Differences in the Olfactory Sensitivity of Ceratitis capitata to Headspace of Some Host Plants in Relation to Sex, Mating Condition and Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
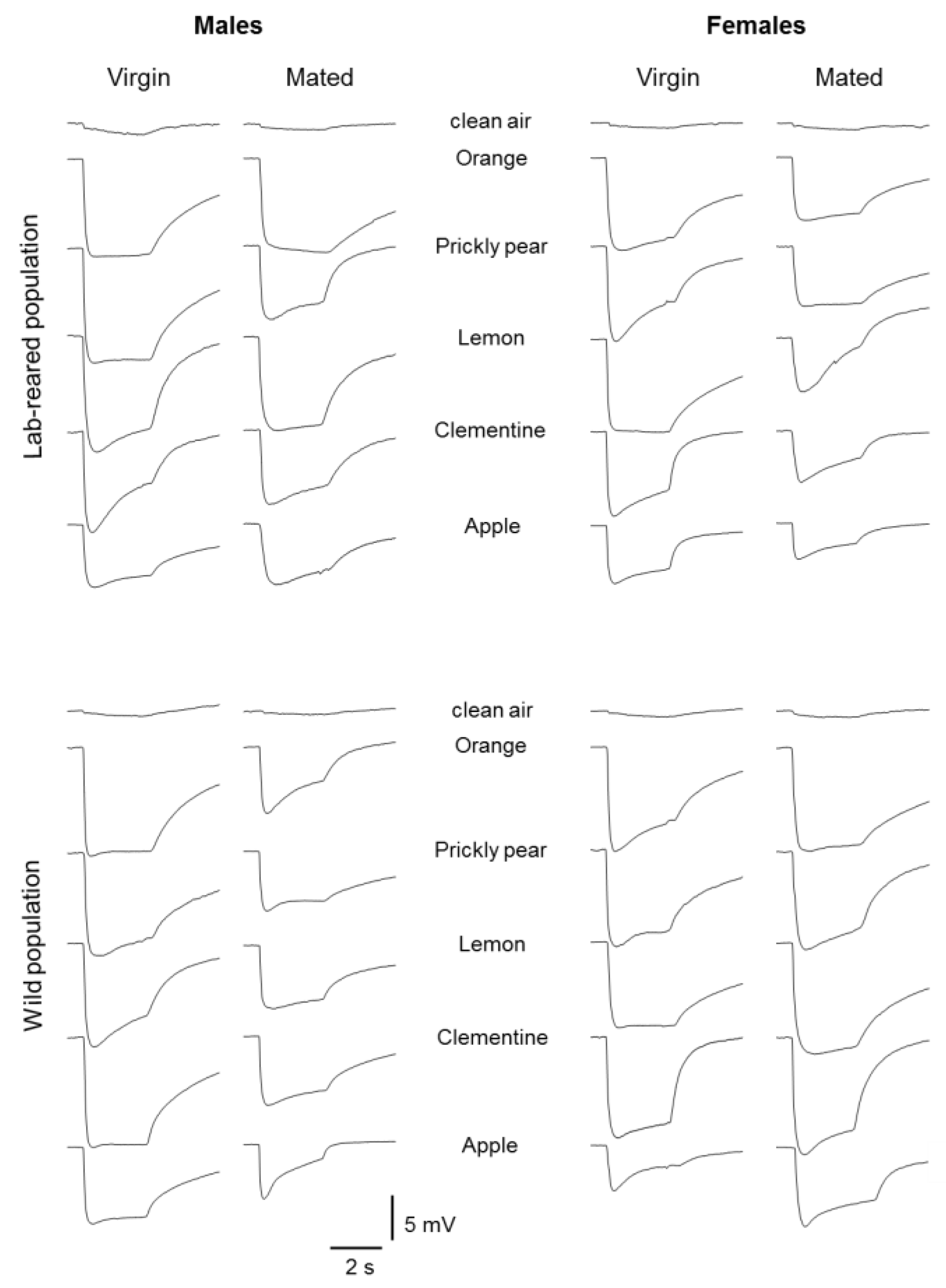
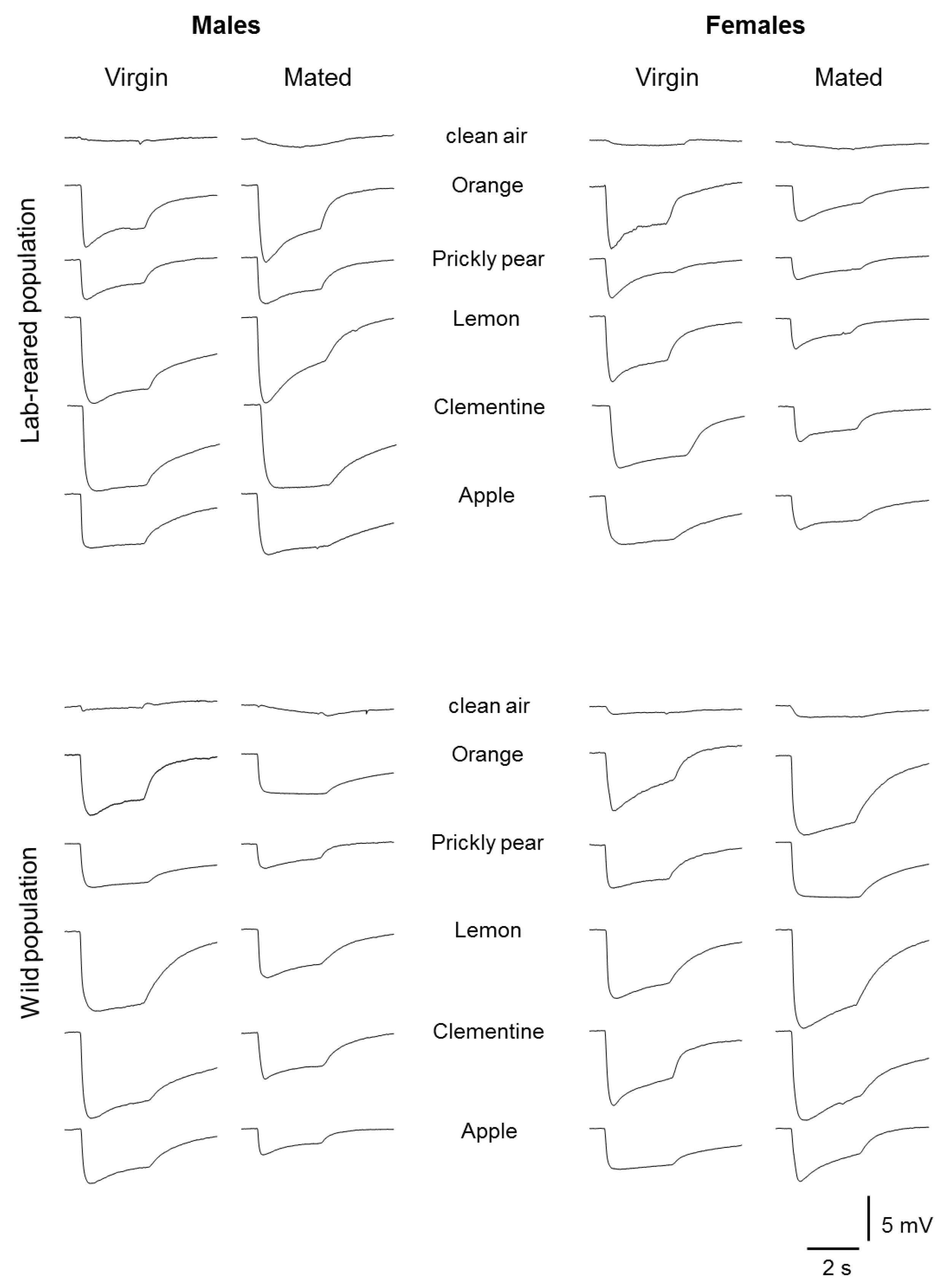
2.2. Electrophysiology
2.3. Stimuli
2.4. Statistical Analysis
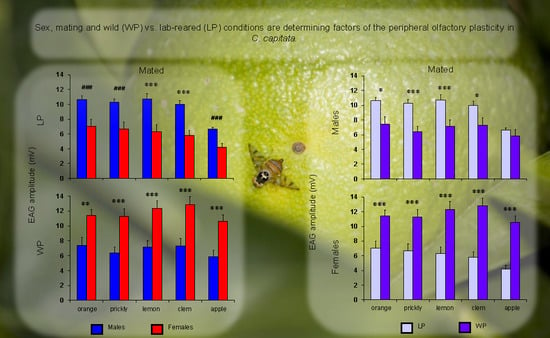
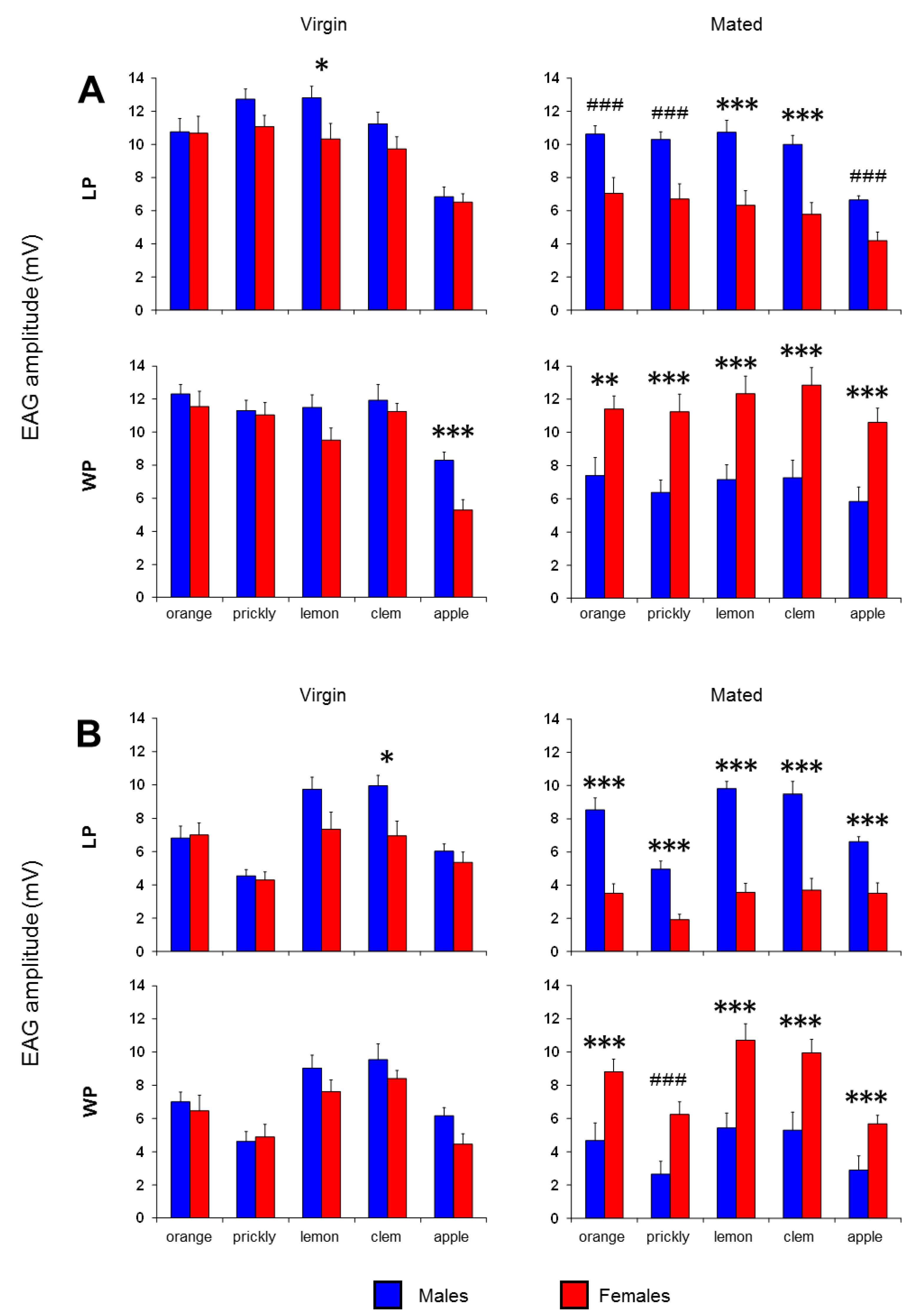
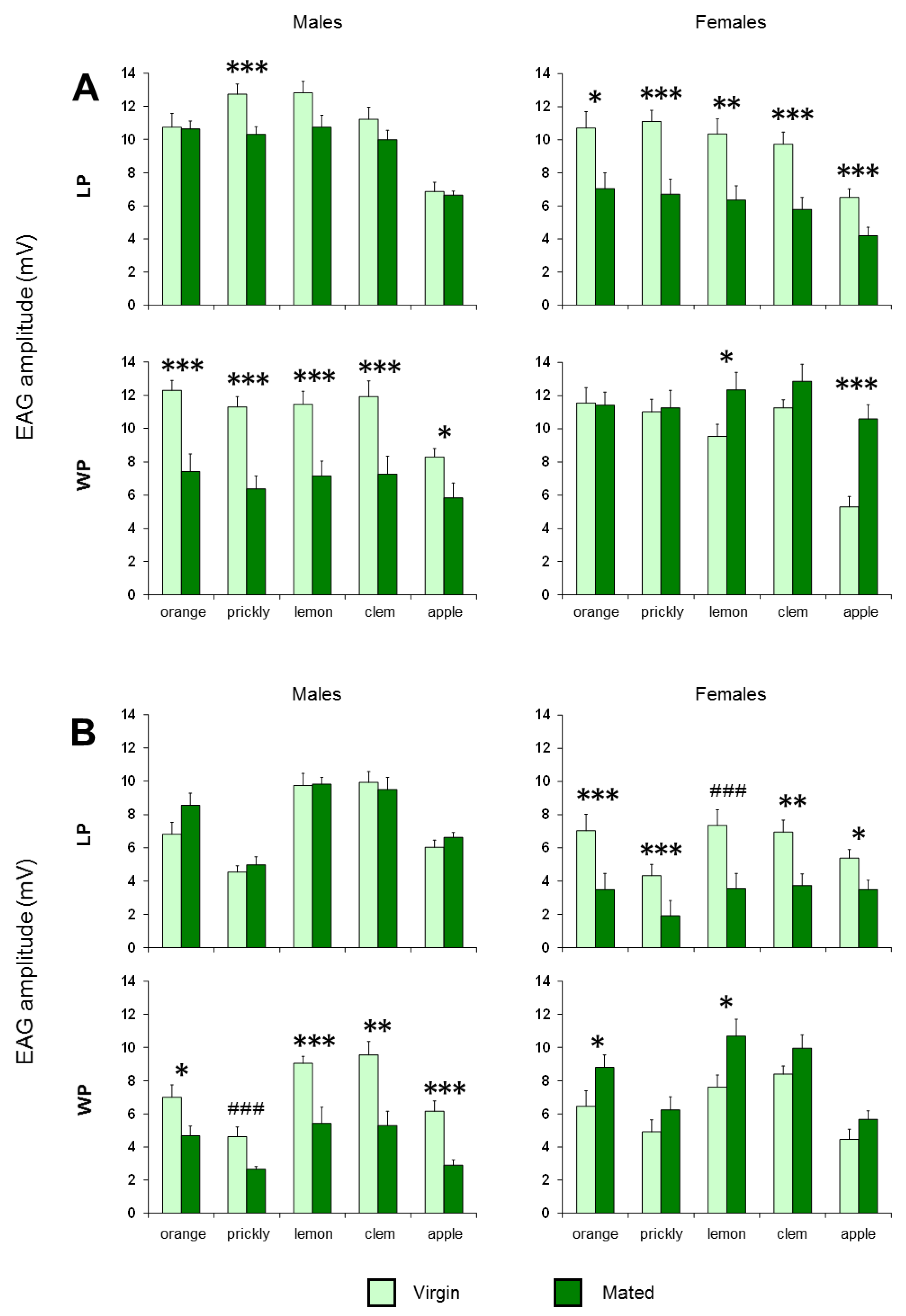
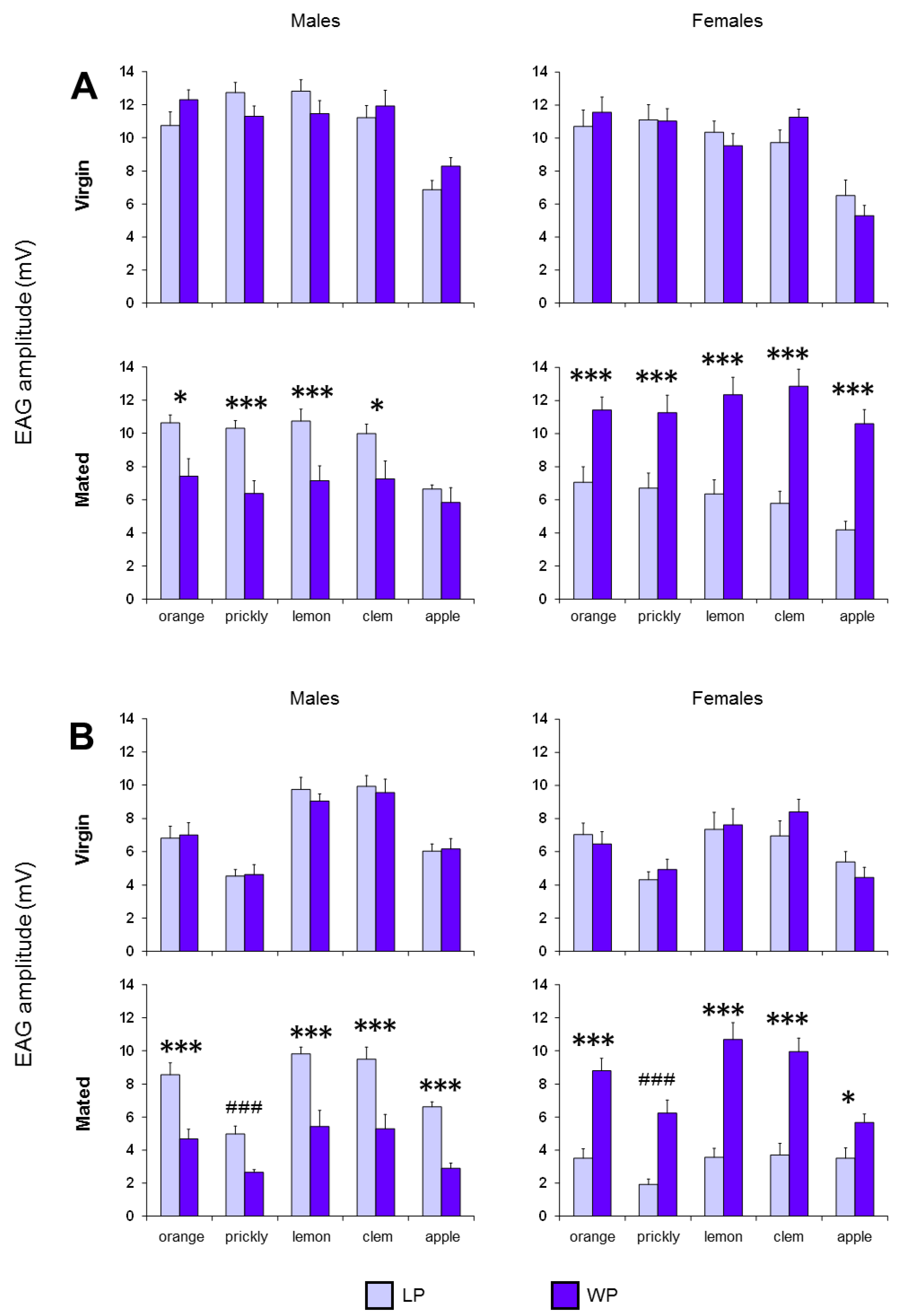
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dethier, V.G. The Hungry Fly; Harvard University Press: Cambridge, MA, USA, 1976. [Google Scholar]
- Merivee, E.; Ploomi, A.; Rahi, M.; Bresciani, J.; Ravn, H.P.; Luik, A.; Sammelselg, V. Antennal sensilla of the ground beetle Bembidion properans Steph. (Coleoptera, Carabidae). Micron 2002, 33, 429–440. [Google Scholar] [CrossRef]
- Masala, C.; Solari, P.; Sollai, G.; Crnjar, R.; Liscia, A. Clonidine effects on protein and carbohydrate electrophysiological responses of labellar and tarsal sensilla in Phormia regina. J. Insect Physiol. 2008, 54, 1193–1199. [Google Scholar] [CrossRef]
- Masala, C.; Solari, P.; Sollai, G.; Crnjar, R.; Liscia, A. Transduction mechanism(s) of Na-saccharin in the blowfly Protophormia terraenovae: Evidence for potassium and calcium conductance involvement. J. Comp. Physiol. A 2009, 195, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J.P. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Solari, P.; Masala, C.; Falchi, A.M.; Sollai, G.; Liscia, A. The sense of water in the blowfly Protophormia terraenovae. J. Insect Physiol. 2010, 56, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Faucher, C.P.; Anderson, A.; Berna, A.Z.; Trowell, S.; Chen, Q.M.; Xia, Q.Y.; Chyb, S. Comparisons of contact chemoreception and food acceptance by larvae of polyphagous Helicoverpa armigera and oligophagous Bombyx mori. J. Chem. Ecol. 2013, 39, 1070–1080. [Google Scholar] [CrossRef]
- Walker, W.B., III; Jacquin-Joly, E.; Hill, S.R. Functional characterization of insect chemoreceptors: Receptivity range, expression and evolution. Front. Ecol. Evol. 2016, 4, 37. [Google Scholar] [CrossRef]
- Biolchini, M.; Murru, E.; Anfora, G.; Loy, F.; Banni, S.; Crnjar, R.; Sollai, G. Fat storage in Drosophila suzukii is influenced by different dietary sugars in relation to their palatability. PLoS ONE 2017, 12, e0183173. [Google Scholar] [CrossRef]
