Limited Cross-Shelf Variation in the Growth of Three Branching Corals on Australia’s Great Barrier Reef
Abstract
1. Introduction
2. Materials and Methods
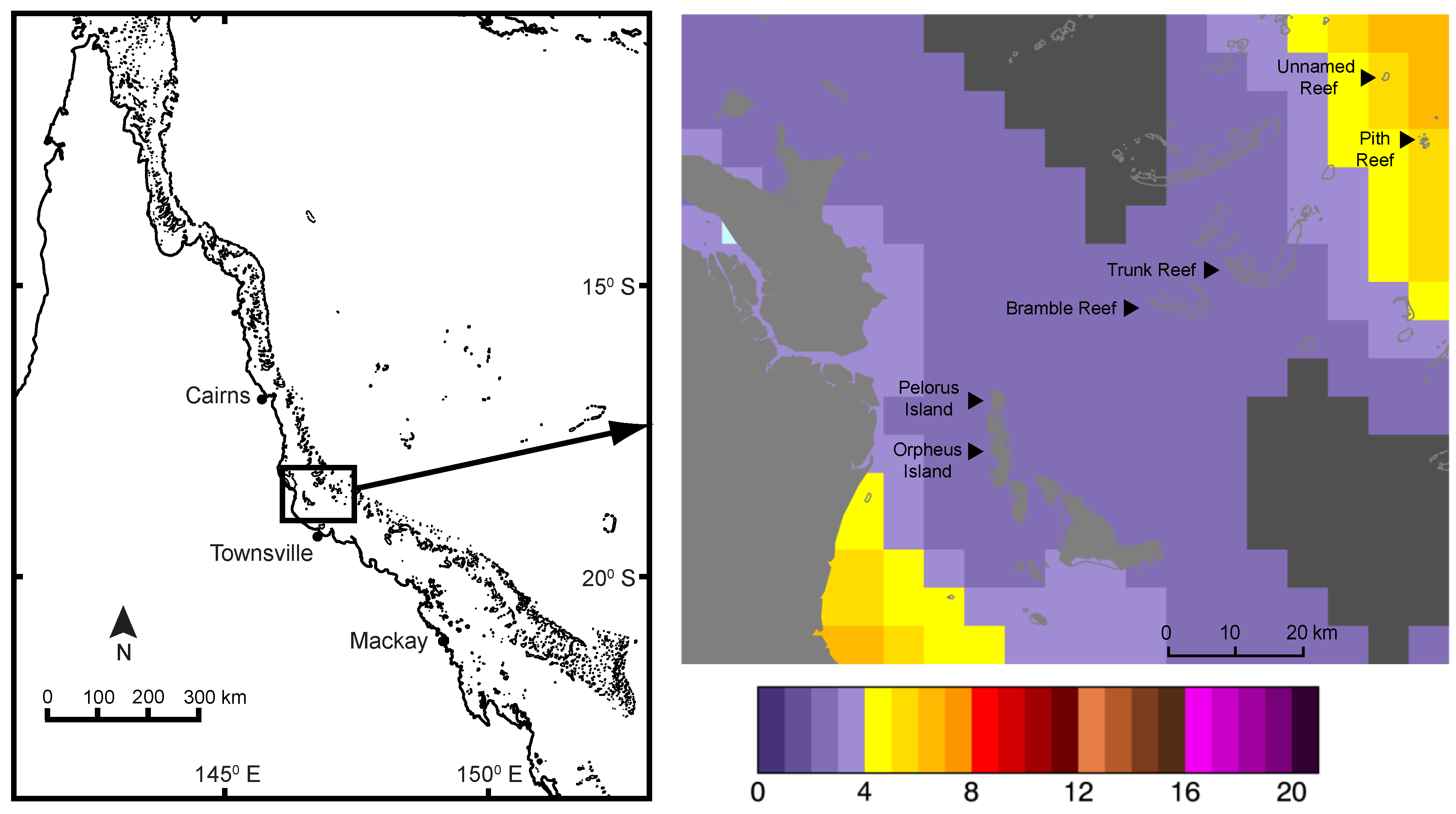
2.1. Field Sampling
2.2. Data Analyses
3. Results
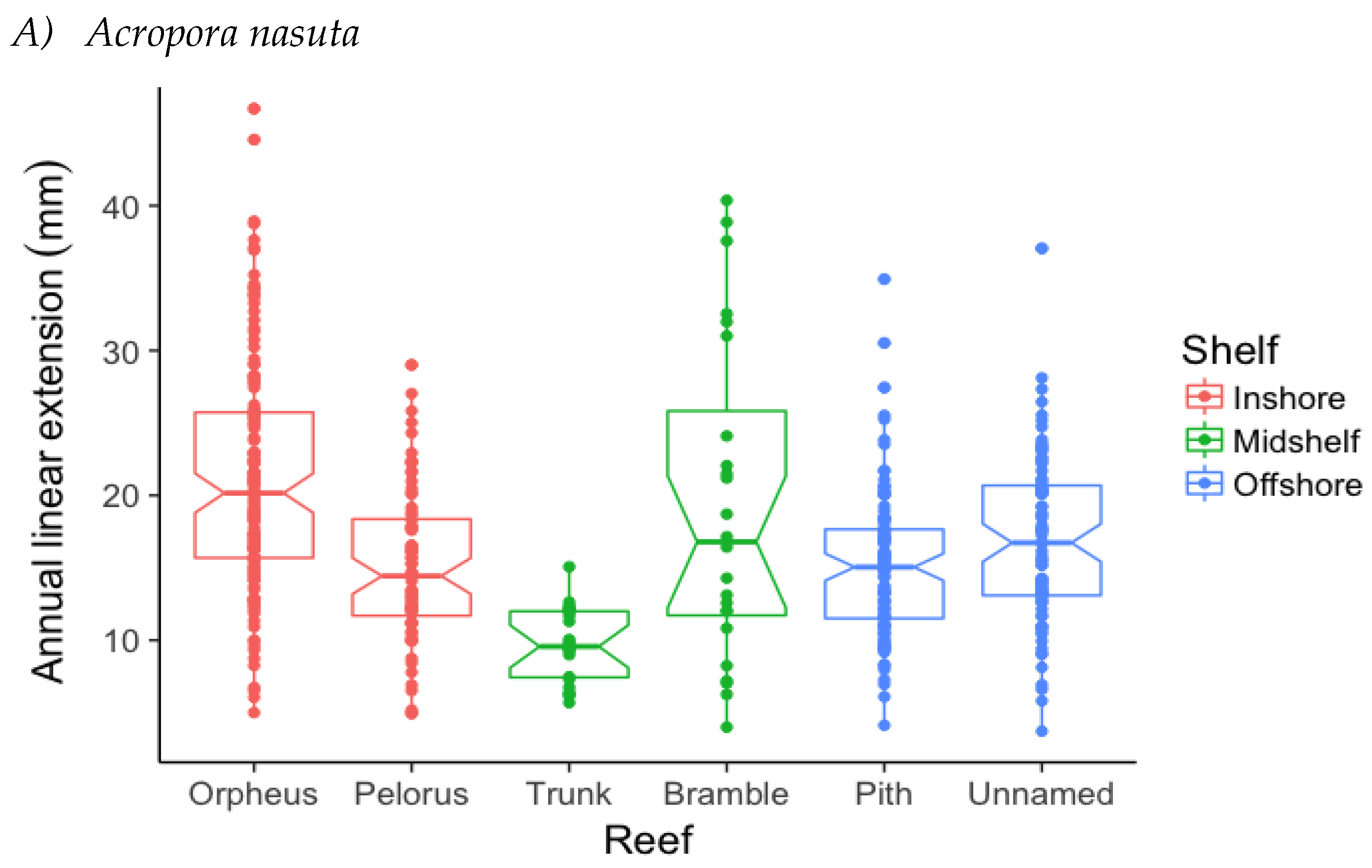
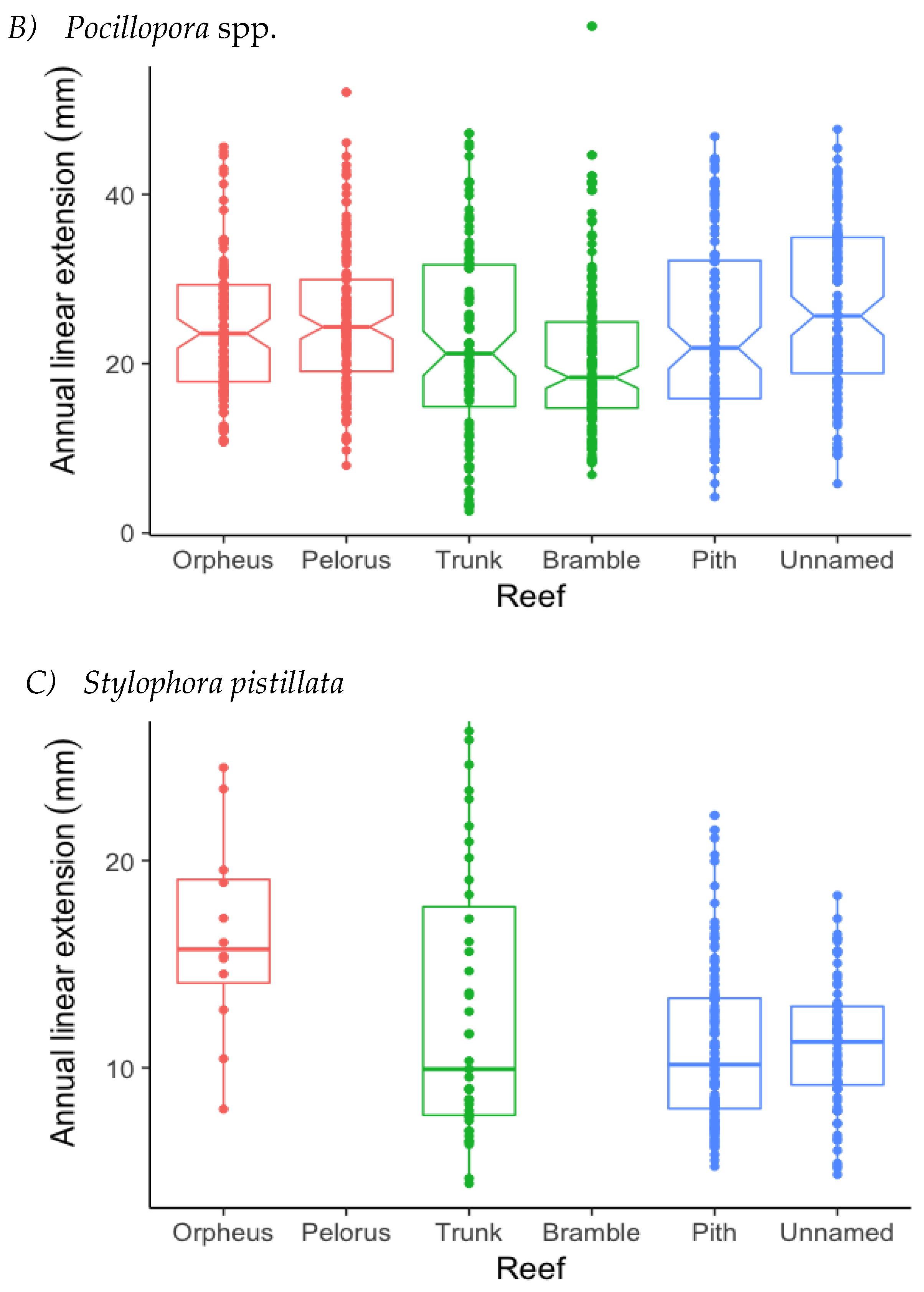
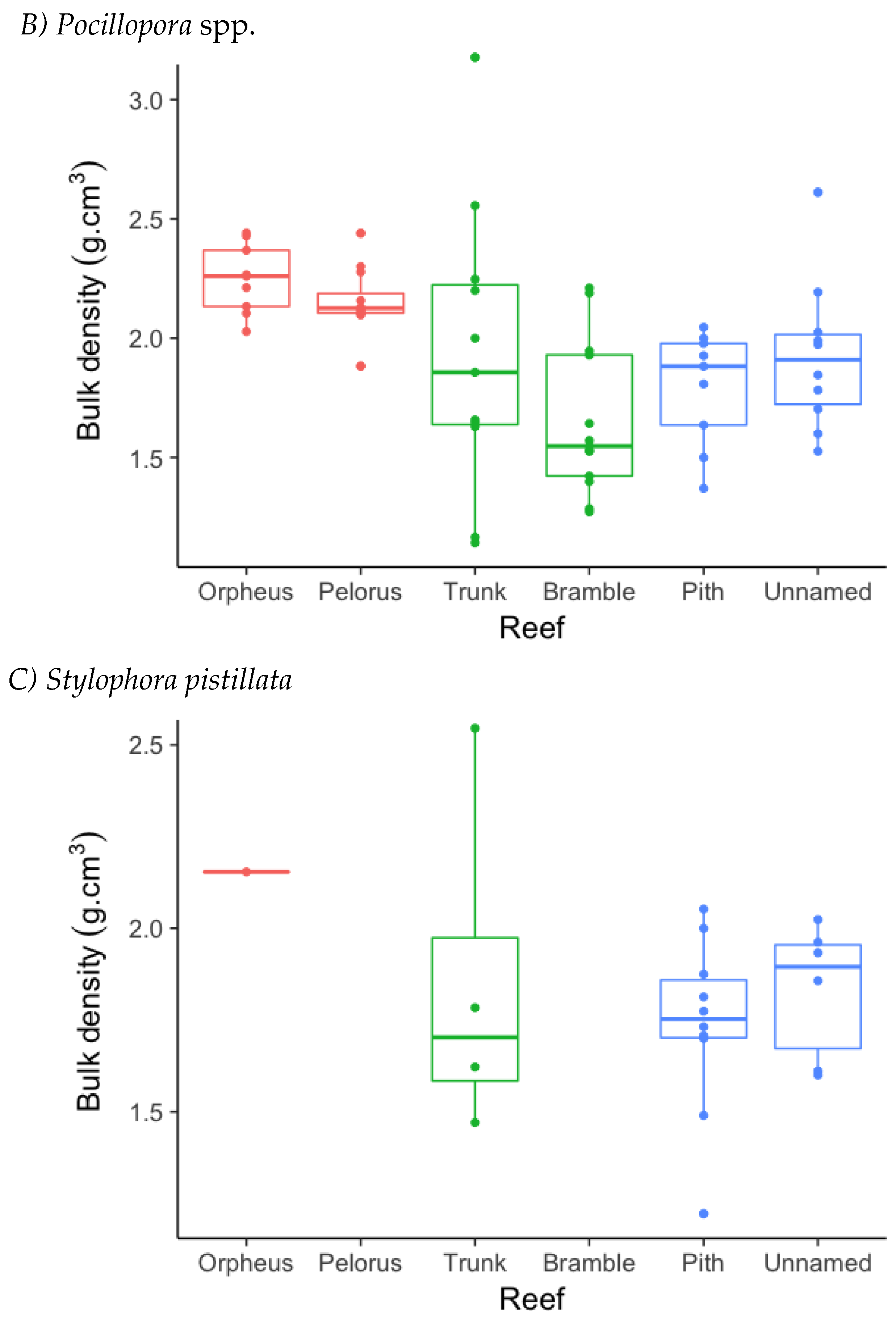
3.1. Annual Linear Extension (ALE)
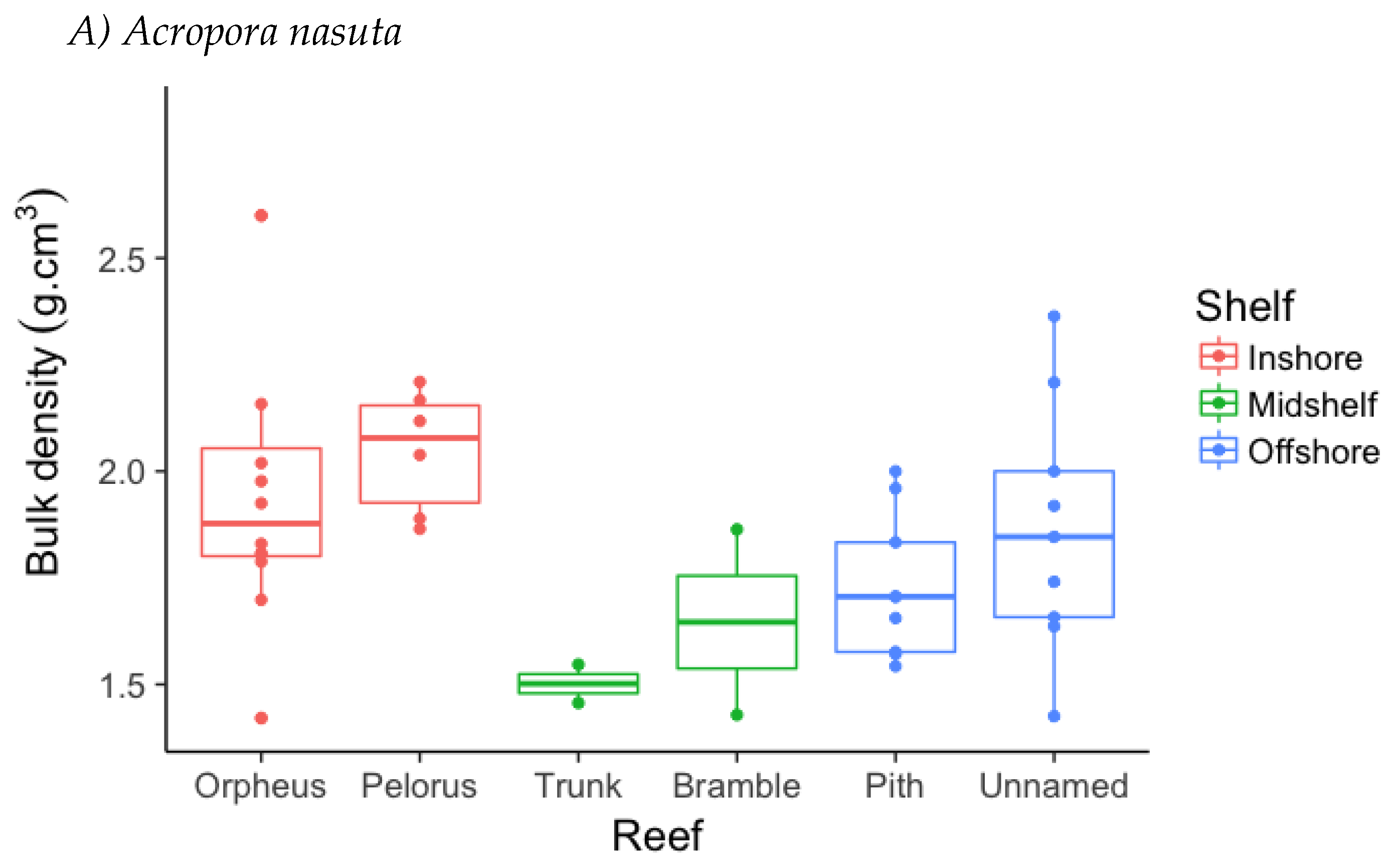
3.2. Skeletal Density
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kleypas, J.A.; McManus, J.W.; Menez, L.A.B. Environmental limits to coral reef development: Where do we draw the line? Am. Zool. 1999, 39, 146–159. [Google Scholar] [CrossRef]
- Done, T.J. Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs 1982, 1, 95–107. [Google Scholar] [CrossRef]
- Harriott, V.J. Coral growth in subtropical eastern Australia. Coral Reefs 1999, 18, 281–291. [Google Scholar] [CrossRef]
- Anderson, K.D.; Cantin, N.E.; Heron, S.F.; Pisapia, C.; Pratchett, M.S. Variation in growth rates of branching corals along Australia’s Great Barrier Reef. Sci. Rep. 2017, 7, 2920. [Google Scholar] [CrossRef] [PubMed]
- Buddemeier, R.W.; Kinzie, R.A., III. Coral growth. Oceanogr. Mar. Biol. Ann. Rev. 1976, 14, 183–225. [Google Scholar]
- Muir, P.R.; Wallace, C.C.; Done, T.; Aguirre, J.D. Limited scope for latitudinal extension of reef corals. Science 2015, 348, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Bellwood, D.R.; Hughes, T.P. Regional-scale assembly rules and biodiversity of coral reefs. Science 2001, 292, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Heron, S.F.; Pratchett, M.S. Species-specific declines in the linear extension of branching corals at a sub-tropical reef, Lord Howe Island. Coral Reefs 2015, 34, 479–490. