Assessing Assemblage Composition of Reproductively Mature Resource Fishes at a Community Based Subsistence Fishing Area (CBSFA)
Abstract
1. Introduction
2. Materials and Methods
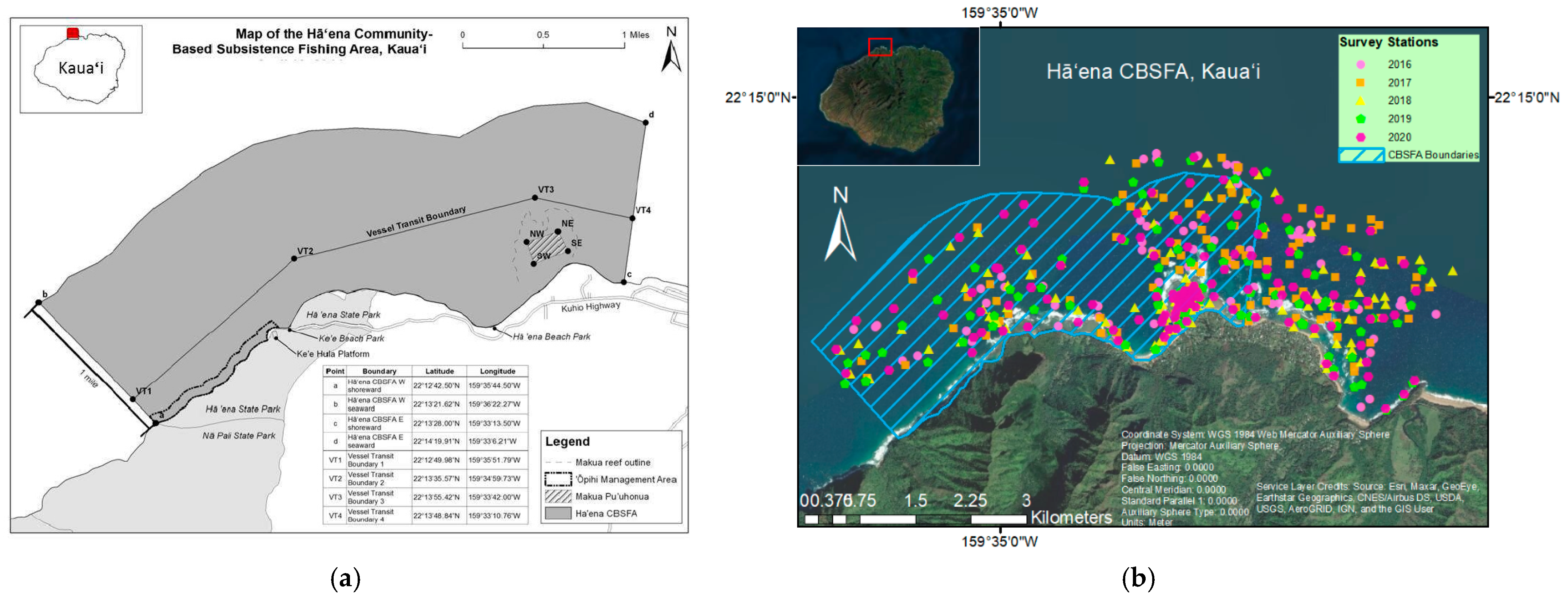
2.1. Sample Design
2.2. Fish and Benthic Surveys
2.3. Assessing Reproductive Maturity
2.4. Statistical Analysis
3. Results
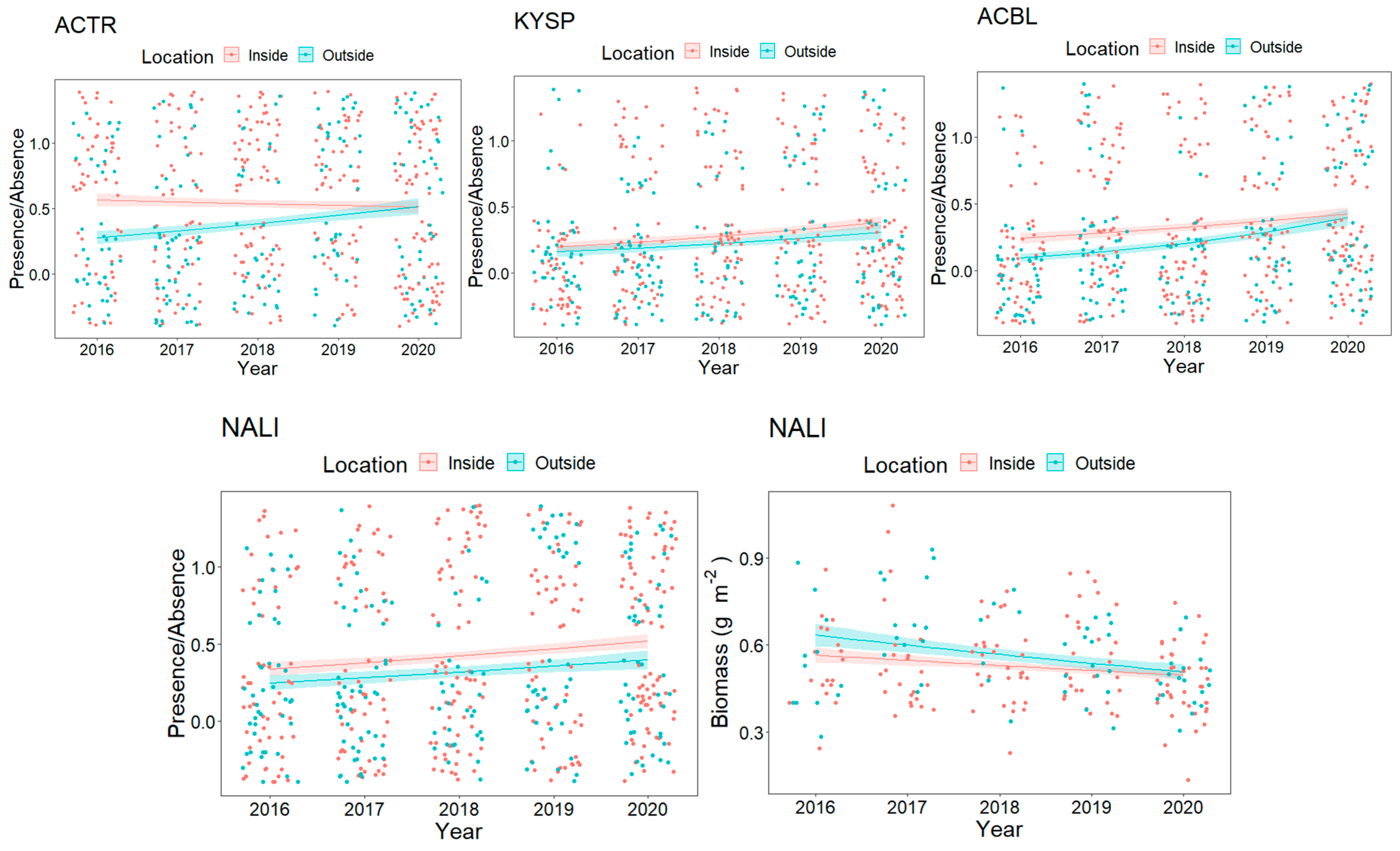
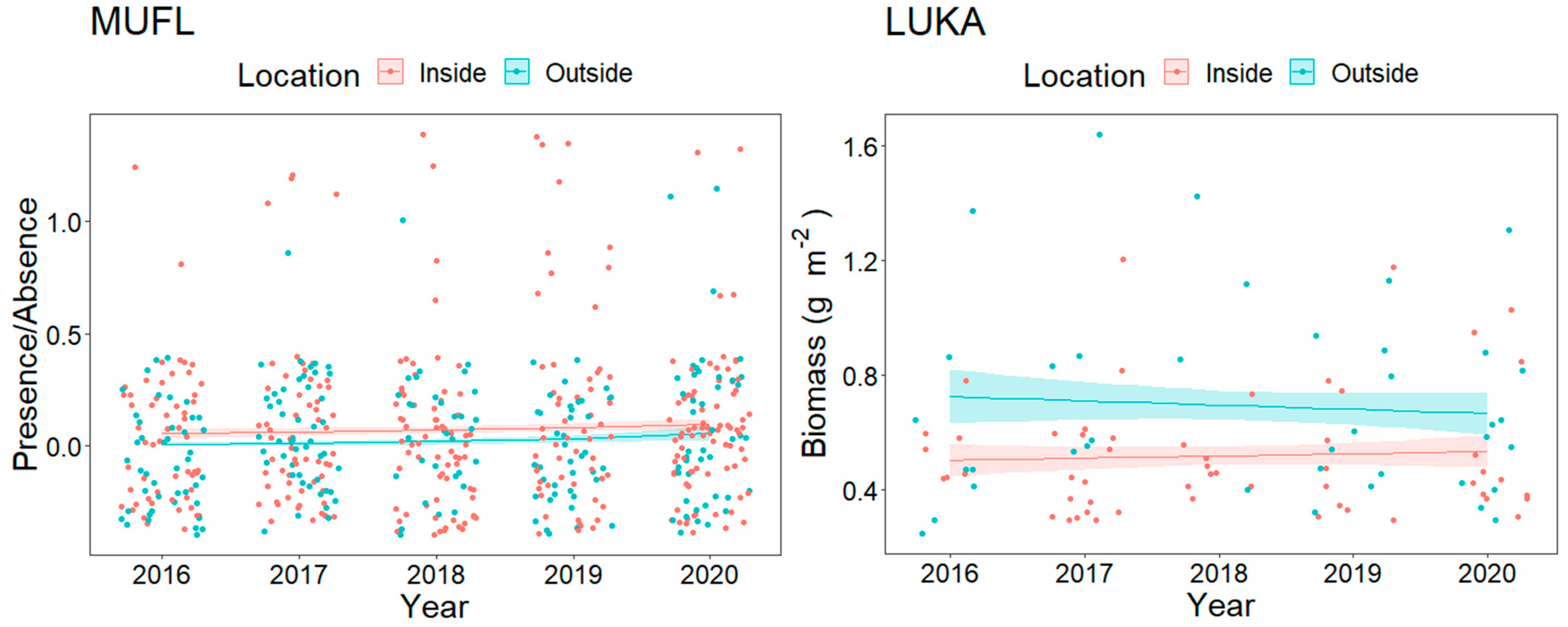
3.1. Overall Fish Assemblage
3.2. Resource Fish Assemblages (above L50)
3.2.1. Trophic Level Assemblage
3.2.2. Fish and Benthic Community Relationships
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Code | Taxon Name | Common | Hawaiian | Endemism | L50 (cm) | Citation |
|---|---|---|---|---|---|---|---|
| Acanthuridae | ACBL | Acanthurus blochii | Ringtail Surgeonfish | pualu | Native | 27.6 | Choat and Robertson, 2002, Kritzer, 2001 |
| ACDU | Acanthurus dussumieri * | Eye-stripe Surgeonfish | palani | Native | 28.2 | Choat and Robertson, 2002, Kritzer, 2001 | |
| ACNR | Acanthurus nigroris * | Bluelined Surgeonfish | maiko | Native | 15.7 | DiBattista et al., 2010 | |
| ACTR | Acanthurus triostegus * | Convict Tang | manini | Endemic | 13.2 | Schemmel and Friedlander, 2017 | |
| NABR | Naso brevirostris | Spotted Unicornfish | kala lolo | Native | 26.9 | Choat and Robertson, 2002, Kritzer, 2001 | |
| NAHE | Naso hexacanthus | Sleek Unicornfish | kala holo | Native | 51.1 | Choat and Robertson, 2002, Kritzer, 2001 | |
| NALI | Naso lituratus | Orangespine Unicornfish | umaumalei | Native | 25 | Kritzer, 2001 | |
| NAUN | Naso unicornis * | Bluespine Unicornfish | kala | Native | 33 | Nadon et al., 2015, Eble et al., 2009 | |
| Carangidae | CAME | Caranx melampygus * | Blue Trevally | ‘omilu | Native | 47.5 | Sudekum et al., 1991, Nadon et al., 2015 |
| CAOR | Carangoides orthogrammus * | Island Jack | ulua | Native | 45.4 | Nadon and Ault, 2016 | |
| SECR | Selar crumenophthalmus * | Big-Eyed Scad | akule | Native | 17 | FishBase | |
| SEDU | Seriola dumerili * | Amberjack | kahala | Native | 99.5 | FishBase | |
