Recommendations for IUCN Red List Conservation Status of the “Dryophytes immaculatus Group” in North East Asia
Abstract
1. Introduction
2. Materials and Methods
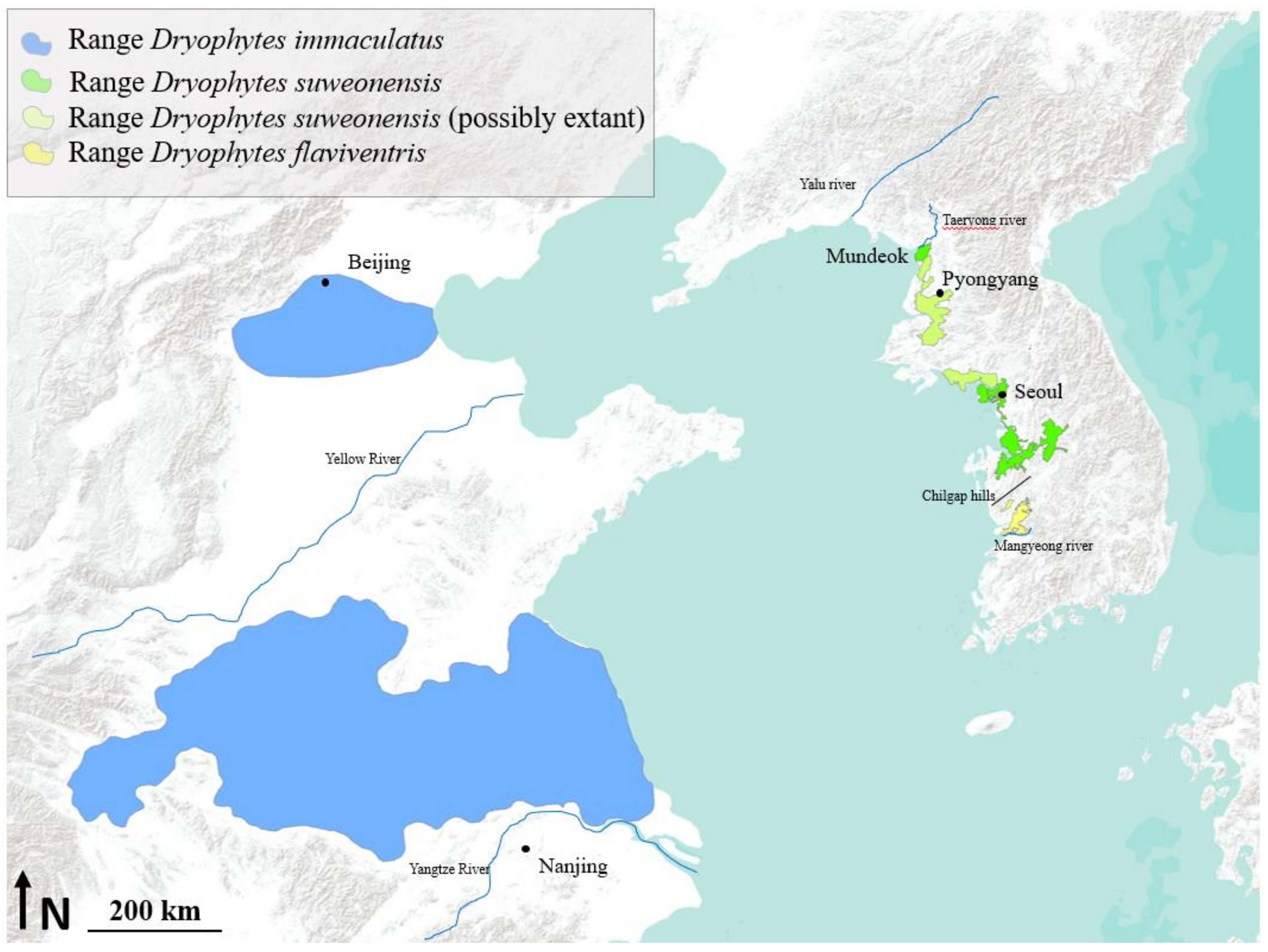
2.1. Species Introduction
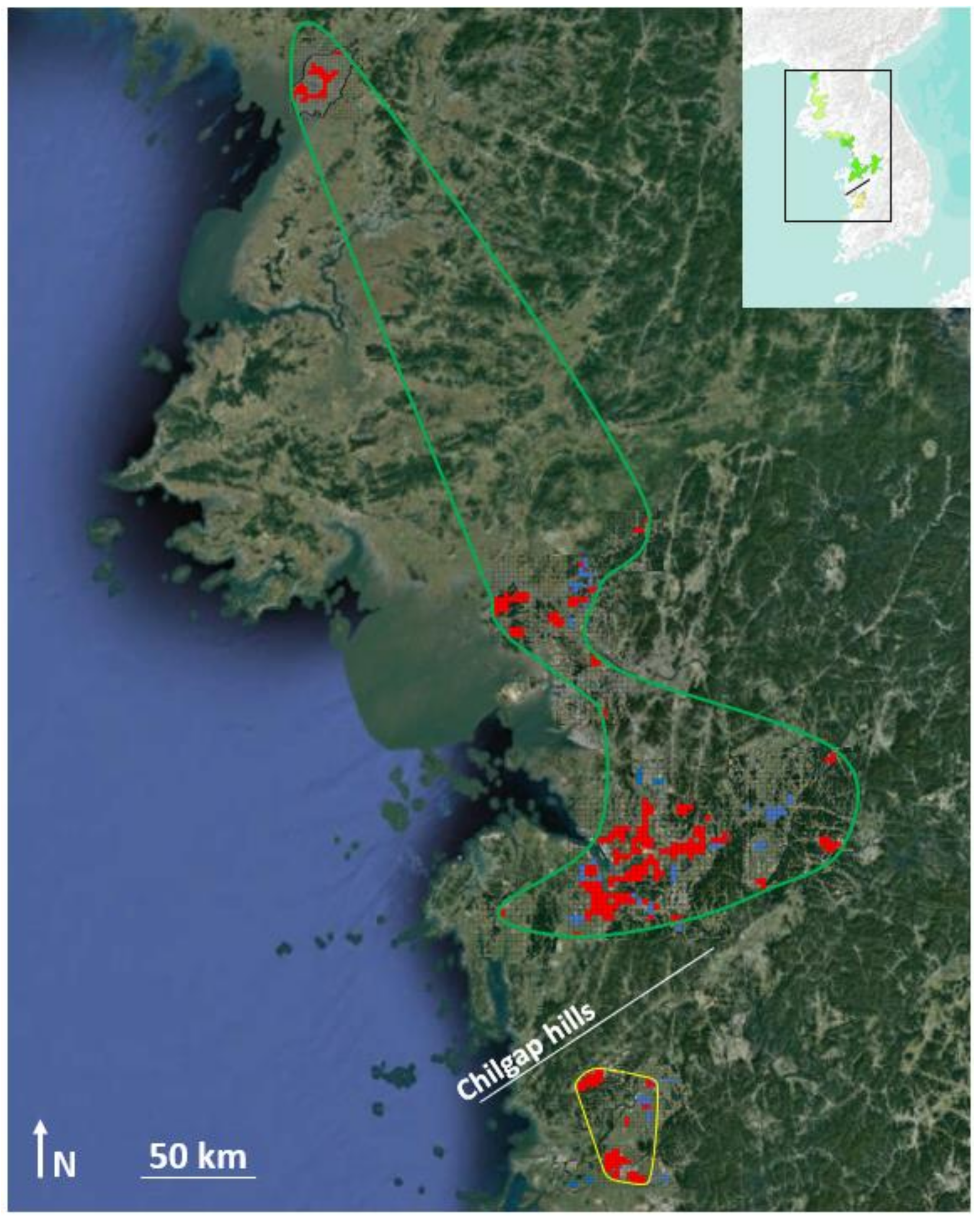
2.2. Field Surveys
2.3. Method for Assessment
2.4. General Introduction and Threats to the Three Species
2.5. IUCN Assessment: Dryophytes immaculatus
2.5.1. Population Size Reduction
2.5.2. Geographic Range
2.5.3. Small Population Size and Decline
2.5.4. Very Small or Restricted Population
2.5.5. Quantitative Analysis
2.6. IUCN Assessment: Dryophytes suweonensis
2.6.1. Population Size Reduction
2.6.2. Geographic Range
2.6.3. Small Population Size and Decline
2.6.4. Very Small or Restricted Population
2.6.5. Quantitative Analysis
2.7. IUCN Assessment: Dryophytes flaviventris
2.7.1. Population Size Reduction
2.7.2. Geographic Range
2.7.3. Small Population Size and Decline
2.7.4. Very Small or Restricted Population
2.7.5. Quantitative Analysis
3. Results
3.1. Extinction Threat Estimate: Dryophytes immaculatus
3.1.1. Population Size Reduction
3.1.2. Geographic Range
3.1.3. Small Population Size and Decline
3.1.4. Very Small or Restricted Population
3.1.5. Quantitative Analysis
3.2. Extinction Threat Estimate: Dryophytes suweonensis
3.2.1. Population Size Reduction
3.2.2. Geographic Range
