Plant-Soil Feedback Effects on Germination and Growth of Native and Non-Native Species Common across Southern California
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Collection
2.2. Experimental Design
2.3. Analyses
3. Results
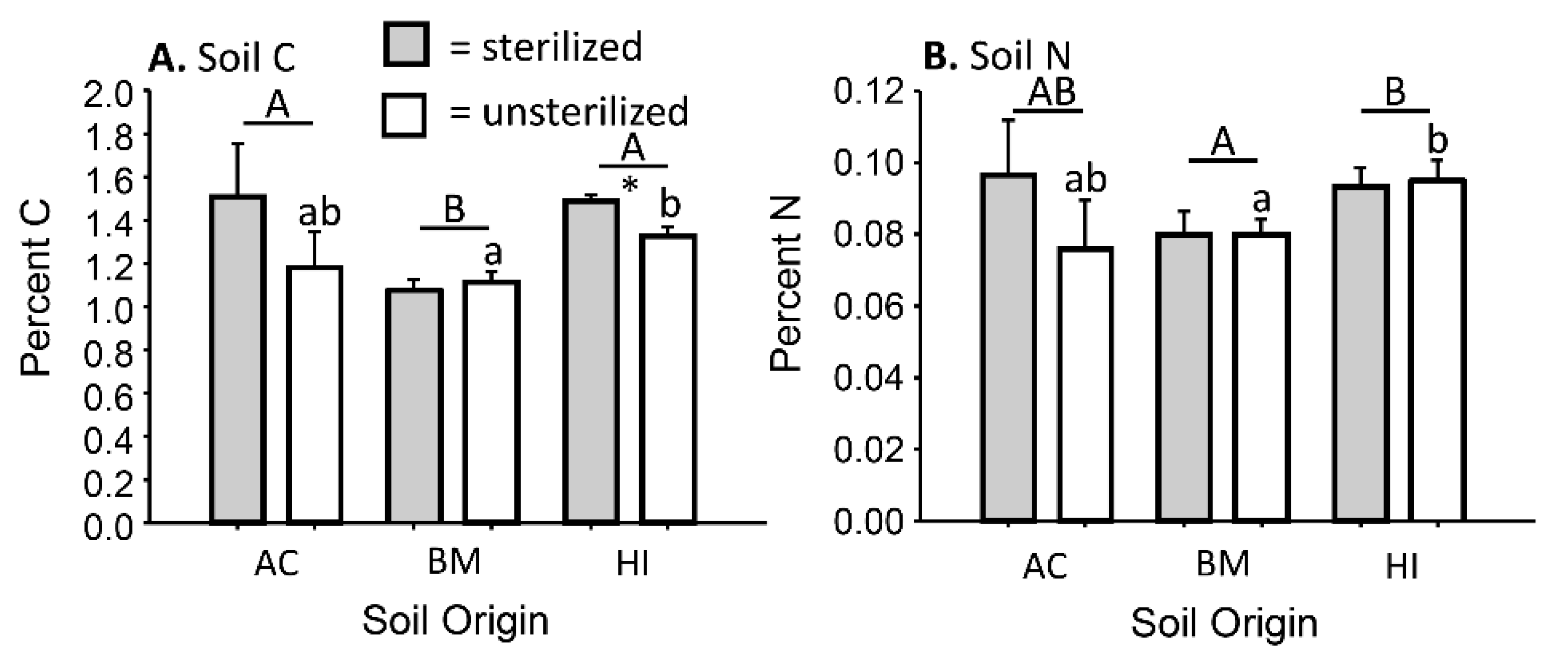
3.1. Impacts of Sterilization on Biotic and Abiotic Soil Conditions
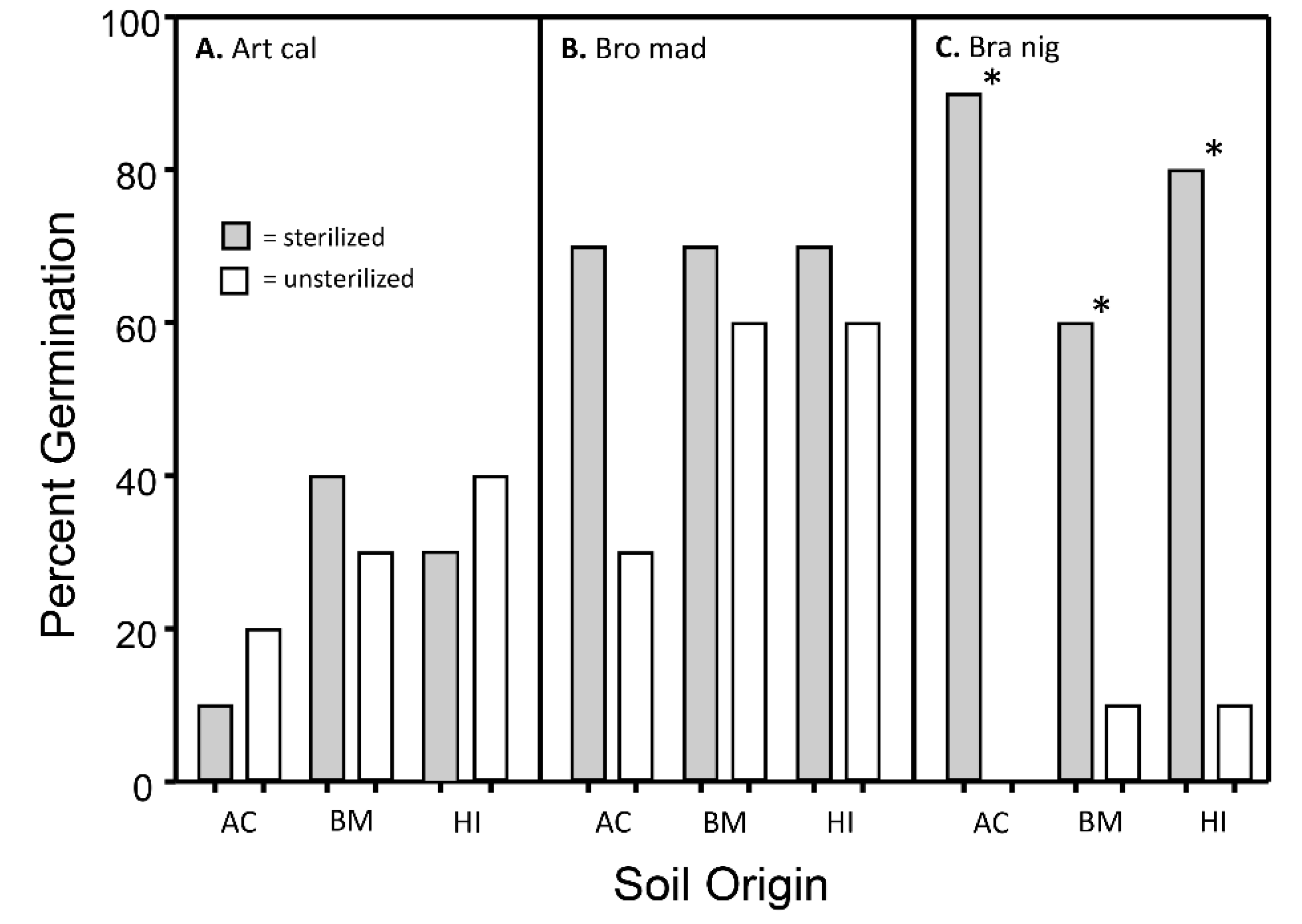
3.2. Influence of Soil Treatment on Germination Rates
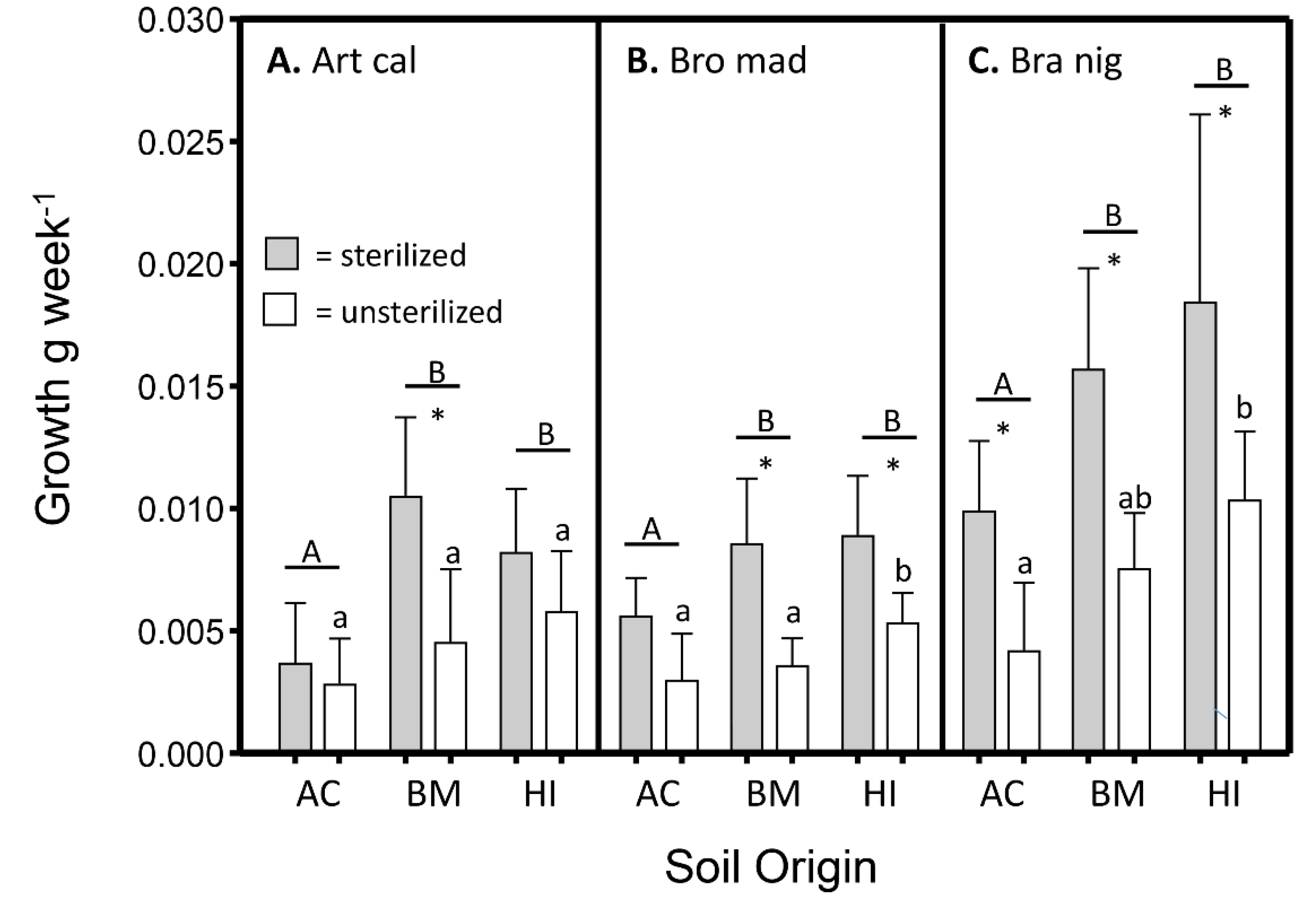
3.3. Differences in Growth among Plant Species, Soil Origins, and Soil Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Caspi, T.; Estrada, L.; Dowling, A.V.; Su, E.; Leshchinskiy, M.; Cavalcanti, A.R.; Crane, E.J.; Robins, C.R.; Meyer, W.M. Carbon and nitrogen in the topsoils of Inceptisols and Mollisols under native sage scrub and non-native grasslands in southern California. Geoderma Reg. 2018, 14, 00172. [Google Scholar] [CrossRef]
- Caspi, T.; Hartz, L.A.; Villa, A.E.S.; Loesberg, J.A.; Robins, C.; Meyer, W.M. Impacts of invasive annuals on soil carbon and nitrogen storage in southern California depend on the identity of the invader. Ecol. Evol. 2019, 9, 4980–4993. [Google Scholar] [CrossRef] [PubMed]
- Pickett, B.; Irvine, I.C.; Bullock, E.; Arogyaswamy, K.; Aronson, E. Legacy effects of invasive grass impact soil microbes and native shrub growth. Invasive Plant Sci. Manag. 2019, 12, 22–35. [Google Scholar] [CrossRef]
- Wheeler, M.M.; Dipman, M.; Adams, T.A.; Ruina, A.V.; Robins, C.R.; Meyer, W.M. Carbon and nitrogen storage in California sage scrub and non-native grassland habitats. J. Arid. Environ. 2016, 129, 119–125. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Wardle, D.A. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change; Oxford University Press: New York, NY, USA, 2010; p. 301. [Google Scholar]
- Miller, E.C.; Perron, G.G.; Collins, C.D. Plant-driven changes in soil microbial communities influence seed germination through negative feedbacks. Ecol. Evol. 2019, 9, 9298–9311. [Google Scholar] [CrossRef] [PubMed]