| Holocentridae | MYBE | Myripristis berndti | Bigscale Soldierfish | ‘u‘u | Native | 17.5 | Murty, 2002, Craig and Franklin, 2008, Kritzer, 2001 |
| Kyphosidae | KYSP | Kyphosus species * | Lowfin Chub | nenue | Native | 25.3 | Longnecker et al., 2013 |
| Lethrinidae | MOGR | Monotaxis grandoculis | Bigeye Emperor | mu | Native | 38.9 | Nadon and Ault, 2016 |
| Lutjanidae | APVI | Aprion virescens | Green Jobfish | uku | Native | 50 | Everson, 1989, Nadon, 2017 |
| LUFU | Lutjanus fulvus | Blacktail Snapper | to‘au | Introduced | 24 | Nadon and Ault, 2016 | |
| LUKA | Lutjanus kasmira | Bluestripe Snapper | ta‘ape | Introduced | 20 | Allen, 1985, Kritzer, 2001 | |
| Mullidae | MUFL | Mulloidichthys flavolineatus * | Yellowstripe Goatfish | weke | Native | 19.9 | Cole, 2009, Nadon, 2017 |
| MUVA | Mulloidichthys vanicolensis * | Yellowfin Goatfish | weke ‘ula | Native | 20.6 | Cole, 2009, Kritzer, 2001 | |
| PACY | Parupeneus cyclostomus | Blue Goatfish | moano kea | Native | 26.9 | Nadon and Ault, 2016 | |
| PAPO | Parupeneus porphyreus * | Whitesaddle Goatfish | kūmū | Endemic | 26.4 | Nadon et al., 2015 | |
| Scaridae | CACA | Calotomus carolinus * | Stareye Parrotfish | Native | 24.3 | DeMartini and Howard, 2016 | |
| CHPE | Chlorurus perspicillatus * | Spectacled Parrotfish | uhu uliuli | Endemic | 34.5 | DeMartini and Howard, 2016 | |
| CHSO | Chlorurus spilurus | Native | 17.2 | DeMartini and Howard, 2016 | |||
| SCDU | Scarus dubius * | Regal Parrotfish | lauia | Endemic | 23.2 | Nadon and Ault, 2016 | |
| SCPS | Scarus psittacus * | Palenose Parrotfish | uhu | Native | 13.9 | DeMartini and Howard, 2016 | |
| SCRU | Scarus rubroviolaceus * | Redlip Parrotfish | pālukaluka | Native | 35 | DeMartini and Howard, 2016 | |
| Epinephelidae | CEAR | Cephalopholis argus | Blue-spotted Grouper | Introduced | 20 | Schemmel et al., 2016 |
References
- Gaines, S.D.; White, C.; Carr, M.H.; Palumbi, S.R. Designing Marine Reserve Networks for Both Conservation and Fisheries Management. Proc. Natl. Acad. Sci. USA 2010, 107, 18286–18293. [Google Scholar] [CrossRef]