3.2.3. Small Population Size and Decline
3.2.4. Very Small or Restricted Population
3.2.5. Quantitative Analysis
3.3. Extinction Threat Estimate: Dryophytes flaviventris
3.3.1. Population Size Reduction
3.3.2. Geographic Range
3.3.3. Small Population Size and Decline
3.3.4. Very Small or Restricted Population
3.3.5. Quantitative Analysis
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, K.; Pressey, R.L.; Newton, A.; Burgman, M.; Possingham, H.; Weston, C. Measuring and incorporating vulnerability into conservation planning. Environ. Manag. 2005, 35, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.M.; Mittermeier, R.A.; da Fonseca, G.A.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.T.; Cowling, R.M. Embracing opportunism in the selection of priority conservation areas. Conserv. Biol. 2007, 21, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Pressey, R.L.; Bottrill, M.C. Opportunism, threats, and the evolution of systematic conservation planning. Conserv. Biol. 2008, 22, 1340–1345. [Google Scholar] [CrossRef]
- Arponen, A. Prioritizing species for conservation planning. Biodivers. Conserv. 2012, 21, 875–893. [Google Scholar] [CrossRef]
- Bradford, D.F.; Tabatabai, F.; Graber, D.M. Isolation of remaining populations of the native frog, Rana muscosa, by introduced fishes in Sequoia and Kings Canyon National Parks, California. Conserv. Biol. 1993, 7, 882–888. [Google Scholar] [CrossRef]
