Abstract
Cactaceae are subject to strong anthropogenic pressure, which motivates the search of strategies for its conservation. This paper aimed to evaluate the survival and growth of eight native species from northeastern Mexico, in order to propose their in-situ conservation, applying three substrates (perlite/peat-moss, zeolite/peat-moss and natural soil), in two sun exposures (west and east). The variability of both survival and growth resulted dependent on substrate type and sun exposure. Astrophytum myriostigma presented higher diameter at eastern aspects (51.20 and 42.63 mm on zeolite/peat-moss and perlite/peat-moss, respectively), and the higher heights were also registered at eastern aspects, with Sclerocactus scheeri (7.38.cm on perlite/peat-moss and 6.98 cm on natural soil) and A. myriostigma (6.8 cm on zeolite/peat-moss and 6.08 cm on perlite/peat-moss). As for survival, the highest value (100%) was recorded with the treatments of West-Zeolite/peat-moss and East-Perlite/peat-moss, in six of the eight species evaluated (A. myriostigma, Acharagma roseana, Escobaria dasyacantha, S. scheeri and Mammillaria prolifera), while the lowest value (0%) was recorded with M. plumosa in the three western combinations. The best growth and survival responses were observed in A. myriostigma and S. scheeri under east exposure and zeolite, being the best combination, to be considered for their optimal establishment.
1. Introduction
Cactaceae are a very diverse family of plants in the arid and semi-arid zones of the Americas, with around 1900 species included in 125 genera [1], of which about 73% at genera and 78% at species levels present a high degree of endemism in Mexico [2]. It is a group of plants with particular biological and ecological characteristics [3], expressed in a great variety of shapes and colors, which have made them extremely attractive for collectors and in great demand in the ornamental plants market [4]. In addition, they are of cultural, food, industrial and medicinal importance [5]. These characteristics make them vulnerable to anthropogenic factors, since they present slow growth rates, with long life cycles [3,6]. Also, they are affected by biotic and abiotic factors: extreme temperatures, variable precipitations, low nutrient availability, competition, positive associations, recruitment of seedlings at sites safe for germination [7] and herbivore pressures [8]. So, many cacti have been lost due to their restricted distribution, destruction or fragmentation of their populations, and natural environments, as well as the excessive collection of specimens by enthusiast and cactophiles, who look for these extraordinary plants for their great commercial value [9]. Thus, anthropogenic threats and the ecological characteristics of cacti species altogether make this group of plants particularly vulnerable, such that all members of the family are included in Appendix II of the Convention of International Trade in Endangered Species (CITES), several in Appendix I of CITES, and many others in the Mexican endangered species list (Norma Official Mexicana NOM-059) and the Red List of the International Union for the Conservation of Nature [10]. A high global proportion of cacti species are threatened, and it is recognized that 1/3 of cactus species are under the risk of extinction [11].
Faced with this ever increasing threats to Cactus diversity, there arises the need to implement strategies in order to maintain this special group of plants. In this way, conservation biology is gaining relevance in a progressive and irreversible way because the society is increasingly aware of the importance of wild flora, which constitutes a key piece [12]. The main basis for the success of this conservation lies in knowledge about the biology and ecology of species, including factors affecting recruitment [13], and techniques for propagation are essential for successful restoration, introduction, or reintroduction [14].
For the Cactaceae, propagation by traditional routes has the disadvantage of low growth and low reproductive rates, which result in years to decades until their reintroduction, for which the ecological restoration programs are prolonged [15]. Efforts must involve the development and improvement of techniques that will guarantee reproductive success, ex-situ and in-situ conservation of cactus populations [16]. An alternative for cactus propagation is to develop new substrates with ideal characteristics for the optimal growth of seedlings. Natural zeolites meet these specific needs, due to their physical and chemical structure, which makes them particularly attractive [17]. Perlite with its high porosity, retains and maintains moisture, providing a water reservoir and adequate support for proper growth of the plants and healthier soil [18]. Additionally, it is true that hillside orientation influences environmental conditions, and is determinant in the development and structure of plant communities [19]. It would be good to elaborate on the conditions present with eastern versus western exposure. However, there is little evidence of its effect on the population structure of cacti [20]. Thus, the present study aims to determine the effect of hillside orientation in combination with experimental substrates in the pattern of growth and seedling survival of eight species of endemic Cactaceae in Northeastern Mexico, in order to facilitate their establishment and restore their natural populations, thus ensuring the conservation of their germplasm.
