River Capture and Freshwater Biological Evolution: A Review of Galaxiid Fish Vicariance
Abstract
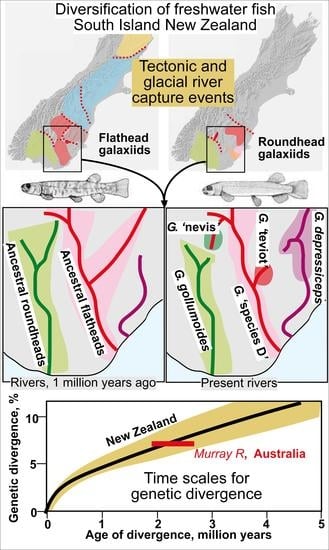
1. Introduction
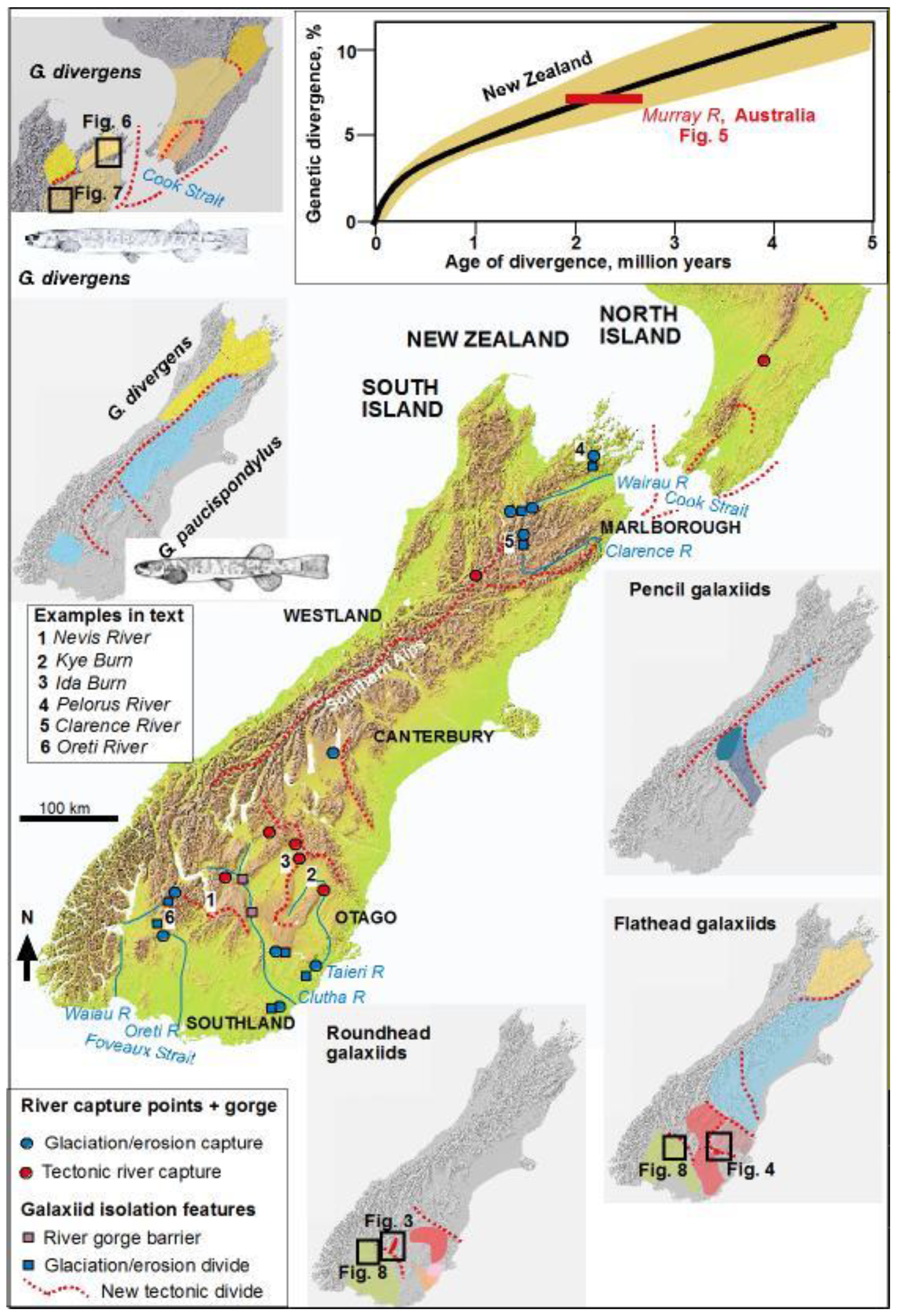
2. The Evolution of New Zealand Landscapes
3. Freshwater Phylogeographic Anomalies: the Biological Legacy of River Capture
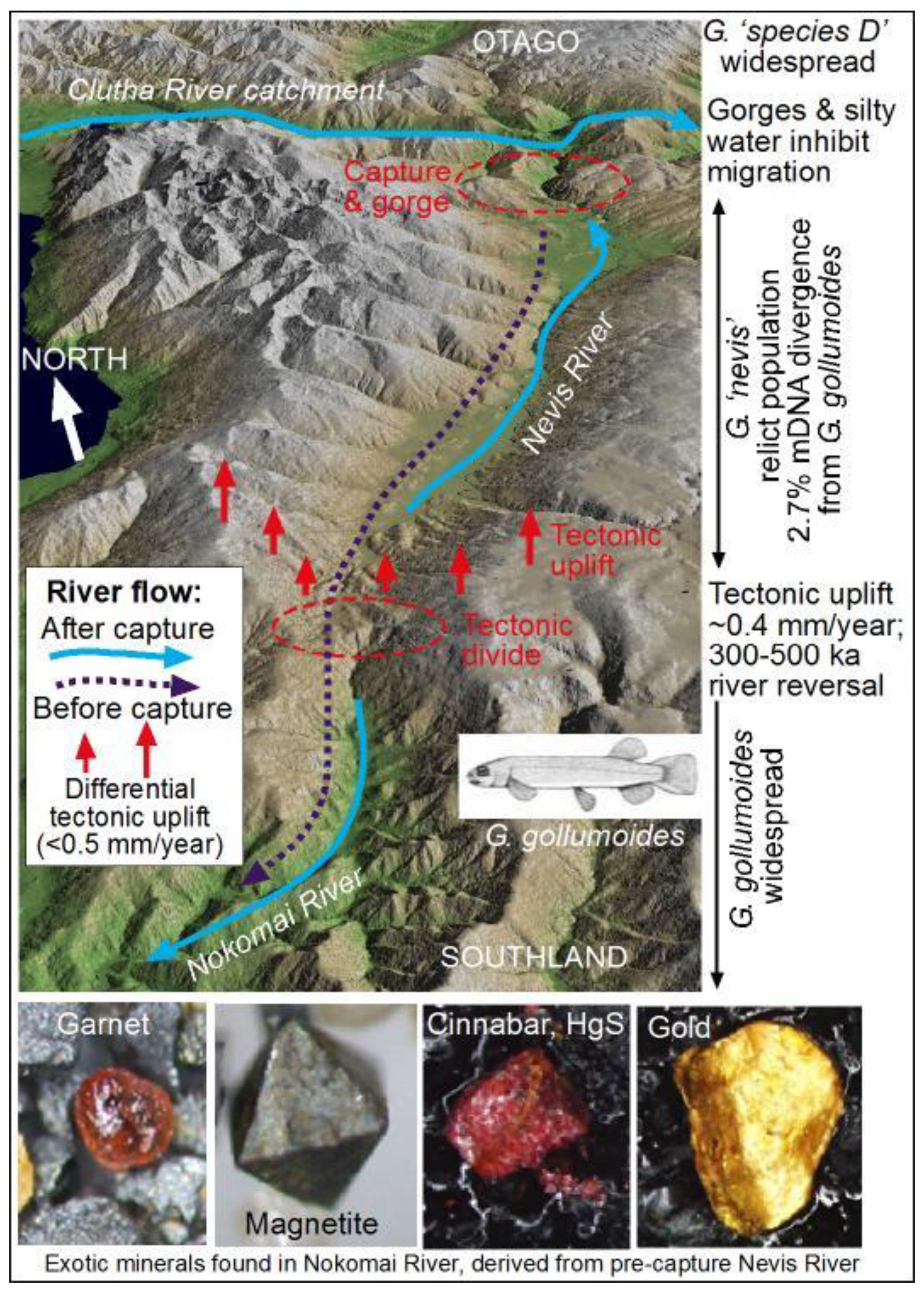
3.1. Nevis River Capture—Isolation of the Nevis Galaxiid
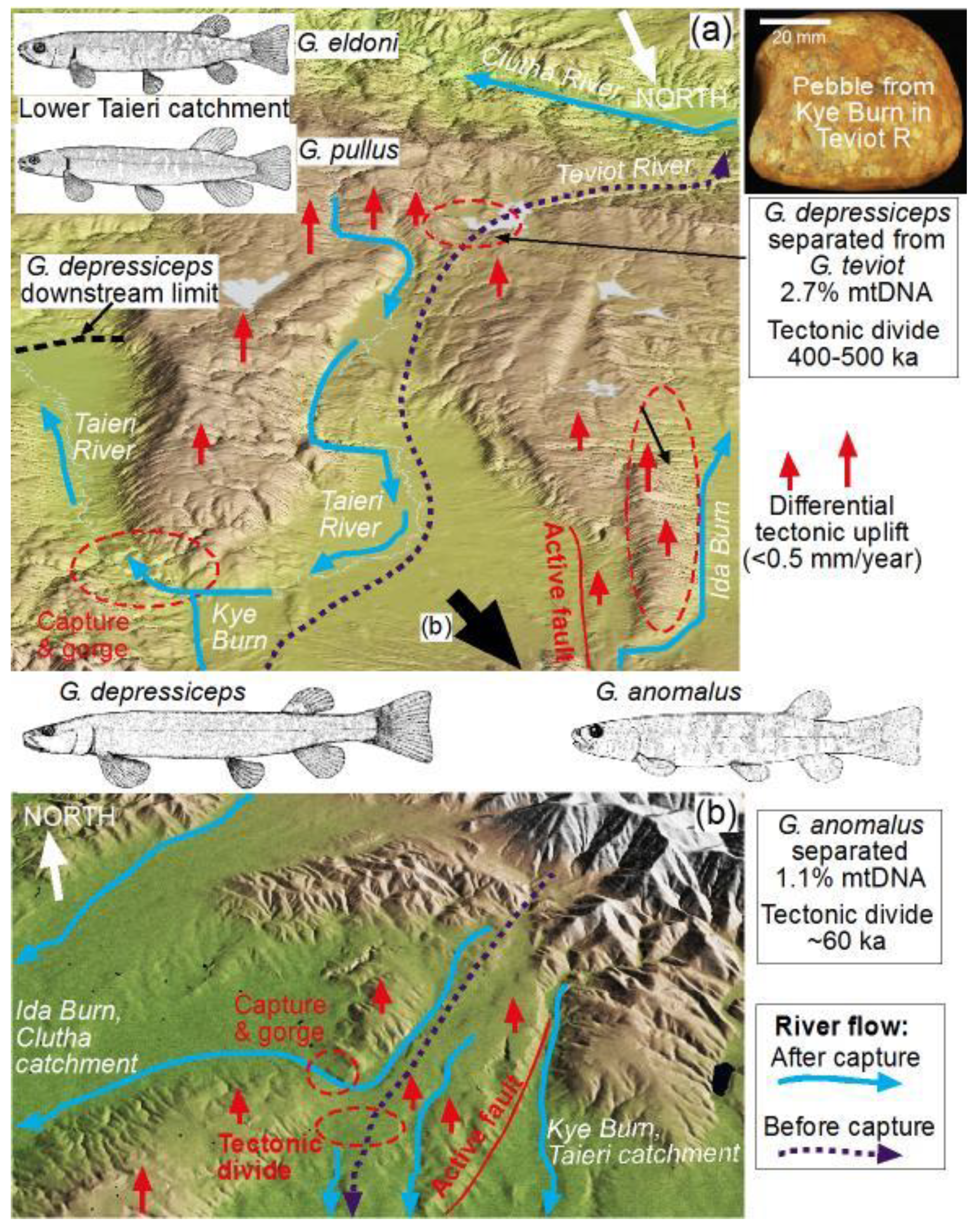
3.2. Capture of the Kye Burn—Isolation of the Teviot Galaxiid
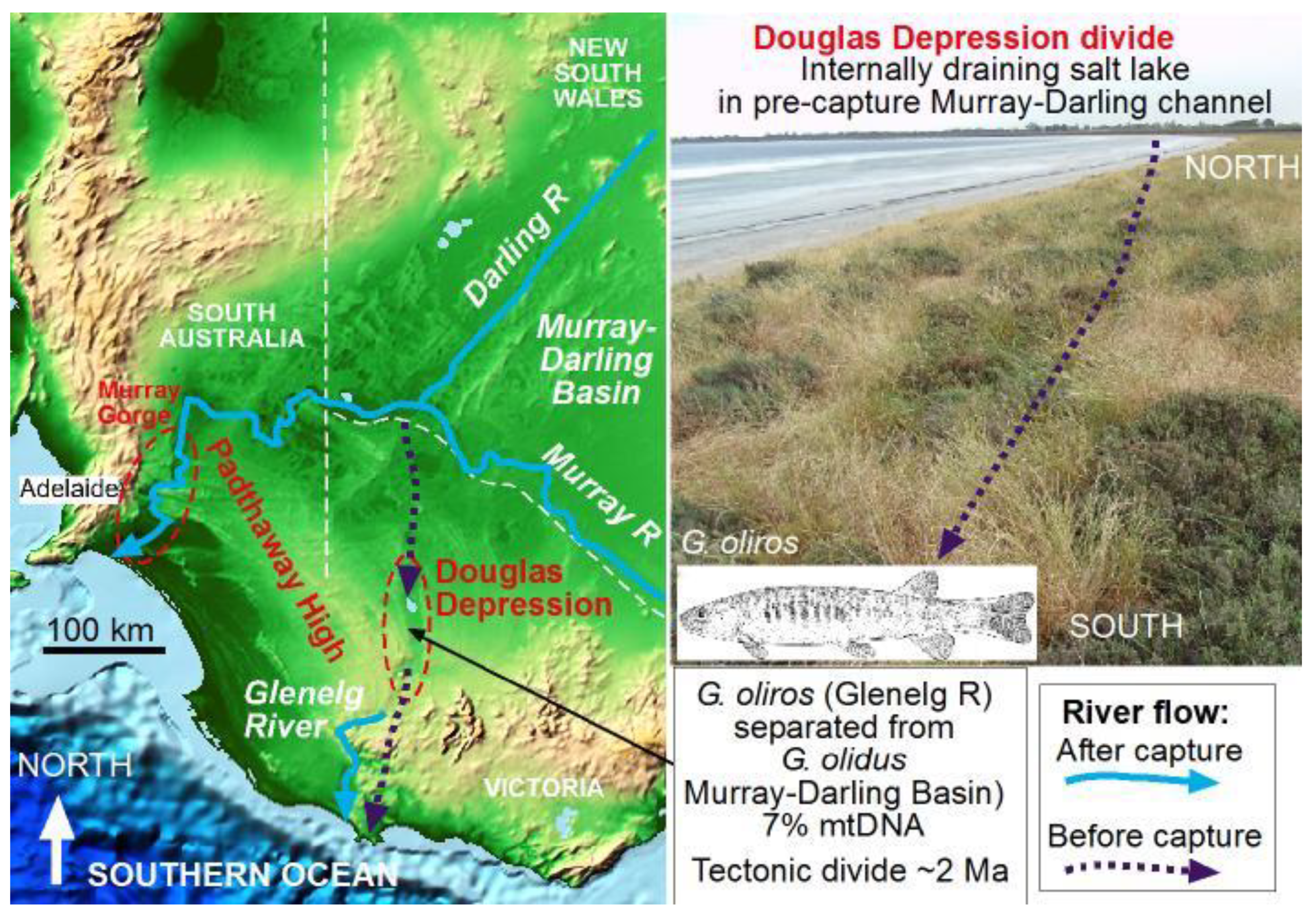
3.3. Rerouting of Australia’s Murray River Isolated the Glenelg River Fauna
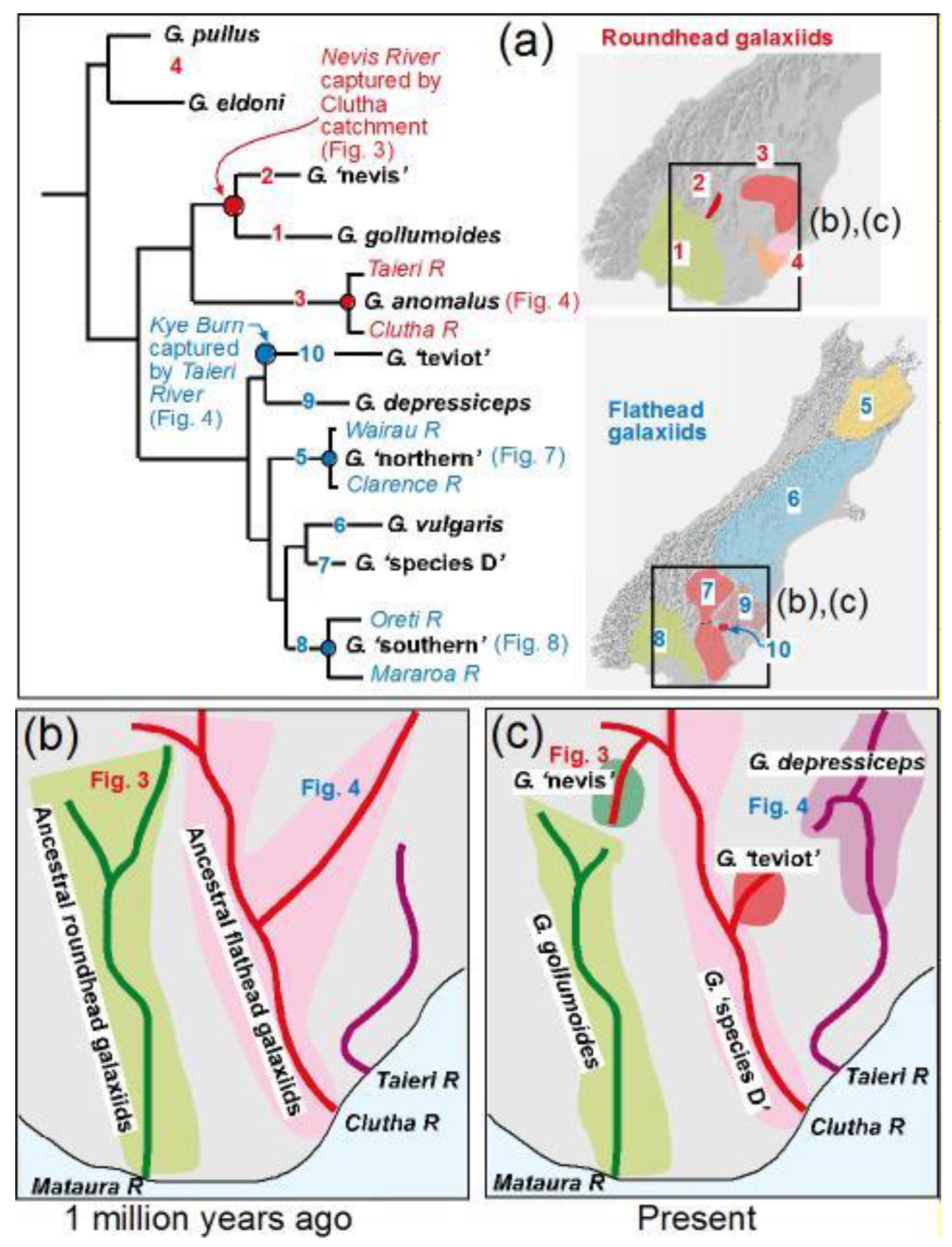
4. River Vicariance Synchronises Population Divergence across Multiple Species
4.1. Isolation of Pelorus–Kaituna
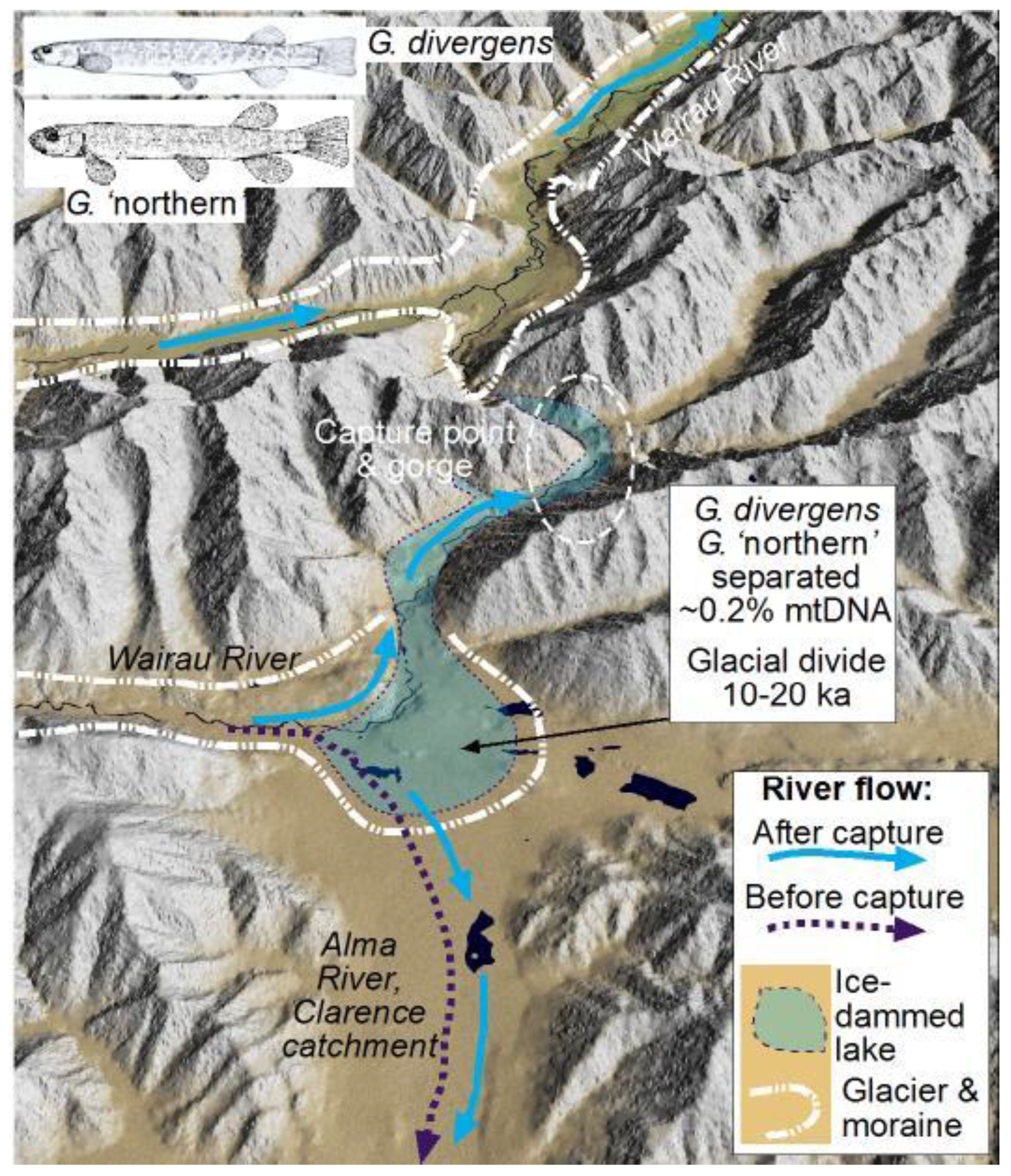
4.2. Capture of Upper Clarence
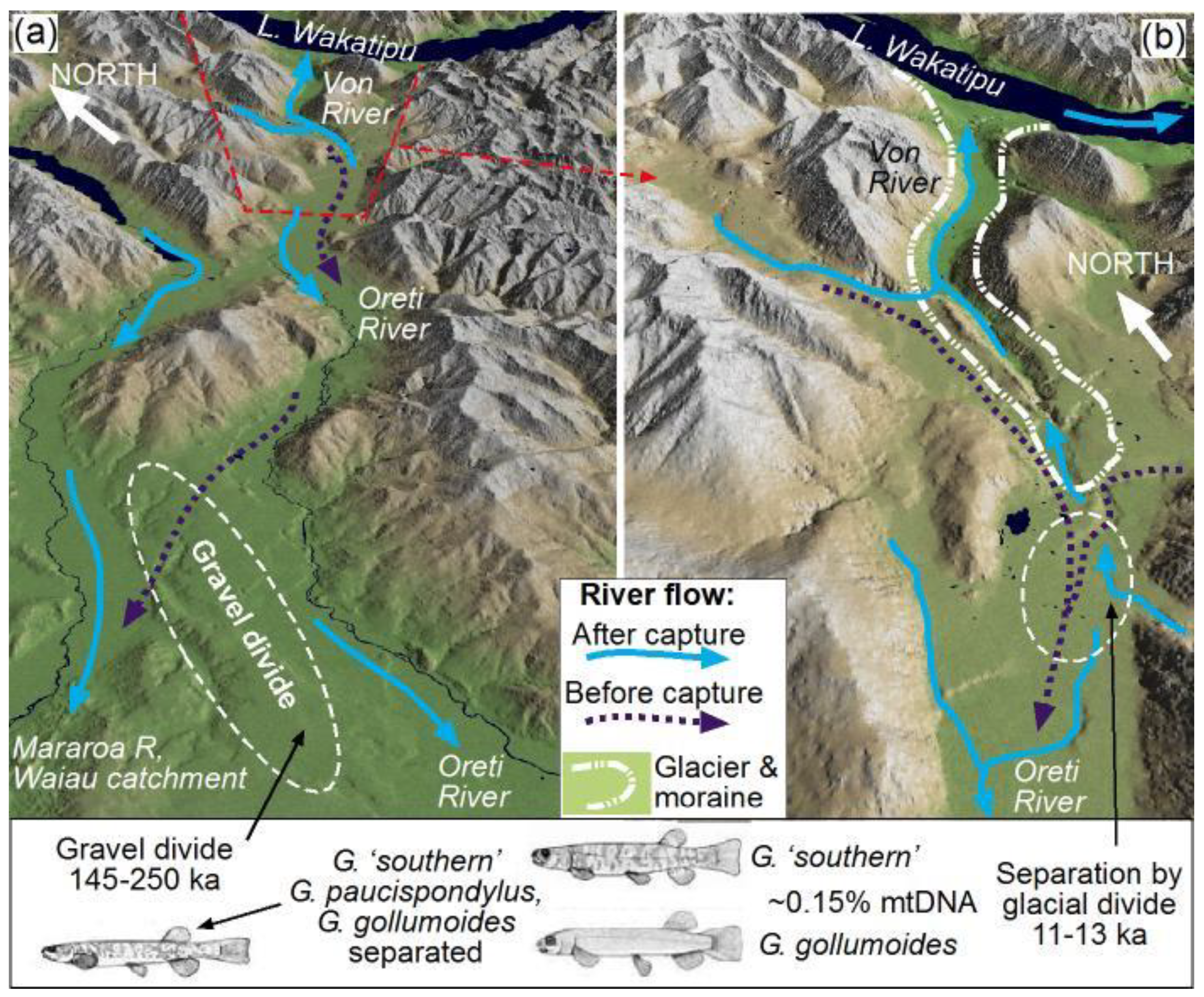
4.3. Isolation of Von-Oreti
5. Discussion and Conclusions
5.1. Persistence of Biological Signatures of River Capture
5.2. Links between Geological and Genetic Change
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hewitt, G.M. The genetic legacy of the Quaternary ice ages. Nature 2000, 405, 907–913. [Google Scholar] [CrossRef]
- Riddle, B.R.; Hafner, D.J.; Alexander, L.F.; Jaeger, J.R. Cryptic vicariance in the historical assembly of a Baja California Peninsular desert biota. Proc. Natl. Acad. Sci. USA 2000, 97, 14438–14443. [Google Scholar] [CrossRef] [PubMed]
