Aggressive Predation Drives Assembly of Adriatic Fish Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Fish and Habitats
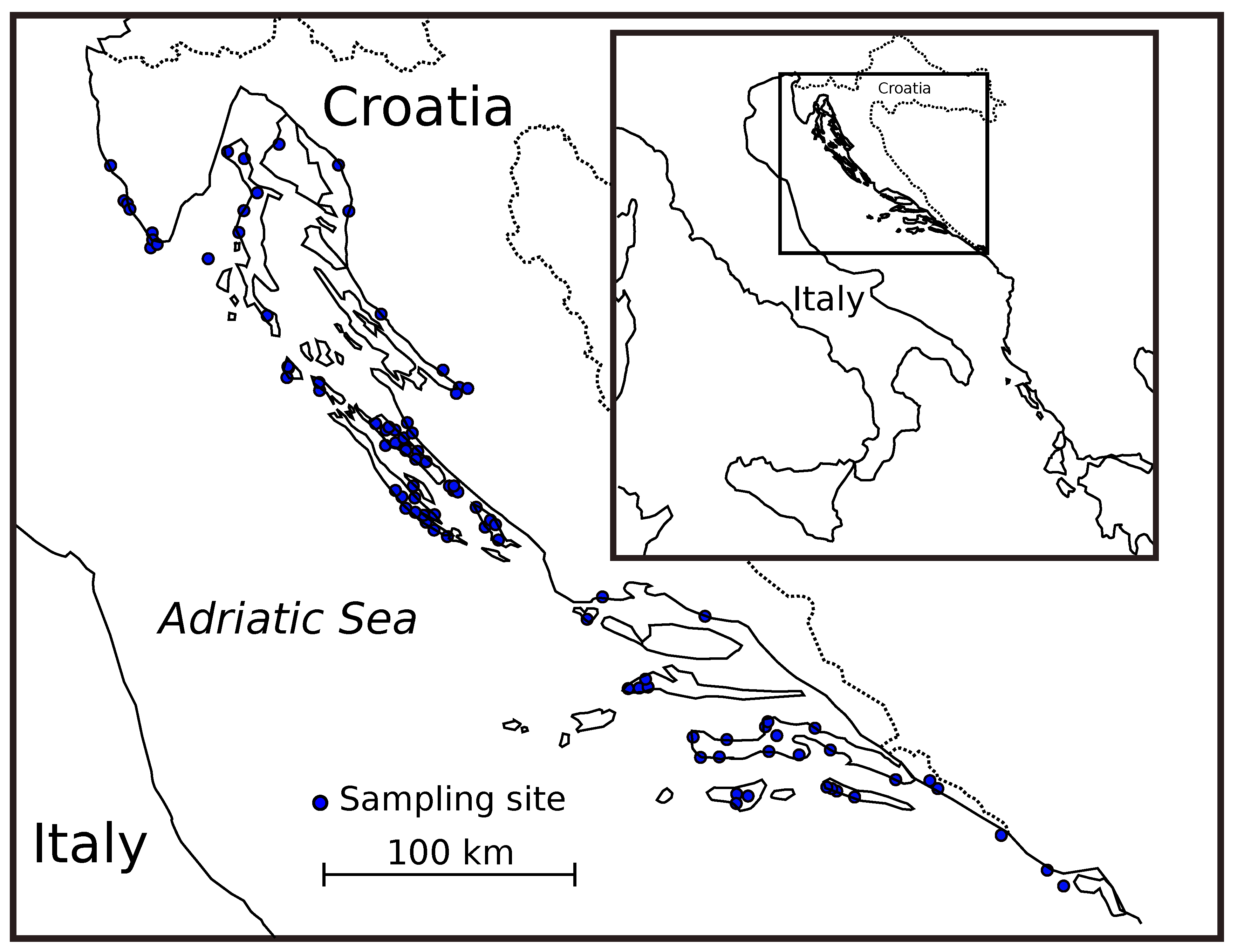
2.2. Sampling Scale and Locations
2.3. Transects
2.4. Homogeneous vs. Heterogeneous Habitats
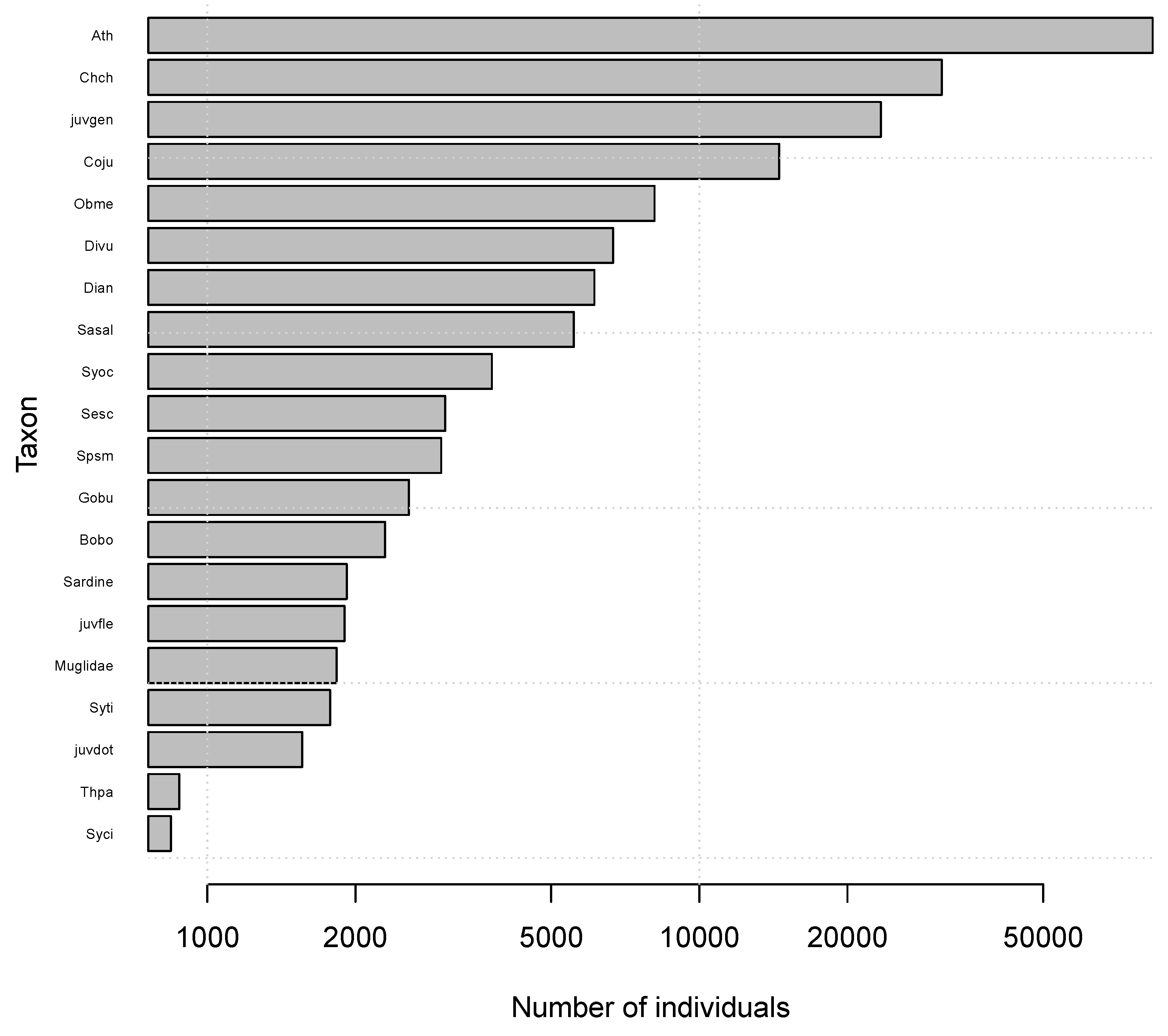
2.5. Fish Functional Groups
2.6. Predator Aggression
2.7. Predation Modes
2.8. Statistical Analysis
3. Results
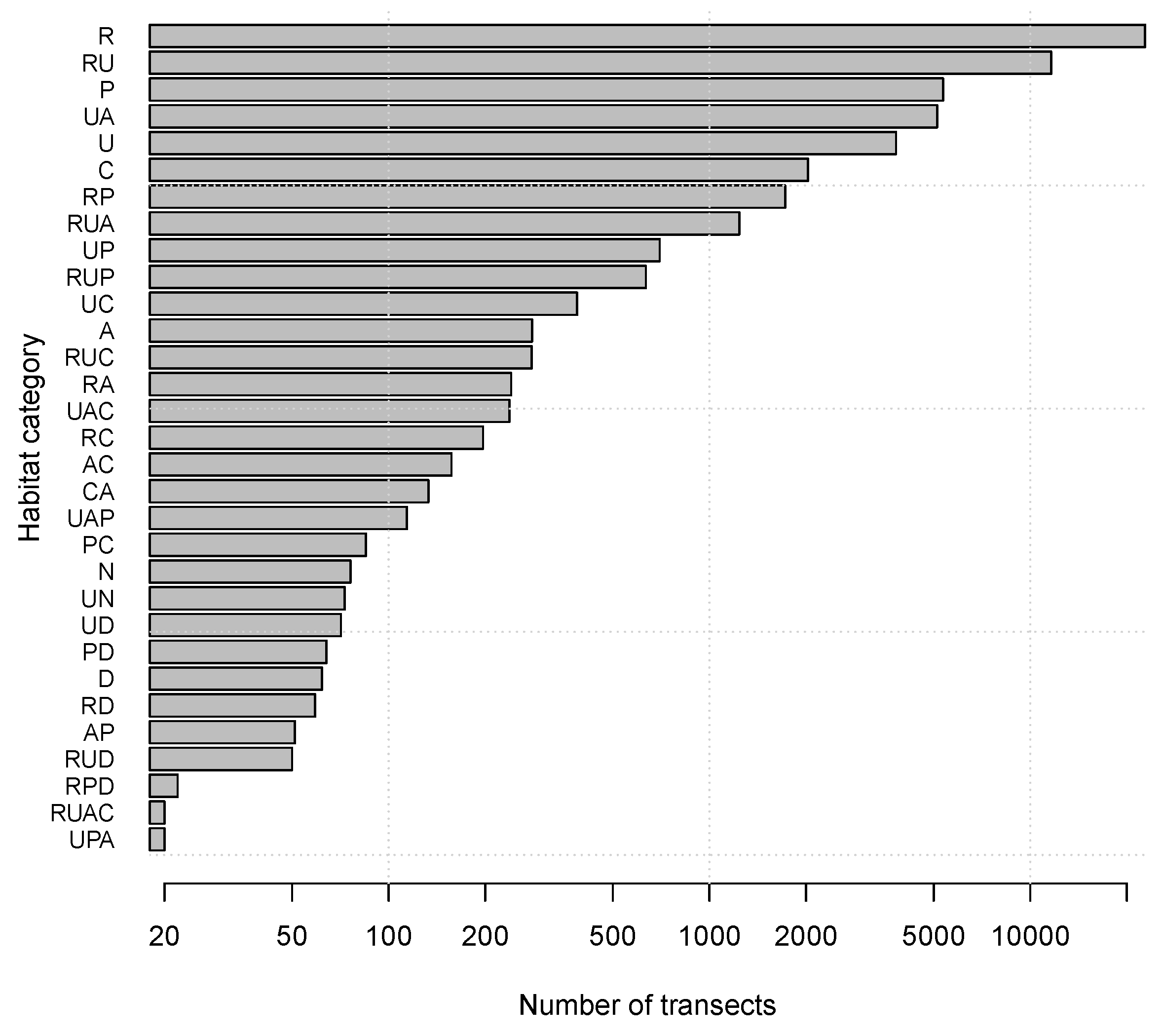
3.1. Spatial Segregation
3.2. Habitat Segregation
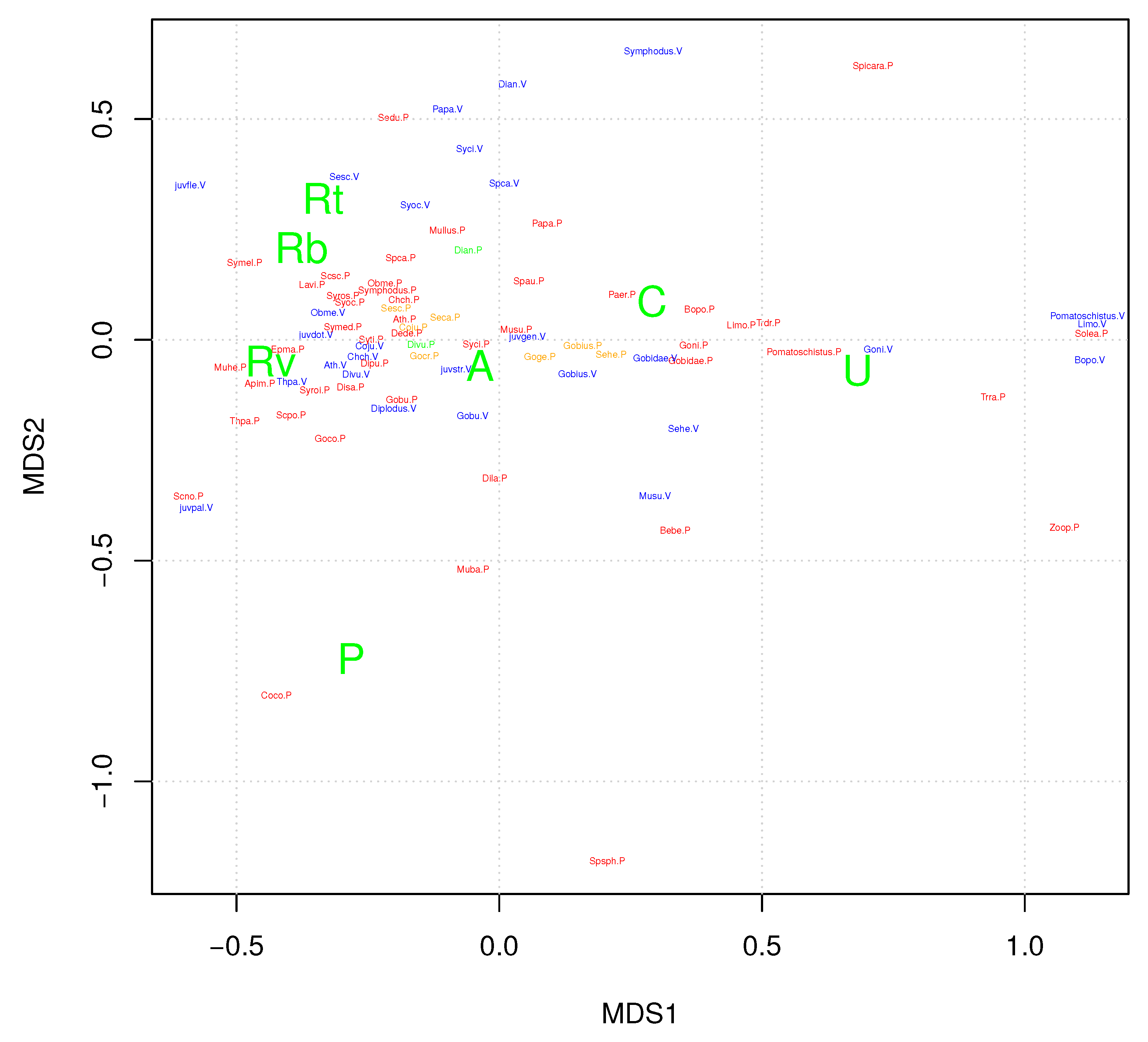
3.3. Ordination
4. Discussion
4.1. Predators and Prey are Negatively Associated at the Local Scale
4.2. Predators Tend to Prefer Habitats that Favor Their Predation Search/Capture Modes
4.3. Aggressive Predators and Prey are Negatively Associated at Larger Scales with Different Habitat Preferences
4.4. Interactions among Predators
5. Caveats and Recommendations for Further Research
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| U | unconsolidated sediment |
| R | rock lacking macroalgae |
| Rv | rock with macroalgae |
| Rt | rock with algal turf and no macroalgae |
| Rb | bare rock |
| A | macroalgae or Active or Ambush |
| C | Cymodocea nodosa seagrass |
| N | Zostera seagrass or less-aggressive predators |
| D | dead Posidonia litter |
| P | Posidonia seagrass or Passive or Predator |
| WCH | wait-chase |
| CRCH | cruise-chase |
| SNA | sneak-attack |
| SNCH | sneak-chase |
| CRDW | cruise-dwelling |
| V | prey |
| PMH | predation mode hypothesis |
References
- Clemente, S.; Hernandez, J.C.; Rodriguez, A.; Brito, A. Identifying keystone predators and the importance of preserving functional diversity in sublittoral rocky-bottom areas. Mar. Ecol. Prog. Ser. 2010, 413, 55–67. [Google Scholar] [CrossRef]
- Atherton, J.A.; McCormick, M.I. Active in the sac: Damselfish embryos use innate recognition of odours to learn predation risk before hatching. Anim. Behav. 2015, 103, 1–6. [Google Scholar] [CrossRef]
- Loennstedt, O.M.; McCormick, M.I. Chemical alarm cues inform prey of predation threat: The importance of ontogeny and concentration in a coral reef fish. Anim. Behav. 2011, 82, 213–218. [Google Scholar] [CrossRef]
- Arnason, E.; Hernandez, U.B.; Kristinsson, K. Intense Habitat-Specific Fisheries-Induced Selection at the Molecular Pan I Locus Predicts Imminent Collapse of a Major Cod Fishery. PLoS ONE 2009, 4, e5529. [Google Scholar] [CrossRef]
- Schultz, S.T.; Kruschel, C.; Bakran-Petricioli, T. Influence of seagrass meadows on predator-prey habitat segregation in an Adriatic lagoon. Mar. Ecol. Prog. Ser. 2009, 374, 85–99. [Google Scholar] [CrossRef]
- Ritchie, E.G.; Johnson, C.N. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009, 12, 982–998. [Google Scholar] [CrossRef]
- Macreadie, P.I.; Geraldi, N.R.; Peterson, C.H. Preference for feeding at habitat edges declines among juvenile blue crabs as oyster reef patchiness increases and predation risk grows. Mar. Ecol. Prog. Ser. 2012, 466, 145–153. [Google Scholar] [CrossRef]
