Antarctic Studies Show Lichens to be Excellent Biomonitors of Climate Change
Abstract
:1. Introduction
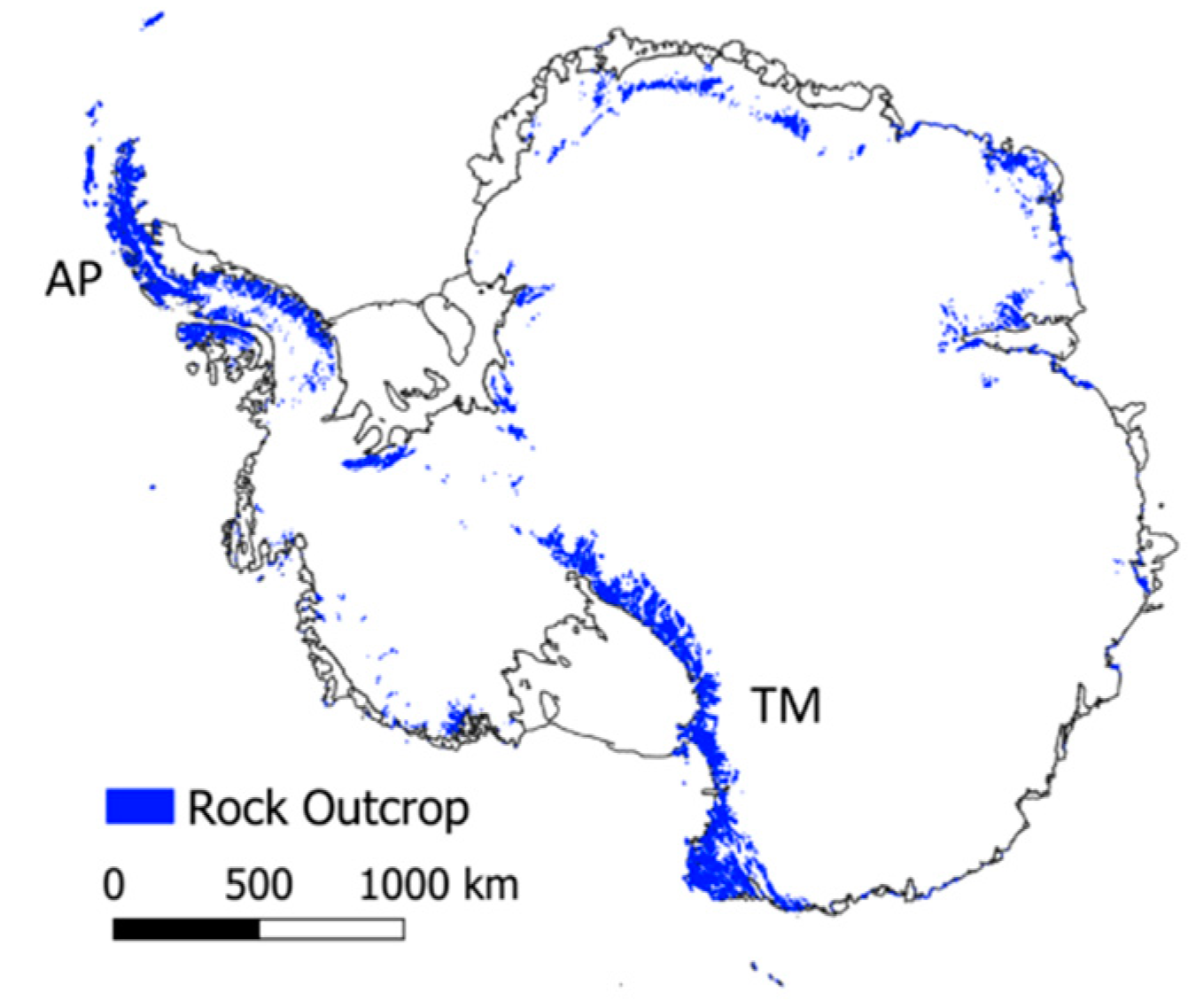
2. Antarctica
2.1. Physical Features
2.2. Human Influence
2.3. Vegetation
3. Gradients
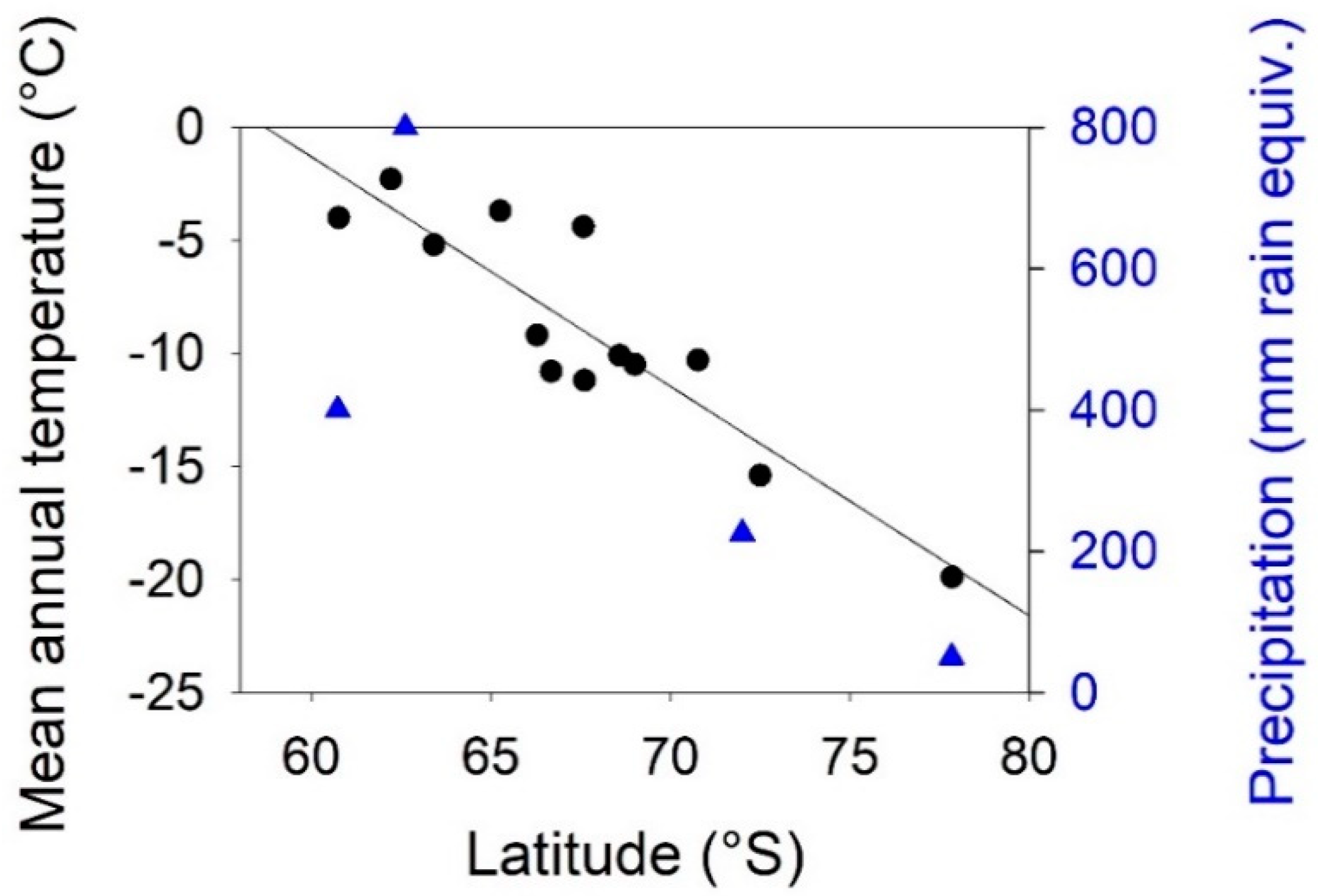
3.1. Environmental Gradients Across Antarctica
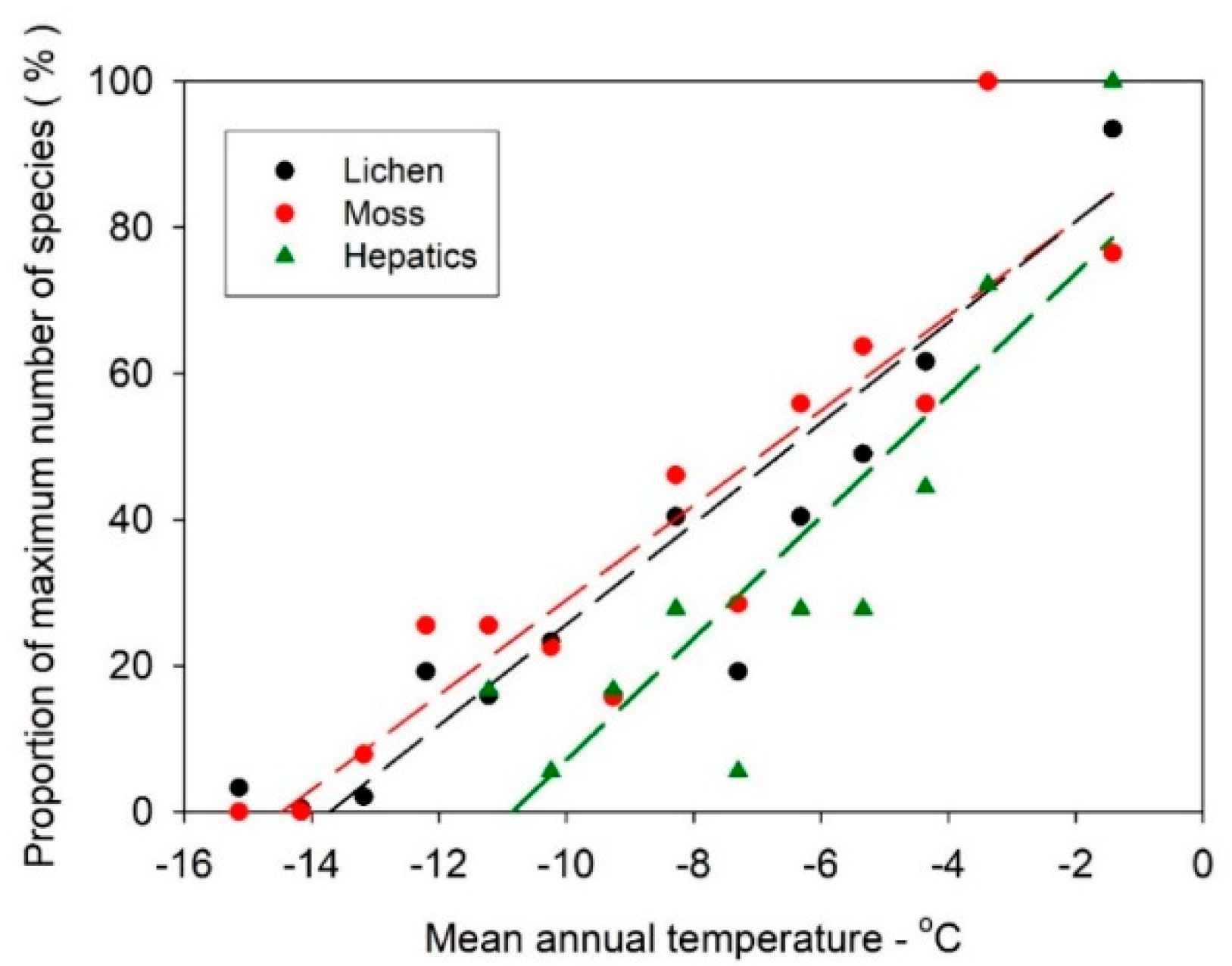
3.2. Lichen and Bryophyte Gradients Across Antarctica
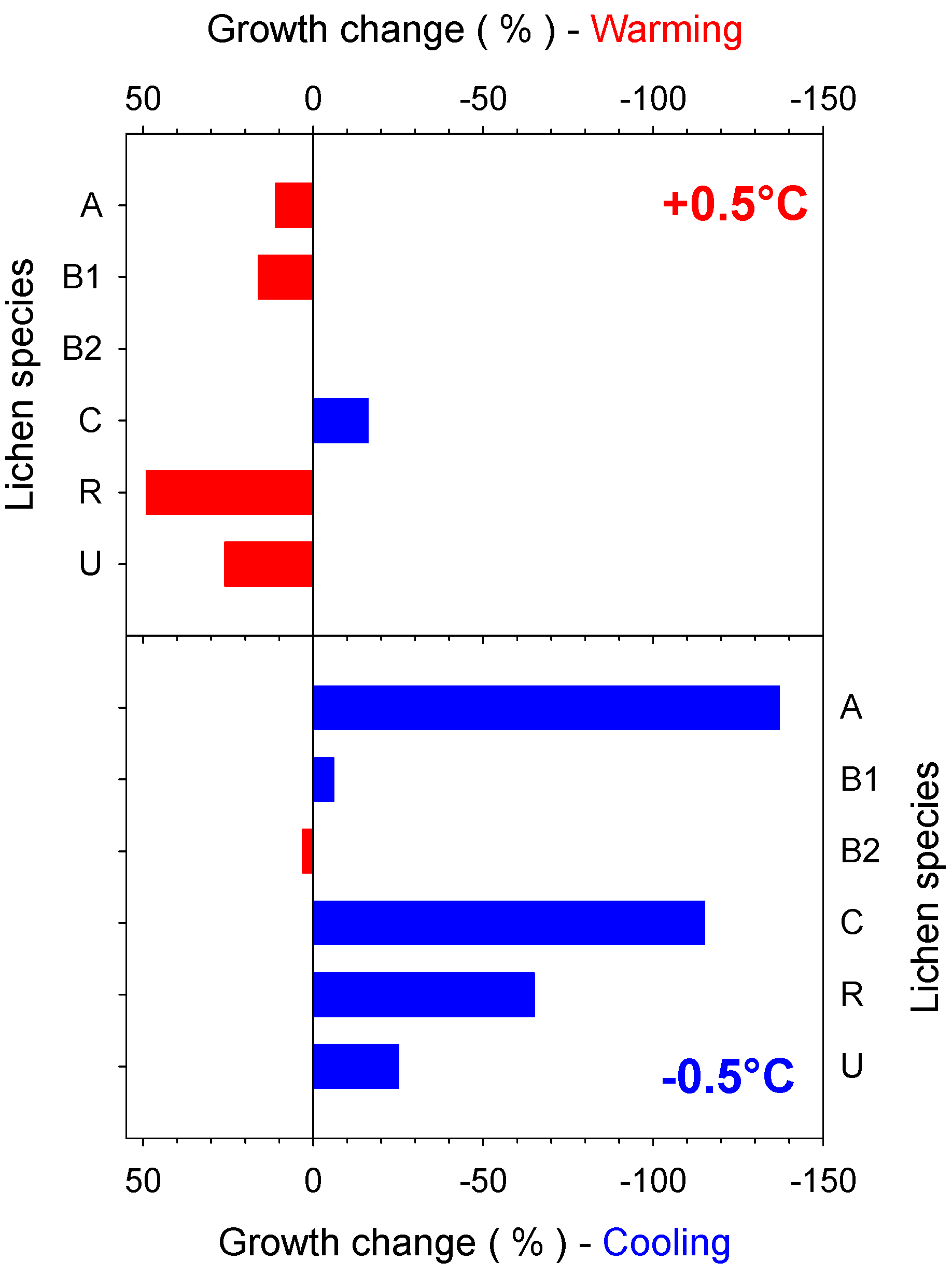
4. Short-Term Effects of Climate on Lichen Growth
4.1. Short-Term Growth Rate Studies
4.2. Predicted Effect of Increasing Temperature for Different Antarctic Lichen Species
5. Discussion
Possible Drivers of Lichen Diversity and Growth
6. Future Possibilities
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nylander, M.W. Les Lichens Du Jardin Du Luxembourg. Bull. Société Bot. Fr. 1866, 13, 364–371. [Google Scholar] [CrossRef]
- Gilbert, O.L. The effect of SO2 on lichens and bryophytes around Newcastle upon Tyne. In Proceedings of the First European Congress on the Influence of Air Pollution on Plants and Animals, Wageningen, The Netherlands, 22–27 April 1968; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1969; pp. 223–233. [Google Scholar]
- Hawksworth, D.L.; Rose, F. Qualitative scale for estimating sulphur dioxide air pollution in England and Wales using epiphytic lichens. Nature 1970, 227, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Cislaghi, C.; Nimis, P.L. Lichens, air pollution and lung cancer. Nature 1997, 387, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Giordani, P.; Brunialti, G. Sampling and interpreting lichen diversity data for biomonitoring purposes. In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; Volume 1, pp. 19–46. [Google Scholar] [CrossRef]
- Kuldeep, S.; Bhattacharya, P. Lichen as a Bioindicator Tool for Assessment of Climate and Air Pollution Vulnerability: Review. Int. Res. J. Environ. Sci. 2015, 4, 107–117. [Google Scholar]
- Leavitt, S.D.; Clair, L.L.S. Bio-monitoring in Western North America: What Can Lichens Tell Us About Ecological Disturbances? In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; Volume 1, pp. 119–138. [Google Scholar] [CrossRef]