2. Materials and Methods
2.1. Study Area
The experimental field is located in the municipality of Linares, Nuevo León, and belongs to the campus of the Faculty of Forestry Sciences of the Universidad Autónoma de Nuevo León (UANL), within the coordinates 24°47″ north latitude and 99°32” west longitude. The site lies in a region of the plain, from 430 to 450 m of altitude in the piedmont of the Sierra Madre Oriental, Mexico [21]. The regional climate is defined as semi-arid and sub-humid [(A) C (Wo)] in the Köppen scheme modified by García [22], with two rainy seasons (June–August and September-) and a dry season between November and April. According to CONAGUA [23], the accumulated rainfall in the Mexican Republic during the year 2013 reached 921 mm, which was 21.2% higher than the long-term average of 760 mm from 1971 to 2000. Soil is of clayey textural type (from 42 to 66% of clays) and of scarce salinity (low electrical conductivity of 44 to 130 µS/cm), with pH close to neutrality (6.2–7.6), and tendencies to alkalinization from 50 cm depth in the proximity of the terrace with a high content of calcium carbonate (CaCO3) [24].
2.2. Biological Material
Seedlings were obtained from previous experiments on the viability and germination of Cacti species. Plants with homogeneous size and vigor were selected (3–5 replicates per experimental unit), as Table 1 shows.

Table 1.
Ecological protection status of the 8 species of Cactaceae under study.
2.3. Acclimatization and Field Transfer of the Species Studied
208 plants were selected and gradually acclimatized to the natural habitat conditions. The acclimatization process consisted of four stages: (1) For seedlings of 0–4 months in a bioclimatic chamber, manual spraying irrigation was applied maintaining humidity close to 100% in the germination and establishment phase; (2) For plants older than 4 months (in greenhouse), irrigation was weekly at field capacity; (3) At two years of age, the size recommended for reintroduction [27], the irrigation frequency was gradually reduced by up to two weeks for two months; (4) The plants were placed in the botanical garden of the Biological Sciences Faculty (UANL), to expose them to direct solar radiation for eight weeks prior to transferal to experimental field. Before the field transfer, the diameter and height of each plant were recorded.
In the experimental field, the plants were placed in pots with three types of substrates (perlite/peat-moss 1:1, zeolite/peat-moss 1:1 and natural soil), under two sun exposures (east and west), inside wooden cages of 1.5 × 0.6 m dimensions with metal mesh of opening 1 × 1 mm, to provide protection against birds predators. The plants were evaluated and irrigated for six months biweekly and monthly, recording height, diameter and mortality.
2.4. Experimental Design
The experimental design was Random Blocks with Factorial Arrangement, consisting of two factors: Factor A, sun exposure with two levels (west and east exposure). Factor B, substrate with three soil types (perlite/peat-moss, zeolite/peat-moss and natural soil). For each of the eight species, six treatments with 3 to 5 replications were evaluated depending on the availability of plants with a homogeneous size. In each sun exposure, there were two cages with about 50 pots of 10 × 10 cm inside (Figure 1).
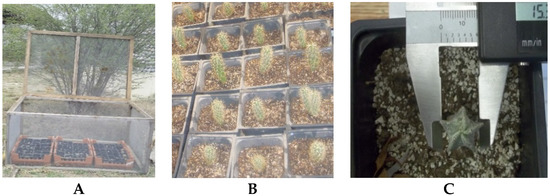
Figure 1.
Field transfer: (A) Plants in three substrate types inside cages; (B) Plants establishment; (C) Measurements.