- Nunes, J.A.C.C.; Leduc, A.; Miranda, R.J.; Cipresso, P.H.; Alves, J.P.; Mariano-Neto, E.; Sampaio, C.L.S.; Barros, F. Refuge choice specificity increases with predation risk in a rocky reef fish. J. Exp. Mar. Biol. Ecol. 2019, 520, UNSP151207. [Google Scholar] [CrossRef]
- Gilliam, J.F.; Fraser, D.F. Habitat Selection under Predation Hazard - Test of a Model with Foraging Minnows. Ecology 1987, 68, 1856–1862. [Google Scholar] [CrossRef]
- Bostrom, C.; Pittman, S.J.; Simenstad, C.; Kneib, R.T. Seascape ecology of coastal biogenic habitats: Advances, gaps, and challenges. Mar. Ecol. Prog. Ser. 2011, 427, 191–217. [Google Scholar] [CrossRef]
- Whitfield, A.K. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Rev. Fish Biol. Fish. 2017, 27, 75–110. [Google Scholar] [CrossRef]
- Kruschel, C.; Schultz, S. Lure-assisted visual census: A new method for quantifying fish abundance, behaviour, and predation risk in shallow coastal habitats. Mar. Freshw. Res. 2010, 61, 1349–1359. [Google Scholar] [CrossRef]
- Heck, K.; Crowder, L. Habitat structure and predator—Prey interactions in vegetated aquatic systems. In Habitat Structure; Springer: Berlin/Heidelberg, Germany, 1991; pp. 281–299. [Google Scholar]
- Kovalenko, K.E.; Thomaz, S.M.; Warfe, D.M. Habitat complexity: Approaches and future directions. Hydrobiologia 2012, 685, 1–17. [Google Scholar] [CrossRef]
- Bartholomew, A.; Diaz, R.J.; Cicchetti, G. New dimensionless indices of structural habitat complexity: Predicted and actual effects on a predator’s foraging success. Mar. Ecol. Prog. Ser. 2000, 206, 45–58. [Google Scholar] [CrossRef]
- Hovel, K.A.; Warneke, A.M.; Virtue-Hilborn, S.P.; Sanchez, A.E. Mesopredator foraging success in eelgrass (Zostera marina L.): Relative effects of epiphytes, shoot density, and prey abundance. J. Exp. Mar. Biol. Ecol. 2016, 474, 142–147. [Google Scholar] [CrossRef]
- Horinouchi, M. Review of the effects of within-patch scale structural complexity on seagrass fishes. J. Exp. Mar. Biol. Ecol. 2007, 350, 111–129. [Google Scholar] [CrossRef]
- Schultz, S.T.; Kruschel, C. Frequency and success of ambush and chase predation in fish assemblages associated with seagrass and bare sediment in an Adriatic lagoon. Hydrobiologia 2010, 649, 25–37. [Google Scholar] [CrossRef]
- Fouzai, N.; Opdal, A.F.; Jorgensen, C.; Fiksen, O. Dying from the lesser of three evils: Facilitation and non-consumptive effects emerge in a model with multiple predators. Oikos 2019, 128, 1307–1317. [Google Scholar] [CrossRef]
- Miller, J.R.B.; Ament, J.M.; Schmitz, O.J. Fear on the move: Predator hunting mode predicts variation in prey mortality and plasticity in prey spatial response. J. Anim. Ecol. 2014, 83, 214–222. [Google Scholar] [CrossRef]
- Peckarsky, B.L. Alternative predator avoidance syndromes of stream-dwelling mayfly larvae. Ecology 1996, 77, 1888–1905. [Google Scholar] [CrossRef]
- Sih, A. Prey Uncertainty and the Balancing of Antipredator and Feeding Needs. Am. Nat. 1992, 139, 1052–1069. [Google Scholar] [CrossRef]