- Knapp, R.A.; Boiano, D.M.; Vredenburg, V.T. Removal of nonnative fish results in population expansion of a declining amphibian (mountain yellow-legged frog, Rana muscosa). Biol. Conserv. 2007, 135, 11–20. [Google Scholar] [CrossRef]
- Matthews, K.R. Response of mountain yellow-legged frogs, Rana muscosa, to short distance translocation. J. Herpetol. 2003, 37, 621–626. [Google Scholar] [CrossRef]
- Vredenburg, V.T.; Knapp, R.A.; Tunstall, T.S.; Briggs, C.J. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc. Natl. Acad. Sci. USA 2010, 107, 9689–9694. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef]
- Longcore, J.E.; Pessier, A.P.; Nichols, D.K. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 1999, 91, 219–227. [Google Scholar] [CrossRef]
- Nelson, A.; Setiyono, T.; Rala, A.B.; Quicho, E.D.; Raviz, J.V.; Abonete, P.J.; Maunahan, A.A.; Garcia, C.A.; Bhatti, H.Z.M.; Villano, L.S.; et al. Towards an operational SAR-based rice monitoring system in Asia: Examples from 13 demonstration sites across Asia in the RIICE project. Remote Sens. 2014, 6, 10773–10812. [Google Scholar] [CrossRef]
- MacDonald, D.; Associates Ltd. (MDA). World Land Cover at 30m Resolution from MDAUS BaseVue. 2013. Available online: http://www.arcgis.com/home/item.html?id=1770449f11df418db482a14df4ac26eb (accessed on 1 June 2020).
