Abstract
Intraspecific trait variation in generalist animals is widespread in nature, yet its effects on community ecology are not well understood. Newts are considered opportunistic feeders that may co-occur in different syntopic conditions and represent an excellent model for studying the role of individual feeding specialization in shaping the population dietary strategy. Here, we investigated the diet of three newt species from central Italy occurring in artificial habitats in different coexistence conditions to test the predictions of the niche width (NW) variation hypotheses. Population NW did not vary among species and between presence and absence of coexisting species. An overall positive relationship between individual specialization and population NW was observed. However, this pattern was disrupted by the condition of syntopy with newt populations showing an individual NW variation invariant with population NW in presence of coexisting species, whereas it was larger in populations occurring alone. The observed pattern of newt behavior was not consistent with any of the proposed scenarios. We found a consistent pattern with the degree of individual specialization being (1) size-dependent (specialized individuals increasing within larger sized species) and (2) assemblage-complexity-dependent (specialized individuals increasing in syntopic populations in comparison to singly populations).
1. Introduction
Urodeles are important elements of freshwater vertebrate communities either as prey or as predators [1,2,3,4] contributing to most of the total predator biomass in specific study areas [5]. They usually perform many “key ecological functions” [6] occurring at the top of the food chain [7,8,9] or, more frequently, at an intermediate level [10,11]. As predators of invertebrates and small vertebrates, they modulate energy pathways by decreasing the abundance of competitively dominant preys and consequently increasing taxa diversity in lower trophic levels [12]. Moreover, through their dual life cycle, they serve as connecting pathways for energy between aquatic and terrestrial environments affecting prey communities in both habitat types [13].
In general, newts are opportunistic feeders [14,15] consuming zooplankton, crustaceans, insects, fish, tadpoles, and even aerial or soil fauna fallen into the water during the aquatic phase [16,17,18,19], as well as isopods, diplopods, insects, and earthworms collected on the ground during the terrestrial phase [19,20,21,22]. Assemblages of newt species of differing body size are common throughout Europe, such as in Italy, where up to three species may co-occur in syntopy in the same aquatic site [16,21,23]. Because of their generalist feeding habits, and because they may co-occur in different sympatric conditions (see below), newts are excellent models for studying the role of individual feeding specialization in the “overall dietary niche” of a generalist feeder species/population. Indeed, it has been postulated that within-population niche variation can stabilize population and community dynamics [24], with environments having greater resource diversity favoring ecological diversity among consumers via disruptive selection or phenotypic and ecological plasticity or, as an alternative mechanism, that niche variation may be a consequence of neutral genomic diversity in more abundant populations [25].
Here, we investigated the diet of three newt species, the Italian newt (Lissotriton italicus), the Italian smooth newt (Lissotriton vulgaris), and the Italian crested newt (Triturus carnifex), in different co-occurring conditions in central Italy in order to test the predictions of the “within-population niche variation” theory across different species and co-presence conditions [26,27]. Except for a few theoretical studies [28,29], the “within-population niche variation” theory has rarely been tested before on natural assemblages of potentially competing species (but see [30,31]), but merely on populations of single species across several taxa (reviewed in [24,32]). Thus, our paper is one of the first to test the theory on an assemblage of species that have been considered in competition for the available resources in previous studies [33].
Specifically, the aims of the present study were to answer the following key questions:
(1) Does newt body size affect the tendency of individuals to partition their trophic niche by specializing the use of food resources towards distinct prey categories? Being newt gap-limited predators [11,33,34], we hypothesize that larger species feed on a large variety of prey items and thus have higher chance to differentiate the diet at the individual level to limit the potential impact of intraspecific competition.
(2a) Does the presence of taxonomically related species (congeneric or belonging to the same family) influence the feeding strategy of target newt populations in terms of trophic niche width and individual feeding behavior (i.e., degree of individual specialization)? We would expect that the co-occurrence of related species should promote differentiation of the trophic spectrum in conspecific individuals to mitigate the likely increased intra- and inter-specific competition [26]. (2b) Does the degree of syntopy (single vs. multispecies systems) affect newt body size that in turn may influence the feeding ecology of the species? We hypothesize that (a) large-bodied species outcompete smaller species through competition and intra guild predation, and (b) body size of smaller species are negatively affected by the occurrence of larger species. Moreover, we would expect that in multispecies conditions, the smaller species would suffer from the co-occurrence of large-bodied species due to the negative effect of intra guild predation (i.e., competition and predation by the same antagonist). Predation might influence individual behavior by constraining individuals to forage in restricted areas based on their boldness (i.e., propensity to forage in the presence of risk; [35]), and thus affecting the magnitude of individual specialization if resources are patchy [24]. Since the effect of interspecific competition and predation on individual specialization remains controversial both in the theoretical and empirical literature [24,30], we would expect species specific response by the various newt species.
In order to answer to the above-mentioned key questions, we surveyed specific artificial aquatic habitats (i.e., wells) characterized by a circular shape, vertical walls, and high depth (up to 6 m), generally associated with traditional agriculture and cattle watering. Indeed, wells represent ideal scale-effective systems to investigate interspecific interactions and community composition being characterized by a higher stability and a more simplified structure (e.g., limited volume, closed physical boundaries, simple and consistent vegetation structure and resource availability, absence of fish predators) in comparison to natural aquatic sites [36]. Moreover, at the study area, these habitats (i) are widespread, (ii) have been in place for a time long enough to enable the establishment of stable communities, (iii) host one up to three newt species syntopically, and (iv) are consistent in shape and size, thus, representing a self-set replicated experimental system [9,36].