2.5. Statistical Analysis
The data were processed with the statistical package Statgraphics ver. 7.0, using an analysis of variance to verify significant differences between growth (diameter, height) and survival variables, with a 95% confidence interval, according to Zar [28].
Since the survival results are percentage values, they were transformed with the sum of square function of p arcsine, where p is the proportion of the dependent variable [29]. Subsequently, normality tests were performed for each variable, using the Kolmogorov Smirnov test.
3. Results
3.1. Survival
Two of the six treatments resulted in total survival (100%) of five species out of the eight evaluated (Figure 2): west exposure–zeolite substrate (Acharagma roseana, Astrophytum myriostigma, Escobaria dasyacantha, Sclerocactus scheeri and Mammillaria prolifera), east exposure–perlite substrate (A. roseana, A. myriostigma, E. dasyacantha, M. prolifera and Turbinicarpus saueri).
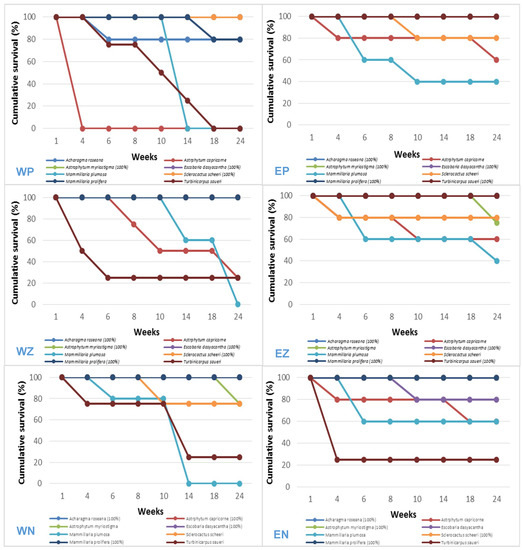
Figure 2.
Cacti survival curves during 24 weeks of evaluation in different treatments: W = West exposure, E = East exposure, P = Perlite, Z = Zeolite, NS = Natural soil.
The perlite substrate at a west exposure, as well as zeolite and natural substrate at an east exposure presented strong survival (80%) with the species A. roseana, M. prolifera, S. scheeri and E. dasyacantha. Moderate survival (60–75%) was observed at east exposure, with the species Astrophytum capricorne and A. myriostigma in the zeolite substrate and M. plumosa in the natural substrate. The low survival (25–40%) was obtained at a west exposure in zeolite, with the species A. capricorne and Turbinicarpus saueri; and at an east exposure in perlite/zeolite with M. plumosa, and an east exposure in natural substrate with M. plumosa and T. saueri.
The different treatments were not favorable for the establishment of M. plumosa, since their individuals survived at the east exposure with only 40% and 60% in perlite, zeolite, and natural substrate, respectively, and 0% at the west exposure, from the 14th week.
3.2. Diameter and Height of Cacti
The analysis of variance of diameter and height data showed highly significant differences (p < 0.01) among growth parameters and sun exposure, and significant differences (p < 0.05) with substrate (Table 2).

Table 2.
Analysis of variance for growth variables.
At the 24th week after reintroduction, the greatest diameter was recorded with A. myriostigma at the eastern sun exposure (51.20 and 42.63 mm, in zeolite/peat moss and perlite/peat moss, respectively), while the smallest value was recorded at the western exposure with T. saueri (8.45 mm in perlite/peat moss), as Figure 3 shows.
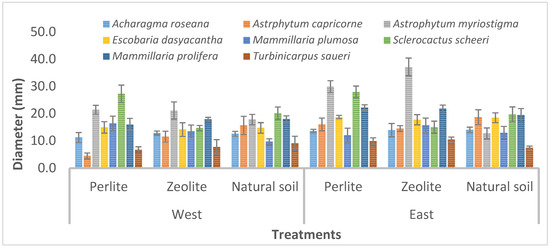
Figure 3.
Diameter of eight cacti species, at 24 weeks in different treatments.
