A New Species of Andean Gymnophthalmid Lizard (Squamata: Gymnophthalmidae) from the Peruvian Andes, and Resolution of Some Taxonomic Problems
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material and Taxon Sampling
2.2. DNA Extraction, Amplification, and Sequencing
2.3. Phylogenetic Reconstruction and Genetic Distances
2.4. Designation of Neotype and Species Descriptions
3. Results
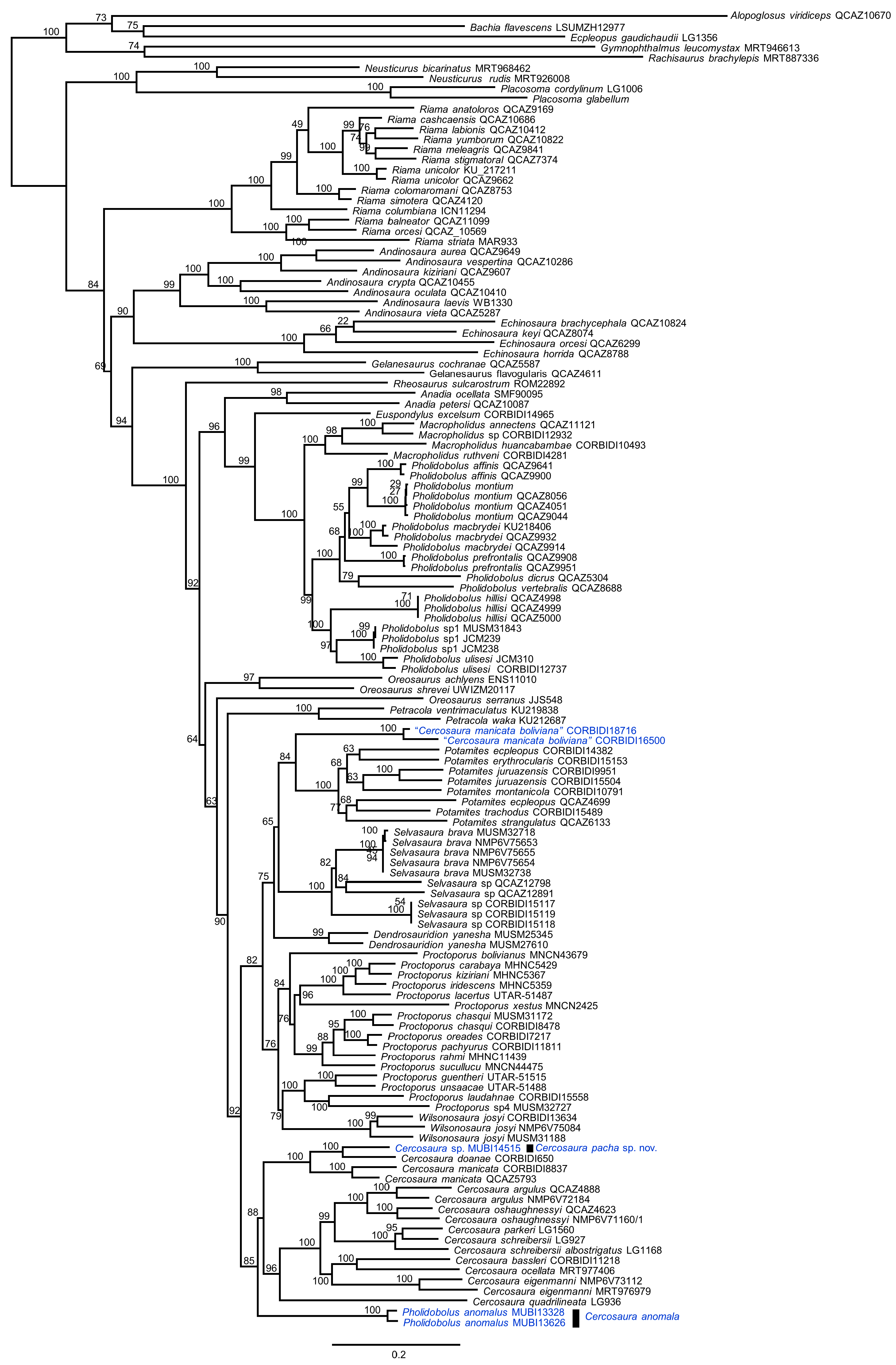
3.1. Phylogenetic Relationships
3.2. Taxonomy
- Cercosaura anomala new. comb. (Müller, 1923)
- Pholidobolus anomalus Müller, 1923
Taxonomic status of Cercosaura manicata boliviana
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Specimens Examined
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Timms, J.; Chaparro, J.C.; Venegas, P.J.; Salazar-Valenzuela, D.; Scrocchi, G.; Cuevas, J.; Leynaud, G.; Carrasco, P. A new species of pitviper of the genus Bothrops (Serpentes: Viperidae: Crotalinae) from the Central Andes of South America. Zootaxa 2019, 4656, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Tejedor, A.; Calatayud, G. Eleven new scaly tree ferns (Cyathea: Cyatheaceae) from Peru. Am. Fern J. 2017, 107, 156–191. [Google Scholar] [CrossRef]
- Rodríguez, L.O.; Mamani, L. A new species of Petracola (Squamata: Gymnophthalmidae) from Río Abiseo National Park, San Martín, Peru. Amphib. Reptil. Conserv. 2020, 14, 140–146. [Google Scholar]
- Lehr, E.; Moravec, J.; Lundberg, M.; Koehler, G.; Catenazzi, A.; Smid, J. A new genus and species of arboreal lizard (Gymnophthalmidae: Cercosaurinae) from the eastern Andes of Peru. Salamandra 2019, 55, 1–13. [Google Scholar]
- Moravec, J.; Šmíd, J.; Štundl, J.; Lehr, E. Systematics of Neotropical microteiid lizards (Gymnophthalmidae, Cercosaurinae), with the description of a new genus and species from the Andean montane forests. ZooKeys 2018, 774, 105–139. [Google Scholar] [CrossRef]