- Mayden, R.L. Vicariance biogeography, parsimony, and evolution in North American freshwater fishes. Syst. Zool. 1988, 37, 329–355. [Google Scholar] [CrossRef]
- Knowlton, N.; Weigt, L.A. New dates and new rates for divergence across the Isthmus of Panama. Proc. R. Soc. Lond., Ser. B: Biol. Sci. 1998, 265, 2257. [Google Scholar] [CrossRef]
- Wallis, G.P.; Waters, J.M.; Upton, P.; Craw, D. Transverse alpine speciation driven by glaciation. Trends Ecol. Evol. 2016, 31, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Craw, D.; Craw, L.; Burridge, C.P.; Wallis, G.P.; Waters, J.M. Evolution of the Taieri River catchment, East Otago, New Zealand. N. Z. J. Geol. Geophys. 2016, 59, 257–273. [Google Scholar] [CrossRef]
- Kozak, K.H.; Blaine, R.A.; Larson, A. Gene lineages and eastern North American palaeodrainage basins: Phylogeography and speciation in salamanders of the Eurycea bislineata species complex. Mol. Ecol. 2006, 15, 191–207. [Google Scholar] [CrossRef]
- Zemlak, T.S.; Habit, E.M.; Walde, S.J.; Battini, M.A.; Adams, E.D.M.; Ruzzante, D.E. Across the southern Andes on fin: Glacial refugia, drainage reversals and a secondary contact zone revealed by the phylogeographical signal of Galaxias platei in Patagonia. Mol. Ecol. 2008, 17, 5049–5061. [Google Scholar] [CrossRef]
- Goodier, S.A.M.; Cotterill, F.P.D.; O’Ryan, C.; Skelton, P.H.; de Wit, M.J. Cryptic diversity of African tigerfish (genus Hydrocynus) reveals palaeogeographic signatures of linked Neogene geotectonic events. PLoS ONE 2011, 6, e28775. [Google Scholar] [CrossRef]