- Lebreton, S.; Borrero-Echeverry, F.; Gonzalez, F.; Solum, M.; Wallin, E.; Hedenström, E.; Hansson, B.S.; Gustavsson, A.; Bengtsson, M.; Birgersson, G.; et al. A Drosophila female pheromone elicits species-specific long-range attraction via an olfactory channel with dual specificity for sex and food. BMC Biol. 2017, 15, 88. [Google Scholar] [CrossRef]
- Sollai, G.; Biolchini, M.; Loy, F.; Solari, P.; Crnjar, R. Taste input from tarsal sensilla is related to egg-laying behavior in Papilio hospiton. Entomol. Exp. Appl. 2017, 165, 38–49. [Google Scholar] [CrossRef]
- Sollai, G.; Biolchini, M.; Crnjar, R. Taste sensitivity and divergence in host plant acceptance between adult female and larvae of Papilio hospiton. Insect Sci. 2018, 25, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Corda, G.; Solari, P.; Dettori, M.A.; Fabbri, D.; Delogu, G.; Crnjar, R.; Sollai, G. Association between olfactory sensitivity and behavioral responses of Drosophila suzukii to naturally occurring volatile compounds. Arch. Ins. Biochem. Physiol. 2020, e2169. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Hepper, P.G. Directional tracking in the domestic dog, Canis familiaris. Appl. Anim. Behav. Sci. 2003, 84, 297–305. [Google Scholar] [CrossRef]
- Laska, M.; Bautista, R.M.; Hofelmann, D.; Sterlemann, V.; Salazar, L.T. Olfactory sensitivity for putrefaction-associated thiols and indols in three species of non-human primate. J. Exp. Biol. 2007, 210, 4169–4178. [Google Scholar] [CrossRef][Green Version]
- Lemmen, J.; Evenden, M. Peripheral and behavioral plasticity of pheromone response and its hormonal control in a long-lived moth. J. Exp. Biol. 2009, 212, 2000–2006. [Google Scholar] [CrossRef]
- Ferdenzi, C.; Roberts, S.C.; Schirmer, A.; Delplanque, S.; Cekic, S.; Porcherot, C.; Cayeux, I.; Sander, D.; Grandjean, D. Variability of affective responses to odors: Culture, gender, and olfactory knowledge. Chem. Senses 2013, 38, 175–186. [Google Scholar] [CrossRef]
- Gadenne, C.; Barrozo, R.B.; Anton, S. Plasticity in insect olfaction: To smell or not to smell? Annu. Rev. Entomol. 2016, 61, 317–333. [Google Scholar] [CrossRef]
- Martin, F.; Riveron, J.; Alcorta, E. Environmental temperature modulates olfactory reception in Drosophila melanogaster. J. Insect Physiol. 2011, 57, 1631–1642. [Google Scholar] [CrossRef]