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Anderson, K.D.; Hoogenboom, M.O.; Widman, E.; Baird, A.H.; Pandolfi, J.M.; Edmunds, P.J.; Lough, J.M. Spatial, temporal and taxonomic variation in coral growth—Implications for the structure and function of coral reef ecosystems. Oceanogr. Mar. Biol. Ann. Rev. 2015, 53, 215–295. [Google Scholar]
- Hoey, A.S.; Pratchett, M.S.; Cvitanovic, C. High macroalgal cover and low coral recruitment undermines the potential resilience of the world’s southernmost coral reef assemblages. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.R.; Bellwood, D.R.; Hughes, T.P. Indo–Pacific biodiversity of coral reefs: Deviations from a mid-domain model. Ecology 2003, 84, 2178–2190. [Google Scholar] [CrossRef]
- Keith, S.A.; Baird, A.H.; Hughes, T.P.; Madin, J.S.; Connolly, S.R. Faunal breaks and species composition of Indo–Pacific corals: The role of plate tectonics, environment and habitat distribution. Proc. R. Soc. B 2013, 280. [Google Scholar] [CrossRef] [PubMed]
- Cleary, D.F.; Becking, L.E.; de Voogd, N.J.; Renema, W.; de Beer, M.; van Soest, R.W.; Hoeksema, B.W. Variation in the diversity and composition of benthic taxa as a function of distance offshore, depth and exposure in the Spermonde Archipelago, Indonesia. Est. Coast. Shelf Sci. 2005, 65, 557–570. [Google Scholar] [CrossRef]
- Wilkinson, C.R.; Cheshire, A.C. Patterns in the distribution of sponge populations across the central Great Barrier Reef. Coral Reefs 1989, 8, 127–134. [Google Scholar] [CrossRef]
- Fabricius, K.; De’Ath, G. Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef. Coral Reefs 2001, 19, 303–309. [Google Scholar] [CrossRef]
- Wismer, S.; Hoey, A.S.; Bellwood, D.R. Cross-shelf benthic community structure on the Great Barrier Reef: Relationships between macroalgal cover and herbivore biomass. Mar. Ecol. Prog. Ser. 2009, 376, 45–54. [Google Scholar] [CrossRef]
