Do Different Teams Produce Different Results in Long-Term Lichen Biomonitoring?
Abstract
1. Introduction
2. Materials and Methods
2.1. Survey Selection and Sampling Design
2.2. Data Analysis
3. Results
4. Discussion
4.1. Quantitative Aspects of Lichen Diversity
4.2. Taxonomic Agreement in Relation to Team Composition
4.3. Recommandations
- Periodic ring test organization. The effectiveness of this activity is achieved only if the intercalibration exercise is regularly repeated over time [34]. In fact, it has been shown that a decrease, even if limited, of taxonomic accuracy can be observed even in taxonomist experts. The effect of the loss of accuracy is obviously much more evident in trained personnel who have not yet reached a high level of experience.
- Calibrations of the operators within the same program. This calibration activity should be done between the two operators composing the team carrying out the same survey and/or among teams involved in different surveys of the same program. Additionally, external skilled personnel could also be involved as a control team to provide a further level of quality assurance. This activity would minimize the differences attributable to non-sampling errors within the same area of study (e.g., between high and low diversity areas) and/or subsequent surveys of the same monitoring program. The main problems in applying these interventions can be identified in the difficulty of planning long-term activities and involving people who have worked at different times.
- Preparatory training aimed at improving the knowledge of local lichen biota. In many cases, the operators involved in the sampling of a study area may not have specific knowledge of the local biota. This is particularly true in case of operators with low level of experience, but even skilled lichenologists may not be able to maintain a high level of taxonomic accuracy without preparatory and intensive training on the local lichen biota.
- Staff training on critical taxonomic groups. On the basis of the results reported in this study, it is evident that specialized training on some critical groups of species (e.g., genera of crustose lichens) can lead to a substantial improvement of the agreement between operators. Although recommendable, the organization of advanced workshops involves a considerable logistical effort in the retrieval of materials, laboratory equipment and the availability of experts able to clarify doubts on critical species. As a further option, experts could be invited to participate in the surveys of the monitoring program, even though this may lead to an increase of the total cost of the program.
Author Contributions
Funding
Conflicts of Interest
References
- Nash, T.H. Lichen Biology; Cambridge University Press: Leiden, The Netherlands, 2006; ISBN 978-0-511-41407-7. [Google Scholar]
- Nimis, P.L.; Scheidegger, C.; Wolseley, P.A. (Eds.) Monitoring with Lichens—Monitoring Lichens; Springer: Dordrecht, The Netherlands, 2002; pp. 1–4. ISBN 978-1-4020-0430-8. [Google Scholar]