- Yelenik, S.; Levine, J.M. The role of plant-soil feedbacks in driving native-species recovery. Ecology 2011, 92, 66–74. [Google Scholar] [CrossRef]
- Chambers, J.C.; Miller, R.F.; Board, D.I.; Pyke, D.; Roundy, B.A.; Grace, J.B.; Schupp, E.W.; Tausch, R.J. Resilience and Resistance of Sagebrush Ecosystems: Implications for State and Transition Models and Management Treatments. Rangel. Ecol. Manag. 2014, 67, 440–454. [Google Scholar] [CrossRef]
- Connolly, B.M.; Carris, L.M.; Mack, R.N. Soil-borne seed pathogens: Contributors to the naturalization gauntlet in Pacific Northwest (USA) forest and steppe communities? Vegetation 2018, 219, 359–368. [Google Scholar] [CrossRef]
- D’Antonio, C.M.; Thomsen, M. Ecological Resistance in Theory and Practice1. Weed Technol. 2004, 18, 1572–1577. [Google Scholar] [CrossRef]
- Han, X.; Dendy, S.P.; Garrett, K.A.; Fang, L.; Smith, M.D. Comparison of damage to native and exotic tallgrass prairie plants by natural enemies. Vegetation 2008, 198, 197–210. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.V.; Wren, I.F.; Herman, D.J.; Firestone, M.K. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 2005, 8, 976–985. [Google Scholar] [CrossRef]
- Suding, K.; Gross, K.L.; Houseman, G.R. Alternative states and positive feedbacks in restoration ecology. Trends Ecol. Evol. 2004, 19, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Pickett, B.; Maltz, M.; Aronson, E. Impacts of Invasive Plants on Soil Fungi and Implications for Restoration. In Diversity and Ecology of Invasive Plants; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Allen, T.A.Z.R.F. The Effects of Organic Amendments on the Restoration of a Disturbed Coastal Sage Scrub Habitat. Restor. Ecol. 1998, 6, 52–58. [Google Scholar] [CrossRef]
- Cox, R.D.; Allen, E. Stability of exotic annual grasses following restoration efforts in southern California coastal sage scrub. J. Appl. Ecol. 2008, 45, 495–504. [Google Scholar] [CrossRef]