- Lirman, D.; Fong, P. Is proximity to land-based sources of coral stressors an appropriate measure of risk to coral reefs? An example from the Florida Reef Tract. Mar. Pollut. Bull. 2007, 54, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M. Patterns in the distribution of fish communities across the central Great Barrier Reef. Coral Reefs 1982, 1, 35–43. [Google Scholar] [CrossRef]
- Williams, D.M.; Hatcher, A.I. Structure of fish communities on outer slopes of inshore, mid-shelf and outer shelf reefs of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 1983, 10, 239–250. [Google Scholar] [CrossRef]
- Russ, G. Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. I. Levels of variability across the entire continental shelf. Mar. Ecol. Prog. Ser. 1984, 20, 23–34. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 2008, 27, 37–47. [Google Scholar] [CrossRef]
- Emslie, M.J.; Pratchett, M.S.; Cheal, A.J.; Osborne, K. Great Barrier Reef butterflyfish community structure: The role of shelf position and benthic community type. Coral Reefs 2010, 29, 705–715. [Google Scholar] [CrossRef]
- Bellwood, D.; Wainwright, P. Locomotion in labrid fishes: Implications for habitat use and cross-shelf biogeography on the Great Barrier Reef. Coral Reefs 2001, 20, 139–150. [Google Scholar] [CrossRef]
- Hughes, T.P.; Day, J.C.; Brodie, J. Securing the future of the Great Barrier Reef. Nat. Clim. Chang. 2015, 5, 508–511. [Google Scholar] [CrossRef]
- Kroon, F.J.; Thorburn, P.; Schaffelke, B.; Whitten, S. Towards protecting the Great Barrier Reef from land-based pollution. Glob. Chang. Biol. 2016, 22, 1985–2002. [Google Scholar] [CrossRef] [PubMed]
- Loya, Y. Effects of water turbidity and sedimentation on the community structure of Puerto Rican corals. Bull. Mar. Sci. 1976, 26, 450–466. [Google Scholar]
