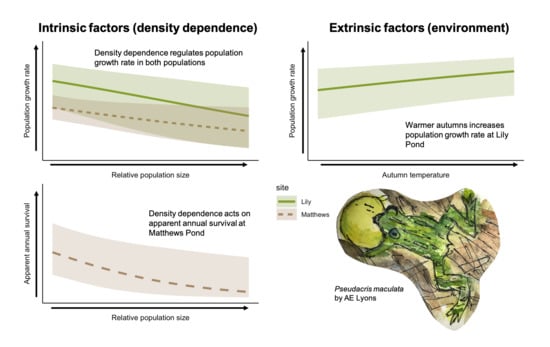
Density Dependence and Adult Survival Drive Dynamics in Two High Elevation Amphibian Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species
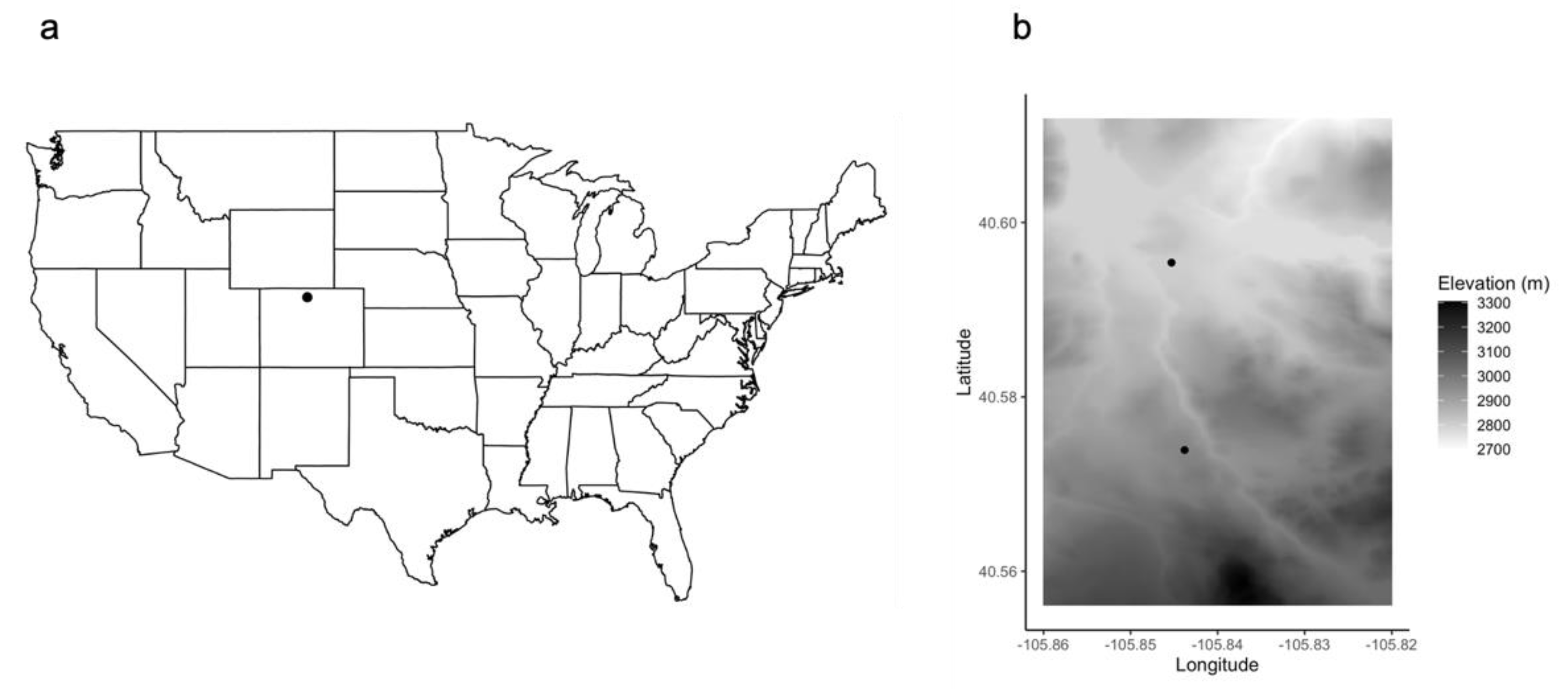
2.2. Data Collection
2.3. Covariate Development
2.4. Data Analysis
3. Results
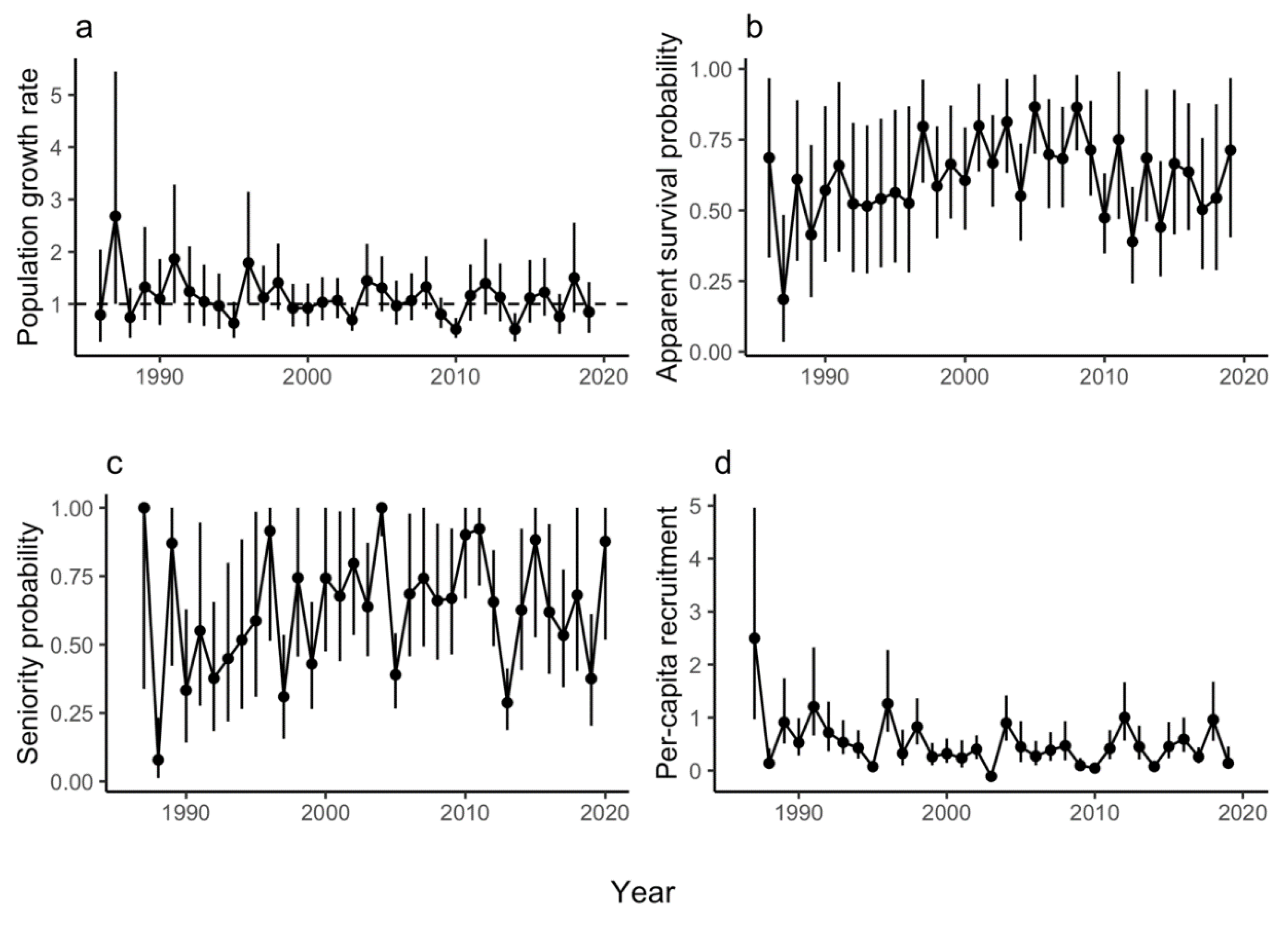
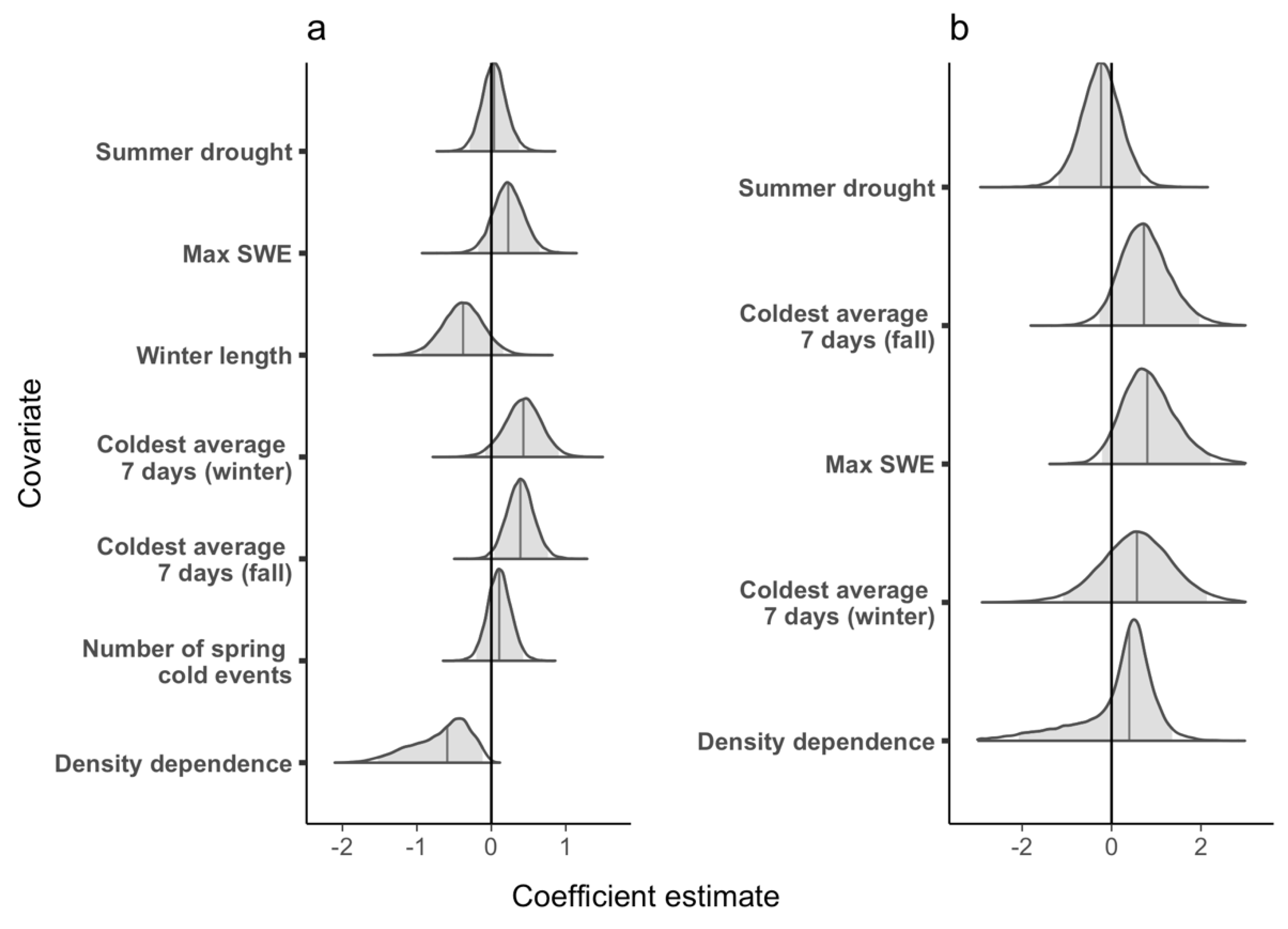
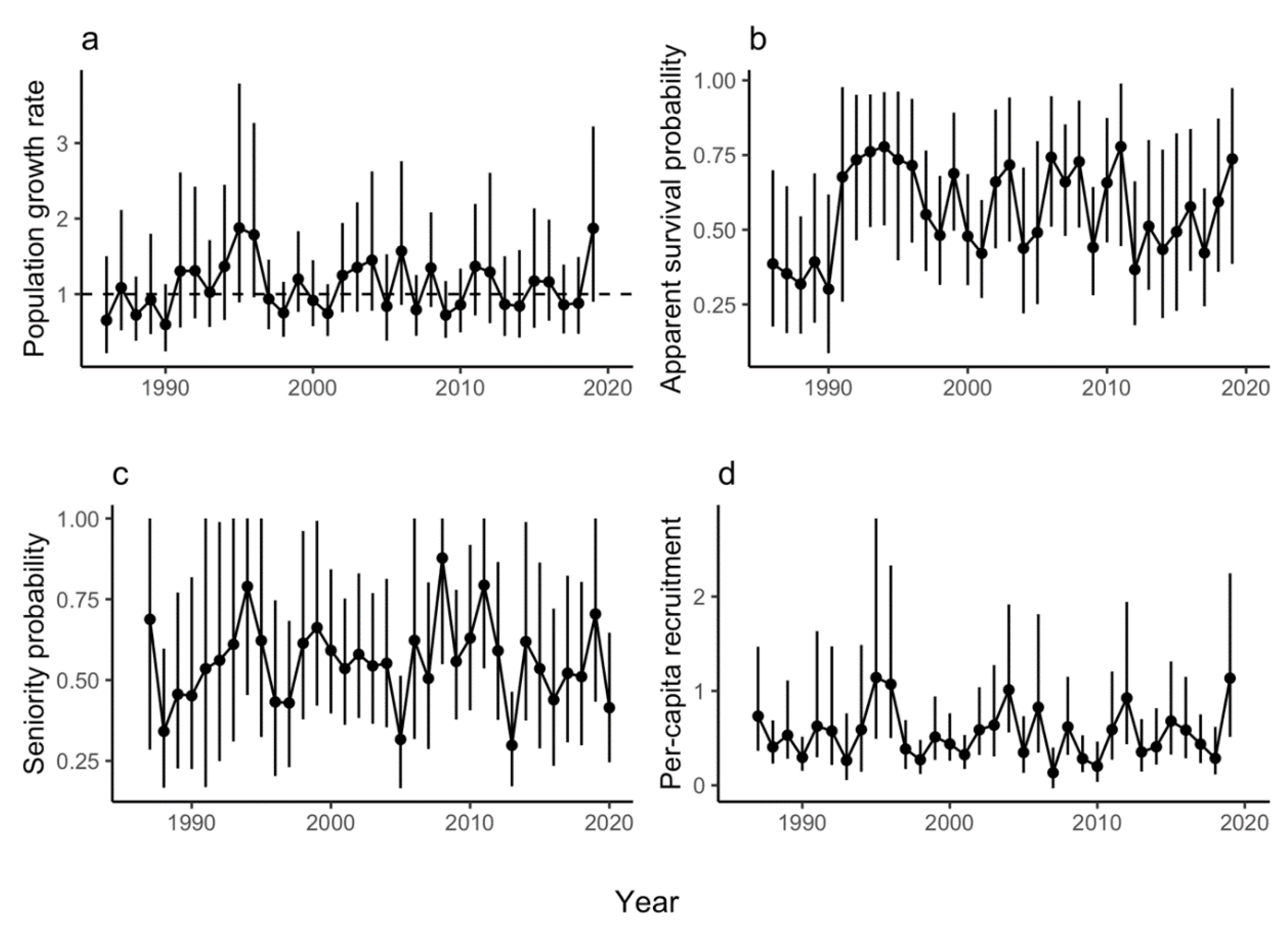
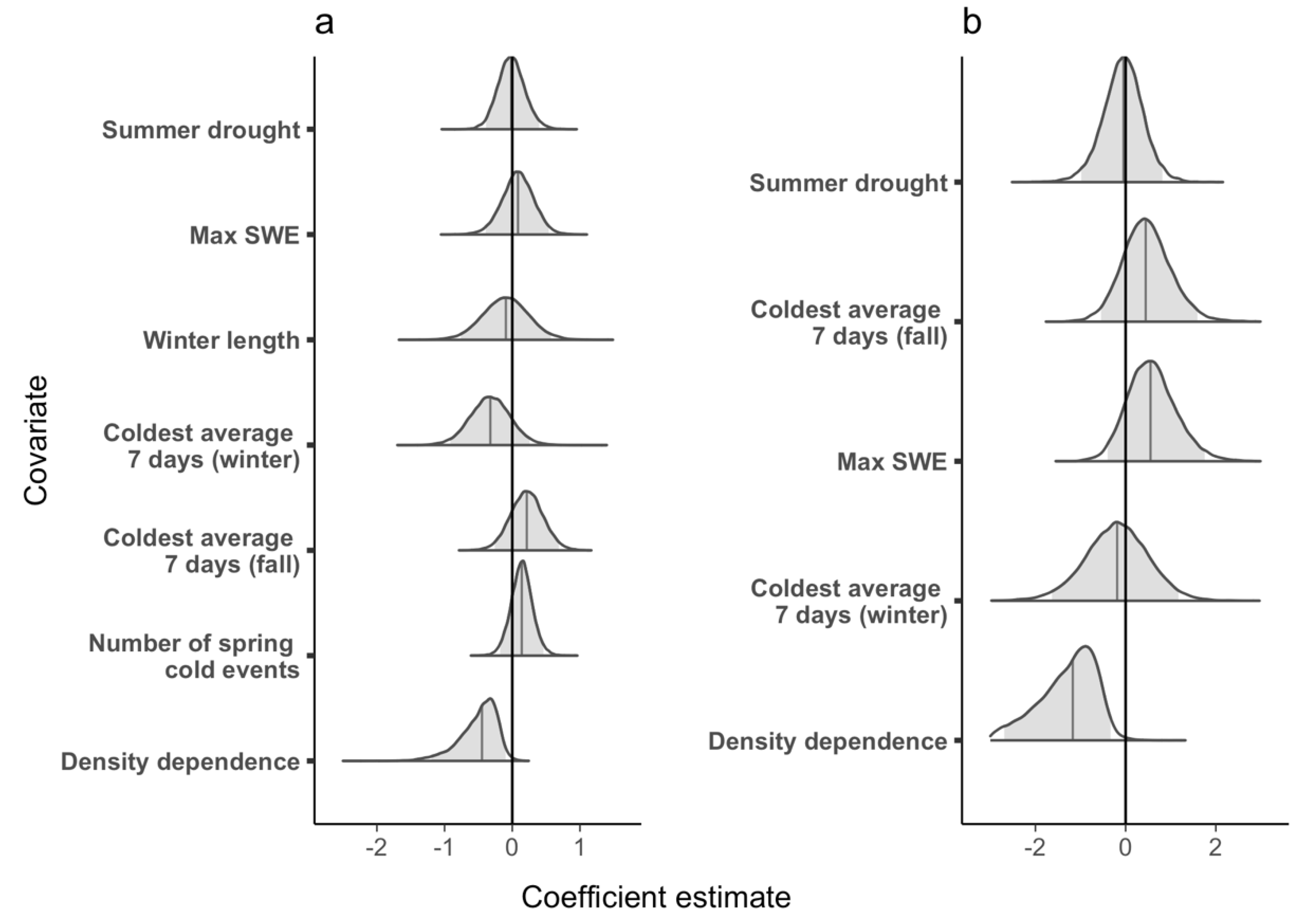
3.1. Population Growth Rate
3.2. Survival
3.3. Additional Parameters
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blaustein, A.R.; Wake, D.B. Declining amphibian populations: A global phenomenon? Trends Ecol. Evol. 1990, 5, 203–204. [Google Scholar] [CrossRef]
- Daszak, P.; Scott, D.E.; Kilpatrick, A.M.; Faggioni, C.; Gibbons, J.W.; Porter, D. Amphibian population declines at Savannah river site are linked to climate, not Chytridiomycosis. Ecology 2005, 86, 3232–3237. [Google Scholar] [CrossRef]
- Knapp, R.A.; Boiano, D.M.; Vredenburg, V.T. Removal of nonnative fish results in population expansion of a declining amphibian (mountain yellow-legged frog, Rana muscosa). Biol. Conserv. 2007, 135, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, E.H.C.; Miller, D.A.W.; Schmidt, B.R.; Adams, M.J.; Amburgey, S.M.; Chambert, T.; Cruickshank, S.S.; Fisher, R.N.; Green, D.M.; Hossack, B.R.; et al. Quantitative evidence for the effects of multiple drivers on continental-scale amphibian declines. Sci. Rep. 2016, 6, 25625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skelly, D.K. Distributions of pond-breeding anurans: An overview of mechanisms. Isr. J. Zool. 2001, 47, 313–332. [Google Scholar] [CrossRef]
- Longo, A.V.; Burrowes, P.A.; Joglar, R.L. Seasonality of Batrachochytrium dendrobatidis infection in direct-developing frogs suggests a mechanism for persistence. Dis. Aquat. Org. 2010, 92, 253–260. [Google Scholar] [CrossRef]
- Garner, T.W.J.; Rowcliffe, J.M.; Fisher, M.C. Climate change, chytridiomycosis or condition: An experimental test of amphibian survival. Glob. Chang. Biol. 2011, 17, 667–675. [Google Scholar] [CrossRef]
- Canessa, S.; Bozzuto, C.; Grant, E.H.C.; Cruickshank, S.S.; Fisher, M.C.; Koella, J.C.; Lötters, S.; Martel, A.; Pasmans, F.; Scheele, B.C.; et al. Decision-making for mitigating wildlife diseases: From theory to practice for an emerging fungal pathogen of amphibians. J. Appl. Ecol. 2018, 55, 1987–1996. [Google Scholar] [CrossRef] [Green Version]