- Lemmen, J.; Evenden, M. Environmental conditions terminate reproductive diapause and influence pheromone perception in the long-lived moth Caloptilia fraxinella. Physiol. Entomol. 2015, 40, 30–42. [Google Scholar] [CrossRef]
- Lemmen, J.K.; Evenden, M.L. The roles of juvenile hormone and biogenic amines on pheromone response plasticity and diapause termination in male Caloptilia fraxinella. Entomol. Exp. Appl. 2016, 158, 184–201. [Google Scholar] [CrossRef]
- Tait, G.; Vezzulli, S.; Sassù, F.; Antonini, G.; Biondi, A.; Baser, N.; Sollai, G.; Cini, A.; Tonina, L.; Ometto, L.; et al. Genetic variability in Italian populations of Drosophila suzukii. BMC Genet. 2017, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Lemmen, J.K.; Wist, T.J.; Evenden, M.L. State-dependent plasticity in response to host-plant volatiles in a long-lived moth, Caloptilia fraxinella (Lepidoptera: Gracillariidae). J. Chem. Ecol. 2018, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Biolchini, M.; Crnjar, R. Taste receptor plasticity in relation to feeding history in two congeneric species of Papilionidae. J. Insect Physiol. 2018, 107, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Solari, P.; Crnjar, R. Olfactory sensitivity to major, intermediate and traces components of sex pheromone in Ceratitis capitata is related to mating and circadian rhythm. J. Insect Physiol. 2018, 110, 23–33. [Google Scholar] [CrossRef]
- Clymans, R.; Van Kerckvoorde, V.; Bangels, E.; Akkermans, W.; Alhmedi, A.; De Clercq, P.; Beliën, T.; Bylemans, D. Olfactory preference of drosophila suzukii shifts between fruit and fermentation cues over the season: Effects of physiological status. Insects 2019, 10, 200. [Google Scholar] [CrossRef]
- Sollai, G.; Melis, M.; Magri, S.; Usai, P.; Hummel, T.; Tomassini Barbarossa, I.; Crnjar, R. Association between the rs2590498 polymorphism of Odorant-binding Protein (OBPIIa) gene and olfactory performance in healthy subjects. Behav. Brain Res. 2019, 372, 112030. [Google Scholar] [CrossRef]
- Tasnin, S.; Merkel, K.; Clarke, A.R. Effects of advanced age on olfactory response of male and female Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). J. Insect Physiol. 2020, 122, 104024. [Google Scholar] [CrossRef]