- Bloom, D.D.; Weir, J.T.; Piller, K.R.; Lovejoy, N.R. Do freshwater fishes diversify faster than marine fishes? A test using state-dependent diversification analyses and molecular phylogenetics of new world silversides (Atherinopsidae). Evolution 2013, 67, 2040–2057. [Google Scholar] [CrossRef]
- Burridge, C.P.; Craw, D.; Jack, D.C.; King, T.M.; Waters, J.M. Does fish ecology predict dispersal across a river drainage divide? Evolution 2008, 62, 1484–1499. [Google Scholar] [CrossRef] [PubMed]
- Burridge, C.P.; Craw, D.; Waters, J.M. River capture, range expansion, and cladogenesis: The genetic signature of freshwater vicariance. Evolution 2006, 60, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Burridge, C.P.; Craw, D.; Waters, J.M. An empirical test of freshwater vicariance via river capture. Mol. Ecol. 2007, 16, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.M.; Wallis, G.P. Cladogenesis and loss of the marine life-history phase in freshwater galaxiid fishes (Osmeriformes: Galaxiidae). Evolution 2001, 55, 587–597. [Google Scholar] [CrossRef]
- Waters, J.M.; Wallis, G.P.; Burridge, C.P.; Craw, D. Geology shapes biogeography: Quaternary river-capture explains New Zealand’s biologically ‘composite’ Taieri River. Quat. Sci. Rev. 2015, 120, 47–56. [Google Scholar] [CrossRef]