- Rundel, P.W. Sage Scrub. In Terrestrial Vegetation of California; Barbour, M.G., Keeler-Wolf, T., Schoenherr, A., Eds.; University of California Press: Berkeley, CA, USA, 2007; pp. 208–228. [Google Scholar]
- Talluto, M.V.; Suding, K. Historical change in coastal sage scrub in southern California, USA in relation to fire frequency and air pollution. Landsc. Ecol. 2008, 23, 803–815. [Google Scholar] [CrossRef]
- Lambert, A.M.; D’Antonio, C.M.; Dudley, T.L. Invasive species and fire in California ecosystems. Fremontia 2010, 38, 29–36. [Google Scholar]
- Minnich, R.A.; Dezzani, R.J. Historical decline of coastal sage scrub in the Riverside-Perris Plain, California. West. Birds 1998, 29, 366–391. [Google Scholar]
- Valliere, J.M.; Bucciarelli, G.M.; Bytnerowicz, A.; Fenn, M.E.; Irvine, I.C.; Johnson, R.F.; Allen, E.B. Declines in native forb richness of an imperiled plant community across an anthropogenic nitrogen deposition gradient. Ecosphere 2020, 11, e03032. [Google Scholar] [CrossRef]
- Kimball, S.; Goulden, M.; Suding, K.; Parker, S. Altered water and nitrogen input shifts succession in a southern California coastal sage community. Ecol. Appl. 2014, 24, 1390–1404. [Google Scholar] [CrossRef]
- D’Antonio, C. Biological Invasions by Exotic Grasses, the Grass Fire Cycle, and Global Change. Annu. Rev. Ecol. Syst. 1992, 23, 63–87. [Google Scholar] [CrossRef]
- Keeley, J.; Brennan, T.J. Fire-driven alien invasion in a fire-adapted ecosystem. Oecologia 2012, 169, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Bozzolo, F.H.; Lipson, D.A. Differential responses of native and exotic coastal sage scrub plant species to N additions and the soil microbial community. Plant Soil 2013, 371, 37–51. [Google Scholar] [CrossRef]
- Valliere, J.M.; Allen, E.B. Nitrogen enrichment contributes to positive responses to soil microbial communities in three invasive plant species. Biol. Invasions 2016, 18, 2349–2364. [Google Scholar] [CrossRef]
