Patterns of Understory Community Assembly and Plant Trait-Environment Relationships in Temperate SE European Forests
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Vegetation Data Collection
2.3. Plant Functional Traits
2.4. Data Analyses
3. Results
3.1. Understory Vegetation and Environmental Conditions Across IM Sites
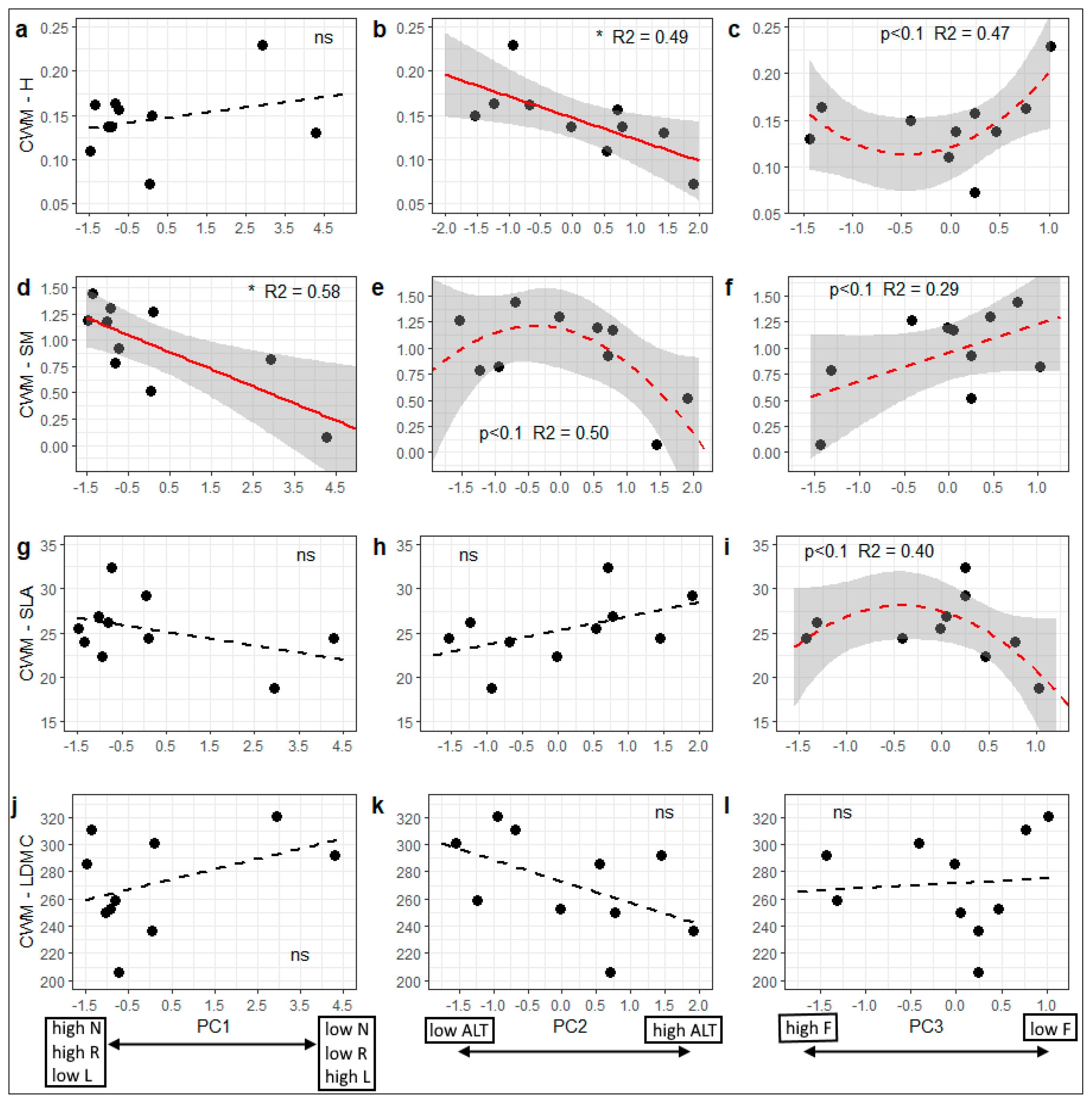
3.2. Variation in Community-Weighted Trait Means Along Environmental Gradients
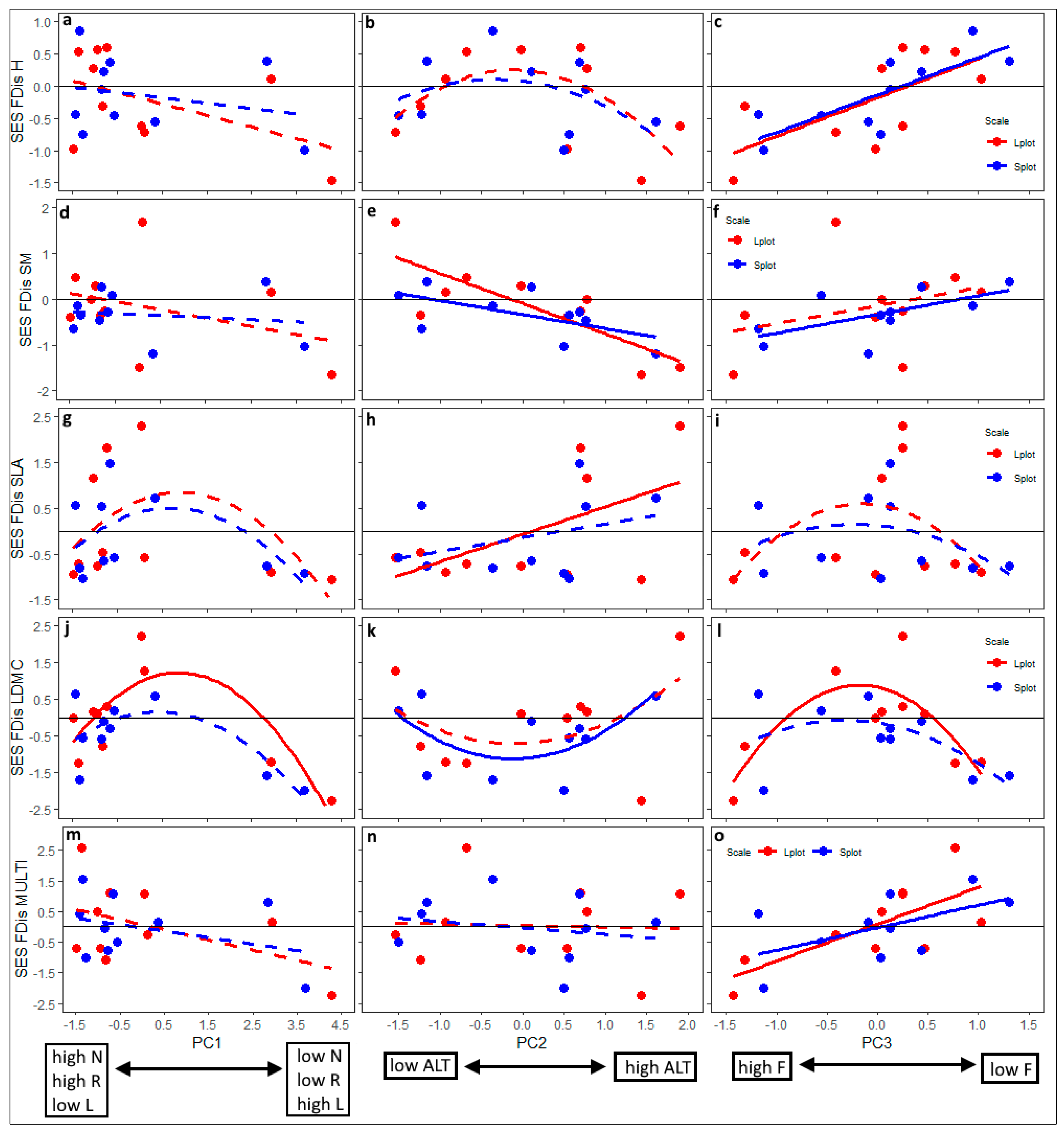
3.3. Patterns of Trait Convergence and Divergence: Dependence on Environmental Gradients, Spatial Scale and Community Attributes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lepš, J.; de Bello, F.; Lavorel, S.; Berman, S. Quantifying and interpreting functional diversity of natural communities: Practical consideration matter. Preslia 2006, 78, 481–501. Available online: https://hal.archives-ouvertes.fr/halsde-00293183/ (accessed on 15 December 2019).
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Herben, T.; Goldberg, D.E. Community assembly by limiting similarity vs. competitive hierarchies: Testing the consequences of dispersion of individual traits. J. Ecol. 2014, 102, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.I.; McEwan, R.W. The Role of Environmental Filtering in Structuring Appalachian Tree Communities: Topographic Influences on Functional Diversity Are Mediated through Soil Characteristics. Forests 2018, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Spasojevic, M.J.; Suding, K.N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 2012, 100, 652–661. [Google Scholar] [CrossRef]
- Mason, N.W.H.; de Bello, F.; Mouillot, D.; Pavoine, S.; Dray, S. A guide for using functional diversity indices to reveal changes in assembly processes along ecological gradients. J. Veg. Sci. 2013, 24, 794–806. [Google Scholar] [CrossRef]
- Chelli, S.; Marignani, M.; Barni, E.; Petraglia, A.; Puglielli, G.; Wellstein, C.; Acosta, A.T.R.; Bolpagni, R.; Bragazza, L.; Campetella, G.; et al. Plant-environment interactions through a functional traits perspective: A review of Italian studies. Plant Biosyst. 2018, 153, 853–869. [Google Scholar] [CrossRef]
- Chelli, S.; Ottaviani, G.; Simonetti, E.; Wellstein, C.; Canullo, R.; Carnicelli, S.; Andreetta, A.; Puletti, N.; Bartha, S.; Cervellini, M.; et al. Climate is the main driver of clonal and bud bank traits in Italian forest understories. Perspect. Plant. Ecol. 2019, 40, 125478. [Google Scholar] [CrossRef] [Green Version]
- Pinho, B.X.; de Melo, F.P.L.; Arroyo-Rodríguez, V.; Pierce, S.; Lohbeck, M.; Tabarelli, M. Soil-mediated filtering organizes tree assemblages in regenerating tropical forests. J. Ecol. 2018, 106, 137–147. [Google Scholar] [CrossRef]
- Weiher, E.; Keddy, P.A. Assembly rules, null models, and trait dispersion: New questions from old patterns. Oikos 1995, 74, 159–164. [Google Scholar] [CrossRef] [Green Version]
- Keddy, P.A. A pragmatic approach to functional ecology. Funct. Ecol. 1992, 6, 621–626. [Google Scholar] [CrossRef]