- Waters, J.M.; Rowe, D.L.; Apte, S.; King, T.M.; Wallis, G.P.; Anderson, L.; Norris, R.J.; Craw, D.; Burridge, C.P. Geological dates and molecular rates: Rapid divergence of rivers and their biotas. Syst. Biol. 2007, 56, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Craw, D.; Burridge, C.; Anderson, L.; Waters, J.M. Late Quaternary river drainage and fish evolution, Southland, New Zealand. Geomorphology 2007, 84, 98–110. [Google Scholar] [CrossRef]
- Craw, D.; Burridge, C.; Norris, R.; Waters, J. Genetic ages for Quaternary topographic evolution: A new dating tool. Geology 2008, 36, 19–22. [Google Scholar] [CrossRef]
- Hurwood, D.A.; Hughes, J.M. Phylogeography of the freshwater fish, Mogurnda adspersa, in streams of northeastern Queensland, Australia: Evidence for altered drainage patterns. Mol. Ecol. 1998, 7, 1507–1517. [Google Scholar] [CrossRef]
- Waters, J.M.; Burridge, C.P.; Craw, D. The lasting biological signature of Pliocene tectonics: Reviewing the re-routing of Australia’s largest river drainage system. J. Biogeogr. 2019, 46, 1494–1503. [Google Scholar] [CrossRef]
- Waters, J.M.; Wallis, G.P. Mitochondrial DNA phylogenetics of the Galaxias vulgaris complex from South Island, New Zealand: Rapid radiation of a species flock. J. Fish. Biol. 2001, 58, 1166–1180. [Google Scholar] [CrossRef]
- Adams, M.; Raadik, T.A.; Burridge, C.P.; Georges, A. Global biodiversity assessment and hyper-cryptic species complexes: More than one species of elephant in the room? Syst. Biol. 2014, 63, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Raadik, T.A. Fifteen from one: A revision of the Galaxias olidus Günther, 1866 complex (Teleostei, Galaxiidae) in south-eastern Australia recognises three previously described taxa and describes 12 new species. Zootaxa 2014, 3898, 1–198. [Google Scholar] [CrossRef] [PubMed]