- Kulmatiski, A. Factorial and ‘self vs. other’ plant soil feedback experiments produce similar predictions of plant growth in communities. Plant Soil 2016, 408, 485–492. [Google Scholar] [CrossRef]
- Fortune, P.M.; Pourtau, N.; Viron, N.; Ainouche, M.L. Molecular phylogeny and reticulate origins of the polyploid Bromusspecies from section Genea(Poaceae). Am. J. Bot. 2008, 95, 454–464. [Google Scholar] [CrossRef]
- Spear, D.; Adams, T.A.; Boyd, E.S.; Dipman, M.; Staubus, W.J.; Meyer, W.M. The effects of development, vegetation-type conversion, and fire on low-elevation Southern California spider assemblages. Invertebr. Biol. 2017, 10, 1230. [Google Scholar] [CrossRef]
- Fenn, M.; Haeuber, R.; Tonnesen, G.; Baron, J.S.; Grossman-Clarke, S.; Hope, D.; Jaffe, D.A.; Copeland, S.; Geiser, L.; Rueth, H.M.; et al. Nitrogen Emissions, Deposition, and Monitoring in the Western United States. Bioscience 2003, 53, 391. [Google Scholar] [CrossRef]
- Sigüenza, C.; Crowley, D.; Allen, E.B. Soil microorganisms of a native shrub and exotic grasses along a nitrogen deposition gradient in southern California. Appl. Soil Ecol. 2006, 32, 13–26. [Google Scholar] [CrossRef]
- Schnürer, J.; Rosswall, T. Fluorescein Diacetate Hydrolysis as a Measure of Total Microbial Activity in Soil and Litter. Appl. Environ. Microbiol. 1982, 43, 1256–1261. [Google Scholar] [CrossRef]
- Marushia, R.G.; Brooks, M.L.; Holt, J.S. Phenology, Growth, and Fecundity as Determinants of Distribution in Closely Related Nonnative Taxa. Invasive Plant Sci. Manag. 2012, 5, 217–229. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Castro, S.P.; Cleland, E.E.; Wagner, R.; Al Sawad, R.; Lipson, D.A. Soil microbial responses to drought and exotic plants shift carbon metabolism. ISME J. 2019, 13, 1776–1787. [Google Scholar] [CrossRef] [PubMed]
- Dickens, S.J.M.; Allen, E.B.; Santiago, L.S.; Crowley, D. Extractable nitrogen and microbial community structure respond to grassland restoration regardless of historical context and soil composition. AoB Plants 2015, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Pakpour, S.; Klironomos, J. The invasive plant, Brassica nigra, degrades local mycorrhizas across a wide geographical landscape. R. Soc. Open Sci. 2015, 2, 150300. [Google Scholar] [CrossRef][Green Version]
