Does Protection Really Matter? A Case Study from Central European Oak Forests
Abstract
1. Introduction
2. Materials and Methods
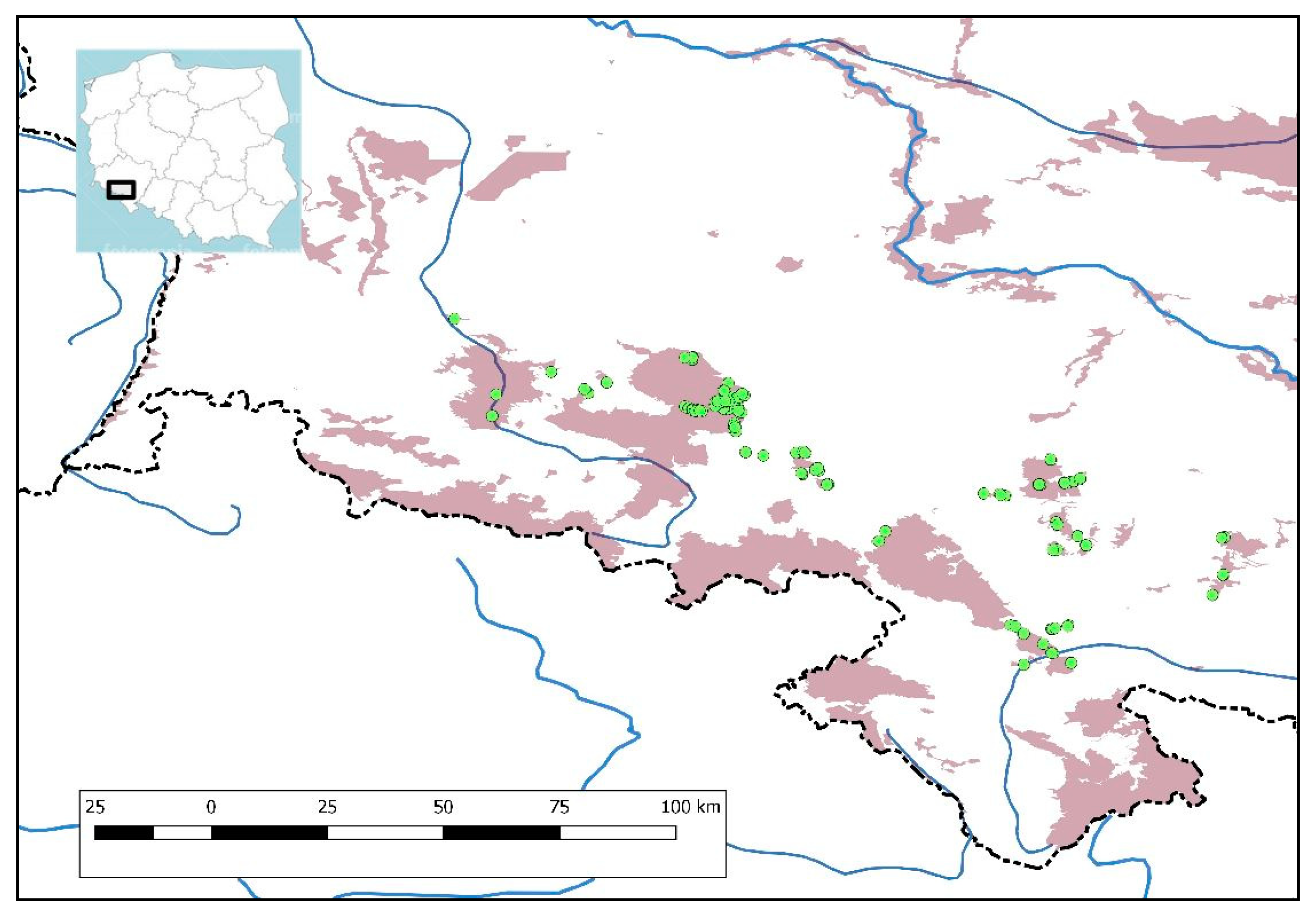
2.1. Subject of Research
2.2. Data Collection
2.3. Data Analysis
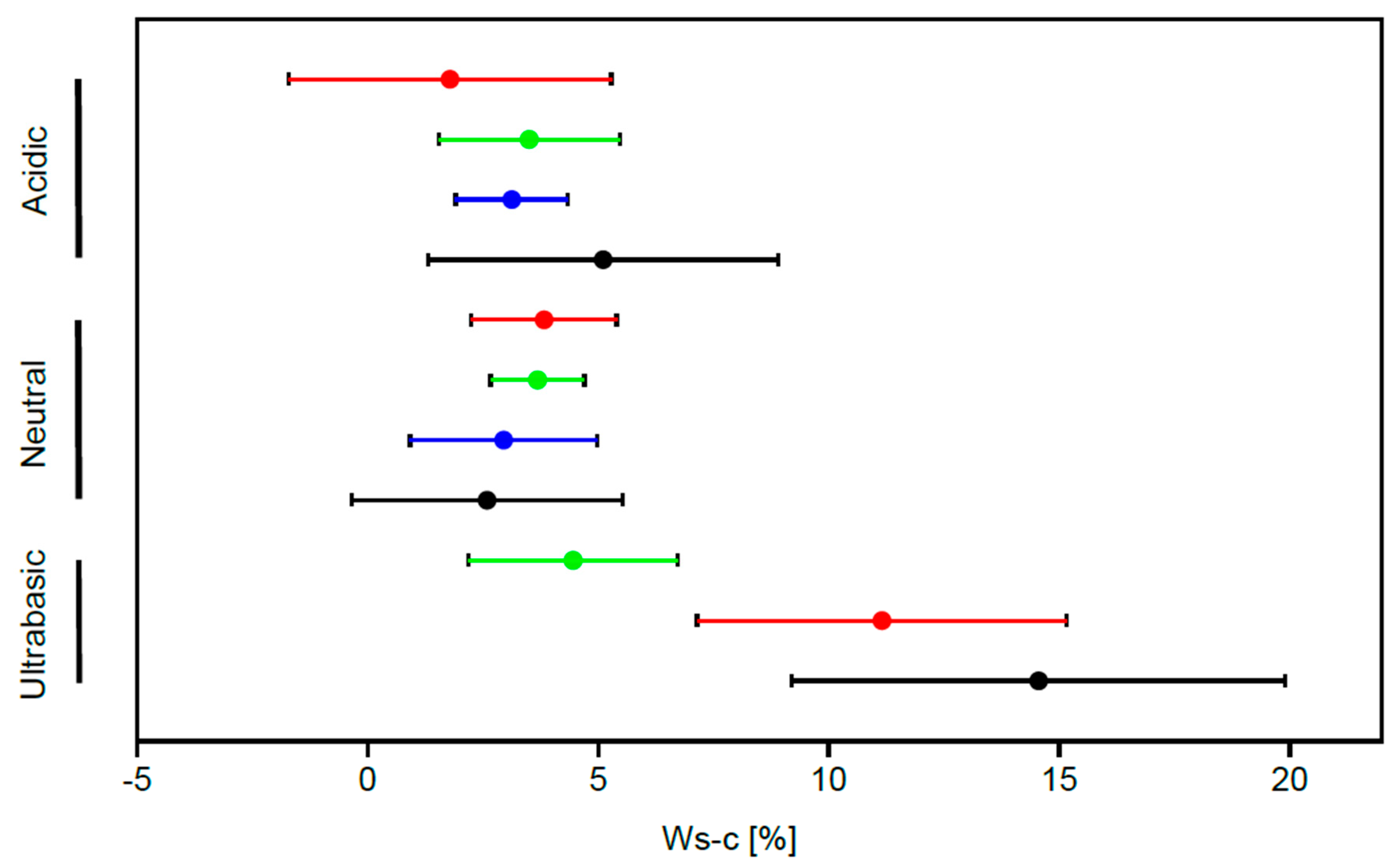
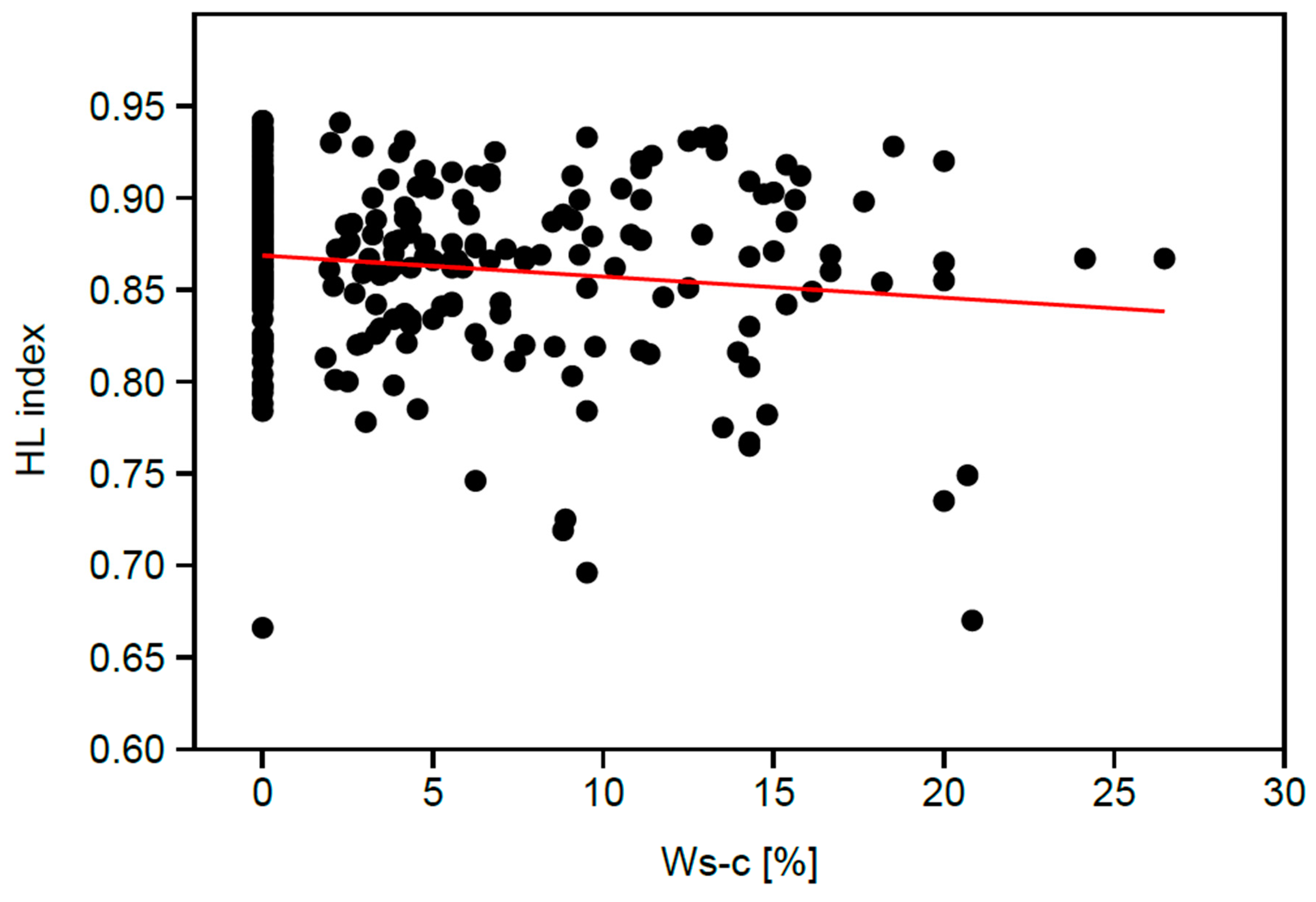
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
- Amelanchier spicata
- Conyza canadensis
- Impatiens parviflora
- Padus serotina
- Pseudotsuga taxifolia
- Quercus rubra
- Robinia pseudacacia
- Fallopia convolvulus
- Solanum luteum
- Vicia hirsuta
- Viola arvensis
- Cotoneaster horizontalis
- Malus domestica
- Sorbus intermedia
- Larix decidua
- Aegopodium podagraria
- Agropyron repens
- Alliaria petiolata
- Allium oleraceum
- Anthriscus sylvestris
- Arenaria serpyllifolia
- Calamagrostis epigejos
- Chaerophyllum aromaticum
- Chamaenerion angustifolium
- Dactylis glomerata
- Galeopsis tetrahit
- Galium aparine
- Geranium robertianum
- Geum urbanum
- Holcus lanatus
- Hypochoeris radicata
- Rubus caesius
- Sambucus nigra
- Sarothamnus scoparius
- Senecio jacobaea
- Taraxacum sect. Ruderalia
- Torilis japonica
- Urtica dioica
- Verbascum nigrum
- Vicia cracca.