- Petrovan, S.O.; Schmidt, B.R. Neglected juveniles; a call for integrating all amphibian life stages in assessments of mitigation success (and how to do it). Biol. Conserv. 2019, 236, 252–260. [Google Scholar] [CrossRef]
- Hellriegel, B. Single- or multistage regulation in complex life cycles: Does it make a difference? Oikos 2000, 88, 239–249. [Google Scholar] [CrossRef]
- Biek, R.; Funk, W.C.; Maxell, B.A.; Mills, L.S. What is missing in amphibian decline research: Insights from ecological sensitivity analysis. Conserv. Biol. 2002, 16, 728–734. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-94-011-2106-4. [Google Scholar]
- Leão, S.M.; Pianka, E.R.; Pelegrin, N. Is there evidence for population regulation in amphibians and reptiles? J. Herpetol. 2018, 52, 28–33. [Google Scholar] [CrossRef]
- Briggs, C.J.; Vredenburg, V.T.; Knapp, R.A.; Rachowicz, L.J. Investigating the population-level effects of chytridiomycosis: An emerging infectious disease of amphibians. Ecology 2005, 86, 3149–3159. [Google Scholar] [CrossRef]
- Reading, C.J. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia 2007, 151, 125–131. [Google Scholar] [CrossRef]
- Bell, B.D.; Carver, S.; Mitchell, N.J.; Pledger, S. The recent decline of a New Zealand endemic: How and why did populations of Archey’s frog Leiopelma archeyi crash over 1996–2001? Biol. Conserv. 2004, 120, 189–199. [Google Scholar] [CrossRef]
- Schmidt, B.R.; Feldmann, R.; Schaub, M. Demographic processes underlying population growth and decline in Salamandra salamandra. Conserv. Biol. 2005, 19, 1149–1156. [Google Scholar] [CrossRef]
- Bell, B.D.; Pledger, S.A. How has the remnant population of the threatened frog Leiopelma pakeka (Anura: Leiopelmatidae) fared on Maud Island, New Zealand, over the past 25 years? Austral Ecol. 2010, 35, 241–256. [Google Scholar] [CrossRef]
- McCaffery, R.M.; Maxell, B.A. Decreased winter severity increases viability of a montane frog population. Proc. Natl. Acad. Sci. USA 2010, 107, 8644–8649. [Google Scholar] [CrossRef] [Green Version]
- Muths, E.; Scherer, R.D.; Pilliod, D.S. Compensatory effects of recruitment and survival when amphibian populations are perturbed by disease. J. Appl. Ecol. 2011, 48, 873–879. [Google Scholar] [CrossRef]
- Muths, E.; Chambert, T.; Schmidt, B.R.; Miller, D.a.W.; Hossack, B.R.; Joly, P.; Grolet, O.; Green, D.M.; Pilliod, D.S.; Cheylan, M.; et al. Heterogeneous responses of temperate-zone amphibian populations to climate change complicates conservation planning. Sci. Rep. 2017, 7, 17102. [Google Scholar] [CrossRef] [Green Version]