2. Materials and Methods
2.1. Study Species
Lissotriton italicus (Peracca, 1898) and L. vulgaris (Linnaeus, 1758) are endemic to the southern Italian peninsula and widespread throughout Europe, respectively [22], whereas T. carnifex (Laurenti, 1768) is distributed through the Italian and northern Balkan peninsulas [22]. All the study species occur in natural permanent and temporary aquatic sites with stagnant or semi-flowing water, but they can also colonize artificial aquatic sites (tanks, drinking troughs, reservoirs, and wells) during the breeding season [22]. They are mostly threatened by fragmentation and loss of wetlands, pollution of aquatic habitats, and the introduction of alien fish [22,37,38]. However, only the Italian crested newt is listed on Appendix II of the Bern Convention and on Annexes II and IV of the Habitats Directive (92/43/EEC), while L. italicus is listed in the Annex IV.
Triturus carnifex attains the largest body size of any Italian newt (with females measuring 120–180 mm and males 100–150 mm in total length) and it often co-occurs with L. vulgaris (60–110 mm for males and females) and L. italicus (with a total length of up to 80 mm for the larger females, this species is considered the smallest of the European newts) [22]. Contrarily, L. italicus rarely lives in syntopy with L. vulgaris because of their mostly allopatric distribution [22]. Triturus carnifex and L. vulgaris are the species less dependent on an aquatic environment [22].
2.2. Study Area
Field work was carried out in the Aurunci Mountains, part of the Volsci range, constituted by the Lepini-Ausoni-Aurunci Mts. and forming a limestone chain parallel to Apennines and close to the Tyrrhenian Sea, located in the southern part of the Latium Region (central Italy). They are part of the “Monti Aurunci Regional Park” (surface: 20,000 ha) established in 1997 and characterized by a mosaic of complex and heterogeneous landscapes [39]. The vegetation includes Mediterranean scrubs (Spartium junceum L., Myrtus communis L., Pistacia lentiscus L., Arbutus unedo L., Calluna vulgaris L., Erica spp.) and woodlands (Quercus ilex L.) in the southern slope, whereas several arboreal species (Ostrya carpinifolia Scopoli, Carpino orientalis Miller, Fraxinus ornus L.) have colonized the northern slope [39,40]. At higher altitudes forests with presence of Fagus sylvatica L. are intermitted with grasslands [39,41].
The area considered in the present study is located between 41°27′ N and 41°18′ N latitude and 12°23′ E–13°45′ E longitude, with an extent of about 400 km2 (Figure 1). A comprehensive survey of wells was conducted by locating them on topographical maps (Google Earth©; Figure 1), and 17 artificial aquatic sites were located and geo-referred in the study area.
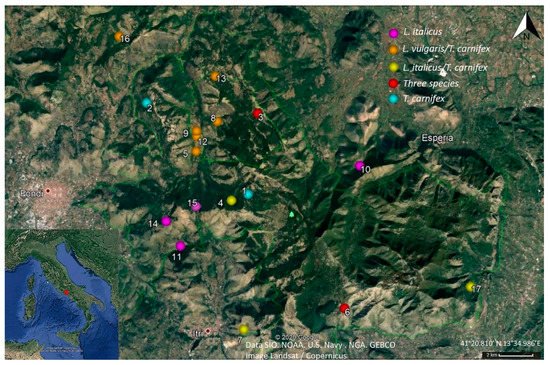
Figure 1.
Map illustrating the distribution of the study wells and the newt species with indication of species coexistence. The numbers on the map refer to the well ID (see Table S1) (Google Earth, earth.google.com/web/).
Wells are characterized by a relatively thick aquatic vegetation, mainly Potamogeton spp. Overall, the macrophytic flora is scant and limited to a few small patches of riparian vegetation and algae. Each aquatic site was sampled at least once during the aquatic phase of newt species (March–July; [22]) (Figure 2).

Figure 2.
A typical well used as water reservoir in the study area. All the sampled wells, characterized by this same structure consisting of vertical stony walls with the upper margin just one small step above the ground, are easily colonized by newts.
At the study area, wells are important elements of the landscape as they are used to help traditional husbandry and agriculture because of the scarcity of natural aquatic systems due to the widespread karst phenomena [42]. The three newt species studied in this paper do occur in wells either alone or in syntopy, but L. vulgaris rarely occurs alone or in exclusive co-presence with L. italicus. In the present paper, new species foraging in the same well, representing a physically closed habitat isolated from the other aquatic sites, were considered as syntopic. On the other hand, species that live in areas with several wetlands, whose borders are arbitrarily defined, often at a larger scale than that perceived by the species, i.e., a lake or river floodplain [8], a forest [43], a mountainous system [44], or a protected area [45], can be defined as sympatric, and thus, by potentially exploiting distinct habitats for reproduction and/or feeding, may not interact at all [19].