- Frye, C.; Wright, D.J.; Nordstrand, E.; Terborgh, C.; Foust, J. Using classified and unclassified land cover data to estimate the footprint of human settlement. Data Sci. J. 2018, 17, 1–12. [Google Scholar] [CrossRef]
- Dong, J.; Xiao, X. Evolution of regional to global paddy rice mapping methods: A review. ISPRS J. Photogramm. Remote Sens. 2016, 119, 214–227. [Google Scholar] [CrossRef]
- Lee, S.-D.; Miller-Rushing, A.J. Degradation, urbanization, and restoration: A review of the challenges and future of conservation on the Korean Peninsula. Biol. Conserv. 2014, 176, 262–276. [Google Scholar] [CrossRef]
- Halstead, B.J.; Rose, J.P.; Reyes, G.A.; Wylie, G.D.; Casazza, M.L. Conservation reliance of a threatened snake on rice agriculture. Glob. Ecol. Conserv. 2019, 19, e00681. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Park, D.S. Habitat use and movement patterns of the viviparous aquatic snake, Oocatochus rufodorsatus, from Northeast Asia. Zool. Sci. 2011, 28, 593–599. [Google Scholar] [CrossRef]
- Katayama, N.; Odaya, Y.; Amano, T.; Yoshida, H. Spatial and temporal associations between fallow fields and Greater Painted Snipe density in Japanese rice paddy landscapes. Agric. Ecosyst. Environ. 2020, 295, 106892. [Google Scholar] [CrossRef]
- Sedlock, J.L.; Stuart, A.M.; Horgan, F.G.; Hadi, B.; Como Jacobson, A.; Alviola, P.A.; Alvarez, J.D. Local-scale bat guild activity differs with rice growth stage at ground level in the Philippines. Diversity 2019, 11, 148. [Google Scholar] [CrossRef]
- Gomez Lutz, M.C.; Kehr, A.I.; Fernández, L.A. Abundancia, diversidad y caracterización de la comunidad de coleópteros acuáticos en una plantación de arroz al noreste de Argentina. Rev. Biol. Trop. 2016, 63, 629–638. [Google Scholar] [CrossRef]
- Fujioka, M.; Lee, S.D.; Kurechi, M.; Yoshida, H. Bird use of rice fields in Korea and Japan. Waterbirds 2010, 33, 8–29. [Google Scholar] [CrossRef]
- Duré, M.I.; Kehr, A.I.; Schaefer, E.F.; Marangoni, F. Diversity of amphibians in rice fields from northeastern Argentina. Interciencia 2008, 33, 528–531. [Google Scholar]
- Hobbs, R.J.; Higgs, E.; Harris, J.A. Novel ecosystems: Implications for conservation and restoration. Trends Ecol. Evol. 2009, 24, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.F.; Maltchik, L. Can management practices in rice fields contribute to amphibian conservation in southern Brazilian wetlands? Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 39–46. [Google Scholar] [CrossRef]
- Magle, S.B.; Hunt, V.M.; Vernon, M.; Crooks, K.R. Urban wildlife research: Past, present, and future. Biol. Conserv. 2012, 155, 23–32. [Google Scholar] [CrossRef]
- Holzer, K.A. Amphibian-Human Coexistence in Urban Areas; University of California Davis: San Diego, CA, USA, 2014. [Google Scholar]
- Holzer, K.A.; Bayers, R.P.; Nguyen, T.T.; Lawler, S.P. Habitat value of cities and rice paddies for amphibians in rapidly urbanizing Vietnam. J. Urban Ecol. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Borzée, A.; Heo, K.; Jang, Y. Relationship between agro-environmental variables and breeding Hylids in rice paddies. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Fujioka, M.; Lane, S.J. The impact of changing irrigation practices in rice fields on frog populations of the Kanto Plain, central Japan. Ecol. Res. 1997, 12, 101–108. [Google Scholar] [CrossRef]
- Naito, R.; Sakai, M.; Natuhara, Y.; Morimoto, Y.; Shibata, S. Microhabitat use by Hyla japonica and Pelophylax porosa brevipoda at levees in rice paddy areas of Japan. Zool. Sci. 2013, 30, 386–391. [Google Scholar] [CrossRef]
- Groffen, J.; Borzée, A.; Jang, Y. Preference for natural borders in rice paddies by two treefrog species. Anim. Cells Syst. 2018, 22, 205–211. [Google Scholar] [CrossRef]