- Will-Wolf, S.; Makholm, M.M.; Nelsen, M.P.; Trest, M.T.; Reis, A.H.; Jovan, S. Element analysis of two common macrolichens supports bioindication of air pollution and lichen response in rural midwestern USA. Bryologist 2015, 118, 371–384. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Air Pollution. Published online at OurWorldInData.org. 2019. Available online: https://ourworldindata.org/air-pollution (accessed on 24 January 2019).
- Gunn, J.; Keller, W.; Negusanti, J.; Potvin, R.; Beckett, P.; Winterhalder, K. Ecosystem recovery after emission reductions: Sudbury, Canada. Water Air Soil Pollut. 1995, 85, 1783–1788. [Google Scholar] [CrossRef]
- Pescott, O.L.; Simkin, J.M.; August, T.A.; Randle, Z.; Dore, A.J.; Botham, M.S. Air pollution and its effects on lichens, bryophytes, and lichen-feeding Lepidoptera: Review and evidence from biological records. Biol. J. Linn. Soc. 2015, 115, 611–635. [Google Scholar] [CrossRef]
- Van Herk, C.M.; Mathijssen-Spiekman, E.A.M.; De Zwart, D. Long distance nitrogen air pollution effects on lichens in Europe. Lichenologist 2003, 35, 347–359. [Google Scholar] [CrossRef]
- Carter, T.S.; Clark, C.M.; Fenn, M.E.; Jovan, S.; Perakis, S.S.; Riddell, J.; Schaberg, P.G.; Greaver, T.L.; Hastings, M.G. Mechanisms of nitrogen deposition effects on temperate forest lichens and trees. Ecosphere 2017, 8, e01717. [Google Scholar] [CrossRef] [Green Version]
- Van Herk, C.V.; Aptroot, A.; van Dobben, H.F. Long-term monitoring in the Netherlands suggests that lichens respond to global warming. Lichenologist 2002, 34, 141–154. [Google Scholar] [CrossRef]
- Aptroot, A.; van Herk, C.M. Further evidence of the effects of global warming on lichens particularly those with Trentepohlia phycobionts. Environ. Pollut. 2007, 146, 293–298. [Google Scholar] [CrossRef]
- Peterson, E.B.; McCune, B. The importance of hotspots for lichen diversity in forests of western Oregon. Bryologist 2003, 106, 246–256. [Google Scholar] [CrossRef]
- Ellis, C.J.; Yahr, R.; Coppins, B.J. Local extent of old-growth woodland modifies epiphyte response to climate change. J. Biogeogr. 2009, 36, 302–313. [Google Scholar] [CrossRef]