- Chavez, G.; Catenazzi, A.; Venegas, P.J. A new species of arboreal microteiid lizard of the genus Euspondylus (Gymnophtalmidae: Cercosaurinae) from the Andean slopes of central Peru with comments on Peruvian Euspondylus. Zootaxa 2017, 4350, 301–316. [Google Scholar] [CrossRef]
- Mamani, L.; Goicoechea, N.; Chaparro, J.C. A new species of Andean lizard Proctoporus (Squamata: Gymnophthalmidae) from montane forest of the Historic Sanctuary of Machu Picchu, Peru. Amphib. Reptil. Conserv. 2015, 9, 1–11. [Google Scholar]
- Goicoechea, N.; Padial, J.M.; Chaparro, J.C.; Castroviejo-Fisher, S.; De la Riva, I. A taxonomic revision of Proctoporus bolivianus Werner (Squamata: Gymnophthalmidae) with the description of three new species and resurrection of Proctoporus lacertus Stejneger. Am. Mus. Novit. 2013, 3786, 1–32. [Google Scholar] [CrossRef][Green Version]
- Goicoechea, N.; Padial, J.M.; Chaparro, J.C.; Castroviejo-Fisher, S.; De la Riva, I. Molecular phylogenetics, species diversity, and biogeography of the Andean lizards of the genus Proctoporus (Squamata: Gymnophthalmidae). Mol. Phylogenet. Evol. 2012, 65, 953–964. [Google Scholar] [CrossRef]
- Torres-Carvajal, O.; Lobos, S.E.; Venegas, P.J. Phylogeny of Neotropical Cercosaura (Squamata: Gymnophthalmidae) lizards. Mol. Phylogenet. Evol. 2015, 93, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Torres-Carvajal, O.; Lobos, S.E.; Venegas, P.J.; Chávez, G.; Aguirre-Peñafiel, V.; Zurita, D.; Echevarría, L.Y. Phylogeny and biogeography of the most diverse clade of South American gymnophthalmid lizards (Squamata, Gymnophthalmidae, Cercosaurinae). Mol. Phylogenet. Evol. 2016, 99, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Sturaro, M.J.; Rodrigues, M.T.; Colli, G.R.; Knowles, L.L.; Avila-Pires, T.C. Integrative taxonomy of the lizards Cercosaura ocellata species complex (Reptilia: Gymnophthalmidae). Zool. Anz. 2018, 275, 37–65. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hošek, J. (Eds.) The Reptile Database. Available online: http://www.reptile-database.org (accessed on 14 April 2020).