- Hossack, B.R.; Adams, M.J.; Pearl, C.A.; Wilson, K.W.; Bull, E.L.; Lohr, K.; Patla, D.; Pilliod, D.S.; Jones, J.M.; Wheeler, K.K.; et al. Roles of patch characteristics, drought frequency, and restoration in long-term trends of a widespread amphibian. Conserv. Biol. 2013, 27, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, H.; Arsovski, D.; Thirion, J.-M.; Bonnaire, E.; Pichenot, J.; Boitaud, S.; Brison, A.-L.; Miaud, C.; Joly, P.; Besnard, A. Contrasting patterns of environmental fluctuation contribute to divergent life histories among amphibian populations. Ecology 2016, 97, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Kissel, A.M.; Palen, W.J.; Ryan, M.E.; Adams, M.J. Compounding effects of climate change reduce population viability of a montane amphibian. Ecol. Appl. 2019, 29, e01832. [Google Scholar] [CrossRef] [PubMed]
- Beebee, T.J.C.; Denton, J.S.; Buckley, J. Factors affecting population densities of adult Natterjack toads Bufo calamita in Britain. J. Appl. Ecol. 1996, 33, 263–268. [Google Scholar] [CrossRef]
- Meyer, A.H.; Schmidt, B.R.; Grossenbacher, K. Analysis of three amphibian populations with quarter–century long time–series. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1998, 265, 523–528. [Google Scholar] [CrossRef]
- Pellet, J.; Schmidt, B.R.; Fivaz, F.; Perrin, N.; Grossenbacher, K. Density, climate and varying return points: An analysis of long-term population fluctuations in the threatened European tree frog. Oecologia 2006, 149, 65–71. [Google Scholar] [CrossRef]
- Băncilă, R.I.; Ozgul, A.; Hartel, T.; Sos, T.; Schmidt, B.R. Direct negative density-dependence in a pond-breeding frog population. Ecography 2016, 39, 449–455. [Google Scholar] [CrossRef]
- Cayuela, H.; Griffiths, R.A.; Zakaria, N.; Arntzen, J.W.; Priol, P.; Léna, J.-P.; Besnard, A.; Joly, P. Drivers of amphibian population dynamics and asynchrony at local and regional scales. J. Anim. Ecol. 2020, 89, 1350–1364. [Google Scholar] [CrossRef]
- Freckleton, R.P.; Watkinson, A.R.; Green, R.E.; Sutherland, W.J. Census error and the detection of density dependence. J. Anim. Ecol. 2006, 75, 837–851. [Google Scholar] [CrossRef] [Green Version]
- Lebreton, J.-D. Assessing density dependence: Where are we left? In Modeling Demographic Processes in Marked Populations; Thomson, D.L., Cooch, E.G., Conroy, M.J., Eds.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009; ISBN 978-0-387-78151-8. [Google Scholar]