- Weiher, E.; Freund, D.; Bunton, T.; Stefanski, A.; Lee, T.; Bentivenga, S. Advances, challenges and a developing synthesis of ecological community assembly theory. Philos. Trans. R. Soc. B 2011, 366, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Götzenberger, L.; de Bello, F.; Bråthen, K.A.; Davison, J.; Dubuis, A.; Guisan, A.; Lepš, J.; Lindborg, R.; Moora, M.; Pärtel, M.; et al. Ecological assembly rules in plant communities—Approaches, patterns and prospects. Biol. Rev. 2012, 87, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Verdier, M.; Navas, M.-L.; Vellend, M.; Violle, C.; Fayolle, A.; Garnier, E. Community assembly along a soil depth gradient: Contrasting patterns of plant trait convergence and divergence in a Mediterranean rangeland. J. Ecol. 2012, 100, 1422–1433. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The Limiting Similarity, Convergence, and Divergence of Coexisting Species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 1388–1391. [Google Scholar] [CrossRef] [Green Version]
- Carlucci, M.B.; Streit, H.; Duarte, L.D.S.; Pillar, V.D. Individual-based trait analyses reveal assembly patterns in tree sapling communities. J. Veg. Sci. 2012, 23, 176–186. [Google Scholar] [CrossRef]
- Sonnier, G.; Johnson, S.E.; Waller, D.M. Fragmentation reduces the importance of niche-based factors relative to dispersal traits in structuring temperate forest understories. J. Veg. Sci. 2020, 31, 75–83. [Google Scholar] [CrossRef]
- Lhotsky, B.; Kovács, B.; Ónodi, G.; Csecserits, A.; Rédei, T.; Lengyel, A.; Kertész, M.; Botta-Dukát, Z. Changes in assembly rules along a stress gradient from open dry grasslands to wetlands. J. Ecol. 2016, 104, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Grime, J.P. Trait convergence and trait divergence in herbaceous plant communities: Mechanisms and consequences. J. Veg. Sci. 2006, 17, 255–260. [Google Scholar] [CrossRef]
- Liu, B.; Chen, H.Y.H.; Yang, J. Understory Community Assembly Following Wildfire in Boreal Forests: Shift from Stochasticity to Competitive Exclusion and Environmental Filtering. Front. Plant Sci. 2018, 9, 1854. [Google Scholar] [CrossRef] [PubMed]
- Cornwell, W.K.; Schwilk, D.; Ackerly, D.D. A trait-based approach for habitat filtering: Convex hull volume. Ecology 2006, 87, 1465–1471. [Google Scholar] [CrossRef]
- Schellenberger Costa, D.; Gerschlauer, F.; Pabst, H.; Kühnel, A.; Huwe, B.; Kiese, R.; Kuzyakov, Y.; Kleyer, M. Community-weighted means and functional dispersion of plant functional traits along environmental gradients on Mount Kilimanjaro. J. Veg. Sci. 2017, 28, 684–695. [Google Scholar] [CrossRef]
- Miedema, L.J.; Capmourteres, V.; Anand, M. Impact of land composition and configuration on the functional trait assembly of forest communities in southern Ontario. Ecosphere 2019, 10, e02633-10. [Google Scholar] [CrossRef] [Green Version]
- Bricca, A.; Conti, L.; Tardella, M.F.; Catorci, A.; Iocchi, M.; Theurillat, J.-P.; Cutini, M. Community assembly processes along a sub-Mediterranean elevation gradient: Analyzing the interdependence of trait community weighted mean and functional diversity. Plant Ecol. 2019, 220, 1139–1151. [Google Scholar] [CrossRef]
- Diamond, J.M. Assembly of species communities. In Ecology and Evolution of Communities; Cody, M.L., Diamond, J.M., Eds.; Belknap Press: Cambridge, MA, USA, 1975; pp. 342–444. [Google Scholar]
- Zhang, H.; Chen, H.Y.H.; Lian, J.; John, R.; Ronghua, L.; Liu, H.; Ye, W.; Berninger, F.; Ye, Q. Using functional trait diversity patterns to disentangle the scale-dependent ecological processes in a subtropical forest. Funct. Ecol. 2018, 32, 1379–1389. [Google Scholar] [CrossRef]
