May the Diversity of Epiphytic Lichens Be Used in Environmental Forensics?
Abstract
1. Introduction
2. Biological Monitoring
3. Lichen Biomonitoring
4. Lichen Diversity
5. Measuring the Diversity of Epiphytic Lichens
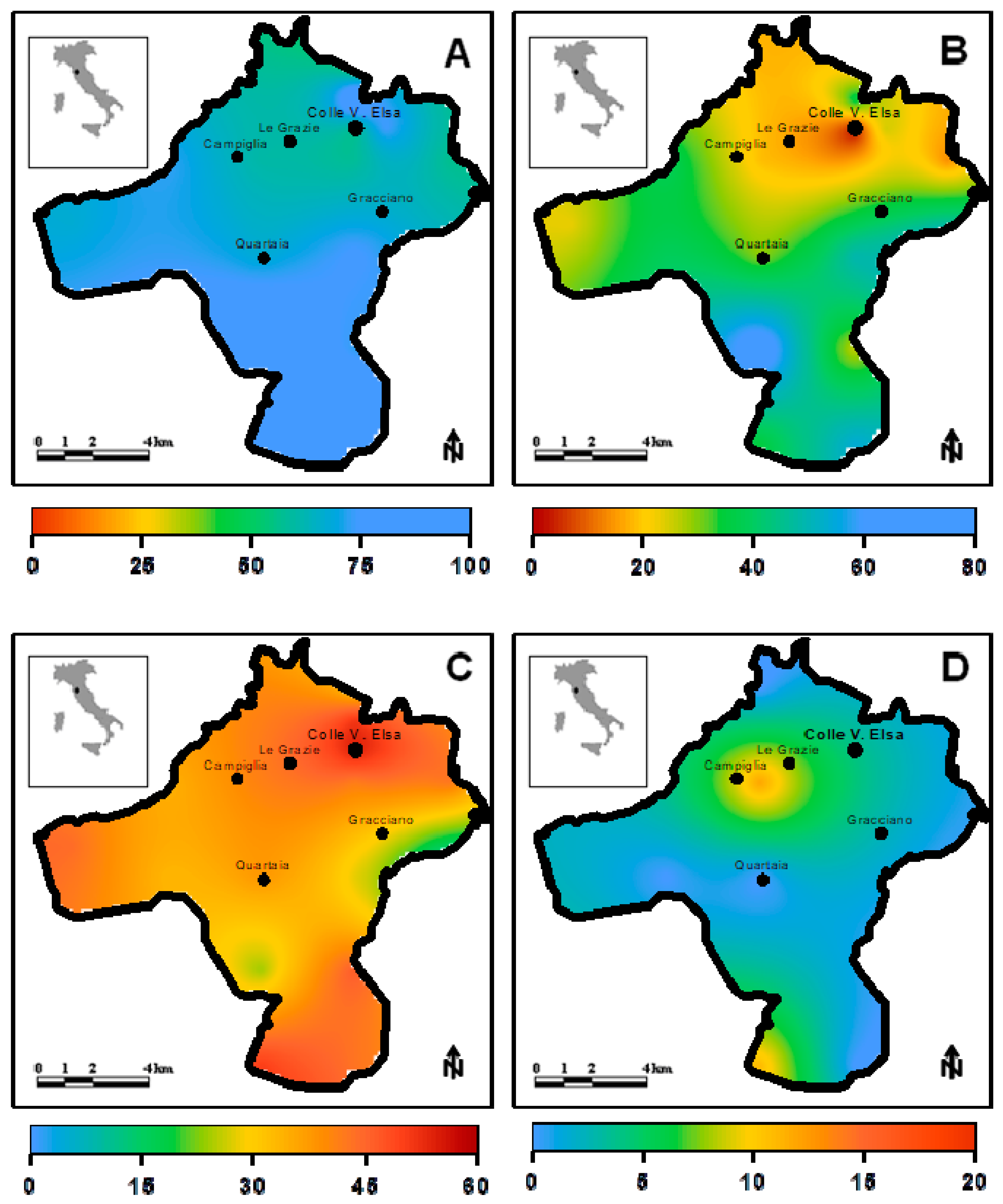
6. Interpretation and Presentation of the Results
7. Lichen Diversity and Environmental Forensics
8. Possible Pitfalls in Interpreting the Results of Lichen Diversity Monitoring
9. Present Challenges in Using Lichen Diversity in Air Pollution Monitoring
10. Monitoring Lichen Diversity under a Climate-Change Scenario
11. Proxies for Lichen Diversity
12. Toward a Worldwide Lichen Diversity Index
13. The Spatial Scale
14. Uncertainty of Lichen Diversity Biomonitoring
15. Sensitivity Analysis
16. Lichen Biomonitoring and Environmental Justice
17. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Murphy, B.L.; Morrison, R.D. Introduction to Environmental Forensics; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Margiotta, G.; Bacaro, G.; Carnevali, E.; Severini, S.; BAcci, M.; Gabrielli, M. Forensic botany as a useful tool in the crime scene: Report of a case. J. Forensic Leg. Med. 2015, 34, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.G. The use of forensic botany and geology in war crimes investigations in NE Bosnia. Forensic Sci. Int. 2006, 163, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D.L.; Wiltshire, P.E.J. Forensic mycology: The use of fungi in criminal investigations. Forensic Sci. Int. 2011, 206, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.E. Biological Monitoring: Theory & Applications. Bioindicators and Biomarkers for Environmental Quality and Human Exposure Assessment; WIT Press: Southampton, UK, 2008. [Google Scholar]
- Markert, B.A.; Breure, A.M.; Zechmeiste, H.G. Bioindicators and Biomonitors. Principles, Concept and Applications; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Loppi, S. Lichens as sentinels for air pollution at remote alpine areas (Italy). Environ. Sci. Pollut. Res. 2014, 21, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Nimis, P.L.; Scheidegger, C.; Wolseley, P.A. Monitoring with Lichens—Monitoring Lichens; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Hale, M.E. The Biology of Lichens; Edward Arnold: London, UK, 1983. [Google Scholar]
- Pirintsos, S.A.; Loppi, S. Biomonitoring atmospheric pollution: The challenge of times in environmental policy on air quality. Environ. Pollut. 2008, 151, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Cislaghi CNiMis, P.L. Lichens, air pollution and lung cancer. Nature 1997, 387, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Bealey, W.J.; Long, S.; Spurgeon, D.J.; Leith, I.; Cape, J.N. Review and Implementation Study of Biomonitoring for Assessment of Air Quality Outcomes; Science Report SC030175/SR2; Environment Agency: Bristol, UK, 2008; pp. 1–170.