2.3. Sampling
Newts were collected (Italian Ministry of the Environment gave LV the permit [0001255/PNM] to conduct stomach flushing and manipulate amphibians) by daytime (from 9:00 a.m. to 6:00 p.m.) during the breeding season (March–July 2019) [22]. Individuals were visually located and captured when surfacing to breathe by using a long-handled dip net (3.5 m in length) from the shore. Immediately after capture, newts were marked by a photograph of the ventral pattern to avoid pseudoreplication, measured (SVL = snout-vent length to the nearest mm) and sexed based on secondary sexual characters [22]. We sampled each well from one to three times to gather enough individuals (n > 7) for each population. Unfortunately, we did not have sufficient recapture data to provide good-enough estimates on density. All the analyses were carried out on adult newts. Stomach contents were collected by stomach flushing [46], individually stored in vials containing 70% ethanol solution and analyzed in the laboratory. Collected newts were temporarily housed in tanks filled with water for approximately two hours after flushing to verify their return to normal activity, and then released at the same point of capture. No mortality was observed during or after stomach flushing. Taxonomic identification of stomach contents was made using a stereomicroscope (Olympus SZX 12. Range of magnification 9–55×). Food items were classified to the lowest taxonomic level possible (usually order or family). Prey composition in newt diet was quantified by estimating the number and occurrence of each food item. The ingestion of plants and minerals was considered accidental and not included in further analyses. We did not estimate prey availability in the environment due to logistic issues. However, it can be assumed that the study wells were homogenous enough in terms of size, structure, and aquatic habitat to limit the variability of trophic resource availability across sites. Although an overall positive effect of ecological opportunity on diet variation is reported for some taxa (i.e., higher prey diversity likely creates more opportunity for individual diet variation; [30,47]), it is very likely that in most habitats, ecological opportunity (i.e., prey availability) may not be limited for generalist predators such as newts, and thus, that it does not represent a sufficient constraint to refrain individual diets from diverging [15,19], so that it may not be biologically important for generalist species in diverse communities [30]. Moreover, the empirical estimate of prey availability should be considered a proxy of, and may diverge from, the actual consumer perception of ecological opportunity due to sampling methods and effort, thus leading to unreliable inferences [30].
2.4. Data Analyses
Only populations represented by n > 7 collected individuals were considered in the analyses. Biometric variables were log-transformed to meet the assumptions of parametric tests. Factorial analysis of variance (ANOVA) was performed to compare SVL among newt species and among study sites and to identify intersexual differences within and among species. ANOVA was also used to test differences in body size for T. carnifex and L. italicus when they occurred as a single species or in syntopy with other newt species. Lissotriton vulgaris was excluded from this latter analysis, since it was always found in syntopy with at least one of the other newt species.
The within-population variation in diet was assessed through the proportional similarity index (PSi) [48,49] that compares the resource use distribution of an individual to that of its population in terms of overlap:
where pij is the frequency of diet category j in the individual i’s diet, and qj is the frequency of diet category j in the population as a whole. PSi equal to qj identified individuals specialized on a single diet item j, whereas PSi values equal to 1 corresponded to conformer individuals (i.e., those consuming resource proportionally to population). The overall prevalence of individual specialization (IS) in the population was expressed by the average PSi value:
IS varies between a value close to 0 (strong individual specialization) and 1 (no individual specialization) [50]. We then represented the population diet variation as V = 1 − IS, which ranges from 0 (all individuals use the full range of resources exploited by the population) to higher decimal values (individuals use only a subset of their population’s diet spectrum) [26].
Population niche breadth was estimated by calculating the total niche width (TNW) of each group quantified by means of the Shannon–Weaver diversity index, following Roughgarden [51]. This index will yield a value of 0 when the entire population uses only a single category of prey, increasing with both the number of prey categories and the evenness with which they are used.
We regressed V on TNW for all the newt populations in order to explore how the relationship between niche width and individual specialization vary among species and in different condition of species co-occurrence. Because the evaluation and comparison (i.e., two populations or the same population at different points) of niche indices are affected by the limitation of arbitrary cut-offs [52], we compared the observed overlap values to an appropriate null model [48]. For each group of individuals, we first pooled all prey counts and determined the frequency of each prey category in the summed population diet. Each individual, observed to have consumed n number of prey items, was then randomly reassigned n items via multinomial sampling from the population diet frequencies. The null degree of IS and diet variation (V) was calculated once all individuals were assigned random diets. For each group, we carried out 999 such resampling estimates. We then regressed the mean resampled V against the observed TNW, to evaluate the null hypothesis that limited individual diet data also generate a positive relationship between these measures. To evaluate whether our observed trend can be explained by this null model alone, we used a General Linear Model (GLM) to test for a difference between the slopes of the observed and simulated V (data type) against TNW (significant interaction term between TNW and “data type” factor indicates that the observed and simulated slopes differ). The same analysis performed on each newt species was conducted on all the species pooled, thus considering all the newt individuals as a model organism irrespective of the species they belong to. This latter analysis allowed to test the overall effect of the degree of syntopy (single species vs. co-occurring species) on the relationship between V and TNW.
The disadvantage of using IS when analyzing different species at distinct locations is represented by the difficulty to interpret high or low values as, respectively, low or high degrees of individual specialization (i.e., arbitrary cut-offs). Indeed, the absolute values index does not unambiguously indicate to what extent the observed variations represent significant differences in individual diet width. The comparison of the observed degree of individual specialization to the distribution of a null population consisting of generalist (i.e., conformer) individuals allows to overcome this potential critical issue. Thus, for those newt species that occurred as single species or syntopic with other species, we used a GLM to test the effect of the species co-occurrence on individual trophic specialization using the percentage of “simulated Psi > observed Psi” as dependent variable.
Statistical tests were performed using STATISTICA software (version 8.0 for Windows) with alpha set at 5%. Both TNW and PSi were calculated in IndSpec1.0 [48].
3. Results
3.1. Body Size
Overall, 562 adult newts were collected from 25 distinct populations with n > 7 (236 T. carnifex, 172 L. vulgaris and 154 L. italicus) (Table S2). Significant differences in SVL among species were found, with T. carnifex > L. vulgaris > L. italicus (Table 1; Figure 3A; for all post hoc comparisons, p < 0.001; refer to Table S2 for a synopsis of newt body size in all sampled sites). Body size differed among study sites and intersexual differences were found within each newt species, with females being larger than males (Table 1; Figure 3A). Both T. carnifex and L. italicus SVL varied significantly in the presence/absence of other newt species (effect of syntopy: Table 1; Figure 3B): T. carnifex was larger when the species occurred in syntopy with other newt species, whereas for L. italicus the opposite was true.

Table 1.