- Reigl, B.; Branch, G.M. Effects of sediment on the energy budgets of four Scleractinian (Bourne 19000) and five Alcyonacean (Lamouroux 1816) corals. J. Exp. Mar. Biol. Ecol. 1995, 186, 259–275. [Google Scholar] [CrossRef]
- Fabricius, K.E. Effects of terrestrial runoff on the ecology of corals and coral reefs: Review and synthesis. Mar. Pollut. Bull. 2005, 50, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; De Beer, D.; Lott, C.; Polerecky, L.; Kohls, K.; Abed, R.M.; Ferdelman, T.G.; Fabricius, K.E. Mechanisms of damage to corals exposed to sedimentation. Proc. Natl. Acad. Sci. USA 2012, 109, E1558–E1567. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.R.; Fabricius, K.E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Biol. Ecol. 2000, 252, 221–253. [Google Scholar] [CrossRef]
- Dodge, R.E.; Vaisnys, J.R. Coral populations and growth patterns: Responses to sedimentation and turbidity associated with dredging. J. Mar. Sci. 1977, 35, 715–730. [Google Scholar]
- Lough, J.M.; Barnes, D.J. Environmental controls on growth of the massive coral Porites. J. Exp. Mar. Biol. Ecol. 2000, 245, 225–243. [Google Scholar] [CrossRef]
- Carricart-Ganivet, J.P.; Merino, M. Growth responses of the reef-building coral Montastraea annularis along a gradient of continental influence in the southern Gulf of Mexico. Bull. Mar. Sci. 2001, 68, 133–146. [Google Scholar]
- Messmer, V.; Jones, G.P.; Munday, P.L.; Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 2011, 92, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Roach, S.; Miller, K.J.; Lundgren, P.; Andreakis, N. With eyes wide open: A revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zool. J. Linn. Soc. 2014, 170, 1–33. [Google Scholar] [CrossRef]