- Friedlander, M.A.; Goodell, W.; Mary, K. Characteristics of Effective Marine Protected Areas in Hawai‘i. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 103–117. [Google Scholar] [CrossRef]
- Burgess, S.C.; Nickols, K.J.; Griesemer, C.D.; Barnett, L.A.K.; Dedrick, A.G.; Satterthwaite, E.V.; Yamane, L.; Morgan, S.G.; White, J.W.; Botsford, L.W. Beyond Connectivity: How Empirical Methods Can Quantify Population Persistence to Improve Marine Protected-Area Design. Ecol. Appl. 2014, 24, 257–270. [Google Scholar] [CrossRef]
- Williams, D.I.; Walsh, W.J.; Miyasaka, A.; Friedlander, A.M. Effects of Rotational Closure on Coral Reef Fishes in Waikiki-Diamond Head Fishery Management Area, Oahu, Hawaii. Mar. Ecol. Prog. Ser. 2006, 310, 139–149. [Google Scholar] [CrossRef]
- Agardy, T. Information Needs for Marine Protected Areas: Scientific and Societal. Bull. Mar. Sci. 2000, 66, 875–888. [Google Scholar]
- Lubchenco, J.; Groud-Colvert, K. Making Waves: The Science and Politics of Ocean Protection. Science 2015, 350, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.D.; White, D.J.; Sparks, R.T.; Lino, K.C.; Zamzow, J.P.; Kelly, E.L.A.; Ramey, H.L. Responses of Herbivorous Fishes and Benthos to 6 Years of Protection at the Kahekili Herbivore Fisheries Management Area, Maui. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Brown, E.K.; Monaco, M.E. Coupling Ecology and GIS to Evaluate Efficacy of Marine Protected Areas in Hawaii. Ecol. Appl. 2007, 17, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, A.M.; Golbuu, Y.; Ballesteros, E.; Caselle, J.E.; Gouezo, M.; Olsudong, D.; Sala, E. Size, Age, and Habitat Determine Effectiveness of Palau’s Marine Protected Areas. PLoS ONE 2017, 12, e0174787. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Stamoulis, K.A.; Kittinger, J.N.; Drazen, J.; Tissot, B.N. Understanding the Scale of Marine Protection in Hawai‘i: From Community-Based Management to the Remote Northwestern Hawaiian Islands Marine National Monument. Adv. Mar. Biol. 2014, 69, 153–203. [Google Scholar] [PubMed]