Effect of site (random factor), species, sex, syntopy condition (yes/no), and interaction terms of fixed factors on newt body size (snout-vent length to the nearest mm; SVL) at the study sites. The effect of syntopy condition (°) was tested on Lissotriton italicus and Triturus carnifex only, because Lissotriton vulgaris was always found in co-occurrence with other species. Abbreviations: SS = sum of squares; MS = mean squares. Significant effects are highlighted in bold.
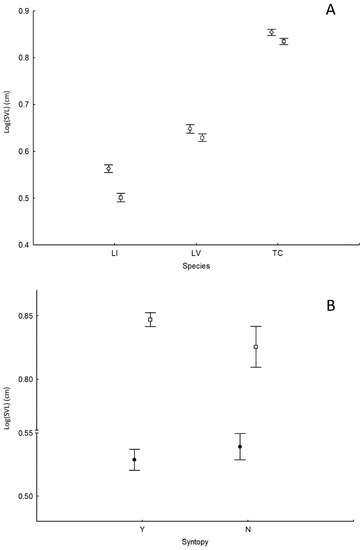
Figure 3.
(A) Effect of species and sex on newt body size (SVL). LI = L. italicus; LV = L. vulgaris; TC = T. carnifex. Circles = females; squares = males. (B) Effect of syntopy on newt body size (SVL). Black circles = L. italicus; empty squares T. carnifex. Y = two or more species co-occurring; N = single species. Lissotriton vulgaris was excluded from this analysis because it was always found in syntopic condition with the remaining species. Vertical bars denote 0.95 confidence intervals.
3.2. Diet Analysis
Overall, few prey categories dominated the diet spectrum of the study species in terms of frequency (Table S3): insect larvae in T. carnifex (69%) and L. italicus (65%), and cladocerans in L. vulgaris (62%). Lissotriton italicus diet was dominated by aquatic insect larvae (32%) followed by cladocerans (23%). In L. vulgaris, the most important food types were cladocerans (72%). Triturus carnifex diet was dominated by cladocerans (48%). Overall, the average number of preys ingested by each newt was 21.53 ± 32.76 (mean ± SD) with minimum and maximum number per stomach ranging 3–383.
3.3. Individual Specialization and the Effect of Coexisting Species on Within-Population Diet Variation
In terms of Total Niche Width (TNW), newt populations did not show any interspecific difference (F2,22 = 1.311, p = 0.290) or any variation in the different condition of syntopy (F1,16 = 0.007, p = 0.935), with consistent behavior among species (F1,16 = 1.724, p = 0.208). Within-population diet variation (V) significantly varied across sites, species (on average, L. vulgaris showed smaller V than the other species), and syntopy condition with V being significantly higher in coexisting populations (Table 2).

Table 2.
Effect of site (random factor), species, sex, syntopy condition (yes/no), newt body size (SVL; covariate), and interaction terms of fixed factors on newt diet variation (V = 1 − IS; see Methods) at the study sites. The effect of syntopy condition (°) was tested on L. italicus and T. carnifex only because L. vulgaris was always found in co-occurrence with other species. Significant effects are highlighted in bold.
Moreover, V increased with population niche breadth (TNW) in all three newt species considered. Linear regression confirmed a significant positive slope in each case for both observed and simulated data, with observed V values always larger than simulated ones, whereas slopes did not differ between observed and simulated data (LI: slopeobs = 0.305, R2obs = 0.743, slopesim = 0.194, R2sim = 0.656, obs ≠ sim F1,12 = 1.426, p = 0.255. LV: slopeobs = 0.269; R2obs = 0.882, slopesim = 0.317, R2sim = 0.938, obs ≠ sim F1,6 = 0.429, p = 0.537; TC: slopeobs = 0.243, R2obs = 0.461, slopesim = 0.248, R2sim = 0.702, obs ≠ sim F1,20 = 0.003, p = 0.956). When we pooled data from all the species, the overall patterns of the relationship between V and TNW mirrored those found in each single species (slopeobs = 0.273, R2obs = 0.611, slopesim = 0.239, R2sim = 0.742, obs ≠ sim F1,48 = 0.401, p = 0.530. Figure 4A). This pattern was apparently disrupted by the effect of syntopy condition: populations occurring as a single species had a significantly stronger and steeper V vs. TNW relationship than the null model (slopeobs = 0.329, R2obs = 0.930, slopesim = 0.231, R2sim = 0.795, obs ≠ sim F1,12 = 4.848, p = 0.049. Figure 4C), whereas populations occurring syntopically with others exhibited a larger diet variation than the null model but with no significant relationship with TNW (slopeobs = −0.014, R2obs = 0.002, slopesim = 0.242, R2sim = 0.700, obs ≠ sim F1,30 = 0.001, p = 0.976. Figure 4B).
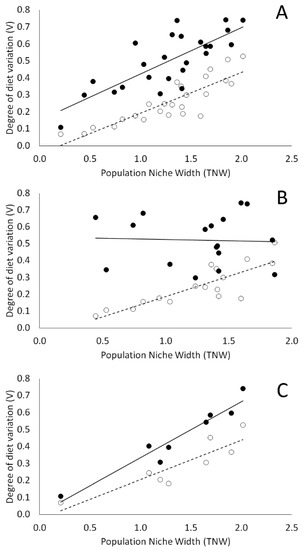
Figure 4.
Correlation between diet variation among individuals (V) and the TNW for all the newt populations (A), for coexisting populations (B), and for population living in isolation (C). The observed values are shown with filled circles (and continue regression line). Empty circles (and the dotted regression line) indicate the expected trend under a null model in which diet variation arises solely by individuals randomly sampling a limited set of prey from a shared prey distribution.
As for the variation of the degree of individual specialization between species and syntopy condition, a clear consistent pattern emerged; the largest species (T. carnifex) showed a higher degree of individual specialization than the smallest one (L. italicus) and both species revealed a significantly higher percentage of specialized individuals in syntopic conditions (Table 3; Figure 5).