- Cornelissen, J.H.C.; Callaghan, T.V.; Alatalo, J.M.; Michelsen, A.; Graglia, E.; Hartley, A.E.; Hik, D.S.; Hobbie, S.E.; Press, M.C.; Robinson, C.H.; et al. Global change and arctic ecosystems: Is lichen decline a function of increases in vascular plant biomass? J. Ecol. 2001, 89, 984–994. [Google Scholar] [CrossRef]
- Upreti, D.K.; Divakar, P.K.; Nayaka, S. Commercial and ethnic use of lichens in India. Econ. Bot. 2005, 59, 269–273. [Google Scholar] [CrossRef]
- Insarov, G.E.; Semenov, S.M.; Insarova, I.D. A system to monitor climate change with epilithic lichens. Environ. Monit. Assess. 1999, 55, 279–298. [Google Scholar] [CrossRef]
- Lewis Smith, R.I. Vascular plants as bioindicators of regional warming in Antarctica. Oecologia 1994, 99, 322–328. [Google Scholar] [CrossRef]
- Convey, P. Antarctic climate change and its influences on terrestrial ecosystems. In Trends in Antarctic Terrestrial and Limnetic Ecosystems; Bergstrom, D., Convey, P., Huiskes, A.H.L., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 253–272. [Google Scholar] [CrossRef]
- Burton-Johnson, A.; Black, M.; Fretwell, P.; Kaluza-Gilbert, J. An automated methodology for differentiating rock from snow, clouds and sea in Antarctica from Landsat 8 imagery: A new rock outcrop map and area estimation for the entire Antarctic continent. Cryosphere 2016, 10, 1665–1677. [Google Scholar] [CrossRef]
- Cabrerizo, A.; Dachs, J.; Barceló, D.; Jones, K.C. Influence of organic matter content and human activities on the occurrence of organic pollutants in Antarctic soils, lichens, grass, and mosses. Environ. Sci. Technol. 2012, 46, 1396–1405. [Google Scholar] [CrossRef]
- Green, T.G.A.; Lange, O.L. Photosynthesis in poikilohydric plants: A comparison of lichens and bryophytes. In Ecophysiology of photosynthesis; Schultze, E.-D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 100, pp. 319–341. [Google Scholar]
- Anderson, J.T.; Panetta, A.M.; Mitchell-Olds, T.M. Evolutionary and ecological responses to anthropogenic climate change. Plant Phys. 2012, 160, 1728–1740. [Google Scholar] [CrossRef]