- Stoks, R.; McPeek, M.A.; Mitchell, J.L. Evolution of prey behavior in response to changes in predation regime: Damselflies in fish and dragonfly lakes. Evolution 2003, 57, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Hesse, J.; Stanley, J.A.; Jeffs, A.G. Relative predation risk in two types of habitat for juvenile Australasian spiny lobsters, Jasus edwardsii. Mar. Biol. Res. 2016, 12, 895–906. [Google Scholar] [CrossRef]
- Zubak, I.; Kruschel, C.; Schultz, S.T. Predators structure fish communities in Posidonia oceanica meadows: Meta-analysis of available data across the Mediterranean basin. Mar. Ecol. Prog. Ser. 2017, 566, 145–157. [Google Scholar] [CrossRef]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef]
- Coll, M.; Santojanni, A.; Palomera, I.; Arneri, E. Food-web changes in the Adriatic Sea over the last three decades. Mar. Ecol. Prog. Ser. 2009, 381, 17–37. [Google Scholar] [CrossRef]
- Boaden, A.E.; Kingsford, M.J. Predators drive community structure in coral reef fish assemblages. Ecosphere 2015, 6, 46. [Google Scholar] [CrossRef]
- Marba, N.; Diaz-Almela, E.; Duarte, C.M. Mediterranean seagrass (Posidonia oceanica) loss between 1842 and 2009. Biol. Conserv. 2014, 176, 183–190. [Google Scholar] [CrossRef]
- Airoldi, L.; Beck, M.W. Loss, status and trends for coastal marine habitats of Europe. Oceanogr. Mar. Biol. 2007, 45, 345–405. [Google Scholar]
- Giakoumi, S.; Cebrian, E.; Kokkoris, G.D.; Ballesteros, E.; Sala, E. Relationships between fish, sea urchins and macroalgae: The structure of shallow rocky sublittoral communities in the Cyclades, Eastern Mediterranean. Estuar. Coast. Shelf Sci. 2012, 109, 1–10. [Google Scholar] [CrossRef]
- Bonaviri, C.; Gianguzza, P.; Pipitone, C.; Hereu, B. Micropredation on sea urchins as a potential stabilizing process for rocky reefs. J. Sea Res. 2012, 73, 18–23. [Google Scholar] [CrossRef]
- VegaFernandez, T.; Milazzo, M.; Badalamenti, F.; D’Anna, G. Comparison of the fish assemblages associated with Posidonia oceanica after the partial loss and consequent fragmentation of the meadow. Estuar. Coast. Shelf Sci. 2005, 65, 645–653. [Google Scholar] [CrossRef]
- Kruschel, C.; Schultz, S.T. Use of a lure in visual census significantly improves probability of detecting wait-ambushing and fast cruising predatory fish. Fish. Res. 2012, 123, 70–77. [Google Scholar] [CrossRef]
- Loya, Y. Monographs on Oceanic Methodology.Coral Reefs: Research Methods; UNESCO: Paris, France, 1978; Chapter Plotless and Transect Methods; pp. 197–218. [Google Scholar]
- Murphy, H.M.; Jenkins, G.P.; Hindell, J.S.; Connolly, R.M. Response of fauna in seagrass to habitat edges, patch attributes and hydrodynamics. Austral Ecol. 2010, 35, 535–543. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Weber, A.; Pipitone, C.; Leopold, M.; Cronin, M.; Scheidat, M.; Doyle, T.K.; Buhl-Mortensen, L.; Buhl-Mortensen, P.; D’Anna, G.; et al. Monitoring marine populations and communities: Methods dealing with imperfect detectability. Aquat. Biol. 2012, 16, 31–52. [Google Scholar] [CrossRef]
- Watson, D.L.; Harvey, E.S. Behaviour of temperate and sub-tropical reef fishes towards a stationary SCUBA diver. Mar. Freshw. Behav. Physiol. 2007, 40, 85–103. [Google Scholar] [CrossRef]