For height, the highest values were recorded in the plants placed at the eastern exposure, with S. scheeri (7.38.cm on perlite/peat moss and 6.98 cm on natural soil) and A. myriostigma (6.8 cm with zeolite/peat-moss and 6.08 cm with perlite/peat-moss). The lowest values were recorded at the western exposure for M. plumosa (0.6 cm on perlite/peat moss, 0.63 cm on natural soil and 0.87 cm on zeolite/peat moss), as shown in Figure 4.
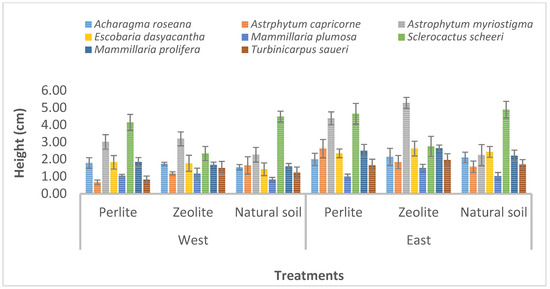
Figure 4.
Height of eight cacti species, at 24 weeks in different combinations of sun exposure with substrate type.
In general, the combination of an east exposure and zeolite substrate was the best for growth in both diameter and height parameters.
3.3. Relationship of Growth Parameters with the Combinations Substrate/Sun Exposure
The growth parameters (diameter and height) presented a similar tendency in all combinations, with a positive relationship (p < 0.01) (Table 3). However, the west exposure (Figure 5IA, IB, IC) showed greater dispersion than the east exposure (Figure 5IIA, IIB, IIC); therefore, the east favors the harmonized growth of both diameter and height. This was reflected in the types of models obtained, being moderate in the west exposure, with r2 values of 63.49, 64.26 and 69.45 for natural soil, zeolite and perlite substrates, respectively. The highest value of r2 was obtained in the east exposure with zeolite (r2 = 80.07), emphasizing a close relationship among the two parameters in these conditions. However, the lowest value was recorded in the east exposure, with the natural soil (r2 = 38.16).

Table 3.
Mathematical models and significance of treatments.
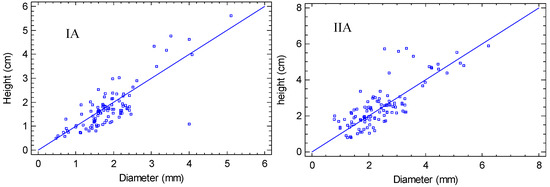
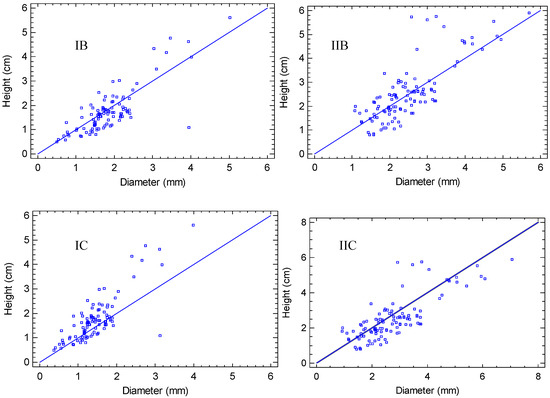
Figure 5.
Regressions of growth variables in function of treatments: I. West exposure, II. East exposure, A. Perlite/peat-moss substrate, B. Zeolite/peat-moss substrate, C. Natural soil substrate.
4. Discussion
4.1. Survival in the Field
The combination of sun exposure and soil type in the experimental treatments west–zeolite and east–perlite allowed the successful establishment of six of the eight evaluated species. In deserts, cactus seedlings (even species with high germination rates) rarely succeed in establishing themselves due to the unpredictable environmental conditions of high temperatures and low soil moisture content and nutrients [30,31], so that, significant losses are recorded when plants are used for reintroduction. The combinations tested in this study are useful to facilitate the establishment of a viable cacti population in the medium term.
Moreover, seedling mortality was less than 5%, similar to that reported by Meiado [16] for Melocactus sergipensis, after 5 months in the greenhouse, favoring seedlings to grow sufficiently to be ready for reintroduction programs.