- Angert, A.L.; Crozier, L.G.; Rissler, L.J.; Gilman, S.E.; Tewksbury, J.J.; Chunco, A.J. Do species’ traits predict recent shifts at expanding range edges? Ecol. Lett. 2011, 14, 677–689. [Google Scholar] [CrossRef]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Elmendorf, S.C.; Henry, G.H.; Hollister, R.D.; Fosaa, A.M.; Gould, W.A.; Hermanutz, L.; Hofgaard, A.; Jónsdóttir, I.S.; Jorgenson, J.C.; Lévesque, E.; et al. Experiment, monitoring, and gradient methods used to infer climate change effects on plant communities yield consistent patterns. Proc. Natl. Acad. Sci. USA 2015, 112, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Green, T.G.A.; Sancho, L.G.; Pintado, A.; Schroeter, B. Functional and spatial pressures on terrestrial vegetation in Antarctica forced by global warming. Polar Biol. 2011, 34, 1643. [Google Scholar] [CrossRef]
- Peat, H.J.; Clarke, A.; Convey, P. Diversity and biogeography of the Antarctic flora. J. Biogeogr. 2007, 34, 132–146. [Google Scholar] [CrossRef]
- Green, T.G.A.; Schroeter, B.; Sancho, L.G. Plant life in Antarctica. In Functional Plant Ecology, 2nd ed.; Pugnaire, F.I., Valladares, F., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 408–453. [Google Scholar] [CrossRef]
- Seppelt, R.D.; Türk, R.; Green, T.G.A.; Moser, G.; Pannewitz, S.; Sancho, L.G.; Schroeter, B. Lichen and moss communities of Botany Bay, Granite Harbour, Ross Sea, Antarctica. Antarct. Sci. 2010, 22, 691–702. [Google Scholar] [CrossRef] [Green Version]
- Sancho, L.G.; Green, T.G.A.; Pintado, A. Slowest to fastest: Extreme range in lichen growth rates supports their use as an indicator of climate change in Antarctica. Flora 2007, 202, 667–673. [Google Scholar] [CrossRef]
- Colesie, C.; Green, T.G.A.; Haferkamp, I.; Büdel, B. Habitat stress initiates changes in composition, CO2 gas exchange and C-allocation as life traits in biological soil crusts. ISME J. 2014, 8, 2104. [Google Scholar] [CrossRef]
- Turner, J.; Lu, H.; White, I.; King, J.C.; Phillips, T.; Hosking, J.S.; Bracegirdle, T.J.; Marshall, G.J.; Mulvaney, R.; Deb, P. Absence of 21st century warming on Antarctic Peninsula consistent with natural variability. Nature 2016, 535, 411–415. [Google Scholar] [CrossRef] [Green Version]
