exp. Mar. Biol. Ecol. 1989, 129, 279–303. [Google Scholar] [CrossRef]
- Goenaga, C.; Canals, M. Island-wide coral bleaching in Puerto Rico. Caribb. J. Sci. 1990, 26, 171–175. [Google Scholar]
- Brown, B.; Dunne, R.; Ambarsari, I.; Le Tissier, M.; Satapoomin, U. Seasonal fluctuations in environmental factors and variations in symbiotic algae and chlorophyll pigments in four Indo-Pacific coral species. Mar. Ecol. Prog. Ser. 1999, 191, 53–69. [Google Scholar] [CrossRef]
- Cleary, D.F.R.; Polónia, A.R.; Renema, W.; Hoeksema, B.W.; Rachello-Dolmen, P.G.; Moolenbeek, R.G.; Budiyanto, A.; Yahmantoro; Tuti, Y.; Giyanto; et al. Variation in the composition of corals, fishes, sponges, echinoderms, ascidians, molluscs, foraminifera and macroalgae across a pronounced in-to-offshore environmental gradient in the Jakarta Bay–Thousand Islands coral reef complex. Mar. Pollut. Bull. 2016, 110, 701–717. [Google Scholar] [CrossRef] [PubMed]
- Polónia, A.R.; Cleary, D.F.R.; de Voogd, N.J.; Renema, W.; Hoeksema, B.W.; Martins, A.; Gomes, N.C.M. Habitat and water quality variables as predictors of community composition in an Indonesian coral reef: A multi-taxon study in the Spermonde Archipelago. Sci. Total Environ. 2015, 537, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Russell, M. Acclimation and growth response of the green sea urchin Strongylocentrotus droebachiensis to fluctuating salinity. In Proceedings of the International Conference on Sea Urchin Fisheries and Aquaculture, Puerto Varas, Chile, 25–27 March 2003; pp. 110–117. [Google Scholar]
- Tsuchiya, M.; Lirdwitayapasit, T. Distribution of intertidal animals on rocky shores of the Sichang Islands, the Gulf of Thailand. Galaxea 1986, 5, 15–25. [Google Scholar]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed]
- Lirman, D. Competition between macroalgae and corals: Effects of herbivore exclusion and increased algal biomass on coral survivorship and growth. Coral Reefs 2001, 19, 392–399. [Google Scholar] [CrossRef]
- Williams, I.; Polunin, N. Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 2001, 19, 358–366. [Google Scholar] [CrossRef]
- Coppard, S.; Campbell, A.C. Grazing preferences of diadematid echinoids in Fiji. Aquat. Bot. 2007, 86, 204–212. [Google Scholar] [CrossRef]
- McClanahan, T.; Polunin, N.; Done, T. Ecological states and the resilience of coral reefs. Conserv. Ecol. 2002, 6. [Google Scholar] [CrossRef]
- Smith, J.E.; Hunter, C.L.; Smith, C.M. The effects of top–down versus bottom–up control on benthic coral reef community structure. Oecologia 2010, 163, 497–507. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nyström, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Hall, W.J. Effects of hurricane Hugo on South Carolina’s marine artificial reefs. Bull. Mar. Sci. 1994, 55, 836–847. [Google Scholar]
- Munks, L.S.; Harvey, E.S.; Saunders, B.J. Storm-induced changes in environmental conditions are correlated with shifts in temperate reef fish abundance and diversity. J. Exp. Mar. Biol. Ecol. 2015, 472, 77–88. [Google Scholar] [CrossRef]
- Rousseau, Y.; Galzin, R.; Marechal, J. Impact of hurricane Dean on coral reef benthic and fish structure of Martinique, French West Indies. Cybium 2010, 34, 243–256. [Google Scholar]
- Walsh, W.J. Stability of a coral reef fish community following a catastrophic storm. Coral Reefs 1983, 2, 49–63. [Google Scholar] [CrossRef]