- Anton, S.; van Loon, J.J.A.; Meijerink, J.; Smid, H.M.; Takken, W.; Rospars, J.P. Central projections of olfactory receptor neurons from single antennal and palpal sensilla in mosquitoes. Arthropod Struct. Dev. 2003, 32, 319–327. [Google Scholar] [CrossRef]
- Galizia, C.G.; Roessler, W. Parallel olfactory systems in insects: Anatomy and function. Annu. Rev. Entomol. 2010, 55, 399–420. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Loy, F.; Masala, C.; Crnjar, R.; Liscia, A. Morpho-functional identification of abdominal olfactory receptors in the midge Culicoides imicola. J. Comp. Physiol. A 2010, 196, 817–824. [Google Scholar] [CrossRef]
- Martin, F.; Boto, T.; Gomez-Diaz, C.; Alcorta, E. Elements of olfactory recepton in adult Drosophila melanogaster. Anat. Rec. 2013, 296, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Strausfeld, N.J.; Hildebrand, J.G. Olfactory systems: Common design, uncommon origins? Curr. Opin. Neurobiol. 1999, 9, 634–639. [Google Scholar] [CrossRef]
- Galizia, C.G. Olfactory coding in the insect brain: Data and conjectures. Eur. J. Neurosci. 2014, 39, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Solari, P.; Corda, V.; Sollai, G.; Kreissl, S.; Galizia, C.G.; Crnjar, R. Morphological characterization of the antennal lobes in the Mediterranean fruit fly Ceratitis capitata. J. Comp. Physiol. A 2016, 202, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Gasperi, G.; Bonizzoni, M.; Gomulski, L.M.; Murelli, V.; Torti, C.; Malacrida, A.R.; Guglielmino, C.R. Genetic differentiation, gene flow and the origin of infestations of the medfly, Ceratitis capitata. Genetica 2002, 116, 125–135. [Google Scholar] [CrossRef]
- De Meyer, M.; Robertson, M.P.; Peterson, A.T.; Mansell, M.W. Ecological niches and potential geographical distributions of Mediterranean fruit fly (Ceratitis capitata) and Natal fruit fly (Ceratitis rosa). J. Biogeogr. 2008, 35, 270–281. [Google Scholar] [CrossRef]
- Diamantidis, A.D.; Carey, J.R.; Nakas, C.T.; Papadopoulos, N.T. Population-specific demography and invasion potential in medfly. Ecol. Evol. 2011, 1, 479–488. [Google Scholar] [CrossRef]
- Christenson, L.D.; Foote, R.H. Biology of fruit flies. Annu. Rev. Entomol. 1960, 5, 171–192. [Google Scholar] [CrossRef]
- Liquido, N.J.; Cunningham, R.T.; Nakagawa, A. Host plants of Mediterranean fruit fly (Diptera: Tephritidae) on the island of Hawaii (1949–1985 survey). J. Econ. Entomol. 1990, 83, 1863–1878. [Google Scholar] [CrossRef]