- Oliver, J.K.; Chalker, B.E.; Dunlap, W.C. Bathymetric adaptations of reef-building corals at Davies Reef, Great Barrier Reef, Australia. I. Long-term growth responses of Acropora formosa (Dana 1846). J. Exp. Mar. Biol. Ecol. 1995, 73, 11–35. [Google Scholar] [CrossRef]
- Bucher, D.J.; Harriott, V.J.; Roberts, L.G. Skeletal micro-density, porosity and bulk density of acroporid corals. J. Exp. Mar. Biol. Ecol. 1998, 228, 117–136. [Google Scholar] [CrossRef]
- Hughes, T.P. Skeletal density and growth form of corals. Mar. Ecol. Prog. Ser. 1987, 35, 259–266. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Carricart-Ganivet, J.P. Sea surface temperature and the growth of the West Atlantic reef-building coral Montastraea annularis. J. Exp. Mar. Biol. Ecol. 2004, 302, 249–260. [Google Scholar] [CrossRef]
- Lough, J.M. Coral calcification from skeletal records revisited. Mar. Ecol. Prog. Ser. 2008, 373, 257–264. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.D.; Cantin, N.E.; Heron, S.F.; Lough, J.M.; Pratchett, M.S. Temporal and taxonomic contrasts in coral growth at Davies Reef, central Great Barrier Reef, Australia. Coral Reefs 2018, 37, 409–421. [Google Scholar] [CrossRef]
- Tomascik, T.; Sander, F. Effects of eutrophication on reef-building corals. Mar. Biol. 1985, 87, 143–155. [Google Scholar] [CrossRef]
- Guzman, H.M.; Cipriani, R.; Jackson, J.B. Historical decline in coral reef growth after the Panama Canal. Ambio 2008, 37, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Sowa, K.; Watanabe, T.; Kan, H.; Yamano, H. Influence of land development on Holocene Porites coral calcification at Nagura bay, Ishigaki island, Japan. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Scoffin, T.P.; Tudhope, A.W.; Brown, B.E.; Chansang, H.; Cheeney, R.F. Patterns and possible environmental controls of skeletogenesis of Porites lutea, South Thailand. Coral Reefs 1992, 11, 1–11. [Google Scholar] [CrossRef]