- CEN. Ambient Air—Biomonitoring with Lichens—Assessing Epiphytic Lichen Diversity; CEN: Brussels, Belgium, 2014. [Google Scholar]
- ANPA. I.B.L. Indice di Biodiversità Lichenica; Manuali e linee guida; ANPA: Roma, Italy, 2001; ISBN 88-448-0256-2. [Google Scholar]
- Asta, J.; Erhardt, W.; Ferretti, M.; Fornasier, F.; Kirschbaum, U.; Nimis, P.L.; Purvis, O.W.; Pirintsos, S.; Scheidegger, C.; Van Haluwyn, C.; et al. Mapping Lichen Diversity as an Indicator of Environmental Quality. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; NATO Science Series; Springer: Dordrecht, The Netherlands, 2002; pp. 273–279. ISBN 978-94-010-0423-7. [Google Scholar]
- Giordani, P.; Brunialti, G. Sampling and Interpreting Lichen Diversity Data for Biomonitoring Purposes. In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; pp. 19–46. ISBN 978-81-322-2180-7. [Google Scholar]
- Kricke, R.; Loppi, S. Bioindication: The I.A.P. Approach. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 21–37. ISBN 978-1-4020-0430-8. [Google Scholar]
- Nimis, P.L.; Purvis, O.W. Monitoring Lichens as Indicators of Pollution. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 7–10. ISBN 978-1-4020-0430-8. [Google Scholar]
- Will-Wolf, S.; Geiser, L.H.; Neitlich, P.; Reis, A.H. Forest lichen communities and environment—How consistent are relationships across scales? J. Veg. Sci. 2009, 17, 171–184. [Google Scholar]
- Will-Wolf, S.; Esseen, P.-A.; Neitlich, P. Monitoring Biodiversity and Ecosystem Function: Forests. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 203–222. ISBN 978-1-4020-0430-8. [Google Scholar]
- Nascimbene, J.; Thor, G.; Nimis, P.L. Effects of forest management on epiphytic lichens in temperate deciduous forests of Europe—A review. For. Ecol. Manag. 2013, 298, 27–38. [Google Scholar] [CrossRef]
- Maes, W.H.; Fontaine, M.; Rongé, K.; Hermy, M.; Muys, B. A quantitative indicator framework for stand level evaluation and monitoring of environmentally sustainable forest management. Ecol. Indic. 2011, 11, 468–479. [Google Scholar] [CrossRef]
- Giordani, P. Assessing the effects of forest management on epiphytic lichens in coppiced forests using different indicators. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2012, 146, 628–637. [Google Scholar] [CrossRef]
- Pinho, P.; Bergamini, A.; Carvalho, P.; Branquinho, C.; Stofer, S.; Scheidegger, C.; Máguas, C. Lichen functional groups as ecological indicators of the effects of land-use in Mediterranean ecosystems. Ecol. Indic. 2012, 15, 36–42. [Google Scholar] [CrossRef]
- Wolseley, P.A.; Stofer, S.; Mitchell, R.; Truscott, A.-M.; Vanbergen, A.; Chimonides, J.; Scheidegger, C. Variation of lichen communities with landuse in Aberdeenshire, UK. Lichenologist 2006, 38, 307. [Google Scholar] [CrossRef]
- Stofer, S.; Bergamini, A.; Aragón, G.; Carvalho, P.; Coppins, B.J.; Davey, S.; Dietrich, M.; Farkas, E.; Kärkkäinen, K.; Keller, C.; et al. Species richness of lichen functional groups in relation to land use intensity. Lichenologist 2006, 38, 331. [Google Scholar] [CrossRef]