- Borzée, A.; Messenger, K.R.; Chae, S.; Andersen, D.; Groffen, J.; Kim, Y.I.; An, J.; Othman, S.; Ri, K.; Nam, T.Y.; et al. Yellow sea mediated segregation between North East Asian Dryophytes species. PLoS ONE 2020, 15, e0234299. [Google Scholar] [CrossRef] [PubMed]
- Borzée, A.; Kim, K.; Heo, K.; Jablonski, P.G.; Jang, Y. Impact of land reclamation and agricultural water regime on the distribution and conservation status of the endangered Dryophytes Suweonensis. PeerJ 2017, 5, e3872. [Google Scholar] [CrossRef]
- Borzée, A.; Jang, Y. Impact of rice and bean harvests on the Suweon Treefrog (Dryophytes suweonensis). Int. J. Curr. Res. 2017, 9, 59620–59623. [Google Scholar]
- Borzée, A.; Jang, Y. Description of a seminatural habitat of the endangered Suweon treefrog, Hyla suweonensis. Anim. Cells Syst. 2015, 19, 216–220. [Google Scholar] [CrossRef]
- IUCN Species Survival Commission. IUCN Red List Categories and Criteria, 2nd ed.; Version 3.1; IUCN: Gland, Switzerland; Cambridge, UK, 2012; p. 32. [Google Scholar]
- Xie, F. Dryophytes immaculatus (amended version of 2014 assessment). In The IUCN Red List of Threatened Species 2017; e.T55512A112714297; ICUN: Gland, Switzerland, 2017. [Google Scholar] [CrossRef]
- IUCN SSC Amphibian Specialist Group. Dryophytes suweonensis (amended version of 2014 assessment). In The IUCN Red List of Threatened Species 2017; e.T55670A112715252; ICUN: Gland, Switzerland, 2017. [Google Scholar] [CrossRef]
- Dufresnes, C.; Litvinchuk, S.N.; Borzée, A.; Jang, Y.; Li, J.-T.; Miura, I.; Perrin, N.; Stöck, M. Phylogeography reveals an ancient cryptic radiation in East-Asian tree frogs (Hyla japonica group) and complex relationships between continental and island lineages. BMC Evol. Biol. 2016, 16, 253. [Google Scholar] [CrossRef]
- Fei, L.; Changyuan, Y.; Jianping, J. Colored Atlas of Chinese Amphibians and Their Distributions; Sichuan Science and Technology Press: Chendu, China, 2012. [Google Scholar]
- Borzée, A.; Santos, J.L.; Sanchez-Ramirez, S.; Bae, Y.; Heo, K.; Jang, Y.; Jowers, M.J. Phylogeographic and population insights of the Asian common toad (Bufo gargarizans) in Korea and China: Population isolation and expansions as response to the ice ages. PeerJ 2017, 5, e4044. [Google Scholar] [CrossRef]
- Kuramoto, M. Mating calls of treefrogs (genus Hyla) in the far east, with description of a new species from Korea. Copeia 1980, 1, 100–108. [Google Scholar] [CrossRef]
- Park, S.; Jeong, G.; Jang, Y. No reproductive character displacement in male advertisement signals of Hyla japonica in relation to the sympatric H. suweonensis. Behav. Ecol. Sociobiol. 2013, 67, 1345–1355. [Google Scholar] [CrossRef]
- Borzée, A.; Oh, S.; Sin, E.; Jang, Y. Spring voices in Korean rice fields: The effect of abiotic variables and syntopic calls on the calling activity of the treefrog Dryophytes suweonensis. Asian Herpetol. Res. 2020, in press. [Google Scholar]
- Borzée, A.; Kosch, T.A.; Kim, M.; Jang, Y. Introduced bullfrogs are associated with increased Batrachochytrium dendrobatidis prevalence and reduced occurrence of Korean treefrogs. PLoS ONE 2017, 12, e0177860. [Google Scholar] [CrossRef]
- Kim, E.; Nugraha, C.A.; Jang, Y.; Borzée, A. Breeding range variation between Korean hylids (Dryophytes sp.). J. Asia-Pac. Biodivers. 2019, 12, 135–138. [Google Scholar] [CrossRef]
- Borzée, A.; Kim, J.Y.; Cunha, M.A.M.d.; Lee, D.; Sin, E.; Oh, S.; Yi, Y.; Jang, Y. Temporal and spatial differentiation in microhabitat use: Implications for reproductive isolation and ecological niche specification. Integr. Zool. 2016, 11, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Borzée, A.; Andersen, D.; Jang, Y. Population trend inferred from aural surveys for calling anurans in Korea. PeerJ 2018, 6, e5568. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.; Isaac, N.J.; Harrower, C.A.; Collen, B.; Van Strien, A.J.; Roy, D.B. The use of opportunistic data for IUCN Red List assessments. Biol. J. Linn. Soc. 2015, 115, 690–706. [Google Scholar] [CrossRef]