- Malacrida, A.R.; Gomulski, L.M.; Bonizzoni, M.; Bertin, S.; Gasperi, G.; Guglielmino, C.R. Globalization and fruit fly invasion and expansion: The medfly paradigm. Genetica 2007, 131, 1–9. [Google Scholar] [CrossRef]
- Carey, J.R.; Liedo, P.; Harshman, L.; Zhang, Y.; Müller, H.G.; Partridge, L.; Wang, J.L. A mortality cost of virginity at older ages in female Mediterranean fruit flies. Exp. Gerontol. 2002, 37, 507–512. [Google Scholar] [CrossRef][Green Version]
- Robinson, A.S. Mutations and their use in insect control. Mutat. Res. 2002, 511, 113–132. [Google Scholar] [CrossRef]
- Light, D.M.; Jang, E.B.; Dickens, J.C. Electroantennogram responses of the Mediterranean fruit fly, Ceratitis capitata, to a spectrum of plant volatiles. J. Chem. Ecol. 1988, 14, 159–180. [Google Scholar] [CrossRef] [PubMed]
- Levinson, H.; Levinson, A.; Muller, K. Influence of some olfactory and optical properties of fruits on host location by the Mediterranean fruit fly (Ceratitis capitataWied.). J. Appl. Entomol. 1990, 109, 44–54. [Google Scholar] [CrossRef]
- Light, D.M.; Jang, E.B.; Flath, R.A. Electroantennogram responses of the Mediterranean fruit fly, Ceratitis capitata, to the volatile constituents of nectarines. Entomol. Exp. Appl. 1992, 63, 13–26. [Google Scholar] [CrossRef]
- Hernandez, M.M.; Sanz, I.; Adelantado, M.; Ballach, S.; Primo, E. Electroantennogram activity from antennae of Ceratitis capitata (Wied.) to fresh orange airborne volatiles. J. Chem. Ecol. 1996, 22, 1607–1619. [Google Scholar] [CrossRef]
- Katsoyannos, B.I.; Kouloussis, N.A.; Papadopoulos, N.T. Response of Ceratitis capitata to Citrus chemicals under semi-natural conditions. Entomol. Exp. Appl. 1997, 82, 181–188. [Google Scholar] [CrossRef]
- Shelly, T.E. Exposure to α-copaene and α-copaene-containing oils enhances mating success of male Mediterranean fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2001, 94, 497–502. [Google Scholar] [CrossRef]
- Barry, J.D.; Shelly, T.E.; McInnis, D.O.; Morse, J.G. Potential for reducing overflooding ratios of sterile mediterranean fruit flies (diptera: Tephritidae) with the use of ginger root oil. Fla. Entomol. 2008, 86, 29–33. [Google Scholar] [CrossRef]