- Allibone, R.M.; Wallis, G.P. Genetic variation and diadromy in some native New Zealand galaxiids (Teleostei, Galaxiidae). Biol. J. Linn. Soc. 1993, 50, 19–33. [Google Scholar] [CrossRef]
- Allibone, R.M.; Crowl, T.A.; Holmes, J.M.; King, T.M.; McDowall, R.M.; Townsend, C.R.; Wallis, G.P. Isozyme analysis of Galaxias species (Teleostei: Galaxiidae) from the Taieri River, South Island, New Zealand: A species complex revealed. Biol. J. Linn. Soc. 2008, 57, 107–127. [Google Scholar] [CrossRef][Green Version]
- McDowall, R.M.; Wallis, G.P. Description and redescription of Galaxias species (Teleostei: Galaxiidae) from Otago and Southland. J. R. Soc. N. Z. 1996, 26, 401–427. [Google Scholar] [CrossRef]
- McDowall, R.M. Two further new species of Galaxias (Teleostei: Galaxiidae) from the Taieri River, southern New Zealand. J. R. Soc. N. Z. 1997, 27, 199–217. [Google Scholar] [CrossRef]
- McDowall, R.M.; Chadderton, W.L. Galaxias gollumoides (Teleostei : Galaxiidae), a new fish species from Stewart Island, with notes on other non-migratory freshwater fishes present on the island. J. R. Soc. N. Z. 1999, 29, 77–88. [Google Scholar] [CrossRef]
- Waters, J.M.; Rowe, D.L.; Burridge, C.P.; Wallis, G.P. Gene trees versus species trees: Reassessing life-history evolution in a freshwater fish radiation. Syst. Biol. 2010, 59, 504–517. [Google Scholar] [CrossRef]
