Species Diversity of Micromycetes Associated with Epipactis helleborine and Epipactis purpurata (Orchidaceae, Neottieae) in Southwestern Poland
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Study Area
2.3. Isolation of Fungi from Host Plant
2.4. Fungal Identification
2.5. Alignment
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hawksworth, D.L. The magnitude of fungal diversity: The 1.5 million species estimate revisited. Mycol. Res. 2001, 105, 1422–1432. [Google Scholar] [CrossRef]
- Ogórek, R.; Dyląg, M.; Kozak, B. Dark stains on rock surfaces in Driny Cave (Little Carpathian Mountains, Slovakia). Extremophiles 2016, 20, 641–652. [Google Scholar] [CrossRef]
- Read, D.J.; Duckett, J.G.; Francis, R.R.; Ligrone, R.; Russe, A. Symbiotic fungal associations in ‘lower’ land plants. Philos. Trans. R. Soc. B 2000, 355, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Bidartondo, M.I.; Read, D.J.; Trappe, J.M.; Merckx, V.; Ligrone, R.; Duckett, J.G. The dawn of symbiosis between plants and fungi. Biol. Lett. 2011, 7, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Ogórek, R.; Lejman, A.; Sobkowicz, P. Effect of the Intensity of Weed Harrowing with Spike-Tooth Harrow in Barley-Pea Mixture on Yield and Mycobiota of Harvested Grains. Agronomy 2019, 9, 103. [Google Scholar] [CrossRef]
- Ogórek, R.; Piecuch, A.; Višňovská, Z.; Cal, M.; Niedźwiecka, K. First report on the occurrence of dermatophytes of Microsporum cookei clade and close affinities to Paraphyton cookei in the Harmanecká Cave (Veľká Fatra Mts., Slovakia). Diversity 2019, 11, 191. [Google Scholar] [CrossRef]
- Leake, J.R. The biology of myco-heterotrophic (‘saprophytic’) plants. New Phytol. 1994, 127, 171–216. [Google Scholar] [CrossRef]
- Pecoraro, L.; Caruso, T.; Cai, L.; Gupta, V.K.; Liu, Z.J. Fungal networks and orchid distribution: New insights from above-and below-ground analyses of fungal communities. IMA Fungus 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Ma, X.; Kang, J.; Nontachaiyapoom, S.; Wen, T.; Hyde, K.D. Non-mycorrhizal endophytic fungi from orchids. Curr. Sci. 2015, 109, 72–87. [Google Scholar]
- Rasmussen, H.N. Terrestrial Orchids: From Seed to Mycotrophic Plant; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Herrera, H.; Valadares, R.; Contreras, D.; Bashan, Y.; Arriagada, C. Mycorrhizal compatibility and symbiotic seed germination of orchids from the Coastal Range and Andes in south central Chile. Mycorrhiza 2017, 27, 175–188. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; O’Neill, J.P.; Becker, J.J.; Werner, S.; Rasmussen, H.N.; Bruns, T.D.; Taylor, D.L. Abundance and distribution of Corallorhiza odonthoriza reflect variations in climate and ectomycorrhizae. Ecol. Monogr. 2009, 79, 619–635. [Google Scholar] [CrossRef]