- Ellis, C.J.; Eaton, S.; Theodoropoulos, M.; Coppins, B.J.; Seaward, M.R.D.; Simkin, J. Response of epiphytic lichens to 21st Century climate change and tree disease scenarios. Biol. Conserv. 2014, 180, 153–164. [Google Scholar] [CrossRef]
- Geiser, L.H.; Neitlich, P.N. Air pollution and climate gradients in western Oregon and Washington indicated by epiphytic macrolichens. Environ. Pollut. 2007, 145, 203–218. [Google Scholar] [CrossRef]
- Giordani, P.; Incerti, G. The influence of climate on the distribution of lichens: A case study in a borderline area (Liguria, NW Italy). Plant Ecol. 2008, 195, 257–272. [Google Scholar] [CrossRef]
- Matos, P.; Geiser, L.; Hardman, A.; Glavich, D.; Pinho, P.; Nunes, A.; Soares, A.M.V.M.; Branquinho, C. Tracking global change using lichen diversity: Towards a global-scale ecological indicator. Methods Ecol. Evol. 2017, 8, 788–798. [Google Scholar] [CrossRef]
- Matos, P.; Pinho, P.; Aragón, G.; Martínez, I.; Nunes, A.; Soares, A.M.V.M.; Branquinho, C. Lichen traits responding to aridity. J. Ecol. 2015, 103, 451–458. [Google Scholar] [CrossRef]
- Ferretti, M.; Brambilla, E.; Brunialti, G.; Fornasier, F.; Mazzali, C.; Giordani, P.; Nimis, P. Reliability of different sampling densities for estimating and mapping lichen diversity in biomonitoring studies. Environ. Pollut. 2004, 127, 249–256. [Google Scholar] [CrossRef]
- Frati, L.; Brunialti, G. Long-Term Biomonitoring with Lichens: Comparing Data from Different Sampling Procedures. Environ. Monit. Assess. 2006, 119, 391–404. [Google Scholar] [CrossRef]
- Giordani, P.; Matteucci, E.; Redana, M.; Ferrarese, A.; Isocrono, D. Unsustainable cattle load in alpine pastures alters the diversity and the composition of lichen functional groups for nitrogen requirement. Fungal Ecol. 2014, 9, 69–72. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Calderisi, M.; Malaspina, P.; Frati, L. Beta diversity and similarity of lichen communities as a sign of the times. Lichenologist 2018, 50, 371–383. [Google Scholar] [CrossRef]
- Ferretti, M.; Erhardt, W. Key Issues in Designing Biomonitoring Programmes. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 111–139. ISBN 978-1-4020-0430-8. [Google Scholar]
- Barkman, J.J. Phytosociology and Ecology of Cryptogamic Epiphytes: Including a Taxonomic Survey and Description of Their Vegetation Units in Europe; Van Gorcum: Assen, The Netherlands, 1958. [Google Scholar]
- Ribeiro, M.C.; Pinho, P.; Branquinho, C.; Llop, E.; Pereira, M.J. Geostatistical uncertainty of assessing air quality using high-spatial-resolution lichen data: A health study in the urban area of Sines, Portugal. Sci. Total Environ. 2016, 562, 740–750. [Google Scholar] [CrossRef]
- Brunialti, G.; Giordani, P.; Isocrono, D.; Loppi, S. Evaluation of data quality in lichen biomonitoring studies: The Italian experience. Environ. Monit. Assess. 2002, 75, 271–280. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L.; Cristofolini, F.; Chiarucci, A.; Giordani, P.; Loppi, S.; Benesperi, R.; Cristofori, A.; Critelli, P.; Di Capua, E.; et al. Can we compare lichen diversity data? A test with skilled teams. Ecol. Indic. 2012, 23, 509–516. [Google Scholar] [CrossRef]