- Sancho, L.G.; Pintado, A.; Navarro, F.; Ramos, M.; Pablo, M.A.; Blanquer, J.M.; Raggio, J.; Valladares, F.; Green, T.G.A. Recent warming and cooling in the Antarctic Peninsula region has rapid and large effects on lichen vegetation. Sci. Rep. 2017, 7, 5689. [Google Scholar] [CrossRef]
- Benedict, J.B. Lichen mortality due to late-lying snow: Results of a transplant study. Arct. Antarct. Alp. Res. 1990, 22, 81–89. [Google Scholar] [CrossRef]
- Armstrong, R.A. The influence of environmental factors on the growth of lichens in the field. In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; Volume 1, pp. 1–18. [Google Scholar] [CrossRef]
- Green, T.G.A.; Brabyn, L.; Beard, C.; Sancho, L.G. Extremely low lichen growth rates in Taylor Valley, Dry Valleys, continental Antarctica. Polar Biol. 2012, 35, 535–541. [Google Scholar] [CrossRef]
- Alatalo, J.M.; Jägerbrand, A.K.; Chen, S.; Molau, U. Responses of lichen communities to 18 years of natural and experimental warming. Ann. Bot. 2017, 120, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Jandt, R.; Joly, K.; Meyers, C.R.; Racine, C. Slow recovery of lichen on burned caribou winter range in Alaska tundra: Potential influences of climate warming and other disturbance factors. Arct. Antarct. Alp. Res. 2008, 40, 89–95. [Google Scholar] [CrossRef]
- Bokhorst, S.; Convey, P.; Huiskes, A.; Aerts, R. Usnea antarctica, an important Antarctic lichen, is vulnerable to aspects of regional environmental change. Polar Biol. 2016, 39, 511–521. [Google Scholar] [CrossRef]
- Bokhorst, S.; Huiskes, A.; Aerts, R.; Convey, P.; Cooper, E.J.; Dalen, L.; Erschbamer, B.; Gudmundsson, J.; Hofgaard, A.; Hollister, R.D.; et al. Variable temperature effects of Open Top Chambers at polar and alpine sites explained by irradiance and snow depth. Glob. Chang. Biol. 2013, 19, 64–74. [Google Scholar] [CrossRef]
- Schroeter, B.; Olech, M.; Kappen, L.; Heitland, W. Ecophysiological investigations of Usnea antarctica in the maritime Antarctic I. Annual microclimatic conditions and potential primary production. Antarct. Sci. 1995, 7, 251–260. [Google Scholar] [CrossRef]