- Kricke, R.; Loppi, S. Bioindication: The IAP approach. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 21–37. [Google Scholar]
- Loppi, S.; Frati, L.; Paoli, L.; Bigagli, V.; Rossetti, C.; Bruscoli, C.; Corsini, A. Biodiversity of epiphytic lichens and heavy metal contents of Flavoparmelia caperata thalli as indicators of temporal variations of air pollution in the town of Montecatini Terme (central Italy). Sci. Total Environ. 2004, 326, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Asta, J.; Erhardt, W.; Ferretti, M.; Fornasier, F.; Kirschbaum, U.; Nimis, P.L.; Purvis, O.W.; Pirintsos, S.; Scheidegger, C.; Van Haluwyn, C.; et al. Mapping lichen diversity as an indicator of environmental quality. In Monitoring with Lichens-Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 273–279. [Google Scholar]
- Loppi, S.; Giordani, P.; Brunialti, G.; Isocrono, D.; Piervittori, R. Identifying deviation from naturality of lichen diversity for bioindication purposes. In Monitoring with Lichens—Monitoring Lichens; Nimis, P.L., Scheidegger, C., Wolseley, P., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 281–284. [Google Scholar]
- Loppi, S.; Giordani, P.; Brunialti, G.; Isocrono, D.; Piervittori, R. A new scale for the interpretation of lichen biodiversity values in the Tyrrhenian side of Italy. Bibl. Lichenol. 2002, 82, 237–243. [Google Scholar]
- Giordani, P. Is the diversity of epiphytic lichens a reliable indicator of air pollution? A case study from Italy. Environ. Pollut. 2007, 146, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Giordani, P.; Brunialti, G.; Alleteo, D. Effects of atmospheric pollution on lichen biodiversity (LB) in a Mediterranean region (Liguria, northwest Italy). Environ. Pollut. 2002, 118, 53–64. [Google Scholar] [CrossRef]
- Rao, D.N.; Leblanc, F. Influence of an iron sintering plant on corticolous epiphytes in Wawa, Ontario. Bryologist 1967, 70, 141–157. [Google Scholar] [CrossRef]
- Nash, T.H. Simplification of the Blue Mountain lichen communities near a zinc factory. Bryologist 1972, 75, 315–324. [Google Scholar]
- Nimis, P.L.; Castello, M.; Periotti, M. Lichens as Biomonitors of Sulphur Dioxide Pollution in La Spezia (Northern Italy). Lichenologist 1990, 22, 333–344. [Google Scholar] [CrossRef]
- Castello, M.; Nimis, P.L.; Alleteo, D.; Bellio, M.G. Biomonitoring of SO2 and metal pollution with lichens and barks in Savona (N ltaly). Bull. Soc. Adriat. Sci. 1994, 75, 61–83. [Google Scholar]
- Showman, R.E. Lichens as indicators of air quality around a coal-fired power generating plant. Bryologist 1975, 78, 1–6. [Google Scholar] [CrossRef]
- Showman, R.E. Lichen recolonization following air quality improvement. Bryologist 1981, 84, 492–497. [Google Scholar] [CrossRef]
- Brunialti, G.; Frati, L.; Incerti, G.; Rizzi, G.; Vinci, M.; Giordani, P. lichen biomonitoring of air pollution: Issues for applications in complex environments. In Air Quality in the 21st Century; Romano, G.C., Conti, A.G., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2008. [Google Scholar]