- Brown, B.E.; Le Tissier, M.D.A.; Scoffin, T.P.; Tudhope, A.W. Evaluation of the environmental impact of dredging on intertidal coral reefs at Ko Phuket, Thailand, using ecological and physiological parameters. Mar. Ecol. Prog. Ser. 1990, 65, 273–281. [Google Scholar] [CrossRef]
- Manzello, D.P.; Enochs, I.C.; Kolodziej, G.; Carlton, R. Recent decade of growth and calcification of Orbicella faveolata in the Florida Keys: An inshore-offshore comparison. Mar. Ecol. Prog. Ser. 2015, 521, 81–89. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Risk, M.J.; Sammarco, P.W. Cross-shelf trends in skeletal density of the massive coral Porites lobata from the Great Barrier Reef. Mar. Ecol. Prog. Ser. 1991, 69, 195–200. [Google Scholar] [CrossRef]
- Lough, J.M.; Barnes, D.J. Comparisons of skeletal density variations in Porites from the central Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 1992, 155, 1–25. [Google Scholar] [CrossRef]
- Humphrey, C.; Weber, M.; Lott, C.; Cooper, T.; Fabricius, K. Effects of suspended sediments, dissolved inorganic nutrients and salinity on fertilisation and embryo development in the coral Acropora millepora (Ehrenberg, 1834). Coral Reefs 2008, 27, 837–850. [Google Scholar] [CrossRef]
- Jones, R.; Bessell-Browne, P.; Fisher, R.; Klonowski, W.; Slivkoff, M. Assessing the impacts of sediments from dredging on corals. Mar. Pollut. Bull. 2016, 102, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Wolanski, E.; Fabricius, K.; Spagnol, S.; Brinkman, R. Fine sediment budget on an inner-shelf coral-fringed island, Great Barrier Reef of Australia. Est. Coast. Shelf Sci. 2005, 65, 153–158. [Google Scholar] [CrossRef]