- Sancho, L.G.; Pintado, A.; Green, T.G.A.; Pannewitz, S.; Schroeter, B. Photosynthetic and morphological variation within and among populations of the Antarctic lichen Umbilicaria aprina: Implications of the thallus size. Bibl. Lichenol. 2003, 86, 299–311. [Google Scholar]
- Pannewitz, S.; Green, T.G.A.; Maysek, K.; Schlensog, M.; Seppelt, R.; Sancho, L.G.; Türk, R.; Schroeter, B. Photosynthetic responses of three common mosses from continental Antarctica. Antarct. Sci. 2005, 17, 341–352. [Google Scholar] [CrossRef]
- Laguna-Defior, C.; Pintado, A.; Green, T.G.A.; Blanquer, J.M.; Sancho, L.G. Distributional and ecophysiological study on the Antarctic lichens species pair Usnea antarctica/Usnea aurantiaco-atra. Polar Biol. 2016, 39, 1183–1195. [Google Scholar] [CrossRef]
- Cavieres, L.A.; Sáez, P.; Sanhueza, C.; Sierra-Almeida, A.; Rabert, C.; Corcuera, L.J.; Alberdi, M.; Bravo, L.A. Ecophysiological traits of Antarctic vascular plants: Their importance in the responses to climate change. Plant Ecol. 2016, 217, 343–358. [Google Scholar] [CrossRef]
- Schroeter, B.; Green, T.G.A.; Pannewitz, S.; Schlensog, M.; Sancho, L.G. Fourteen degrees of latitude and a continent apart: Comparison of lichen activity over two years at continental and maritime Antarctic sites. Antarct. Sci. 2010, 22, 681–690. [Google Scholar] [CrossRef]
- Robinson, S.A.; King, D.H.; Bramley-Alves, J.; Waterman, M.J.; Ashcroft, M.B.; Wasley, J.; Turnbull, J.D.; Miller, R.E.; Ryan-Colton, E.; Benny, T.; et al. Rapid change in East Antarctic terrestrial vegetation in response to regional drying. Nat. Clim. Chang. 2018, 8, 879–884. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–2027. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, M.J.; Grytnes, J.A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E.; et al. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cooper, D.J.; Yang, J.; Zhang, X.; Wang, X. Rapid warming induces the contrasting growth of Yezo spruce (Picea jezoensis var. microsperma) at two elevation gradient sites of northeast China. Dendrochronologia 2018, 50, 52–63. [Google Scholar] [CrossRef]