- Shelly, T.E.; Holler, T.C.; Stewart, J.L. Mating competitiveness of mass-reared males of the mediterranean fruit fly (Diptera: Tephritidae) from eclosion towers. Fla. Entomol. 2006, 89, 380–387. [Google Scholar] [CrossRef]
- Shelly, T.E.; Cowan, A.N.; Edu, J.; Pahio, E. Mating success of male mediterranean fruit flies following exposure to two sources of α-copaene, manuka oil and mango. Fla. Entomol. 2008, 91, 9–15. [Google Scholar] [CrossRef]
- Jang, E.B. Effects of mating and accessory gland injections on olfactory-mediated behavior in the female Mediterranean fruit fly, Ceratitis capitata. J. Insect Physiol. 1995, 41, 705–710. [Google Scholar] [CrossRef]
- Jang, E.B. Physiology of mating behavior in Mediterranean fruit fly (Diptera: Tephritidae): Chemoreception and male accessory gland fluids in female post-mating behavior. Fla. Entomol. 2002, 85, 89–93. [Google Scholar] [CrossRef]
- Vanickova, L.; do Nascimento, R.R.; Hoskovec, M.; Jezkova, Z.; Brizova, R.; Tomcala, A.; Kalikova, B. Are the wild and laboratory insect populations different in semiochemical emission? The case of the medfly sex pheromone. J. Agric. Food Chem. 2012, 60, 7168–7176. [Google Scholar] [CrossRef]
- Crnjar, R.; Scalera, G.; Liscia, A.; Angioy, A.M.; Bigiani, A.; Pietra, P.; Tomassini Barbarossa, I. Morphology and EAG mapping of the antennal olfactory receptors in Dacus oleae. Entomol. Exp. Appl. 1989, 51, 77–85. [Google Scholar] [CrossRef]
- Willhoeft, U.; Franz, G. Identification of the sex-determining region of the Ceratitis capitata Y chromosome by deletion mapping. Genetics 1996, 144, 737–745. [Google Scholar]
- Solari, P.; Crnjar, R.; Frongia, A.; Sollai, G.; Secci, F.; Spiga, M.; Masala, C.; Liscia, A. Oxaspiropentane derivatives as effective sex pheromone analogues in the gypsy moth: Electrophysiological and behavioral evidence. Chem. Senses 2007, 32, 755–763. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Masala, C.; Crnjar, R.; Liscia, A. Effects of avermectins on olfactory responses of Culicoides imicola (Diptera: Ceratopogonidae). J. Med. Entomol. 2007, 44, 656–659. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Masala, C.; Liscia, A.; Crnjar, R. A K+/H+ P-ATPase transport in the accessory cell membrane of the blowfly taste chemosensilla sustains the transepithelial potential (TEP). J. Comp. Physiol. A 2008, 194, 981–988. [Google Scholar] [CrossRef]