- Leahy, S.M.; McCormick, M.I.; Mitchell, M.D.; Ferrari, M.C.O. To fear or to feed: The effects of turbidity on perception of risk by a marine fish. Biol. Lett. 2011, 7, 811–813. [Google Scholar] [CrossRef]
- Kultz, D. Physiological mechanisms used by fish to cope with salinity stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Bacheler, N.M.; Shertzer, K.W.; Cheshire, R.T.; Macmahan, J.H. Tropical storms influence the movement behavior of a demersal oceanic fish species. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Locascio, J.V.; Mann, D.A. Effects of Hurricane Charley on fish chorusing. Biol. Lett. 2005, 1, 362–365. [Google Scholar] [CrossRef]
- Lassig, B.R. The effects of a cyclonic storm on coral reef fish assemblages. Environ. Biol. Fishes 1983, 9, 55–63. [Google Scholar] [CrossRef]
- Jones, G.P.; Syms, C. Disturbance, habitat structure and the ecology of fishes on coral reefs. Austral. Ecol. 1998, 23, 287–297. [Google Scholar] [CrossRef]
- Rodgers, K.; Stender, Y.O.; Dona, A.R.; Han, J.H.; Graham, A.; Stamoulis, K.A.; Delevaux, J. 2018 Long-Term Monitoring and Assessment of the Hā‘ena, Kaua‘i Community Based Subsistence Fishing Area; State of Hawai‘i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2019; p. 78. [Google Scholar]
- Jokiel, P.L.; Hill, E.; Farrell, F.; Brown, E.K.; Rodgers, K. Reef Coral Communities at Pila’a Reef in Relation to Environmental Factors; Hawai‘i Institute of Marine Biology, University of Hawai‘i: Mānoa, Honolulu, HI, USA, 2002. [Google Scholar]
- Rodgers, K.S.; Bahr, K.D.; Stender, K.; Stender, Y.O.; Dona, A.R.; Weible, R.; Tsang, A.; Han, J.H.; McGowan, A.E. 2016 Recovery Assessment and Long-Term Monitoring of Reef Coral Communities at Pila‘a Reef, Kaua‘i; State of Hawai‘i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2017; p. 60. [Google Scholar]
- Rodgers, K.S.; Stender, K.; Stender, Y.O.; Dona, A.R.; Tsang, A.; Han, J.H.; Prouty, N. 2017 Recovery Assessment and Long-Term Monitoring of Reef Coral Communities at Pila‘a Reef, Kaua‘i; State of Hawai‘i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2018; p. 85. [Google Scholar]
- Rodgers, K.S.; Stender, Y.O.; Dona, A.R.; Tsang, A.; Han, J.H.; Graham, A. 2018 Recovery Assessment and Long-Term Monitoring of Reef Coral Communities at Pila‘a Reef, Kaua‘i; State of Hawai‘i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2019; p. 82. [Google Scholar]
- Rodgers, K.S.; Stender, Y.O.; Tsang, A.; Graham, A.; Dona, A.R. 2019 Recovery Assessment and Long-Term Monitoring of Reef Coral Communities at Pila‘a Reef, Kaua‘i; State of Hawai‘i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2020; p. 88. [Google Scholar]
- Davis, S.A. Some Aspects of the Biology of Anthelia edmondsoni (Verrill); University of Hawai’i: Honolulu, HI, USA, 1977. [Google Scholar]
- Rodgers, K.; Graham, A.; Han, J.H.; Stefanak, M.; Stender, Y.O.; Tsang, A.; Stamoulis, K.A.; Delevaux, J. 2019 Long-Term Monitoring and Assessment of the Hā‘ena, Kaua‘i Community Based Subsistence Fishing Area; State of Hawai’i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2020; p. 75. [Google Scholar]
- Walsh, W.; Cotton, S.; Barnett, C.; Couch, C.; Preskitt, L.; Tissot, B.; Osada-D’Avella, K. Long-Term Monitoring of Coral Reefs of the Main Hawaiian Islands; NA09NOS4260100; 2009 NOAA Coral Reef Conservation Program: Silver Spring, MD, USA, 2013; p. 97.