- Jacquemyn, H.; Brys, R.; Honnay, O.; Roldán-Ruiz, I.; Lievens, B.; Wiegand, T. Non-random spatial structuring of orchids in a hybrid zone of three Orchis species. New Phytol. 2012, 193, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Brys, R.; Merckx, V.S.; Waud, M.; Lievens, B.; Wiegand, T. Co-existing orchid species have distinct mycorrhizal communities and display strong spatial segregation. New Phytol. 2014, 202, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Jacquemyn, H.; Waud, M.; Lievens, B.; Brys, R. Differences in mycorrhizal communities between Epipactis palustris, E. helleborine and its presumed sister species E. neerlandica. Ann. Bot. 2016, 118, 105–114. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.K.; Taylor, D.L.; Juhaszova, K.; Burnett, R.K.J.R.; Whigham, D.F.; O’Neill, J.P. Limitations on orchid recruitment: Not a simple picture. Mol. Ecol. 2012, 21, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.K.; Jacquemyn, H. What constrains the distribution of orchid populations? New Phytol. 2014, 202, 392–400. [Google Scholar] [CrossRef]
- Salmia, A. Endomycorrhizal fungus in chlorophyll-free and green forms of the terrestrial orchid Epipactis helleborine. Karstenia 1988, 28, 3–18. [Google Scholar] [CrossRef]
- Schneider-Maunoury, L.; Leclercq, S.; Clément, C.; Covès, H.; Lambourdière, J.; Sauve, M.; Richard, F.; Selosse, M.A.; Taschen, E. Is Tuber melanosporum colonizing the roots of herbaceous, nonectomycorrhizal plants? Fungal Ecol. 2018, 31, 59–68. [Google Scholar] [CrossRef]
- Schneider-Maunoury, L.; Deveau, A.; Moreno, M.; Todesco, F.; Belmondo, S.; Murat, C.; Courty, P.E.; Jąkalski, M.; Selosse, M.A. Two ectomycorrhizal truffles, Tuber melanosporum and T. aestivum, endophytically colonise roots of non-ectomycorrhizal plants in natural environments. New Phytol. 2020, 225, 2542–2556. [Google Scholar] [CrossRef]
- May, M.; Jąkalski, M.; Novotná, A.; Dietel, J.; Ayasse, M.; Lallemand, F.; Figura, T.; Minasiewicz, J.; Selosse, M.A. Three-year pot culture of Epipactis helleborine reveals autotrophic survival, without mycorrhizal networks, in a mixotrophic species. Mycorrhiza 2020, 30, 51–61. [Google Scholar] [CrossRef]
- Hansen, K.; Perry, B.A.; Dranginis, A.W.; Pfister, D.H. A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected life history traits. Mol. Phylogenet. Evol. 2013, 67, 311–333. [Google Scholar] [CrossRef] [PubMed]
- Jumpponen, A. Dark septate endophytes—Are they mycorrhizal? Mycorrhiza 2013, 11, 207–211. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W.; Martos, F.; Selosse, M.A. 12 orchid mycorrhizas: Molecular ecology, physiology, evolution and conservation aspects. In Fungal Associations. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Hock, B., Ed.; Springer: Berlin, Germany, 2012; pp. 207–230. [Google Scholar]