- Gilbert, B.; Lechowicz, M.J. Neutrality, niches, and dispersal in a temperate forest understory. Proc. Natl. Acad. Sci. USA 2004, 101, 7651–7656. [Google Scholar] [CrossRef] [Green Version]
- Wilson, P.J.; Thompson, K.; Hodgson, J.G. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999, 143, 155–162. [Google Scholar] [CrossRef]
- Luo, Y.-H.; Cadotte, M.W.; Burgess, K.S.; Liu, J.; Tan, S.-L.; Xu, K.; Li, D.-Z.; Gao, L.-M. Forest community assembly is driven by different strata-dependent mechanisms along an elevational gradient. J. Biogeogr. 2019, 46, 2174–2187. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001; 375p. [Google Scholar]
- De Bello, F.; Vandewalle, M.; Reitalu, T.; Lepš, J.; Prentice, H.C.; Lavorel, S.; Sykes, M.T. Evidence for scale- and disturbance-dependent trait assembly patterns in dry semi-natural grasslands. J. Ecol. 2013, 101, 1237–1244. [Google Scholar] [CrossRef]
- Long, W.; Schamp, B.S.; Zang, R.; Ding, Y.; Huang, Y.; Xiang, Y. Community assembly in a tropical cloud forest related to specific leaf area and maximum species height. J. Veg. Sci. 2015, 26, 513–523. [Google Scholar] [CrossRef]
- Jager, M.M.; Richardson, S.J.; Bellingham, P.J.; Clearwater, M.J.; Laughlin, D.C. Soil fertility induces coordinated responses of multiple independent functional traits. J. Ecol. 2014, 103, 374–385. [Google Scholar] [CrossRef]
- De la Riva, E.G.; Tosto, A.; Pérez-Ramos, I.M.; Navarro-Fernández, C.M.; Olmo, M.; Anten, N.P.R.; Marañón, T.; Villar, R. A plant economics spectrum in Mediterranean forests along environmental gradients: Is there coordination among leaf, stem and root traits? J. Veg. Sci. 2016, 27, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Liu, J.; Tan, S.; Cadotte, M.W.; Xu, K.; Gao, L.; Li, D. Trait variation and functional diversity maintenance of understory herbaceous species coexisting along an elevational gradient in Yulong Mountain, Southwest China. Plant Divers. 2016, 38, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Su, X.; Wang, M.; Huang, Z.; Fu, S.; Chen, H.Y.H. Forest Understory Vegetation: Colonization and the Availability and Heterogeneity of Resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef] [Green Version]
- Valladares, F.; Laanisto, L.; Niinemets, Ü.; Zavala, M.A. Shedding light on shade: Ecological perspectives of understory plant life. Plant Ecol. Divers. 2016, 9, 237–251. [Google Scholar] [CrossRef] [Green Version]
- Ottaviani, G.; Götzenberger, L.; Bacaro, G.; Chiarucci, A.; de Bello, F.; Marcantonio, M. A multifaceted approach for beech forest conservation: Environmental drivers of understory plant diversity. Flora 2019, 256, 85–91. [Google Scholar] [CrossRef]
- Schuster, B.; Diekmann, M. Changes in species density along the soil pH gradient—Evidence from German plant communities. Folia Geobot. 2003, 38, 367–379. [Google Scholar] [CrossRef]
- Landuyt, D.; Perring, M.P.; Seidl, R.; Taubert, F.; Verbeeck, H.; Verheyen, K. Modelling understorey dynamics in temperate forests under global change-Challenges and perspectives. Perspect. Plant Ecol. 2018, 31, 44–54. [Google Scholar] [CrossRef] [Green Version]