The number of live plants at the end of the experimentation was higher in the west exposure, corroborating the research of Valiente and Ezcurra [32], who reported that the inclination of the sun is a factor that influences the establishment of Neobuxbumia tetetzo seedlings in the arid and semiarid zones in central-southern Mexico. It is well known that in the northern hemisphere, the sunshine throughout the year is greater on the southern exposure, particularly the southwest, giving it drier conditions. On the other hand, the northern exposure, especially the northeast, receives less solar radiation throughout the year and is consequently more humid. This is true for both the mountain slopes and the sides of a rock. Hence, the placement of small rocks of about 20 cm or more in height helps to maintain a little more humidity towards northeast side and keeps its temperatures a little lower under its shade than in the exposed areas, representing a better microenvironment for the cactus planted there [33]. The chances of survival are strongly favored by the conditions of sun exposure, in accordance with Castro et al. [34], who found that survival probabilities are strongly favored by shade conditions, regardless of soil type. However, these authors used seedlings of 4 months, and at that stage of development the roots still do not allow the adequate absorption of nutrients. For this reason, in the present study, it was decided to use plants 2 years of age, since the roots allow the taking of nutrients at this older age. Solar radiation is presented as the most important factor in the establishment of cactus seedlings, which survive better at an eastern exposure.
The zeolites used as fertilizers maintain a prolonged effect by the slow release of the macronutrients added to the porous structure, and the retention of water inside the pores [35]. Zeolites are crystal alumina silicates that have a negative charge, which is balanced by one or two positively charged valence cations [36]. Other properties of zeolites include their high absorption level, water retaining and releasing, high cation exchange capacity (CEC), and high buffering against pH change [37].
4.2. Growth in Natural Habitat
The Cactaceae established with zeolite reached the greater diameter and height, showing that this substrate provides a greater availability of nutrients and facilitates the absorption of water, favoring the growth of plants, supporting the observations of Manolov et al. [38] and López et al. [39].
The decrement and subsequent recuperation observed in diameter and height is due to the adaptation of this type of succulent plant, which has the capacity to change the moisture levels in their tissues under water stress conditions, such as when reintroducing the plants to the changing environmental conditions of their natural habitat [40].
Another aspect to consider is the reintroduction season. Cervera et al. [41] found that during the dry season, all Mammillaria gaumeri plants died, as their establishment is limited to years with higher rainfall seasons. The present investigation was carried out in an abundant rains season according to the CONAGUA [23], which likely facilitated the establishment of seedlings.
Predation is another factor that might influence the results obtained in a reintroduction study, and has been reported in several previous studies; for example, Ríos [42] mentioned that only 11% of the seeds available for dispersion of Neobuxbaumia macrocephala reached to ground due to predation by frugivorous birds and bats. However, in this study, the reintroduced seedlings were protected by the wooden cages and metal mesh, guaranteeing their establishment without influencing the predation factor.
The growth in height was remarkably higher for S. scheeri because it is a species with a cylindrical growth form, according to the classification proposed by Vázquez et al. [43] who described different forms of growth that Cactaceae may present. In some cases, growth form may be globose (if the stem is about the same height as diameter, for example: Mammillaria plumosa, Sclerocactus scheeri and Turbinicarpus saueri) or cylindrical (if it grows more in height than in diameter, but the height does not reach more than twice its diameter, for example: Acharagma roseana, Astrophytum capricorne, Astrophytum myriostigma, Escobaria dasyacantha and Mammillaria prolifera). In this way, the results obtained in the evaluation of growth are related to the form of each evaluated species. The lowest ranks were recorded for all species in the combination west exposure/natural soil, so that the natural soil in the west exposure is not indicated for the growth in height of the Cactaceae evaluated.