- Veldre, V.; Abarenkov, K.; Bahram, M.; Martos, F.; Selosse, M.A.; Tamm, H.; Kõljalg, U.; Tedersoo, L. Evolution of Nutritional Modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as Revealed from Publicly Available ITS Sequences. Fungal Ecol. 2013, 6, 256–268. [Google Scholar] [CrossRef]
- Weiß, M.; Waller, F.; Zuccaro, A.; Selosse, M.A. Sebacinales—One thousand and one interactions with land plants. New Phytol. 2016, 211, 20–40. [Google Scholar] [CrossRef]
- Lallemand, F.; Robionek, A.; Courty, P.E.; Selosse, M.A. The 13C content of the orchid Epipactis palustris (L.) Crantz responds to light as in autotrophic plants. Bot. Lett. 2018, 165, 265–273. [Google Scholar] [CrossRef]
- Selosse, M.A.; Faccio, A.; Scappaticci, G.; Bonfante, P. Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles. Microb. Ecol. 2004, 47, 416–426. [Google Scholar] [CrossRef]
- Redman, R.S.; Sheehan, K.B.; Stout, R.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Khan, S.A.; Hamayun, M.; Yoon, H.; Kim, H.Y.; Suh, S.J.; Hwang, S.K.; Kim, J.M.; Lee, I.J.; Choo, Y.S.; Yoon, U.H.; et al. Plant growth promotion and Penicillium citrinum. BMC Microbiol. 2008, 8, 231. [Google Scholar] [CrossRef]
- Xing, Y.M.; Chen, J.; Cui, J.L.; Chen, X.M.; Guo, S.X. Antimicrobial activity and biodiversity of endophytic fungi in Dendrobium devonianum and Dendrobium thyrsiflorum from Vietman. Curr. Microbiol. 2011, 62, 1218–1224. [Google Scholar] [CrossRef]
- Rifai, M.A. A revision of the genus Trichoderma. Mycol. Pap. 1969, 116, 1–56. [Google Scholar]
- Schipper, M.A.A. A study on variability in Mucor hiemalis and related species. Stud. Mycol. 1973, 4, 1–40. [Google Scholar]
- Seifert, K. Fuskey: Fusarium Interactive Key. Agric. Agric. Food Can. 1996, 1–65. [Google Scholar]
- Ho, H.-M.; Chuang, S.-C.; Chen, S.-J. Notes on Zygomycetes of Taiwan (IV): Three Absidia species (Mucoraceae). Fung. Sci. 2004, 19, 125–131. [Google Scholar]
- Peterson, S.W. Multilocus DNA sequence analysis shows that Penicillium biourgeianum is a distinct species closely to P. brevicompactum and P. olsonii. Mycol. Res. 2004, 108, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Taxonomy of Penicillium section Citrina. Stud. Mycol. 2011, 70, 53–138. [Google Scholar] [CrossRef]