- Weible, R.M. An In-Depth Investigation of Resource Fishes within and Surrounding a Community-Based Subsistence Fishing Area at Hā‘ena, Kaua‘i. M.S. Thesis, University of Hawai‘i, Mānoa, HI, USA, 2019. [Google Scholar]
- Goodell, W.; Stamoulis, K.A.; Friedlander, A.M. Coupling Remote Sensing with In Situ Surveys to Determine Reef Fish Habitat Associations for the Design of Marine Protected Areas. Mar. Ecol. Prog. Ser. 2017, 588, 121–134. [Google Scholar] [CrossRef]
- Allen, G.R. 1985. Snappers of the World: An Annotated and Illustrated Catalogue of Lutjanid Species Known to Date. FAO Fish. Synop. 1985, 6, 208. [Google Scholar]
- Choat, J.H.; Robertson, D.R. Age-Based Studies. In Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem; Academic Press: San Diego, CA, USA, 2002; pp. 57–80. [Google Scholar]
- Cole, K.S. Size-Dependent and Age-Based Female Fecundity and Reproductive Output for Three Hawaiian Goatfish (Family Mullidae) Species, Mulloidichthys flavolineatus (Yellowstripe Goatfish), M. vanicolensis (Yellowfin Goatfish), and Parupeneus porphyreus (Whitesaddle Goatfish); Report; Division of Aquatic Resources Dingell-Johnson Sport Fish Restoration: Honolulu, HI, USA, 2009. [Google Scholar]
- Craig, M.T.; Franklin, E.C. Life History of Hawaiian Redfish: A Survey of Age and Growth in ‘Aweoweo (Priacanthus meeki) and U‘u (Myripristis berndti); Hawaii Institute of Marine Biology: Kaneohe, HI, USA, 2008. [Google Scholar]
- DeMartini, E.E.; Howard, K.G. Comparisons of Body Sizes at Sexual Maturity and at Sex Change in the Parrotfishes of Hawaii: Input Needed for Management Regulations and Stock Assessments. J. Fish Biol. 2016, 88, 523–541. [Google Scholar] [CrossRef]
- DiBattista, J.D.; Wilcox, C.; Craig, M.T.; Rocha, L.A.; Bowen, B.W. Phylogeography of the Pacific Blueline Surgeonfish, Acanthurus nigroris, Reveals High Genetic Connectivity and a Cryptic Endemic Species in the Hawaiian Archipelago. J. Mar. Biol. 2011, 2011. [Google Scholar] [CrossRef]