- Nali, C.; Francini, A.; Lorenzini, G. BiologAssessing the quality of biomonitoring via signal-to-noise ratio analysisical monitoring of ozone: The twenty-year Italian experience. J. Environ. Monit. 2006, 8, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Will-Wolf, S. Structure of corticolous lichen communities before and after exposure to emissions from a “clean” coal-fired generating station. Bryologist 1980, 83, 281–295. [Google Scholar] [CrossRef]
- Paoli, L.; Corsini, A.; Bigagli, V.; Vannini, J.; Bruscoli, C.; Loppi, S. Long-term biological monitoring of environmental quality around a solid waste landfill assessed with lichens. Environ. Pollut. 2012, 161, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Loppi, S. Lichen biomonitoring as a tool for assessing air quality in geothermal areas. In Proceedings of the World Geothermal Congress 2000, Kyushu-Tohoku, Japan, 28 May–10 June 2000; Iglesis, E., Blackwell, D., Hunt, T., Kund, J., Tamanyu, S., Kimbara, K., Eds.; University of Siena: Siena, Italy, 2000; pp. 645–648. [Google Scholar]
- Loppi, S. Lichens as bioindicators of geothermal air pollution in central Italy. Bryologist 1996, 99, 41–48. [Google Scholar] [CrossRef]
- Loppi, S.; Nascimbene, J. Lichen bioindication of air quality in the Mt. Amiata geothermal area (central Italy). Geothermics 1997, 27, 295–304. [Google Scholar] [CrossRef]
- Loppi, S.; Destito, G.; Pirintsos, S.A.; De Dominicis, V. Temporal variation of air pollution in a geothermal area of central Italy: Assessment by the biodiversity of epiphytic lichens. Isr. J. Plant Sci. 2002, 50, 45–50. [Google Scholar] [CrossRef]
- Loppi, S.; Frati, L.; Benedettini, G.; Pirintsos, S.A.; Leonzio, C. Geothermal energy and air pollution at Larderello (Tuscany, central Italy): Biodiversity of epiphytic lichens as indicator. Isr. J. Plant Sci. 2002, 50, 119–126. [Google Scholar] [CrossRef]
- Loppi, S.; Paoli, L.; Gaggi, C. Diversity of epiphytic lichens and Hg contents of Xanthoria parietina thalli as monitors of geothermal air pollution in the Mt. Amiata area (central Italy). J. Atmos. Chem. 2006, 53, 93–105. [Google Scholar] [CrossRef]
- Paoli, L.; Loppi, S. A biological method to monitor early effects of the air pollution caused by the industrial exploitation of geothermal energy. Environ. Pollut. 2008, 155, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Loppi, S.; Nascimbene, J. Monitoring H2S air pollution caused by the industrial exploitation of gethoermal energy: The pitfall of using lichens as bioindicators. Environ. Pollut. 2010, 158, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Department for Environment, Food & Rural Affairs. Emissions of Air Pollutants in the UK, 1970 to 2016; Defra National Statistics Release; Department for Environment, Food & Rural Affairs: London, UK, 2018.