- Pechmann, J.H.K.; Scott, D.E.; Semlitsch, R.D.; Caldwell, J.P.; Vitt, L.J.; Gibbons, J.W. Declining amphibian populations: The problem of separating human impacts from natural fluctuations. Science 1991, 253, 892–895. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, D.B.; Likens, G.E.; Andersen, A.; Bowman, D.; Bull, C.M.; Burns, E.; Dickman, C.R.; Hoffmann, A.A.; Keith, D.A.; Liddell, M.J.; et al. Value of long-term ecological studies. Austral Ecol. 2012, 37, 745–757. [Google Scholar] [CrossRef]
- Hughes, B.B.; Beas-Luna, R.; Barner, A.K.; Brewitt, K.; Brumbaugh, D.R.; Cerny-Chipman, E.B.; Close, S.L.; Coblentz, K.E.; de Nesnera, K.L.; Drobnitch, S.T.; et al. Long-term studies contribute disproportionately to ecology and policy. BioScience 2017, 67, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D. Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef]
- Dayton, P.K.; Tegner, M.J.; Edwards, P.B.; Riser, K.L. Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecol. Appl. 1998, 8, 309–322. [Google Scholar] [CrossRef]
- Pechmann, J.H.K.; Wilbur, H.M. Putting declining amphibian populations in perspective: Natural fluctuations and human impacts. Herpetologica 1994, 50, 65–84. [Google Scholar]
- Lindenmayer, D.B.; Cunningham, R.B. Longitudinal patterns in bird reporting rates in a threatened ecosystem: Is change regionally consistent? Biol. Conserv. 2011, 144, 430–440. [Google Scholar] [CrossRef]
- Hammerson, G.A. Amphibians and Reptiles in Colorado, Revised Edition, 2nd ed.; University Press of Colorado: Niwot, CO, USA, 1999; ISBN 978-0-87081-534-8. [Google Scholar]
- Ricklefs, R.E. The Economy of Nature by Ricklefs, 5th ed.; W. H. Freeman: New York, NY, USA, 2001. [Google Scholar]
- Pradel, R. Utilization of capture-mark-recapture for the study of recruitment and Population growth rate. Biometrics 1996, 52, 703–709. [Google Scholar] [CrossRef]
- Tenan, S.; Tavecchia, G.; Oro, D.; Pradel, R. Assessing the effect of density on population growth when modeling individual encounter data. Ecology 2019, 100, e02595. [Google Scholar] [CrossRef]
- Moriarty-Lemmon, E.; Lemmon, A.R.; Collins, J.T.; Lee-Yaw, J.A.; Cannatella, D.C. Phylogeny-based delimitation of species boundaries and contact zones in the trilling chorus frogs (Pseudacris). Mol. Phylogenet. Evol. 2007, 44, 1068–1082. [Google Scholar] [CrossRef]
- Moriarty, E.C.; Cannatella, D.C. Phylogenetic relationships of the North American chorus frogs (Pseudacris: Hylidae). Mol. Phylogenet. Evol. 2004, 30, 409–420. [Google Scholar] [CrossRef]