- Sturaro, M.J.; Avila-Pires, T.C.S.; Rodrigues, M.T. Molecular phylogenetic diversity in the widespread lizard Cercosaura ocellata (Reptilia: Gymnophthalmidae) in South America. Syst. Biodivers. 2017, 15, 532–540. [Google Scholar] [CrossRef]
- Echevarría, L.Y.; Barboza, A.C.; Venegas, P.J. A new species of montane gymnophthalmid lizard, genus Cercosaura (Squamata: Gymnophthalmidae), from the Amazon slope of northern Peru. Amphib. Reptil. Conserv. 2015, 9, 34–44. [Google Scholar]
- Doan, T.M.; Lamar, W.W. A new montane species of Cercosaura (Squamata: Gymnophthalmidae) from Colombia, with notes on the distribution of the genus. Zootaxa 2012, 3565, 44–54. [Google Scholar] [CrossRef]
- Doan, T.M. A new phylogenetic classification for the Gymnophthalmid genera Cercosaura, Pantodactylus and Prionodactylus (Reptilia: Squamata). Zool. J. Linnean Soc. 2003, 137, 101–115. [Google Scholar] [CrossRef]
- Castoe, T.A.; Doan, T.M.; Parkinson, C.L. Data partitions and complex models in Bayesian analysis: The phylogeny of gymnophthalmid lizards. Syst. Biol. 2004, 53, 448–469. [Google Scholar] [CrossRef]
- Doan, T.M.; Castoe, T.A. Phylogenetic taxonomy of the Cercosaurini (Squamata: Gymnophthalmidae), with new genera for species of Neusticurus and Proctoporus. Zool. J. Linnean Soc. 2005, 143, 405–416. [Google Scholar] [CrossRef]
- Müller, L. Neue oder seltene Reptilien und Batrachier der Zoologischen Sammlung des bayrischen Staates. Zool. Anz. 1923, 57, 49–61. [Google Scholar]
- Franzen, M.; Glaw, F. Type catalogue of reptiles in the Zoologische Staatssammlung München. Spixiana 2007, 30, 201–274. [Google Scholar]
- Köhler, G.; Lehr, E. Comments on Euspondylus and Proctoporus (Squamata: Gymnophthalmidae) from Peru, with the description of three new species and a key to the Peruvian species. Herpetologica 2004, 60, 501–518. [Google Scholar] [CrossRef]
- Doan, T.M.; Cusi, J.C. Geographic distribution of Cercosaura vertebralis O’Shaughnessy, 1879 (Reptilia: Squamata: Gymnophthalmidae) and the status of Cercosaura ampuedai (Lancini, 1968). Check List 2014, 10, 1195–1200. [Google Scholar] [CrossRef]
- Montanucci, R.R. Systematics and evolution of the Andean lizard genus Pholidobolus (Sauria: Teiidae). Univ. Kans. Mus. Nat. Hist. Misc. Publ. 1973, 59, 1–52. [Google Scholar]
- Reeder, T.W. A new species of Pholidobolus (Squamata: Gymnophthalmidae) from the Huancabamba depression of northern Peru. Herpetologica 1996, 52, 282–289. [Google Scholar]
- Torres-Carvajal, O.; Mafla-Endara, P. Evolutionary history of Andean Pholidobolus and Macropholidus (Squamata: Gymnophthalmidae) lizards. Mol. Phylogenet. Evol. 2013, 68, 212–217. [Google Scholar] [CrossRef]
- Hurtado-Gómez, J.P.; Arredondo, J.C.; Sales-Nunes, P.M.; Daza, J.M. A new species of Pholidobolus (Squamata: Gymnophthalmidae) from the paramo ecosystem in the northern Andes of Colombia. S. Am. J. Herpetol. 2018, 13, 271–286. [Google Scholar] [CrossRef]
- Parra, V.; Nunes, P.M.S.; Torres-Carvajal, O. Systematics of Pholidobolus lizards (Squamata, Gymnophthalmidae) from southern Ecuador, with descriptions of four new species. Zookeys 2020, 954, 109–156. [Google Scholar] [CrossRef]
- Uzzell, T.M. A revision of lizard of the genus Prionodactylus, with a new genus for P. leucotictus and notes on the genus Euspondylus (Sauria, Teiidae). Postilla 1973, 150, 1–67. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Pellegrino, K.C.M.; Rodrigues, M.T.; Yonenaga-Yassuda, Y.; Sites, J.W. A molecular perspective on the evolution of microteiid lizard (Squamata, Gymnophthalmidae), and a new classification for the family. Biol. J. Linnean Soc. 2001, 74, 315–338. [Google Scholar] [CrossRef]
- Blair, C.; Méndez de La Cruz, F.R.; Ngo, A.; Lindell, J.; Lathrop, A.; Murphy, R.W. Molecular phylogenetics and taxonomy of leaf-toed geckos (Phyllodactylidae: Phyllodactylus) inhabiting the peninsula of Baja California. Zootaxa 2009, 2027, 28–42. [Google Scholar] [CrossRef]
- Arevalo, E.; Davis, S.K.; Sites, J.W., Jr. Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in central Mexico. Syst. Biol. 1994, 43, 387–418. [Google Scholar] [CrossRef]
- Saint, K.M.; Austin, C.C.; Donnellan, S.C.; Hutchinson, M.N. C-mos, a nuclear marker useful for squamate phylogenetic analysis. Mol. Phylogenet. Evol. 1998, 10, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.61. 2019. Available online: http://www.mesquiteproject.org (accessed on 21 March 2020).