5. Conclusions
The eight Cacti species selected for this study were successfully established in Northeast Mexico, showing different growth responses depending on the sun exposure, the substrate type and especially on the species, since they have very particular biological form. Therefore, the soil conditions together with sun exposure have a significant influence on the relationship among the growth parameters diameter and height. The best responses in growth and survival were observed in A. myriostigma and S. scheeri under east exposure and zeolite substrate, which could be considered as the optimal establishment of the studied Cacti. These factors provide the conditions for a successful reintroduction of these species of Cactaceae, which can be applied in programs of ecological restoration and/or management and conservation of biodiversity, in order to preserve the integrity of the species of this important family.
Author Contributions
Conceptualization, S.C.L.R. and F.P.R.; methodology, S.C.L.R. and F.P.R.; validation, S.C.L.R., F.P.R. and D.J.L.; formal analysis, S.C.L.R., N.H.M. and F.P.R.; investigation, S.C.L.R.; resources, S.C.L.R., D.J.L. and F.P.R.; data curation, S.C.L.R.; writing—original draft preparation, S.C.L.R.; writing—review and editing, F.P.R., D.J.L. and N.H.M.; visualization, S.C.L.R. and N.H.M.; supervision, F.P.R. and D.J.L.; project administration, D.J.L. and F.P.R.; funding acquisition, D.J.L., S.C.L.R. and F.P.R.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT), Grant No. 377994.
Acknowledgments
Authors would like to thank Perla Galván for her valuable technical support in experimental monitoring.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Areces, A. Cactaceae. In Flowering Plants of the Neotropics; Smith, N., Mori, S.A., Henderson, A., Stevenson, D.W., Heald, S.V., Eds.; Princeton University Press: Princeton, NJ, USA, 2004; pp. 73–76. [Google Scholar]
- Durán, G.R.; Méndez, G.M.E. Cactáceas. In Biodiversidad y Desarrollo Humano en Yucatán; Durán, R., Méndez, M., Eds.; SEDUMA: Mérida, México, 2010; p. 496. [Google Scholar]
- Hernández, H.; Godínez, H. Contribución al conocimiento de las cactáceas mexicanas amenazadas. Act. Bot. Mex. 1994, 26, 33–52. [Google Scholar] [CrossRef]
- Sánchez, M.H. Some Prehispanic Uses of Cacti among the Indians of Mexico; Goberment [ie Government] of Mexico State, Secretaría de Desarrollo Agropecuario, Dirección de Recursos Naturales: México City, México, 1982.
- Alanís, G.; Velazco, C. Cactáceas de Nuevo León. Available online: https://www.naturalista.mx/guides/1424 (accessed on 02 June 2017).
- Godínez, A.H.; Valverde, T.; Ortega, B.P. Demographic trends in the Cactaceae. Bot. Rev. 2003, 69, 173–203. [Google Scholar] [CrossRef]
- Holland, J.N.; Molina, F.F. Hierarchical effects of rainfall, nurse plants, granivory and seed banks on cactus recruitment. J. Veg. Sci. 2013, 24, 1053–1061. [Google Scholar] [CrossRef]
- Mandujano, M.C.; Montaña, C.; Méndez, I.; Golubov, J. The relative contributions of sexual reproduction and propagation in Opuntia rastrera from two habitats in the Chihuahuan Desert. J. Ecol. 1998, 86, 911–921. [Google Scholar] [CrossRef]
- Becerra, R. Las cactáceas, plantas amenazadas por su belleza. Biodiversitas 2000, 32, 1–5. [Google Scholar]
- Carrillo-Angeles, I.G.; Suzán-Azpiri, H.; Mandujano, M.C.; Golubov, J.; Martínez-Ávalos, J.G. Niche breadth and the implications of climate change in the conservation of the genus Astrophytum (Cactaceae). J. Arid Environ. 2016, 124, 310–317. [Google Scholar] [CrossRef]
- Goettsch, B.; Hilton-Taylor, C.; Cruz-Piñón, G.; Duffy, J.P.; Frances, A.; Hernández, H.M.; Inger, R.; Pollock, C.; Schipper, J.; Superina, M.; et al. High proportion of cactus species threatened with extinction. Nat. Plants 2015, 1, 15142. [Google Scholar] [CrossRef] [PubMed]
- Quiala, E.; Montalvo, G.; Matos, J. Empleo de la Biotecnología vegetal para la propagación de cactáceas amenazadas. Biotecnol. Veg. 2004, 4, 195–199. [Google Scholar]
- Palmer, M.E. A critical look at rare plant monitoring in the United States. Biol. Conserv. 1987, 39, 113–127. [Google Scholar] [CrossRef]
- Iriondo, A.J.M. Conservación de Germoplasma de Especies raras y Amenazadas (Revisión). Available online: http://www.inia.es/gcontrec/pub/germoplasma_1161158274546.pdf (accessed on 19 January 2018).