- Watson, J.J.; Kerley, G.I.H. Survey of the dune-breeding birds in the Eastern Cape, South Africa. Ostrich 1995, 66, 15–20. [Google Scholar] [CrossRef]
- Bussotti, S.; Guidetti, P. Timing and habitat preferences for settlement of juvenile fishes in the Marine Protected Area of Torre Guaceto (south-eastern Italy, Adriatic Sea). Ital. J. Zool. 2011, 78, 243–254. [Google Scholar] [CrossRef]
- García-Rubies, A.; Macpherson, E. Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral. Mar. Biol. 1995, 124, 35–42. [Google Scholar] [CrossRef]
- Raventos, N.; Macpherson, E. Planktonic larval duration and settlement marks on the otoliths of Mediterranean littoral fishes. Mar. Biol. 2001, 138, 1115–1120. [Google Scholar]
- Harmelin-Vivien, M.; Harmelin, J.; Chauvet, C.; Duval, C.; Galzin, R.; Lejeune, P.; Barnabe, G.; Blanc, F.; Chevalier, R.; Duclerc, J.; et al. The underwater observation of fish communities and fish populations. Methods and problems. Revue d’Ecologie (France) 1985, 40, 467–539. [Google Scholar]
- Nagelkerken, I.; van der Velde, G.; Gorissen, M.W.; Meijer, G.J.; van’t Hof, T.; den Hartog, C. Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar. Coast. Shelf Sci. 2000, 51, 31–44. [Google Scholar] [CrossRef]
- Schmidt, T.W.; Thompson, M.J. Validation of the species/time random count technique sampling fish assemblages. In Proceedings of the Third International Symposium of Coral Reefs, Miami, FL, USA, May 1977; Volume 1, pp. 283–288. [Google Scholar]
- Gerking, S. Feeding Ecology of Fishes; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- LIMA, S.L.; DILL, L.M. Behavioral Decisions Made under the Risk of Predation—A Review and Prospectus. Can. J. Zool. -Rev. Can. De Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of Fear: Spatial Patterns of Risk Perception and Response. Trends Ecol. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.C.O.; Wisenden, B.D.; Chivers, D.P. Chemical ecology of predator-prey interactions in aquatic ecosystems: A review and prospectus. Can. J. Zool. 2010, 88, 698–724. [Google Scholar] [CrossRef]
- Broom, M.; Higginson, A.D.; Ruxton, G.D. Optimal investment across different aspects of anti-predator defences. J. Theor. Biol. 2010, 263, 579–586. [Google Scholar] [CrossRef]
- Kruschel, C.; Schultz, S.T. Juvenile Gobius niger avoids seagrass in the presence and uncertain absence of seagrass-inhabiting predators. J. Exp. Mar. Biol. Ecol. 2011, 409, 240–246. [Google Scholar] [CrossRef]
- Kruschel, C.; Harras, J.; Blindow, I.; Schultz, S.T. Do fish assemblages at sites featuring man-made concrete walls differ from those at natural rocky-reef sites? Ann. Ser. Hist. Nat. 2017, 27, 167–180. [Google Scholar]
- Stergiou, K.I.; Karpouzi, V.S. Feeding habits and trophic levels of Mediterranean fish. Rev. Fish Biol. Fish. 2001, 11, 217–254. [Google Scholar] [CrossRef]
- Como, S.; Magni, P.; Baroli, M.; Casu, D.; De Falco, G.; Floris, A. Comparative analysis of macrofaunal species richness and composition in Posidonia oceanica, Cymodocea nodosa and leaf litter beds. Mar. Biol. 2008, 153, 1087–1101. [Google Scholar] [CrossRef]