Table 3.
Effect of species, syntopy, and interaction terms of fixed factors on the percentage of resampling PSi < observed Psi of L. italicus and T. carnifex populations (see Material and Methods). Lissotriton vulgaris was excluded from the analysis since it was always found in syntopic condition with other species. Significant effects are highlighted in bold.
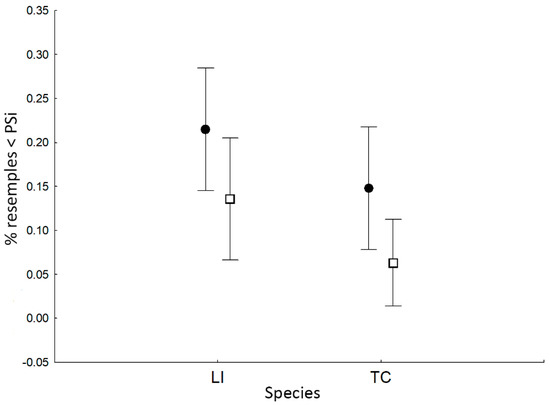
Figure 5.
Effect of species and syntopy on the percentage of resampling PSi < observed Psi of the newt populations (see Methods). Lissotriton vulgaris was excluded from the analysis because this species was always found in syntopic condition with other newt species. Black circles = species alone; empty squares = two or more coexisting species; LI = L. italicus; TC = T. carnifex. Vertical bars denote 0.95 confidence intervals.
4. Discussion
4.1. Body Size and Diet
Newts are considered opportunistic and generalist predators [11,53]. Overall, our results confirm this suggestion as in our study all the three species exploited a large variety of resources with a clear prevalence of aquatic insects (larvae and pupae) and other aquatic invertebrates (mainly cladocerans).
The three studied newt species relied on the same prey types and their dietary similarity was high, as already shown in earlier studies [14,15,19,54]. Our findings are also consistent with previous studies documenting that syntopic species generally show overlap in the use of resources during the aquatic phase [19,54,55]. Newts also generally exhibit a seasonal dietary plasticity [21], which allows them to consume prey opportunistically in relation to their availability and local abundance [18,56].
As expected, the three species vary significantly in SVL, with T. carnifex being the largest and L. italicus the smallest [22]. Although this result is not surprising per se, it is, however, intriguing that T. carnifex grew larger when found in syntopy with one or two smaller newt species than elsewhere. It is possible that this enlarged body size may merely depend on a more abundant prey availability, allowing two or three species to live together. We reject this hypothesis; whereas prey availability should have affected all the species belonging to the same assemblage if the hypothesis was to be supported, we found that, instead, this pattern was not consistent across species (see below for L. italicus). Moreover, this pattern could also be due to an advantage of growing bigger as a larger body size may minimize niche overlap with the smaller species, by widening the trophic spectrum (i.e., preying on bigger prey items; [33]), or even to increase the ability to directly prey on the smaller ones (we found a subadult of L. vulgaris in a T. carnifex stomach; [19,57]). Our data are not sufficient to disentangle the true reasons behind the above-mentioned pattern. Italian newts were larger in absence of the other two species. Since the three wells where we found L. italicus as single species were very small and similar between each other, we can exclude the absolute size of the site as the factor. It is possible that interspecific interactions, such as competition for trophic resources, can impact on the body size of L. italicus, on the other hand smaller sizes may allow the usage of resources not reachable by the larger species (e.g., refugees to escape predation, etc.).
4.2. Individual Specialization and the Effect of Coexisting Species on Within-Population Diet Variation
In our study system, we assumed that a newt population living in absence of other newt species can access resources that may otherwise be depleted or monopolized by competitors, which thus can experience a niche expansion via “ecological release” [27]. This niche width (NW) variation may occur at both the population and the individual levels. Bolnick et al. [27] illustrated three scenarios with (1) a population NW varying together with individual NWs (parallel release), (2) a population NW varying while individual NWs remain constant (NVH; [58]), (3) a population NW remaining constant and individual NWs expanding (“individual release”). In our study system, newt populations did not show interspecific differences in their population NW, which in turn did not vary between presence and absence of coexisting species (i.e., competitors). Overall, the within-population diet variation (V) increased with population NW in all three newt species examined, showing values of V significantly larger than null populations but with no significant slopes. This would mean that the observed pattern could be explained by the null model alone. The increasing values of V at larger values of TNW was potentially due to the small number of preys recorded per individual, which may have underestimated the diversity of preys actually consumed, and therefore, overestimated the variation among individuals [27]. However, it is unlikely that the high number of prey per stomach recorded in all the three species did not properly represent the actual diversity of prey consumed by newts. In addition, the observed level of diet variation, always significantly larger than expected by chance, indicated a high degree of individual specialization not explained by the null model (random sampling from a common diet distribution) [26].
The observed positive relationship between the degree of diet variation and the population diet width was consistent among all the populations considered, but this pattern was disrupted by the condition of syntopy. In presence of coexisting species, newt populations showed an individual diet variation invariant with TNW. This would suggest that interspecific competition could limit the degree of individual diet variation by setting a maximum threshold. In this condition, the increase of TNW is accomplished by the increase of individual NW. Conversely, when newt species did not face potential interspecific competitors, individuals showed larger diet variations, with the relationship with TNW being significantly steeper than the null model. L. italicus and T. carnifex showed a consistent pattern of higher individual specialization in absence of coexisting heterospecific newts, and therefore, a comparatively lower individual NW. Theoretical models predict that niche variation between individuals should weaken coexistence by reducing (a) species-level niche differentiation and (if coupled with demographic stochasticity) (b) the likelihood of long-term coexistence by favoring abundant competitors over species recovering from small population sizes [29]. Our short-term study does not exactly mirror these predictions but revealed that interspecific competition could limit the degree of trophic niche variation between individuals and thus favoring species coexistence. By and large, the observed pattern of newt behavior (stable TNW and increased individual specialization) was not consistent with any of the proposed scenarios.