- Lanfear, R.; Calcott, B.; Ho, S.Y.; Guindon, S. PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Barbour, T. Reptiles collected by the Yale Peruvian expedition of 1912. Proc. Acad. Nat. Sci. Phila. 1913, 65, 505–507. [Google Scholar]
- Amaral, A. New genera and species of snakes. Proc. N. Engl. Zool. Club 1923, 8, 85–105. [Google Scholar] [CrossRef]
- ICZN. The International Trust for Zoological Nomenclature; Natural History Museum: London, UK, 1999; p. 306. [Google Scholar]
- Uzzell, T.M. Teiid lizards of the genus Proctoporus from Bolivia and Peru. Postilla 1970, 142, 1–39. [Google Scholar]
- Kizirian, D.A. A review of Ecuadorian Proctoporus (Squamata: Gymnophthalmidae) with descriptions of nine new species. Herpetol. Monogr. 1996, 10, 85–155. [Google Scholar] [CrossRef]
- Avila-Pires, T.C.S. Lizard of Brazilian Amazonia (Reptilia: Squamata); Zoologische Verhandelingen: Leiden, The Netherlands, 1995; p. 706. [Google Scholar]
- Gorzula, S.; Senaris, J.C. Contribution to the Herpetofauna of the Venezuelan Guayana; Scientia Guaianae: Caracas, Venezuela, 1999; p. 269. [Google Scholar]
- Soares-Barreto, D.; Martin Valdão, R.; Nogueira, C.; Potter de Castro, C.; Ferrerira, V.L.; Strüssman, C. New locality records, geographical distribution, and morphological variation in Cercosaura parkeri (Ruibal, 1952) (Squamata: Gymnophthalmidae) from western Brazil. Check List 2012, 8, 1365–1369. [Google Scholar] [CrossRef]
- Tedesco, M.E. Una nueva especie de Pantodactylus (Squamata, Gymnophthalmidae) de la provincia de Corrientes, República de Argentina. Facena 1998, 14, 53–62. [Google Scholar]
- Sánchez-Pacheco, S.J.; Torres-Carvajal, O.; Aguirre-Peñafiel, V.; Nunes, P.M.S.; Verrastro, L.; Rivas, G.A.; Rodrigues, M.T.; Grant, T.; Murphy, R.W. Phylogeny of Riama (Squamata: Gymnophthalmidae), impact of phenotypic evidence on molecular datasets, and the origin of the Sierra Nevada de Santa Marta endemic fauna. Cladistics 2018, 34, 260–291. [Google Scholar] [CrossRef]
- Vásquez-Restrepo, J.D.; Ibáñez, R.; Sánchez-Pacheco, S.J.; Daza, J.M. Phylogeny, taxonomy and distribution of the Neotropical lizard genus Echinosaura (Squamata: Gymnophthalmidae), with the recognition of two new genera in Cercosaurinae. Zool. J. Linnean Soc. 2019, 189, 287–314. [Google Scholar] [CrossRef]
- Fisher-Reid, M.C.; Wiens, J.J. What are the consequences of combining nuclear and mitochondrial data for phylogenetic analysis? Lessons from Plethodon salamanders and 13 other vertebrate clades. BMC Evol. Biol. 2011, 11, 300. [Google Scholar] [CrossRef]
- Werner, F. Beschreibung neuer Reptilien und Batrachier. Zool. Anz. 1899, 22, 479–484. [Google Scholar]
- O’Shaughnessy, A.W.E. An Account of the Collection of Lizards made by Mr. Buckley in Ecuador, and now in the British Museum, with descriptions of the new Species. Proc. Zool. Soc. Lond. 1881, 49, 227–245. [Google Scholar] [CrossRef]