- Sollai, G.; Solari, P.; Corda, V.; Masala, C.; Crnjar, R. The spike generator in the labellar of the blowfly is differentially affected by 4-aminopyridine and 5-hydroxytryptamine. J. Insect Physiol. 2012, 58, 1686–1693. [Google Scholar] [CrossRef]
- Stensmyr, M.C.; Larsson, M.C.; Bice, S.; Hansson, B.S. Detection of fruit- and flower-emitted volatiles by olfactory receptor neurons in the plyphagous fruit chafer Pachnoda marginata (Coleoptera: Cetoniinae). J. Comp. Physiol. A 2001, 187, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Tomassini Barbarossa, I.; Usai, P.; Hummel, T.; Crnjar, R. Association between human olfactory performance and ability to detect single compounds in complex chemical mixtures. Physiol. Behav. 2020, 217, 112820. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Tomassini Barbarossa, I.; Masala, C.; Solari, P.; Crnjar, R. Gustatory sensitivity and food acceptance in two phylogenetically closely related Papilionid species: Papilio hospiton and Papilio machaon. PLoS ONE 2014, 9, e100675. [Google Scholar] [CrossRef] [PubMed]
- Sollai, G.; Tomassini Barbarossa, I.; Solari, P.; Crnjar, R. Taste discriminating capability to different bitter compounds by the larval styloconic sensilla in the insect herbivore Papilio hospiton (Géné). J. Insect Physiol. 2015, 74, 45–55. [Google Scholar] [CrossRef]
- Sollai, G.; Biolchini, M.; Solari, P.; Crnjar, R. Chemosensory basis of larval performance of Papilio hospiton on different host plants. J. Insect Physiol. 2017, 99, 47–57. [Google Scholar] [CrossRef]
- Siciliano, P.; Scolari, F.; Gomulski, L.M.; Falchetto, M.; Manni, M.; Gabrieli, P.; Field, L.M.; Zhou, J.-J.; Gasperi, G.; Malacrida, A. Sniffing out chemosensory genes from the Mediterranean fruit fly, Ceratitis capitata. PLoS ONE 2014, 9, e85523. [Google Scholar] [CrossRef][Green Version]
- Jang, E.B.; Light, D.M.; Binder, R.G.; Flath, R.A.; Carvalho, L.A. Electroantennogram response of the Mediterranean fruit fly, Ceratitis capitata, to identified constituents from calling males. Entomol. Exp. Appl. 1989, 50, 7–19. [Google Scholar] [CrossRef]
- Demirel, N. Behavior paradigms in the Mediterranean fruit fly, Ceratitis capitata (Weidemann). J. Entomol. 2007, 4, 129–135. [Google Scholar]
- Fein, B.L.; Reissig, W.H.; Roelofs, W.L. Identification of apple volatiles attractive to the apple magot, Rhagoletis pomonella. J. Chem. Ecol. 1982, 8, 1473–1487. [Google Scholar] [CrossRef]