- Cabral, A.; Groenewald, J.Z.; Rego, C.; Oliveira, H.; Crous, P.W. Cylindrocarpon root rot: Multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol. Prog. 2012, 11, 655–688. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z. A phylogenetic re-evaluation of Arthrinium. IMA Fungus 2013, 4, 133–154. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Ogórek, R.; Kozak, B.; Višňovská, Z.; Tancinová, D. Phenotypic and genotypic diversity of airborne fungalspores in Demänovská Ice Cave (Low Tatras, Slovakia). Aerobiologia 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Dyląg, M.; Sawicki, A.; Ogórek, R. Diversity of species and susceptibility phenotypes toward commercially available fungicides of cultivable fungi colonizing bones of Ursus spelaeus on display in Niedźwiedzia Cave (Kletno, Poland). Diversity 2019, 11, 224. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Dearnaley, J.D.W. Further advances in orchid mycorrhizal research. Mycorrhiza 2007, 17, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Bidartondo, M.I.; Burghardt, B.; Gebauer, G.; Bruns, T.D.; Read, D.J. Changing partners in the dark: Isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. Lond. Ser. B 2004, 271, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Ogura-Tsujita, Y.; Yukawa, T. High mycorrhizal specificity in a widespread mycoheterotrophic plant, Eulophia zollingeri (Orchidaceae). Am. J. Bot. 2008, 95, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Těšitelová, T.; Těšitel, J.; Jersáková, J.; RÍhová, G.; Selosse, M.A. Symbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am. J. Bot. 2012, 99, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; Maherali, H.; Antunes, P.M. Plant geographic origin and phylogeny as potential drivers of community structure in root-inhabiting fungi. J. Ecol. 2019, 107, 1720–1736. [Google Scholar] [CrossRef]
- Větrovský, T.; Kohout, P.; Kopecký, M.; Machac, A.; Man, M.; Bahnmann, B.D.; Brabcová, V.; Choi, J.; Meszárošová, L.; Human, Z.R.; et al. A meta-analysis of global fungal distribution reveals climate-driven patterns. Nat. Commun. 2019, 10, 5142. [Google Scholar] [CrossRef]
- Pasanen, A.L. A review: Fungal exposure assessment in in-door environments. Indoor Air 2001, 11, 87–98. [Google Scholar] [CrossRef]
- MacNeil, L.; Kauri, T.; Robertson, W. Molecular techniques and their potential application in monitoring the microbiological quality of indoor air. Can. J. Microbiol. 1995, 41, 657–675. [Google Scholar] [CrossRef]
- Wu, P.C.; Su, H.J.J.; Ho, H.M. A comparison of sampling media for environmental viable fungi collected in a hospital environment. Environ. Res. 2000, 82, 253–257. [Google Scholar] [CrossRef]
- Macher, J.M. Review of methods to collect settled dust and isolate culturable microorganisms. Indoor Air 2001, 11, 99–110. [Google Scholar] [CrossRef]
- Ogórek, R.; Višňovská, Z.; Tančinová, D. Mycobiota of underground habitats: Case study of Harmanecká Cave in Slovakia. Microb. Ecol. 2016, 71, 87–99. [Google Scholar] [CrossRef]