- Díaz, M.I.; Ttito, A.; Mamani, L. Una nueva localidad y reexaminación de la serie tipo de la lagartija Andina de Machu Picchu Proctoporus machupicchu Mamani, Goicoechea y Chaparro, 2015 (Squamata: Gymnophthalmidae). Rev. Peru. Biol. 2019, 26, 503–508. [Google Scholar] [CrossRef]
- Chávez, G.; Chávez-Arribasplata, J.C. Distribution and natural history notes on the Peruvian lizard Proctoporus laudahnae (Squamata: Gymnophthalmidae). Phyllomedusa J. Herpetol. 2016, 15, 147–154. [Google Scholar] [CrossRef]
- Marques-Souza, S.; Prates, I.; Fouquet, A.; Camacho, A.; Kok, P.J.; Nunes, P.M.; Vechio, F.D.; Recorder, R.S.; Mejia, N.; Junior, M.T.; et al. Reconquering the water: Evolution and systematics of South and Central American aquatic lizards (Gymnophthalmidae). Zool. Scr. 2018, 47, 255–265. [Google Scholar] [CrossRef]
- Grizante, M.B.; Brandt, R.; Kohlsdorf, T. Evolution of body elongation in gymnophthalmid lizards: Relationships with climate. PLoS ONE 2012, 7, e49772. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.T.; Santos, E.M.D. A new genus and species of eyelid-less and limb reduced gymnophthalmid lizard from northeastern Brazil (Squamata, Gymnophthalmidae). Zootaxa 2008, 1873, 50–60. [Google Scholar] [CrossRef]
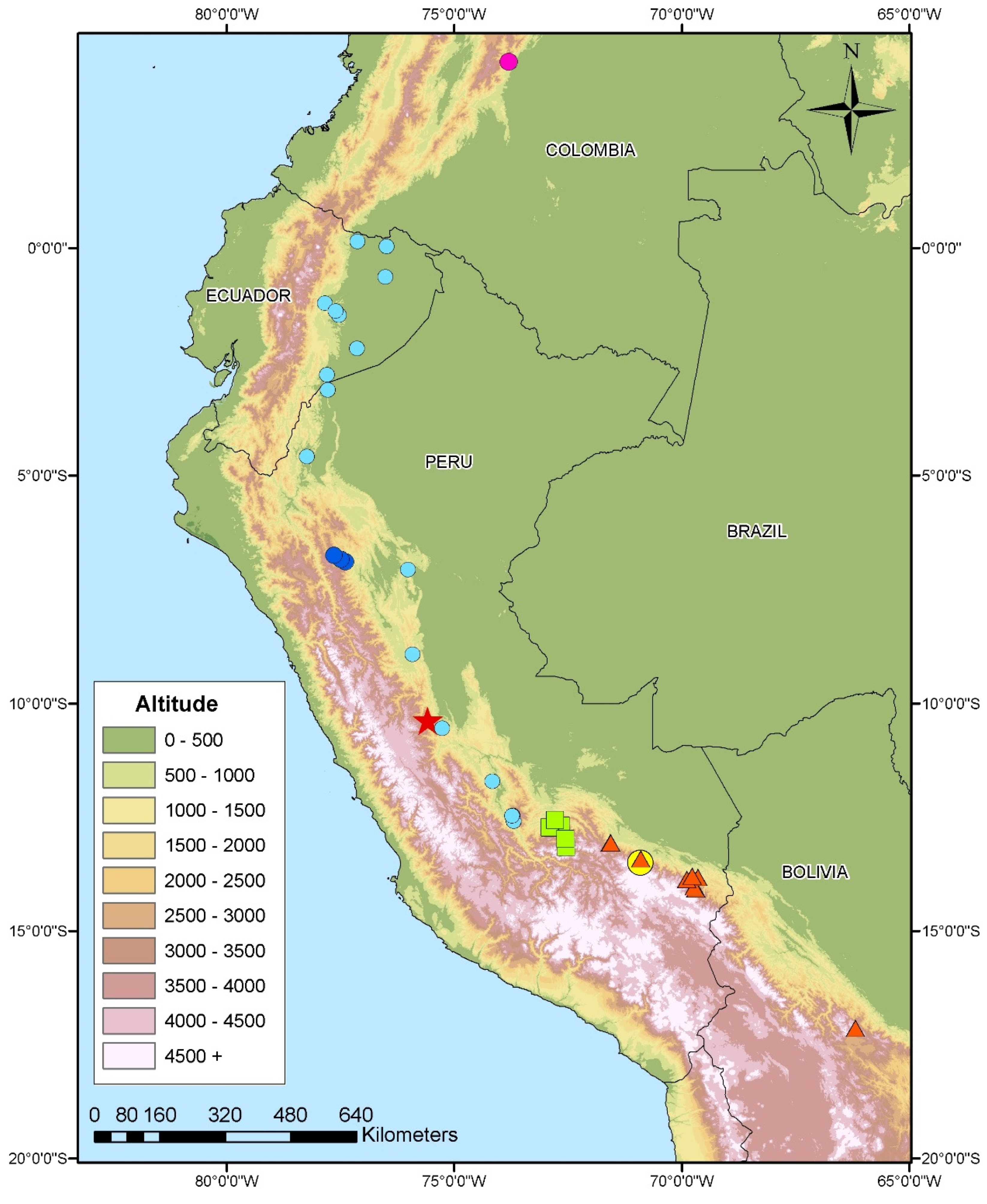







| Species | Locality | Coordinates | Voucher | 12S | 16S | ND4 | Cytb | c-mos |
|---|---|---|---|---|---|---|---|---|
| Pholidobolus anomalus | Tucantinas, La Convención, Cusco | 12°44′16″ S/72°53′29″ W | MUBI 13328 | MT531384 | MT524454 | MT522845 | - | MT512508 |
| Pholidobolus anomalus | Urusayhua, La Convención, Cusco | 12°41′32″ S/72°39′18″ W | MUBI 13626 | MT531385 | MT524455 | MT522846 | MT512513 | MT512509 |
| Cercosaura manicata boliviana | San Pedro, Parque nacional del Manu, Paucartambo, Cusco | 13°4′4″ S/71°33′45″ W | CORBIDI 16500 | MT531386 | MT524452 | MT522849 | - | MT512512 |
| Cercosaura manicata boliviana | Santo Domingo, Limbani, Sandia, Puno | 13°50′1″ S/69°38′29″ W | CORBIDI 18716 | MT531387 | MT524453 | MT522848 | MT512515 | MT512511 |
| Cercosaura sp. (*) | Lanturachi, Huancabamba, Oxapampa | 10°23′01″ S/75°34′49″ W | MUBI 14515 | MT531388 | MT524456 | MT522847 | MT512514 | MT512510 |
| Gene | Primer | Primers Sequence (5′–3′) | PCR Cycle | Reference |
|---|---|---|---|---|
| 12S | 12S1L 12S2H | CAAACTGGGATTAGATACCCCACTAT AGGGTGACGGGCGGTGTGT | 94 °C/3 min; 33 × (95 °C/30 s, 57 °C/30 s, 72 °C/90 s); 72 °C/10 min | [31] |
| 16S | 16sF.0 16sR.0 | CTGTTTACCAAAAACATMRCCTYTAGC TAGATAGAAACCGACCTGGATT | 96 °C/3 min; 40 × (95 °C/30 s, 51 °C/60 s, 72 °C/60 s); 72 °C/10 min | [31,32] |
| ND4 | ND412931L ND413824H | CTACCAAAAGCTCATGTAGAAGC CATTACTTTTACTTGGATTTGCACCA | 96 °C/3 min; 40 × (95 °C/30 s, 52 °C/60 s, 72°C/60 s); 72 °C/10 min | [33,34] |