- Mabrouk, L.; Ben Brahim, M.; Hamza, A.; Bradai, M.N. Diversity and temporal fluctuations of epiphytes and sessile invertebrates on the rhizomes Posidonia oceanica in a seagrass meadow off Tunisia. Mar. Ecol. - Evol. Perspect. 2014, 35, 212–220. [Google Scholar] [CrossRef]
- Sanchez-Jerez, P.; Cebrian, C.B.; Espla, A.A.R. Comparison of the epifauna spatial distribution in Posidonia oceanica, Cymodocea nodosa and unvegetated bottoms: Importance of meadow edges. Acta Oecologica-Int. J. Ecol. 1999, 20, 391–405. [Google Scholar] [CrossRef]
- Scipione, M.B.; Zupo, V. Crustacean amphipods from the seagrasses Zostera marina, Cymodocea nodosa and Posidonia oceanica in the Adriatic Sea (Italy): A first comparison. Zool. Baetica 2010, 21, 15–32. [Google Scholar]
- Hussein, C.; Verdoit-Jarraya, M.; Pastor, J.; Ibrahim, A.; Saragoni, G.; Pelletier, D.; Mahevas, S.; Lenfant, P. Assessing the impact of artisanal and recreational fishing and protection on a white seabream (Diplodus sargus sargus) population in the north-western Mediterranean Sea using a simulation model. Part 1: Parameterization and simulations. Fish. Res. 2011, 108, 163–173. [Google Scholar] [CrossRef]
- Sala, E.; Ballesteros, E. Partitioning of space and food resources by three fish of the genus Diplodus (Sparidae) in a Mediterranean rocky infralittoral ecosystem. Mar. Ecol. Prog. Ser. 1997, 152, 273–283. [Google Scholar] [CrossRef]
- Macpherson, E.; Biagi, F.; Francour, P.; Garcia-Rubies, A.; Harmelin, J.; Harmelin-Vivien, M.; Jouvenel, J.Y.; Planes, S.; Vigliola, L.; Tunesi, L. Mortality of juvenile fishes of the genus Diplodus in protected and unprotected areas in the western Mediterranean Sea. Mar. Ecol. Prog. Ser. 1997, 160, 135–147. [Google Scholar] [CrossRef]
- Cuadros, A.; Basterretxea, G.; Cardona, L.; Cheminee, A.; Hidalgo, M.; Moranta, J. Settlement and post-settlement survival rates of the white seabream (Diplodus sargus) in the western Mediterranean Sea. PLoS ONE 2018, 13, e0190278. [Google Scholar] [CrossRef]
- Bouchoucha, M.; Darnaude, A.M.; Gudefin, A.; Neveu, R.; Verdoit-Jarraya, M.; Boissery, P.; Lenfant, P. Potential use of marinas as nursery grounds by rocky fishes: Insights from four Diplodus species in the Mediterranean. Mar. Ecol. Prog. Ser. 2016, 547, 193–209. [Google Scholar] [CrossRef]
- Northfield, T.D.; Barton, B.T.; Schmitz, O.J. A spatial theory for emergent multiple predator-prey interactions in food webs. Ecol. Evol. 2017, 7, 6935–6948. [Google Scholar] [CrossRef]
- Schmitz, O.J. Predator diversity and trophic interactions. Ecology 2007, 88, 2415–2426. [Google Scholar] [CrossRef]
- Teckentrup, L.; Kramer-Schadt, S.; Jeltsch, F. The risk of ignoring fear: Underestimating the effects of habitat loss and fragmentation on biodiversity. Landsc. Ecol. 2019, 34, 2851–2868. [Google Scholar] [CrossRef]
- McGhee, K.E.; Pintor, L.M.; Bell, A.M. Reciprocal Behavioral Plasticity and Behavioral Types during Predator-Prey Interactions. Am. Nat. 2013, 182, 704–717. [Google Scholar] [CrossRef] [PubMed]