- Li, Y.; Schichtel, B.; Walker, J.; Schwede, D.; Chen, X.; Lehmann, C.; Puchalski, M.; Gay, D.; Collett, J. The increasing importance of deposition of reduced nitrogen in the United States. Proc. Nat. Acad. Sci. USA 2016, 113, 5874–5879. [Google Scholar] [CrossRef] [PubMed]
- Munzi, S.; Pisani, T.; Loppi, S. The integrity of lichen cell membrane as a suitable parameter for monitoring biological effects of acute nitrogen pollution. Ecotoxicol. Environ. Saf. 2009, 72, 2009–2012. [Google Scholar] [CrossRef] [PubMed]
- Munzi, S.; Pisani, T.; Paoli, L.; Loppi, S. Time- and dose-dependency of the effects of nitrogen pollution on lichens. Ecotoxicol. Environ. Saf. 2010, 73, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Van Herk, C.M. Mapping of ammonia pollution with epiphytic lichens in The Netherlands. Lichenologist 1999, 31, 9–20. [Google Scholar]
- Van Dobben, H.F.; Ter Braak, C.J.F. Effects of atmospheric NH3 on epiphytic lichens in the Netherlands: The pitfalls of biological monitoring. Atmos. Environ. 1998, 32, 551–557. [Google Scholar] [CrossRef]
- Sparrius, L.B. Response of epiphytic lichen communities to decreasing ammonia air concentrations in a moderately polluted area of The Netherlands. Environ. Pollut. 2007, 146, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Van Herk, C.M.; Mathijssen-Spiekman, E.A.M.; de Zwart, D. Long distance nitrogen air pollution effects on lichens in Europe. Lichenologist 2003, 35, 347–359. [Google Scholar] [CrossRef]
- Loppi, S.; De Dominicis, V. Effects of agriculture on epiphytic lichen vegetation in central Italy. Isr. J. Plant Sci. 1996, 44, 297–307. [Google Scholar] [CrossRef]
- Loppi, S.; Pirintsos, S.A. Effect of dust on epiphytic lichen vegetation in the Mediterranean area (Italy and Greece). Isr. J. Plant Sci. 2000, 48, 91–95. [Google Scholar] [CrossRef]
- Loppi, S.; Pirintsos, S.A.; De Dominicis, V. Analysis of the distribution of epiphytic lichens on Quercus pubescens along an altitudinal gradient in a Mediterranean area (Tuscany, central Italy). Isr. J. Plant Sci. 1997, 45, 53–58. [Google Scholar] [CrossRef]
- Frati, L.; Brunialti, G.; Loppi, S. Effects of reduced nitrogen compounds on epiphytic lichen communities in Mediterranean Italy. Sci. Total Environ. 2008, 407, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Pinho, P.; Augusto, S.; Martins-Loucao, M.A.; Pereira, M.J.; Soares, A.; Maguas, C.; Branquinho, C. Causes of change in nitrophytic and oligotrophic lichen species in a Mediterranean climate: Impact of land cover and atmospheric pollutants. Environ. Pollut. 2008, 154, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Giordani, P.; Brunialti, G.; Bacaro, G.; Nascimbene, J. Functional traits of epiphytic lichens as potential indicators of environmental conditions in forest ecosystems. Ecol. Indic. 2012, 18, 413–420. [Google Scholar] [CrossRef]
- Loppi, S. Mapping the effects of air pollution, nitrogen deposition, agriculture and dust by the diversity of epiphytic lichens. In Lichens in a Changing Pollution Environment; English Nature Research Report No. 525; Lambley, P., Wolseley, P., Eds.; English Nature: Peterborough, UK, 2004; pp. 37–41. [Google Scholar]