- Cristofolini, F.; Brunialti, G.; Giordani, P.; Nascimbene, J.; Cristofori, A.; Gottardini, E.; Frati, L.; Matos, P.; Batič, F.; Caporale, S.; et al. Towards the adoption of an international standard for biomonitoring with lichens—Consistency of assessment performed by experts from six European countries. Ecol. Indic. 2014, 45, 63–67. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Benesperi, R.; Rizzi, G.; Frati, L.; Modenesi, P. Rapid biodiversity assessment in lichen diversity surveys: Implications for quality assurance. J. Environ. Monit. 2009, 11, 730. [Google Scholar] [CrossRef]
- Mccune, B.; Dey, J.P.; Peck, J.E.; Cassell, D.; Heiman, K.; Will-Wolf, S.; Neitlich, P.N. Repeatability of community data: Species richness versus gradient scores in large-scale lichen studies. Bryologist 1997, 100, 40–46. [Google Scholar] [CrossRef]
- Ferretti, M. Quality assurance: A vital need in ecological monitoring. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2011, 6. [Google Scholar] [CrossRef]
- Stribling, J.B.; Moulton, S.R.; Lester, G.T. Determining the quality of taxonomic data. J. N. Am. Benthol. Soc. 2003, 22, 621–631. [Google Scholar] [CrossRef]
- Stribling, J.B.; Pavlik, K.L.; Holdsworth, S.M.; Leppo, E.W. Data quality, performance, and uncertainty in taxonomic identification for biological assessments. J. N. Am. Benthol. Soc. 2008, 27, 906–919. [Google Scholar] [CrossRef]
- Contardo, T.; Giordani, P.; Paoli, L.; Vannini, A.; Loppi, S. May lichen biomonitoring of air pollution be used for environmental justice assessment? A case study from an area of N Italy with a municipal solid waste incinerator. Environ. Forensics 2018, 0, 1–12. [Google Scholar] [CrossRef]
- Loppi, S. May the Diversity of Epiphytic Lichens Be Used in Environmental Forensics? Diversity 2019, 11, 36. [Google Scholar] [CrossRef]
- Podani, J.; Schmera, D. A new conceptual and methodological framework for exploring and explaining pattern in presence—Absence data. Oikos 2011, 120, 1625–1638. [Google Scholar] [CrossRef]
- Akaike, H. A Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 1979, 66, 237–242. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B. Package ‘lme4’; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- R Core team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Nimis, P.L. The Lichens of Italy. A Second Annotated Catalogue; EUT: Trieste, Italy, 2016; ISBN 978-88-8303-754-2. [Google Scholar]
- Ellis, C.J.; Coppins, B.J. Taxonomic survey compared to ecological sampling: Are the results consistent for woodland epiphytes? Lichenologist 2017, 49, 141–155. [Google Scholar] [CrossRef]
- Shukla, V.; Upreti, D.K.; Bajpai, R. Lichens to Biomonitor the Environment; Springer: New Delhi, India, 2014. [Google Scholar]
- van Herk, C.M. Bark pH and susceptibility to toxic air pollutants as independent causes of changes in epiphytic lichen composition in space and time. Lichenologist 2001, 33, 419–441. [Google Scholar] [CrossRef]