- Schreiner, R.P.; Koide, R.T. Mustards, mustard oils and mycorrhizas. New Phytol. 2006, 123, 107–113. [Google Scholar] [CrossRef]
- Sigüenza, C.; Corkidi, L.; Allen, E. Feedbacks of Soil Inoculum of Mycorrhizal Fungi Altered by N Deposition on the Growth of a Native Shrub and an Invasive Annual Grass. Plant Soil 2006, 286, 153–165. [Google Scholar] [CrossRef]
- Staubus, W.J.; Boyd, E.S.; Adams, T.A.; Spear, D.; Dipman, M.; Meyer, W.M. Ant communities in native sage scrub, non-native grassland, and suburban habitats in Los Angeles County, USA: Conservation implications. J. Insect Conserv. 2015, 19, 669–680. [Google Scholar] [CrossRef]
- Dipman, M.M.; Meyer, W.M. Type conversion from native California sage scrub to non-native grassland accelerates decomposition processes. Appl. Soil Ecol. 2019, 144, 68–71. [Google Scholar] [CrossRef]
- Bennett, J.A.; Klironomos, J. Mechanisms of plant–soil feedback: Interactions among biotic and abiotic drivers. New Phytol. 2018, 222, 91–96. [Google Scholar] [CrossRef]
- Haynes, R. Nitrification. In Mineral Nitrogen in the Plant-Soil System; Haynes, R., Ed.; Academic Press: New York, NY, USA, 1996; pp. 127–165. [Google Scholar]
- Sahrawat, K.L. Nitrification in some tropical soils. Plant Soil 1982, 65, 281–286. [Google Scholar] [CrossRef]
- Dickens, S.J.M.; Allen, E.B. Soil nitrogen cycling is resilient to invasive annuals following restoration of coastal sage scrub. J. Arid Environ. 2014, 110, 12–18. [Google Scholar] [CrossRef]
- De Boer, W.; Hundscheid, M.P.J.; Schotman, J.M.T.; Troelstra, S.R.; Laanbroek, H.J.; Boer, W. In situ net N transformations in pine, fir, and oak stands of different ages on acid sandy soil, 3 years after liming. Biol. Fertil. Soils 1993, 15, 120–126. [Google Scholar] [CrossRef]