| Prey Search Mode | Search Activity | Interactions with Substrate | Predator Size | Interactions with Prey | Pursuit Mode | Pursuit Activity | Category Names | Taxa |
|---|---|---|---|---|---|---|---|---|
| Lie-in-wait or sit-and wait, no lure | P | Motionless, aggressive and/or protective resemblance | Small to medium | Suction and Strike | No chase | P | A (A) ambush | Bopo, Goco, Gopa, Solea, Zoop Sysa, Trla, Trdr, Trra, Scorpaena, Scno, Scpo, Scsc |
| Lie-in-wait or sit-and wait, no lure | P | Hovering inside caves | Medium to large | Suction and strike | No chase | P | A (A) | Apim, Epma, Scorpaena, Scno, Scpo, Scsc |
| Lie-in-wait or sit-and wait, no lure | P | Hovering above substrate aggressive and/or protective resemblance | Medium | Rushing forward, C-start or S-start | Burst chase | A | WCH | Coju, Epma, Spsph, Seca, Sesc, Sehe, Spsph |
| Lie-in-wait or sit-and wait no lure | P | On or inside substrate, motionless, aggressive and/or protective resemblance | Medium | Burst chase | A | WCH Wait-chase | Coju, Gocr, Gobidae, Gobius, Goge, Goni, Lame, Epma, Spsph, Lavi, Seca, Sesc, Sehe | |
| Stalking and sneaking | A | Slow motion in contact with substrate | Small to medium | Hopping forward use suction | No chase | P | SNA (A) Sneak attack | Scorpaena, Scno, Scpo. Scsc, |
| Stalking and sneaking | A | Slow motion in contact with substrate | Medium to large | chase and strike | Burst chase | A | SNCH (A) | Coco, Muhe, Spsph, Scpo Scorpaena, Scno, |
| Cruising | A | Constant swimming | Medium to large | Sustained fast cruise swimming | Sustained chase | A | CRCH (CH) Cruise-chase | Epal, Bebe, Dila, Racl, Sedu, |
| Cruising | A | Constant swimming | Medium | Sustained patrol swimming | Burst chases | A | CRCH | Dede, Dian, Diplodus, Dipu, Disa, Divu, Papa, Spau, Spca, Limo, Coco, Epal, Bebe, Dila, Sedu |
| Substrate dwelling: | A | Constant swimming | Small to medium | Slow swimming, picking off surface | No chase | A | CRDW (DW) Cruise-dwelling | Syti |
| Sand probers rock, algae, seagrass | A | Constant swimming | Small to medium | probing habitat, suction, biting | No chase | P | CRDW | Limo, Muba, Mullus, Musu, |
| Scrapers | A | Constant swimming | Small to medium | probing habitat suction, biting | No chase | P | CRDW | Spcr |
| Sand scoopers | A | Constant swimming | Small to medium | probing habitat, suction, filtering | No chase | P | CRDW | Limo, Mugilidae, Muba, Mullus, Musu, |
| Detritivores and herbivores | A | Constant swimming | Small to medium | probing, suction, filtering, biting | No chase | P | CRDW | Musu, Sasal, Pasa, Mugilidae |
| Family | Binomial | Abbrev | Family | Binomial | Abbrev |
|---|---|---|---|---|---|
| Apogonidae | Apogon imberbis | Apim | Moronidae | Dicentrarchus labrax | Dila |
| Atherinidae | Atherina boyeri/hepsetus | Ath | Mullidae | Mullus barbatus | Muba |
| Belonidae | Belone belone | Bebe | Mullidae | Mullus surmuletus | Musu |
| Blennidae | Aidablenius sphynx | Aisp | Muraenidae | Muraena helena | Muhe |
| Blennidae | Microlipophrys canevae | Mica | Pomacentridae | Chromis chromis | Chch |
| Blennidae | Microlipophrys nigroceps | Mini | Rajidae | Raja clavata | Racl |