- Domaschke, S.; Vivas, M.; Sancho, L.G.; Printzen, C. Ecophysiology and genetic structure of polar versus temperate populations of the lichen Cetraria aculeata. Oecologia 2013, 173, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Raggio, J.; Green, T.G.A.; Sancho, L.G. In situ monitoring of microclimate and metabolic activity in lichens from Antarctic extremes: A comparison between South Shetland Islands and the McMurdo Dry Valleys. Polar Biol. 2016, 39, 113–122. [Google Scholar] [CrossRef]
- Pannewitz, S.; Green, T.G.A.; Schlensog, M.; Seppelt, R.; Sancho, L.G.; Schroeter, B. Photosynthetic performance of Xanthoria mawsonii CW Dodge in coastal habitats, Ross Sea region, continental Antarctica. Lichenologist 2006, 38, 67–81. [Google Scholar] [CrossRef]
- Schroeter, B.; Green, T.G.A.; Pannewitz, S.; Schlensog, M.; Sancho, L.G. Summer variability, winter dormancy: Lichen activity over 3 years at Botany Bay, 77 S latitude, continental Antarctica. Polar Biol. 2011, 34, 13–22. [Google Scholar] [CrossRef]
- Breen, K.; Levesque, E. Proglacial succession of biological soil crusts and vascular plants: Biotic interactions in the High Arctic. Can. J. Bot. 2006, 84, 1714–1731. [Google Scholar] [CrossRef]
- Colesie, C.; Green, T.G.A.; Raggio, J.; Büdel, B. Summer Activity Patterns of Antarctic and High Alpine Lichen dominated Biological Soil Crusts—Similar but Different? Arct. Antarct. Alp. Res. 2016, 48, 449–460. [Google Scholar] [CrossRef]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Thomas, H.J.; Alatalo, J.M.; Alexander, H.; Anadon-Rosell, A.; Angers-Blondin, S.; Bai, Y.; et al. Tundra Trait Team: A database of plant traits spanning the tundra biome. Glob. Ecol. Biogeogr. 2018, 27, 1402–1411. [Google Scholar] [CrossRef]
- Green, T.G.A.; Pintado, A.; Raggio, J.; Sancho, L.G. The lifestyle of lichens in soil crusts. Lichenologist 2018, 50, 397–410. [Google Scholar] [CrossRef]
- Jones, T.C.; Hogg, I.D.; Wilkins, R.J.; Green, T.G.A. Photobiont selectivity for lichens and evidence for a possible glacial refugium in the Ross Sea Region, Antarctica. Polar Biol. 2013, 36, 767–774. [Google Scholar] [CrossRef]