- Fabricius, K.; McCorry, D. Changes in octocoral communities and benthic cover along a water quality gradient in the reefs of Hong Kong. Mar. Pollut. Bull. 2006, 52, 22–23. [Google Scholar] [CrossRef]
- Hernández-Muñoz, D.; Alcolado, P.M.; Hernández-González, M. Effects of a submarine discharge of urban waste on octocoral (Octocorallia: Alcyonacea) communities in Cuba. Rev. Biol. Trop. 2008, 56, 64–75. [Google Scholar]
- Baker, D.M.; Webster, K.L.; Kim, K. Caribbean octocorals record changing carbon and nitrogen sources from 1862 to 2005. Glob. Chang. Biol. 2010, 16, 2701–2710. [Google Scholar] [CrossRef]
- Pugh, A. The Growth Response of the Hawaiian Blue Octocoral, Sarcothelia Edmondsoni, to Various Nitrate Concentrations; University of Hawai’i at Hilo: Hilo, HI, USA, 2019. [Google Scholar]
- Rodgers, K.; Bahr, K.D.; Stender, Y.O.; Stender, K.; Dona, A.R.; Weible, R.; Tsang, A.; Han, J.H.; McGowan, A.E. 2016 Long-Term Monitoring and Assessment of the Hā‘ena, Kaua‘i Community Based Subsistence Fishing Area; State of Hawai‘i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2017; p. 58. [Google Scholar]
- Rodgers, K.; Stender, Y.O.; Dona, A.R.; Tsang, A.; Han, J.H. 2017 Long-Term Monitoring and Assessment of the Hā‘ena, Kaua‘i Community Based Subsistence Fishing Area; State of Hawai‘i Department of Land and Natural Resources: Division of Aquatic Resources: Honolulu, HI, USA, 2018; p. 69. [Google Scholar]
- Beijbom, O.; Edmunds, P.J.; Kline, D.I.; Mitchell, B.G.; Kriegman, D. Automated annotation of coral reef survey images. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Providence, RI, USA, 16–21 June 2012. [Google Scholar]
- Beijbom, O.; Edmunds, P.J.; Roelfsema, C.; Smith, J.; Kline, D.I.; Neal, B.P.; Dunlap, M.J.; Moriarty, V.; Fan, T.-Y.; Tan, C.-J.; et al. Towards automated annotation of benthic survey images: Variability of human experts and operational modes of automation. PLoS ONE 2015, 10, e0130312. [Google Scholar] [CrossRef]
- Williams, I.D.; Couch, C.S.; Beijbom, O.; Oliver, T.A.; Vargas-Angel, B.; Schumacher, B.D.; Brainard, R.E. Leveraging automated image analysis tools to transform our capacity to assess status and trends of coral reefs. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Maragos, J.E. Description of reefs and corals for the 1988 Protected Area Survey of the Northern Marshall Islands. Atoll Res. Bull. 1994, 419, 1–88. [Google Scholar] [CrossRef]
- Lucas, M.; Longman, R.J.; Giambelluca, T.W.; Frazier, A.; Lee, J. Mapping 30-years of monthly rainfall using an automated kriging approach. 2020. under review. [Google Scholar]
- Folk, R.L. Petrology of Sedimentary Rocks; University of Texas, Hernphill Publishing Co.: Austin, TX, USA, 1974. [Google Scholar]
- Parker, J. A comparison of methods used for the measurement of organic matter in marine sediment. Chem. Ecol. 1983, 1, 201–209. [Google Scholar] [CrossRef]
- Craft, C.; Seneca, E.; Broome, S. Loss on ignition and Kjeldahl digestion for estimating organic carbon and total nitrogen in estuarine marsh soils: Calibration with dry combustion. Estuaries 1991, 14, 175–179. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE: Los Angeles, CA, USA, 2019; p. 577. [Google Scholar]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA); Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; Wiley StatsRef: Statistics Reference Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Austral. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; OHara, R.; Simpson, G.; Solymos, P. Vegan: Community Ecology Package 0: 1–292; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Ripley, B.; Venables, B.; Bates, D.M.; Hornik, K.; Gebhardt, A.; Firth, D. Package ‘MASS’. 2013. Available online: ftp://192.218.129.11/pub/CRAN/web/packages/MASS/MASS.pdf (accessed on 4 February 2021).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, aka Least-Squares Means, version 1.4.5; 2020. Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 4 February 2021).