| Blennidae | Parablennius gattorugine | Paga | Sciaenidae | Sciaena umbra | Scum |
| Blennidae | Parablennius incognitus | Pain | Scorpaenidae | Scorpaena notata | Scno |
| Blennidae | Parablennius rouxi | Coco | Scorpaenidae | Scorpaena porcus | Scpo |
| Blennidae | Parablennius sanuinolentus | Pasa | Scorpaenidae | Scorpaena scrofa | Scsc |
| Blennidae | Parablennius tentacularis | Pate | Serranidae | Epinephelus marginatus | Epma |
| Blennidae | Parablennius zvonimiri | Pazv | Serranidae | Serranus cabrilla | Seca |
| Blennidae | Salaria pavo | Sapa | Serranidae | Serranus hepatus | Sehe |
| Bothidae | Bothus podas | Bopo | Serranidae | Serranus scriba | Sesc |
| Carangidae | Seriola dumerili | Sedu | Sparidae | Boops boops | Bobo |
| Congridae | Conger conger | Coco | Sparidae | Dentex dentex | Dede |
| Gobiesocidae | Lepadogaster lepadogaster | Lele | Sparidae | Diplodus annularis | Dian |
| Gobiidae | Gobius bucchichi | Gobu | Sparidae | Diplodus puntazzo | Dipu |
| Gobiidae | Gobius cobitis | Goco | Sparidae | Diplodus sargus | Disa |
| Gobiidae | Gobius cruentatus | Gocr | Sparidae | Diplodus vulgaris | Divu |
| Gobiidae | Gobius fallax | Gofa | Sparidae | Lithognathus mormyrus | Limo |
| Gobiidae | Gobius geniporus | Goge | Sparidae | Oblada melanura | Obme |
| Gobiidae | Gobius auratus | Goau | Sparidae | Pagellus acarne | Paar |
| Gobiidae | Gobius niger | Goni | Sparidae | Pagellus erythrinus | Paer |
| Gobiidae | Gobius paganellus | Gopa | Sparidae | Pagrus pagrus | Papa |
| Gobiidae | Gobius vitatus | Govi | Sparidae | Sarpa salpa | Sasal |
| Gobiidae | Zebrus zebrus | Zeze | |||
| Labridae | Coris julis | Coju | Sepidae | Sepia officinalis | Seof |
| Labridae | Labrus merula | Lame | Octopodidae | Octopus vulgaris | Ocvu |
| Labridae | Labrus viridis | Lavi |
| Species | Abbr. | Mode | Abundance | Prey Pursued | Prey Caught |
|---|---|---|---|---|---|
| Coris julis | Coju | WCH | 14,528 | 10,024 | 436 |
| Serranus scriba | Sesc | WCH | 3044 | 1826 | 396 |
| Diplodus annularis | Dian | CRCH | 6119 | 4406 | 245 |
| Serranus cabrilla | Seca | WCH | 515 | 458 | 160 |
| Diplodus vulgaris | Divu | CRCH | 6679 | 3072 | 67 |
| Gobius geniporus | Goge | WCH | 268 | 134 | 16 |
| Serranus hepatus | Sehe | WCH | 693 | 319 | 14 |
| Gobius spp. | Gobius | WCH | 458 | 156 | 9 |
| Gobius cruentatus | Gocr | WCH | 125 | 125 | 4 |
| Model | Df | Deviance | Resid. Df | Resid. Dev | Pr(>Chi) |
|---|---|---|---|---|---|
| V ∼ P | 1 | 2217 | 19676 | 343101 | <0.001 |
| V ∼ N | 1 | 253 | 19676 | 345066 | <0.001 |
| N ∼ P | 1 | 270 | 19676 | 711294 | <0.001 |
| Species | Most Preferred | Most Avoided | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Coris julis | UA | RUA | RU | U | C | P |
| Serranus scriba | UA | RU | P | U | C | R |
| Diplodus annularis | P | PC | UA | R | U | RU |
| Serranus cabrilla | RD | UA | RUA | R | U | C |
| Diplodus vulgaris | UA | RU | RUA | P | U | C |
| Gobius geniporus | RU | UA | U | R | P | C |
| Serranus hepatus | UA | RUAD | C | R | P | RP |
| Gobius spp. | U | RU | UC | R | P | RP |