Costa-Pereira et al. [30], in a field study on coexisting frogs in central Brazil, suggested that individual niche specialization can be strongly context-dependent within and across species. Thus, the hierarchies of individual variation among coexisting species are not necessarily consistent across communities. Similarly, Cloyed and Eason [31] found inconsistent patterns for the effect of heterospecific density on individual trophic specialization. Our study was partially in disagreement with this conclusion, as in our newt assemblages there was a consistent pattern with the degree of individual specialization being (1) size-dependent (percentage of specialized individuals increasing within larger sized species) and (2) assemblage-complexity-dependent (percentage of specialized individuals increasing when a given species does occur syntopically with other species in comparison when it occurs singly in the environment). Therefore, our study provided more consistent evidence with the experimental study on freshwater fish by Bolnick et al. [27] in showing a heterogeneous and contrasting effect of competition on the individual specialization and on the niche width of the whole population.
5. Conclusions
Population niche breadth is thought to represent a balance between the diversifying effect of intraspecific competition and the constraints imposed by interspecific competition [51]. Our study system, consisting of several replicates of the same habitat hosting different co-occurring species combinations, represents a unique opportunity to study the causes and consequences of population and individual niche variation in natural communities. Indeed, our study elucidates important aspects of how individual niche specialization varies across similar coexisting species. While our findings support some predictions of current theory (i.e., the diffuse occurrence of individual specialization [32]), they also provide novel insights into how individual niche variation in closely related and ecologically similar species diverges in different conditions of syntopy. Moreover, while most studies concluded that the ecological parameters driving the development of individual specialization are not consistent across potentially competing species [30,31], we found consistent pattern of increased individual specialization across species in presence of interspecific competition.
Since all the studies published so far consisted of only few sympatric species (up to three in our study case, up to four in Costa-Pereira et al. [30], up to five in Cloyed and Eason [31], and even just two in the experiments by Bolnick et al. [27]), it remains unstudied how much the “intensity” and the “regime” of the interspecific specialization may vary in natural species-rich communities and at different levels of the trophic chain. Without more studies on species-rich communities, it will remain impossible to effectively understand whether the full NVH may be accepted or rejected. In addition, in our study we did not analyze newt density and prey availability as factors potentially influencing the observed patterns. However, since some of the patterns described in this paper may be density dependent [30] or affected by ecological opportunity [59], further studies are needed to examine the effects of population densities and prey availability on population and individual niche variation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/5/181/s1, Table S1: List of the surveyed sites with the recorded species and geographic coordinates. Table S2: Synopsis of the all sampled population in all surveyed sites in the study area. Species codes: Li = Lissotriton italicus; Lv = Lissotriton vulgaris; Tc = Triturus carnifex. Sex codes: M = males; F = females; N = number of sampled individuals; SVL = average snout-vent-length (cm). Table S3: Number (N) and frequency of occurrence (Freq) of prey items ingested by the three species studied. Species codes: Li = Lissotriton italicus; Lv = Lissotriton vulgaris; Tc = Triturus carnifex. The number of analyzed stomachs per species is reported in brackets.
Author Contributions
Conceptualization, L.V.; methodology, L.V.; software, L.V. and J.M.; validation, A.M.B. and L.L.; formal analysis, L.V. and J.M.; investigation, J.M. and L.S.; resources, L.S. and L.V.; data curation, J.M. and A.M.B.; writing—original draft preparation, A.M.B., L.L., and L.V.; writing—review and editing, M.A.B., L.S., and J.M.; visualization, A.M.B.; supervision, L.V.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Grant to Department of Science, Roma Tre University (MIUR-Italy Dipartimenti di Eccellenza, ARTICOLO 1, COMMI 314–337 LEGGE 232/2016).