- Signorell, A.; Aho, K.; Alfons, A.; Anderegg, N.; Aragon, T. DescTools: Tools for Descriptive Statistics, version 0.99.38; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Martinez-Arbizu, P. pairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. 2020. Available online: https://rdrr.io/github/gauravsk/ranacapa/man/pairwise_adonis.html (accessed on 4 February 2021).
- Friedlander, A.; Aeby, G.; Brainard, R.; Brown, E.; Chaston, K.; Clark, A.; McGowan, P.; Montgomery, T.; Walsh, W.; Williams, I. The State of Coral Reef Ecosystems of the Main Hawaiian Islands; NCCOS: Silver Spring, MD, USA, 2008; pp. 222–269.
- Rodgers, K. Evaluation of Nearshore Coral Reef Condition and Identification of Indicators in the Main Hawaiian Islands; University of Hawai’i: Honolulu, HI, USA, 2005. [Google Scholar]
- Coles, S.L.; Bahr, K.D.; Rodgers, K.U.S.; May, S.L.; McGowan, A.E.; Tsang, A.; Bumgarner, J.; Han, J.H. Evidence of acclimatization or adaptation in Hawaiian corals to higher ocean temperatures. PeerJ 2018, 6, e5347. [Google Scholar] [CrossRef]
- Weber, M.; Lott, C.; Fabricius, K. Sedimentation stress in a scleractinian coral exposed to terrestrial and marine sediments with contrasting physical, organic and geochemical properties. J. Exp. Mar. Biol. Ecol. 2006, 336, 18–32. [Google Scholar] [CrossRef]
- Storlazzi, C.D.; Field, M.E.; Bothner, M.H.; Presto, M.; Draut, A.E. Sedimentation processes in a coral reef embayment: Hanalei Bay, Kauai. Mar. Geol. 2009, 264, 140–151. [Google Scholar] [CrossRef]
- PacIOOS. Wave Observations: Hanalei, Kaua‘i; U.S. Integrated Ocean Observing System (IOOS®): Silver Spring, MD, USA, 2019.
- Stickle, W.B.; Diehl, W.J. Effects of Salinity on Echinoderms; Jangoux, M., Lawrence, J.M., Eds.; Balkema: Rotterdam, NL, USA, 1987; Volume 2, pp. 235–285. [Google Scholar]
- Drouin, G.; Himmelman, J.H.; Béland, P. Impact of tidal salinity fluctuations on echinoderm and mollusc populations. Can. J. Zool. 1985, 63, 1377–1387. [Google Scholar] [CrossRef]
- Binyon, J. Salinity tolerance and ionic regulation. In Physiology of Echinodermata; Boolootion, R.A., Ed.; Interscience Publishers: New York, NY, USA, 1966; pp. 359–377. [Google Scholar]
- Walker, J.W. Effects of fine sediments on settlement and survival of the sea urchin Evechinus chloroticus in northeastern New Zealand. Mar. Ecol. Prog. Ser. 2007, 331, 109–118. [Google Scholar] [CrossRef]
- Sutherland, R.A. Distribution of organic carbon in bed sediments of Manoa Stream, O’ahu, Hawai’i. Earth Surf. Process. Landf. 1999, 24, 571–583. [Google Scholar] [CrossRef]
- Pagano, G.; Thomas, P.; Guida, M.; Palumbo, A.; Romano, G.; Oral, R.; Trifuoggi, M. Sea urchin bioassays in toxicity testing: II. Sediment evaluation. Expert Opin. Environ. Biol. 2017, 6. [Google Scholar] [CrossRef]