- Carmona, C.P.; de Bello, F.; Mason, N.W.H.; Leps, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 2016, 31, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Kraft, J.B.N.; Ackerly, D.D. Functional trait and phylogenetic tests of community assembly across spatial scales in an Amazonian forest. Ecol. Monogr. 2010, 80, 410–422. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, G.; Ci, X.; Swenson, N.G.; Cao, M.; Sha, L.; Li, J.; Baskin, C.C.; Slik, J.W.F.; Lin, L. Functional and phylogenetic assembly in a Chinese tropical tree community across size classes, spatial scales and habitats. Funct. Ecol. 2014, 28, 520–529. [Google Scholar] [CrossRef]
- Weiher, E.; Clarke, G.D.P.; Keddy, P.A. Community assembly rules, morphological dispersion, and the coexistence of plant species. Oikos 1998, 81, 309–322. [Google Scholar] [CrossRef]
- Chalmandrier, L.; Münkmüller, T.; Gallien, L.; de Bello, F.; Mazel, F.; Lavergne, S.; Thuiller, W. A family of null models to distinguish between environmental filtering and biotic interactions in functional diversity patterns. J. Veg. Sci. 2013, 24, 853–864. [Google Scholar] [CrossRef]
- Siefert, A. Spatial patterns of functional divergence in old-field plant communities. Oikos 2012, 121, 907–914. [Google Scholar] [CrossRef]
- Mayfield, M.M.; Levine, J.M. Opposing effects of competitive exclusion on the phylogenetic structure of communities. Ecol. Lett. 2010, 13, 1085–1093. [Google Scholar] [CrossRef]
- De Bello, F. The quest for trait convergence and divergence in community assembly: Are null-models the magic wand? Global Ecol. Biogeogr. 2012, 21, 312–317. [Google Scholar] [CrossRef]
- Götzenberger, L.; Botta-Dukát, Z.; Lepš, J.; Pärtel, M.; Zobel, M.; de Bello, F. Which randomizations detect convergence and divergence in trait-based community assembly? A test of commonly used null models. J. Veg. Sci. 2016, 27, 1275–1287. [Google Scholar] [CrossRef]
- Kutnar, L. Intensive Monitoring of Vegetation of Forest Ecosystems in Slovenia. In Monitoring the Management of Forests and Forested Landscapes; Hladnik, D., Ed.; Studia Forestalia Slovenica, Biotehniška Fakulteta, Oddelek za Gozdarstvo in Obnovljive Gozdne Vire: Ljubljana, Slovenia, 2006; pp. 277–290. ISBN1 9616020420. ISBN2 9789616020428. (In Slovenian with English Summary). [Google Scholar]
- Kutnar, L. Diversity of woody species on forest monitoring plots in Slovenia. GozdVestn 2011, 69, 271–278, (In Slovenian with English Summary). [Google Scholar]
- Kutnar, L.; Nagel, T.A.; Kermavnar, J. Effects of Disturbance on Understory Vegetation across Slovenian Forest Ecosystems. Forests 2019, 10, 1048. [Google Scholar] [CrossRef] [Green Version]
- De Vries, W.; Vel, E.M.; Reinds, G.J.; Deelstra, H.; Klap, J.M.; Leeters, E.E.J.M.; Hendriks, C.M.A.; Kerkvoorden, M.; Landmann, G.; Herkendell, J.; et al. Intensive monitoring of forest ecosystems in Europe. 1. Objectives, set-up and evaluation strategy. For. Ecol. Manag. 2003, 174, 77–95. [Google Scholar] [CrossRef]
- Canullo, R.; Starlinger, F.; Granke, O.; Fischer, R.; Aamlid, D.; Neville, P. ICP Forests Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; Part VII.1: Assessment of Ground Vegetation; UNECE ICP Forests Programme Coordinating Centre: Hamburg, Germany, 2011; p. 19. [Google Scholar]