- McDowall, R.M. New Zealand Freshwater Fishes: A Natural History and Guide; MAF Publishing Group: Auckland, New Zealand, 1990. [Google Scholar]
- Waters, J.M.; Craw, D.; Youngson, J.H.; Wallis, G.P. Genes meet geology: Fish phylogeographic pattern reflects ancient, rather than modern, drainage connections. Evolution 2001, 55, 1844–1851. [Google Scholar] [CrossRef]
- Craw, D.; Upton, P.; Burridge, C.P.; Wallis, G.P.; Waters, J.M. Rapid biological speciation driven by tectonic evolution in New Zealand. Nat. Geosci. 2016, 9, 140–144. [Google Scholar] [CrossRef]
- Craw, D.; King, T.M.; McCulloch, G.A.; Upton, P.; Waters, J.M. Biological evidence constraining river drainage evolution across a subduction-transcurrent plate boundary transition, New Zealand. Geomorphology 2019, 336, 119–132. [Google Scholar] [CrossRef]
- Waters, J.M.; Craw, D.; Burridge, C.P.; Kennedy, M.; King, T.M.; Wallis, G.P. Within-river genetic connectivity patterns reflect contrasting geomorphology. J. Biogeogr. 2015, 42, 2452–2460. [Google Scholar] [CrossRef]
- Dunn, N.; Allibone, R.; Closs, G.; Crow, S.; David, B.; Goodman, J.; Griffiths, M.; Jack, D.; Ling, N.; Waters, J. , et al. Conservation Status of New Zealand Freshwater Fishes, 2017; Department of Conservation: Wellington, New Zealand, 2018. [Google Scholar]
- Allibone, R.; David, B.; Hitchmough, R.; Jellyman, D.; Ling, N.; Ravenscroft, P.; Waters, J. Conservation status of New Zealand freshwater fish, 2009. N. Z. J. Mar. Freshwat. Res. 2010, 44, 271–287. [Google Scholar] [CrossRef]