- Light, D.M.; Jang, E.B. Electroantennogram responses of the oriental fruit fly, Dacus dorsalis to a spectrum of alcohol and aldehyde plant volatiles. Entomol. Exp. Appl. 1987, 45, 55–64. [Google Scholar] [CrossRef]
- Huang, A.P.; Bao, X.C.; Liu, B.Y.; Wang, Y.J.; Zhou, L.Y.; Ning, J.; Han, B.Y. Electroantennogram responses of the tea slug moth, Iragoides fasciata to some plant volatiles associated with tea, Camellia Sinensis. J. Insect Sci. 2012, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.H. Host Odor perception in phytophagous insects. Annu. Rev. Entomol. 1986, 31, 121–144. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.A. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef]
- Arita, L.H.; Kaneshiro, K.Y. Sexual selection and lek behavior in the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Pac. Sci. 1989, 43, 135–143. [Google Scholar]
- Eberhard, W. Sexual behavior and sexual selection in the Mediterranean fruit fly, Ceratitis capitata (Dacinae: Ceratitidini). In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 457–489. [Google Scholar]
- Whittier, T.S.; Kaneshiro, K.Y.; Prescott, L.D. Mating-behavior of Mediterranean fruit flies (Diptera, Tephritidae) in a natural environment. Ann. Entomol. Soc. Am. 1992, 85, 214–218. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sollai, G.; Solari, P.; Crnjar, R. Differences in the Olfactory Sensitivity of Ceratitis capitata to Headspace of Some Host Plants in Relation to Sex, Mating Condition and Population. Diversity 2020, 12, 207. https://doi.org/10.3390/d12050207
Sollai G, Solari P, Crnjar R. Differences in the Olfactory Sensitivity of Ceratitis capitata to Headspace of Some Host Plants in Relation to Sex, Mating Condition and Population. Diversity. 2020; 12(5):207. https://doi.org/10.3390/d12050207
Chicago/Turabian StyleSollai, Giorgia, Paolo Solari, and Roberto Crnjar. 2020. "Differences in the Olfactory Sensitivity of Ceratitis capitata to Headspace of Some Host Plants in Relation to Sex, Mating Condition and Population" Diversity 12, no. 5: 207. https://doi.org/10.3390/d12050207
APA StyleSollai, G., Solari, P., & Crnjar, R. (2020). Differences in the Olfactory Sensitivity of Ceratitis capitata to Headspace of Some Host Plants in Relation to Sex, Mating Condition and Population. Diversity, 12(5), 207. https://doi.org/10.3390/d12050207