- Pettus, D.; Angleton, G.M. Comparative reproductive biology of montane and piedmont chorus frogs. Evolution 1967, 21, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Funk, W.C.; Murphy, M.A.; Hoke, K.L.; Muths, E.; Amburgey, S.M.; Lemmon, E.M.; Lemmon, A.R. Elevational speciation in action? Restricted gene flow associated with adaptive divergence across an altitudinal gradient. J. Evol. Biol. 2016, 29, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Muths, E.; Scherer, R.D.; Amburgey, S.M.; Matthews, T.; Spencer, A.W.; Corn, P.S. First estimates of the probability of survival in a small-bodied, high-elevation frog (Boreal Chorus Frog, Pseudacris maculata), or how historical data can be useful. Can. J. Zool. 2016, 94, 599–606. [Google Scholar] [CrossRef]
- Corn, P.S.; Muths, E. Variable breeding phenology affects the exposure of amphibian embryos to ultraviolet radiation. Ecology 2004, 83, 2958–2963. [Google Scholar] [CrossRef]
- Watts, A.G.; Schlichting, P.; Billerman, S.; Jesmer, B.; Micheletti, S.; Fortin, M.-J.; Funk, C.; Hapeman, P.; Muths, E.L.; Murphy, M.A. How spatio-temporal habitat connectivity affects amphibian genetic structure. Front. Genet. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendall, W.L.; Pollock, K.H.; Brownie, C. A likelihood-based approach to capture-recapture estimation of demographic parameters under the robust design. Biometrics 1995, 51, 293–308. [Google Scholar] [CrossRef]
- Muths, E.; Scherer, R.D.; Amburgey, S.M.; Corn, P.S. Twenty-nine years of population dynamics in a small-bodied montane amphibian. Ecosphere 2018, 9, e02522. [Google Scholar] [CrossRef]
- snotelr: Calculate and Visualize “SNOTEL” Snow Data and Seasonality. Available online: https://CRAN.R-project.org/package=snotelr (accessed on 1 October 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 1 October 2020).
- A Wrapper around “rjags” to Streamline “JAGS” Analyses. Available online: https://CRAN.R-project.org/package=jagsUI (accessed on 1 October 2020).
- Brooks, S.P.; Gelman, A. general methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998, 7, 434–455. [Google Scholar] [CrossRef] [Green Version]
- Hobbs, N.T.; Hooten, M.B. Bayesian Models: A Statistical Primer for Ecologists; Princeton University Press: Princeton, NJ, USA, 2015; ISBN 978-1-4008-6655-7. [Google Scholar]
- Morris, W.F.; Doak, D.F. Quantitative Conservation Biology: Theory and Practice of Population Viability Analysis, 1st ed.; Sinauer Associates is an imprint of Oxford University Press: Sunderland, MA, USA, 2002; ISBN 978-0-87893-546-8. [Google Scholar]