- Eble, J.A.; Langston, R.; Bowen, B.W. Growth and Reproduction of Hawaiian Kala, Naso unicornis; Fisheries Local Action Strategy, Final Report; Division of Aquatic Resources: Honolulu, HI, USA, 2009. [Google Scholar]
- Everson, A.R.; Williams, H.A.; Ito, B.M. Maturation and Reproduction in Two Hawaiian Eteline Snappers, Uku, Aprion virescens, and Onaga, Etelis coruscans. Fish. Bull. 1989, 87, 877–888. [Google Scholar]
- Kritzer, J.P.; Davies, C.R.; Mapstone, B.D. Characterizing Fish Populations: Effects of Sample Size and Population Structure on the Precision of Demographic Parameter Estimates. Can. J. Fish. Aquat. Sci. 2001, 58, 1557–1568. [Google Scholar] [CrossRef]
- Murty, V.S. Marine Ornamental Fish Resources of Lakshadweep. CMFRI Spec. Publ. 2002, 72, 1–134. [Google Scholar]
- Nadon, M.O.; Ault, J.S.; Williams, I.D.; Smith, S.G.; DiNardo, G.T. Length-Based Assessment of Coral Reef Fish Populations in the Main and Northwestern Hawaiian Islands. PLoS ONE 2015, 10, e0133960. [Google Scholar] [CrossRef] [PubMed]
- Nadon, M.O.; Ault, J.S. A Stepwise Stochastic Simulation Approach to Estimate Life History Parameters for Data-Poor Fisheries. Can. J. Fish. Aquat. Sci. 2016, 73, 1874–1884. [Google Scholar] [CrossRef]
- Schemmel, E.M.; Donovan, M.K.; Wiggins, C.; Anzivino, M.; Friedlander, A.M. Reproductive Life History of the Introduced Peacock Grouper Cephalopholis argus in Hawaii. J. Fish Biol. 2016, 89, 1271–1284. [Google Scholar] [CrossRef]
- Schemmel, E.M.; Friedlander, A.M. Participatory Fishery Monitoring is Successful for Understanding the Reproductive Biology Needed for Local Fisheries Management. Environ. Biol. Fishes 2017, 100, 171–185. [Google Scholar] [CrossRef]
- Sudekum, A.E.; Parrish, J.D.; Radtke, R.L.; Ralston, S. Life History and Ecology of Large Jacks in Undisturbed Shallow Oceanic Communities. Fish. Bull. 1991, 88, 493–513. [Google Scholar]