| Gobius cruentatus | RU | RUC | AC | P | U | C |
| Habitat | Occurrence | Chi-Square Residuals | ||||
|---|---|---|---|---|---|---|
| V | N | P | V | N | P | |
| UA | 213 | 1891 | 3021 | −9.03 | −12.12 | 16.58 |
| RUA | 36 | 485 | 718 | −6.03 | −4.83 | 7.62 |
| RUP | 16 | 219 | 399 | −4.66 | −5.11 | 7.35 |
| UP | 29 | 268 | 403 | −3.35 | −3.95 | 5.58 |
| A | 6 | 162 | 112 | −3.33 | 2.19 | −0.92 |
| RP | 96 | 661 | 965 | −3.11 | −6.14 | 7.79 |
| N | 0 | 51 | 25 | −2.41 | 2.3 | −1.42 |
| R | 1651 | 12695 | 8458 | −2.21 | 15.05 | −14.98 |
| D | 0 | 18 | 44 | −2.18 | −2.22 | 3.26 |
| RUD | 1 | 30 | 19 | −1.44 | 1.14 | −0.6 |
| RUAC | 0 | 7 | 13 | −1.24 | −0.88 | 1.45 |
| UPA | 0 | 7 | 13 | −1.24 | −0.88 | 1.45 |
| UD | 3 | 28 | 40 | −1.04 | −1.12 | 1.62 |
| RUAP | 0 | 7 | 6 | −1 | 0.27 | 0.14 |
| AP | 2 | 19 | 30 | −0.96 | −1.17 | 1.64 |
| PA | 0 | 1 | 6 | −0.73 | −1.31 | 1.68 |
| APC | 0 | 1 | 5 | −0.68 | −1.12 | 1.47 |
| CA | 8 | 95 | 30 | −0.68 | 3.75 | −3.68 |
| RAP | 0 | 2 | 3 | −0.62 | −0.28 | 0.55 |
| PC | 5 | 27 | 53 | −0.59 | −2.24 | 2.61 |
| PCD | 0 | 0 | 4 | −0.55 | −1.4 | 1.71 |
| RPC | 0 | 0 | 4 | −0.55 | −1.4 | 1.71 |
| RUAD | 0 | 0 | 3 | −0.48 | −1.21 | 1.48 |
| UCA | 0 | 2 | 1 | −0.48 | 0.45 | −0.27 |
| CD | 0 | 1 | 1 | −0.39 | 0.03 | 0.14 |
| AU | 0 | 2 | 0 | −0.39 | 1.04 | −0.93 |
| UCD | 0 | 2 | 0 | −0.39 | 1.04 | −0.93 |
| UPC | 0 | 2 | 0 | −0.39 | 1.04 | −0.93 |
| AN | 0 | 1 | 0 | −0.28 | 0.73 | −0.66 |
| UAD | 0 | 1 | 0 | −0.28 | 0.73 | −0.66 |
| UN | 5 | 38 | 30 | −0.25 | 0.41 | −0.33 |
| RD | 4 | 15 | 40 | −0.24 | −2.56 | 2.81 |
| RPU | 1 | 5 | 9 | −0.14 | −0.85 | 0.96 |
| PD | 5 | 21 | 38 | 0.05 | −1.82 | 1.91 |
| RAC | 1 | 6 | 3 | 0.27 | 0.51 | −0.65 |
| UAP | 10 | 38 | 66 | 0.44 | −2.35 | 2.3 |
| RU | 907 | 4909 | 5802 | 0.63 | −9.97 | 10.27 |
| CN | 1 | 0 | 1 | 2.17 | −0.99 | 0.14 |
| RC | 24 | 113 | 60 | 2.3 | 1.74 | −2.8 |
| U | 332 | 2359 | 1125 | 2.36 | 11.6 | −13.24 |
| RPD | 5 | 6 | 11 | 2.56 | −1.44 | 0.45 |
| UC | 44 | 192 | 151 | 2.65 | 0.25 | −1.38 |
| UCN | 1 | 0 | 0 | 3.34 | −0.7 | −0.66 |
| RA | 34 | 85 | 122 | 3.63 | −2.99 | 1.64 |
| UPD | 6 | 4 | 8 | 3.94 | −1.61 | 0.05 |
| P | 532 | 2460 | 2359 | 6.08 | −2.87 | 0.49 |
| UAC | 47 | 51 | 140 | 6.75 | −6.03 | 3.55 |
| AC | 36 | 64 | 57 | 6.93 | −1.43 | −1.39 |
| RUC | 55 | 114 | 110 | 7.29 | −1.88 | −1.07 |
| C | 304 | 999 | 725 | 11.96 | 0.35 | −5.38 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruschel, C.; Schultz, S.T. Aggressive Predation Drives Assembly of Adriatic Fish Communities. Diversity 2020, 12, 130. https://doi.org/10.3390/d12040130
Kruschel C, Schultz ST. Aggressive Predation Drives Assembly of Adriatic Fish Communities. Diversity. 2020; 12(4):130. https://doi.org/10.3390/d12040130
Chicago/Turabian StyleKruschel, Claudia, and Stewart T. Schultz. 2020. "Aggressive Predation Drives Assembly of Adriatic Fish Communities" Diversity 12, no. 4: 130. https://doi.org/10.3390/d12040130
APA StyleKruschel, C., & Schultz, S. T. (2020). Aggressive Predation Drives Assembly of Adriatic Fish Communities. Diversity, 12(4), 130. https://doi.org/10.3390/d12040130