- Ruisi, S.; Zucconi, L.; Fornasier, F.; Paoli, L.; Frati, L.; Loppi, S. Mapping environmental effects of agriculture with epiphytic lichens (central Italy). Isr. J. Plant Sci. 2005, 53, 115–124. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Formentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Psarras, G. Global change effects on crop photosynthesis and production in Mediterranean: The case of Crete, Greece. Agric. Ecosyst. Environ. 2005, 106, 147–157. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Van Herk, C.M.; Aptroot, A.; van Dobben, H.F. Long-term monitoring in the Netherlands suggests that lichens respond to global warming. Lichenologist 2002, 34, 141–154. [Google Scholar] [CrossRef]
- Bjerke, J.W.; Zielke, M.; Solheim, B. Long-term impacts of simulated climatic change on secondary metabolism, thallus structure and nitrogen fixation activity in two cyanolichens from the Arctic. New Phytol. 2003, 159, 361–367. [Google Scholar] [CrossRef]
- Pirintsos, S.A.; Paoli, L.; Loppi, S.; Kotzabasis, K. Photosynthetic performance of lichen transplants as early indicator of climatic stress along an altitudinal gradient in the arid Mediterranean area. Clim. Chang. 2011, 107, 305–328. [Google Scholar] [CrossRef]
- Insarov, G.; Insarova, I. Assessment of lichen sensitivity to climate change. Isr. J. Plant Sci. 1996, 44, 309–334. [Google Scholar] [CrossRef]
- Insarov, G.; Schroeter, B. Lichen Monitoring and Climate Change; Nimis, P.L., Scheidegger, C., Wolseley, P.A., Eds.; Kluwer: Dordrecht, The Netherlands, 2002; pp. 183–201. [Google Scholar]
- Semenov, S.; Insarova, I. A system to monitor climate change with epilithic lichens. Environ. Monit. Assess. 1999, 55, 279–298. [Google Scholar]
- Loppi, S.; Pirintsos, S.A.; Sforzi, B.; De Dominicis, V. Effects of climate and agriculture on epiphytic lichen vegetation in the Mediterranean area (Tuscany, Central Italy). Acta Bot. Croat. 1998, 55–56, 17–27. [Google Scholar]
- Matos, P.; Geiser, L.; Hardman, A.; Glavich, D.; Pinho, P.; Nunes, A.; Soares, A.M.V.M.; Branquinho, C. Tracking global change using lichen diversity: Towards a global-scale ecological indicator. Methods Ecol. Evol. 2017, 8, 788–798. [Google Scholar] [CrossRef]
- Brunialti, G.; Giordani, P.; Isocrono, D.; Loppi, S. Evaluation of data quality in lichen biomonitoring studies: The Italian experience. Environ. Monit. Assess. 2002, 75, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Giordani, P.; Brunialti, G.; Benesperi, R.; Rizzi, G.; Frati, L.; Modenesi, P. Rapid biodiversity assessment in lichen diversity surveys: Implications for quality assurance. J. Environ. Monit. 2009, 11, 730–735. [Google Scholar] [CrossRef] [PubMed]
- USDA. Forest Inventory and Analysis National Core Field Guide Vol. 1: Field Data Collection Procedures for Phase 2 Plots; ver 7.0; USDA Forest Service: Arlington, VA, USA, 2011.