- Pagano, G.; Anselmi, B.; Dinnel, P.A.; Esposito, A.; Guida, M.; Laccarino, M.; Melluso, G.; Pascale, M.; Trieff, N.M. Effects on sea urchin fertilization and embryogenesis of water and sediment from two rivers in Campania, Italy. Arch. Environ. Contam. Toxicol. 1993, 25, 20–26. [Google Scholar] [CrossRef]
- Baker, E.T.; Lavelle, J.W. The effect of particle size on the light attenuation coefficient of natural suspensions. J. Geophys. Res. Oceans 1984, 89, 8197–8203. [Google Scholar] [CrossRef]
- Te, F.T. Responses of Hawaiian Scleractinian Corals to Different Levels of Terrestrial and Carbonate Sediment; University of Hawai‘i at Mānoa: Honolulu, HI, USA, 2001. [Google Scholar]
- Storlazzi, C.; Norris, B.; Rosenberger, K.J. The influence of grain size, grain color, and suspended-sediment concentration on light attenuation: Why fine-grained terrestrial sediment is bad for coral reef ecosystems. Coral Reefs 2015, 34, 967–975. [Google Scholar] [CrossRef]
- Erftemeijer, P.; Riegl, B.; Hoeksema, B.W.; Todd, P.A. Environmental impacts of dredging and other sediment disturbances on corals: A review. Mar. Pollut. Bull. 2012, 64, 1737–1765. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.; Bessell-Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef]
- Couch, C.; Burns, J.; Steward, K.; Gutlay, T.; Liu, G.; Geiger, E.; Eakin, C.; Kosaki, R. Causes and Consequences of the 2014 Mass coral Bleaching Event in Papahanaumokuakea Marine National Monument; Technical Report NOAA PMNM; NOAA: Silver Spring, MD, USA, 2016.
- Bongaerts, P.; Ridgway, T.; Sampayo, E.M.; Hoegh-Guldberg, O. Assessing the ‘deep reef refugia’ hypothesis: Focus on Caribbean reefs. Coral Reefs 2010, 29, 309–327. [Google Scholar] [CrossRef]
- Couch, C.S.; Burns, J.H.R.; Liu, G.; Steward, K.; Gutlay, T.N.; Kenyon, J.; Eakin, C.M.; Kosaki, R.K. Mass coral bleaching due to unprecedented marine heatwave in Papahānaumokuākea Marine National Monument (Northwestern Hawaiian Islands). PLoS ONE 2017, 12, e0185121. [Google Scholar] [CrossRef] [PubMed]
- Nir, O.; Gruber, D.F.; Shemesh, E.; Glasser, E.; Tchernov, D. Seasonal mesophotic coral bleaching of Stylophora pistillata in the Northern Red Sea. PLoS ONE 2014, 9, e84968. [Google Scholar] [CrossRef]
- Venegas, R.M.; Oliver, T.; Liu, G.; Heron, S.F.; Clark, S.J.; Pomeroy, N.; Young, C.; Eakin, C.M.; Brainard, R.E. The rarity of depth refugia from coral bleaching heat stress in the Western and Central Pacific Islands. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Frade, P.R.; Bongaerts, P.; Englebert, N.; Rogers, A.; Gonzalez-Rivero, M.; Hoegh-Guldberg, O. Deep reefs of the Great Barrier Reef offer limited thermal refuge during mass coral bleaching. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Cleary, D.F.R.; DeVantier, L.; Giyanto; Vail, L.; Manto, P.; de Voogd, N.J.; Rachello-Dolmen, P.G.; Tuti, Y.; Budiyanto, A.; Wolstenholme, J.; et al. Relating variation in species composition to environmental variables: A multi-taxon study in an Indonesian coral reef complex. Aquat. Sci. 2008, 70, 419–431. [Google Scholar] [CrossRef]