References
- Soizic Le, S.; Michael, H.; Yichuan, S.; Adrian, H.; Cyril, B.; Thomas, M.B.; Bastian, B.; Stuart, H.M.B.; Simon, N.S.; Tim, B.; et al. Protected areas and effective biodiversity conservation. Science 2013, 80, 803–805. [Google Scholar] [CrossRef]
- David, V.; Zuzana, V.H.; Helena, K.; Kateřina, S. Human transformation of ecosystems: Comparing protected and unprotected areas with natural baselines. Ecol. Indic. 2016, 66, 321–328. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.B.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S.L. Global biodiversity conservation priorities. Science 2006, 80, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.J.; Martín-López, B.; López, E.; Plieninger, T.; Alcaraz-Segura, D.; Vaughn, C.C.; Cabello, J. Do protected areas networks ensure the supply of ecosystem services? Spatial patterns of two nature reserve systems in semi-arid Spain. Appl. Geogr. 2015, 60, 1–9. [Google Scholar] [CrossRef]
- Eastwood, A.; Brooker, R.; Irvine, R.J.; Artz, R.R.E.; Norton, L.R.; Bullock, J.M.; Ross, L.; Fielding, D.; Ramsay, S.; Roberts, J.; et al. Does nature conservation enhance ecosystem services delivery? Ecosyst. Serv. 2016, 17, 152–162. [Google Scholar] [CrossRef]
- Lecina-Diaz, J.; Alvarez, A.; De Cáceres, M.; Herrando, S.; Vayreda, J.; Retana, J. Are protected areas preserving ecosystem services and biodiversity? Insights from Mediterranean forests and shrublands. Landscape Ecol. 2019, 34, 2307–2321. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Available online: https://www.cbd.int/sp/targets/ (accessed on 20 November 2019).
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 2013, 161, 230–238. [Google Scholar] [CrossRef]
- Wiersma, Y.F.; Nudds, T.D. Efficiency and effectiveness in representative reserve design in Canada: The contribution of existing protected areas. Biol. Conserv. 2009, 142, 1639–1646. [Google Scholar] [CrossRef]
- García-Bañuelos, P.; Rovito, S.M.; Pineda, E. Representation of Threatened Biodiversity in Protected Areas and Identification of Complementary Areas for Their Conservation: Plethodontid Salamanders in Mexico. Trop. Conserv. Sci. 2019, 12, 1940082919834156. [Google Scholar] [CrossRef]
- Bosso, L.; Ancillotto, L.; Smeraldo, S.; D’Arco, S.; Migliozzi, A.; Conti, P.; Russo, D. Loss of potential bat habitat following a severe wildfire: A model-based rapid assessment. Int. J. Wildland Fire 2018, 27, 756–769. [Google Scholar] [CrossRef]
- Clark, N.E.; Boakes, E.H.; McGowan, P.J.K.; Mace, G.M.; Fuller, R.A. Protected areas in South Asia have not prevented habitat loss: A study using historical models of land-use change. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Joppa, L.N.; Pfaff, A. High and far: Biases in the location of protected areas. PLoS ONE 2009, 4, e8273. [Google Scholar] [CrossRef] [PubMed]
- Rodrıíguez, N.; Armenteras, D.; Retana, J. Effectiveness of protected areas in the Colombian Andes: Deforestation, fire and land-use changes. Reg. Environ. Change 2013, 13, 423–435. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Kučera, T. Inclusion of native and alien species in temperate nature reserves: A historical study from Central Europe. Conserv. Biol. 2003, 17, 1414–142. [Google Scholar] [CrossRef]
- Pyšek, P.; Jarošík, V.; Kučera, T. Patterns of invasion in temperate nature reserves. Biol. Conserv. 2002, 104, 13–24. [Google Scholar] [CrossRef]
- Lambdon, P.W.; Pyšek, P.; Basnou, C.; Hejda, M.; Arianoutsou, M.; Essl, F.; Jarošík, V.; Pergl, J.; Winter, M.; Anastasiu, P.; et al. Alien flora of Europe: Species diversity, temporal trends, geographical patterns and research needs. Preslia 2008, 80, 101–149. [Google Scholar]
- Plant Invasions in Protected Areas; Foxcroft, L.C., Pyšek, P., Richardson, D.M., Genovesi, P., Eds.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; New York, NY, USA; London, UK, 2014; p. 656. [Google Scholar] [CrossRef]
- Reczyńska, K. Udział gatunków synantropijnych w zbiorowiskach z klasy Quercetea robori-petraeae Br.-Bl. et R.Tx. 1943 w Sudetach i na ich Przedgórzu. Acta Bot. Sil. 2011, 7, 113–123. [Google Scholar]
- Reczyńska, K. Diversity and ecology of oak forests in SW Poland (Sudetes Mts). Phytocoenologia 2015, 45, 85–106. [Google Scholar] [CrossRef]
- Interpretation Manual of European Union Habitats—EUR28. Available online: https://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf (accessed on 16 December 2019).