- Purvis, A.; Hector, A. Getting the measure of biodiversity. Nature 2000, 405, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wolterbeek, H.T.; Bode, P.; Verburg, T.G. Assessing the quality of biomonitoring via signal-to-noise ratio analysis. Sci. Total Environ. 1996, 180, 107–116. [Google Scholar] [CrossRef]
- Ferretti, M.; Brambilla, E.; Brunialti, G.; Fornasier, F.; Mazzali, C.; Giordani, P.; Nimis, P.L. Reliability of different sampling densities for estimating and mapping lichen diversity in biomonitoring studies. Environ. Pollut. 2004, 127, 249–256. [Google Scholar] [CrossRef]
- Giordani, P.; Brunialti, G.; Frati, L.; Incerti, G.; Ianesch, L.; Vallone, E.; Bacaro, G.; Maccherini, S. Spatial scales of variation in lichens: Implications for sampling design in biomonitoring surveys. Environ. Monit. Assess. 2013, 185, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Brunialti, G.; Frati, L.; Cristofolini, F.; Chiarucci, A.; Giordani, P.; Loppi, S.; Benesperi, R.; Cristofori, A.; Critelli, P.; Di Capua, E.; et al. Can we compare lichen diversity data? A test with skilled teams. Ecol. Indic. 2012, 23, 509–516. [Google Scholar]
- Burgass, M.J.; Halpern, B.S.; Nicholson, E.; Milner-Gulland, E.J. Navigating uncertainty in environmental composite indicators. Ecol. Indic. 2017, 75, 268–278. [Google Scholar] [CrossRef]
- Milner-Gulland, E.J.; Shea, K. Embracing uncertainty in applied ecology. J. Appl. Ecol. 2017, 54, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studiesof therapeutics. Pharmacoepidemiol. Drug Saf. 2006, 15, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Thabane, L.; Mbuagbaw, L.; Zhang, S.; Samaan, Z.; Marcucci, M.; Ye, C.; Thabane, M.; Giangregorio, L.; Dennis, B.; Kosa, D.; et al. A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med. Res. Methodol. 2013, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Brulle, R.J.; Pellow, D.N. Environmental justice: Human health and environmental inequalities. Annu. Rev. Public Health 2006, 27, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Billionnet, C.; Sherrill, D.; Annesi-Maesano, I. Estimating the health effects of exposure to multi-pollutant mixture. Ann. Epidemiol. 2012, 22, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Occelli, F.; Bavdek, R.; Deram, A.; Hellequin, A.P.; Cuny, M.A.; Zwarterook, I.; Cuny, D. Using lichen biomonitoring to assess environmental justice at a neighbourhood level in an industrial area of Northern France. Ecol. Ind. 2016, 60, 781–788. [Google Scholar] [CrossRef]
- Contardo, T.; Giordani, P.; Paoli, L.; Vannini, A.; Loppi, S. May lichen biomonitoring of air pollution be used for environmental justice assessment? A case study from an area of N Italy with a municipal solid waste incinerator. Environ. Forensics 2018, 265–276. [Google Scholar] [CrossRef]
- Lanier, C.; Deram, A.; Cuny, M.A.; Cuny, D.; Occedlli, F. Spatial analysis of environmental inequalities caused by multiple air pollutants: A cumulative impact screening method, applied to the north of France. Ecol. Ind. 2019, 99, 91–100. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loppi, S. May the Diversity of Epiphytic Lichens Be Used in Environmental Forensics? Diversity 2019, 11, 36. https://doi.org/10.3390/d11030036
Loppi S. May the Diversity of Epiphytic Lichens Be Used in Environmental Forensics? Diversity. 2019; 11(3):36. https://doi.org/10.3390/d11030036
Chicago/Turabian StyleLoppi, Stefano. 2019. "May the Diversity of Epiphytic Lichens Be Used in Environmental Forensics?" Diversity 11, no. 3: 36. https://doi.org/10.3390/d11030036
APA StyleLoppi, S. (2019). May the Diversity of Epiphytic Lichens Be Used in Environmental Forensics? Diversity, 11(3), 36. https://doi.org/10.3390/d11030036