- Frati, L.; Santoni, S.; Nicolardi, V.; Gaggi, C.; Brunialti, G.; Guttova, A.; Gaudino, S.; Pati, A.; Pirintsos, S.A.; Loppi, S. Lichen biomonitoring of ammonia emission and nitrogen deposition around a pig stockfarm. Environ. Pollut. 2007, 146, 311–316. [Google Scholar] [CrossRef]
- Pinho, P.; Theobald, M.R.; Dias, T.; Tang, Y.S.; Cruz, C.; Martins-Loução, M.A.; Máguas, C.; Sutton, M.; Branquinho, C. Critical loads of nitrogen deposition and critical levels of atmospheric ammonia for semi-natural Mediterranean evergreen woodlands. Biogeosciences 2012, 9, 1205–1215. [Google Scholar] [CrossRef]
- Pinho, P.; Dias, T.; Cruz, C.; Sim Tang, Y.; Sutton, M.A.; Martins-Loução, M.-A.; Máguas, C.; Branquinho, C. Using lichen functional diversity to assess the effects of atmospheric ammonia in Mediterranean woodlands. J. Appl. Ecol. 2011, 48, 1107–1116. [Google Scholar] [CrossRef]
- Davies, L.; Bates, J.W.; Bell, J.N.B.; James, P.W.; Purvis, O.W. Diversity and sensitivity of epiphytes to oxides of nitrogen in London. Environ. Pollut. 2007, 146, 299–310. [Google Scholar] [CrossRef]
- Munzi, S.; Cruz, C.; Branquinho, C.; Pinho, P.; Leith, I.D.; Sheppard, L.J. Can ammonia tolerance amongst lichen functional groups be explained by physiological responses? Environ. Pollut. 2014, 187, 206–209. [Google Scholar] [CrossRef]
- Llop, E.; Pinho, P.; Matos, P.; Pereira, M.J.; Branquinho, C. The use of lichen functional groups as indicators of air quality in a Mediterranean urban environment. Ecol. Indic. 2012, 13, 215–221. [Google Scholar] [CrossRef]
- Pinho, P.; Llop, E.; Ribeiro, M.C.; Cruz, C.; Soares, A.; Pereira, M.J.; Branquinho, C. Tools for determining critical levels of atmospheric ammonia under the influence of multiple disturbances. Environ. Pollut. 2014, 188, 88–93. [Google Scholar] [CrossRef]
- Munzi, S.; Correia, O.; Silva, P.; Lopes, N.; Freitas, C.; Branquinho, C.; Pinho, P. Lichens as ecological indicators in urban areas: Beyond the effects of pollutants. J. Appl. Ecol. 2014, 51, 1750–1757. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Bacaro, G.; Nascimbene, J. Functional traits of epiphytic lichens as potential indicators of environmental conditions in forest ecosystems. Ecol. Indic. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Ellis, C.J.; Coppins, B.J. Contrasting functional traits maintain lichen epiphyte diversity in response to climate and autogenic succession. J. Biogeogr. 2006, 33, 1643–1656. [Google Scholar] [CrossRef]
- Van Herk, C.M.; Mathijssen-Spiekman, E.A.M.; De Zwart, D. Long distance nitrogen air pollution effects on lichens in Europe. Lichenologist 2003, 35, 347–359. [Google Scholar] [CrossRef]
- Cristofolini, F.; Giordani, P.; Gottardini, E.; Modenesi, P. The response of epiphytic lichens to air pollution and subsets of ecological predictors: A case study from the Italian Prealps. Environ. Pollut. 2008, 151, 308–317. [Google Scholar] [CrossRef]
- Hauck, M. Susceptibility to acidic precipitation contributes to the decline of the terricolous lichens Cetraria aculeata and Cetraria islandica in central Europe. Environ. Pollut. 2008, 152, 731–735. [Google Scholar] [CrossRef]