- Roncero, M.I.G.; Hera, C.; Ruiz-Rubio, M.; García Maceira, F.I.; Madrid, M.P.; Caracuel, Z.; Calero, F.; Delgado-Jarana, J.; Roldán-Rodríguez, R.; Martínez-Rocha, A.L.; et al. Fusarium as a model for studying virulence in soil borne plant pathogens. Physiol. Mol. Plant Pathol. 2003, 62, 87–98. [Google Scholar] [CrossRef]
- Tan, X.M.; Zhou, Y.Q.; Zhou, X.L.; Xia, X.H.; Wei, Y.; He, L.L.; Tang, H.Z.; Yu, L.Y. Diversity and bioactive potential of culturable fungal endophytes of Dysosma versipellis; a rare medicinal plant endemic to China. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ivanová, H.; Hrehová, Ľ.; Pristaš, P. First confirmed report on Fusarium sporotrichioides on Pinus ponderosa var. jeffreyi in Slovakia. Plant Protect. Sci. 2016, 52, 250–253. [Google Scholar]
- Srivastava, S.; Kadooka, C.; Uchida, J.Y. Fusarium species as pathogen on orchids. Microbiol. Res. 2018, 207, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bungtongdee, N.; Sopalun, K.; Laosripaiboon, W.; Iamtham, S. The chemical composition, antifungal, antioxidant and antimutagenicity properties of bioactive compounds from fungal endophytes associated with Thai orchids. J. Phytopathol. 2019, 167, 56–64. [Google Scholar] [CrossRef]
- Latiffah, Z.; Hayati, M.Z.N.; Baharuddin, S.; Maziah, Z. Identification and pathogenicity of Fusarium species associated with root rot and stem rot of Dendrobium. Asian J. Plant Pathol. 2009, 3, 14–21. [Google Scholar] [CrossRef]
- Swett, C.L.; Uchida, J.Y. Characterization of Fusarium diseases on commercially grown orchids in Hawaii. Plant Pathol. 2015, 64, 648–654. [Google Scholar] [CrossRef]
- Thongkamngam, T.; Jaenaksorn, T. Fusarium oxysporum (F221-B) as biocontrol agent against plant pathogenic fungi in vitro and in hydroponics. Plant Protect. Sci. 2017, 53, 85–95. [Google Scholar]
- Gong, L.J.; Guo, S.X. Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afr. J. Biotechnol. 2009, 8, 731–736. [Google Scholar]
- Parthibhan, S.; Rao, M.V.; Kumar, T.S. Culturable fungal endophytes in shoots of Dendrobium aqueum Lindley–An imperiled orchid. Ecol. Genet. Genom. 2017, 3, 18–24. [Google Scholar] [CrossRef]
- Bernard, N. L’évolution dans la symbiose. Le orchidées et leurs champignons commensaux. Ann. Sci. Nat. Bot. 1909, 9, 1–19. [Google Scholar]
- Bayman, P.; Otero, J.T. Microbial endophytes of orchid roots. In Microbial Root Endophytes. Soil Biology; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin, Germany, 2006; pp. 153–177. [Google Scholar]
- Vujanovic, V.; St.-Arnaud, M.; Barabé, D.; Thibeault, G. Viability testing of orchid seed and the promotion of colouration and germination. Ann. Bot. 2000, 86, 79–86. [Google Scholar] [CrossRef]