- Rackham, O. Woodlands; Collins: London, UK, 2006; p. 608. [Google Scholar]
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: New York, NY, USA, 2009; p. 756. [Google Scholar]
- Szabó, P. Driving forces of stability and change in woodland structure: A case study from the Czech lowlands. Forest Ecol. Manag. 2010, 259, 650–656. [Google Scholar] [CrossRef]
- Szymura, T.H. Tradycyjna gospodarka odroślowa w Europie Środkowej i jej wpływ na różnorodność biologiczną. Sylwan 2010, 154, 545–551. [Google Scholar]
- Hédl, R.; Šipoš, J.; Chudomelová, M.; Utinek, D. Dynamics of herbaceous vegetation during four years of experimental coppice introduction. Folia Geobot. 2017, 52, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Reprint; The Blackburn Press: Caldwell, NJ, USA, 2002; p. 547. [Google Scholar]
- Chytrý, M.; Otýpková, Z. Plot sizes used for phytosociological sampling of European vegetation. J. Veg. Sci. 2003, 14, 563–570. [Google Scholar] [CrossRef]
- Kącki, Z.; Śliwiński, M. The Polish Vegetation Database: Structure, resources and development. Acta Soc. Bot. Pol. 2012, 81, 75–79. [Google Scholar] [CrossRef]
- McCune, B.; Keon, D. Equations for potential annual direct incident radiation and heat load. J. Veg. Sci. 2002, 13, 603–606. [Google Scholar] [CrossRef]
- Bolewski, A.; Parachoniak, W. Petrografia, 3rd ed.; Wydawnictwa Geologiczne: Warszawa, Poland, 1988; p. 656. [Google Scholar]
- Jackowiak, B. Anthropogenic changes in the flora of vascular plants of Poznań. Wyd Nauk UAM Ser. Biol 1990, 42, 1–234. [Google Scholar]
- Sudnik-Wójcikowska, B. Synantropization indices of urban floras: An attempt at definition and assessment. Acta Soc. Bot. Pol. 1991, 60, 163–185. [Google Scholar] [CrossRef]
- Chmiel, J. Flora of vascular plants of the eastern part of the Gniezno Lake District and its anthropogenic transformations in the 19th and 20th centuries, part 1. Prace Zakładu Taksonomii Roślin UAM w Poznaniu 1993, 1, 1–202. [Google Scholar]
- Falencka-Jabłońska, M. Species of synantropic plants as indicators of the transformation level of forest biocenosis. Studia i Materiały Centrum Edukacji Przyrodniczo-Leśnej 2007, 9, 279–287. [Google Scholar]
- Zając, M.; Zając, A. A tentative list of segetal and ruderal apophyte in Poland. Zesz. Nauk. UJ. Pr. Bot. 1992, 24, 7–23. [Google Scholar]
- Mirek, Z.; Piękoś-Mirkowa, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland. A Checklist; W. Szafer Institute of Botany Polish Academy of Sciences: Kraków, Poland, 2002; p. 442. [Google Scholar]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Alien Plants in Poland with Particular Reference to Invasive Species; Generalna Dyrekcja Ochrony Środowiska: Warszawa, Poland, 2012; p. 197. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001, 4, 1–9. [Google Scholar]
- Kiedrzyński, M.; Zielińska, K.; Grzelak, P. Transformation of forest vegetation after 40 years of protection in the Tomczyce Nature Reserve (Central Poland). Folia Biol. Oecologica 2011, 7, 207–227. [Google Scholar] [CrossRef]
- Wilczek, Z.; Wika, S.; Gorczyca, M.; Bregin, M. Flora roślin naczyniowych rezerwatu przyrody Skarpa Wiślicka na Pogórzu Śląskim. Acta Bot. Silesiaca 2014, 10, 99–118. [Google Scholar]
- Stachnowicz, W.; Rusińska, A.; Pawlik, A. Synanthropization of flora and vegetation of transitional peat bog remaining under human pressure–the “Żurawiniec” Nature Reserve in Poznań. Rocz nauk Pol Tow Ochr Przyr “Salamandra” 2003, 7, 27–63. [Google Scholar]