- Pence, V. The Application of Biotechnology for the Conservation of Endangered Plants. In Plant Conservation Biotechnology; Benson, E., Ed.; Taylor & Francis: London, UK, 1999; pp. 227–250. [Google Scholar]
- Meiado, M.V. Seed germination of Melocactus sergipensis NP Taylor & MV Meiado, the newest Brazilian cactus destined for extinction. Plant Spec. Biol. 2016, 31, 296–299. [Google Scholar]
- Urbina, S.E.; Baca, C.G.; Núñez, E.R.; Colinas, L.M.; Tijerina, C.L.; Tirado, T.J. Cultivo hidropónico de plántulas de jitomate en zeolita cargada con K+, Ca2+ o Mg2+ y diferente granulometría. Agrociencia-Mexico 2006, 40, 419–429. [Google Scholar]
- Markoska, V.; Lisichkov, K.; Boev, B.; Gulaboski, R. The influence of the perlite as a substrate for improving on some water properties on the fluvial soil with an aplication of retentional curves. J. Agric. Plant Sciences 2018, 16, 73–82. [Google Scholar]
- Ortiz, P.R.; Rico, G.V. Seed dispersal of Bursera fagaroides (Burseraceae): The effect of linking environmental factors. Southwest Nat. 2006, 51, 11–21. [Google Scholar] [CrossRef]
- López, G.V.; Zedillo, A.P.; Anaya, H.S.; González, L.E.; Cano, S.Z. Efecto de la orientación de la ladera sobre la estructura poblacional y ecomorfología de Neobuxbaumia tetetzo (Cactaceae). Bot. Sci. 2012, 90, 453–457. [Google Scholar] [CrossRef]
- Foroughbakhch, R.; Alvarado-Vazquez, M.A.; Hernandez-Pinero, J.L.; Rocha-Estrada, A.; Guzman-Lucio, M.A.; Trevino-Garza, E.J. Establishment, growth and biomass production of 10 tree woody species introduced for reforestation and ecological restoration in northeastern Mexico. Forest Ecol. Manag. 2006, 235, 194–201. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Koppen Para Adaptarlo a las Condiciones de la República Mexicana. Available online: http://www.igeograf.unam.mx/sigg/utilidades/docs/pdfs/publicaciones/geo_siglo21/serie_lib/modific_al_sis.pdf (accessed on 20 April 2018).
- Comisión Nacional del Agua (CONAGUA). Estadísticas del Agua en México edición 2014. Available online: http://www.conagua.gob.mx/CONAGUA07/Publicaciones/Publicaciones/EAM2014.pdf (accessed on 20 April 2018).
- Llorente, S.M. Caracterización Física y Química de Vertisoles del Noreste de México Sometidos a Distintas Formas de Manejo. Master’s Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, México, December 2004. [Google Scholar]
- Guzmán, U.; Arias, S.; Dávila, P. Catálogo de Cactáceas Mexicanas. Available online: http://bioteca.biodiversidad.gob.mx/janium/Documentos/ETAPA01/PDF/3315/3315.pdf (accessed on 30 March 2018).
- Salas-Cruz, L.R. Aplicación de Zeolitas en la Propagación Aclimatación y Reintroducción de Cactáceas en dos Zonas ecológicas del Noreste de México. Ph.D. Thesis, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, México, September 2014. [Google Scholar]
- Reyes, S. Conservación y Restauración de Cactáceas y otras Plantas Suculentas Mexicanas, Manual Práctico; Comisión Nacional Forestal: Zapopan, Mexico, 2007.