- Wirtz, N.; Lumbsch, H.T.; Green, T.A.; Türk, R.; Pintado, A.; Sancho, L.; Schroeter, B. Lichen fungi have low cyanobiont selectivity in maritime Antarctica. New Phytol. 2003, 160, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Pisa, S.E.; Biersma, E.M.; Convey, P.E.; Patiño, J.A.; Vanderpoorten, A.L.; Werner, O.L.; Ros, R.M. The cosmopolitan moss Bryum argenteum in Antarctica: Recent colonisation or in situ survival? Polar Biol. 2014, 37, 1469–1477. [Google Scholar] [CrossRef]
- Jones, T.C.; Hogg, I.D.; Wilkins, R.J.; Green, T.G.A. Microsatellite analyses of the Antarctic endemic lichen Buellia frigida Darb. (Physciaceae) suggest limited dispersal and the presence of glacial refugia in the Ross Sea region. Polar Biol. 2015, 38, 941–949. [Google Scholar] [CrossRef]
- Bennett, K.R.; Hogg, I.D.; Adams, B.J.; Hebert, P.D. High levels of intraspecific genetic divergences revealed for Antarctic springtails: Evidence for small-scale isolation during Pleistocene glaciation. Biol. J. Linn. Soc. 2016, 119, 166–178. [Google Scholar] [CrossRef]
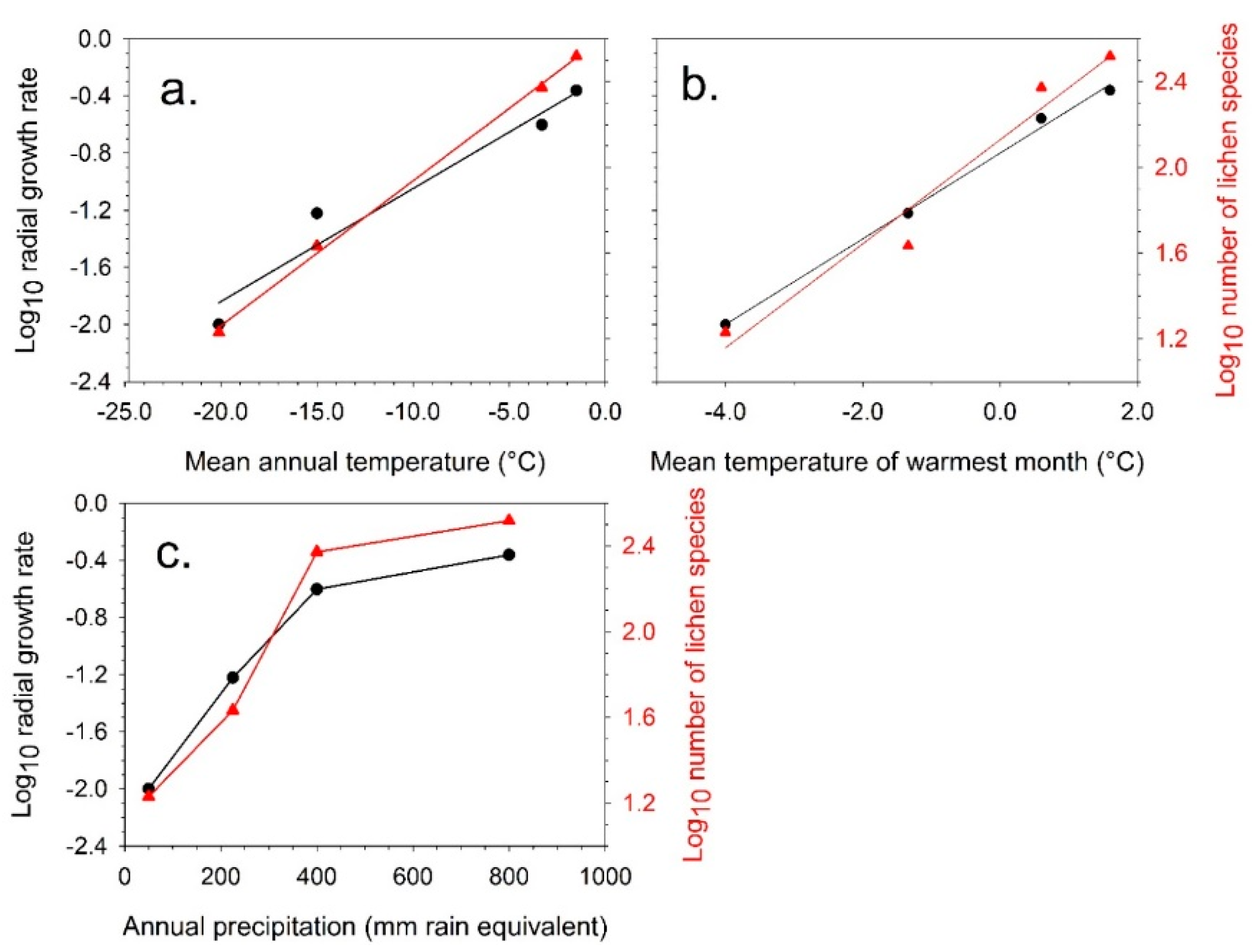
| Location | Growth Rate Radial (mm y−1) | Warmest Month (Mean T °C) | Coldest Month (Mean T °C) | Annual Mean (T °C) | Precipitation (mm Rain Equivalent) |
|---|---|---|---|---|---|
| Dry Valleys (*) | 0.01 | −4.8 | −30.5 | −20.0 | 50 |
| Cape Hallett (*) | 0.07 | −1.4 | −26.4 | −15.0 | 120 |
| Signy Island (**) | 0.25 | 1.3 | −9.0 | −3.3 | 400 |
| Livingston Island (**) | 0.44 | 1.3 | −7.0 | −1.5 | 800 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancho, L.G.; Pintado, A.; Green, T.G.A. Antarctic Studies Show Lichens to be Excellent Biomonitors of Climate Change. Diversity 2019, 11, 42. https://doi.org/10.3390/d11030042
Sancho LG, Pintado A, Green TGA. Antarctic Studies Show Lichens to be Excellent Biomonitors of Climate Change. Diversity. 2019; 11(3):42. https://doi.org/10.3390/d11030042
Chicago/Turabian StyleSancho, Leopoldo G., Ana Pintado, and T. G. Allan Green. 2019. "Antarctic Studies Show Lichens to be Excellent Biomonitors of Climate Change" Diversity 11, no. 3: 42. https://doi.org/10.3390/d11030042
APA StyleSancho, L. G., Pintado, A., & Green, T. G. A. (2019). Antarctic Studies Show Lichens to be Excellent Biomonitors of Climate Change. Diversity, 11(3), 42. https://doi.org/10.3390/d11030042