- Giordani, P.; Malaspina, P. Do tree-related factors mediate the response of lichen functional groups to eutrophication? Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2017, 151, 1062–1072. [Google Scholar] [CrossRef]
- Archaux, F.; Camaret, S.; Dupouey, J.-L.; Ulrich, E.; Corcket, E.; Bourjot, L.; Brêthes, A.; Chevalier, R.; Dobremez, J.-F.; Dumas, Y.; et al. Can We Reliably Estimate Species Richness with Large Plots? An Assessment through Calibration Training. Plant Ecol. 2009, 203, 303–315. [Google Scholar] [CrossRef]
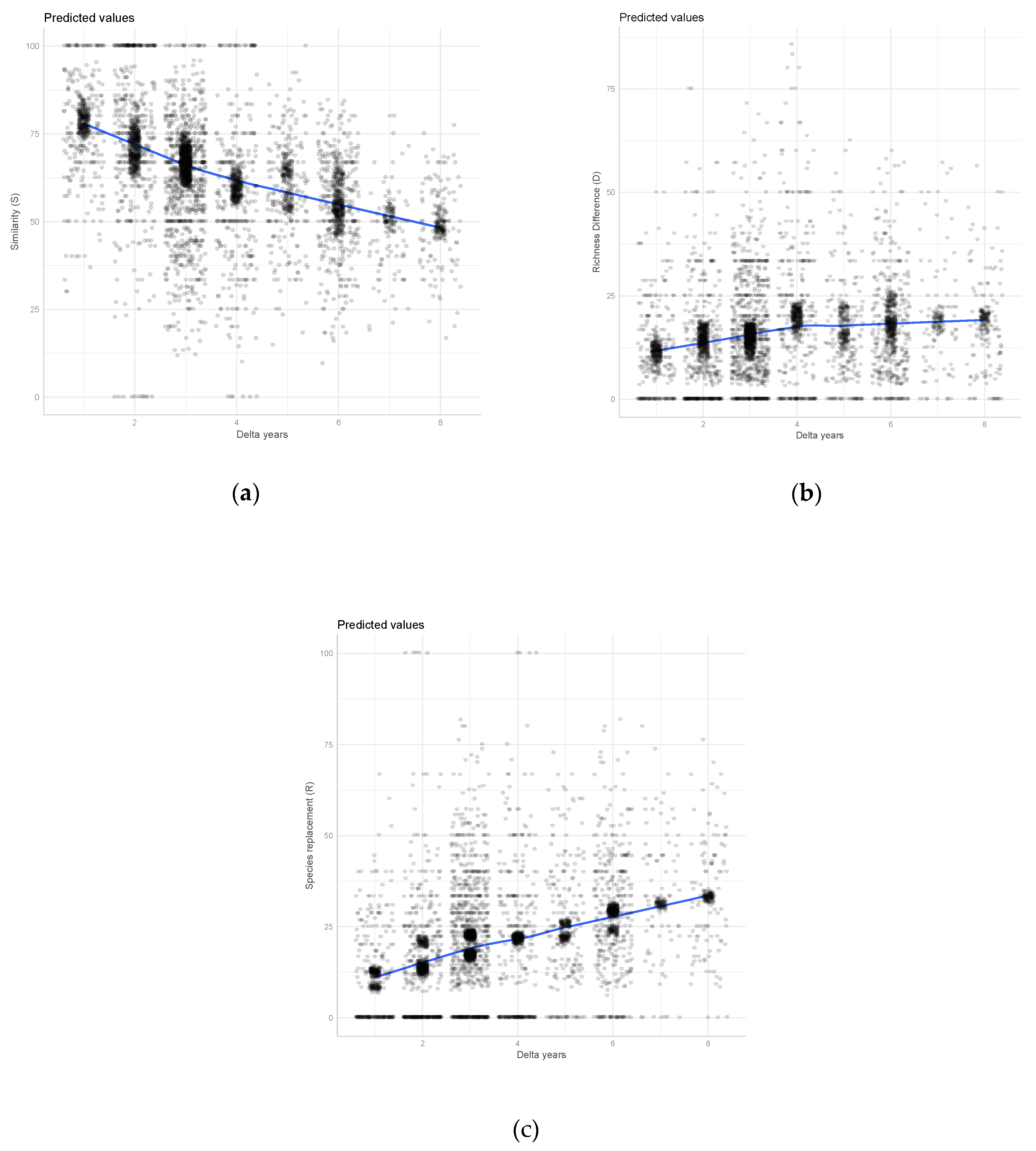
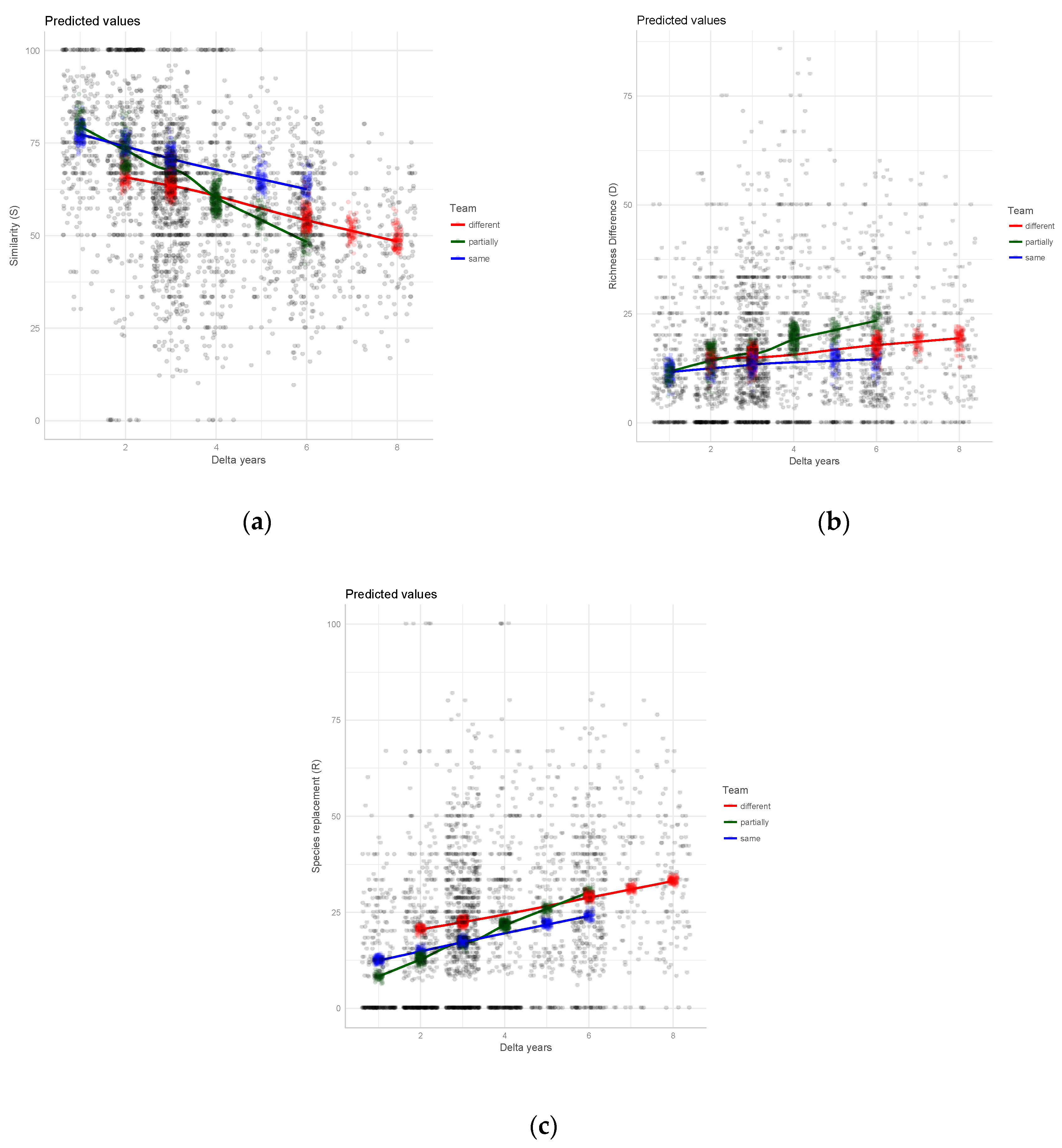
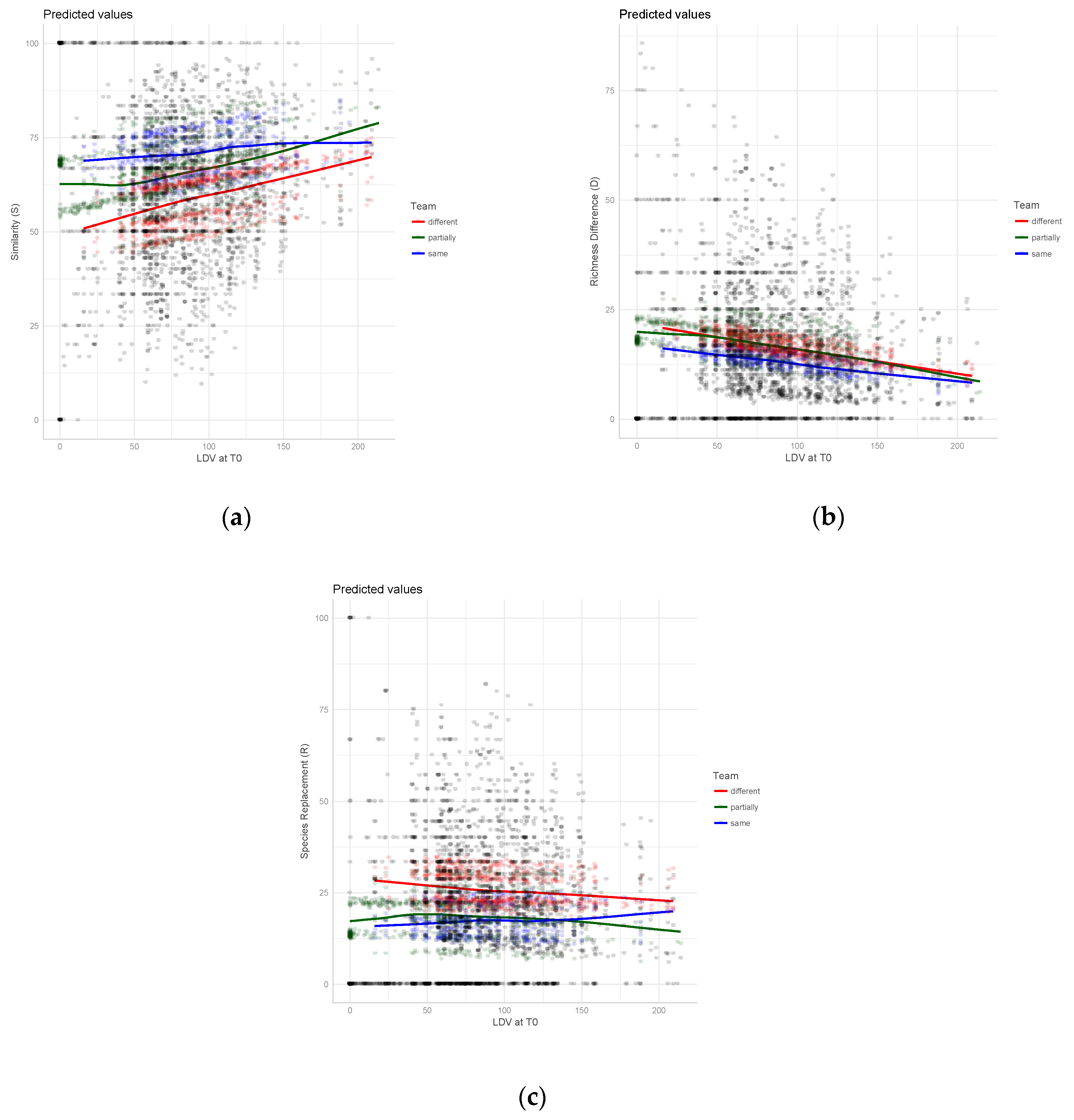
| Study Area | N Trees | N Plots | Gamma Diversity (N Species) | Survey Pair (Years of Surveys) | Team Composition in the Surveys | Delta Years | Av. Similarity (S) | Av. Richness Difference (D) | Av. Species Replacement (R) |
|---|---|---|---|---|---|---|---|---|---|
| A | 78 | 26 | 84 | 2008 versus 2009 | same | 1 | 72 | 12 | 15 |
| 2008 versus 2011 | partially | 3 | 52 | 20 | 28 | ||||
| 2008 versus 2012 | partially | 4 | 48 | 22 | 30 | ||||
| 2008 versus 2014 | partially | 6 | 47 | 23 | 31 | ||||
| 2008 versus 2015 | different | 7 | 46 | 20 | 34 | ||||
| 2009 versus 2011 | partially | 2 | 63 | 17 | 20 | ||||
| 2009 versus 2012 | partially | 3 | 56 | 19 | 25 | ||||
| 2009 versus 2014 | partially | 5 | 53 | 18 | 29 | ||||
| 2009 versus 2015 | different | 6 | 52 | 20 | 28 | ||||
| 2011 versus 2012 | same | 1 | 74 | 11 | 15 | ||||
| 2011 versus 2014 | same | 2 | 64 | 14 | 22 | ||||
| 2011 versus 2015 | partially | 3 | 61 | 18 | 21 | ||||
| 2012 versus 2014 | same | 2 | 69 | 15 | 17 | ||||
| 2012 versus 2015 | partially | 3 | 65 | 18 | 17 | ||||
| 2014 versus 2015 | partially | 1 | 82 | 12 | 6 | ||||
| B | 108 | 36 | 119 | 2007 versus 2009 | different | 2 | 72 | 11 | 17 |
| 2007 versus 2012 | same | 5 | 62 | 14 | 24 | ||||
| 2007 versus 2015 | different | 8 | 49 | 18 | 34 | ||||
| 2009 versus 2012 | different | 3 | 70 | 11 | 19 | ||||
| 2009 versus 2015 | different | 6 | 54 | 15 | 30 | ||||
| 2012 versus 2015 | different | 3 | 55 | 16 | 29 | ||||
| C | 135 | 39 | 55 | 2012 versus 2016 | partially | 4 | 58 | 25 | 17 |
| D | 73 | 21 | 98 | 2010 versus 2013 | different | 3 | 57 | 21 | 22 |
| 2010 versus 2016 | same | 6 | 62 | 14 | 24 | ||||
| 2013 versus 2016 | different | 3 | 53 | 18 | 30 | ||||
| E | 71 | 24 | 83 | 2009 versus 2012 | same | 3 | 71 | 13 | 15 |
| F | 135 | 42 | 94 | 2014 versus 2016 | partially | 2 | 81 | 9 | 10 |
| S | D | R | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AIC | −1441.79 | −2532.52 | −1485.97 | ||||||
| Estimates | Std Error | t-value | Estimates | Std Error | t-value | Estimates | Std Error | t-value | |
| Random effect | |||||||||
| (Plot/Area) St. dev. | 0.099 | 0.036 | 0.055 | ||||||
| Residuals | 0.166 | 0.137 | 0.169 | ||||||
| Fixed effects | |||||||||
| (Intercept) | 0.653 | 0.021 | 31.472 ** | 0.176 | 0.015 | 11.872 ** | 0.173 | 0.019 | 9.155 ** |