- Barkman, J.J.; Doing, H.; Segal, S. Kritische Bemerkungen und Vorschläge zur quantitativen Vegetationsanalyse. Acta Bot. Neerl. 1964, 13, 394–419. [Google Scholar] [CrossRef]
- Londo, G. The decimal scale for relevés of permanent quadrats. Vegetatio 1976, 33, 61–64. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1964–1980; Volume 1. [Google Scholar]
- Tutin, T.G.; Burges, N.A.; Chater, A.O.; Edmondson, J.R.; Heywood, V.H.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea, 2nd ed.; Cambridge University Press: Cambridge, UK, 1993. [Google Scholar]
- Martinčič, A.; Wraber, T.; Jogan, N.; Podobnik, A.; Turk, B.; Vreš, B.; Ravnik, V.; Frajman, B.; Strgulc Krajšek, S.; Trčak, B.; et al. Mala Flora Slovenije: Ključ za Določanje Praprotnic in Semenk; Tehniška Založba Slovenije: Ljubljana, Slovenia, 2007; p. 967. ISBN 978-961-251-026-8. [Google Scholar]
- Westoby, M. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 1998, 199, 213–227. [Google Scholar] [CrossRef]
- Keddy, P.A.; Shipley, B. Competitive Hierarchies in Herbaceous Plant Communities. Oikos 1989, 54, 234–241. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant ecological strategies: Some leading dimensions of variation between species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef] [Green Version]
- Pakeman, R.J.; Garnier, E.; Lavorel, S.; Ansquer, P.; Castro, C.; Cruz, P.; Dolezal, J.; Eriksson, O.; Freitas, H.; Golodets, C.; et al. Impact of abundance weighting on the response of seed traits to climate and land use. J. Ecol. 2008, 96, 355–366. [Google Scholar] [CrossRef]
- Joner, F.; Anand, M.; Pillar, V.D. Trait-convergence and divergence assembly patterns in a temperate forest herbaceous layer along the gradient of canopy closure. Community Ecol. 2012, 13, 178–184. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Roumet, C.; Cruz, P.; Blanchard, A.; Autran, P.; Garnier, E. Evidence for a “plant community economics spectrum” driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J. Ecol. 2012, 100, 1315–1327. [Google Scholar] [CrossRef] [Green Version]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Klotz, S.; Kühn, I.; Durka, W. BIOLFLOR—Eine Datenbank Mit Biologisch-Ökologischen Merkmalen Zur Flora Von Deutschland [A Database with Biological-Ecological Characteristics for the Flora of Germany]. Bundesamt fur Naturschutz Bonn, Bonn, Germany. 2002. Available online: http://www.vifabio.de/iqfBio/detail/5415 (accessed on 15 December 2019).
- Hintze, C.; Heydel, F.; Hoppe, C.; Cunze, S.; König, A. D3: Dispersal and Diaspore Database—Baseline data and statistics on seed dispersal. Perspect. Plant. Ecol. 2013, 15, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Pakeman, R.J. Functional trait metrics are sensitive to the completeness of the species’ trait data? Methods Ecol. Evol. 2014, 5, 9–15. [Google Scholar] [CrossRef]
- Garnier, E.; Cortez, J.; Billès, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology 2004, 85, 2630–2637. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 15 December 2019).
- Laliberté, E.; Legendre, P.; Shipley, B. Package “FD”: Measuring Functional Diversity (FD) from Multiple Traits, and Other Tools for Functional Ecology, R Package Version 1.0–12. 2015. Available online: https://cran.r-project.org/web/packages/FD/FD.pdf (accessed on 4 December 2019).