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2010; p. 662. [Google Scholar]
- Schefler, W. Bioestadística; Fondo Educativo Interamericano: México City, México, 1981; p. 676. [Google Scholar]
- Mandujano, M.C.; Montaña, C.; Franco, M.; Golubov, J.; Flores-Martínez, A. Integration of demographic annual variability in a clonal desert cactus. Ecology 2001, 82, 344–359. [Google Scholar] [CrossRef]
- García, O.; Malda, G. Conservación in situ y ex situ de Mammilllaria mathildae, cactácea endémica en peligro de extinción de la ciudad de Querétaro. Ciencia@UAQ 2009, 2, 3–16. [Google Scholar]
- Valiente-Banuet, A.; Ezcurra, E. Shade as a cause of the association between the cactus Neobuxbaumia tetetzo and the nurse plant Mimosa luisana in the Tehuacan Valley, Mexico. J. Ecol. 1991, 79, 961–971. [Google Scholar] [CrossRef]
- Ramírez, C.A.; Rodríguez, T.D.A. Efecto de calidad de planta, exposición y micrositio en una plantación de Quercus rugosa. Rev Chapingo Ser Cie 2004, 10, 5–11. [Google Scholar]
- Castro, C.V.; Eyzaguirre, P.R.; Ceroni, S.A. Supervivencia de plántulas de Melocactus peruvianus Vaupel y Haageocereus pseudomelanostele subsp. aureispinus (Rauh y Backeberg) Ostolaza, en condiciones experimentales. Cerro Umarcata, Valle del Río Chillón, Lima. Ecol. Apl. 2006, 5, 61–66. [Google Scholar] [CrossRef]
- Bansiwal, A.; Rayalu, S.; Labhasetwar, N.; Juwarkar, A.; Devotta, S. Surfactant-modified zeolite as a slow release fertilizer for phosphorus. J. Agric. Food Chem. 2006, 54, 4773–4779. [Google Scholar] [CrossRef] [PubMed]
- Mumpton, F.A. La roca mágica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.R.; Ming, D. Recent progress in the use of natural zeolites in agronomy and horticulture. Nat. Zeolites 1995, 93, 477–490. [Google Scholar]
- Manolov, I.; Antonov, D.; Stoilov, G.; Tsareva, I.; Baev, M. Jordanian zeolitic tuff as a raw material for the preparation of substrates used for plant growth. J. Cent. Eur. Agric. 2005, 6, 485–494. [Google Scholar]
- López, M.; Hernández, M.; Barahona, C.; Martínez, M.; Portillo, R.; Rojas, F. Propiedades fisicoquímicas de la clinoptilolita tratada con fertilizantes a usar como aditivo en el cultivo de Pleurotus ostreatus. Terra Latinoam. 2010, 28, 247–254. [Google Scholar]
- Mauseth, D.J. Structure Function Relationships in Highly Modified Shoots of Cactaceae. Ann. Bot. 2006, 98, 901–926. [Google Scholar] [CrossRef] [PubMed]
- Cervera, J.C.; Andrade, J.L.; Sima, J.L.; Grahamy, E.A. Microhabitats, germination, and establishment for Mammillaria gaumeri (Cactaceae), a rare species from Yucatan. Int. J. Plant Sci. 2006, 167, 311–319. [Google Scholar] [CrossRef]
- Ríos, R.M.M. Limitaciones en el Reclutamiento de Neobuxbaumia Macrocephala: Un análisis de Las Interacciones a Través de su ciclo Reproductivo. Master’s Thesis, Universidad Nacional Autónoma de México, México City, México, January 2009. [Google Scholar]
- Vázquez, S.M.; Terrazas, T.; Arias, S. El hábito y la forma de crecimiento en la tribu Cacteae (Cactaceae, Cactoideae). Bot. Sci. 2012, 90, 97–108. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).