- Hoogenboom, M.O.; Frank, G.E.; Chase, T.J.; Jurriaans, S.; Álvarez-Noriega, M.; Peterson, K.; Critchell, K.; Berry, K.L.E.; Nicolet, K.J.; Ramsby, B.; et al. Environmental drivers of variation in bleaching severity of Acropora species during an extreme thermal anomaly. Front. Mar. Sci. 2017, 4. [Google Scholar] [CrossRef]
- Carlson, R.R.; Foo, S.A.; Asner, G.P. Land use impacts on coral reef health: A ridge-to-reef perspective. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Smith, T.D.; Lillycrop, L.S. Hawai‘i Regional Sediment Management Needs Assessment; TR-14-4; US Army Corps of Engineers: Engineer Research and Development Center, Coastal and Hydraulics Lab: Vicksburg, MS, USA, 2014; p. 95. [Google Scholar]




| Year (n) | |||||||
|---|---|---|---|---|---|---|---|
| Site | Sector/Depth | Variables | Data Collection Method | 2016 | 2017 | 2018 | 2019 |
| Hā‘ena | Shallow (≤7 m) | Fish abundance and biomass | Transect surveys | 42 | 112 | 73 | 58 |
| Sea urchins | Transect surveys | 42 | 57 | 73 | 58 | ||
| Coral bleaching | Transect surveys and CoralNet | 42 | 57 | 73 | 58 | ||
| Sediments | Bulk grab sample | 9 | N/D | 19 | N/D | ||
| Deep (>7 m) | Fish abundance and biomass | Transect surveys | 56 | 99 | 37 | 40 | |
| Sea urchins | Transect surveys | 56 | 51 | 37 | 40 | ||
| Coral bleaching | Transect surveys and CoralNet | 56 | 51 | 37 | 40 | ||
| Pila‘a | East (1–2 m) | Sea urchins | Transect surveys | 37 | 48 | 39 | 23 |
| Coral bleaching and Sarcothelia abundance | Transect surveys and CoralNet | 37 | 48 | 39 | 23 | ||
| Temperature | Loggers | 5 | 1 | 4 | N/D | ||
| Sediments | Bulk grab sample | 7 | N/D | 5 | N/D | ||
| West (1–2 m) | Sea urchins | Transect surveys | 13 | 34 | 21 | 27 | |
| Coral bleaching and Sarcothelia abundance | Transect surveys and CoralNet | 13 | 34 | 21 | 27 | ||
| Temperature | Loggers | 3 | 1 | 2 | N/D | ||
| Sediments | Bulk grab sample | 3 | N/D | 3 | N/D | ||
| Site | Year | Coarse | Medium | Fine | Silt/Clay |
|---|---|---|---|---|---|
| Pila‘a East | 2016 | 84.9 ± 4.7 | 7.9 ± 2.0 | 6.1 ± 3.4 | 1.2 ± 0.5 |
| Pila‘a East | 2018 | 65.8 ± 7.9 * | 15.0 ± 6.3 | 9.1 ± 1.1 | 10.1 ± 0.8 * |
| Pila‘a West | 2016 | 88.7 ± 2.2 | 8.6 ± 2.6 | 2.1 ± 0.5 | 0.6 ± 0.2 |
| Pila‘a West | 2018 | 65.9 ± 7.5 * | 17.0 ± 5.3 | 8.1 ± 2.3 * | 8.9 ± 0.9 † |
| Hā‘ena | 2016 | 71.6 ± 11.4 | 17.5 ± 6.0 | 10.5 ± 6.1 | 0.4 ± 0.1 |
| Hā‘ena | 2018 | 61.5 ± 5.1 | 24.7 ± 4.6 † | 7.7 ± 1.6 | 6.2 ± 0.3 * |
| Depth | Years | Z-Ratio | SE | Sig. |
|---|---|---|---|---|
| Shallow | 2016–2017 | −3.548 | 0.085 | 0.001 * |
| 2016–2018 | 0.380 | 0.155 | 0.704 | |
| 2016–2019 | −1.412 | 0.122 | 0.189 | |