| Cytb | L14841 H15149 | AAAAAGCTTCCATCCAACATCTCAGCATGATGAAA AAACTGCAGCCCCTCAGAATGATATTTGTCCTCA | 94 °C/5 min; 30 × (94 °C/60 s, 50 °C/60 s, 72 °C/60 s); 72 °C/10 min | [31] |
| c-mos | G73 G74 | GCGGTAAAGCAGGTGAAGAAA TGAGCATCCAAAGTCTCCAATC | 96 °C/3 min; 35 × (95 °C/25 s, 52 °C/60 s, 72 °C/120 s), 72 °C/10 min | [35] |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cercosaura anomala MUBI 13626 | 0.073 | 0.095 | 0.06 | 0.121 | 0.065 | 0.069 | 0.086 | 0.078 | 0.073 | 0.06 | 0.082 | 0.073 | 0.069 | 0.065 | |
| 2. Cercosaura argulus QCAZ 4888 | 0.065 | 0.073 | 0.073 | 0.103 | 0.095 | 0.091 | 0.069 | 0.043 | 0.039 | 0.082 | 0.039 | 0.034 | 0.108 | 0.082 | |
| 3. Cercosaura bassleri CORBIDI 11218 | 0.078 | 0.06 | 0.099 | 0.112 | 0.112 | 0.108 | 0.06 | 0.082 | 0.06 | 0.103 | 0.078 | 0.073 | 0.121 | 0.082 | |
| 4. Cercosaura doanae CORBIDI 650 | 0.067 | 0.073 | 0.071 | 0.116 | 0.052 | 0.052 | 0.095 | 0.086 | 0.091 | 0.069 | 0.099 | 0.091 | 0.052 | 0.073 | |
| 5. Cercosaura eigenmanni MRT 976979 | 0.071 | 0.058 | 0.048 | 0.08 | 0.121 | 0.116 | 0.099 | 0.108 | 0.108 | 0.121 | 0.108 | 0.116 | 0.125 | 0.121 | |
| 6. Cercosaura manicata CORBIDI 8837 | 0.056 | 0.065 | 0.082 | 0.067 | 0.089 | 0.022 | 0.116 | 0.091 | 0.095 | 0.073 | 0.095 | 0.086 | 0.047 | 0.078 | |
| 7. Cercosaura manicata QCAZ 5793 | 0.037 | 0.048 | 0.069 | 0.054 | 0.071 | 0.03 | 0.112 | 0.086 | 0.082 | 0.06 | 0.082 | 0.073 | 0.034 | 0.073 | |
| 8. Cercosaura ocellata MRT 977406 | 0.095 | 0.067 | 0.065 | 0.08 | 0.054 | 0.095 | 0.082 | 0.078 | 0.073 | 0.112 | 0.065 | 0.078 | 0.095 | 0.065 | |
| 9. Cercosaura oshaughnessyi QCAZ 4623 | 0.076 | 0.054 | 0.052 | 0.078 | 0.054 | 0.078 | 0.06 | 0.069 | 0.047 | 0.078 | 0.047 | 0.052 | 0.095 | 0.056 | |
| 10. Cercosaura parkeri LG 1560 | 0.082 | 0.058 | 0.063 | 0.086 | 0.06 | 0.073 | 0.063 | 0.067 | 0.058 | 0.069 | 0.017 | 0.013 | 0.091 | 0.069 | |
| 11. Cercosaura quadrilineata LG 936 | 0.086 | 0.069 | 0.084 | 0.089 | 0.069 | 0.084 | 0.069 | 0.071 | 0.08 | 0.076 | 0.082 | 0.073 | 0.078 | 0.065 | |
| 12. Cercosaura schreibersii albostrigatus LG 1168 | 0.063 | 0.054 | 0.056 | 0.078 | 0.048 | 0.076 | 0.056 | 0.071 | 0.052 | 0.041 | 0.069 | 0.022 | 0.091 | 0.078 | |
| 13. Cercosaura schreibersii schreibersii LG 927 | 0.069 | 0.06 | 0.056 | 0.08 | 0.054 | 0.078 | 0.058 | 0.076 | 0.054 | 0.043 | 0.08 | 0.026 | 0.082 | 0.073 | |
| 14. Cercosaura sp. MUBI 14515 | 0.06 | 0.069 | 0.067 | 0.032 | 0.073 | 0.058 | 0.041 | 0.073 | 0.067 | 0.071 | 0.065 | 0.065 | 0.067 | 0.065 | |
| 15. Cercosaura manicata boliviana CORBIDI 18716 | 0.056 | 0.063 | 0.071 | 0.069 | 0.067 | 0.065 | 0.045 | 0.078 | 0.06 | 0.071 | 0.082 | 0.058 | 0.065 | 0.067 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cercosaura anomala MUBI 13626 | 0.196 | 0.188 | 0.187 | 0.187 | 0.195 | 0.185 | 0.198 | 0.187 | 0.176 | 0.18 | 0.18 | 0.179 | 0.176 | 0.176 | |