- Carrea, C.; Anderson, L.V.; Craw, D.; Waters, J.M.; Burridge, C.P. The significance of past interdrainage connectivity for studies of diversity, distribution and movement of freshwater-limited taxa within a catchment. J. Biogeogr. 2014, 41, 536–547. [Google Scholar] [CrossRef]
- McLaren, S.; Wallace, M.W.; Gallagher, S.J.; Miranda, J.A.; Holdgate, G.R.; Gow, L.J.; Snowball, I.; Sandgren, P. Palaeogeographic, climatic and tectonic change in southeastern Australia: The Late Neogene evolution of the Murray Basin. Quat. Sci. Rev. 2011, 30, 1086–1111. [Google Scholar] [CrossRef]
- McCulloch, G.A.; Wallis, G.P.; Waters, J.M. Onset of glaciation drove simultaneous vicariant isolation of alpine insects in New Zealand. Evolution 2010, 64, 2033–2043. [Google Scholar] [CrossRef]
- Kreger, K.M.; Shaban, B.; Wapstra, E.; Burridge, C.P. Phylogeographic parallelism: Concordant patterns in closely related species illuminate underlying mechanisms in the historically glaciated Tasmanian landscape. J. Biogeogr. 2020, in press. [Google Scholar] [CrossRef]
- Craw, D.; Waters, J. Geological and biological evidence for regional drainage reversal during lateral tectonic transport, Marlborough, New Zealand. J. Geol. Soc. 2007, 164, 785–793. [Google Scholar] [CrossRef]
- Knowles, L.L.; Maddison, W.P. Statistical phylogeography. Mol. Ecol. 2002, 11, 2623–2635. [Google Scholar] [CrossRef]