- Azam, M.; Shahid, A.A.; Majeed, R.A.; Ali, M.; Ahmad, N.; Haider, M.S. First report of Penicillium biourgeianum causing post-harvest fruit rot of apple in Pakistan. Plant Dis. 2016, 100, 1778. [Google Scholar] [CrossRef]
- Li, S.; Mou, Q.; Xu, X.; Qi, S.; Leung, P.H.M. Synergistic antibacterial activity between penicillenols and antibiotics against methicillin-resistant Staphylococcus aureus. R. Soc. Open Sci. 2018, 5, 172466. [Google Scholar] [CrossRef]
- Baute, M.A.; Deffieux, G.; Baute, R.; Neveu, A. New antibiotics from the fungus Epicoccum nigrum. J. Antibiot. Res. 1978, 31, 1099–1101. [Google Scholar] [CrossRef]
- Ogórek, R.; Pląskowska, E. Epicoccum nigrum for biocontrol agents in vitro of plant fungal pathogens. Commun. Agric. Appl. Biol. Sci. 2011, 76, 691–697. [Google Scholar]
- Kukreja, N.; Sridhara, S.; Singh, B.P.; Arora, N. Effect of proteolytic activity of Epicoccum purpurascens major allergen, Epi p 1 in allergic inflammation. Clin. Exp. Immunol. 2008, 154, 162–171. [Google Scholar] [CrossRef]
- Fávaro, L.C.L.; De Melo, F.L.; Aguilar-Vildoso, C.I.; Araújo, W.L. Polyphasic analysis of intraspecific diversity in Epicoccum nigrum warrants reclassification into separate species. PLoS ONE 2011, 6, e14828. [Google Scholar] [CrossRef]
- Vaz, A.B.; Mota, R.C.; Bomfim, M.R.Q.; Vieira, M.L.; Zani, C.L.; Rosa, C.A.; Rosa, L.H. Antimicrobial activity of endophytic fungi associated with Orchidaceae in Brazil. Can. J. Microbiol. 2009, 55, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Simmons, E.G. Alternaria: An Identification Manual; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007; pp. 500–502. [Google Scholar]
- Chełkowski, J.; Visconti, A. Alternaria: Biology, Plant Diseases and Metabolites; Elsevier Science Limited: Amsterdam, The Netherlands, 1992; pp. 364–365. [Google Scholar]
- Ibarrola, I.; Suárez-Cervera, M.; Arilla, M.C.; Martínez, A.; Monteseirín, J.; Conde, J.; Asturias, J.A. Production profile of the major allergen Alt a 1 in Alternaria alternata cultures. Ann. Allergy Asthma Immunol. 2004, 93, 589–593. [Google Scholar] [CrossRef]
- Pastor, F.J.; Guarro, J. Alternaria infections: Laboratory diagnosis and relevant clinical features. Clin. Microbiol. Infect. 2008, 14, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.X.; Li, Y.C.; Li, C.F. Optimization of homoharringtonine fermentation conditions for Alternaria tenuissima CH1307, an endophytic fungus of Cephalotaxus mannii Hook. f. J. Trop. Org. 2012, 3, 236–242. [Google Scholar]
- Greven, S.; Egberts, F.; Buchner, M.; Beck-Jendroschek, V.; Voss, K.; Brasch, J. Cutaneous chromomycosis caused by Arthrinium arundinis. J. Dtsch. Dermatol. Ges. 2018, 5, 621–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.; Yuan, X.L.; Du, Y.M.; Wang, B.G.; Zhang, Z.F. Antifungal prenylated diphenyl ethers from Arthrinium arundinis, an endophytic fungus isolated from the leaves of Tobacco (Nicotiana tabacum L.). Molecules 2018, 23, 3179. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-opportunistic, a virulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