| 2. Cercosaura argulus QCAZ 4888 | 0.033 | 0.167 | 0.174 | 0.169 | 0.177 | 0.193 | 0.164 | 0.122 | 0.153 | 0.179 | 0.147 | 0.159 | 0.201 | 0.214 | |
| 3. Cercosaura bassleri CORBIDI 11218 | 0.033 | 0.023 | 0.187 | 0.167 | 0.177 | 0.171 | 0.179 | 0.184 | 0.176 | 0.187 | 0.185 | 0.174 | 0.196 | 0.192 | |
| 4. Cercosaura doanae CORBIDI 650 | 0.016 | 0.039 | 0.036 | 0.164 | 0.129 | 0.127 | 0.185 | 0.179 | 0.161 | 0.176 | 0.156 | 0.161 | 0.093 | 0.166 | |
| 5. Cercosaura eigenmanni MRT 976979 | 0.029 | 0.02 | 0.01 | 0.033 | 0.18 | 0.184 | 0.192 | 0.167 | 0.166 | 0.192 | 0.151 | 0.166 | 0.182 | 0.221 | |
| 6. Cercosaura manicata CORBIDI 8837 | 0.02 | 0.042 | 0.036 | 0.01 | 0.036 | 0.09 | 0.19 | 0.192 | 0.184 | 0.195 | 0.164 | 0.176 | 0.135 | 0.187 | |
| 7. Cercosaura manicata QCAZ 5793 | 0.02 | 0.042 | 0.036 | 0.01 | 0.036 | 0 | 0.184 | 0.182 | 0.176 | 0.169 | 0.158 | 0.182 | 0.127 | 0.179 | |
| 8. Cercosaura ocellata MRT 977406 | 0.039 | 0.029 | 0.013 | 0.042 | 0.016 | 0.036 | 0.036 | 0.177 | 0.161 | 0.193 | 0.184 | 0.167 | 0.188 | 0.2 | |
| 9. Cercosaura oshaughnessyi QCAZ 4623 | 0.042 | 0.039 | 0.036 | 0.039 | 0.033 | 0.036 | 0.036 | 0.036 | 0.135 | 0.174 | 0.153 | 0.159 | 0.196 | 0.203 | |
| 10. Cercosaura parkeri LG 1560 | 0.033 | 0.016 | 0.013 | 0.029 | 0.01 | 0.033 | 0.033 | 0.02 | 0.029 | 0.176 | 0.113 | 0.092 | 0.182 | 0.18 | |
| 11. Cercosaura quadrilineata LG 936 | 0.046 | 0.049 | 0.046 | 0.042 | 0.042 | 0.046 | 0.046 | 0.052 | 0.049 | 0.039 | 0.203 | 0.198 | 0.188 | 0.176 | |
| 12. Cercosaura schreibersii albostrigatus LG 1168 | 0.033 | 0.016 | 0.013 | 0.029 | 0.01 | 0.033 | 0.033 | 0.02 | 0.029 | 0 | 0.039 | 0.118 | 0.169 | 0.203 | |
| 13. Cercosaura schreibersii schreibersii LG 927 | 0.033 | 0.016 | 0.013 | 0.029 | 0.01 | 0.033 | 0.033 | 0.02 | 0.029 | 0 | 0.039 | 0 | 0.182 | 0.166 | |
| 14. Cercosaura sp. MUBI 14515 | 0.01 | 0.039 | 0.029 | 0.007 | 0.026 | 0.01 | 0.01 | 0.036 | 0.039 | 0.029 | 0.042 | 0.029 | 0.029 | 0.179 | |
| 15. Cercosaura manicata boliviana CORBIDI 18716 | 0.023 | 0.052 | 0.042 | 0.026 | 0.039 | 0.029 | 0.029 | 0.049 | 0.052 | 0.042 | 0.055 | 0.042 | 0.042 | 0.02 |
| Measurements (mm) | Cercosaura anomala (all adults) | Cercosaura pacha sp. nov. | ||||||
|---|---|---|---|---|---|---|---|---|
| MUBI 641 Male | MUBI 5277 Male | MUBI 16169 Male | MUBI 13626 Female | MUBI 640 Female | MUBI 13328 Female | MUBI 14515 Female | MUBI 14512 Subadult Female | |
| Snout-vent length | 61.7 | 60.69 | 56.2 | 68.22 | 70.08 | 72.05 | 49.7 | 32.4 |
| Tail length | 72.6 | 126.44 | 146.2 | 130.3 | 107.3 | 40.87 | 98.1 | 41 |
| Head length (chin to eardrum) | 13.3 | 13.22 | 13.1 | 14.31 | 14.18 | 14.75 | 10.8 | 7.8 |
| Head width | 10 | 8.95 | 8 | 9.37 | 9.58 | 9.09 | 7.8 | 5.4 |
| Postoculars | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Superciliars | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/5 | 3/3 |