Acknowledgments
The authors are indebted to the Aurunci Natural Park, which authorized this research, with special thanks to G. De Marchis and C. Esposito for their help. We also thank G. Simbula for her help in the field.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petranka, J.W. Fish predation: A factor affecting the spatial distribution of a stream-breeding salamander. Copeia 1983, 1983, 624–628. [Google Scholar] [CrossRef]
- Resetarits, W.J. Differences in an ensemble of streamside salamanders (Plethodontidae) above and below a barrier to brook trout. Amphibia-Reptilia 1997, 18, 15–25. [Google Scholar] [CrossRef]
- Wilkins, R.N.; Peterson, N.P. Factors related to amphibian occurrence and abundance in headwater streams draining second-growth Douglas-fir forests in southwestern Washington. For. Ecol. Manag. 2000, 139, 79–91. [Google Scholar] [CrossRef]
- Sánchez-Hernández, J. Reciprocal role of salamanders in aquatic energy flow pathways. Diversity 2020, 12, 32. [Google Scholar] [CrossRef]
- Murphy, M.L.; Hall, J.D. Vaired effects of clear-cut logging on predators and their habitat in small streams of the Cascade Mountains, Oregon. Can. J. Fish. Aq. Sci. 1981, 38, 137–145. [Google Scholar] [CrossRef]
- Marcot, B.G.; Vander Heyden, M. Key ecological functions of wildlife species. In Wildlife-Habitat Relationships in Oregon and Washington; Johnson, D.H., O’Neil, T.A., Eds.; Technical Coordinators Oregon State University Press: Corvallis, OR, USA, 2001. [Google Scholar]
- Schabetsberger, R.; Jersabek, C.D. Alpine newts (Triturus alpestris) as top predators in a high-altitude karst lake: Daily food consumption and impact on the copepod Arctodiaptomus Alpinus. Freshw. Biol. 1995, 33, 47–61. [Google Scholar] [CrossRef]
- Cogălniceanu, D.; Palmer, M.; Ciubuc, C. Feeding in anuran communities on islands in the Danube floodplain. Amphibia-Reptilia 2001, 22, 1–19. [Google Scholar]
- Buono, V.; Bissattini, A.M.; Vignoli, L. Can a cow save a newt? The role of cattle drinking troughs in amphibian conservation. Aquat. Conserv. 2019, 29, 964–975. [Google Scholar] [CrossRef]
- Semlitsch, R.D.; O’Donnell, K.M.; Thompson, F.R., III. Abundance, biomass production, nutrient content, and the possible role of terrestrial salamanders in Missouri Ozark forest ecosystems. Can. J. Zool. 2014, 92, 997–1004. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Davic, R.D.; Welsh JR, H.H. On the ecological roles of salamanders. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 405–434. [Google Scholar] [CrossRef]
- Polis, G.A.; Strong, D.R. Food web complexity and community dynamics. Am. Nat. 1996, 147, 813–846. [Google Scholar] [CrossRef]
- Avery, R.A. Food and feeding relations of three species of Triturus (Amphibia Urodela) during the aquatic phases. Oikos 1968, 19, 408–412. [Google Scholar] [CrossRef]
- Griffiths, R.A. Feeding niche overlap and food selection in smooth and palmate newts, Triturus vulgaris and T. helveticus, at a pond in mid-Wales. J. Anim. Ecol. 1986, 55, 201–214. [Google Scholar] [CrossRef]
- Joly, P.; Giacoma, C. Limitation of similarity and feeding habits in three syntopic species of newts (Triturus, Amphibia). Ecography 1992, 15, 401–411. [Google Scholar] [CrossRef]
- Petranka, J.W. Salamanders of the United States and Canada; Smithsonian Institution Press: Washington, DC, USA, 1998. [Google Scholar]
- Vignoli, L.; Bologna, M.A.; Luiselli, L. Seasonal patterns of activity and community structure in an amphibian assemblage at a pond network with variable hydrology. Acta Oecol. 2007, 31, 185–192. [Google Scholar] [CrossRef]
- Vignoli, L.; Luiselli, L.; Bologna, M.A. Dietary patterns and overlap in an amphibian assemblage at a pond in Mediterranean Central Italy. Vie Milieu 2009, 59, 47–57. [Google Scholar]
- Kopecký, O.; Novak, K.; Vojar, J.; Šusta, F. Food composition of alpine newt (Ichthyosaura alpestris) in the post-hibernation terrestrial life stage. North-West. J. Zool. 2016, 12, 299–303. [Google Scholar]
- Fasola, M.; Canova, L. Feeding habits of Triturus vulgaris, T. cristatus and T. alpestris (Amphibia, Urodela) in the northern Apennines (Italy). Ital. J. Zool. 1992, 59, 273–280. [Google Scholar]
- Lanza, B.; Andreone, F.; Bologna, M.A.; Corti, C.; Razzetti, E. Amphibia; Edizioni Calderini: Bologna, Italy, 2007. [Google Scholar]
- Bologna, M.A.; Capula, M.; Carpaneto, G.M. Anfibi e Rettili Del Lazio; Palombi Editori: Rome, Italy, 2000. [Google Scholar]
- Araújo, M.S.; Bolnick, D.I.; Layman, C.A. The ecological causes of individual specialisation. Ecol. Lett. 2011, 14, 948–958. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Ballare, K.M. Resource diversity promotes among-individual diet variation, but not genomic diversity, in lake stickleback. Ecol. Lett. 2020, 23, 495–505. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Araújo, M.S.; Persson, L. Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proc. Natl. Acad. Sci. USA 2007, 104, 10075–10079. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Ingram, T.; Stutz, W.E.; Snowberg, L.K.; Lau, O.L.; Paull, J.S. Ecological release from interspecific competition leads to decoupled changes in population and individual niche width. Proc. R. Soc. B 2010, 277, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Barabás, G.; D’Andrea, R. The effect of intraspecific variation and heritability on community pattern and robustness. Ecol. Lett. 2016, 19, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Hart, S.P.; Schreiber, S.J.; Levine, J.M. How variation between individuals affects species coexistence. Ecol. Lett. 2016, 19, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pereira, R.; Rudolf, V.H.; Souza, F.L.; Araújo, M.S. Drivers of individual niche variation in coexisting species. J. Anim. Ecol. 2018, 87, 1452–1464. [Google Scholar] [CrossRef] [PubMed]