| DeltaYears | −0.029 | 0.003 | −9.710 ** | 0.008 | 0.002 | 3.261 ** | 0.021 | 0.003 | 7.169 ** |
| Team ‘partially’ (versus ‘different’) | 0.159 | 0.025 | 6.480 ** | −0.048 | 0.018 | −2.692 ** | −0.126 | 0.023 | −5.524 ** |
| Team ‘same’ (versus ‘different’) | 0.091 | 0.027 | 3.347 ** | −0.025 | 0.019 | −1.294 * | −0.063 | 0.025 | −2.554 * |
| LDV at T0 | 0.001 | 0.000 | 6.772 ** | −0.001 | 0.000 | −6.260 ** | 0.000 | 0.000 | −1.078 |
| DeltaYears:Team ‘partially’ (versus different) | −0.036 | 0.005 | −6.999 ** | 0.017 | 0.004 | 4.204 ** | 0.023 | 0.005 | 4.561 ** |
| DeltaYears:Team ‘same’ (versus ‘different’) | −0.003 | 0.006 | −0.505 | 0.000 | 0.005 | 0.051 | 0.003 | 0.006 | 0.488 |
| Species | Average Percentage Agreement | |||
|---|---|---|---|---|
| Total | Team “different” | Team “partially” | Team “same” | |
| Ramalina fraxinea (L.) Ach. | 39 | 19 a | 48 b | 51 b |
| Amandinea punctata (Hoffm.) Coppins & Scheid | 40 | 22 a | 48 b | 49 b |
| Candelariella xanthostigma (Ach.) Lettau | 42 | 41 a | 43 a | 44 a |
| Caloplaca ferruginea (Huds.) Th. Fr. | 45 | 50 a | 44 a | 39 a |
| Evernia prunastri (L.) Ach. | 45 | 52 a | 40 a | 46 a |
| Physcia biziana (A. Massal.) Zahlbr. var. biziana | 46 | 20 a | 73 b | 39 a |
| Candelariella reflexa (Nyl.) Lettau | 46 | 44 a | 50 a | 43 a |
| Ramalina fastigiata (Pers.) Ach. | 47 | 50 a | 51 a | 34 a |
| Phlyctis argena (Spreng.) Flot. | 47 | 63 b | 34 a | 51 ab |
| Pertusaria pustulata (Ach.) Duby | 54 | 32 a | 59 b | 61 b |
| Lecanora expallens Ach. | 54 | 41 a | 65 b | 53 ab |
| Normandina pulchella | 56 | 46 a | 68 a | 47 a |
| Lepra amara (Ach.) Hafenller | 59 | 60 a | 58 a | 57 a |
| Flavoparmelia soredians (Nyl.) Hale | 60 | 46 a | 67 a | 66 a |
| Candelaria concolor (Dicks.) Stein | 61 | 56 a | 63 a | 61 a |
| Physconia grisea (Lam.) Poelt | 64 | 54 a | 75 b | 58 a |
| Melanelixia subaurifera (Nyl) O. Blanco, A. Crespo, Divakar, Essl., D. Hawksw. & Lumbsch | 66 | 60 a | 71 a | 67 a |
| Physcia aipolia (Humb.) Fürnr | 67 | 55 a | 73 b | 71 b |
| Punctelia subrudecta (Nyl.) Krog | 68 | 68 a | 68 a | 67 a |
| Parmelina tiliacea Taylor | 70 | 73 a | 67 a | 70 a |
| Lecanora chlarotera Nyl. | 71 | 69 a | 68 a | 77 a |
| Parmotrema perlatum (Huds.) M. Choisy | 73 | 69 a | 76 a | 71 a |
| Parmelia sulcata (Taylor) | 74 | 77 a | 70 a | 75 a |
| Pertusaria albescens (Huds.) M. Choisy & Werner | 75 | 68 a | 100 b | 82 ab |
| Lecidella elaeochroma (Ach.) M. Choisy | 75 | 73 a | 74 a | 81 a |
| Physconia distorta (With.) J.R. Laundon | 76 | 75 a | 75 a | 77 a |
| Xanthoria parietina (L.) Th. Fr. | 78 | 73 a | 82 a | 76 a |
| Hyperphyscia adglutinata (Flörke) H. Mayrhofer & Poelt | 78 | 64 a | 88 b | 79 b |
| Flavoparmelia caperata (L.) Hale | 82 | 85 a | 79 a | 84 a |
| Physcia adscendens H. Oliver | 87 | 83 a | 89 a | 87 a |
| Species | Average Agreement | |||
|---|---|---|---|---|
| Total | Team “different” | Team “partially” | Team “same” | |
| Caloplaca pyracea (Ach.) Zwackh. | 10 | 10 a | 11 a | 8 a |
| Physcia tenella (Scop.) DC. | 14 | 0 a | 21 a | 8 a |