| Palpebral disc | entire | entire | entire | entire | entire | entire | divided | 1/2 |
| Prefrontal scales | present | present | present | present | present | present | present | present |
| Nasoloreal suture | present | present | present | present | present | present | present | present |
| Supralabials | 7/8 | 8/8 | 7/8 | 8/8 | 7/7 | 7/8 | 8/8 | 7/7 |
| Supralabials anterior to the posteroventral angle of the subocular | 4/5 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 |
| Suboculars | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Infralabials | 6/7 | 7/7 | 7/7 | 7/7 | 8/7 | 7/7 | 6/6 | 6/6 |
| Femoral pores | 9/9 | 9/9 | 9/10 | 5/6 | 4/5 | 4/4 | 7/7 | 6/6 |
| Loreal | present | present | present | present | present | present | present | present |
| Supraoculars | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Genials in contact | 5 | 5 | 6 | 6 | 6 | 6 | 5 | 4 |
| Gular rows | 9 | 9 | 8 | 9 | 9 | 9 | 8 | 7 |
| Postparietals | 5 | 5 | 5 | 5 | 5 | 3 | 3 | 2 |
| Scales around midbody | 38 | 35 | 39 | 39 | 40 | 40 | 38 | 37 |
| Longitudinal dorsal count | 24 | 27 | 31 | 27 | 24 | 25 | 30 | 29 |
| Longitudinal ventral count | 9 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Transversal dorsal count | 34 | 36 | 34 | 35 | 33 | 33 | 35 | 32 |
| Transversal ventral count | 22 | 21 | 19 | 19 | 21 | 19 | 20 | 19 |
| Lamellae under finger IV | 14 | 13 | 14 | 13 | 14 | 13 | 13 | 12 |
| Lamellae under toe IV | 18 | 18 | 19 | 19 | 19 | 18 | 18 | 18 |
| Anal plate | 6 | 6 | 5 | 6 | 6 | 5 | 5 | 5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamani, L.; Chaparro, J.C.; Correa, C.; Alarcón, C.; Salas, C.Y.; Catenazzi, A. A New Species of Andean Gymnophthalmid Lizard (Squamata: Gymnophthalmidae) from the Peruvian Andes, and Resolution of Some Taxonomic Problems. Diversity 2020, 12, 361. https://doi.org/10.3390/d12090361
Mamani L, Chaparro JC, Correa C, Alarcón C, Salas CY, Catenazzi A. A New Species of Andean Gymnophthalmid Lizard (Squamata: Gymnophthalmidae) from the Peruvian Andes, and Resolution of Some Taxonomic Problems. Diversity. 2020; 12(9):361. https://doi.org/10.3390/d12090361
Chicago/Turabian StyleMamani, Luis, Juan C. Chaparro, Claudio Correa, Consuelo Alarcón, Cinthya Y. Salas, and Alessandro Catenazzi. 2020. "A New Species of Andean Gymnophthalmid Lizard (Squamata: Gymnophthalmidae) from the Peruvian Andes, and Resolution of Some Taxonomic Problems" Diversity 12, no. 9: 361. https://doi.org/10.3390/d12090361
APA StyleMamani, L., Chaparro, J. C., Correa, C., Alarcón, C., Salas, C. Y., & Catenazzi, A. (2020). A New Species of Andean Gymnophthalmid Lizard (Squamata: Gymnophthalmidae) from the Peruvian Andes, and Resolution of Some Taxonomic Problems. Diversity, 12(9), 361. https://doi.org/10.3390/d12090361