- Sauer, J.R.; Link, W.A. Analysis of the North American breeding bird survey using hierarchical models. Auk 2011, 128, 87–98. [Google Scholar] [CrossRef]
- Tenan, S.; Pradel, R.; Tavecchia, G.; Igual, J.M.; Sanz-Aguilar, A.; Genovart, M.; Oro, D. Hierarchical modelling of population growth rate from individual capture–recapture data. Methods Ecol. Evol. 2014, 5, 606–614. [Google Scholar] [CrossRef]
- Doak, D.F.; Morris, W.F. Demographic compensation and tipping points in climate-induced range shifts. Nature 2010, 467, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Vonesh, J.R.; De la Cruz, O. Complex life cycles and density dependence: Assessing the contribution of egg mortality to amphibian declines. Oecologia 2002, 133, 325–333. [Google Scholar] [CrossRef]
- Govindarajulu, P.; Altwegg, R.; Anholt, B.R. Matrix model investigation of invasive species control: Bullfrogs on Vancouver Island. Ecol. Appl. 2005, 15, 2161–2170. [Google Scholar] [CrossRef]
- Mote, P.W.; Li, S.; Lettenmaier, D.P.; Xiao, M.; Engel, R. Dramatic declines in snowpack in the western US. Npj Clim. Atmos. Sci. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Amburgey, S.; Funk, W.C.; Murphy, M.; Muths, E. Effects of hydroperiod duration on survival, developmental rate, and size at metamorphosis in Boreal chorus frog tadpoles (Pseudacris maculata). Herpetologica 2012, 68, 456–467. [Google Scholar] [CrossRef]
- Ryan, M.E.; Palen, W.J.; Adams, M.J.; Rochefort, R.M. Amphibians in the climate vise: Loss and restoration of resilience of montane wetland ecosystems in the western US. Front. Ecol. Environ. 2014, 12, 232–240. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Ryan, M.E.; Hamlet, A.F.; Palen, W.J.; Lawler, J.J.; Halabisky, M. Projecting the hydrologic impacts of climate change on montane wetlands. PLoS ONE 2015, 10, e0136385. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Abatzoglou, J.T.; Williams, A.P. Impact of anthropogenic climate change on wildfire across western US forests. Proc. Natl. Acad. Sci. USA 2016, 113, 11770–11775. [Google Scholar] [CrossRef] [Green Version]
- Pickrell, J.; Pennisi, E. Record, U.S. and Australian fires raise fears for many species. Science 2020, 370, 18–19. [Google Scholar] [CrossRef]