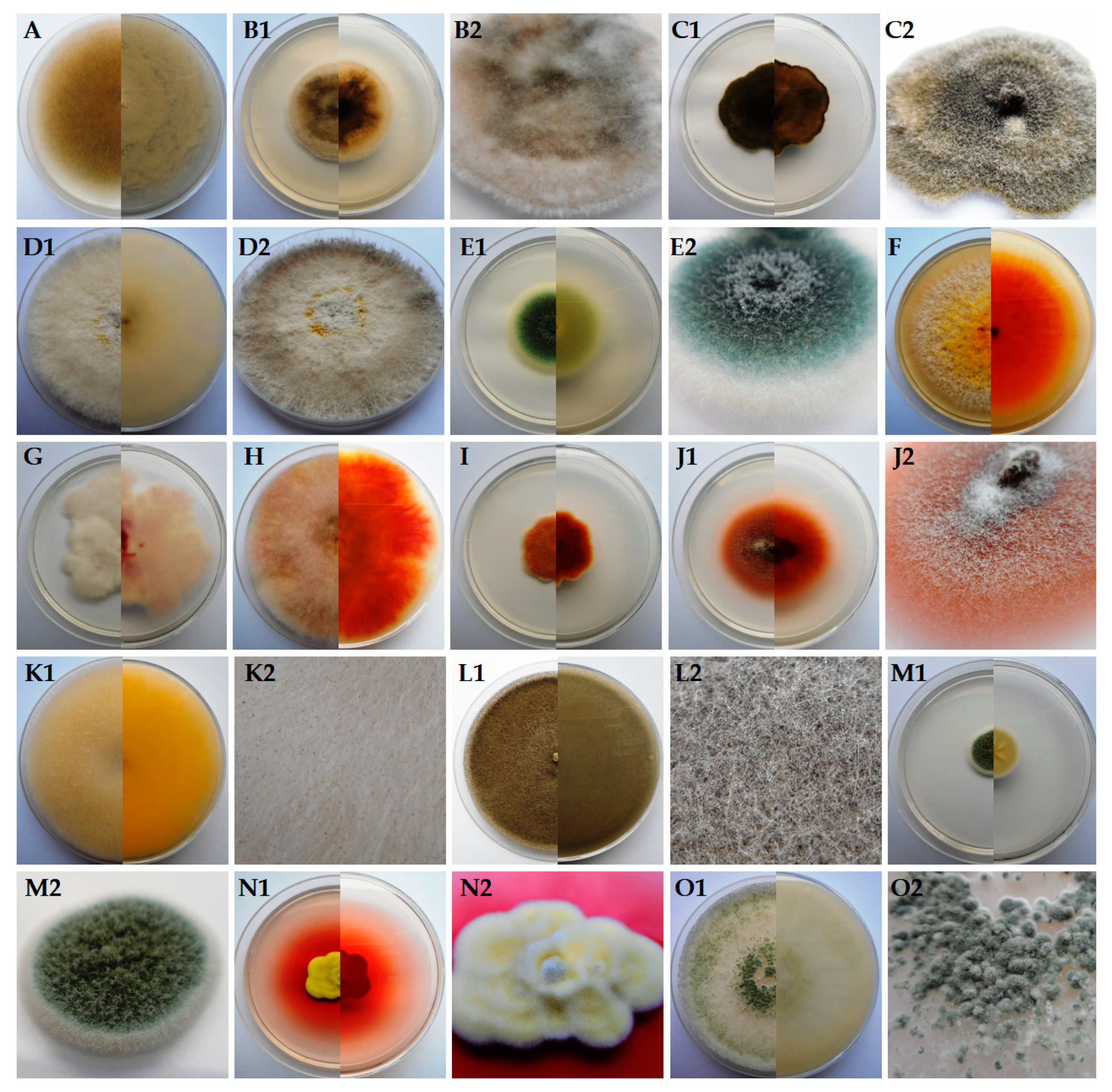
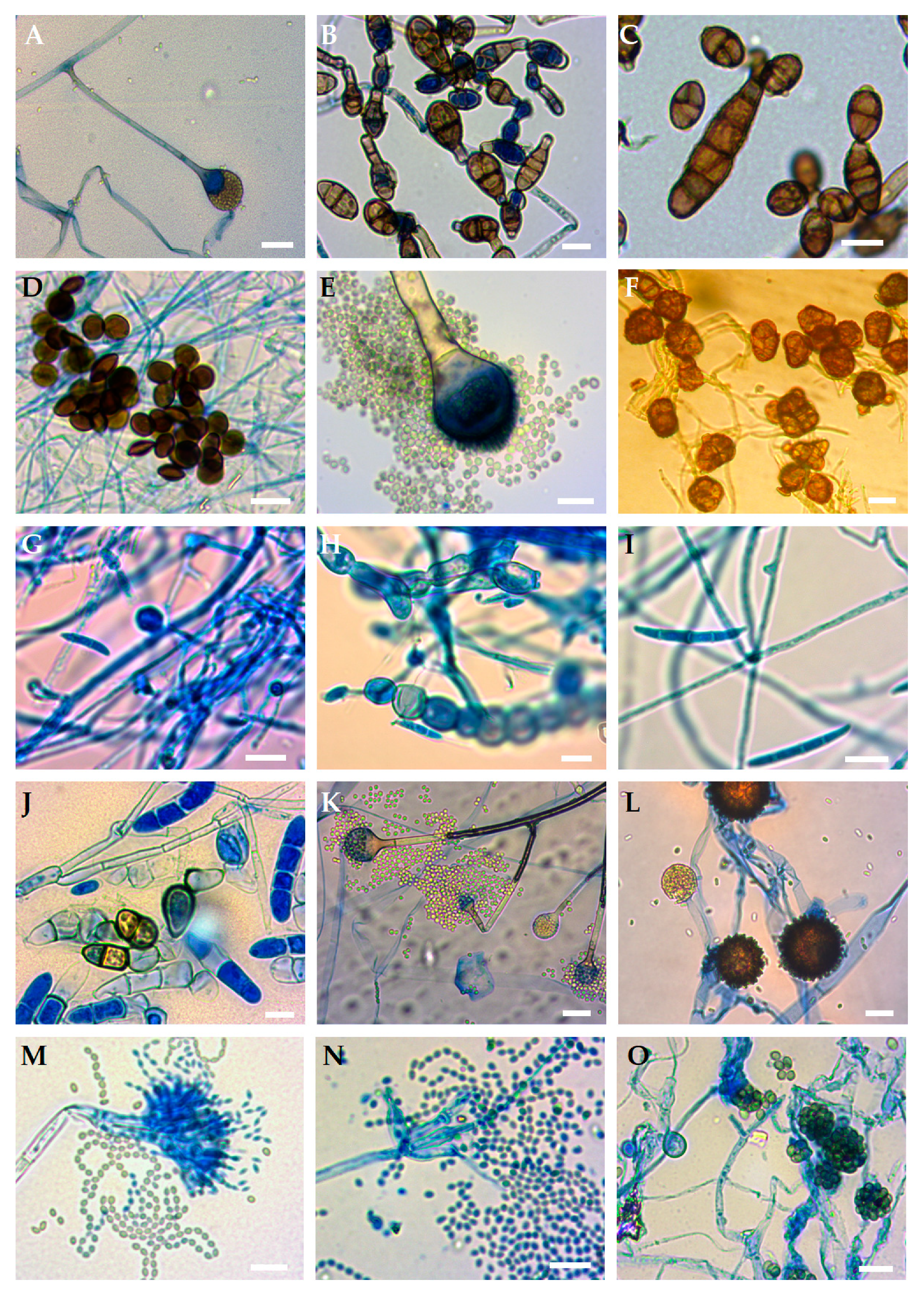

| Fungi Isolated from Orchids | Identity with Sequence from GenBank | |||||
|---|---|---|---|---|---|---|
| Isolate Number | Identified Species | GenBank Accession No. | The Sequence Length (bp) | Query Cover, % | Identity, % | Accession |
| UWR_170 | Absidia cylindrospora | MN817778.1 | 514 | 99 | 95.34 | JN205822.1 |
| UWR_171 | Alternaria alternata | MN817779.1 | 391 | 100 | 98.72 | KF996773.1 |
| UWR_172 | A. tenuissima | MN817780.1 | 487 | 100 | 100.00 | MN712241.1 |
| UWR_173 | Arthrinium arundinis | MN817781.1 | 451 | 100 | 100.00 | MN593205.1 |
| UWR_174 | Aspergillus fumigatus | MN817782.1 | 539 | 100 | 99.07 | MN178808.1 |
| UWR_175 | Epicoccum nigrum | MN817783.1 | 454 | 100 | 95.59 | MG736195.1 |
| UWR_176 | Fusarium oxysporum | MN817784.1 | 473 | 100 | 100.00 | MN240928.1 |
| UWR_177 | F. sporotrichioides | MN817785.1 | 435 | 100 | 99.77 | MK595076.1 |
| UWR_178 | F. tricinctum | MN817786.1 | 497 | 100 | 100.00 | MK102644.1 |
| UWR_179 | Ilyonectria robusta | MN817787.1 | 456 | 100 | 100.00 | MK602790.1 |
| UWR_180 | Mucor hiemalis | MN817788.1 | 530 | 100 | 100.00 | MH794214.1 |
| UWR_181 | M. moelleri | MN817789.1 | 570 | 100 | 100.00 | MH857827.1 |
| UWR_182 | Penicillium biourgeianum | MN817790.1 | 506 | 100 | 100.00 | KX067821.1 |
| UWR_183 | P. manginii | MN817791.1 | 481 | 100 | 99.17 | MH858641.1 |
| UWR_184 | Trichoderma viride | MN817792.1 | 552 | 100 | 100.00 | KX379164.1 |
| Fungal Species | Roots | Rhizomes | Stems | Leaves | Inflorescences | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | ND | D | ND | D | ND | D | ND | D | ND | ||
| Fruiting plants 2018 | Alternaria tenuissima | + | + | + | + | + | |||||
| Epicoccum nigrum | + | + | + | + | + | ||||||
| Fusarium oxysporum | + | + | |||||||||
| Fusarium sporotrichioides | + | + | + | + | + | + | + | ||||
| Fusarium tricinctum | + | + | + | + | + | ||||||
| Ilyonectria robusta | + | + | + | ||||||||
| Mucor hiemalis | + | + | + | + | |||||||
| M. moelleri | + | + | + | + | + | + | |||||
| Penicillium biourgeianum | + | + | + | + | + | ||||||
| P. manginii | + | ||||||||||
| Trichoderma viride | + | + | |||||||||
| In total | 6 | 6 | 2 | 6 | 3 | 5 | 4 | 6 | 2 | 5 | |
| Flowering plants 2019 | Absidia cylindrospora | + | + | + | + | + | |||||
| Alternaria alternata | + | ||||||||||
| A. tenuissima | + | + | + | ||||||||
| Arthrinium arundinis | + | + | + | ||||||||