- Gotelli, N.J.; Graves, G.R. Null Models in Ecology; Smithsonian Institution Press: Washington, DC, USA, 1996. [Google Scholar]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [Green Version]
- Gotelli, N.J.; McCabe, D.J. Species co-occurrence: A meta-analysis of J. M. Diamond’s assembly rules model. Ecology 2002, 83, 2091–2096. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa. 2. verbesserte und erweiterte Auflage. Scr. Geobot. 1992, 18, 1–258. [Google Scholar]
- Szymura, T.H.; Szymura, M.; Macioł, A. Bioindication with Ellenberg’s indicator values: A comparison with measured parameters in Central European oak forests. Ecol. Indic. 2014, 46, 495–503. [Google Scholar] [CrossRef]
- Bartelheimer, M.; Poschlod, P. Functional characterization of Ellenberg indicator values—A review on ecophysiological determinants. Funct. Ecol. 2016, 30, 506–516. [Google Scholar] [CrossRef]
- Shipley, B.; Belluau, M.; Kühn, I.; Soudzilovskaia, N.A.; Bahn, M.; Penuelas, J.; Kattge, J.; Sack, L.; Cavender-Bares, J.; Ozinga, W.A.; et al. Predicting habitat affinities of plant species using commonly measured functional traits. J. Veg. Sci. 2017, 28, 1082–1095. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Brusa, G.; Pierce, S.; Zanzottera, M.; Cerabolini, B.E.L. Plant trait variation along environmental indicators to infer global change impacts. Flora 2019, 254, 113–121. [Google Scholar] [CrossRef]
- Maes, S.L.; Perring, M.P.; Depauw, L.; Bernhardt-Römermann, M.; Blondeel, H.; Brūmelis, G.; Brunet, J.; Decocq, G.; den Ouden, J.; Govaert, S.; et al. Plant functional trait response to environmental drivers across European temperate forest understorey communities. Plant Biol. 2019. Early View. [Google Scholar] [CrossRef] [PubMed]
- Le Roux, P.C.; Pellissier, L.; Wisz, M.S.; Luoto, M. Incorporating dominant species as proxies for biotic interactions strengthens plant community models. J. Ecol. 2014, 102, 767–775. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package Vegan Community Ecology Package. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 20 December 2019).
- Moles, A.T.; Warton, D.I.; Warman, L.; Swenson, N.G.; Laffan, S.W.; Zanne, A.E.; Pitman, A.; Hemmings, F.A.; Leishman, M.R. Global patterns in plant height. J. Ecol. 2009, 97, 923–932. [Google Scholar] [CrossRef]
- Moles, A.T.; Ackerly, D.D.; Tweddle, J.C.; Dickie, J.B.; Smith, R.; Leishman, M.R.; Mayfield, M.M.; Pitman, A.; Wood, J.T.; Westoby, M. Global patterns in seed size. Glob. Ecol. Biogeogr. 2007, 16, 109–116. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, I.C.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Bruelheide, H.; Dengler, J.; Purschke, O.; Lenoir, J.; Jiménez-Alfaro, B.; Hennekens, S.M.; Botta-Dukát, Z.; Chytrý, M.; Field, R.; Jansen, F.; et al. Global trait-environment relationships of plant communities. Nat. Ecol. Evol. 2018, 2, 1906–1917. [Google Scholar] [CrossRef]
- Jafari, S.M.; Zarre, S.; Alavipanah, S.K.; Ghahremaninejad, F. Functional turnover from lowland to montane forest: Evidence from the Hyrcanian forest in Northern Iran. iForest 2015, 8, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Urbančič, M.; Kutnar, L.; Kobal, M.; Žlindra, D.; Marinšek, A.; Simončič, P. Soil and vegetation characteristics on Intensive Monitoring Plots of forest ecosystems. GozdVestn 2016, 74, 3–27, (In Slovenian with English Summary). [Google Scholar]
- Funk, J.L.; Larson, J.E.; Ames, G.M.; Butterfield, B.J.; Cavender-Bares, J.; Firn, J.; Laughlin, D.C.; Sutton-Grier, A.E.; Williams, L.; Wright, J. Revisiting the Holy Grail: Using plant functional traits to understand ecological processes. Biol. Rev. 2017, 92, 1156–1173. [Google Scholar] [CrossRef] [PubMed]