- Ward, M.; Tulloch, A.I.T.; Radford, J.Q.; Williams, B.A.; Reside, A.E.; Macdonald, S.L.; Mayfield, H.J.; Maron, M.; Possingham, H.P.; Vine, S.J.; et al. Impact of 2019–2020 mega-fires on Australian fauna habitat. Nat. Ecol. Evol. 2020, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Hossack, B.R.; Corn, P.S. Responses of pond-breeding amphibians to wildfire: Short-term patterns in occupancy and colonization. Ecol. Appl. 2007, 17, 1403–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossack, B.R.; Pilliod, D.S. Amphibian responses to wildfire in the Western United States: Emerging Patterns from Short-Term Studies. Fire Ecol. 2011, 7, 129–144. [Google Scholar] [CrossRef]
- Potvin, D.A.; Parris, K.M.; Date, K.L.S.; Keely, C.C.; Bray, R.D.; Hale, J.; Hunjan, S.; Austin, J.J.; Melville, J. Genetic erosion and escalating extinction risk in frogs with increasing wildfire frequency. J. Appl. Ecol. 2017, 54, 945–954. [Google Scholar] [CrossRef] [Green Version]
| Covariate | Derivation | Hypothesis (ρ) | Hypothesis (φ) |
|---|---|---|---|
| Density dependence (Pi) | The number of individuals at time i relative to the number of individuals in the population at time 2 (Ni/N2). | Population growth rate decreases as population size increases due to competition for resources via decreased recruitment or decreased survival. | Apparent survival decreases as population sizes increase due to competition for resources via increased mortality of adults or permanent emigration. |
| Number of spring cold events (sprevnt) | The total number of times that the minimum temperature was below −2 °C for 1 or more days after SWE was 50% of maximum for the year. | Increasing numbers of freezing events in the spring (e.g., early in the active season) increases mortality at multiple life stages decreasing recruitment and subsequently population growth rate. | NA |
| Coldest average 7 days in the fall (tmin7FL) | The coldest week (i.e., 7 day period) between October 1 and the development of persistent snow (SWE> = 2). | Colder temperatures in the fall (e.g., late in the active season) increase mortality at multiple life stages decreasing recruitment and subsequently population growth rate. | Colder temperatures in the fall (e.g., late in the active season) increase mortality at the adult stage. |
| Coldest average 7 days in the winter (tmin7wn) | The coldest week (i.e., 7-day period) between October 1 and March 31 | Colder temperatures in the winter increase mortality at multiple life stages decreasing recruitment and subsequently population growth rate. | Colder temperatures in the winter increase mortality at the adult stage. |