- Rodgers, K.; Bahr, K.; Dona, A.R.; Weible, R.; Tsang, A.; Han, J.H.; McGowan, A. 2016 Long-Term Monitoring and Assessment of the Hā‘ena, Kaua‘i Community Based Subsistence Fishing Area; Report; The Hawai‘i Coral Reef Assessment and Monitoring Program: Honolulu, HI, USA, 2017. [Google Scholar]
- Brock, V.E. A Preliminary Report on a Method of Estimating Reef Fish Populations. J. Wildl. Manag. 1954, 18, 297–308. [Google Scholar] [CrossRef]
- Nadon, M.O. Improving Stock Assessment Capabilities for the Coral Reef Fishes of Hawai‘i and the Pacific Region. Ph.D. Thesis, University of Miami, Miami, FL, USA, 2014. [Google Scholar]
- Hixon, M.A.; Johnson, D.W.; Sogard, S.M. BOFFFFs: On the Importance of Conserving Old-Growth Age Structure in Fishery Populations. Ices J. Mar. Sci. 2014, 71, 2171–2185. [Google Scholar] [CrossRef]
- Nadon, M.O. Stock Assessment of the Coral Reef Fishes of Hawaii, 2016; Report; National Oceanic Atmospheric Administration: Washington, DC, USA, 2017. [Google Scholar]
- Longenecker, K.R.; Langston, R.; Bolick, H.; Kondio, U. Size and Reproduction of Exploited Reef Fishes at Kamiali Wildlife Management Area, Papua New Guinea; Bishop Museum Press: Honolulu, HI, USA, 2013. [Google Scholar]
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Friedlander, A.M.; Donovan, M.K.; Stamoulis, K.A.; Williams, I.D.; Brown, E.K.; Conklin, E.J.; DeMartini, E.E.; Rodgers, K.S.; Sparks, R.T.; Walsh, W.J. Human-Induced Gradients of Reef Fish Declines in the Hawaiian Archipelago Viewed through the Lens of Traditional Management Boundaries. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 146–157. [Google Scholar] [CrossRef]
- Magris, R.A.; Treml, E.A.; Pressey, R.L.; Weeks, R. Integrating Multiple Species Connectivity and Habitat Quality into Conservation Planning for Coral Reefs. Ecography 2016, 39, 649–664. [Google Scholar] [CrossRef]
- Rodgers, K.S.; Stefanak, M.P.; Tsang, A.O.; Han, J.J.; Graham, A.T.; Stender, Y.O. Impact to Coral Reef Populations at Hā‘ena and Pila‘a, Kaua‘i, Following a Record 2018 Freshwater Flood Event. Diversity 2021, 13, 66. [Google Scholar] [CrossRef]