| Site | Altitude (m) | Slope (°) | Rockiness (%) | Dominant Tree Species | Tree Layer Cover (%) | Tree Layer Richness a | Shrub Layer Cover (%) | Five Most Abundant (Frequent) Understory Species |
|---|---|---|---|---|---|---|---|---|
| 1-KK (Krucmanove konte) | 1397 | 9.0 (1.4) | 8.8 (7.8) | Norway spruce (Picea abies) | 60.0 (14.1) | 1 | 1.1 (1.9) | Aposeris foetida, Picea abies, Hieracium murorum, Oxalis acetosella, Vaccinium myrtillus |
| 2-FO (Fondek) | 827 | 10.9 (3.6) | 40.6 (25.3) | European beech (Fagus sylvatica) | 95.3 (10.4) | 4 | 3.4 (1.8) | Sesleria autumnalis, Anemone trifolia, Vinca minor, Cyclamen purpurascens, Mercurialis perennis |
| 3-GB (Gropajski bori) | 420 | 8.3 (2.1) | 39.5 (28.6) | Austrian pine (Pinus nigra) | 94.3 (3.0) | 5 | 8.3 (5.4) | Sesleria autumnalis, Brachypodium rupestre, Hedera helix, Frangula rupestris, Helleborus multifidus |
| 4-BR (Brdo) | 471 | 3.0 (2.5) | 0.0 (0.0) | Scots pine (Pinus sylvestris) | 45.6 (30.1) | 3 | 5.4 (8.1) | Vaccinium myrtillus, Pteridium aquilinum, Molinia caerulea subsp. arundinacea, Calluna vulgaris, Vaccinium vitis-idaea |
| 5-BO (Borovec) | 705 | 13.3 (6.5) | 14.1 (13.8) | European beech (Fagus sylvatica) | 98.8 (3.5) | 6 | 11.1 (7.4) | Omphalodes verna, Acer pseudoplatanus, Fagus sylvatica, Pteridium aquilinum, Sanicula europaea |
| 6-KL (Kladje) | 1304 | 4.8 (1.0) | 0.0 (0.0) | Norway spruce (Picea abies) | 62.5 (5.0) | 1 | 0.4 (0.3) | Calamagrostis villosa, Deschampsia flexuosa, Picea abies, Luzula pilosa, Melamyprum pratense |
| 8-LO (Lontovž) | 958 | 26.6 (1.8) | 3.1 (1.8) | European beech (Fagus sylvatica) | 99.4 (1.8) | 3 | 4.1 (2.8) | Mercurialis perennis, Hacquetia epipactis, Acer pseudoplatanus, Polygonatum verticillatum, Galium sylvaticum |
| 9-GO (Gorica) | 955 | 14.0 (1.6) | 2.5 (1.7) | European beech (Fagus sylvatica) | 88.8 (19.3) | 5 | 17.5 (15.5) | Acer pseudoplatanus, Mercurialis perennis, Omphalodes verna, Fagus sylvatica, Hacquetia epipactis |
| 10-KG (Krakovski gozd) | 160 | 0.0 (0.0) | 0.0 (0.0) | European hornbeam (Carpinus betulus), Pedunculate oak (Quercus robur) | 96.3 (2.5) | 7 | 16.3 (9.5) | Carex brizoides, Galeobdolon montanum, Quercus robur, Circea lutetiana, Aegopodium podagraria |
| 11-MŠ (Murska šuma) | 170 | 0.1 (0.4) | 0.0 (0.0) | Pedunculate oak (Quercus robur), European hornbeam (Carpinus betulus) | 100.0 (0.0) | 8 | 10.3 (16.3) | Galeobdolon montanum, Galeopsis tetrahit, Galium odoratum, Aegopodium podagraria, Asarum europaeum |
| IM site | Species Richness | Cover (%) | Species Evenness | |||
|---|---|---|---|---|---|---|
| Lplots | Splots | Lplots | Splots | Lplots | Splots | |
| 1-KK | 40.5 (9.3) | 20.7 (4.8) | 76.3 (20.6) | 79.5 (12.1) | 0.65 (0.13) | 0.61 (0.13) |
| 2-FO | 25.5 (5.7) | 10.9 (3.0) | 75.9 (19.3) | 70.5 (23.0) | 0.51 (0.05) | 0.57 (0.14) |
| 3-GB | 25.3 (1.7) | 8.5 (3.5) | 75.0 (28.0) | 77.5 (26.5) | 0.45 (0.07) | 0.43 (0.20) |
| 4-BR | 10.9 (2.4) | 7.7 (1.4) | 100.0 (0.0) | 100.0 (0.0) | 0.62 (0.07) | 0.67 (0.08) |
| 5-BO | 43.5 (4.7) | 18.0 (3.3) | 50.6 (16.4) | 43.5 20.8 | 0.87 (0.02) | 0.84 (0.08) |
| 6-KL | 10.3 (2.9) | 6.9 (1.7) | 85.0 (12.2) | 91.2 (9.5) | 0.19 (0.04) | 0.36 (0.16) |
| 8-LO | 50.4 (3.5) | 27.1 (3.0) | 75.6 (6.8) | 48.5 (21.2) | 0.83 (0.03) | 0.81 (0.12) |
| 9-GO | 55.0 (3.2) | 26.5 (3.6) | 86.3 (8.5) | 56.0 (20.4) | 0.86 (0.05) | 0.85 (0.03) |
| 10-KG | 29.8 (1.5) | 25.3 (4.5) | 80.0 (11.5) | 70.5 (17.2) | 0.77 (0.07) | 0.74 (0.07) |
| 11-MŠ | 22.3 (6.1) | 18.7 (3.1) | 81.9 (13.1) | 84.0 (17.3) | 0.49 (0.23) | 0.62 (0.06) |
| Total (average) | 31.1 (15.4) | 17.0 (8.3) | 78.0 (19.0) | 72.1 (24.7) | 0.64 (0.22) | 0.65 (0.19) |
| Functional Trait | PC1 (PC12) | PC2 (PC22) | PC3 (PC32) | Whole Model R2 (%) |