- Mace, G.M.; Collar, N.J.; Gaston, K.J.; Hilton-Taylor, C.; Akçakaya, H.R.; Leader-Williams, N.; Milner-Gulland, E.J.; Stuart, S.N. Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv. Biol. 2008, 22, 1424–1442. [Google Scholar] [CrossRef]
- Lamoreux, J.; Akçakaya, H.R.; Bennun, L.; Collar, N.J.; Boitani, L.; Brackett, D.; Bräutigam, A.; Brooks, T.M.; da Fonseca, G.A.; Mittermeier, R.A. Value of the IUCN red list. Trends Ecol. Evol. 2003, 18, 214–215. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Pilgrim, J.D.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef]
- Mace, G.M. Classifying threatened species: Means and ends. Phil. Trans. R. Soc. Lond. B 1994, 344, 91–97. [Google Scholar]
- Hermoso, V.; Kennard, M.J.; Linke, S. Evaluating the costs and benefits of systematic data acquisition for conservation assessments. Ecography 2015, 38, 283–292. [Google Scholar] [CrossRef]
- Keller, V.; Bollmann, K. From red lists to species of conservation concern. Conserv. Biol. 2004, 18, 1636–1644. [Google Scholar] [CrossRef]
- Simaika, J.P.; Samways, M.J. Reserve selection using Red Listed taxa in three global biodiversity hotspots: Dragonflies in South Africa. Biol. Conserv. 2009, 142, 638–651. [Google Scholar] [CrossRef]
- Butchart, S.H.; Akcakaya, H.R.; Kennedy, E.; Hilton-Taylor, C. Biodiversity indicators based on trends in conservation status: Strengths of the IUCN Red List Index. Conserv. Biol. 2006, 20, 579–581. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Y.; Wang, Y.; Adams, M.J. Diet of introduced Bullfrogs (Rana catesbeiana): Predation on and diet overlap with native frogs on Daishan Island, China. J. Herpetol. 2005, 39, 668–674. [Google Scholar] [CrossRef]
- Bai, C.; Garner, T.W.; Li, Y. First evidence of Batrachochytrium dendrobatidis in China: Discovery of chytridiomycosis in introduced American bullfrogs and native amphibians in the Yunnan Province, China. EcoHealth 2010, 7, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Liu, X.; Fisher, M.C.; Garner, T.W.; Li, Y. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Divers. Distrib. 2012, 18, 307–318. [Google Scholar] [CrossRef]
- Borzée, A.; Kwon, S.; Kyo Soung, K.; Jang, Y. Policy recommendation on the restriction on amphibian trade toward the Republic of Korea. Front. Environ. Sci. 2020, 8, 129. [Google Scholar] [CrossRef]
- Koo, K.S.; Park, H.R.; Choi, J.H.; Sung, H.C. Present status of non-native amphibians and reptiles traded in Korean online pet shops. Korean J. Environ. Ecol. 2020, 3, 106–114. [Google Scholar] [CrossRef]
- Small, C.; Cohen, J.E. Continental Physiography, Climate, and the Global Distribution of Human Population. Curr. Anthropol. 2004, 45, 269–277. [Google Scholar] [CrossRef]
- Huston, M. Biological diversity, soils, and economics. Science 1993, 262, 1676–1679. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Gosselink, J.G. Wetlands; John Wiley & Sons, Inc: Hoboken, NJ, USA, 2007. [Google Scholar]
- Roh, G.; Borzée, A.; Jang, Y. Spatiotemporal distributions and habitat characteristics of the endangered treefrog, Hyla suweonensis, in relation to sympatric H. japonica. Ecol. Inform. 2014, 24, 78–84. [Google Scholar] [CrossRef]
- Song, W. Habitat analysis of Hyla suweonensis in the breeding season using species distribution modeling. J. Korea Soc. Environ. Restor. Technol. 2015, 18, 71–82. [Google Scholar] [CrossRef][Green Version]
- COP21. Paris Climate Agreement; United Nations: Paris, France, 2015. [Google Scholar]
- Mundaca, L.; Ürge-Vorsatz, D.; Wilson, C. Demand-side approaches for limiting global warming to 1.5 °C. Energy Effic. 2019, 12, 343–362. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- IPCC. Summary for Policymakers. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufouma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; World Meteorological Organization: Geneva, Switzerland, 2018; p. 32. [Google Scholar]