- Browne, N.K.; Smithers, S.G.; Perry, C.T. Spatial and temporal variations in turbidity on two inshore turbid reefs on the Great Barrier Reef, Australia. Coral Reefs 2013, 32, 195–210. [Google Scholar] [CrossRef]
- Morgan, K.M.; Kench, P.S. Skeletal extension and calcification of reef-building corals in the central Indian Ocean. Mar. Environ. Res. 2012, 81, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Figueira, W.F.; Pratchett, M.S.; Boube, T.; Adam, A.; Kobelkowsky-Vidrio, T.; Doo, S.S.; Atwood, T.B.; Byrne, M. 3D photogrammetry quantifies growth and external erosion of individual coral colonies and skeletons. Sci. Rep. 2017, 7, 16737. [Google Scholar] [CrossRef] [PubMed]
- Browne, N.K.; Smithers, S.G.; Perry, C.T. Coral reefs of the turbid inner-shelf of the Great Barrier Reef, Australia: An environmental and geomorphic perspective on their occurrence, composition and growth. Earth Sci. Rev. 2012, 115, 1–20. [Google Scholar] [CrossRef]
- Anthony, K.R. Enhanced energy status of corals on coastal, high-turbidity reefs. Mar. Ecol. Prog. Ser. 2006, 319, 111–116. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Cantin, N.E.; Cohen, A.L.; Karnauskas, K.B.; Tarrant, A.M.; McCorkle, D.C. Ocean warming slows coral growth in the central Red Sea. Science 2010, 329, 322–325. [Google Scholar] [CrossRef] [PubMed]
| Shelf Position-Reef | Acropora nasuta | Pocillopora spp. | Stylophora pistillata | Total |
|---|---|---|---|---|
| Inshore–Orpheus Island | 15/15 (100%) | 10/12 (83%) | 1/3 * (33%) | 26/30 (87%) |
| Inshore–Pelorus Island | 6/13 (46%) | 12/12(100%) | 0/0 * | 18/25 (72%) |
| Mid-shelf–Bramble Reef | 2/12 (17%) | 13/13 (100%) | 0/11 (0%) | 15/36 (42%) |
| Mid-shel –Trunk Reef | 2/12 (17%) | 11/12 (92%) | 4/11 (36%) | 17/35 (49%) |
| Offshore–Pith Reef | 9/12 (75%) | 9/12 (75%) | 10/13 (77%) | 28/37 (76%) |
| Offshore–Unnamed Reef | 9/11 (82%) | 10/12 (83%) | 6/12 (50%) | 25/35 (71%) |