|---|---|---|---|---|
| H | 0.010 m | −0.026 * | 0.016 | 74.6 * |
| SM | −0.129 ** | −0.146 * | 0.179 m | 81.1 ** |
| SLA | −1.106 m | 1.813 m | −1.468 | 59.3 |
| LDMC | 10.022 | −17.998 m | 8.021 | 52.6 |
| Dependent Variable | Explanatory Variable | Gradient 1 (PC1) | Gradient 2 (PC2) | Gradient 3 (PC3) |
|---|---|---|---|---|
| SES-H (plant height) | PC | 2.82 | 1.11 | 17.34 *** |
| PC2 | / | 4.30 m | / | |
| Scale | 0.03 | 0.01 | 0.03 | |
| PC:Scale | 0.36 | 0.09 | 0.00 | |
| PC2:Scale | / | 0.19 | / | |
| Model R2 (%) | 16.7 | 28.9 | 52.1 ** | |
| SES-SM (seed mass) | PC | 1.46 | 19.72 *** | 3.37 m |
| PC2 | / | / | / | |
| Scale | 0.31 | 0.92 | 0.35 | |
| PC:Scale | 0.49 | 2.42 | 0.00 | |
| PC2:Scale | / | / | / | |
| Model R2 (%) | 12.3 | 59.1 ** | 18.9 | |
| SES-SLA (specific leaf area) | PC | 1.50 | 4.79 * | 0.01 |
| PC2 | 3.21 m | / | 3.67 m | |
| Scale | 0.16 | 0.03 | 0.07 | |
| PC:Scale | 0.06 | 0.48 | 0.01 | |
| PC2:Scale | 0.01 | / | 0.47 | |
| Model R2 (%) | 26.1 | 24.9 | 23.2 | |
| SES-LDMC (leaf dry matter content) | PC | 10.21 ** | 0.32 | 0.02 |
| PC2 | 12.56 ** | 4.35 m | 15.23 ** | |
| Scale | 2.01 | 0.22 | 1.05 | |
| PC:Scale | 0.44 | 0.01 | 0.10 | |
| PC2:Scale | 0.50 | 0.13 | 2.50 | |
| Model R2 (%) | 64.8 ** | 26.3 | 57.4 * | |
| SES-MULTI (all 4 traits combined) | PC | 3.59 m | 0.19 | 11.93 ** |
| PC2 | / | / | / | |
| Scale | 0.04 | 0.02 | 0.07 | |
| PC:Scale | 0.18 | 0.08 | 0.68 | |
| PC2:Scale | / | / | / | |
| Model R2 (%) | 19.2 | 1.8 | 44.2 * |
| IM Site | H | SM | SLA | LDMC | MULTI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Splots (test 1) | Lplots (test 2) | Splots (test 1) | Lplots (test 2) | Splots (test 1) | Lplots (test 2) | Splots (test 1) | Lplots (test 2) | Splots (test 1) | Lplots (test 2) | |
| 1-KK | −0.56 ** | −0.62 | −1.19 ** | −1.50 m | 0.74 * | 2.30 * | 0.61 | 2.23 * | 0.14 | 1.06 * |
| 2-FO | −0.74 ** | −0.98 * | −0.36 * | −0.39 | −1.03 ** | −0.95 | −0.56 m | 0.00 | −1.00 * | −0.70 |
| 3-GB | 0.86 m | 0.53 | −0.13 | 0.48 | −0.81 ** | −0.71 | −1.68 ** | −1.22 | 1.54 | 2.57 |
| 4-BR | 0.39 ** | 0.11 * | 0.38 ** | 0.16 * | −0.75 ** | −0.90 | −1.56 ** | −1.21 ** | 0.81 * | 0.16 ** |
| 5-BO | 0.22 | 0.57 | 0.28 | 0.29 | −0.64 ** | −0.75 | −0.09 | 0.11 | −0.78 ** | −0.70 |
| 6-KL | −0.98 ** | −1.46 ** | −1.03 ** | −1.64 ** | −0.93 ** | −1.05 | −1.98 ** | −2.25 | −1.99 ** | −2.23 |
| 8-LO | 0.37 | 0.60 | −0.27 | −0.25 | 1.48 ** | 1.82 | −0.27 ** | 0.32 ** | 1.07 * | 1.09 |
| 9-GO | −0.05 | 0.27 | −0.45 m | 0.01 | 0.54 | 1.16 * | −0.57 * | 0.17 * | −0.05 | 0.48 * |
| 10-KG | −0.45 ** | −0.72 | 0.09 | 1.68 * | −0.58 ** | −0.58 | 0.19 | 1.28 * | −0.51 ** | −0.25 |
| 11-MŠ | −0.44 ** | −0.32 | −0.63 m | −0.36 | 0.57 ** | −0.46 ** | 0.66 ** | −0.77 ** | 0.43 ** | −1.08 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kermavnar, J.; Kutnar, L. Patterns of Understory Community Assembly and Plant Trait-Environment Relationships in Temperate SE European Forests. Diversity 2020, 12, 91. https://doi.org/10.3390/d12030091
Kermavnar J, Kutnar L. Patterns of Understory Community Assembly and Plant Trait-Environment Relationships in Temperate SE European Forests. Diversity. 2020; 12(3):91. https://doi.org/10.3390/d12030091
Chicago/Turabian StyleKermavnar, Janez, and Lado Kutnar. 2020. "Patterns of Understory Community Assembly and Plant Trait-Environment Relationships in Temperate SE European Forests" Diversity 12, no. 3: 91. https://doi.org/10.3390/d12030091
APA StyleKermavnar, J., & Kutnar, L. (2020). Patterns of Understory Community Assembly and Plant Trait-Environment Relationships in Temperate SE European Forests. Diversity, 12(3), 91. https://doi.org/10.3390/d12030091