- Botsford, L.W.; Brumbaugh, D.R.; Grimes, C.; Kellner, J.B.; Largier, J.; O’Farrell, M.R.; Ralston, S.; Soulanille, E.; Wespestad, V. Connectivity, Sustainability, and Yield: Bridging the Gap between Conventional Fisheries Management and Marine Protected Areas. Rev. Fish Biol. Fish. 2009, 19, 69–95. [Google Scholar] [CrossRef]
- Barneche, D.R.; Kulbicki, M.; Floeter, S.R.; Friedlander, A.M.; Maina, J.; Allen, A.P. Scaling Metabolism from Individuals to Reef-Fish Communities at Broad Spatial Scales. Ecol. Lett. 2014, 17, 1067–1076. [Google Scholar] [CrossRef]
- DeMartini, E.E.; Anderson, T.W.; Friedlander, A.M.; Beets, J.P. Predator Biomass, Prey Density, and Species Composition Effects on Group Size in Recruit Coral Reef Fishes. Mar. Biol. 2011, 158, 2437–2447. [Google Scholar] [CrossRef]
- Green, A.L.; Maypa, A.P.; Almany, G.R.; Rhodes, K.L.; Weeks, R.; Abesamis, R.A.; Gleason, M.G.; Mumby, P.J.; White, A.T. Larval Dispersal and Movement Patterns of Coral Reef Fishes, and Implications for Marine Reserve Network Design. Biol. Rev. 2015, 90, 1215–1247. [Google Scholar] [CrossRef]
- Weeks, R.; Green, A.L.; Joseph, E.; Peterson, N.; Terk, E. Using Reef Fish Movement to Inform Marine Reserve Design. J. Appl. Ecol. 2016, 54, 145–152. [Google Scholar] [CrossRef]
- Fromentin, J.M.; Fonteneau, A. Fishing Effects and Life History Traits: A Case Study Comparing Tropical versus Temperate Tunas. Fish. Res. 2001, 53, 133–150. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Blyth-Skyrme, R.E.; Hart, P.J.B.; Edwards-Jones, G.; Palmer, D. Evidence for Greater Reproductive Output per Unit Area in Areas Protected from Fishing. Can. J. Fish. Aquat. Sci. 2007, 64, 1284–1289. [Google Scholar] [CrossRef]
- Tissot, B.N.; Walsh, W.J.; Hixon, M.A. Hawaiian Islands Marine Ecosystem Case Study: Ecosystem-and Community-Based Management in Hawaii. Coast. Manag. 2009, 37, 255–273. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Rodgers, K.S.; Walsh, W.J.; Polhemus, D.A.; Wilhelm, T.A. Marine Resource Management in the Hawaiian Archipelago: The Traditional Hawaiian System in Relation to the Western Approach. J. Mar. Biol. 2011, 2011, 1–16. [Google Scholar] [CrossRef]