| 2017–2018 | 4.566 | 0.209 | <0.001 * | |
| 2017–2019 | 2.244 | 0.167 | 0.050 * | |
| 2018–2019 | −2.012 | 0.102 | 0.066† | |
| Deep | 2016–2017 | 0.034 | 0.120 | 0.973 |
| 2016–2018 | 1.703 | 0.195 | 0.265 | |
| 2016–2019 | 0.970 | 0.179 | 0.498 | |
| 2017–2018 | 1.918 | 0.170 | 0.265 | |
| 2017–2019 | 1.073 | 0.157 | 0.498 | |
| 2018–2019 | −0.658 | 0.147 | 0.612 |
| Sector | Years | SE | z-Ratio | Sig. | Depth | Years | SE | z-Ratio | Sig. |
|---|---|---|---|---|---|---|---|---|---|
| East | 2016–2017 | 0.094 | −3.760 | <0.001 * | Shallow | 2016–2017 | 0.364 | 0.741 | 0.550 |
| 2016–2018 | 0.178 | −1.796 | 0.109 | 2016–2018 | 1.035 | 4.524 | <0.001 * | ||
| 2016–2019 | 0.210 | −1.475 | 0.168 | 2016–2019 | 0.938 | 3.860 | <0.001 * | ||
| 2017–2018 | 0.664 | 2.307 | 0.063† | 2017–2018 | 0.737 | 4.255 | <0.001 * | ||
| 2017–2019 | 0.779 | 2.149 | 0.063† | 2017–2019 | 0.676 | 3.500 | <0.001 * | ||
| 2018–2019 | 0.342 | 0.116 | 0.908 | 2018–2019 | 0.224 | −0.548 | 0.584 | ||
| West | 2016–2017 | 0.295 | −0.840 | 0.481 | Deep | 2016–2017 | 0.211 | −1.029 | 0.495 |
| 2016–2018 | 0.452 | 0.077 | 0.939 | 2016–2018 | 0.235 | −0.882 | 0.495 | ||
| 2016–2019 | 0.690 | 1.192 | 0.430 | 2016–2019 | 0.386 | 0.819 | 0.495 | ||
| 2017–2018 | 0.534 | 1.065 | 0.430 | 2017–2018 | 0.306 | 0.061 | 0.952 | ||
| 2017–2019 | 0.799 | 2.502 | 0.074† | 2017–2019 | 0.503 | 1.827 | 0.313 | ||
| 2018–2019 | 0.576 | 1.289 | 0.430 | 2018–2019 | 0.536 | 1.625 | 0.313 |
| Sector | Years | F | Sig. |
|---|---|---|---|
| 2016–2017 | 0.921 | 0.755 | |
| 2016–2018 | 3.872 | 0.045 * | |
| East | 2016–2019 | 0.847 | 0.758 |
| 2017–2018 | 4.967 | 0.023 * | |
| 2017–2019 | N/A | N/A | |
| 2018–2019 | 4.565 | 0.031 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodgers, K.S.; Stefanak, M.P.; Tsang, A.O.; Han, J.J.; Graham, A.T.; Stender, Y.O. Impact to Coral Reef Populations at Hā‘ena and Pila‘a, Kaua‘i, Following a Record 2018 Freshwater Flood Event. Diversity 2021, 13, 66. https://doi.org/10.3390/d13020066
Rodgers KS, Stefanak MP, Tsang AO, Han JJ, Graham AT, Stender YO. Impact to Coral Reef Populations at Hā‘ena and Pila‘a, Kaua‘i, Following a Record 2018 Freshwater Flood Event. Diversity. 2021; 13(2):66. https://doi.org/10.3390/d13020066
Chicago/Turabian StyleRodgers, Ku’ulei S., Matthew P. Stefanak, Anita O. Tsang, Justin J. Han, Andrew T. Graham, and Yuko O. Stender. 2021. "Impact to Coral Reef Populations at Hā‘ena and Pila‘a, Kaua‘i, Following a Record 2018 Freshwater Flood Event" Diversity 13, no. 2: 66. https://doi.org/10.3390/d13020066
APA StyleRodgers, K. S., Stefanak, M. P., Tsang, A. O., Han, J. J., Graham, A. T., & Stender, Y. O. (2021). Impact to Coral Reef Populations at Hā‘ena and Pila‘a, Kaua‘i, Following a Record 2018 Freshwater Flood Event. Diversity, 13(2), 66. https://doi.org/10.3390/d13020066