- Sperry, L.J.; Belnap, J.; Evans, R.D. Bromus Tectorum Invasion Alters Nitrogen Dynamics in an Undisturbed Arid Grassland Ecosystem. Ecology 2006, 87, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Halsey, R.W. In Search of Allelopathy: An Eco-Historical View of the Investigation of Chemical Inhibition in California Coastal Sage Scrub and Chamise Chaparral. J. Torrey Bot. Soc. 2004, 131, 343. [Google Scholar] [CrossRef]
- Halligan, J.P. Toxic Terpenes from Artemisia Californica. Ecology 1975, 56, 999–1003. [Google Scholar] [CrossRef]
- Litle, J.; Quon, L.H.; Antill, M.L.; Questad, E.J.; Meyer, W.M. Vertebrate herbivory on shrub seedlings in California sage scrub: Important but understudied interactions. Vegetation 2019, 220, 523–528. [Google Scholar] [CrossRef]
| Active or Total Fungi Soil Condition | Soil Origin | ||
|---|---|---|---|
| A. californica | B. madritensis | H. incana | |
| Active Fungi | |||
| Unsterilized | 7.83 | 2.94 | 3.91 |
| Sterilized | 0 | 0 | 0 |
| Total Fungi | |||
| Unsterilized | 240.71 | 406.21 | 228.68 |
| Sterilized | 114.31 | 122.13 | 121.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Meyer, W.M., III. Plant-Soil Feedback Effects on Germination and Growth of Native and Non-Native Species Common across Southern California. Diversity 2020, 12, 217. https://doi.org/10.3390/d12060217
Singh M, Meyer WM III. Plant-Soil Feedback Effects on Germination and Growth of Native and Non-Native Species Common across Southern California. Diversity. 2020; 12(6):217. https://doi.org/10.3390/d12060217
Chicago/Turabian StyleSingh, Manya, and Wallace M. Meyer, III. 2020. "Plant-Soil Feedback Effects on Germination and Growth of Native and Non-Native Species Common across Southern California" Diversity 12, no. 6: 217. https://doi.org/10.3390/d12060217
APA StyleSingh, M., & Meyer, W. M., III. (2020). Plant-Soil Feedback Effects on Germination and Growth of Native and Non-Native Species Common across Southern California. Diversity, 12(6), 217. https://doi.org/10.3390/d12060217