| Buellia griseovirens (Sm.) Almb. | 15 | 5 a | 24 a | 20 a |
| Leprocaulon microscopicum (Vill.) Gams | 16 | 20 a | 19 a | 0 a |
| Physcia leptalea (Ach.) DC. | 17 | 23 a | 11 a | 22 a |
| Naetrocymbe punctiformis (Pers.) R.C. Harris | 20 | 14 a | 25 a | 16 a |
| Physconia perisidiosa (Erichsen) Moberg | 24 | 25 a | 20 a | 32 a |
| Lecanora argentata (Ach.) Malme | 27 | 5 a | 32 b | 39 b |
| Gyalecta truncigena (Ach.) Hepp | 27 | 38 b | 11 a | 44 ab |
| Tephromela atra (Huds.) Hafellner | 27 | 13 a | 33 b | 37 b |
| Melanelixia fuliginosa (Duby) O. Blanco, A. Crespo, Divakar, Essl., D. Hawksw. and Lumbsch | 30 | 38 a | 28 a | 25 a |
| Lecanora hagenii (Ach.) Ach. | 30 | 20 a | 41 a | 28 a |
| Ramalina farinacea (L.) Ach. | 31 | 47 b | 17 a | 29 ab |
| Pertusaria hymenea (Ach.) Schaer | 31 | 40 a | 17 a | 47 a |
| Bacidia rubella (Hoffm.) A. Massal | 35 | 37a | 40 a | 21 a |
| Physconia servitii (Nádv.) Poelt | 35 | 18 a | 47 b | 39 ab |
| Phaeophyscia orbicularis (Neck.) Moberg | 36 | 17 a | 46 b | 47 b |
| Lecanora horiza (Ach.) Linds. | 39 | 28 a | 44 a | 43 a |
| Phaeophyscia hirsuta (Mereschk.) Moberg | 40 | 45 b | 27 a | 58 b |
| Collema furfuraceum Du Rietz | 48 | 40 a | 49 a | 58 a |
| Pertusaria pertusa (L.) Tuck. | 48 | 46 a | 47 a | 53 a |
| Caloplaca cerinelloides (Erichsen) Poelt | 49 | 29 a | 62 a | 50 a |
| Physcia clementei (Turner) Lynge | 49 | 27 a | 53 a | 58 a |
| Pertusaria flavida (DC.) J.R. Laundon | 50 | 27 a | 56 b | 71 b |
| Lecanora carpinea (L.) Vain. | 50 | 48 a | 56 a | 45 a |
| Dendrographa decolorans (Sm.) Ertz and Tehler | 52 | 35 a | 58 b | 48 ab |
| Pleurosticta acetabulum (Neck.) Elix & Lumbsch | 53 | 45 a | 67 a | 39 a |
| Diploicia canescens (Dicks.) A. Massal. | 55 | 38 a | 60 a | 74 a |
| Lecanora symmicta (Ach.) Ach. | 55 | 39 a | 67 b | 58 ab |
| Heterodermia obscurata (Nyl.) Trevis. | 60 | 33 a | 76 b | 63 b |
| Chrysothrix candelaris (L.) J.R. Laundon | 61 | 35 a | 80 b | 67 b |
| Parmotrema reticulatum (Taylor) M. Choisy | 64 | 66 a | 61 a | 68 a |
| Opegrapha niveoatra (Borrer) J.R. Laundon | 98 | 100 a | 97 a | 100 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunialti, G.; Frati, L.; Malegori, C.; Giordani, P.; Malaspina, P. Do Different Teams Produce Different Results in Long-Term Lichen Biomonitoring? Diversity 2019, 11, 43. https://doi.org/10.3390/d11030043
Brunialti G, Frati L, Malegori C, Giordani P, Malaspina P. Do Different Teams Produce Different Results in Long-Term Lichen Biomonitoring? Diversity. 2019; 11(3):43. https://doi.org/10.3390/d11030043
Chicago/Turabian StyleBrunialti, Giorgio, Luisa Frati, Cristina Malegori, Paolo Giordani, and Paola Malaspina. 2019. "Do Different Teams Produce Different Results in Long-Term Lichen Biomonitoring?" Diversity 11, no. 3: 43. https://doi.org/10.3390/d11030043
APA StyleBrunialti, G., Frati, L., Malegori, C., Giordani, P., & Malaspina, P. (2019). Do Different Teams Produce Different Results in Long-Term Lichen Biomonitoring? Diversity, 11(3), 43. https://doi.org/10.3390/d11030043