- Piórek, K.; Krechowski, J. Synantropizacja flory rezerwatu jodłowego Rudka Sanatoryjna (woj. Mazowieckie). Chrońmy Przyrodę Ojczystą 2007, 63, 82–96. [Google Scholar]
- Fałtynowicz, W.; Halama, M.; Kmiecik, M. Flora roślin naczyniowych rezerwatu “Cisy” koło Barda (południowo-zachodnia Polska). Acta Bot. Sil. 2004, 1, 35–48. [Google Scholar]
- Obidziński, T.; Symonides, E. The influence of the groundlayer structure on the invasion of small balsam (Impatiens parviflora DC.) to natural and degraded forests. Acta Soc. Bot. Pol. 2000, 69, 1–8. [Google Scholar] [CrossRef]
- Chmura, D.; Sierka, E. The occurrence of invasive alien plant species in selected forest nature reserves in southern Poland as a conservation problem. Nat. Conserv. 2006, 62, 3–11. [Google Scholar]
- Łysik, M. Ten years of change in ground-layer vegetation of European beech forest in the protected area (Ojców National Park, South Poland). Pol. J. Ecol. 2008, 56, 17–31. [Google Scholar]
- Levine, J.M. Species Diversity and Biological Invasions: Relating Local Process to Community Pattern. Science 2000, 288, 852–864. [Google Scholar] [CrossRef]
- Sołtys-Lelek, A.; Barabasz-Krasny, B.; Możdżeń, K. Synanthropization of riparian plant communities in the Ojców National Park (Southern Poland). Biodiv. Res. Conserv. 2016, 44, 35–53. [Google Scholar] [CrossRef][Green Version]
- Jaroszewicz, B.; Cholewińska, O.; Gutowski, J.J.; Samojlik, T.; Zimny, M.; Latałowa, M. Białowieża Forest—A Relic of the High Naturalness of European Forests. Forests 2019, 10, 849. [Google Scholar] [CrossRef]
- Sallustio, L.; De Toni, A.; Strollo, A.; Di Febbraro, M.; Gissi, E.; Casella, L.; Geneletti, D.; Munafo, M.; Vizzarri, M.; Marchetti, M. Assessing habitat quality in relation to the spatial distribution of protected areas in Italy. J. Environ. Manag. 2017, 201, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Gawenda-Kempczyńska, D.; Paszek, I.; Załuski, T. Regeneration of vegetation in manor park in Laskowice (Dąbrowa Forest District). Ecol. Quest. 2017, 27, 39–52. [Google Scholar] [CrossRef]
- Cox, R.L.; Underwood, E.C. The importance of conserving biodiversity outside of protected areas in Mediterranean ecosystems. PLoS ONE 2011, 6, e14508. [Google Scholar] [CrossRef] [PubMed]
| - | ρ |
|---|---|
| Duration and Type of Protection | - |
| Duration of protection (0–58 years) | 0.072 n.s. |
| Nature Reserves | 0.060 n.s. |
| N2000 sites | 0.004 n.s. |
| Landscape Parks | −0.102 n.s. |
| Unprotected areas | 0.098 n.s. |
| Bedrock | - |
| Ultrabasic | 0.352 *** |
| Acidic | −0.145 * |
| Basic | −0.113 * |
| Topography | - |
| HL index | −0.145 *. |
| Aspect | 0.017 n.s. |
| Slope [o] | 0.002 n.s. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reczyńska, K.; Świerkosz, K. Does Protection Really Matter? A Case Study from Central European Oak Forests. Diversity 2020, 12, 6. https://doi.org/10.3390/d12010006
Reczyńska K, Świerkosz K. Does Protection Really Matter? A Case Study from Central European Oak Forests. Diversity. 2020; 12(1):6. https://doi.org/10.3390/d12010006
Chicago/Turabian StyleReczyńska, Kamila, and Krzysztof Świerkosz. 2020. "Does Protection Really Matter? A Case Study from Central European Oak Forests" Diversity 12, no. 1: 6. https://doi.org/10.3390/d12010006
APA StyleReczyńska, K., & Świerkosz, K. (2020). Does Protection Really Matter? A Case Study from Central European Oak Forests. Diversity, 12(1), 6. https://doi.org/10.3390/d12010006