| Winter Length (winlength) | The number of days between persistent snowpack (Snow Water Equivalent (SWE)> = 2) and 50% of maximum SWE in the spring | Longer winters decrease recruitment (and subsequently population growth rate) because sub-adults may not have the resources to sustain them through the season. | Longer winters decrease survival because adults may not have the resources to sustain them through the season. |
| Maximum Snow Water Equivalent (maxswe) | The maximum SWE measurement for the breeding year. | Higher snowpack results in improved breeding conditions and summer habitat, resulting in increased recruitment and population growth rate. | Higher snowpack results in improved breeding conditions and summer habitat, resulting in increased survival. |
| Summer Drought (phdism) | PHDI value for estimating the month of metamorphosis (50 days past 50% of maximum SWE). | Increased summer drought results in decreased water availability on the landscape and increased mortality (via desiccation) at multiple life stages, reducing recruitment and population growth rate. | Increased summer drought results in decreased water availability on the landscape and increased mortality (via desiccation) at multiple life stages, adult survival. |
| Active season length (actseas) | The number of days between 50% of max SWE and the earliest date on which minimum temperature drops below −2 °C at the end of the growing season prior to the current breeding season (e.g., we used the active season in 2019 as a covariate on rho/phi in 2020). | Longer growing seasons increase recruitment/growth rate because individuals have more time to procure fat reserves prior to hibernation. | Longer growing seasons increase adult survival because individuals have more time to procure fat reserves prior to hibernation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kissel, A.M.; Tenan, S.; Muths, E. Density Dependence and Adult Survival Drive Dynamics in Two High Elevation Amphibian Populations. Diversity 2020, 12, 478. https://doi.org/10.3390/d12120478
Kissel AM, Tenan S, Muths E. Density Dependence and Adult Survival Drive Dynamics in Two High Elevation Amphibian Populations. Diversity. 2020; 12(12):478. https://doi.org/10.3390/d12120478
Chicago/Turabian StyleKissel, Amanda M., Simone Tenan, and Erin Muths. 2020. "Density Dependence and Adult Survival Drive Dynamics in Two High Elevation Amphibian Populations" Diversity 12, no. 12: 478. https://doi.org/10.3390/d12120478