| TOTAL | 43/75 (57%) | 65/73 (89%) | 21/50 (42%) | 129/198 (65%) |
| Coral Taxa | Parameter | Model | AICc | df |
|---|---|---|---|---|
| Acropora nasuta | Growth | ~Shelf + Colony | 3029.63 | 5 |
| ~Reef + Colony | 2875.40 | 8 | ||
| ~DHW + Colony | 3033.57 | 4 | ||
| Density | ~Shelf | 21.66 | 4 | |
| ~Reef | 28.56 | 7 | ||
| Pocilloporaspp. | Growth | ~Shelf + Colony | 5597.42 | 5 |
| ~Reef + Colony | 5424.05 | 8 | ||
| ~DHW + Colony | 5438.64 | 4 | ||
| Density | ~Shelf | 51.19 | 4 | |
| ~Reef | 51.34 | 7 | ||
| Stylophora pistillata | Growth | ~Shelf + Colony | 1568.08 | 5 |
| ~Reef + Colony | 1427.73 | 6 | ||
| ~DHW + Colony | 1429.35 | 4 | ||
| Density | ~Shelf | 15.75 | 4 | |
| ~Reef | 16.33 | 5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burn, D.; Pratchett, M.S.; Heron, S.F.; Thompson, C.A.; Pratchett, D.J.; Hoey, A.S. Limited Cross-Shelf Variation in the Growth of Three Branching Corals on Australia’s Great Barrier Reef. Diversity 2018, 10, 122. https://doi.org/10.3390/d10040122
Burn D, Pratchett MS, Heron SF, Thompson CA, Pratchett DJ, Hoey AS. Limited Cross-Shelf Variation in the Growth of Three Branching Corals on Australia’s Great Barrier Reef. Diversity. 2018; 10(4):122. https://doi.org/10.3390/d10040122
Chicago/Turabian StyleBurn, Deborah, Morgan S. Pratchett, Scott F. Heron, Cassandra A. Thompson, Deborah J. Pratchett, and Andrew S. Hoey. 2018. "Limited Cross-Shelf Variation in the Growth of Three Branching Corals on Australia’s Great Barrier Reef" Diversity 10, no. 4: 122. https://doi.org/10.3390/d10040122
APA StyleBurn, D., Pratchett, M. S., Heron, S. F., Thompson, C. A., Pratchett, D. J., & Hoey, A. S. (2018). Limited Cross-Shelf Variation in the Growth of Three Branching Corals on Australia’s Great Barrier Reef. Diversity, 10(4), 122. https://doi.org/10.3390/d10040122