- Xu, Y.; Zhou, B.T.; Wu, J.; Han, Z.Y.; Zhang, Y.X.; Wu, J. Asian climate change under 1.5–4 C warming targets. Adv. Clim. Chang. Res. 2017, 8, 99–107. [Google Scholar] [CrossRef]
- Katzenberger, M.; Hammond, J.; Duarte, H.; Tejedo, M.; Calabuig, C.; Relyea, R.A. Swimming with predators and pesticides: How environmental stressors affect the thermal physiology of tadpoles. PLoS ONE 2014, 9, e98265. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.T. Crop Evolution, Adaptation and Yield; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1996. [Google Scholar]
- Chuang, M.F.; Borzée, A.; Jang, Y. Impact of environmental variables on the breeding phenology of a South Korean treefrog, Dryophytes suweonensis. In Proceedings of the International Long Term Ecological Research Network, Taiwan, 14–19 October 2018. [Google Scholar]
- Zhao, J.; Fan, Y.; Mu, Y. Sea level prediction in the Yellow Sea from satellite altimetry with a combined least squares-neural network approach. Mar. Geod. 2019, 42, 344–366. [Google Scholar] [CrossRef]
- Kulp, S.A.; Strauss, B.H. New elevation data triple estimates of global vulnerability to sea-level rise and coastal flooding. Nat. Commun. 2019, 10, 1–12. [Google Scholar]
- Gomez-Mestre, I.; Tejedo, M. Local adaptation of an anuran amphibian to osmotically stressful environments. Evol. Int. J. Org. Evol. 2003, 57, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, R.J. Planning for the impacts of sea level rise. Oceanography 2011, 24, 144–157. [Google Scholar] [CrossRef]
- Wu, C.S.; Yang, W.K.; Lee, T.H.; Gomez-Mestre, I.; Kam, Y.C. Salinity acclimation enhances salinity tolerance in tadpoles living in brackish water through increased Na+, K+-ATPase expression. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2014, 321, 57–64. [Google Scholar] [CrossRef]
- Heo, K.; Kim, Y.I.; Bae, Y.; Jang, Y.; Amaël, B. First report of Dryophytes japonicus tadpoles in saline environment. Russ. J. Herpetol. 2019, 26, 87–90. [Google Scholar] [CrossRef]
- Auffarth, J.; Krug, A.; Proehl, H.; Jehle, R. A genetically-informed Population Viability Analysis reveals conservation priorities for an isolated population of Hyla arborea. Salamandra 2017, 53, 171–182. [Google Scholar]
- Moen, D.S.; Irschick, D.J.; Wiens, J.J. Evolutionary conservatism and convergence both lead to striking similarity in ecology, morphology and performance across continents in frogs. Proc. R. Soc. Lond. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef] [PubMed]
- Borzée, A.; Fong, J.J.; Nguyen, H.; Jang, Y. Large-scale hybridisation as an extinction threat to the Suweon treefrog (Hylidae: Dryophytes suweonensis). Animals 2020, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Borzée, A.; Kim, J.Y.; Jang, Y. Asymmetric competition over calling sites in two closely related treefrog species. Sci. Rep. 2016, 6, 32569. [Google Scholar] [CrossRef]
- Borzée, A.; Jang, Y. Interference competition driven by hydric stress in Korean Hylids. Nat. Conserv. Res. 2018, 3, 120–124. [Google Scholar] [CrossRef]
- Borzée, A.; Yu, A.-Y.; Jang, Y. Variation in the persistence of two Hylid species in relation to behavioural and physiological traits. Ethol. Ecol. Evol. 2018, 30, 515–533. [Google Scholar] [CrossRef]
- Borzée, A.; Kyong, C.N.; Kil, H.K.; Jang, Y. Impact of water quality on the occurrence of two endangered Korean anurans: Dryophytes suweonensis and Pelophylax chosenicus. Herpetologica 2018, 74, 1–7. [Google Scholar] [CrossRef]
- Deng, N.; Grassini, P.; Yang, H.; Huang, J.; Cassman, K.G.; Peng, S. Closing yield gaps for rice self-sufficiency in China. Nat. Commun. 2019, 10, 1–9. [Google Scholar] [CrossRef]
- NBSC. China Statistical Yearbook 1980–2016; China Statistics Press: Beijing, China, 2019. [Google Scholar]