| Aspergillus fumigatus | + | + | + | + | |||||||
| Epicoccum nigrum | + | + | |||||||||
| Fusarium tricinctum | + | ||||||||||
| Ilyonectria robusta | + | ||||||||||
| Mucor hiemalis | + | + | |||||||||
| M. moelleri | + | ||||||||||
| Penicillium biourgeianum | + | + | + | + | + | ||||||
| P. manginii | + | + | + | + | + | ||||||
| Trichoderma viride | + | + | |||||||||
| In total | 4 | 5 | 2 | 4 | 2 | 4 | 3 | 5 | 3 | 4 | |
| Fungal Species | Roots | Rhizomes | Stems | Leaves | Inflorescences | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | ND | D | ND | D | ND | D | ND | D | ND | ||
| Fruiting plants 2018 | Alternaria tenuissima | + | + | + | + | + | |||||
| Epicoccum nigrum | + | + | |||||||||
| Fusarium sporotrichioides | + | + | + | ||||||||
| F. tricinctum | + | + | + | + | + | ||||||
| Ilyonectria robusta | + | + | + | + | |||||||
| Mucor hiemalis | + | + | + | + | + | ||||||
| M. moelleri | + | + | + | + | + | + | |||||
| Penicillium biourgeianum | + | + | + | ||||||||
| Trichoderma viride | + | + | |||||||||
| In total | 4 | 5 | 3 | 5 | 2 | 4 | 3 | 3 | 2 | 4 | |
| Flowering plants 2019 | Absidia cylindrospora | + | + | + | |||||||
| Alternaria alternata | + | + | + | + | + | ||||||
| A. tenuissima | + | + | + | + | + | + | |||||
| Aspergillus fumigatus | + | + | + | ||||||||
| Epicoccum nigrum | + | + | |||||||||
| Fusarium tricinctum | + | + | + | + | |||||||
| Penicillium biourgeianum | + | + | + | + | + | ||||||
| P. manginii | + | + | |||||||||
| In total | 2 | 3 | 3 | 4 | 2 | 4 | 2 | 3 | 3 | 4 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogórek, R.; Kurczaba, K.; Łobas, Z.; Żołubak, E.; Jakubska-Busse, A. Species Diversity of Micromycetes Associated with Epipactis helleborine and Epipactis purpurata (Orchidaceae, Neottieae) in Southwestern Poland. Diversity 2020, 12, 182. https://doi.org/10.3390/d12050182
Ogórek R, Kurczaba K, Łobas Z, Żołubak E, Jakubska-Busse A. Species Diversity of Micromycetes Associated with Epipactis helleborine and Epipactis purpurata (Orchidaceae, Neottieae) in Southwestern Poland. Diversity. 2020; 12(5):182. https://doi.org/10.3390/d12050182
Chicago/Turabian StyleOgórek, Rafał, Klaudia Kurczaba, Zbigniew Łobas, Elżbieta Żołubak, and Anna Jakubska-Busse. 2020. "Species Diversity of Micromycetes Associated with Epipactis helleborine and Epipactis purpurata (Orchidaceae, Neottieae) in Southwestern Poland" Diversity 12, no. 5: 182. https://doi.org/10.3390/d12050182
APA StyleOgórek, R., Kurczaba, K., Łobas, Z., Żołubak, E., & Jakubska-Busse, A. (2020). Species Diversity of Micromycetes Associated with Epipactis helleborine and Epipactis purpurata (Orchidaceae, Neottieae) in Southwestern Poland. Diversity, 12(5), 182. https://doi.org/10.3390/d12050182