- Townsend, C.R. Invasion biology and ecological impacts of brown trout Salmo trutta in New Zealand. Biol. Conserv. 1996, 78, 13–22. [Google Scholar] [CrossRef]
- Waters, J.M. Competitive exclusion: phylogeography’s ‘elephant in the room’? Mol. Ecol. 2011, 20, 4388–4394. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.M.; Fraser, C.I.; Hewitt, G.M. Founder takes all: Density-dependent processes structure biodiversity. Trends Ecol. Evol. 2013, 28, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Burridge, C.P.; Craw, D.; Fletcher, D.; Waters, J.M. Geological dates and molecular rates: Fish DNA sheds light on time dependency. Mol. Biol. Evol. 2008, 25, 624–633. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waters, J.M.; Burridge, C.P.; Craw, D. River Capture and Freshwater Biological Evolution: A Review of Galaxiid Fish Vicariance. Diversity 2020, 12, 216. https://doi.org/10.3390/d12060216
Waters JM, Burridge CP, Craw D. River Capture and Freshwater Biological Evolution: A Review of Galaxiid Fish Vicariance. Diversity. 2020; 12(6):216. https://doi.org/10.3390/d12060216
Chicago/Turabian StyleWaters, Jonathan M., Christopher P. Burridge, and Dave Craw. 2020. "River Capture and Freshwater Biological Evolution: A Review of Galaxiid Fish Vicariance" Diversity 12, no. 6: 216. https://doi.org/10.3390/d12060216
APA StyleWaters, J. M., Burridge, C. P., & Craw, D. (2020). River Capture and Freshwater Biological Evolution: A Review of Galaxiid Fish Vicariance. Diversity, 12(6), 216. https://doi.org/10.3390/d12060216