- Yan, T.; Wang, J.; Huang, J. Urbanization, agricultural water use, and regional and national crop production in China. Ecol. Model. 2015, 318, 226–235. [Google Scholar] [CrossRef]
- Wang, L.; Anna, H.; Zhang, L.; Xiao, Y.; Wang, Y.; Xiao, Y.; Liu, J.; Ouyang, Z. Spatial and temporal changes of arable land driven by urbanization and ecological restoration in China. Chin. Geogr. Sci. 2019, 29, 809–819. [Google Scholar] [CrossRef]
- KOSIS (Ed.) Statistics Korea. In Statistical Annual Report (1999/2019); KOSIS: Daejeon, Korea, 2020. [Google Scholar]
- Jeong, S.; Ko, J.; Yeom, J.M. Nationwide projection of rice yield using a crop model integrated with geostationary satellite imagery: A case study in South Korea. Remote Sens. 2018, 10, 1665. [Google Scholar] [CrossRef]
- Borzée, A. Why Are Anurans Threatened? The Case of Dryophytes suweonensis. Ph.D Thesis, Seoul National University, Seoul, Korea, 2018. [Google Scholar]
- Burgman, M.A.; Fox, J.C. Bias in species range estimates from minimum convex polygons: Implications for conservation and options for improved planning. Anim. Conserv. 2003, 6, 19–28. [Google Scholar] [CrossRef]
- Groffen, J.; Kong, S.; Jang, Y.; Borzée, A. The invasive American bullfrog (Lithobates catesbeianus) in the Republic of Korea: History and recommendation for population control. Manag. Biol. Invasions 2019, 10, 517–535. [Google Scholar] [CrossRef]
- Borzée, A. A complicated situation: Population Viability Analysis for an endangered treefrog endemic to the Korean Peninsula, Dryophytes suweonensis. In Why Are Anurans Threatened? The Case of Dryophytes Suweonensis (PhD Thesis); Seoul National University: Seoul, Korea, 2017. [Google Scholar]
- Wang, Y.; Li, Y. Habitat selection by the introduced American bullfrog (Lithobates catesbeianus) on Daishan Island, China. J. Herpetol. 2009, 43, 205–211. [Google Scholar] [CrossRef]
- Borzée, A.; Jang, Y. Policy recommendation for the conservation of the Suweon Treefrog (Dryophytes suweonensis) in the Republic of Korea. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]

| Criterion | Critically Endangered | Endangered | Vulnerable | Dryophytes immaculatus | Dryophytes suweonensis | Dryophytes flaviventris |
|---|---|---|---|---|---|---|
| A. Population size reduction | ||||||
| A1 | ≥90% | ≥70% | ≥50% | n. a. | n. a. | n. a. |
| A2 | ≥80% | ≥50% | ≥30% | 27.5% | 15.9% | 15.9% |
| A3 | ≥80% | ≥50% | ≥30% | 27.5% | 15.9% | 15.9% |
| A4 | ≥80% | ≥50% | ≥30% | 27.5% | 15.9% | 15.9% |
| B. Geographic range | ||||||
| B1 | <100 km2 | <5000 km2 | <20,000 km2 | >20,000 km2 | 18,409 km2 | 876 km2 |
| B2 | <10 km2 | <500 km2 | <2000 km2 | 412 + 160 km2 | 268 km2 | |
| C. Small population size and decline | ||||||
| C1 | <250 | <2500 | <10,000 | 405,000 ? | ||
| C2 | <250 | <2500 | <10,000 | ca. 2500 | ca. 552 | |
| D. Very small or restricted population | ||||||
| D1 | <50 | <250 | <1000 | 405,000 ? | ca. 2500 | ca. 552 |
| D2 | – | – | AOO < 20 km2 | |||
| E. Quantitative analysis | ||||||
| E | ≥50% | ≥20% | ≥10% | 50.5% R Korea | 61.92% | |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzée, A. Recommendations for IUCN Red List Conservation Status of the “Dryophytes immaculatus Group” in North East Asia. Diversity 2020, 12, 336. https://doi.org/10.3390/d12090336
Borzée A. Recommendations for IUCN Red List Conservation Status of the “Dryophytes immaculatus Group” in North East Asia. Diversity. 2020; 12(9):336. https://doi.org/10.3390/d12090336
Chicago/Turabian StyleBorzée, Amaël. 2020. "Recommendations for IUCN Red List Conservation Status of the “Dryophytes immaculatus Group” in North East Asia" Diversity 12, no. 9: 336. https://doi.org/10.3390/d12090336
APA StyleBorzée, A. (2020). Recommendations for IUCN Red List Conservation Status of the “Dryophytes immaculatus Group” in North East Asia. Diversity, 12(9), 336. https://doi.org/10.3390/d12090336