| Df | SS | MS | Pseudo-F | R2 | p | |
|---|---|---|---|---|---|---|
| Location | 1 | 0.58 | 0.58 | 2.114 | 0.004 | 0.052 |
| Year | 4 | 2.52 | 0.63 | 2.292 | 0.019 | 0.001 |
| Location:Year | 4 | 0.97 | 0.24 | 0.879 | 0.007 | 0.613 |
| Residuals | 433 | 119.11 | 0.28 | 0.911 |
| Pairs | F.Model | R2 | p-Value | p-Adjusted |
|---|---|---|---|---|
| 2016 vs.2017 | 3.520 | 0.021 | 0.001 | 0.010 |
| 2016 vs. 2018 | 2.151 | 0.012 | 0.046 | 0.276 |
| 2016 vs.2019 | 5.565 | 0.034 | 0.001 | 0.010 |
| 2016 vs. 2020 | 2.932 | 0.015 | 0.014 | 0.112 |
| 2017 vs. 2018 | 1.929 | 0.011 | 0.071 | 0.355 |
| 2017 vs. 2019 | 0.859 | 0.005 | 0.500 | 0.752 |
| 2017 vs. 2020 | 1.711 | 0.008 | 0.115 | 0.460 |
| 2018 vs. 2019 | 2.503 | 0.014 | 0.020 | 0.140 |
| 2018 vs. 2020 | 1.045 | 0.005 | 0.376 | 0.752 |
| 2019 vs. 2020 | 1.422 | 0.007 | 0.199 | 0.597 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weible, R.M.; Rodgers, K.S.; Friedlander, A.M.; Hunter, C.L. Assessing Assemblage Composition of Reproductively Mature Resource Fishes at a Community Based Subsistence Fishing Area (CBSFA). Diversity 2021, 13, 114. https://doi.org/10.3390/d13030114
Weible RM, Rodgers KS, Friedlander AM, Hunter CL. Assessing Assemblage Composition of Reproductively Mature Resource Fishes at a Community Based Subsistence Fishing Area (CBSFA). Diversity. 2021; 13(3):114. https://doi.org/10.3390/d13030114
Chicago/Turabian StyleWeible, Rebecca M., Ku‘ulei S. Rodgers, Alan M. Friedlander, and Cynthia L. Hunter. 2021. "Assessing Assemblage Composition of Reproductively Mature Resource Fishes at a Community Based Subsistence Fishing Area (CBSFA)" Diversity 13, no. 3: 114. https://doi.org/10.3390/d13030114
APA StyleWeible, R. M., Rodgers, K. S., Friedlander, A. M., & Hunter, C. L. (2021). Assessing Assemblage Composition of Reproductively Mature Resource Fishes at a Community Based Subsistence Fishing Area (CBSFA). Diversity, 13(3), 114. https://doi.org/10.3390/d13030114