- Cloyed, C.S.; Eason, P.K. Different ecological conditions support individual specialization in closely related, ecologically similar species. Evol. Ecol. 2016, 30, 379–400. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Svanbäck, R.; Fordyce, J.A.; Yang, L.H.; Davis, J.M.; Hulsey, C.D.; Forister, M.L. The ecology of individuals: Incidence and implications of individual specialization. Am. Nat. 2003, 161, 1–28. [Google Scholar] [CrossRef]
- Vignoli, L.; Bissattini, A.M.; Luiselli, L. Food partitioning and the evolution of non-randomly structured communities in tailed amphibians: A worldwide systematic review. Biol. J. Lin. Soc. 2017, 120, 489–502. [Google Scholar] [CrossRef]
- Cloyed, C.S.; Eason, P.K. Feeding limitations in temperate anurans and the niche variation hypothesis. Amphibia-Reptilia 2017, 38, 473–482. [Google Scholar] [CrossRef]
- Toscano, B.J.; Gownaris, N.J.; Heerhartz, S.M.; Monaco, C.J. Personality, foraging behavior and specialization: Integrating behavioral and food web ecology at the individual level. Oecologia 2016, 182, 55–69. [Google Scholar] [CrossRef]
- Cerini, F.; Bologna, M.A.; Vignoli, L. Dragonflies community assembly in artificial habitats: Glimpses from field and manipulative experiments. PLoS ONE 2019, 14. [Google Scholar] [CrossRef] [PubMed]
- Scillitani, G.; Scalera, R.; Carafa, M.; Tripepi, S. Conservation and biology of Triturus italicus in Italy (Amphibia, Salamandridae). It. J. Zool. 2004, 71, 45–54. [Google Scholar] [CrossRef]
- Razzetti, E.; Bernini, F. Triturus vulgaris. In Atlas of Italian Amphibians and Reptiles Societas Herpetologica Italica; Sindaco, R., Doria, G., Razzetti, E., Bernini, F., Eds.; Edizioni Polistampa: Firenze, Italy, 2006. [Google Scholar]
- Novellino, D. An account of basket weaving and the use of fibre plants in the Mount Aurunci Regional Park (Central Italy). In Proceedings of the Fourth International Conference of Ethnobotany (ICEB 2005), Istanbul, Turkey, 21–26 August 2005. [Google Scholar]
- Anzalone, B. Prodromo Della Flora Romana: Elenco Preliminare Delle Piante Vascolari Spontanee Del Lazio; Regione Lazio: Rome, Italy, 1984. [Google Scholar]
- Minutillo, F.; Moraldo, B.; Ross, W. Segnalazioni floristiche italiane. Inform. Bot. Ital. 1985, 17, 124–127. [Google Scholar]
- Romano, A.; Montinaro, G.; Mattoccia, M.; Sbordoni, V. Amphibians of the Aurunci Mountains (Latium, Central Italy). Checklist and conservation guidelines. Acta Herpetol. 2007, 2, 17–25. [Google Scholar]
- Salvidio, S.; Sindaco, R.; Emanueli, L. Feeding habits of sympatric Discoglossus montalentii, Discoglossus sardus and Euproctus montanus during the breeding season. Herpetol. J. 1999, 9, 163–167. [Google Scholar]
- Lizana, M.; Perez-Mellado, V.; Ciudad, M.J. Analysis of the structure of an amphibian community in the central system of Spain. Herpetol. J. 1990, 1, 435–446. [Google Scholar]
- Reques, R.; Tejedo, M. Fenología y hábitats reproductivos de una comunidad de anfibios en la Sierra de Cabra (Córdoba). Rev. Esp. Herp. 1991, 6, 49–54. [Google Scholar]
- Solé, M.; Beckmann, O.; Pelz, B.; Kwet, A.; Engels, W. Stomach-flushing for diet analysis in anurans: An improved protocol evaluated in a case study in Araucaria forests, southern Brazil. Stud. Neotrop. Fauna Environ. 2005, 40, 23–28. [Google Scholar] [CrossRef]
- Newsome, S.D.; Tinker, M.T.; Gill, V.A.; Hoyt, Z.N.; Doroff, A.; Nichol, L.; Bodkin, J.L. The interaction of intraspecific competition and habitat on individual diet specialization: A near range-wide examination of sea otters. Oecologia 2015, 178, 45–59. [Google Scholar] [CrossRef]
- Bolnick, D.I.; Yang, L.H.; Fordyce, J.A.; Davis, J.M.; Svanbäck, R. Measuring individual-level resource specialization. Ecology 2002, 83, 2936–2941. [Google Scholar] [CrossRef]
- Schoener, T.W. The Anolis lizards of Bimini: Resource partitioning in a complex fauna. Ecology 1968, 49, 704–726. [Google Scholar] [CrossRef]
- Svanbäck, R.; Persson, L. Individual diet specialization, niche width and population dynamics: Implications for trophic polymorphisms. J. Anim. Ecol. 2004, 73, 973–982. [Google Scholar] [CrossRef]
- Roughgarden, J. Evolution of niche width. Am. Nat. 1972, 106, 683–718. [Google Scholar] [CrossRef]
- Feinsinger, P.; Spears, E.E.; Poole, R.W. A simple measure of niche breadth. Ecology 1981, 62, 27–32. [Google Scholar] [CrossRef]
- Griffiths, R.A. Newts and Salamanders of Europe; T & AD Poyser: London, UK, 1996. [Google Scholar]
- Dolmen, D.; Koksvik, J.I. Food and feeding habits of Triturus vulgaris (L.) and T. cristatus (Laurenti) (Amphibia) in two bog tarns in central Norway. Amphibia-Reptilia 1983, 4, 17–24. [Google Scholar] [CrossRef]
- Griffiths, R.A.; Mylotte, V.J. Microhabitat selection and feeding relations of smooth and warty newts, Triturus vulgaris and T. cristatus, at an upland pond in mid-Wales. Ecography 1987, 10, 1–7. [Google Scholar] [CrossRef]
- Vignoli, L.; Bombi, P.M.; D’Amen, M.; Bologna, M.A. Seasonal variation in the trophic niche of a heterochronic population of Triturus alpestris apuanus from the south-western Alps. Herpetol. J. 2007, 17, 183–191. [Google Scholar]
- Fasola, M. Resource partitioning by three species of newts during their aquatic phase. Ecography 1993, 16, 73–81. [Google Scholar] [CrossRef]
- Van Valen, L. Morphological variation and width of ecological niche. Am. Nat. 1965, 99, 377–390. [Google Scholar] [CrossRef]
- Salvidio, S.; Crovetto, F.; Costa, A. Individual trophic specialisation in the Alpine newt increases with increasing resource diversity. Ann. Zool. Fenn. 2019, 56, 17–24. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).